Abstract
Background
A comprehensive evaluation of the independent and combined associations of estimated glomerular filtration rate (eGFR) and albuminuria with mortality is required for assessment of the impact of kidney function on risk in the general population, with implications for improving the definition and staging of chronic kidney disease (CKD).
Methods
A collaborative meta-analysis of general population cohorts was undertaken to pool standardized data for all-cause and cardiovascular mortality. The two kidney measures and potential confounders from 14 studies (105,872 participants; 730,577 person-years) with urine albumin-to-creatinine ratio (ACR) measurements and seven studies (1,128,310 participants; 4,732,110 person-years) with urine protein dipstick measurements were modeled.
Findings
In ACR studies, mortality risk was unrelated to eGFR between 75-105 ml/min/1·73 m2 and increased at lower eGFR. Adjusted hazard ratios (HRs) for all-cause mortality at eGFR 60, 45, and 15 (versus 95) ml/min/1·73 m2 were 1·18 (95% CI: 1·05-1·32), 1·57 (1·39-1·78), and 3·14 (2·39-4·13), respectively. ACR was associated with mortality risk linearly on the log-log scale without threshold effects. Adjusted HRs for all-cause mortality at ACR 10, 30, and 300 (versus 5) mg/g were 1·20 (1·15-1·26), 1·63 (1·50-1·77), and 2·22 (1·97-2·51). eGFR and ACR were multiplicatively associated with mortality without evidence of interaction. Similar findings were observed for cardiovascular mortality and in dipstick studies.
Interpretation
Lower eGFR (<60 ml/min/1·73 m2) and higher albuminuria (ACR ≥10 mg/g) were independent predictors of mortality risk in the general population. This study provides quantitative data for using both kidney measures for risk evaluation and CKD definition and staging.
Introduction
Chronic kidney disease (CKD) is recognized as a major global public health problem.1, 2 CKD affects 10 to 16% of the adult population in Asia, Australia, Europe, and USA.3-6 and increases the risk of all-cause mortality and cardiovascular disease (CVD) as well as progression to kidney failure, even after accounting for traditional risk factors such as hypertension and diabetes mellitus.1, 7
The 2002 Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines2 define CKD as persistent kidney damage, usually marked by albuminuria, or reduced glomerular filtration rate (GFR), and assign CKD stages based on level of GFR. The KDOQI guidelines have encouraged research into the prognostic impact of CKD and have contributed to an increase in its awareness.8, 9 However, substantial controversy surrounds the use of GFR and albuminuria to define and stage CKD. Some have proposed a lower GFR threshold (e.g., GFR <45 vs. <60 ml/min/1·73 m2) or age- or sex-specific GFR thresholds to define CKD, higher urine albumin-to-creatinine ratio (ACR) as a marker of kidney damage (ACR ≥300 vs. ≥30 mg/g), combining stages with GFR ≥60 ml/min/1·73 m2, or incorporating albuminuria into all stages determined by GFR.2, 8-11
Numerous studies have reported the relationship of estimated GFR (eGFR) or albuminuria to clinical outcomes in the general population. However, most studies have investigated only one measure at a time, analyzed broad eGFR categories (e.g., < or ≥60 ml/min/1·73 m2), and have not evaluated age-specific associations. A few studies have dealt with some, but not all, of these issues.3, 12-16 Thus, a comprehensive examination adjusting for each kidney measure, testing for their interactions on risk, using fine categories and examining the association separately in younger and older individuals is needed.
The Kidney Disease: Improving Global Outcomes (KDIGO)17 has taken the initiative in establishing the CKD Prognosis Consortium. The Consortium is tasked with compiling and meta-analyzing the best available data to provide a more comprehensive evaluation of the independent and combined associations of eGFR and albuminuria with mortality and kidney outcomes, with implications for improving the definition and staging of CKD. The Consortium currently consists of 45 cohorts, which arise from either general, high-risk, or CKD populations. Separate reports will describe mortality and kidney outcomes by population type. This report describes the findings from a collaborative meta-analysis of the 21 general population cohorts for all-cause and cardiovascular mortality.
Methods
Search strategy and selection criteria
Electronic searches, not restricted to the English language, were conducted in PubMed for studies published between 1966 and July 2009 using the following combination of terms: (eGFR OR GFR OR glomerular filtration rate OR kidney function OR renal function) AND (albuminuria OR albumin to creatinine ratio OR ACR OR urinary albumin concentration OR UAC OR dipstick) AND (mortality OR ESRD OR end stage renal disease OR progressive chronic kidney disease OR acute kidney injury) AND (adult[MeSH]) AND (Humans[Mesh]). The search was performed independently by two investigators (PEdJ and RTG). Any study that was considered relevant based on its title was retrieved in abstract form, and if relevant, in full text. Disagreement over eligibility was resolved by discussion. We extended our search by reviewing references from articles that were obtained. Further studies and unpublished data were sought by discussion between collaborators, nephrologists, and CVD epidemiologists. This was enhanced by a call for participation through a published position statement of KDOQI and KDIGO17 and the KDIGO website (www.kdigo.org).
To be included in the meta-analysis for this manuscript, the study had to have at least 1,000 subjects selected from a general population, information at baseline on eGFR and urine albumin levels, and either of our two study outcomes, all-cause or cardiovascular mortality, with a minimum of 50 events. As recommended in clinical guidelines,2, 10 we preferred urine ACR as the measure of albuminuria. However, we also accepted urine albumin excretion as well as a qualitative measurement using dipstick, since a positive result is primarily due to increased albumin excretion and in some settings dipstick screening is widely used.2 Studies that selected participants based on CVD risk factors or CVD were excluded.
Study variables
GFR was estimated using the Modification of Diet in Renal Disease (MDRD) Study equation using age, sex, race, and serum creatinine concentration.18 CVD history was defined as a history of myocardial infarction, coronary revascularization, heart failure or stroke. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or use of antihypertensive medication. Hypercholesterolemia was defined as total cholesterol ≥5.0 mmol/L in case of positive history of CVD and as ≥6.0 mmol/L in case of negative history of CVD. Diabetes mellitus was defined as fasting glucose ≥7.0 mmol/L or non-fasting glucose ≥11.1 mmol/L or use of glucose lowering drugs or self-reported diabetes. Smoking was dichotomised as current versus former/non-smokers. Cardiovascular mortality was defined as death due to myocardial infarction, heart failure, sudden cardiac death, or stroke. We selected cardiovascular mortality as a specific cause of death since it is the leading cause of death in individuals with CKD.7
Statistical analysis
Investigators from each study analyzed their data following an a priori analytic plan using standard computer programs. All analyses were conducted using Stata version 10 or 11 (Stata Corp, College Station, Texas, USA), SAS version 9 (SAS Institute, Inc., Cary, North Carolina, USA), or R version 2.9.2 (R Foundation for Statistical Computing, Vienna, Austria). Cox proportional hazards models were used to estimate the hazard ratios (HRs) of all-cause and cardiovascular mortality associated with eGFR and albuminuria, adjusted for age, sex, race (blacks vs. non-blacks), history of CVD, systolic blood pressure (continuous), diabetes, serum total cholesterol concentration (continuous), and smoking. The distributed data analysis overview and analytic notes for individual studies are described in webappendix p 26.
We first evaluated the independent association of eGFR and albuminuria as continuous variables with mortality risk. We modeled eGFR and ACR using linear splines with knots at 45, 60, 75, 90, and 105 ml/min/1·73 m2 and 10, 30, and 300 mg/g (to convert to mg/mmol multiply by 0.113), respectively. eGFR 95 ml/min/1·73 m2 and ACR 5 mg/g were treated as reference points, respectively. Subgroup analyses divided participants into groups split at age 65 years. Interaction between eGFR and age was evaluated by likelihood-ratio tests in individual studies (see webappendix p 29 for statistical model details).
Subsequently, we investigated the joint association of eGFR and albuminuria with mortality risk in several analyses. We compared the risk in 32 categories of eGFR (<15, 15-29, 30-44, 45-59, 60-74, 75-89, 90-104, ≥105 ml/min/1·73 m2) and albuminuria (ACR: <10, 10-29, 30-299, ≥300 mg/g; dipstick: negative [−], trace [±], +, ≥++). The category with eGFR 90-104 ml/min/1·73 m2 and the lowest albuminuria was used as the reference group. The interaction between eGFR and albuminuria was tested by likelihood-ratio tests between the models incorporating spline eGFR and categorical albuminuria (ACR: <30, 30-299, and ≥300 mg/g; dipstick: −/±, +, and ≥++) with and without their interaction terms. Based on the model with the interaction terms, we estimated HRs at 23 points determined by the combination of eGFR (15, 45, 60, 75, 90, 95, 105, 120 ml/min/1·73 m2) and three categories of albuminuria compared to the reference of eGFR 95 ml/min/1·73 m2 plus ACR <30 mg/g or dipstick −/±.
Pooled estimates of the HR and 95% CI were obtained from a random effects meta-analysis. Heterogeneity was estimated using the χ2 test for heterogeneity and the I2 statistic.19 Meta-analyses were conducted separately for studies with ACR and dipstick measures. Since there were few participants (0.1%) with eGFR <15 ml/min/1·73 m2, we only reported results from participants with eGFR ≥15 ml/min/1·73 m2. We conducted meta-regression analysis with a random-effects model to explore sources of heterogeneity. In all analyses, a P-value of less than 0.05 was considered statistically significant.
Role of the funding sources
The KDIGO planning committee and NKF staff participated in study design and, data collection. The analytic team had full access to all the analyses conducted for each study and the writing committee had final responsibility for the decision to submit for publication informed by discussions with the collaborators.
Results
Study selection
We identified 767 potential articles from our search of published articles. Review of the title, abstract, or full text yielded 23 potentially eligible articles (figure 1). We found one study by citation tracing and eight large studies with unpublished, eligible data through contacts with experts. The investigators of these 32 studies were asked whether they would be willing to participate in the Consortium. Of these, 11 studies were excluded, because they did not meet the inclusion criteria or their investigators were not able to provide data, resulting in 21 cohorts for analysis.
Figure 1.
Flow chart for selection of articles.
Study characteristics
Of the 21 studies, nine were from North America, six from Europe, five from Asia, and one from Australia (table 1). The median follow-up time was 7.9 years (range 2.1-11.6) (table 1). Overall, 1,234,182 participants (ACR studies: 105,872 participants; dipstick studies: 1,128,310 participants) were followed up for 5,462,687 person-years (ACR: 730,577 person-years and dipstick: 4,732,110 person-years). The mean age for ACR studies had a median of 61 years (range 47-78); 62 years (range 42-81) for dipstick studies. The distribution of participants and outcomes according to the categories of eGFR and albuminuria is shown in the webappendix p 1-7. During follow-up, there were 45,584 deaths. Fifteen of the 21 cohorts reported data on 9,637 CVD deaths.
Table 1.
Characteristics of included studies
| Region | n | Mean Age (years) | Male (%) | Black (%) | Smoking (%) | CVD (%) | HC (%) | HT (%) | DM (%) | Mean eGFR (ml/min/1.73 m2) | Median ACR (mg/g) | Mean follow up (years) | deaths |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| total | CVD | ||||||||||||||
| ACR studies | |||||||||||||||
| ARIC | US | 11,408 | 63 | 44 | 22 | 15 | 9 | 35 | 48 | 17 | 83 | 3.7 | 8.0 | 1,235 | 443 |
| AusDiab* | Australia | 11,244 | 52 | 45 | 0 | 16 | 8 | 71 | 33 | 8 | 79 | 4.9 | 7.9 | 667 | 166 |
| Beijing | China | 1,563 | 60 | 50 | 0 | 24 | 18 | 29 | 47 | 28 | 85 | 2.8 | 3.9 | 59 | NA |
| CHS | US | 3,230 | 78 | 40 | 16 | 8 | 29 | 31 | 50 | 15 | 79 | 8.8 | 7.6 | 1,487 | 562 |
| COBRA | Pakistan | 2,872 | 52 | 48 | 0 | 39 | 9 | 35 | 44 | 21 | 110 | 5.2 | 4.1 | 212 | 95 |
| Framingham | US | 2,956 | 59 | 47 | 0 | 15 | 6 | 24 | 40 | 10 | 87 | 6.4 | 10.5 | 301 | 93 |
| Gubbio | Italy | 1,684 | 55 | 45 | 0 | 31 | 5 | 47 | 39 | 5 | 78 | 8.6 | 10.7 | 119 | NA |
| HUNT | Norway | 9,525 | 62 | 45 | 0 | 20 | 23 | 61 | 83 | 18 | 84 | 7.5 | 9.3 | 1,916 | 981 |
| MESA | US | 6,705 | 62 | 47 | 28 | 13 | 0 | 9 | 45 | 13 | 81 | 5.3 | 4.7 | 222 | NA |
| NHANES III | US | 15,853 | 47 | 47 | 27 | 25 | 11 | 62 | 40 | 7 | 103 | 6.2 | 8.5 | 2,119 | 910 |
| PREVEND | Netherlands | 8,370 | 49 | 50 | 1 | 38 | 5 | 36 | 33 | 3 | 81 | 7.0 | 7.2 | 438 | 132 |
| Rancho Bernardo | US | 1,759 | 71 | 39 | 0 | 7 | 11 | 29 | 56 | 12 | 77 | 11.6 | 10.4 | 587 | 233 |
| REGARDS | US | 27,583 | 65 | 45 | 42 | 15 | 23 | 59 | 60 | 22 | 85 | 7.5 | 4.0 | 1,380 | NA |
| ULSAM | Sweden | 1,120 | 71 | 100 | 0 | 20 | 42 | 56 | 75 | 11 | 75 | 7.6 | 11.6 | 467 | 208 |
| Dipstick studies | |||||||||||||||
| AKDN | Canada | 690,680 | 47 | 45 | NA | NA | 2 | NA | 20 | 6 | 81 | NA | 2.3 | 14,628 | NA |
| Beaver Dam | US | 4,926 | 62 | 44 | 0 | 20 | 15 | 54 | 51 | 10 | 76 | NA | 11.6 | 1,576 | 709 |
| ESTHER | Germany | 9,350 | 62 | 45 | 0 | 16 | 17 | 46 | 60 | 11 | 88 | NA | 2.1 | 171 | NA |
| MRC Older People | UK | 12,158 | 81 | 39 | 0 | 11 | 17 | NA | 73 | 8 | 59 | NA | 6.4 | 6,927 | 2,936 |
| Ohasama | Japan | 1,466 | 63 | 34 | 0 | 14 | 3 | 18 | 37 | 10 | 80 | NA | 10.5 | 201 | 61 |
| Severance | Korea | 42,637 | 46 | 51 | 0 | 31 | 5 | 12 | 25 | 6 | 84 | NA | 8.8 | 1,291 | 239 |
| Taiwan | Taiwan | 367,093 | 42 | 50 | 0 | 24 | 3 | 14 | 18 | 5 | 84 | NA | 7.1 | 9,581 | 1,869 |
Included also in dipstick analyses
CVD: cardiovascular disease, HC: hypercholesterolemia, HT: hypertension, DM: diabetes mellitus, ACR: urine albumin-to-creatinine ratio.
The study acronyms and abbreviations are listed in webappendix p 25.
Independent continuous associations of eGFR and albuminuria with mortality risk
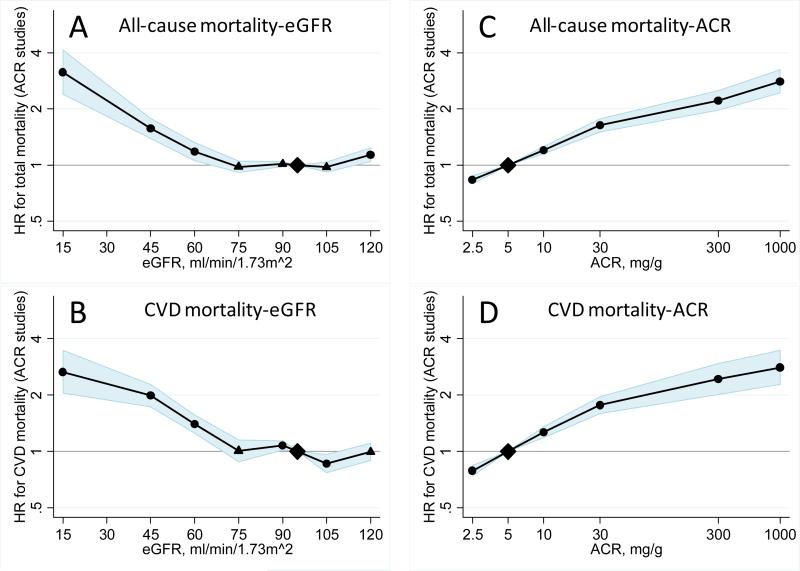
Pooled HRs of all-cause and cardiovascular mortality according to eGFR and ACR adjusted for each other and covariates in the 14 studies with ACR data are shown in figure 2. Mortality risk was relatively constant at eGFR 75-105 ml/min/1·73 m2 and was higher at lower eGFR. As compared to eGFR 95 ml/min/1·73 m2, HRs for all-cause mortality at eGFR 60, 45, and 15 ml/min/1·73 m2 were 1·18 (95% CI: 1·05-1·32), 1·57 (1·39-1·78), and 3·14 (2·39-4·13) for all-cause mortality. Similar findings were observed for cardiovascular mortality (figure 2, panel B). A U-shaped association with higher mortality risk at eGFR >105 ml/min/1·73 m2 was observed for all-cause mortality but was less evident for cardiovascular mortality. The U-shape was more pronounced in the dipstick studies than in the ACR studies, where the risk became statistically significant at eGFR <45 ml/min/1·73 m2 for all-cause mortality and at eGFR 60 for cardiovascular mortality (webappendix p 20).
Figure 2.
HRs and 95% CIs for all-cause and cardiovascular mortality according to spline eGFR and ACR adjusted for each other, age, gender, race, CVD history, systolic blood pressure, diabetes, smoking, and total cholesterol. The reference was eGFR 95 ml/min/1·73 m2 and ACR 5 mg/g (0·6 mg/mmol), respectively. Dots represent statistically significant and triangles represent not significant.
The relationships of ACR to the risks of all-cause and cardiovascular mortality were monotonic on the log-log scale. As compared to ACR 5 mg/g, HRs for all-cause mortality at ACRs of 10, 30, and 300 mg/g were 1·20 (95% CI: 1·15-1·26), 1·63 (1·50-1·77), and 2·22 (1·97-2·51) (figure 2, panel C). Similar findings were observed for cardiovascular mortality (figure 2, panel D).
The HRs for lower eGFR were seemingly higher in people aged <65 years compared to those older than 65 years, particularly for all-cause mortality at eGFR between 30 and 60 ml/min/1·73 m2 (webappendix p 21). For example, the HRs associated with eGFR of 45 compared with 95 ml/min/1·73 m2 in younger vs. older people were 2·14 (1·56-2·92) vs. 1·60 (1·46-1·75), respectively. However, the shapes of the associations of eGFR and all-cause and cardiovascular risk were largely similar across the two age populations (webappendix p 21). The tests for interaction between eGFR and age were not statistically significant in most studies (webappendix p 8). Similar findings were observed for dipstick studies (webappendix p 22). In contrast, HRs per eight-fold higher ACR (e.g., 40 vs. 5 mg/g) were quantitatively similar below vs. above age 65 years (1·49 [95% CI, 1·40-1·59] vs. 1·52 [1·45-1·61] for all-cause mortality and 1·65 [1·47-1·85] vs. 1·53 [1·40-1·67] for cardiovascular mortality).
Joint associations of eGFR and albuminuria with mortality risk
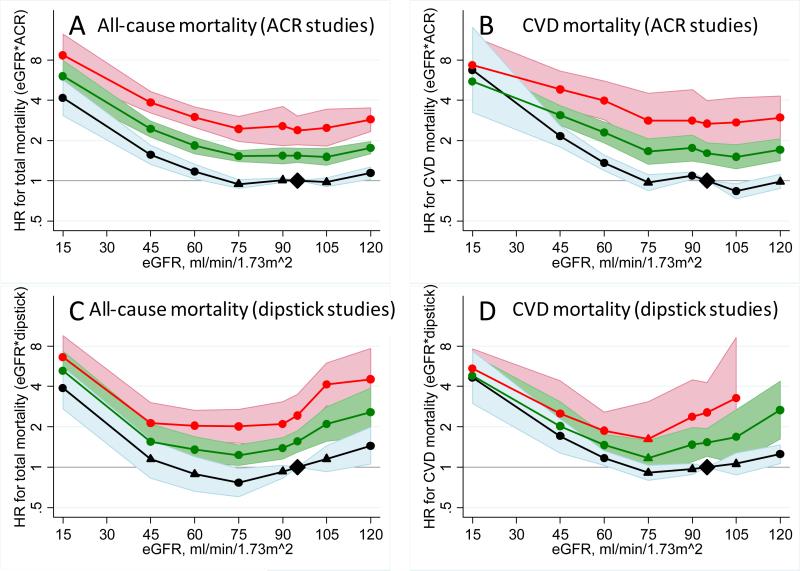
Pooled HRs for all-cause and cardiovascular mortality of the 28 categories of eGFR and albuminuria for ACR studies are shown in table 2. Lower eGFR showed a similar association with risk across all levels of ACR and vice versa, indicating multiplicative independent mortality risk factors (parallel lines in figure 3). Higher ACR categories were associated with more than two-fold mortality risk within all eGFR categories but the lowest (table 3). Dipstick ± compared to negative was significantly associated with increased risk for all-cause and cardiovascular mortality at eGFR 90-104 ml/min/1·73 m2 with similar association at other eGFR levels. Although HRs tended to be higher in those younger vs. older than age 65 years (particularly for all-cause mortality), eGFR and albuminuria were multiplicatively associated with mortality risk below and above age 65 y (webappendix p 10-13).
Table 2.
Pooled estimates of adjusted HRs (95% CI) for all-cause and cardiovascular mortality according to categories of eGFR and ACR
| ACR (mg/g) |
||||
|---|---|---|---|---|
| eGFR (ml/min/1.73 m2) | <10 | 10-29 | 30-299 | ≥300 |
| all-cause mortality | ||||
| ≥105 | 1.14 (1.02 - 1.27) | 1.52 (1.28 - 1.81) | 2.32 (2.00 - 2.70) | 5.26 (2.80 - 9.85) |
| 90-104 | reference | 1.48 (1.29 - 1.69) | 1.61 (1.39 - 1.87) | 3.65 (2.13 - 6.27) |
| 75-89 | 1.00 (0.91 - 1.09) | 1.40 (1.26 - 1.55) | 1.78 (1.58 - 2.01) | 2.50 (1.89 - 3.31) |
| 60-74 | 1.02 (0.92 - 1.15) | 1.49 (1.34 - 1.66) | 1.95 (1.67 - 2.27) | 3.09 (2.56 - 3.72) |
| 45-59 | 1.28 (1.05 - 1.57) | 1.95 (1.73 - 2.20) | 2.51 (2.16 - 2.90) | 4.10 (3.39 - 4.95) |
| 30-44 | 1.97 (1.59 - 2.43) | 2.65 (2.19 - 3.22) | 3.66 (2.91 - 4.60) | 5.08 (4.20 - 6.15) |
| 15-29 | 5.39 (3.30 - 8.80) | 3.66 (2.43 - 5.50) | 4.85 (3.26 - 7.21) | 6.96 (5.28 - 9.19) |
| cardiovascular mortality | ||||
| ≥105 | 0.93 (0.74 - 1.16) | 1.33 (1.04 - 1.72) | 2.46 (1.88 - 3.23) | 2.69 (1.36 - 5.32) |
| 90-104 | reference | 1.63 (1.20 - 2.19) | 1.82 (1.36 - 2.45) | 4.77 (3.16 - 7.22) |
| 75-89 | 1.03 (0.85 - 1.24) | 1.48 (1.23 - 1.78) | 1.73 (1.29 - 2.32) | 4.01 (2.62 - 6.14) |
| 60-74 | 1.09 (0.92 - 1.29) | 1.58 (1.31 - 1.91) | 2.18 (1.58 - 3.02) | 4.23 (2.95 - 6.06) |
| 45-59 | 1.52 (1.18 - 1.97) | 2.38 (1.91 - 2.96) | 3.13 (2.32 - 4.22) | 4.97 (3.70 - 6.66) |
| 30-44 | 2.40 (1.80 - 3.21) | 3.07 (1.73 - 5.44) | 4.12 (2.84 - 5.98) | 6.10 (4.08 - 9.10) |
| 15-29 | 13.51 (4.89 - 37.35) | 7.99 (1.95 - 32.81) | 5.60 (3.66 - 8.57) | 9.49 (4.97 - 18.10) |
Adjusted for age, race, gender, CVD history, systolic blood pressure, diabetes, smoking, total cholesterol.
Abbreviations: eGFR: estimated glomerular filtration rate; ACR: urine albumin-to-creatinine ratio; CVD: cardiovascular disease.
Figure 3.
HRs and 95% CIs for all-cause and cardiovascular mortality according to spline eGFR and categorical albuminuria (ACR: <30 [black], 30-299 [green], and ≥300 [red] mg/g; dipstick: −/± [black], + [green], and ≥++ [red]) with their interaction terms adjusted for age, gender, race, CVD history, systolic blood pressure, diabetes, smoking, and total cholesterol. The reference was eGFR 95 ml/min/1·73 m2 plus ACR <30 mg/g or dipstick −/±. Dots represent statistically significant and triangles represent not significant. The estimated HR and 95% CI at eGFR 120 with dipstick ≥++ for CVD mortality were omitted, since only two studies contributed to reliable estimation. To convert ACR in mg/g to mg/mmol multiply 0.113.
Table 3.
Pooled estimates of adjusted HRs (95% CI) for all-cause and cardiovascular mortality according to categories of eGFR and dipstick urinalysis for proteinuria
| Dipstick |
||||
|---|---|---|---|---|
| eGFR (ml/min/1.73 m2) | − | ± | + | ≥++ |
| all-cause mortality | ||||
| ≥105 | 1.35 (0.94 - 1.92) | 2.34 (1.17 - 4.69) | 2.59 (1.32 - 5.07) | 4.40 (2.79 - 6.93) |
| 90-104 | reference | 1.47 (1.09 - 2.00) | 1.88 (1.27 - 2.77) | 2.44 (1.53 - 3.89) |
| 75-89 | 0.81 (0.65 - 1.01) | 1.44 (1.31 - 1.57) | 1.69 (1.50 - 1.89) | 2.43 (1.94 - 3.04) |
| 60-74 | 0.81 (0.59 - 1.10) | 1.27 (1.17 - 1.37) | 1.53 (1.32 - 1.78) | 1.88 (1.56 - 2.25) |
| 45-59 | 0.94 (0.66 - 1.32) | 1.38 (1.06 - 1.78) | 1.71 (1.52 - 1.92) | 2.41 (1.77 - 3.29) |
| 30-44 | 1.47 (0.97 - 2.22) | 1.94 (1.26 - 2.98) | 2.35 (1.66 - 3.33) | 2.86 (1.88 - 4.36) |
| 15-29 | 2.30 (1.61 - 3.29) | 4.37 (2.29 - 8.36) | 3.21 (2.00 - 5.13) | 5.98 (3.59 - 9.97) |
| cardiovascular mortality | ||||
| ≥105 | 0.96 (0.72 - 1.29) | 2.13 (1.22 - 3.74) | 3.27 (1.50 - 7.09) | 3.70 (1.17 - 11.68) |
| 90-104 | reference | 1.88 (1.32 - 2.69) | 1.68 (0.82 - 3.43) | 3.63 (1.58 - 8.32) |
| 75-89 | 0.82 (0.69 - 0.97) | 1.78 (1.33 - 2.37) | 2.08 (1.40 - 3.09) | 2.38 (1.40 - 4.04) |
| 60-74 | 0.99 (0.84 - 1.17) | 1.43 (1.13 - 1.82) | 1.84 (1.15 - 2.95) | 2.01 (1.26 - 3.19) |
| 45-59 | 1.38 (1.16 - 1.65) | 1.89 (1.47 - 2.44) | 2.40 (1.73 - 3.33) | 2.67 (1.58 - 4.50) |
| 30-44 | 2.42 (1.92 - 3.05) | 2.99 (2.13 - 4.20) | 2.73 (1.83 - 4.08) | 3.06 (2.00 - 4.70) |
| 15-29 | 3.29 (1.72 - 6.31) | 4.25 (2.28 - 7.93) | 7.66 (1.24 - 47.22) | 5.72 (1.66 - 19.73) |
Adjusted for age, race, gender, CVD history, systolic blood pressure, diabetes, smoking, total cholesterol.
Abbreviations: eGFR: estimated glomerular filtration rate; CVD: cardiovascular disease.
While general population cohorts are not optimal for studying severely reduced GFR, the mortality risk associated with elevated albuminuria appeared somewhat weaker among people with eGFR <30 ml/min/1·73 m2 (table 2 and 3). However, this difference was very subtle for all-cause mortality and 95% CIs were wide for cardiovascular mortality (figure 3). Indeed, the majority of the studies did not report statistically significant interaction between eGFR and ACR (webappendix p 9).
Source of heterogeneity
The results from individual cohorts are shown in webappendix (p 14-19 and 23). We found moderate but statistically significant heterogeneity in the HRs of eGFR in the ACR studies in the range of eGFR ≤60 ml/min/1·73 m2 only for all-cause mortality (I2=35%-56%). In the dipstick studies, we found large heterogeneity for all-cause mortality across the entire eGFR range (I2 ≥92%). We observed mild heterogeneity in the HRs of ACR in the entire range tested for all-cause mortality (I2=33%-40%).
We conducted meta-regression analysis for the ACR studies using variables listed in table 1 to seek potential sources of heterogeneity at eGFR 45 (vs. 95 ml/min/1·73 m2) and ACR 30 (vs. 5 mg/g) for all-cause mortality. These two levels were selected due to their clinical relevance. Only the proportion of blacks was borderline significant (p=0.064) and positively associated with variation of log-HR at eGFR 45 ml/min/1·73 m2 (webappendix, p 24). For ACR 30 mg/g, none of the variables tested was significantly associated with log-HR for all-cause mortality across studies (data not shown).
Discussion
The current meta-analyses based on more than one hundred thousand individuals with ACR data and 1.1 million participants with dipstick data from 21 general population cohorts demonstrated that eGFR and albuminuria were associated with all-cause and cardiovascular mortality independently of each other and traditional cardiovascular risk factors. The consistency in both continuous and categorical models for eGFR and ACR demonstrates that our findings are robust. With 21 studies from 14 countries of Asia, Europe, North America, and Oceania, our study shows the range of association as well as summary estimates across the globe.
We observed an exponential increase in mortality risk at low eGFR. The risk became statistically significant around eGFR 60 ml/min/1·73 m2 and was 2-fold higher around eGFR 30-45 as compared to optimal eGFR levels independently of albuminuria and potential confounders – although there was some variation across outcomes, models, and age groups tested. These findings argue against the concept that mild-to-moderate reduction in eGFR is not associated with adverse clinical consequences.11
Mortality risk was relatively constant at eGFR 75-105 ml/min/1·73 m2 and, as previously reported,20, 21 was higher at eGFR >105 ml/min/1·73 m2 especially for all-cause mortality. However, these findings should be interpreted with caution. The MDRD Study equation is known to underestimate measured GFR at the range of GFR ≥60 ml/min/1.73m2 in healthy individuals22 and overestimate measured GFR in individuals with reduced muscle mass due to ill health, the latter potentially contributing to the U-shaped association of GFR with mortality. The fact that such a U-shaped curve was less apparent for cardiovascular mortality supports this explanation for the U-shaped association, as does the more linear association in studies where GFR was estimated from cystatin C.20, 23 Thus, further studies using a more accurate creatinine-based equation24 or equations using other biomarkers such as cystatin C25 may provide better estimates for clinical risk at GFR ≥60 ml/min/1.73m2.
The relationship of albuminuria to mortality was linear on the log-log scale, with 2-fold higher risk at ACR of approximately 100 mg/g (within the range usually described as microalbuminuria [30-299 mg/g])2 as compared to an optimal ACR level (5 mg/g) independently of eGFR and conventional risk factors. Of note, the risk was statistically significant at an ACR of 10 mg/g compared to 5 mg/g. Furthermore, mortality gradient conferred by higher albuminuria within all but the lowest eGFR category was more than two-fold. This is greater than the risk between adjacent CKD stages based on eGFR suggesting that albuminuria provides additional prognostic information beyond eGFR.
Clinical and laboratory guidelines recommend ACR as the preferred measure of albuminuria for the definition and staging of CKD.2, 10, 26 The urine dipstick is often used for initial screening because it is less expensive and can be performed at the point of care.2, 27 Our findings demonstrated that even a trace (±) urine protein on dipstick was associated with an increase in mortality risk, consistent with the fact that 60% of individuals with trace on dipstick have microalbuminuria.28 These findings suggest that dipstick is useful for risk stratification despite being a less precise measure of albuminuria.
The HRs for lower eGFR with all-cause and cardiovascular mortality tended to be higher in younger than older populations. Similar findings have been reported for traditional cardiovascular risk factors.29 However, the shape of the eGFR-mortality associations was largely similar below and above age 65 years. Additionally, tests for interaction for eGFR and age in the majority of studies were not statistically significant. The HRs for higher ACR were quantitatively similar in people younger and older than 65 years.
We found significant heterogeneity for the association between lower eGFR and all-cause mortality, but not for cardiovascular mortality. Studies with higher proportion of blacks tended to have higher HR for all-cause mortality. While not statistically significant, this is consistent with previous studies demonstrating that lower eGFR is more strongly associated with adverse outcomes in blacks as compared with whites.30 For ACR, none of baseline variables tested were significantly associated with the variation of mortality risk. While the reasons for statistically significant quantitative heterogeneity will need further study, the patterns of risk association were qualitatively similar across a wide range of studies (webappendix p. 23).
Altogether the findings in this analysis are consistent with the current KDOQI thresholds for eGFR <60 ml/min/1·73 m2 and ACR ≥30 mg/g as indicative of increased mortality risk. They also suggest including albuminuria stages in CKD staging independent of GFR stages. Nevertheless, the final decisions regarding which levels of GFR and albuminuria are used for the definition and staging of CKD should incorporate a wide range of considerations including prevalence, incidence of targeted outcomes, risk classification, and the cost-effectiveness associated with management directed at each kidney measure.31-33 This is particularly the case for ACR, since we observed a continuous association with mortality.
Although this is the most comprehensive assessment of the relation between eGFR, albuminuria and mortality yet performed, some limitations of the present study should be discussed. The distributed data analysis consortium we used provides superior uniformity than a review of the literature but falls short of having a uniform study protocol and centralized laboratories across all cohorts. Measurements of creatinine and albuminuria were not standardized in all studies. Some studies measured creatinine and albuminuria in fresh samples and others used frozen samples. Analyses required complete data on both eGFR and albuminuria. We could only test interactions between eGFR, albuminuria, and age in each cohort. Most of the participants recorded as blacks were from studies in the USA. Although some of the participating studies confirmed the proportional hazards assumption for mortality with eGFR and/or albuminuria as predictors,3, 30, 34, 35 we cannot confirm the validity of this assumption for every study. Thus, the pooled HRs we obtain estimate the average hazard ratio over follow-up time.
In conclusion, eGFR <60 ml/min/1·73 m2 and ACR ≥10 mg/g were significantly associated with an increased risk of all-cause and cardiovascular mortality independently of each other and traditional risk factors. These findings provide a quantitative basis for including these two kidney measures in risk evaluation in the general population and CKD definition and staging.
Supplementary Material
Acknowledgments
The CKD Prognosis Consortium is supported by Kidney Disease: Improving Global Outcomes (KDIGO) and the US National Kidney Foundation. The meta-analyses work conducted jointly at Johns Hopkins School of Public Health, Baltimore, USA and University Medical Center Groningen, Groningen, The Netherlands, was supported by the US National Kidney Foundation and the Dutch Kidney Foundation, respectively. KDIGO helped support the 2009 meeting of collaborators.
Footnotes
Contributors
All members of the writing committee contributed to the collection and analysis of the data, and to the preparation of the report. All collaborators were sent the paper as prepared for submission and given the opportunity to comment on the draft manuscript. The writing committee accepts full responsibility for the content of this paper.
CKD Prognosis Consortium
Writing Committee: Kunihiro Matsushita, Marije van der Velde, Brad C. Astor, Mark Woodward, Andrew S. Levey, Paul E. de Jong, Josef Coresh, Ron T. Gansevoort.
KDIGO Controversies Conference Planning Committee: Andrew S. Levey, Meguid El-Nahas, Paul E. de Jong, Josef Coresh, Kai-Uwe Eckardt, Bertram L. Kasiske.
CKD Prognosis Consortium investigators/collaborators (The study acronyms/abbreviations are listed in webappendix p 25): AKDN: Marcello Tonelli, Brenda Hemmelgarn; ARIC: Josef Coresh, Brad C. Astor, Kunihiro Matsushita, Yaping Wang; AusDiab: Robert C. Atkins, Kevan R. Polkinghorne, Steven J. Chadban; Beaver Dam: Anoop Shankar, Ronald Klein, Barbara EK Klein; Beijing: HaiYan Wang, Fang Wang, Luxia Zhang, Lisheng Liu; CHS: Michael Shlipak, Mark J. Sarnak, Ronit Katz, Linda P. Fried; COBRA: Tazeen Jafar, Muhammad Islam, Juanita Hatcher, Neil Poulter, Nish Chaturvedi; ESTHER: Dietrich Rothenbacher, Hermann Brenner, Elke Raum, Wolfgang Koenig; Framingham: Caroline S. Fox, Shih-Jen Hwang, James B. Meigs; Gubbio: Massimo Cirillo; HUNT: Stein Hallan, Stian Lydersen, Jostein Holmen; MESA: Michael Shlipak, Mark J. Sarnak, Ronit Katz, Linda P. Fried; MRC Older People: Paul Roderick, Dorothea Nitsch, Astrid Fletcher, Christopher Bulpitt; NHANES III: Brad Astor, Josef Coresh; Ohasama: Takayoshi Ohkubo, Hirohito Metoki, Masaaki Nakayama, Masahiro Kikuya, Yutaka Imai; PREVEND: Ron T. Gansevoort, Paul E. de Jong, Marije van der Velde; Rancho Bernardo: Simerjot Kaur Jassal, Elizabeth Barrett-Connor, Jaclyn Bergstrom; REGARDS: David G. Warnock, Paul Muntner, Suzanne Judd, William M. McClellan, Mary Cushman, George Howard, Leslie McClure; Severance: Sun Ha Jee, Heejin Kimm, Ji Eun Yun; Taiwan: Chi-Pang Wen, Sung-Feng Wen, Chwen-Keng Tsao, Min-Kuang Tsai; ULSAM: Johan Ärnlöv.
CKD Prognosis Consortium Analytic Team: Brad C. Astor, Priscilla Auguste, Josef Coresh, Ron T. Gansevoort, Paul E de Jong, Kunihiro Matsushita, Marije van der Velde, Kasper Veldhuis, Yaping Wang, Mark Woodward.
CKD Prognosis Consortium Administration Staff: Laura Camarata, Beverly Thomas.
National Kidney Foundation Staff: Tom Manley.
Conflict of interest statement
The members of the Writing Committee declare that they have no conflict of interests. A variety of sources have supported the cohorts contributing to the CKD Prognosis Consortium and are described in its publications.
Contributor Information
Kunihiro Matsushita, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Marije van der Velde, Department of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Brad C Astor, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Mark Woodward, George Institute, University of Sydney, Sydney, Australia.
Andrew S Levey, Division of Nephrology, Tufts Medical Center, Boston, MA, USA.
Paul E de Jong, Department of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Josef Coresh, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Ron T Gansevoort, Department of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands..
References
- 1.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–59. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 3.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–82. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 4.Chadban SJ, Briganti EM, Kerr PG, Dunstan DW, Welborn TA, Zimmet PZ, et al. Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J Am Soc Nephrol. 2003;14:S131–8. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- 5.Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17:2275–84. doi: 10.1681/ASN.2005121273. [DOI] [PubMed] [Google Scholar]
- 6.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 7.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 8.Bauer C, Melamed ML, Hostetter TH. Staging of chronic kidney disease: time for a course correction. J Am Soc Nephrol. 2008;19:844–6. doi: 10.1681/ASN.2008010110. [DOI] [PubMed] [Google Scholar]
- 9.Gansevoort RT, de Jong PE. The case for using albuminuria in staging chronic kidney disease. J Am Soc Nephrol. 2009;20:465–8. doi: 10.1681/ASN.2008111212. [DOI] [PubMed] [Google Scholar]
- 10.Crowe E, Halpin D, Stevens P, on behalf of the Guideline Development G Early identification and management of chronic kidney disease: summary of NICE guidance. BMJ. 2008;337:a1530. doi: 10.1136/bmj.a1530. [DOI] [PubMed] [Google Scholar]
- 11.Winearls CG, Glassock RJ. Dissecting and refining the staging of chronic kidney disease. Kidney Int. 2009;75:1009–14. doi: 10.1038/ki.2009.49. [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 13.O'Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758–65. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 14.Hallan S, Astor B, Romundstad S, Aasarod K, Kvenild K, Coresh J. Association of Kidney Function and Albuminuria With Cardiovascular Mortality in Older vs Younger Individuals: The HUNT II Study. Arch Intern Med. 2007;167:2490–6. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 15.Astor BC, Hallan SI, Miller ER, 3rd, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167:1226–34. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 16.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–9. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 17.Eckardt KU, Berns JS, Rocco MV, Kasiske BL. Definition and classification of CKD: the debate should be about patient prognosis--a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53:915–20. doi: 10.1053/j.ajkd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 19.Woodward M. Epidemiology: study design and data analysis. 2nd ed. Chapman & Hall/CRC; Boca Raton: 2005. [Google Scholar]
- 20.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–60. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD-EPI equation as compared to the MDRD Study equation for estimated glomerular filtration rate: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010;55:648–59. doi: 10.1053/j.ajkd.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–57. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 23.Reese PP, Feldman HI. More evidence that cystatin C predicts mortality better than creatinine. J Am Soc Nephrol. 2009;20:2088–90. doi: 10.1681/ASN.2009080832. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, McQueen MJ, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55:24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
- 27.Robert DT. Microalbuminuria: Definition, Detection, and Clinical Significance. The Journal of Clinical Hypertension. 2004;6:2–7. doi: 10.1111/j.1524-6175.2004.4064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jong PE, van der Velde M, Gansevoort RT, Zoccali C. Screening for chronic kidney disease: where does Europe go? Clin J Am Soc Nephrol. 2008;3:616–23. doi: 10.2215/CJN.04381007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krumholz HM, Seeman TE, Merrill SS, de Leon CFM, Vaccarino V, Silverman DI, et al. Lack of Association Between Cholesterol and Coronary Heart Disease Mortality and Morbidity and All-Cause Mortality in Persons Older Than 70 Years. JAMA. 1994;272:1335–40. [PubMed] [Google Scholar]
- 30.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–15. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 31.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 32.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–90. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 33.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up Report on the Diagnosis of Diabetes Mellitus. Diabetes Care. 2003;26:3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 34.White SL, Polkinghorne KR, Atkins RC, Chadban SJ. Comparison of the Prevalence and Mortality Risk of CKD in Australia Using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR Estimating Equations: The AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis. 2010;55:660–70. doi: 10.1053/j.ajkd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Soveri I, Arnlov J, Berglund L, Lind L, Fellstrom B, Sundstrom J. Kidney function and discrimination of cardiovascular risk in middle-aged men. J Intern Med. 2009;266:406–13. doi: 10.1111/j.1365-2796.2009.02122.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.