Abstract
BACKGROUND
Adding the measurement of cystatin C to that of serum creatinine to determine the estimated glomerular filtration rate (eGFR) improves accuracy, but the effect on detection, staging, and risk classification of chronic kidney disease across diverse populations has not been determined.
METHODS
We performed a meta-analysis of 11 general-population studies (with 90,750 participants) and 5 studies of cohorts with chronic kidney disease (2960 participants) for whom standardized measurements of serum creatinine and cystatin C were available. We compared the association of the eGFR, as calculated by the measurement of creatinine or cystatin C alone or in combination with creatinine, with the rates of death (13,202 deaths in 15 cohorts), death from cardiovascular causes (3471 in 12 cohorts), and end-stage renal disease (1654 cases in 7 cohorts) and assessed improvement in reclassification with the use of cystatin C.
RESULTS
In the general-population cohorts, the prevalence of an eGFR of less than 60 ml per minute per 1.73 m2 of body-surface area was higher with the cystatin C–based eGFR than with the creatinine-based eGFR (13.7% vs. 9.7%). Across all eGFR categories, the reclassification of the eGFR to a higher value with the measurement of cystatin C, as compared with creatinine, was associated with a reduced risk of all three study outcomes, and reclassification to a lower eGFR was associated with an increased risk. The net reclassification improvement with the measurement of cystatin C, as compared with creatinine, was 0.23 (95% confidence interval [CI], 0.18 to 0.28) for death and 0.10 (95% CI, 0.00 to 0.21) for end-stage renal disease. Results were generally similar for the five cohorts with chronic kidney disease and when both creatinine and cystatin C were used to calculate the eGFR.
CONCLUSIONS
The use of cystatin C alone or in combination with creatinine strengthens the association between the eGFR and the risks of death and end-stage renal disease across diverse populations. (Funded by the National Kidney Foundation and others.)
The estimated glomerular filtration rate (eGFR) is the clinical standard for the assessment of kidney function.1–3 The eGFR thresholds for the definition and staging of chronic kidney disease are based on risk,3 but measurement of creatinine to determine the eGFR has limitations in risk prediction, particularly in patients with reduced muscle mass.4 Cystatin C has received much attention as an alternative filtration marker with stronger and more linear risk relationships than creatinine.5–7 Several studies have suggested that the addition of cystatin C measurements to creatinine measurements in calculating the eGFR significantly improves the risk classification for death, cardiovascular disease, and end-stage renal disease.8–10
However, existing evidence has been limited by the lack of a reference standard for cystatin C to calibrate the measures across studies and by the absence of cystatin C–based equations that are derived from a broad population with a wide range of kidney function. The development of an international reference standard for cystatin C and the publication of improved GFR-estimating equations by the Chronic Kidney Disease Epidemiology Collaboration11 prompted the current meta-analysis of 16 studies across a diverse population of participants. The objectives of this study, which was conducted by the Chronic Kidney Disease Prognosis Consortium (CKD-PC), were to determine whether the addition of an eGFR that was calculated with the use of the recently developed cystatin C equations would strengthen the relationships between various eGFR categories and adjusted risks of death from any cause, death from cardiovascular causes, and end-stage renal disease, as compared with the use of creatinine-based eGFR.
METHODS
STUDY DESIGN AND PARTICIPANTS
Detailed descriptions of the CKD-PC have been reported previously.12–15 To be eligible for inclusion in the meta-analysis, studies had to include at least 1000 participants (with the exception of studies that enrolled only patients with chronic kidney disease)14 for whom data on baseline serum creatinine and albuminuria were available. In addition, studies had to include reports of at least 50 events for any outcome of interest. This meta-analysis included 11 general-population studies (with 90,750 participants) and 5 studies of patients with chronic kidney disease (with 2960 participants) for whom measurements of serum cystatin C were available.
We calculated the eGFR using the latest equations from the Chronic Kidney Disease Epidemiology Collaboration, with measurement of serum creatinine, cystatin C, or both creatinine and cystatin C (combination).11,16–18 All three eGFR equations incorporated kidney-filtration markers (serum creatinine or cystatin C) as well as age, sex, and race (black vs. nonblack), except for the cystatin C–based eGFR, for which data on race were not required.
For each study cohort, we attempted to calibrate cystatin C measurements to the reference standard by evaluating the year and measurement method used (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).11,19 We first used the cystatin C–based eGFR to reclassify participants who were initially classified according to the creatinine-based eGFR. Comparing the cystatin C–based eGFR with the creatinine-based eGFR had the advantage of providing the clearest contrast, since each equation includes only one filtration marker. We then compared the creatinine equation with the combination equation.
STUDY OUTCOMES
The outcomes of interest were death from any cause, death from cardiovascular causes, and end-stage renal disease. Death from cardiovascular causes was defined as death from myocardial infarction, heart failure, or stroke. End-stage renal disease was defined by the need for renal-replacement therapy or death from chronic kidney disease.
We first performed analyses within each study cohort and then performed meta-analyses across all studies, using random-effects models. We restricted our analysis to participants who were at least 18 years of age. Primary analyses were conducted in the general-population cohorts, since participants were not selected on the basis of serum creatinine levels. Secondary analyses were performed in the cohorts with chronic kidney disease.
STUDY OVERSIGHT
The study was designed by the CKD-PC steering committee. Data were collected within each of the 16 cohorts and analyzed centrally at the coordinating center.
STATISTICAL ANALYSIS
We first evaluated the distributions for each eGFR equation. Subsequently, we constructed Cox proportional-hazards models fitted with eGFR linear splines, with adjustment for age, sex, race (black vs. nonblack), smoking status, status with respect to a history of cardiovascular disease, systolic blood pressure, presence or absence of diabetes, total cholesterol level, body-mass index, and the level of albuminuria. From these models, we computed and pooled hazard ratios for each increment in the eGFR of 1 ml per minute per 1.73 m2 of body-surface area for eGFR values from 15 to 120, with a reference point at 95 ml per minute per 1.73 m2 (50 ml per minute per 1.73 m2 for cohorts with chronic kidney disease), as in previous CKD-PC meta-analyses.12–14,16,20,21 The reference point was moved to 65 ml per minute per 1.73 m2 for the end point of end-stage renal disease in the general-population cohorts in order to ensure that there were sufficient events at an eGFR value above the reference point. Details about the statistical analysis are provided in Appendix 1 in the Supplementary Appendix.
We cross-tabulated the eGFR that was calculated by means of each equation, using clinical categories of volume per minute per 1.73 m2 (<15 ml, 15 to 29 ml, 30 to 44 ml, 45 to 59 ml, 60 to 89 ml, and ≥90 ml).1,22 We then evaluated the proportion of participants in each creatinine-calculated eGFR category for whom the eGFR was reclassified on the basis of the cystatin C measurement or the combined measurement.1,16,22–25 For each outcome, we used multivariable Cox proportional-hazards models to assess risks of adverse outcomes among participants for whom the eGFR was reclassified to a higher value (i.e., to higher categories of cystatin C–calculated eGFR or combination-calculated eGFR) or a lower eGFR value (i.e., to lower categories of the two sets of GFR estimates), as compared with participants for whom the eGFR was not reclassified.
We assessed the overall improvement in reclassification on the basis of clinical eGFR categories by applying the net-reclassification-improvement approach,25,26 a method involving the use of predefined risk categories to assess improved risk prediction. To assess generalizability, we calculated the net reclassification improvement in subgroups according to age (<65 vs. ≥65 years), sex, race (black vs. nonblack), eGFR category, and status with respect to diabetes, hypertension, and albuminuria, with a correction for bias when appropriate.27 All analyses were conducted with the use of Stata/MP software, version 11.2.
RESULTS
STUDY OUTCOMES
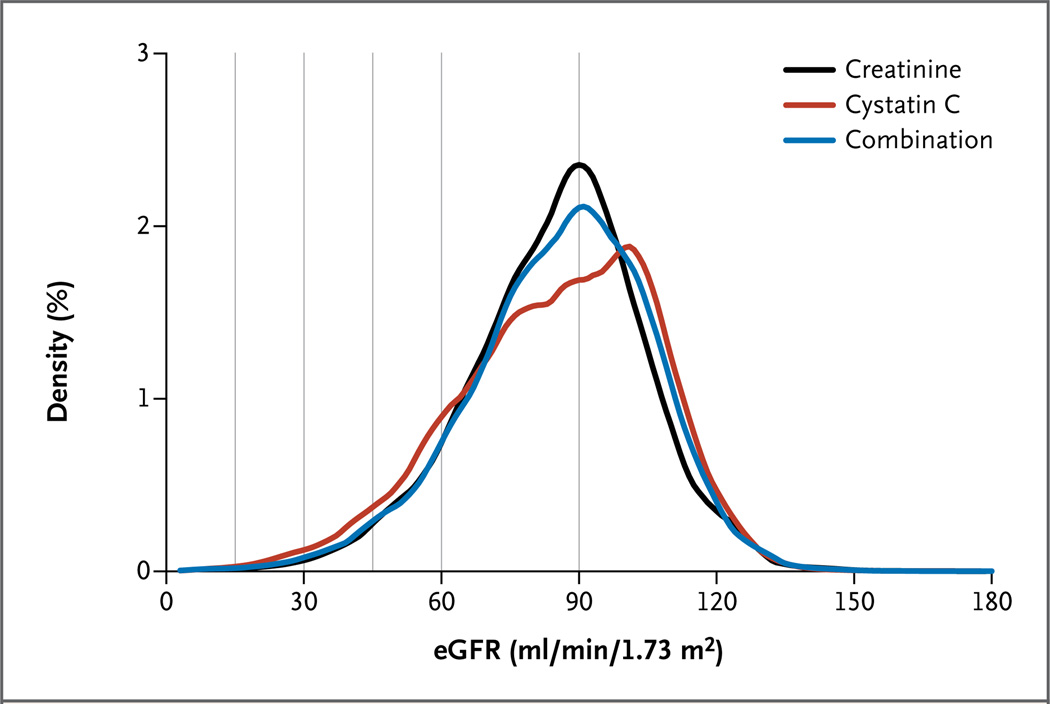
The mean values for the eGFR as measured with creatinine, cystatin C, and a combination of both for volume per minute per 1.73 m2 were 85 ml, 85 ml, and 84 ml, respectively, among the general-population cohorts and 38 ml, 39 ml, and 38 ml, respectively, among the cohorts with chronic kidney disease (Table 1).28–43 In the general-population cohorts, the prevalence of an eGFR of less than 60 ml per minute per 1.73 m2 was 9.7% with the creatinine equation, 13.7% with the cystatin C equation, and 10.0% with the combination (Fig. 1), values that were consistent across subgroups (Fig. S1 in the Supplementary Appendix).
Table 1.
Baseline Characteristics of Participants in 11 General-Population Studies and 5 Studies of Chronic Kidney Disease.*
| Study | Country | No. of Participants |
Mean Age | Female Sex |
Black Race |
Estimated | Glomerular | Filtration Rate | Albumin uria† | Smoking (%) |
History of CVD |
Hypercholes- terolemia |
Hyper- tension |
Diabetes | Mean Follow-up |
Death | End-Stage Renal Disease |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| yr | % of participants | Creatinine | Cystatin C ml/min/1.73 m2 |
Combination | % of participants | yr | Any Cause | CVD no. of participants |
||||||||||
| General population | ||||||||||||||||||
| All studies | 90,750 | 60 | 54 | 20 | 85 | 85 | 84 | 12 | 18 | 15 | 32 | 50 | 16 | 7.7 | 12,351 | 3193 | 357 | |
| ARIC28 | United States | 11,215 | 63±6 | 56 | 22 | 84±16 | 83±18 | 85±16 | 8 | 15 | 14 | 34 | 48 | 17 | 10.6 | 1,884 | 383 | 186 |
| AusDiab29 | Australia | 10,507 | 51±14 | 55 | 0 | 86±17 | 90±20 | 88±17 | 6 | 16 | 8 | 44 | 32 | 6 | 9.9 | 832 | 172 | NA |
| Beaver Dam CKD30 | United States | 4,617 | 62±11 | 56 | <1 | 80±18 | 79±21 | 79±19 | 4 | 20 | 15 | 54 | 50 | 10 | 11.5 | 1,462 | 647 | NA |
| CHS31 | United States | 2,984 | 78±5 | 59 | 17 | 74±17 | 63±19 | 69±18 | 20 | 8 | 31 | 38 | 64 | 16 | 8.4 | 1,729 | 655 | NA |
| ESTHER32 | Germany | 9,607 | 62±7 | 55 | 0 | 84±20 | 83±18 | 84±17 | 12 | 16 | 17 | 46 | 60 | 19 | 5.0 | 487 | 159 | NA |
| Framingham33 | United States | 2,596 | 58±10 | 54 | 0 | 89±18 | 83±19 | 86±17 | 11 | 15 | 6 | 22 | 39 | 9 | 10.7 | 186 | 92 | NA |
| MESA34 | United States | 6,693 | 62±10 | 53 | 28 | 82±16 | 89±20 | 86±18 | 10 | 13 | 0 | 28 | 45 | 12 | 6.2 | 322 | 70 | NA |
| NHANES III35 | United States | 6,748 | 56±20 | 52 | 25 | 90±25 | 84±28 | 87±26 | 16 | 22 | 16 | NA | 42 | 16 | 8.0 | 1,438 | 635 | NA |
| PREVEND36 | Netherlands | 7,982 | 49±13 | 50 | 1 | 89±16 | 103±21 | 97±19 | 11 | 34 | 5 | 39 | 34 | 4 | 9.7 | 602 | 170 | NA |
| REGARDS37 | United States | 26,698 | 64±10 | 54 | 43 | 87±21 | 84±21 | 84±21 | 16 | 16 | 22 | 16 | 60 | 23 | 5.0 | 2,947 | NA | 171 |
| ULSAM38 | Sweden | 1,103 | 71±1 | 0 | 0 | 76±11 | 59±14 | 67±12 | 16 | 20 | 36 | 58 | 75 | 19 | 11.6 | 462 | 210 | NA |
| Chronic kidney disease | ||||||||||||||||||
| All studies | 2,960 | 55 | 37 | 37 | 38 | 39 | 38 | 78 | 19 | 30 | 52 | 97 | 9 | 9.3 | 851 | 278 | 1297 | |
| AASK39 | United States | 949 | 55±11 | 39 | 100 | 46±15 | 45±18 | 44±15 | 61 | 29 | 51 | 43 | 100 | 0 | 8.8 | 216 | NA | 273 |
| CRIB40 | United Kingdom | 292 | 62±14 | 34 | 6 | 22±11 | 22±12 | 21±11 | 86 | 14 | 44 | 57 | 95 | 18 | 6.1 | 110 | 55 | 138 |
| MASTERPLAN41‡ | Netherlands | 473 | 59±13 | 31 | 3 | 36±14 | 46±21 | 40±17 | 86 | 23 | 25 | 72 | 95 | 32 | 4.1 | 52 | NA | 66 |
| MDRD42 | United States | 1,044 | 52±13 | 39 | 10 | 35±15 | 32±14 | 32±14 | 84 | 10 | 13 | NA | NA | 6 | 14.0 | 473 | 223 | 749 |
| MMKD43 | Austria | 202 | 47±12 | 34 | 0 | 47±30 | 50±33 | 48±32 | 95 | 21 | 12 | 39 | 89 | 0 | 4.0 | NA | NA | 71 |
Plus–minus values are means ±SD. AASK denotes African American Study of Kidney Disease and Hypertension, ARIC Atherosclerosis Risk in Communities, AusDiab Australian Diabetes, Obesity, and Lifestyle, Beaver Dam CKD Beaver Dam Chronic Kidney Disease Study, CHS Cardiovascular Health Study, CRIB Chronic Renal Impairment in Birmingham, CVD cardiovascular disease, ESTHER Epidemiologische Studie zu Chancen der Verhütung, Früherkennung und optimierten Therapie chronischer Erkrankungen in der älteren Bevölkerung, Framingham Framingham Heart Study, MASTERPLAN Multifactorial Approach and Superior Treatment Efficacy in Renal Patients with the Aid of a Nurse Practitioner, MDRD Modification of Diet in Renal Disease, MESA Multi-Ethnic Study of Atherosclerosis, MMKD Mild to Moderate Kidney Disease, NA not available, NHANES III Third U.S. National Health and Nutrition Examination Survey, PREVEND Prevention of Renal and Vascular End-Stage Disease, REGARDS Reasons for Geographic and Racial Differences in Stroke, and ULSAM Uppsala Longitudinal Study of Adult Men.
Listed are the proportions of participants with an albumin:creatinine ratio of 30 or more (with albumin measured in milligrams per deciliter and creatinine in grams per deciliter), a protein:creatinine ratio of 50 or more (with protein measured in milligrams per deciliter and creatinine in grams per deciliter), or a dipstick protein value of 1+ or higher.
Five participants were excluded from the analysis of incident end-stage renal disease.
Figure 1. Distribution of the Estimated Glomerular Filtration Rate (eGFR) as Calculated with the Measurement of Creatinine, Cystatin C, or Both in 11 General-Population Cohort Studies.
A total of 90,750 participants were included in the meta-analysis of 11 studies, with a kernel-density estimate showing the smoothed frequency for each 1 ml of the eGFR value. The vertical lines indicate current clinical thresholds for eGFR categories.
Death from Any Cause
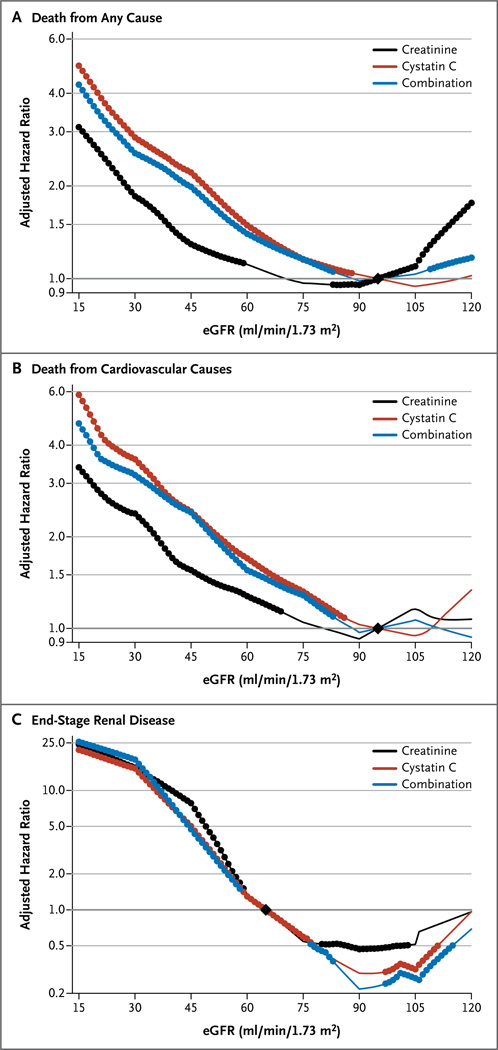
In the 11 general-population cohorts, 12,351 of 90,750 participants (13.6%) died during a mean follow-up of 7.7 years. With a cystatin C–based eGFR, the risk of death from any cause was increased at eGFR values that were below the reference point of 95 ml per minute per 1.73 m2, with a threshold of 88 ml per minute per 1.73 m2 (i.e., the point at which the risk was significantly higher than the risk at the reference point) (Fig. 2A). The corresponding thresholds were 59 ml and 83 ml per minute per 1.73 m2 for the creatinine-based eGFR and the combination-based eGFR, respectively.. There was a reverse J-shaped association for the creatinine and combination measurements of eGFR, with a significantly elevated risk of death at higher eGFR values, as has been reported previously.6
Figure 2. Adjusted Hazard Ratios for the Three Study Outcomes in the General-Population Cohort Studies.
Shown are hazard ratios for death from any cause (Panel A), death from cardiovascular causes (Panel B), and end-stage renal disease (Panel C), according to whether the eGFR was calculated with the measurement of creatinine, cystatin C, or both. The graphs show associations by plotting the adjusted hazard ratio versus the reference points, which are indicated by black diamonds (at 95 ml per minute per 1.73 m2of body-surface area for death from any cause and death from cardiovascular causes and at 65 ml per minute per 1.73 m2for end-stage renal disease). The hazard ratios were adjusted for age, sex, race, body-mass index, systolic blood pressure, total cholesterol, presence or absence of a history of cardiovascular disease, smoking status, presence or absence of diabetes, and level of albuminuria. In each panel, solid circles indicate that the adjusted hazard ratio at the indicated eGFR level was significant, as compared with the reference point. For death from any cause, the meta-analysis included 11 general-population cohorts with 90,750 participants, of whom 12,351 died during follow-up. For death from cardiovascular causes, the meta-analysis included 10 general-population cohorts with 64,010 participants, of whom 3193 died from cardiovascular causes during follow-up. For incident end-stage renal disease, the meta-analysis included 2 general-population cohorts with 37,872 participants, in 357 of whom end-stage renal disease occurred during follow-up. Because there were fewer events of end-stage renal disease than deaths, several eGFR levels had nonsignificant associations with the outcome, despite point estimates that were similar to those for other eGFR levels that had significant associations with end-stage renal disease.
Among the cohorts of patients with chronic kidney disease, eGFR values based on cystatin C and combination measurements also had more linear associations with the risk of death than did the values based on creatinine measurement, although no reverse J-shaped association was observed for the creatinine-based eGFR values (Fig. S2A in the Supplementary Appendix).
In the general-population cohorts, across all the creatinine-based eGFR categories, reclassification to a lower eGFR was associated with a significantly higher adjusted risk of death (Table 2). For example, among participants with a creatinine-based eGFR of 60 to 89 ml per minute per 1.73 m2 (48% of participants overall), 14% were reclassified to a cystatin C–based eGFR of less than 60 ml per minute per 1.73 m2 and had a relative increase of 57% in the adjusted risk of death during follow-up. Within the smaller category of a createnine-based eGFR of 60 to 74 ml per minute per 1.73 m2 (18% of all participants), the 23% who were reclassified to a cystatin C–based eGFR of less than 60 ml per minute per 1.73 m2 also had a significantly higher adjusted risk of death (hazard ratio, 1.54; 95% confidence interval [CI], 1.33 to 1.79) (Table S2 in the Supplementary Appendix).
Table 2.
Risk of Clinical Outcomes According to Reclassification of the eGFR with the Measurement of Cystatin C, as Compared with Creatinine, in the 11 General-Population Cohorts.*
| Outcome and Category of Creatinine- Based eGFR (ml/min/1.73 m2) |
Total No. of Participants Evaluated |
Reclassification to Higher eGFR | No Reclassification | Reclassification to Lower eGFR | |||
|---|---|---|---|---|---|---|---|
| %of Participants |
Adjusted Hazard Ratio (95% CI) |
%of Participants |
Adjusted Hazard Ratio (95% CI) |
%of Participants |
Adjusted Hazard Ratio (95% CI) |
||
| Death from any cause | 1260 events |
6110 events |
4981 events |
||||
| ≥90 | 38,287 | NA | NA | 70 | Reference | 30 | 1.36 (1.24–1.48) |
| 60–89 | 43,630 | 32 | 0.88 (0.76–1.01) | 55 | Reference | 14 | 1.57 (1.39–1.78) |
| 45–59 | 6,358 | 42 | 0.66 (0.57–0.77) | 34 | Reference | 24 | 1.67 (1.49–1.88) |
| 30–44 | 1,917 | 38 | 0.77 (0.61–0.98) | 44 | Reference | 19 | 1.72 (1.24–2.37) |
| 15–29 | 427 | 24 | 0.60 (0.27–1.36) | 65 | Reference | 11 | 3.04 (1.87–4.95) |
| 0–14 | 131 | 20 | 0.44 (0.14–1.36) | 80 | Reference | NA | NA |
| Death from cardiovascular causes | 340 events |
1572 events |
1281 events |
||||
| ≥90 | 25,691 | NA | NA | 71 | Reference | 29 | 1.37(1.09–1.71) |
| 60–89 | 32,283 | 33 | 0.94 (0.70–1.28) | 54 | Reference | 13 | 1.44(1.19–1.74) |
| 45–59 | 4,458 | 47 | 0.79 (0.61–1.04) | 33 | Reference | 21 | 1.60 (1.30–1.95) |
| 30–44 | 1,276 | 43 | 0.66 (0.42–1.02) | 39 | Reference | 18 | 1.19(0.77–1.83) |
| 15–29 | 231 | 23 | 0.15 (0.03–0.74) | 66 | Reference | 12 | 2.83 (0.79–10.18) |
| 0–14 | 71 | 17 | ND | 83 | Reference | NA | NA |
| End-stage renal disease | 52 events |
198 events |
107 events |
||||
| ≥90 | 16,898 | NA | NA | 68 | Reference | 32 | 3.37(0.93–12.15) |
| 60–89 | 17,453 | 29 | 0.71 (0.34–1.48) | 58 | Reference | 13 | 2.66 (1.52–4.67) |
| 45–59 | 2,468 | 35 | 0.20 (0.08–0.53) | 37 | Reference | 27 | 1.72 (0.91–3.27) |
| 30–44 | 758 | 28 | 0.39 (0.16–0.90) | 53 | Reference | 20 | 2.04 (1.14–3.65) |
| 15–29 | 220 | 26 | 7.15 (0.04–1257)† | 64 | Reference | 10 | 2.23 (0.74–6.78) |
| 0–14 | 75 | 20 | 2.39 (0.72–7.87) | 80 | Reference | NA | NA |
Reclassification of the eGFR to a higher value with the measurement of cystatin C was associated with a decreased risk of a study outcome. Conversely, reclassification of the eGFR to a lower value was associated with an increased risk. Hazard ratios were adjusted for age, sex, race, smoking status, systolic blood pressure, total cholesterol level, presence or absence of diabetes, presence or absence of a history of cardiovascular disease, body-mass index, and the level of albuminuria. NA denotes not applicable, and ND not done because of small sample size.
The 95% confidence interval is very wide in this category.
Conversely, across all categories of creatinine-based eGFR values, reclassification to a higher eGFR with the measurement of cystatin C was associated with a reduction in the adjusted risk of death, as compared with the group with eGFR values that were not reclassified, although the findings were significant only for creatinine-based eGFR categories of 30 to 44 ml and 45 to 59 ml per minute per 1.73 m2. In the latter category, 42% of participants were reclassified to a cystatin C–based eGFR of 60 ml per minute per 1.73 m2 or more and had a relative reduction of 34% in the adjusted risk of death. Similar results were observed when findings were unadjusted or further adjusted for the creatinine-based eGFR (Tables S3 and S4 in the Supplementary Appendix).
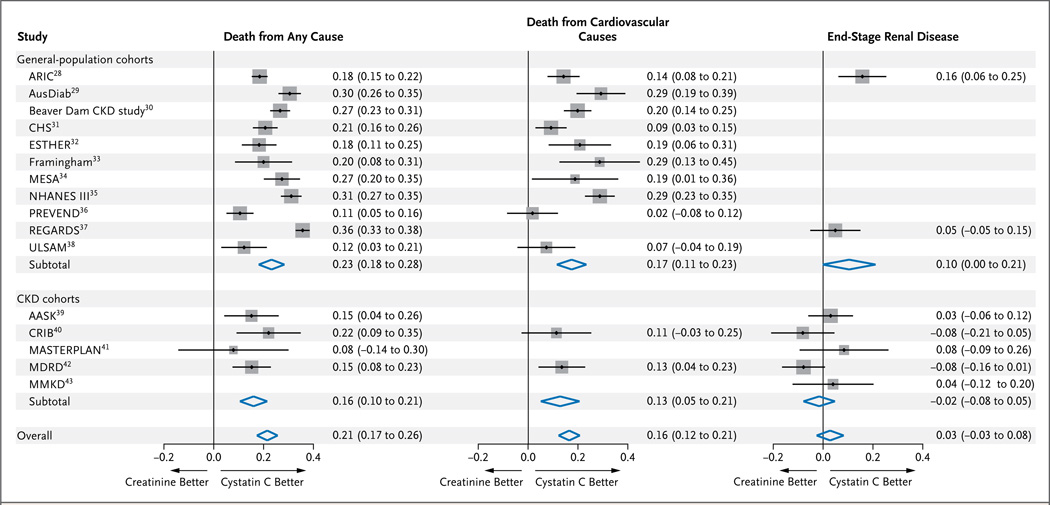
Across all categories of creatinine-based eGFR values, the overall net reclassification improvement for death from any cause was 0.21 (95% CI, 0.17 to 0.26; P<0.001) and was greater in the general-population cohorts than in those with chronic kidney disease (Fig. 3). Among the 15 cohorts for which mortality data were available, all had positive net reclassification improvements. The bias-corrected estimates of net reclassification improvement were highest for the creatinine-based eGFR categories of 45 to 59 ml per minute per 1.73 m2 (0.28; 95% CI, 0.19 to 0.36), 30 to 44 ml per minute per 1.73 m2 (0.32; 95% CI, 0.25 to 0.39), and 15 to 29 ml per minute per 1.73 m2 (0.27; 95% CI, 0.18 to 0.37) (Table S5 in the Supplementary Appendix). In sensitivity analyses that were stratified according to age, sex, race, and status with respect to diabetes, hypertension, and albuminuria, estimates of net reclassification improvement varied minimally (Fig. S3 and Table S6 in the Supplementary Appendix). Meta-regression analyses showed no cohort-specific factors that were significantly associated with the net reclassification improvement (Table S7 in the Supplementary Appendix).
Figure 3. Net Reclassification Improvement in eGFR Based on the Measurement of Cystatin C, as Compared with Creatinine-Based eGFR, for the Three Study Outcomes in the 16 Study Cohorts.
Shown are values for net reclassification improvement, a measure of the overall improvement in the association between the reclassified eGFR based on the measurement of cystatin C, as compared with the creatinine-based eGFR, and the study outcomes of death from any cause, death from cardiovascular causes, and end-stage renal disease. The size of the squares is proportional to the inverse of the variance of the net reclassification improvements. The horizontal line indicates the 95% confidence interval. A similar analysis of the net reclassification improvement in eGFR as calculated with the measurement of both creatinine and cystatin C (combination group) is provided in Figure S4 in the Supplementary Appendix. The full study names are provided in Table 1. CKD denotes chronic kidney disease.
Death from Cardiovascular Causes
The 10 general-population cohorts with data on deaths from cardiovascular causes included 64,010 participants, with 3193 events during a mean follow-up period of 8.8 years. For the creatinine-based eGFR, there was no reverse J-shaped association for the risk of death from cardiovascular causes, but the risk gradient was steeper for eGFR values based on cystatin C measurements and those based on combined measurements than for the creatinine-based eGFR values; threshold values for significant elevations in risk were 69 ml per minute per 1.73 m2 for the creatinine equation, 86 ml per minute per 1.73 m2 for the cystatin C equation, and 83 ml per minute per 1.73 m2 for the combination equation (Fig. 2B).
In analyses according to eGFR category, reclassification to a lower eGFR was consistently associated with an increased risk in each creatinine-based eGFR category, but point estimates were significant only for the creatinine-based eGFR categories of 45 to 59 ml, 60 to 89 ml, and 90 ml per minute per 1.73 m2 or more. Reclassification to a higher eGFR was associated with a decreased risk in each category of creatinine-based eGFR but was significant only for a creatinine-based eGFR of 15 to 29 ml per minute per 1.73 m2 (Table 2). Overall, the net reclassification improvement was 0.16 (95% CI, 0.12 to 0.21; P<0.001), including the two cohorts with chronic kidney disease, and point estimates favored the cystatin C–based eGFR within each cohort (Fig. 3) and within each subgroup tested (Fig. S3 and Table S7 in the Supplementary Appendix).
End-Stage Renal Disease
In two of the general-population cohorts, with a total of 37,872 participants, there were 357 events of incident end-stage renal disease, and in the five cohorts with chronic kidney disease, comprising 2955 participants, there were 1297 such events over a mean follow-up of 9.3 years. The risk associations for creatinine-based eGFR, cystatin C–based eGFR, and eGFR based on combined measurements were similar in the general-population cohorts (Fig. 2C) and cohorts with chronic kidney disease (Fig. S2B in the Supplementary Appendix). Nonetheless, in all categories of creatinine-based eGFR, reclassification to a lower eGFR with measurement of cystatin C was associated with an increased risk of end-stage renal disease, and reclassification to a higher eGFR was generally associated with a lower risk (Table 2). The overall net reclassification improvement was not significant (0.03; 95% CI, −0.03 to 0.08; P = 0.46), although it had borderline significance in the general-population cohorts (0.10; 95% CI, 0.00 to 0.21; P = 0.05) (Fig. 3).
Use of Combined Calculations
Reclassification to a lower eGFR with the combination equation was strongly associated with an increased risk of all three outcomes (Table S8 in the Supplementary Appendix), with effect sizes that were similar to those for cystatin C–based eGFR, but smaller proportions of participants were reclassified with the combination equation. Similarly, reclassification to a higher eGFR with the combination equation was associated with reductions in risks that were similar to those with the cystatin C equation.
The net reclassification improvement with the combination eGFR equation over the creatinine equation was smaller than the improvement with the cystatin C equation for death from any cause (0.13; 95% CI, 0.12 to 0.15; P<0.001) and death from cardiovascular causes (0.11; 95% CI, 0.09 to 0.13; P<0.001) but was larger for end-stage renal disease (0.07; 95% CI, 0.02 to 0.12; P = 0.01) (Fig. 3, and Fig. S4 in the Supplementary Appendix). We found that the net reclassification improvement with the cystatin C equation, as compared with the combination equation, was moderately strong and significant for death from any cause (0.13; 95% CI, 0.09 to 0.18; P<0.001) and for death from cardiovascular causes (0.10; 95% CI, 0.06 to 0.14; P<0.001) but was essentially equivalent for end-stage renal disease (−0.02; 95% CI, 0.16 to 0.13; P = 0.82).
DISCUSSION
Accurate detection and staging of chronic kidney disease are integral components of clinical medicine, since such evaluations have a major effect on disease labeling, interventions, drug doses, and risk stratification for clinical procedures.3 Our study provides evidence that the use of cystatin C improves the role of eGFR in risk categorization, as judged by the risk of death from any cause and to a lesser extent the risks of death from cardiovascular causes and end-stage renal disease. Most notably, reduced values for cystatin C–based eGFR and eGFR based on combined measurements of creatinine and cystatin C had a consistent linear association with increased risks of death from any cause and from cardiovascular causes for all eGFR levels below approximately 85 ml per minute per 1.73 m2, which is well above the threshold of 60 ml per minute per 1.73 m2 for the detection of chronic kidney disease with a creatinine-based eGFR. These findings show that eGFR equations that are based on the measurement of cystatin C can be used to detect increased risks of adverse outcomes that are not detected with creatinine-based calculation of the eGFR.
Differences among the eGFR values with respect to risk relationships probably reflect confounding by non-GFR determinants of the filtration markers. We observed that the creatinine-based eGFR had the weakest association with death from any cause among the three GFR estimates and had a marked reverse J-shaped association. It is well known that the non-GFR determinants of serum creatinine, including muscle mass, diet, and physical activity, can confound the associations between the creatinine-based eGFR and outcomes.44 The hypothesized mechanism is that serum creatinine levels are lower than expected for the level of GFR in patients who are in poor health and who are most likely to die. Non-GFR determinants of cystatin C also exist, though they are quantifiably smaller than those of creatinine.45 It is therefore possible that confounding by non-GFR determinants of cystatin C may enhance the association between cystatin C–based eGFR and the risk of death.29 Hypothesized mechanisms include the potential influences of obesity, inflammation, and diabetes in raising cystatin C levels.46–48
Recent studies have shown that calculation of the eGFR with a combination of creatinine and cystatin C more accurately reflects measured GFR than either marker alone, findings that are probably due to the lesser overall effects of non-GFR determinants of either marker when both markers are included.11,49 However, we found that eGFR calculated with the use of both markers had a weaker association with the risk of death from any cause than did the cystatin C–based eGFR. Because analyses of prognosis are most heavily influenced by the events (in this case, deaths), the confounding by non-GFR determinants of creatinine in persons who are most susceptible to illness may particularly weaken the association between eGFR based on combined measurements and longitudinal outcomes as compared with the effect in cross-sectional comparisons with measured GFR. Thus, calculation of the eGFR with the use of combined measurements may provide the most accurate eGFR overall but not in some subgroups of patients in whom creatinine levels are reduced and risk is high. Alternatively, if non-GFR determinants of cystatin C augment its association with the risk of death, then they will have a greater effect on the cystatin C–based eGFR than on the eGFR that is based on combined measurements. We cannot distinguish among these possibilities, since the strength of our study is in establishing firm associations, with limited ability to determine causal mechanisms.
Recent guidelines suggest the use of cystatin C to validate the diagnosis of chronic kidney disease in patients who are currently considered to have chronic kidney disease solely on the basis of a creatinine-based eGFR of less than 60 ml per minute per 1.73 m2, without albuminuria or other markers of kidney damage.3 In our study, 42% of participants with a creatinine-based eGFR of 45 to 59 ml per minute per 1.73 m2 had a cystatin C–based eGFR of 60 ml per minute per 1.73 m2 or more, and those participants had a 34% reduction in the risk of death and an 80% reduction in the risk of end-stage renal disease, as compared with participants for whom the eGFR was not reclassified. Persons with a creatinine-based eGFR of 45 to 59 ml per minute per 1.73 m2 in the absence of albuminuria account for 4% of all persons in the United States and for 54% of patients with a creatinine-based eGFR of less than 60 ml per minute per 1.73 m2 (the typical threshold for a diagnosis of chronic kidney disease).50 Confirmatory testing with the use of cystatin C could allow substantial reclassification in this group, with more appropriate resource utilization for patients at increased risk for complications of chronic kidney disease. Although the use of two different calculations of the eGFR (the creatinine-based eGFR and the cystatin C–based eGFR) allows for an examination of the average of the two values as well as the difference between them, our results indicate that a single calculation of the eGFR based on the combined measurements performs well for risk classification in addition to its proven advantage in GFR estimation.
Our study examined prognosis on the basis of eGFR values calculated with the use of cystatin C as compared with creatinine-based eGFR values in more than 90,000 study participants. We examined a variety of clinically useful methods for GFR estimation, included a broad range of kidney function, and standardized the measurements of cystatin C and creatinine across studies to the extent possible. Nonetheless, we had less information to evaluate reclassification according to the cystatin C–based eGFR for end-stage renal disease. The available results suggest that cystatin C adds less value to creatinine for the prediction of end-stage renal disease than for the prediction of death, but these findings should be interpreted with caution, since the diagnosis of end-stage renal disease is based on the serum creatinine level in addition to signs and symptoms of uremia.
We acknowledge additional limitations of our study. First, the GFR was not measured. However, we are aware of no diverse, population-based cohort study that has measured GFR. Methods for measurement of creatinine and cystatin C varied across the studies, as did the efforts to calibrate these measures to reference standards. We cannot determine whether any measurement bias would have favored creatinine or cystatin C, nor can we determine the effects of any bias on the thresholds reported for significant elevations in risks. In addition, although the general-population cohorts were broadly representative, the cohorts with chronic kidney disease were not completely generalizable, since only two of the five cohorts included patients with diabetes and none included kidney-transplant recipients. Our results may be influenced by the presence of residual confounding, which could have an effect on the eGFR thresholds for elevated risk. Finally, most participants were either white or black; therefore, caution should be used in extrapolating our results to other racial or ethnic groups.
In conclusion, the use of cystatin C to calculate the eGFR strengthened the associations between eGFR categories and the risks of death and end-stage renal disease across diverse populations. We also found that the risk of death was increased when values for both cystatin C–based eGFR and eGFR based on combined creatinine and cystatin C measurements were below a threshold of approximately 85 ml per minute per 1.73 m2.
Supplementary Material
Acknowledgments
Supported by a program grant (to the CKD-PC data coordinating center) from the National Kidney Foundation, whose funding sources include Abbott and Amgen. Funders of the studies included in the meta-analysis are provided in Appendix 3 in the Supplementary Appendix.
Dr. Inker reports receiving grant support through her institution from Gilead and Pharmalink; Dr. Rothenbacher, serving on a steering committee for Novartis; Dr. Coresh, receiving consulting fees from Amgen and Merck and grant support through his institution from Amgen; and Dr. Levey, receiving grant support through his institution from Amgen, Pharmalink, and Gilead. No other potential conflict of interest relevant to this article was reported.
APPENDIX
The authors’ affiliations are as follows: the Division of General Internal Medicine, San Francisco Veterans Affairs Medical Center, and the Departments of Medicine, Epidemiology, and Biostatistics, University of California San Francisco — both in San Francisco (M.G.S.); the Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore (K.M., J.C.); the Department of Public Health and Caring Sciences/Geriatrics Uppsala University, Uppsala, and the School of Health and Social Studies, Dalarna University, Falun — both in Sweden (J.Ä.); the Division of Nephrology, Tufts Medical Center, Boston (L.A.I., M.J.S., A.S.L.); the University of Washington, Seattle (R.K.); the Department of Nephrology, Monash Medical Centre, and the Department of Medicine, Monash University, Melbourne, VIC, Australia (K.R.P.); the Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, and Institute of Epidemiology and Medical Biometry, Ulm University, Ulm — both in Germany (D.R.); the Department of Population Health Sciences, University of Wisconsin School of Medicine and Public Health, Madison (B.C.A.); and the Department of Nephrology, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands (R.T.G.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 2.National Collaborating Centre for Chronic Conditions. Chronic kidney disease: national clinical guideline for early identification and management in adults in primary and secondary care. NICE clinical guideline 73. London: Royal College of Physicians; 2008. [PubMed] [Google Scholar]
- 3.Kidney Disease. Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(Suppl):1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 5.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 6.Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. 2009;20:2214–2222. doi: 10.1681/ASN.2008090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waheed S, Matsushita K, Sang Y, et al. Combined association of albuminuria and cystatin C-based estimated GFR with mortality, coronary heart disease, and heart failure outcomes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2012;60:207–216. doi: 10.1053/j.ajkd.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlipak MG, Praught ML, Sarnak MJ. Update on cystatin C: new insights into the importance of mild kidney dysfunction. Curr Opin Nephrol Hypertens. 2006;15:270–275. doi: 10.1097/01.mnh.0000222694.07336.92. [DOI] [PubMed] [Google Scholar]
- 9.Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albuminto-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peralta CA, Katz R, Sarnak MJ, et al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol. 2011;22:147–155. doi: 10.1681/ASN.2010050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [Errata, N Engl J Med 2012;367:681, 2060.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality: a collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 14.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease: a collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes: a collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med. 2012;156:785–795. doi: 10.7326/0003-4819-156-11-201203200-00391. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [Erratum, Ann Intern Med 2011; 155:408.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Dunn W, Breaud A, Elliott D, So-koll LJ, Clarke W. Analytical performance of 4 automated assays for measurement of cystatin C. Clin Chem. 2010;56:1336–1339. doi: 10.1373/clinchem.2009.141531. [DOI] [PubMed] [Google Scholar]
- 20.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [Erratum, Lancet 2013;381: 374.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmoodi BK, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. 2012;380:1649–1661. doi: 10.1016/S0140-6736(12)61272-0. [Erratum, Lancet 2012;380:1648.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowe E, Halpin D, Stevens P. Early identification and management of chronic kidney disease: summary of NICE guidance. BMJ. 2008;337:a1530. doi: 10.1136/bmj.a1530. [DOI] [PubMed] [Google Scholar]
- 23.Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD. Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am Heart J. 2011;162:548–554. doi: 10.1016/j.ahj.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Stevens LA, Li S, Kurella Tamura M, et al. Comparison of the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) study equations: risk factors for and complications of CKD and mortality in the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2011;57(Suppl 2):S9–S16. doi: 10.1053/j.ajkd.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010;55:648–659. doi: 10.1053/j.ajkd.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pencina MJ, D’Agostino RB, D’Agos-tino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclas-sification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. 207-12. [DOI] [PubMed] [Google Scholar]
- 27.Paynter NP, Cook NR. A bias-corrected net reclassification improvement for clinical subgroups. Med Decis Making. 2013;33:154–162. doi: 10.1177/0272989X12461856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20:2617–2624. doi: 10.1681/ASN.2009010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White SL, Polkinghorne KR, Atkins RC, Chadban SJ. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis. 2010;55:660–670. doi: 10.1053/j.ajkd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Shankar A, Klein R, Klein BE. The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol. 2006;164:263–271. doi: 10.1093/aje/kwj173. [DOI] [PubMed] [Google Scholar]
- 31.Shlipak MG, Katz R, Kestenbaum B, et al. Rate of kidney function decline in older adults: a comparison using creati-nine and cystatin C. Am J Nephrol. 2009;30:171–178. doi: 10.1159/000212381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang QL, Koenig W, Raum E, Stegmaier C, Brenner H, Rothenbacher D. Epidemiology of chronic kidney disease: results from a population of older adults in Germany. Prev Med. 2009;48:122–127. doi: 10.1016/j.ypmed.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Parikh NI, Hwang S-J, Larson MG, Levy D, Fox CS. Chronic kidney disease as a predictor of cardiovascular disease (from the Framingham Heart Study) Am J Cardiol. 2008;102:47–53. doi: 10.1016/j.amjcard.2008.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bui AL, Katz R, Kestenbaum B, et al. Cystatin C and carotid intima-media thickness in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2009;53:389–398. doi: 10.1053/j.ajkd.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Astor BC, Hallan SI, Miller ER3rd, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167:1226–1234. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 36.Hillege HL, Fidler V, Diercks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 37.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 38.Ingelsson E, Sundström J, Lind L, et al. Low-grade albuminuria and the incidence of heart failure in a community-based cohort of elderly men. Eur Heart J. 2007;28:1739–1745. doi: 10.1093/eurheartj/ehm130. [DOI] [PubMed] [Google Scholar]
- 39.Wright JT, Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [Erratum, JAMA 2006;295: 2726.] [DOI] [PubMed] [Google Scholar]
- 40.Landray MJ, Thambyrajah J, McGlynn FJ, et al. Epidemiological evaluation of known and suspected cardiovascular risk factors in chronic renal impairment. Am J Kidney Dis. 2001;38:537–546. doi: 10.1053/ajkd.2001.26850. [DOI] [PubMed] [Google Scholar]
- 41.van Zuilen AD, Bots ML, Dulger A, et al. Multifactorial intervention with nurse practitioners does not change cardiovascular outcomes in patients with chronic kidney disease. Kidney Int. 2012;82:710–717. doi: 10.1038/ki.2012.137. [DOI] [PubMed] [Google Scholar]
- 42.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 43.Kronenberg F, Kuen E, Ritz E, et al. Lipoprotein(a) serum concentrations and apolipoprotein(a) phenotypes in mild and moderate renal failure. J Am Soc Nephrol. 2000;11:105–115. doi: 10.1681/ASN.V111105. [DOI] [PubMed] [Google Scholar]
- 44.Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans RO, Bakker SJ. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207:534–540. doi: 10.1016/j.atherosclerosis.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathisen UD, Melsom T, Ingebretsen OC, et al. Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J Am Soc Nephrol. 2011;22:927–937. doi: 10.1681/ASN.2010050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST. Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int. doi: 10.1038/ki.2013.7. 2013 February 20 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson AH, Yang W, Hsu CY, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157:471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 50.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.