Abstract
SUMMARY
Streptococcus pyogenes, also known as group A Streptococcus (GAS), causes mild human infections such as pharyngitis and impetigo and serious infections such as necrotizing fasciitis and streptococcal toxic shock syndrome. Furthermore, repeated GAS infections may trigger autoimmune diseases, including acute poststreptococcal glomerulonephritis, acute rheumatic fever, and rheumatic heart disease. Combined, these diseases account for over half a million deaths per year globally. Genomic and molecular analyses have now characterized a large number of GAS virulence determinants, many of which exhibit overlap and redundancy in the processes of adhesion and colonization, innate immune resistance, and the capacity to facilitate tissue barrier degradation and spread within the human host. This improved understanding of the contribution of individual virulence determinants to the disease process has led to the formulation of models of GAS disease progression, which may lead to better treatment and intervention strategies. While GAS remains sensitive to all penicillins and cephalosporins, rising resistance to other antibiotics used in disease treatment is an increasing worldwide concern. Several GAS vaccine formulations that elicit protective immunity in animal models have shown promise in nonhuman primate and early-stage human trials. The development of a safe and efficacious commercial human vaccine for the prophylaxis of GAS disease remains a high priority.
INTRODUCTION
In 1909, Meakins reported serotype-specific immunity stimulated by experimental vaccination of humans against streptococci. One 24-year-old male subject, presenting with endocarditis and a history of scarlatina and acute rheumatic fever, received 16 doses of vaccine over a 3-month period, prepared from streptococci isolated from the subject's own blood, yet died 7 days after the final dose (1). Over 100 years later, a safe and effective commercial vaccine against Streptococcus pyogenes (group A Streptococcus [GAS]) is still not licensed for human use (2–4).
GAS causes a diverse range of human infections, both benign and serious, which include pharyngitis, impetigo, cellulitis, scarlet fever, puerperal sepsis, bacteremia, pneumonia, streptococcal toxic shock syndrome (STSS), necrotizing fasciitis, and endocarditis. In addition, GAS infection can trigger serious postinfectious immune-mediated disorders, including acute poststreptococcal glomerulonephritis (APSGN), acute rheumatic fever (ARF), and rheumatic heart disease (RHD) (5–9). Global disease burden figures reported by the World Health Organization (WHO) rank GAS as the ninth leading infectious cause of human mortality, with the majority of deaths being attributable to invasive infections and RHD, primarily in nonindustrialized countries (5, 10). Several studies had noted a reduction in GAS disease burden in industrialized countries in the mid-20th century (11–14). However, in the last 50 years, there have been widespread reports of significant outbreaks of ARF (15, 16), APSGN (17, 18), GAS invasive disease (14, 19–21), puerperal sepsis (22–24), and scarlet fever (25, 26).
Treatment regimens for GAS infections naturally center on the use of appropriate antibiotics. GAS remains exquisitely and universally sensitive to penicillin, while antibiotics such as cephalosporins, macrolides, and clindamycin are also used clinically (27–29). In some regions of the world, GAS resistance to antibiotics such as macrolides, clindamycin, and lincosamide has become an increasing concern (25–28, 30), and epidemiological vigilance is required to ensure that treatment matches the antibiotic sensitivity profile of circulating GAS strains. The human population is the only known natural reservoir for GAS, and thus, a safe and effective human vaccine holds the promise of reducing disease burden and blocking transmission and even has the potential to eradicate this important human pathogen. Hurdles for the development of a safe human vaccine include significant genetic diversity and antigenic variability among GAS strains and, crucially, the prerequisite to ensure that any vaccine antigen does not trigger autoimmune sequelae such as ARF and APSGN (2–4, 31, 32).
Significant progress has been made in the understanding of the molecular mechanisms underlying GAS disease pathogenesis. Recently, this work has been accelerated by publications of numerous GAS genome sequences (33–41), which have greatly facilitated molecular investigations of virulence. A large number of GAS virulence determinants have been characterized, many of which exhibit functional redundancy in the processes of adhesion and colonization, resistance to innate immunity, and the capacity to spread within the human host. Based on such molecular data, disease models have been formulated for progression to severe disease outcomes such as invasive infection, STSS, ARF, and APSGN. Unraveling the contribution of GAS virulence factors to specific disease processes will provide an improved basis for targeted therapeutic intervention.
EPIDEMIOLOGY, DISEASE BURDEN, AND OUTBREAKS
GAS colonizes epithelial surfaces, primarily of the throat and skin, but also colonizes other surfaces such as the vagina and rectum, from where it can cause a remarkably wide array of superficial, invasive, and immune-mediated diseases. In 2005, the WHO reported a global estimate of 18.1 million cases of severe GAS disease, with 1.78 million new cases of severe disease and 517,000 deaths per year (5). In addition, there were >111 million prevalent cases of GAS pyoderma and >616 million incident cases of GAS pharyngitis per year (5). An overview of the GAS disease spectrum and global burden is given in Table 1.
TABLE 1.
Clinical symptoms and epidemiology of the major group A Streptococcus infections
| Disease | Sign(s) and/or symptom(s) | Estimated global incidencea | Associated M type(s)b | Reference(s) |
|---|---|---|---|---|
| Superficial | ||||
| Pharyngitis | Sore throat, malaise, fever | >600 million/yr | 1, 3, 5, 6, 12, 14, 17, 19, 24 | 6, 46, 58, 62, 63 |
| Scarlet fever | Deep red rash, “strawberry tongue,” exudative pharyngitis | 61, 64–66 | ||
| Impetigo | Skin pustules that mature into honey-colored scabs | 111 million | 33, 41, 42, 52, 53, 70 | 6, 69, 75 |
| Sequelae | ||||
| Acute rheumatic fever | Polyarthritis, carditis, rapid and jerky movements, rash, subcutaneous nodules | >471,000/yr | 1, 3, 5, 6, 11, 12, 14, 17, 18, 19, 24, 27, 29, 30, 32, 41 | 72, 73, 589–591 |
| Rheumatic heart disease | Mitral and/or aortic regurgitation with possible stenosis over time | 15.6 million–19.6 million | 66 | |
| Acute poststreptococcal glomerulonephritis | Edema, hypertension, urinary sediment abnormalities, complement deficiency | >470,000/yr | 1, 4, 12, 49, 55, 57, 60 | 66, 75, 591 |
| Invasive | ||||
| Bacteremia | High fever, nausea, vomiting | 660,000 cases and 160,000 deaths/yr (all invasive diseases) | 592 | |
| Puerperal sepsis | Fever, chills, abdominal pain in a pregnant or early postpartum woman | 28 | 593 | |
| Cellulitis | Acute, tender, erythematous, and swollen area of skin | 594 | ||
| Necrotizing fasciitis | Fever, exquisitely tender skin lesions, vomiting, diarrhea, toxemia, tissue destruction | 1, 3, 28 | 285 | |
| Streptococcal toxic shock syndrome | High fever, rapid-onset hypotension, accelerated multisystem failure | 1, 3 | 291 |
Epidemiology of GAS Infections
Historically, GAS isolates were typed by using serotype-specific antiserum raised against the M protein, an immunodominant surface antigen and key virulence determinant (6). GAS strains are now more commonly typed based on the sequence of the 5′ variable region of the emm gene encoding the M protein, of which there are over 200 emm types (http://www.cdc.gov/ncidod/biotech/strep/strepindex.htm). Large-scale epidemiological studies have shown a remarkable difference in the distribution of emm types in geographically and socioeconomically distinct regions of the world (2–4, 31, 32, 42). In industrialized societies, a significant percentage of GAS isolates belong to a few emm types, most notably emm types 1, 3, 12, and 28, which account for approximately 40% of disease in these countries (4, 43–47). These types are also the types most commonly isolated from asymptomatic carriers (43). In contrast, these emm types are less commonly isolated from cases of human disease in Africa and are rarely isolated from indigenous populations in the Pacific region and the Indian subcontinent (4, 48–50).
While certain emm types are responsible for a significant percentage of infections in industrialized societies, large-scale epidemiological analyses have revealed significant temporal and geographic variability in the isolation rates of individual emm types and subtypes, with prevalent emm types in a particular year being replaced by other emm types in subsequent years (46, 51–54). Furthermore, substantial variability is observed even within individual emm types. For example, Hoe et al. observed extensive allelic variation in streptococcal inhibitor of complement-mediated lysis (SIC) alleles in a large collection of emm 1.0 isolates (55). Likewise, Beres et al. demonstrated that successive waves of M3 infections in Canada were attributed to different subclones that arose primarily by horizontal phage transfer (56). Together, these observations suggest that, rather than being isolated at constant rates, individual emm types and subtypes display features of epidemic behavior, constantly expanding and subsiding as a result of host immune selective pressure.
The isolation frequency for emm types from different GAS diseases generally parallels their rate of asymptomatic carriage in the same population (57). However, there are significant associations of some emm types with particular disease manifestations (Table 1). Examples include the association of emm types 1, 3, and 49 with invasive disease and emm types 2, 4, 6, 12, and 44/61 with superficial disease (46, 58, 59). Additionally, some emm types are associated with particular GAS disease manifestations such as ARF (e.g., emm types 1, 3, 5, 6, 11, 12, 14, 17, 18, 19, 24, 27, 29, 30, 32, and 41), APSGN (e.g., emm types 1, 4, 12, 49, 55, 57, and 60), and puerperal sepsis (emm type 28) (60).
Burden of Superficial GAS Infections
The vast majority of GAS diseases are superficial infections of the throat and skin, resulting in pharyngitis and impetigo, respectively (6, 61).
Pharyngitis.
GAS is the most common bacterial cause of pharyngitis, with over 600 million cases per year (5). The clinical symptoms of GAS pharyngitis include a sudden-onset fever accompanying a sore throat, which frequently manifests physically as inflammation of the pharynx and tonsils, often with patchy exudates and cervical lymph node adenopathy. Other common symptoms include malaise, fever, headache, nausea, abdominal pain, and vomiting (62, 63). Uncomplicated GAS pharyngitis is generally self-limiting. Pharyngitis is spread by person-to-person contact, presumably via nasal secretion or saliva droplets from carriers or infected individuals (63, 64). As such, the incidence of pharyngitis is highest in places of crowding, such as schools and military training facilities. Approximately 15% of schoolchildren and 4 to 10% of adults may suffer an episode of GAS pharyngitis each year in developed countries (5), whereas incidence rates in developing countries are 5 to 10 times higher (5).
Scarlet fever.
Occasionally, GAS pharyngitis is accompanied by scarlet fever, which is thought to result from pharyngeal infection with a GAS strain that secretes bacteriophage-encoded streptococcal pyrogenic exotoxins, most notably SpeA (64–66). Also known as scarlatina, scarlet fever manifests as a deep red, finely papular, erythematous rash; “strawberry tongue”; and exudative pharyngitis (61). While scarlet fever was a significant cause of childhood morbidity and mortality in the 19th and early 20th centuries, global rates have steadily declined over the last 150 to 200 years such that it was considered a relatively rare disease until recently (67, 68). However, recent outbreaks of scarlet fever in Hong Kong and mainland China illustrate that scarlet fever remains a significant health problem (see below).
Impetigo.
Impetigo is a contagious infection of the skin that manifests as pustules that gradually enlarge and rupture, forming thick, honey-colored scabs (64, 69). The disease is spread through direct skin contact and most commonly affects children living in tropical and subtropical climates in areas with poor hygiene and crowded living conditions (69, 70). The annual global disease burden is estimated to be 111 million cases (5).
Burden of Immune Sequelae
Prior GAS infection may result in a number of postinfectious sequelae, which include ARF/RHD, APSGN, and, debatably, pediatric autoimmune neuropsychiatric disorders.
Acute rheumatic fever.
Acute rheumatic fever (ARF) is a systemic disorder that can follow untreated GAS pharyngeal infection (71). Diagnosis of ARF is based on updated Jones criteria and consists of fulfilling a certain number of major and minor disease criteria (72). The major manifestations are inflammation of the joints (arthritis) (60 to 80% of cases), inflammation of the heart (carditis) (30 to 45% of cases), and/or neurological symptoms (e.g., Sydenham chorea) (10% of cases). Less common manifestations of the skin include erythema marginatum (2% of cases) or, rarely, subcutaneous nodules (73). ARF is a major source of morbidity and mortality worldwide, particularly as it may result in long-term damage to the heart, termed RHD, with an estimated 15.6 million cases worldwide in 2005, including 282,000 new cases and 233,000 deaths each year (5). This disease burden includes over 2.4 million cases in patients aged 5 to 14 years, making RHD the most common cause of pediatric heart disease worldwide (73). ARF is a particularly serious problem in indigenous populations and developing nations, where the highest rates of disease are observed. In a 2005 study, the highest rates of ARF were found in sub-Saharan Africa (5.7 cases per 1,000), in the Pacific Islander and indigenous minority populations of Australia and New Zealand (3.5 cases per 1,000), and south central Asia (United Nations regional classification) (2.2 cases per 1,000) (5). Due to the high rates of impetigo and low rates of GAS pharyngitis in the indigenous Australian population, it has been proposed that ARF can also occur as a complication of impetigo, although this has not been confirmed (74).
Acute poststreptococcal glomerulonephritis.
APSGN is an immune complex-mediated disorder of the kidneys, resulting in symptoms such as edema, hypertension, urinary sediment abnormalities, and decreased levels of complement components in the serum (66, 75). Globally, there are over 470,000 annual cases, resulting in approximately 5,000 deaths worldwide (5). APSGN rates are highest in children in less developed countries, with incidence rates as high as 94.3/1,000 being reported in the Northern Territory of Australia (76). Unlike ARF, APSGN tends to occur in outbreaks associated with “nephritogenic” strains of GAS (e.g., emm types 1, 4, 12, 49, 55, 57, and 60) and contributing risk factors such as crowding, poor hygiene, and poverty (75–77). With proper supportive care, long-term renal damage as a result of APSGN is rare.
Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections.
GAS infection has been hypothesized to be temporally associated with rare obsessive-compulsive disorders and Tourette's syndrome in children, termed pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). However, the relationship of GAS to these behavioral disorders remains highly controversial, and recent studies have failed to provide a conclusive link of GAS infection to the exacerbation of disease symptoms (78, 79). As such, the pathogenesis of PANDAS remains unknown, but it has been hypothesized that GAS infection results in antineuronal antibodies (79).
Burden of Invasive Disease
Less common than the superficial diseases and the immune sequelae described above, GAS has the capacity to breach epithelial barriers and cause a variety of invasive diseases, with high rates of morbidity and mortality. Approximately 8 to 23% of patients with GAS invasive disease die within 7 days of infection (7, 80, 81). The most common invasive diseases are bacteremia and cellulitis. GAS may also less commonly cause necrotizing fasciitis, septic arthritis, pneumonia, meningitis, abscess, osteomyelitis and other focal infections, endocarditis, and peritonitis. GAS invasive disease may be complicated by the development of STSS, which increases mortality rates; 23 to 81% of STSS patients die within 7 days postinfection (7, 80–82). There has been a notable global increase in the percentage of invasive GAS infections that are severe since the late 1980s (19, 20, 83, 84). A 2005 WHO study estimated that at least 663,000 cases of invasive GAS disease occur each year, resulting in 163,000 deaths (5). Similar rates of disease are observed in a number of developed countries (2 to 3 per 100,000), which corresponds to approximately 10,000 cases per year in the United States (7, 80, 81, 85, 86). As with other GAS diseases, the rates of invasive GAS disease are higher in less developed regions, with the highest rates being observed in indigenous populations in Australia (13.2 to 82.5 per 100,000 [87–89]), Africa (13.0 per 100,000 [90]), and the Pacific Islands (9.9 to 11.6 per 100,000 [91, 92]).
Outbreaks of GAS Diseases
While GAS is traditionally considered a pathogen in which disease results as a consequence of asymptomatic carriage or recent acquisition from a close contact, it also has the capacity to cause outbreaks. Small-scale localized outbreaks of GAS pharyngitis have been described in the literature and appear to occur as a result of transmission of a GAS strain to susceptible individuals through either a common source or, particularly in crowded settings, person-to-person spread. Examples include outbreaks in military training centers as a result of crowding (93, 94), multiple outbreaks following consumption of contaminated foods (95–102), and hospital-acquired puerperal sepsis outbreaks (22–24).
Large-scale GAS outbreaks also occur as a result of the emergence of dominant clones, which may arise through the horizontal acquisition of DNA that encodes toxins or antibiotic resistance determinants (19, 26, 40, 103), or through more subtle genetic changes (104, 105). The increase in the percentage of severe invasive GAS disease since the 1980s has paralleled the emergence of the M1T1 clone. This clone has disseminated globally and accounts for a significant proportion of clinical isolates throughout the developed world. The M1T1 clone has acquired three regions of heterologous DNA: a 36-kb chromosomal region that encodes the toxins streptolysin O (SLO) and NAD-glycohydrolase and two bacteriophages that encode the DNase Sda1 and the superantigen SpeA (19, 20, 40, 103). Similarly, outbreaks of M3 GAS disease in Canada have been attributed to the rapid expansion of clonal GAS strains following multiple genetic changes, including the acquisition of prophages encoding phospholipase A2 and SpeA and the duplication of 4 amino acids in the N-terminal region of the M3 protein (105–107). An emm 59 GAS outbreak of invasive disease in North America beginning in 2004 has been reported (21, 108).
A large ongoing outbreak of scarlet fever in Hong Kong and mainland China is being monitored (109–111). Starting in 2011, there was an alarming increase in the number of scarlet fever cases, including >1,500 cases in Hong Kong (110) and over 6,000 cases in Beijing (111). The Chinese Ministry of Health reported a combined 110,000 cases of scarlet fever in mainland China in 2011 to 2012. The genome sequence of 2 GAS emm 12 strains from Hong Kong revealed the acquisition of a 64.9-kb integrative and conjugative element encoding tetracycline and macrolide resistance and a 46.4-kb prophage encoding the superantigens streptococcal superantigen (SSA) and SpeC and the DNase Spd1 (26). However, broader molecular analysis identified multiclonal outbreak isolates that were likely circulating in the population prior to 2011. Thus, the reason for the rapid increase in the number of scarlet fever cases in Hong Kong and Beijing may be related to either population immune status and/or other unknown environmental factors (26).
GAS TRANSMISSION, ADHESION, AND TISSUE TROPISM
Transmission of GAS
The nasopharyngeal mucosa and skin are the principal sites of GAS asymptomatic colonization. These sites are also the most common sources of infections such as pharyngitis and impetigo. These tissue sites represent the primary reservoirs responsible for the maintenance and transmission of GAS to a new host. The characterized ability of GAS to overcome the innate and acquired immune mechanisms present in saliva allows the bacterium to remain viable for long periods (112), permitting transmission from infected persons or asymptomatic carriers via respiratory droplets. Similarly, the ability of GAS to colonize and persist in skin tissue permits transmission through person-to-person skin contact. Therefore, family members or close contacts of primary cases are at greater risk than the general population for subsequent infection. While GAS is transmitted primarily from person to person, there have been numerous reports of disease outbreaks caused by food-borne GAS (95–102).
Adherence of GAS
Lipoteichoic acid (LTA) and several proteins on the cell surface of GAS are important for the adherence of these bacteria to cultured human cells and to extracellular matrix (ECM) proteins (reviewed in reference 113). Initial bacterial attachment is hypothesized to be a two-stage process involving weak and/or long-range interactions followed by more specific, high-affinity binding (114). LTA, a membrane-bound amphiphilic polymer of glycerol phosphate containing glucose and d-alanine substitutes (115), may contribute to the initial adherence of GAS to host surfaces by establishing weak hydrophobic interactions between bacterial cells and host components (114). This weak interaction permits longer-distance first-attachment events to be mediated through long surface appendages such as pili, allowing the second stage of adherence to occur, involving multiple, higher-affinity binding events such as protein-protein or lectin-carbohydrate interactions.
The cell surface proteome of GAS includes numerous protein adhesins that permit bacterial interactions with multiple host components, which allow GAS to colonize diverse tissue sites in the human body (Table 2). GAS surface proteins are attached to the bacterial surface by three known mechanisms: covalently to the cell wall peptidoglycan anchored via a C-terminal LPxTG (where x is any amino acid) motif, which is recognized by sortase A (116–118); covalently bound to the cell membrane through N-terminal modifications with lipid (lipoproteins) (113, 118); and through noncovalent interactions with cell surface components (113).
TABLE 2.
Adhesins of S. pyogenesa
| Protein group | Protein(s) | Cell surface linkageb | Substrate(s) and/or function(s) | M type distributionc | Reference(s) |
|---|---|---|---|---|---|
| Pili | Spy0130, Spy0128, Cpa | LPxTG | Pilus structural proteins; collagen binding, gp340 binding, bacterial aggregation, biofilm formation | FCT-specific M type | 119, 126, 130 |
| M and M-like proteins | M1, M3, M6, protein H | LPxTG | Fibronectin binding, fibrinogen binding | M-type specific | 135, 141, 151 |
| AgI/II family | AspA | LPxTG | gp340; bacterial aggregation | M2, M28 | 595, 596 |
| Fibronectin binding | PrtF1/SfbI | LPxTG | Fibronectin binding | FCT-specific M type (FCT 1, 4, 5, 7, 8, 9) | 126, 169, 597 |
| PrtF2/PFBP/FbaB | LPxTG | Fibronectin binding | FCT-specific M type (FCT 3, 4, 6, 7, 8) | 126, 598–600 | |
| SOF/SfbII | LPxTG | Fibronectin binding, fibrinogen binding | In 55% of GAS strains | 168, 171 | |
| SfbX | LPxTG | Fibronectin binding | In 55% of GAS strains | 601 | |
| Fbp54 | Anchorless | Fibronectin binding, fibrinogen binding | In 100% of GAS strains | 172, 566, 602 | |
| FbaA | LPxTG | Fibronectin binding | M1, M2, M4, M9, M13, M22, M28, M44, M49, M60, M67, M75, M77, M79, M80, M82, M87, and M89 | 143 | |
| Collagen-like | Scl1, Scl2 | LPxTG | Laminin binding and α2β1 and α11β1 integrin binding | In 100% of GAS strains | 153, 159, 603, 604 |
| Laminin binding | Lbp | LXXC/XXGC | Laminin binding | In 100% of GAS strains | 155 |
| Shr | Anchorless | Laminin binding, fibronectin binding | In 100% of GAS strains | 154, 605 | |
| Plg binding | GAPDH/Plr | Anchorless | Fibronectin binding, plasminogen binding | In 100% of GAS strains | 606 |
| SEN | Anchorless | Plasminogen binding | In 100% of GAS strains | 343 |
Abbreviations: FCT, fibronectin binding, collagen binding, T antigen region.
LPxTG, sortase A motif; LXXC/XXGC, lipoprotein consensus sequence.
One or more M types can be associated with a specific FCT variant, but strains belonging to a specific M type carry the same FCT region (184).
GAS pili.
Discovered in 2005, GAS pili, or fimbriae, appear as long, flexible rods protruding up to 3 μm from the cell surface (119). GAS pili are heteropolymeric structures consisting of a pilus shaft made up of the major pilin protein subunit (Spy0128), which is assembled by a series of transpeptidase reactions catalyzed by a class B accessory sortase, SrtC (Spy0129) (120–122). Additionally, this class B sortase also mediates the attachment of the minor pilin proteins, including the adhesin Cpa (Spy0125), which attaches to the tip of the pilus, and the basal pilin Spy0130, which acts as a linker protein for attachment to the cell wall (122, 123). Sortase A is responsible for linking the pilus structure to the cell wall (124). The pilus biosynthesis and sortase genes are grouped together as a pathogenicity island located in the FCT region of the GAS genome, which encodes fibronectin binding proteins, collagen binding proteins, and T antigens (pilus subunit genes) (125). Among GAS isolates, this region displays considerable genetic diversity, with nine different FCT variants identified (126, 127). Pili contribute to GAS pathogenesis through direct involvement in bacterial adhesion. Pili mediate the attachment of M1 GAS (strain SF370) to human tonsillar epithelium and to primary human keratinocytes (128). Additionally, pili contribute to microcolony formation on human cells and the formation of biofilm (129). GAS pili also bind the salivary scavenger receptor glycoprotein gp340 to promote bacterial cell aggregation in saliva. This aggregation reduces bacterial adhesion to pharyngeal cells and therefore may serve as a bacterial clearance mechanism (130).
Role of M proteins in adherence.
The M protein is a major surface protein and virulence factor of GAS, which is anchored to the cell wall peptidoglycan through an LPxTG motif (117, 131). The M protein exists as a dimer consisting of two polypeptide chains complexed into an α-helical coiled-coil configuration that extends beyond the GAS surface as hairlike projections (132). The M protein typically consists of four repeat regions (designated regions A to D) that vary in size and amino acid composition. The C terminus of the molecule (including the C and D repeat regions) is highly conserved among GAS strains. The surface-exposed N terminus of the M protein usually consists of a hypervariable region (encompassing the A repeat region) and a semivariable B repeat region (reviewed in reference 133). While the genetic variability of M proteins is used as an epidemiological tool, such variability also imparts a diverse range of physiological functions. Numerous studies have demonstrated that GAS strains utilize M proteins for adherence to and internalization into epithelial cells and keratinocytes (134–139). M proteins (M1, M3, and M6) may promote bacterial colonization by binding directly to components of the ECM, such as fibronectin (140, 141). Additionally, ECM proteins such as fibronectin, which also bind to the host cell surface integrin α5β1, can act as bridging molecules between bacterial cells and host cells that express integrins on the cell surface. This interaction also promotes integrin-mediated internalization of GAS into host cells (135, 139, 142–144). Additionally, the M6 protein binds directly to ligands present on host cells, such as the keratinocyte membrane cofactor CD46 (145), and the M1 protein can bind to surface-expressed glycosaminoglycans, including dermatan sulfate, heparan sulfate, and heparin (146). Furthermore, the M1 protein promotes interbacterial aggregation to enhance bacterial adherence to and invasion of epithelial cells (147).
Other GAS proteins that bind ECM components.
Binding to ECM components is a common strategy used by numerous pathogens for host colonization. Cell surface adhesins that recognize ECM components have been termed MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) (148), which interact directly with ECM components such as fibronectin, collagen, and laminin. In GAS, 11 fibronectin binding proteins have been characterized, with the majority of these proteins being anchored to the cell wall through an LPxTG motif (Table 2).
Fibronectin binding proteins can be divided into two types: those that contain fibronectin binding repeats (PrtF1/SfbI, FbaA, PrtF2/FbaB/PFBP [S. pyogenes fibronectin-binding protein], SfbII/SOF [serum opacity factor], SfbX, and Fbp54) and those with no repeats (M proteins, protein H, glyceraldehyde-3-phosphate dehydrogenase [GAPDH]/Plr, Shr, and Scl1) (reviewed in reference 149). Fibronectin binding repeat sequences are generally located toward the C-terminal end of the protein and can vary in length and number (149). Each fibronectin binding repeat can potentially bind one fibronectin dimer via a specific protein-protein interaction called a “tandem β-zipper,” whereby a repeat sequence forms additional antiparallel β-strands on sequential β-sheet motifs located at the N terminus of fibronectin (150).
Other multifunctional surface adhesins possess the ability to bind to fibronectin despite lacking fibronectin binding repeat domains. These adhesins include M proteins (serotypes M1, M3, and M6) (135, 141), protein H (151), GAPDH (152), the hemoprotein binding protein Shr, and the collagen-like protein Scl1, which can bind both fibronectin and laminin (153, 154). Additionally, GAS strains possess a laminin binding lipoprotein, Lbp, which plays a role in epithelial cell adherence (155).
Epithelial cell adherence and internalization.
Many of the cell surface adhesins expressed by GAS are also involved in internalization into epithelial cells. Bacteria may utilize ECM proteins bound to MSCRAMMs as bridging molecules to bind to integrins present on the surface of host cells. This interaction activates cellular signaling pathways that lead to the rearrangement of cytoskeletal actin in host cells and the uptake of invading bacteria. The most well-characterized example of this signaling mechanism involves MSCRAMM-bound fibronectin and its subsequent interaction with integrin α5β1 (139, 144). When fibronectin binds to MSCRAMMs, it undergoes a conformational change which exposes an RGD motif allowing it to interact with the α5β1 integrin (149) and results in integrin clustering and the formation of focal complexes on the host cell surface (156), followed by internalization into the endocytic pathway via a “zipper-like” mechanism (156–158). Focal adhesion kinase (FAK); integrin-linked kinase (ILK); Rac; CDC42; and the Src kinases Src, Yes, and Fyn have also been shown to be involved in α5β1-mediated GAS internalization (144, 156). Similarly, the collagen-like protein Scl1 can also mediate epithelial cell adherence and internalization by directly binding to integrins α2β1 and α11β1 through their collagen-like domains (159, 160). Once internalized, some GAS strains are susceptible to autophagy following escape from endosomes into the cytoplasm (161, 162). However, expression of the secreted cysteine protease SpeB results in proteolytic clearance of autophagy components from the bacterial surface, allowing intracellular proliferation (161).
Role for human plasminogen in adhesion and internalization.
Plasminogen bound to the cell surface of GAS can also act as a bridging molecule for bacterial interactions with host cells. Plasminogen bound to the GAS cell surface can interact with keratinocytes via the cell-exposed integrins α1β1 and α5β1 (137). This interaction promotes integrin-mediated binding and internalization of GAS in a process which is similar to that of bacterial bound-fibronectin-mediated pathways.
GAS Tissue Tropism
Since GAS bacteria possess a large repertoire of adhesins, there is the possibility of a high degree of functional redundancy. However, the majority of adhesins are not present in all GAS serotypes. Of the adhesins listed in Table 2, only Fbp54, GAPDH, streptococcal surface enolase (SEN), and Lbp genes are present in all GAS strains examined. Additionally, the expression of GAS adhesins is highly regulated in response to environmental factors (163). Therefore, the production of functionally similar adhesins by GAS could be regarded as an adaptation mechanism that allows bacteria to successfully colonize the different tissue environments encountered during the infection process.
Numerous epidemiological studies support the existence of throat-tropic and skin-tropic GAS strains; i.e., certain emm types have a strong tendency to cause throat infection but not superficial skin infection, while other emm types are often recovered from cases of impetigo but rarely from cases of pharyngitis (164). The chromosomal arrangement of emm and emm-like genes is a genetic marker for tissue site preference, and these arrangements, referred to as emm patterns, consist of five major groups, patterns A to E (165). emm pattern A to C isolates are considered throat specialists, whereas emm pattern D strains are skin specialists. Furthermore, emm pattern E isolates are considered generalists and can be found at both tissue sites (166). Risk factors for skin infection include crowding, poor living conditions, and high rates of scabies infection, which may explain the relative prevalence of emm pattern D strains in indigenous populations, while crowding in places such as schools is the major risk factor for pharyngeal infection with GAS strains belonging to emm patterns A to C (42).
Attempts have been made to identify the specific adaptations responsible for niche specialization, and several GAS genes that display strong linkage disequilibrium with the emm pattern groupings have been identified (127, 167). Most of the linked genes currently identified are clustered within two genomic regions: the emm region itself and the FCT region (125). Genes with a differential presence or absence among tissue-tropic strains include cpa (encoding a type I collagen binding protein) (168), prtF1 (encoding the fibronectin binding protein PrtF1) (169), sof (encoding serum opacity factor, which contains a fibronectin binding domain) (170, 171), and prtF2 (encoding the fibronectin binding protein PrtF2) (172). Loci that form discrete phylogenetic lineages, which can then be associated with tissue-specific emm patterns, include the transcriptional regulators mga (a global regulator of numerous virulence factors) (173), rofA and nra (mutually exclusive transcriptional regulators of the genes in the FCT region) (167), and ska (encoding the plasminogen-activating protein streptokinase) (174, 175). While it is conceivable that tissue site preferences for infection in the throat or skin might be explained by the expression and/or regulation of tissue-specific colonization factors such as adhesins, the exact mechanisms responsible for tissue tropism are yet to be fully elucidated.
RESISTANCE TO HOST IMMUNE DEFENSE SYSTEMS
As a human-adapted pathogen, GAS is protected by a multitude of surface-bound and secreted virulence factors that subvert host innate immune defenses. These factors include enhanced resistance to phagocytosis, complement deposition, antibody opsonization, antimicrobial peptides (AMPs), and neutrophil killing mechanisms (Fig. 1).
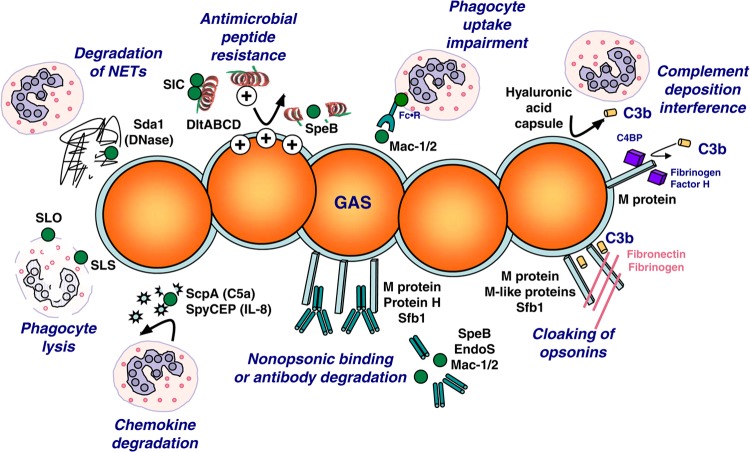
FIG 1.
The arsenal of virulence factors expressed by GAS to thwart the host innate immune response. The secreted proteases SpyCEP/ScpC and ScpA degrade the chemokines IL-8 and C5a, respectively, hindering phagocyte recruitment to the site of infection. Surface-associated M protein binds Fc domains of Ig and the complement-regulatory proteins C4BP and factor H to interfere with complement deposition. Mac-1/2 degrades Ig and binds phagocyte Fc receptors to block phagocytosis. Antimicrobial peptide resistance is mediated by hyaluronic acid capsule, d-alanylation of surface lipoteichoic acid by DltABCD, inactivation though SIC binding, and degradation by the cysteine protease SpeB. Ig and antimicrobial peptides are degraded by SpeB to facilitate the establishment of GAS infection in vivo. Secreted Sda1 DNase activity degrades NETs to promote neutrophil survival. The cytolysins SLS and SLO mediate lysis and apoptosis of neutrophils and macrophages.
Resistance to Opsonophagocytosis
In order to facilitate local invasion and systemic spread, GAS must resist opsonophagocytic killing by neutrophils, which represent the main host clearance mechanism for extracellular pathogens. GAS strains possess an extensive repertoire of virulence factors that play a crucial role in resistance to opsonophagocytosis, including complement inhibitors (M protein, hyaluronic acid capsule, and SIC), leukocidal toxins (SLO and streptolysin S [SLS]), immunoglobulin (Ig) binding proteins (PrtF1/SfbI, FbaA, and Sib), and Ig-degrading enzymes (IdeS, endo-β-N-acetylglucosaminidase of streptococci [EndoS], and SpeB).
Inhibitors of complement deposition and activation.
The M protein is expressed on the surface of all GAS isolates and plays a critical role in GAS resistance to opsonophagocytic clearance (176). Complement-inhibitory proteins, including C4b binding protein (C4BP) (177–179), factor H (180), and factor H-like protein 1 (FHL-1) (181), are bound by the M protein to thwart complement deposition and efficient opsonophagocytosis. Factor H binding accelerates the decay of the C3b opsonin deposited on the bacterial surface and limits the activation of the alternative complement pathway, thereby allowing GAS to persist in infected tissues (180). M-protein- and Mrp-mediated (182) fibrinogen binding confers resistance to phagocytosis by preventing complement C3 convertase deposition on the bacterial surface (183–185). Similarly, M-protein-mediated plasminogen binding prevents deposition of the opsonin C3b, preventing phagocytic uptake of GAS by neutrophils (186). Furthermore, the M protein impairs phagosome maturation by inhibiting the fusion of azurophilic granules within the phagosome (187).
Strains of most GAS serotypes are encased within a capsule composed of hyaluronic acid, a surface shield that further promotes resistance to opsonophagocytosis (188, 189). Capsule expression is upregulated in human blood (190, 191), and hyperencapsulated (mucoid) strains are frequently isolated from patients with GAS invasive disease (192). Encoded by the highly conserved hasABC synthase operon (193, 194), the capsule is a high-molecular-mass polymer of glucuronic β-1,3-N-acetylglucosamine (195) and is structurally identical to the hyaluronic acid expressed on human cell surfaces and connective tissue, allowing GAS to subvert the host immune response through molecular mimicry (6). The membrane-associated hyaluronate synthase, encoded by the first gene in the operon, hasA, is essential for hyaluronic acid biosynthesis (196, 197) and forms the linear hyaluronic acid polymer by the alternate addition of glucuronic acid and β-1,3-linked N-acetylglucosamine residues (196, 198). In serotype M1T1 GAS strains, the second gene in the operon, hasB, is nonessential due to the presence of HasB2, a secondary UDP-glucose 6-dehydrogenase encoded by hasB2 that is located outside hasABC (199). The third gene in the operon, hasC, is not essential for GAS capsule biosynthesis (196, 197).
The GAS capsule blocks antibody access to surface epitopes (200), inhibits complement deposition (188), promotes resistance to opsonophagocytosis (188, 189), enhances survival within neutrophil extracellular traps (NETs) (201), and is necessary for full virulence in mouse (202–205) and nonhuman primate (206) models of invasive GAS infection. Nonencapsulated GAS mutants are susceptible to phagocytic clearance in vitro and have dramatically reduced virulence in murine models of invasive GAS disease (202, 205). GAS emm 4 and emm 22 strains lack the hasABC operon, are nonencapsulated (207), and express a hyaluronic acid-degrading enzyme, hyaluronate lyase (HylA) (208). Despite the lack of capsule, emm 4 GAS strains proliferate in whole human blood ex vivo (207).
Streptococcal inhibitor of complement-mediated lysis (SIC) is a secreted 31-kDa virulence factor uniquely expressed by serotype M1 and M57 GAS strains (209) that binds and inhibits the complement C5b67 complex to prevent formation of the membrane attack complex (209, 210), which is generally ineffective against the thick and highly cross-linked Gram-positive bacterial cell wall. SIC also contributes to epithelial cell adherence (211) and mucosal colonization (212) and binds innate immune factors, including secretory leukocyte protease inhibitor, lysozyme, human α-defensin-1, and human cathelicidin antimicrobial peptide LL-37 (213–215). The sic gene is highly polymorphic, suggesting that it is under strong immune selective pressure to avoid host neutralizing antibodies (55). Variants of SIC, termed CRS (closely related to SIC) and DRS (distantly related to SIC), also bind complement proteins C6 and C7 (216, 217).
The fibronectin binding protein FbaA is encoded in the genome of strains of GAS serotypes 1, 2, 4, 22, 28, and 49 and is positively transcribed by Mga (143). FbaA binds the human complement-regulatory proteins factor H and FHL-1, preventing the deposition of C3b on the GAS cell surface, and promotes survival in whole human blood (218).
Leukocidins.
To avoid host defense mechanisms, GAS bacteria express extracellular toxins to directly attack immune cells or induce harmful by-products. SLO is a 69-kDa cholesterol-dependent and oxygen-labile cytolysin that contributes to beta-hemolysis under the surface of blood agar medium (219). SLO oligomerizes to form large pores (∼25 to 30 nm) in host cell membranes (220). SLO promotes resistance to phagocytic killing by disrupting the integrity of host cell membranes (220), inducing rapid caspase-dependent apoptosis in neutrophils, macrophages (221), and epithelial cells (222). SLO expression facilitates GAS escape from the endosome after host cell invasion (223) and reduces host tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) inflammatory cytokine responses, promoting virulence in mouse models of GAS invasive disease (221). Nicotinamide dehydrogenase (NAD-glycohydrolase) is coexpressed with SLO and transported into the cytoplasm of target cells through SLO-induced pores to deplete intracellular energy stores and augment host cell injury (224). In addition to protecting GAS from phagocytic killing, SLO contributes to tissue damage at the site of infection (221, 222). SLO has also been shown to facilitate penetration of stratified squamous cell mucosa by superantigens (225), enhancing the level of SLO-associated tissue damage during infection. Furthermore, for serotype M1 GAS strains, SLO has been shown to induce platelet-neutrophil aggregation (226), contributing to vascular occlusion and tissue damage in invasive disease. The central importance of SLO in GAS disease pathogenesis is supported by findings that SLO mutants are attenuated for virulence in subcutaneous, intravenous, and intraperitoneal mouse models of invasive GAS disease (221, 227, 228).
SLS is a potent membrane-active beta-hemolysin secreted by virtually all GAS isolates during stationary-phase growth, with structural similarities to the bacteriocin family of microbial toxins (229). SLS is encoded by a conserved nine-gene operon, comprised of the genes sagA to sagI (SLS-associated gene), which is responsible for the characteristic zone of beta-hemolysis surrounding GAS colonies on the surface of blood agar medium (229, 230). SLS is an oxygen-stable cytolytic toxin that enhances GAS resistance to phagocytosis by forming hydrophilic pores in neutrophil cell membranes (231). SLS contributes to GAS virulence and pathogenesis by lysing a broad spectrum of host cells, including lymphocytes, erythrocytes, platelets, and several other cells (232). The impairment of local inflammatory cells by SLS may promote the dissemination of GAS throughout the host (233). In vitro studies suggest that SLS may contribute to pathogenesis by direct cytotoxicity, inflammatory activation, and inhibition of neutrophil phagocytosis (234). Furthermore, the synergistic interaction between SLS, SpeB, and neutrophil-derived proteases has been shown to produce cellular injury in vitro (234). In a mouse model of streptococcal necrotizing soft tissue infection, wild-type GAS strains displayed enhanced virulence compared to SLS-deficient mutants, as measured by bacterial proliferation, neutrophilic inflammation, vascular injury, and tissue necrosis (230). These data suggest that SLS contributes to the rapid tissue necrosis that is characteristic of invasive GAS soft tissue infection. The expression of both SLO and SLS enhanced GAS virulence in a mouse model of bacterial sepsis (235).
Ig binding proteins.
Invading microorganisms are bound with immunoglobulin to facilitate killing by phagocytosis, complement fixation, or both. IgA is found predominantly in mucosal secretions and is most effective at restricting bacterial adhesion. IgG is located in the bloodstream and injured tissues, facilitating bacterium-phagocyte contact via the Fc receptors on phagocytes. GAS isolates are commonly associated with the expression of surface-associated Ig binding proteins that bind the Fc region of human IgA or IgG (236). The GAS Ig binding M proteins, M-related proteins (Mrp), and M-like proteins (Mlp or Enn) are classified into the M protein family (237). M and Mlp typically bind the Fc region of IgA, while Mrp binds the Fc region of IgG, preventing complement activation on the GAS cell surface (238, 239).
The fibronectin binding repeats of PrtF1/SfbI bind the Fc region of human IgG in a nonimmune fashion, which prevents phagocytosis by macrophages and antibody-dependent cell-mediated toxicity (240, 241). The secreted immunoglobulin binding protein from GAS, SibA, is a conserved 45-kDa protein that is encoded in the genomes of most GAS strains. SibA binds the Fc and Fab regions of IgG, IgA, and IgM antibodies (236). While SibA lacks sequence homology with M or M-related proteins, it has an N-terminal alpha-helical secondary structure, which is implicated in M protein Ig binding (236).
Ig-degrading enzymes.
The IgG-degrading enzyme of S. pyogenes (IdeS), also known as Mac-1, Sib35, and MspA, inhibits phagocytosis, neutrophil activation, and the release of reactive oxygen species by binding Fc receptor CD16 (FcγRIIIB) on the surface of neutrophils (242, 243). IdeS exhibits cysteine protease activity and contributes to opsonophagocytosis resistance in whole human blood by cleaving the lower Fc region of surface-bound human IgG (244). However, isogenic mutant studies found that IdeS is not essential for phagocyte resistance or mouse virulence in serotype M1T1 GAS strains (245). Mac-2, a related IgG endopeptidase, binds Fcγ receptors and prevents the recognition of IgG bound to GAS by host phagocytes (246).
Encoded by the ndoS gene in serotype M1 GAS strains, the endo-β-N-acetylglucosaminidase of streptococci (EndoS) is a secreted endoglycosidase that hydrolyzes the chitobiose core of the asparagine-linked glycan on the heavy chain of native human IgG (247), impairing recognition by Fc receptors, complement activation, and phagocytosis (248). The heterologous expression of EndoS in strains of non-M1 serotypes enhances virulence in a mouse model of invasive GAS infection (249). EndoS2, encoded by ndoS2, is a unique and conserved endoglycosidase of serotype M49 GAS with 37% identity to the EndoS protein. EndoS2 hydrolyzes biantennary and sialylated glycans on α1-acid glycoprotein (AGP) and all N-linked glycans on the heavy chain of IgG to impair immune effector functions (250).
Streptococcal pyrogenic exotoxin B (SpeB) is a broad-spectrum cysteine protease secreted by most GAS isolates (251) from late logarithmic to stationary phase (252). Encoded by the ubiquitous and conserved speB gene, SpeB is produced as an inactive 40-kDa zymogen and undergoes sequential N-terminal autocatalytic activation to the active 28-kDa protease (253). SpeB expression impedes the initial host immune response and is required for the establishment of localized skin infections (254), proliferation in human saliva (112), and full virulence in a humanized plasminogen mouse model of GAS infection (255). SpeB degrades IgG, IgA, IgM, IgD, and IgE (247).
Resistance to Antimicrobial Peptides
Antimicrobial peptides (AMPs) are small cationic molecules produced by host cells, often exhibiting potent bactericidal activity. GAS resistance to AMPs is mediated through degradation (SpeB and plasmin), electrostatic repulsion (DltA), or binding/inactivation (SIC).
Degradation or inactivation of AMPs.
SpeB cleaves and inactivates human cathelicidin LL-37 (256), a small cationic antimicrobial peptide of the innate immune system produced by skin keratinocytes, mucosal epithelial cells, and neutrophils with bactericidal activity and electrostatic affinity for negatively charged bacterial surfaces (257). The acquisition of plasmin protease activity on the GAS cell surface plays a key role in GAS pathogenesis (258). GAS surface-bound plasmin activity was recently demonstrated to promote degradation of human cathelicidin LL-37 (259). SIC also binds and inhibits antimicrobial peptide LL-37 (213–215).
Electrostatic repulsion of AMPs.
The d-alanine–d-alanyl carrier protein ligase, DltA, is required for d-alanylation of lipoteichoic acid (LTA) (260), a surface-bound amphiphilic polyglycerolphosphate polymer important for GAS epithelial cell adherence (261, 262). The d-alanine esterification of LTA, coordinated by the dltABCD operon (263), increases the net positive surface charge, improving GAS resistance to LL-37, acidic pH, lysozyme, and neutrophil-mediated killing (264). Targeted mutagenesis of dltA reduces expression levels of the M protein and SIC, rendering the bacteria more susceptible to C3b complement deposition and killing in whole human blood (265).
Impairment of Neutrophil Killing Mechanisms
Degradation of neutrophil attractants.
The proteolytic degradation of chemotactic substances such as IL-8 and C5a by S. pyogenes cell envelope protease (SpyCEP) and ScpA, respectively, impairs neutrophil recruitment, impeding phagocytosis at the site of infection and promoting GAS survival in vivo.
SpyCEP, also known as ScpC, is a cell wall-associated peptidase that perturbs neutrophil recruitment to the infection site by cleaving and inactivating the potent CXC chemokine IL-8 (266). SpyCEP activity increases GAS resistance to neutrophil-mediated killing (267) and enhances virulence a murine model of systemic infection (268).
The streptococcal C5a peptidase, also known as SCPA, is a highly conserved and immunogenic 125-kDa proteolytic enzyme located on the cell surface of all GAS strains that prevents phagocytic activity at the site of GAS infection (269–271). The gene encoding C5a peptidase, scpA, is ubiquitous among clinical GAS isolates and is located within the mga regulon (269). C5a peptidase is a subtilisin-like serine protease that interferes with the host immune system by specifically cleaving the polymorphonuclear leukocyte (PMN) binding site of anaphylotoxin C5a (272), a potent 11-kDa chemotactic peptide component of the complement system involved in neutrophil recruitment and stimulation (273). This cleavage inactivates the chemoattractant activity of C5a and impairs the recruitment of phagocytes to the site of GAS infection (269, 271).
The GAS surface protein GAPDH/Plr/SDH binds human C5a to facilitate its degradation by SCPA, subsequently inhibiting neutrophil chemotaxis and H2O2 production (274).
Resistance to neutrophil extracellular traps.
NETs are DNA-based structures containing microbicidal effectors, including histones, the granule proteases elastase and myeloperoxidase, and cathelicidin antimicrobial peptide LL-37, that are released by neutrophils at the site of infection to ensnare and kill bacteria (275). Serotype M1T1 GAS secretes the bacteriophage-encoded DNase Sda1, also known as SdaD2 (276), which degrades NETs and protects serotype M1T1 strains from neutrophil-mediated killing (277, 278). Sda1 also suppresses Toll-like receptor 9 (TLR9)-mediated innate immune responses and macrophage bactericidal activity by inhibiting the release of alpha interferon (IFN-α) and TNF-α (279).
Cell wall-anchored nuclease A (SpnA) also degrades NETs, promotes survival in whole human blood and neutrophil assays, and is required for full virulence in a mouse model of infection (280). The expression of the M protein and hyaluronic acid capsule enhances GAS survival within NETs by increasing bacterial resistance to the antimicrobial peptide LL-37 (201).
MOLECULAR MECHANISMS OF INVASIVE DISEASE
Severe GAS infection results from the ability of the organism to migrate to normally sterile sites, such as the bloodstream and deep tissues. The interplay between host and pathogen factors underlies the coordinated expression of multiple GAS virulence factors, ultimately leading to tissue destruction, bacterial dissemination, and hyperinflammation (Fig. 2). The mechanisms underlying invasive GAS disease, central to which are the processes of bacterial transcriptome modification, virulence factor expression, and dysregulation of host immune homeostasis, remain the subject of intense research.
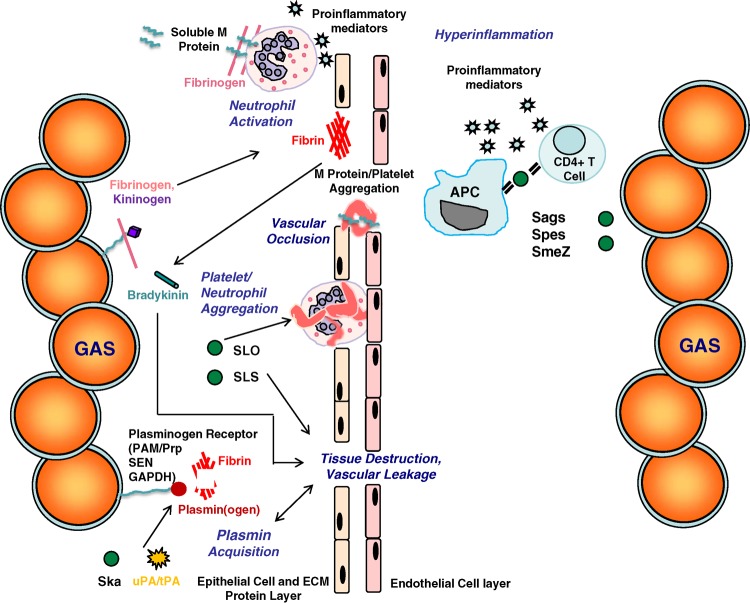
FIG 2.
The interplay between host and bacterial factors leads to tissue destruction, vascular leakage, and hyperinflammation in invasive GAS disease. Plasminogen is recruited to the GAS cell surface directly (PAM/Prp, SEN, and GAPDH) or indirectly (fibrinogen receptors). Activation of plasminogen is mediated by bacterial (Ska) or host (uPA/tPA) plasminogen activators. Subsequent plasmin activity contributes to fibrin degradation, tissue destruction, and vascular leakage. Complexes of soluble M protein and fibrinogen cross-link to β2-integrins on the neutrophil surface, triggering the release of proinflammatory mediators. Soluble M protein fragments also mediate the activation of the extrinsic pathway of coagulation by triggering tissue factor synthesis and platelet aggregation. Contact activation at the GAS cell surface (M protein, fibrinogen, and kininogen) leads to the formation of a fibrin network and bradykinin generation, contributing to vascular leakage. The secreted toxins SLO and SLS trigger apoptosis of host cells, leading to tissue destruction. SLO also mediates neutrophil platelet aggregation. Secreted superantigens (Sags, Spes, and SmeZ) bind to the beta-chain of antigen-presenting cells (APC) and CD4+ T cells, triggering the release of proinflammatory mediators and overstimulation of the immune response.
Types of Invasive Infections Caused by GAS
Invasive infection is defined as the entry of GAS into usually sterile sites of the body (e.g., blood or organs). Invasive GAS infections range from less severe forms (e.g., cellulitis) to diseases with high mortality rates (e.g., necrotizing fasciitis or STSS).
Cellulitis and bacteremia.
Cellulitis and bacteremia are the most common invasive infections caused by GAS, each accounting for 20 to 40% of invasive disease cases. In addition to impetigo (pyoderma), GAS infection of the skin may penetrate the epidermis to cause cellulitis or erysipelas. Cellulitis is an infection of the subcutaneous tissues and is characterized by redness and inflammation of the skin with associated pain and swelling, while a well-circumscribed infection that does not extend beyond the superficial layers is termed erysipelas (6, 281). Bacteremia is the presence of GAS in the bloodstream and is often characterized by high fever, nausea, and vomiting as a result of a robust proinflammatory cytokine response. GAS strains may be introduced directly into the bloodstream as a result of childbirth (puerperal sepsis) or injury or may be introduced transiently as a consequence of superficial infection or colonization of the throat or skin (282).
Necrotizing fasciitis.
Necrotizing fasciitis is a severe GAS infection of the skin, subcutaneous and deep soft tissue, and muscle. Due to the rapidly progressive nature of the disease, mortality rates associated with necrotizing fasciitis are high. In the United States and Europe, case fatality rates are reported to be 24% and 32%, respectively (7, 81), while in developing countries, the rates may be even higher (283). Furthermore, in the absence of surgical treatment, mortality rates are as high as 85.7% (284). This “flesh-eating” disease occurs as a result of rapid GAS growth and spread along the fascial sheaths that separate adjacent muscle groups, which in turn are breached, resulting in severe necrosis of the adjacent tissues (285). The pathogenesis of necrotizing fasciitis is thought to involve host and bacterial proteases (plasmin and SpeB), spreading factors (e.g., phospholipase), tissue-damaging enzymes released by activated host neutrophils, and a proinflammatory cytokine cascade arising from uncontrolled T-cell responses to superantigens (20, 61, 85, 282, 286). Blunt trauma is a major risk factor for the development of necrotizing fasciitis, presumably as a result of increased vimentin expression, which is thought to tether circulating GAS to the injured muscle (287, 288). Epidemiologically, host major histocompatibility complex (MHC) class II haplotype DR11/DQ3 is associated with necrotizing fasciitis, while haplotype DR3/DQ2 may protect against disease (289, 290).
Streptococcal toxic shock syndrome.
STSS can occur in conjunction with other GAS invasive diseases in response to superantigen production, resulting in a rapidly progressing illness characterized by high fever, rapid-onset hypotension, and accelerated multiorgan failure (291). In STSS, GAS superantigens simultaneously engage T-cell receptor beta-chain variable regions and MHC class II, activating large numbers of T cells in an antigen-independent manner. The result is a massive cytokine response by T cells (lymphotoxin-α, IL-2, and IFN-γ) and antigen-presenting cells (TNF-α, IL-1β, and IL-6), which causes widespread tissue damage, disseminated intravascular thrombosis, and organ dysfunction (7, 81, 291, 292). There is strong epidemiological and genetic evidence that the host MHC class II haplotype influences host susceptibility, with haplotype DR15/DQ6 being less commonly associated with STSS disease and haplotype DR14/DQ5 being more commonly associated with this disease (289, 290).
Role of Gene Regulators in GAS Invasive Infections
There are approximately 13 two-component regulatory systems (TCSs) and >100 putative standalone transcriptional regulators in the GAS genomes sequenced to date (34, 35, 39, 293). Both ex vivo and in vivo studies demonstrate that controlled expression of bacterial genes in response to changes in the host environment facilitates the transition from superficial to invasive infection (20, 294, 295).
The ihk-irr two-component regulatory system.
The ihk-irr two-component regulatory system is comprised of the isp-adjacent response regulator (irr) and the isp-adjacent histidine kinase (ihk) (296). Exposure of GAS to neutrophil reactive oxygen species (ROS) or antibacterial peptides results in the activation of ihk-irr, which regulates approximately 20% of the GAS genome (297). Inactivation of this system in an M6 background significantly reduces GAS virulence in a mouse model of bacteremia (298). The contribution of ihk-irr to GAS invasive disease is likely due to the control of genes involved in cell wall synthesis and oxidative stress resistance, rendering GAS less susceptible to neutrophil and macrophage killing and promoting bacterial dissemination (298).
The mga regulon.
The standalone multiple-gene regulator (mga) activates the transcription of several virulence genes, including the M protein family (emm, mrp, arp, and enn), scpA, sof, sic, and sclA (299–304). The role of Mga in GAS virulence has been demonstrated in numerous models of GAS disease, including several invasive disease models (143, 305). Mga activity has been linked to carbohydrate metabolism (143, 303, 305), and variation in the phosphorylation of Mga in response to differences in sugar availability alters Mga function (306). Phosphorylation of Mga in vivo by the GAS phosphotransferase system (PTS) inactivates Mga, effectively downregulating the expression of key GAS colonization factors. In a carbohydrate-rich setting, where a colonization phenotype is desirable, PTS phosphorylation of sugars may prevent Mga phosphorylation, promoting the expression of key adherence factors. In contrast, low carbohydrate availability may signal GAS to disseminate into deep tissue sites; thus, phosphorylation of Mga would result in a downregulation of Mga-regulated adherence factors (306). The inhibition of Mga phosphorylation significantly decreased virulence in a mouse model of invasive infection (306), and thus, the ability to modulate Mga activity appears to contribute to invasive disease initiation.
The covRS two-component system and SpeB expression.
The control of virulence regulatory system (covRS), also known as the capsule synthesis regulon (csrRS) (296, 307, 308), encodes a membrane-bound sensor kinase (CovS) and a DNA binding response regulator (CovR) (309). CovS appears to mediate a general stress response in GAS, whereby specific environmental stimuli such as increased temperature, acidic pH, high salt concentrations, and supraphysiological concentrations of Mg2+ signal CovS to alter the phosphorylation state of CovR (309–311). CovR binds upstream of specific genes, thereby repressing gene expression. It has been hypothesized that phosphorylated CovR represses the expression of certain genes (speA, hasA, and ska), while nonphosphorylated CovR represses the expression of another, distinct set of genes, including speB and grab (312). The covRS regulon directly or indirectly regulates the expression of ∼10 to 15% of genes in the GAS genome (294, 295, 313) and is required for GAS survival under general environmental stress conditions (309). CovRS positively regulates the expression of the secreted cysteine protease SpeB and negatively regulates multiple virulence factors, including hyaluronic acid capsule, streptokinase (Ska), SLO, IgG-degrading enzyme of S. pyogenes (IdeS), and the DNase Sda1 (294, 308). Spontaneous and unidirectional mutations within covRS affect the expression of numerous virulence factors important for impeding the host innate immune response (294, 314) and the initiation and progression of GAS invasive disease (20, 277, 294). Multiple neutrophil resistance factors are upregulated in covRS mutants, including the hyaluronic acid capsule, SIC, IdeS/Mac-1/MspA, Sda1, SpeA, Ska, and C5a peptidase (294). Consequently, GAS isolates with mutations in either covR or covS are more resistant to phagocytosis and killing by human neutrophils (294) and are hypervirulent in mouse models of systemic GAS infection (277, 315). Less frequently, mutations in the standalone regulator ropB (alternatively known as rgg) have also been identified in invasive GAS isolates (316, 317). SpeB expression is strongly downregulated in both covRS mutants (277, 294) and ropB mutants (316, 318). The loss of SpeB activity prevents the degradation of key host proteins (plasminogen and fibrinogen) and GAS virulence factors (Ska, M protein, GAPDH, and SEN), allowing the sequestration of human plasmin protease activity on the GAS cell surface (255, 314). The surface accumulation of plasmin activity allows the pathogen to degrade host tissue barriers and spread systemically (255, 277). Serotype M1T1 GAS is the most frequently isolated serotype from severe invasive human infections worldwide (103), but strains of non-M1 serotypes also cause invasive GAS infections (319, 320). The frequency of covRS mutations in strains of non-M1T1 GAS serotypes is lower than that in M1T1 strains, which may explain why strains of non-M1 serotypes are less frequently isolated from human invasive infections (314). It is important to note that SpeB expression is required for full virulence in mouse models of invasive GAS disease (254, 255, 316, 321, 322), and covRS mutants of GAS are less able to establish infection due to enhanced capsule expression, which may explain the maintenance of the wild-type allele in the GAS population (323, 324).
Subversion of the Plasminogen Activation System
GAS has evolved numerous mechanisms to interact with the host plasminogen activation system. Epidemiological studies suggest that GAS strains associated with invasive disease bind plasminogen more avidly than those associated with benign infection (319), and the ability to accumulate cell surface plasmin is a prerequisite for systemic infection by multiple GAS serotypes (255, 258, 325). The glycoprotein plasminogen is found in plasma and extracellular fluids at concentrations of approximately 2 μM. Plasminogen activation generates the serine protease plasmin, which can degrade fibrin clots, connective tissue, ECM components, and adhesion proteins. Additionally, plasmin activation of prometalloproteases results in the degradation of the collagen structural components of the ECM, further facilitating widespread tissue destruction (326). Conversion of plasminogen to plasmin in the host is mediated by urokinase plasminogen activator (uPA) and tissue-type plasminogen activator (tPA). The broad proteolytic activity of plasmin necessitates tight regulation of this system. The major circulating inhibitor of plasmin is α2-antiplasmin; however, plasmin bound to cell surface receptors, or surfaces such as fibrin, is partially protected from inactivation by α2-antiplasmin (327). Sequestration of the proteolytic activity of plasmin by GAS can facilitate fibrinolysis, thus promoting the release of bacteria from a formed clot or preventing clot formation (328, 329). Plasmin degradation of fibrinogen can also initiate the release of products that enhance blood vessel permeability and the accumulation of inflammatory cells (330, 331). Thus, a major pathogenic consequence of plasminogen acquisition by GAS is unregulated tissue destruction and overstimulation of the inflammatory response.
Streptokinase.
GAS secretes the plasminogen activator streptokinase (Ska), which is highly specific for human plasminogen (332). Ska is a single-chain, 414-amino-acid protein composed of three distinct domains (333). Phylogenetic studies of the most divergent ska sequences have revealed two main sequence clusters (cluster types 1 and 2), with evidence of smaller subclusters observed in cluster type 2 sequences (cluster types 2a and 2b) (174, 175). Ska variants display significant functional differences, with cluster type 2, but not type 1, streptokinase requiring fibrinogen to efficiently activate plasminogen (175). The species specificity of Ska impacts the design and utility of small-animal models of GAS infection (334). To overcome this, a humanized plasminogen mouse model of GAS infection has been developed to clearly demonstrate that GAS exploits plasminogen in invasive disease. Following subcutaneous infection with a covS mutant GAS isolate, humanized plasminogen mice displayed 75% mortality, compared with 20% mortality for wild-type mice. In contrast, infection of humanized plasminogen mice with a ska deletion mutant resulted in only 27% mortality, clearly demonstrating the importance of Ska expression in this model (258). Ska expression is negatively regulated by covRS, and SpeB readily degrades and inactivates streptokinase (255, 294). Thus, covRS mutations enhance the ability of an isolate to activate plasminogen (255). In addition, it has recently been demonstrated that serotype M1T1 GAS strains utilize the host activator uPA to acquire cell surface plasmin in the absence of Ska and that this interaction also plays a role in a mouse model of invasive GAS disease (335).
Plasminogen binding proteins.
Approximately 15% of GAS isolates express plasminogen binding M proteins (PAM/Prp), and similar proteins have been identified in other streptococcal species (319, 336). These proteins bind plasminogen and plasmin directly with high affinity (337–339). Plasminogen binding to GAS M proteins is dependent on the presence of an internal plasminogen binding repeat domain comprised of positively charged lysine, arginine, and histidine residues (339–341). Once bound to the M protein, plasminogen can be readily activated to plasmin by both host activators (tPA and uPA) and Ska (337). For GAS strains that express a plasminogen binding M protein, targeted elimination of plasminogen binding reduces the capacity of GAS to cause lethal infection in the humanized plasminogen mouse model (258, 325, 342).
In addition to specialized plasminogen receptors, GAS bacteria secrete the glycolytic pathway enzymes α-enolase/SEN (343) and GAPDH/Plr/SDH (152), which themselves display plasminogen binding abilities. While the mechanisms underlying the translocation of glycolytic proteins, which lack secretion and surface-anchoring signals, to the GAS cell surface are not defined, it has been confirmed in multiple bacterial species (152, 344–346). Due to the metabolic function of these enzymes, their role as plasminogen receptors in invasive disease has been difficult to characterize using traditional molecular biology techniques involving gene deletion. For GAS strains expressing plasminogen binding M proteins, the contribution of glycolytic enzymes to plasmin recruitment appears to be limited (325). However, a number of GAS strains demonstrate human plasminogen-dependent virulence in the absence of a plasminogen binding M protein (255, 258), and α-enolase/SEN and GAPDH/Plr/SDH may provide an alternate pathway of plasminogen recruitment for these strains.
Fibrinogen binding proteins.
The acquisition of plasmin activity by some GAS isolates requires human fibrinogen in addition to plasminogen and Ska. According to this model, fibrinogen bound to the GAS cell surface provides a target for the lysine-dependent binding of a preassembled plasminogen-Ska complex (328). The trimolecular complex of plasminogen, fibrinogen, and Ska possesses plasmin activity, in addition to activating fluid-phase plasminogen in the presence of host inhibitors (347). Thus, plasminogen acquisition via the fibrinogen-dependent pathway results in the creation of an unregulated surface protease and an immobilized plasminogen activator (348). For GAS strains that do not possess high-affinity plasminogen binding proteins but do express fibrinogen binding proteins such as PrtF1 and PrtF2 variants (349), M protein variants (141), and the lipoprotein Spy0591 (350), this mechanism of plasmin acquisition may be central to plasminogen-mediated virulence. The interaction between GAS, fibrinogen, Ska, and plasminogen confers a stable cell-associated enzymatic activity which can lyse fibrin clots despite the presence of the plasmin inhibitor α2-antiplasmin (328). It has been demonstrated that GAS isolates associated with invasive infection bind significantly higher levels of plasminogen via the indirect pathway than do isolates associated with noninvasive infection (319).
Fibrinogen binding M proteins also mediate inflammation and vascular leakage via direct interactions with fibrinogen and fibrinogen fragments. Cleavage of the M protein from the GAS cell surface by SpeB results in the presence of soluble M protein fragments at the site of infection (351). Binding of soluble M1 to fibrinogen promotes neutrophil activation following cross-linking of β2-integrins on the neutrophil surface. Subsequent neutrophil degranulation leads to the release of heparin binding protein and other inflammatory mediators (352). This process mimics the hyperinflammatory state that is characteristic of invasive GAS infections, and M1 protein/fibrinogen complexes have been identified in biopsy specimens from patients with necrotizing fasciitis and septic shock (352).
GAS Superantigens
Severe GAS infections such as necrotizing fasciitis and STSS are hyperinflammatory states mediated in part by streptococcal superantigens (353). Clinical features of these conditions include increases in levels of inflammatory markers such as interleukin-1β, -2, and -6; IFN-γ; and TNF-α (354). Serum levels of proinflammatory mediators are strongly linked to the severity of infection (282, 289, 354). Multiple GAS superantigens have been identified. These include the streptococcal pyrogenic exotoxins (SpeA, SpeC, and SpeG to SpeM), the streptococcal superantigen (SSA), and streptococcal mitogenic exotoxin Z (SmeZ). Of these superantigens, SpeG and SmeZ are chromosomally encoded, while the remaining superantigen genes are located on prophages in the GAS genome (355). These low-molecular-weight, secreted proteins have a highly conserved tertiary structure consisting of an N-terminal β-barrel globular domain, a β-grasp motif located within the C terminus, and 2 conserved amino acid motifs at the interface between the N- and C-terminal domains of the molecule (356). Superantigens bind to the beta-chain of CD4+ T cells and MHC class II molecules on B cells, monocytes, and dendritic cells, resulting in the overstimulation of the inflammatory response and subsequent systemic toxicity, tissue necrosis, organ failure, and shock (357). Each superantigen is specific for a distinct repertoire of Vβ gene products and can therefore activate up to 20% of circulating naive T cells (358). Rampant T-cell activation has also been shown to occur following the uptake of superantigens by dendritic cells in vivo, further contributing to the proinflammatory cascade (359). In addition, in vitro studies suggest that SpeS can induce a strong proinflammatory response from stratified, squamous epithelial cells (225), suggesting a broader role for superantigens in amplifying the host inflammatory response to GAS. Most superantigens contain a low-affinity N-terminal binding site that recognizes the α-chain of MHC class II molecules. In addition, binding of SpeA and SSA to the β-chain of MHC class II molecules may be mediated by a high-affinity zinc binding site located in the C terminus (356, 360–362). Finally, SpeA, SpeI, SSA, SmeZ, SpeH, and SpeO contain a conserved CD28 binding site that recognizes the CD28 molecule on T cells (363, 364).
Numerous retrospective studies have attempted to make associations between specific GAS superantigens and disease severity. Early studies of STSS in which GAS serotypes M1 and M3 were associated significantly with infection suggested that there may be an association between the superantigen gene repertoire and invasive GAS disease (365–367); however, targeted mutagenesis of SpeA has a limited effect on the mitogenic activity of toxigenic GAS in vitro (368), and several recent studies indicate that there is no association between superantigen gene profile and disease outcome (369–371). Numerous allelic variants have been identified for SpeA, SpeC, SpeG, SSA, and SmeZ (355). This variation may have confounded early studies that screened clinical isolates for the superantigen repertoire, as primer sequences may not have been optimized to detect multiple allelic variants of the one superantigen. Thus, the large amount of functional redundancy between superantigens both highlights the biological importance of these molecules and suggests that host factors contribute significantly to the outcome of GAS infection, as discussed above in the context of MHC class II variation and susceptibility to STSS.
Dysregulation of the Coagulation System
Coagulopathy is another well-recognized clinical feature of invasive human infections, and clot formation may prevent bacterial dissemination. Transgenic mice lacking clotting factor V, deficient for thrombin generation, or lacking fibrinogen are more susceptible to invasive disease following subcutaneous challenge with GAS (372), highlighting the protective effect of proper coagulation in GAS infection. However, vascular thrombosis can promote hypoxia-induced tissue damage in necrotizing fasciitis (373). GAS activates the clotting cascade via both the intrinsic (contact activation) and extrinsic (tissue factor) pathways. Activation of the intrinsic pathway at the bacterial cell surface is mediated by the M protein, which facilitates the recruitment of clotting factors, including kininogen and fibrinogen (374). Kininogen binding has been demonstrated for the M1, M6, and M46 proteins (374). Activation of these clotting factors leads to the formation of a fibrin network, the generation of the proinflammatory peptide bradykinin, vasodilation, and vascular leakage (375). Activation of the intrinsic coagulation pathway occurs in animal models of severe streptococcal infection and also in patients suffering from invasive infection (376–378).
Serotype-dependent activation of the extrinsic pathway of coagulation has been demonstrated in vitro, with heat-killed serotype M1 and M3, but not serotype M6, GAS strains triggering tissue factor synthesis on isolated human monocytes and cultured human umbilical vein endothelial cells, resulting in subsequent induction of procoagulant activity (373, 379). Unlike the activation of the intrinsic pathway, which occurs at the cell surface, the activation of the extrinsic pathway is thought to occur via the interaction of the M protein with TLR2 following the release of M protein from the bacterial cell wall by bacterial or host-derived proteases (380–382). Soluble M protein also mediates platelet activation and aggregation in vitro (383), which may contribute to the aggregated platelet deposition seen in invasive GAS infections (383, 384). In an invasive model of GAS infection, disease severity was significantly reduced in platelet-depleted mice, implying a role for platelets in the migration of GAS from the local site of infection or in the survival of bacteria in the blood (384). Mutation of the mga regulon significantly reduced the ability of serotype M1 GAS bacteria to form complexes with platelets and decreased bacterial dissemination in this model. It was hypothesized that GAS may utilize platelet binding to facilitate passage through the blood to other organs or that bacteria in complex with platelets may be protected from the host immune response (384).
Soluble M protein may also initiate the activation of coagulation by both the intrinsic and extrinsic pathways following the release of procoagulant microvesicles from peripheral blood mononuclear cells (385). The level of procoagulant microvesicles increases significantly in a mouse model of invasive streptococcal infection, and these microvesicles appear to bind to the streptococcal surface, leading to the entrapment of the bacteria within a dense fibrin network (386). Thus, the coagulation system and the early immune response to GAS infection are tightly linked, and dysregulation of this system by GAS appears to contribute significantly to invasive pathogenesis.
MOLECULAR MECHANISMS OF IMMUNE SEQUELAE
GAS infection is associated with several immune-mediated conditions affecting a diverse set of organs and tissues, including the heart, kidneys, joints, brain, eyes, and skin.
Heart
Major criteria for diagnosis of ARF include carditis, migratory polyarthritis, Sydenham chorea, erythema marginatum, and subcutaneous nodules. In addition to one or two of these major clinical conditions, evidence for antecedent GAS infection is one of the important laboratory diagnostic criteria for ARF. Due to the latency of 1 to 5 weeks between infection and the onset of ARF, evidence for antecedent streptococcal infection often relies on serological investigation. If untreated, ARF may progress to RHD. It is well known that the host genetic makeup contributes to a predisposition for developing ARF and RHD. Individuals with certain class II human leukocyte antigens (e.g., DRB1, DRB4, and DQA1) and certain single-nucleotide polymorphisms for TNF-α and mannan binding lectin genes were found to exhibit a greater predilection for ARF and RHD (6, 387, 388).
There is strong support for ARF as an autoimmune disease with an involvement of cross-reactive antibodies and T-cell clones (387–389). Several polyreactive streptococcal antibodies have been shown to react with host tissue autoantigens. For instance, monoclonal antibodies to streptococcal antigens were found to react with both M proteins and sections of myocardium, and the major autoantigen was found to be myosin (390, 391). In the M5 and M6 proteins, the epitope responsible for eliciting this response was found to be the amino acid sequence QKSKQ (390). Likewise, a subset of antistreptococcal monoclonal antibodies was found to cross-react with N-acetyl-β-d-glucosamine (GlcNAc) in the GAS cell wall constituent group A carbohydrate (GAC) and heart proteins including myosin in the endothelium at the valve surface (392). Collectively, these findings provide strong evidence for antibody-mediated deleterious autoimmune reactions as possible primary triggers in the pathogenesis of RHD.
In addition, several lines of investigation revealed an involvement of cross-reactive T-cell clones in the pathogenesis of RHD (6, 393). Epitopes within M proteins as well as cardiac myosin are capable of activating these T-cell clones. An array of chemokines is expressed in myocardium and valvular tissue lesions, among which CXCL9/Mig was shown to play a role in mediating T-cell recruitment to the site of inflammation in the heart (394). Cunningham proposed that antibody mimicries trigger oligoclonal T-cell expansion, followed by their extravasation into the valve through valvular endothelial cells (395). This endothelial damage in ARF patients exposes valve collagen, which, on its own or after aggregating on ARF-associated GAS isolates, elicits elevated anticollagen responses (396, 397). Consistent with this hypothesis, several GAS M proteins, notably those associated with ARF and RHD, are known to bind collagen IV through a motif called PARF (peptide associated with rheumatic fever) (397). PARF-containing M or M-like proteins stimulate an immune response to collagen IV. Interestingly, these antibodies are distinct populations from anti-M-protein antibodies (398) and hence are not likely to be cross-reactive. Although it is possible that not all aspects of ARF/RHD disease pathogenesis could be attributable to cross-reactivity (399), it is believed that the anticollagen antibodies and recurring stimulation of T cells at the valve may result in tissue scarring. Taken together, both humoral and cellular immune responses to streptococcal antigens may contribute to the pathogenesis of RHD (389).
Brain
Sydenham chorea, an immune-mediated neurological disorder, is a definitive manifestation of ARF and RHD. Monoclonal antibodies produced by human hybridomas derived from Sydenham chorea patients are known to cross-react with GlcNAc and neuronal gangliosides (400). A similar group of neurological disorders, called pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS), is hypothesized to be a consequence of GAS infection. The diagnostic criteria for PANDAS include obsessive-compulsive disorder (OCD) or tic disorder, prepubertal onset of symptoms, abnormal choreiform movements, and a temporal relationship between GAS infection and exacerbated OCD behavior. In the early stages of PANDAS onset, it is difficult to differentiate this disorder from Sydenham chorea. However, chorea is associated with RHD, whereas PANDAS is not associated with RHD. The literature on whether GAS infection has a role in PANDAS and related neurological disorders has recently been critically reviewed (401). A definitive association between PANDAS and antecedent GAS infection is still quite debatable (402, 403). Although there may be an overlap in the immunopathogenic mechanisms of Sydenham chorea and the hypothesized PANDAS (reviewed in reference 404), a large number of studies suggest that these two neuropsychiatric diseases are separate entities (405–408).
Joints
Migratory polyarthritis is one of the major presentations of ARF. However, nonmigratory polyarthritis can also occur as a complication of poststreptococcal infection independent of ARF (409, 410). The disease is referred to as poststreptococcal reactive arthritis and is a distinct entity from ARF (411). Unlike ARF, poststreptococcal reactive arthritis is rarely associated with carditis; a long-term follow-up study (412) did not reveal cardiac involvement in poststreptococcal reactive arthritis patients. The latency period after the antecedent GAS infection is shorter than that for ARF. Finally, poststreptococcal reactive arthritis is less responsive to treatment than arthritis in ARF and is persistent. Occasionally, poststreptococcal reactive arthritis can co-occur with APSGN (413). There is also evidence for an association between poststreptococcal reactive arthritis and antecedent Streptococcus dysgalactiae subsp. equisimilis infection (414). The molecular mechanism for the pathogenesis of poststreptococcal reactive arthritis is poorly understood. However, epitopes shared between GAS M proteins and antigens in cartilage and synovium (415) suggest a role for autoimmunity in poststreptococcal reactive arthritis.
Kidneys
The clinical presentation of APSGN includes hematuria, proteinuria, edema, hypertension, elevated serum creatinine levels, hypocomplementemia, and general malaise. Antecedent GAS infections and increased titers to streptococcal antigens are other diagnostic criteria. The time lag between GAS infection and the onset of APSGN (1 to 6 weeks), high antibody titers to several GAS antigens in acute-phase sera, and the presence in the early stages of APSGN of glomerular deposits of IgG, complement C3, and immune complexes containing GAS antigens are consistent with an immune-mediated etiology of APSGN. However, the pathogenic mechanism of APSGN remains incompletely understood (416). Furthermore, despite several excellent correlations between outbreaks of APSGN and community-wide spread of specific GAS types (417), the identity of nephritogenic components in GAS are still undefined.
GAPDH (also referred to as nephritis-associated plasminogen receptor [NAPlr]), SpeB, and streptokinase have been claimed to be possible nephritis-associated antigens (416, 418–420). However, for each of these proteins, there are counterarguments expressing doubts over their involvement in APSGN pathogenesis (421). Interestingly, the unifying feature of all the above-mentioned proteins is the ability to bind plasmin and plasminogen. It is possible that multiple factors potentially initiate glomerular damage through the acquisition of plasmin activity. Such a mechanism has been proposed (422, 423).
The prognosis of APSGN is excellent among children, with long-term complications being rare. However, there have been reports (424–427) suggesting that a history of APSGN is an independent and strong risk factor for chronic kidney disease and end-stage renal disease. In the indigenous Australian population, the mean age of onset of overt albuminurea for GAS-seropositive patients is 15 to 30 years earlier than that for GAS-seronegative patients (428), suggesting that past GAS infection is a risk factor for chronic kidney disease in this population.
Four of the major nephritis-associated GAS serotypes (serotypes M1, M12, M55, and M57) produce highly antigenic secretory proteins called streptococcal inhibitor of complement (SIC) and distantly related to SIC (DRS). Moreover, antibodies against these proteins are persistent (209, 217, 429–431). In the indigenous Australian population, a greater proportion of individuals with a history of APSGN than those without were seropositive for DRS (431). A similar study showed an association between APSGN and SIC seropositivity in a Swedish pediatric population (432). More recently, such serological observations were extended to chronic kidney disease and end-stage renal disease patients in India (Mumbai), where both GAS diseases and chronic renal disease are endemic (433). Interestingly, in this population, SIC seropositivity was associated with chronic kidney disease and end-stage renal disease. Moreover, the prognosis of chronic kidney disease for SIC-seropositive patients was worse than that for SIC-seronegative patients.
TREATMENT OF GROUP A STREPTOCOCCAL INFECTIONS
Treatment of GAS Pharyngitis
Although GAS pharyngitis is typically a self-limiting disease, antibiotic treatment is recommended for individuals with symptomatic pharyngitis once GAS has been confirmed by throat culture or a rapid antigen detection test (RADT) (e.g., latex agglutination), to prevent progression to ARF (434). Additional benefits of treatment of GAS pharyngitis include amelioration of clinical symptoms, a rapid decrease in contagiousness for close contacts, and prevention of suppurative complications, including peritonsillar abscess, cervical lymphadenitis, and, possibly, invasive infections (434).
Where clinical and epidemiological findings strongly suggest GAS, antibiotic treatment can be started pending microbiological confirmation, provided that such treatment is discontinued if culture or RADT results are negative. Antibiotics shorten the clinical course of GAS pharyngitis (435); however, most signs and symptoms spontaneously resolve within 3 to 4 days, even without antibiotic treatment (436). Furthermore, the start of antibiotic treatment can be delayed for up to 9 days after the onset of pharyngitis and can still prevent the development of ARF (437). Thus, there is flexibility in the initiation of antibiotic treatment during careful evaluation of a patient with suspected GAS pharyngitis.
Most advisory groups recommend penicillin as the treatment of choice for GAS pharyngitis (434, 438), because of its narrow spectrum and because GAS has remained exquisitely susceptible to β-lactam drugs (439). Amoxicillin, with efficacy equal to that of penicillin, is often used in children because of the acceptable taste of suspension formulations. Orally administered macrolides, e.g., clarithromycin and azithromycin, are often effective. First-generation cephalosporins have excellent activity and are acceptable in patients who are allergic to penicillin and who do not manifest immediate-type hypersensitivity to β-lactam antibiotics or may be appropriate for patients at high risk for complications, with severe symptoms, or with a suspected treatment failure or relapse. Trimethoprim-sulfamethoxazole and tetracyclines are not effective and should not be used for GAS pharyngitis.
Penicillin resistance has not occurred in GAS anywhere in the world (440), and the baseline level of macrolide resistance is approximately 5 to 10%, although significant outbreaks of macrolide-resistant strains have appeared in specific communities (441–444), such that clinicians should be aware of local resistance patterns. Oral penicillin must be administered 2 to 4 times a day for 10 days in order to achieve maximal rates of GAS eradication (445). Several antimicrobial agents, including clarithromycin, cefuroxime, and cefixime, can successfully eradicate GAS from the pharynx when administered for ≤5 days (446, 447), and effective eradication with once-daily dosing has been described for amoxicillin, azithromycin, and various cephalosporins (448, 449). Importantly, the endpoints of these studies are generally the eradication of GAS and not symptomatic improvement or prevention of ARF, the two main clinical reasons for treatment. Also, such antibiotic alternatives tend to be more expensive than standard therapy and, with broader spectra of activity than penicillin, may produce greater perturbations to the normal flora (450).
Chronic carriers have GAS in their pharynx but are asymptomatic and have little or no immunologic response to the organism. Carriers are unlikely to spread GAS to their close contacts and are at very low, if any, risk for developing suppurative or nonsuppurative complications (451, 452). Treatment is generally not recommended for the carrier state, although it may be considered for close contacts in scenarios such as invasive disease outbreaks (453). Tonsillectomy is not recommended solely for the purpose of reducing GAS pharyngitis (434).
Impetigo/Pyoderma Management
Superficial GAS skin infections are managed with gentle cleansing, using an antibiotic soap and a washcloth to remove the honey-colored crust, along with antibiotic treatment, either topically or topically, in combination with oral therapy. Empirical therapy should recognize that Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA), is also a potential cause of impetigo. Topical mupirocin or retapamulin ointments are effective for focal disease, but systemic antibiotics are indicated for diffuse involvement and outbreaks among athletic teams, child care centers, or multiple family members (454). Clindamycin and linezolid provide dual coverage, including MRSA, but a β-lactam antibiotic is preferred when GAS is the proven infectious agent (434).
Treatment of Invasive Disease
Initial management of severe invasive GAS infection includes hemodynamic stabilization and specific antimicrobial therapy (455). When necrotizing fasciitis is suspected, prompt surgical exploration is required, and debridement or fasciotomy is almost always necessary (456). Parenteral antimicrobial therapy should include coverage of both GAS and S. aureus until the results of bacteriological studies are available. Once GAS has been identified, intravenously (i.v.) administered penicillin G (3 to 4 million units i.v. every 4 h) plus clindamycin (600 to 900 mg i.v. every 6 to 8 h) for 10 to 14 days is the drug regimen of choice (457, 458). A mouse model of streptococcal myositis indicated that clindamycin can be more effective than penicillin in eradicating GAS in severe, invasive infections; in part, this may result from the “Eagle effect,” wherein GAS reduces the expression of penicillin binding proteins (PBPs) during the stationary growth phase (459). As an additional potential benefit, clindamycin is a protein synthesis inhibitor that may limit the production of important GAS virulence factors such as the M protein or superantigens. A number of case reports and small series have described the use of intravenous immunoglobulin (IVIG) therapy in patients with severe invasive GAS infections. An observational cohort study in Canada reported reduced mortality among patients treated with IVIG (460), and a European, multicenter, placebo-controlled trial designed to evaluate the efficacy and safety of IVIG showed a slight trend (not statistically significant) toward reduced mortality rates (461). While IVIG is sometimes considered for patients with invasive GAS who do not respond promptly to other therapeutic measures, current Infectious Diseases Society of America guidelines remind us that the levels of toxin-neutralizing antibodies vary considerably among different IVIG preparations and that additional studies of efficacy are needed before a definitive recommendation for IVIG therapy can be made (457).
Management of ARF
The management of ARF includes antibiotic and anti-inflammatory therapy, symptomatic treatment, and prophylaxis to prevent further GAS infections (73). Following a diagnosis of ARF, an antibiotic is used to eradicate GAS from the upper respiratory tract, regardless of throat culture results. Patients should receive either a single intramuscular injection of benzathine penicillin, 10 days of orally administered penicillin or amoxicillin, or a macrolide for patients allergic to penicillin. Salicylate therapy (50 to 75 mg/kg of body weight/day) should be initiated for 2 to 4 weeks and then tapered over the next 4 to 6 weeks for patients with definite arthritis and those who have carditis without cardiomegaly or congestive heart failure. The response of ARF arthritis to salicylates is usually rapid, and other nonsteroidal anti-inflammatory drugs have not demonstrated greater efficacy. Corticosteroids (e.g., oral prednisone at 2 mg/kg/day for 2 weeks, with a slow taper over 4 to 6 weeks) are often used to treat patients with carditis and cardiomegaly or congestive heart failure, but the benefits of anti-inflammatory therapy for cardiac outcomes remain unproven (462, 463). Additional supportive therapies for patients with carditis include fluid and salt restriction, diuretics, oxygen, angiotensin-converting enzyme inhibitors, or digoxin. If the lesions of RHD are persistent and hemodynamically significant, catheterization or surgery may be required to repair or replace defective valves (463).
Secondary Prevention of Rheumatic Heart Disease
Antibiotic prophylaxis is used to avoid recurrences of GAS pharyngitis that increase the likelihood and severity of RHD (464). Such prophylaxis may be required into adulthood and perhaps for life for patients who have had carditis with the initial episode of ARF. Antibiotic prophylaxis can be discontinued for patients who have a relatively low risk of carditis after 5 years or when they reach the age of 21 years, whichever comes later. However, the discontinuation of antibiotic prophylaxis should include careful consideration of epidemiological factors that may influence the risk of further GAS infections. The treatment of choice for secondary prevention of GAS infection is a single injection of 1.2 million units of benzathine penicillin G every 4 weeks or continuous oral prophylaxis (e.g., penicillin twice daily) for patients who are likely to be compliant (465).
Treatment of Glomerulonephritis
Antibiotic therapy does not affect the clinical course of APSGN. However, antibiotic treatment may clear the nephritogenic GAS strain and reduce the risk of patient-to-patient transmission. Penicillin is the antibiotic of choice, but a macrolide is usually prescribed for patients who are allergic to penicillin. Similarly to ARF, immunosuppressant or anti-inflammatory therapy has not been shown to influence the resolution of APSGN renal lesions. Consequently, treatment is directed at the complications of the disease and involves supportive measures. More than 95% of children with APSGN spontaneously and completely resolve symptoms and edema and regain renal function. Urinalysis results are normal for >95% of patients 15 years following the acute episode (466).
ANTIBIOTIC RESISTANCE
Penicillin Sensitivity
GAS remains exquisitely sensitive to penicillin despite being the drug of choice for most GAS infections. The penicillin susceptibility of GAS has not altered significantly in over 50 years of use of penicillin as a treatment for GAS infection (467). Reported MIC90 values of penicillin are a median of 0.016 mg/liter and a range of 0.0025 to 0.032 mg/liter (467–471). There has been only a single report of naturally occurring penicillin-tolerant GAS (472); however, this report has not been independently confirmed. Despite universal sensitivity to penicillin, GAS infections may fail to respond to penicillin therapy, leading to persistent throat carriage and recurrent infections (473, 474). It has been suggested that GAS may escape penicillin treatment by entering epithelial cells, which are poorly penetrated by penicillin (475–477), or by forming a biofilm (478, 479), or alternatively, failure of penicillin treatment may possibly be due to the protection of GAS by other β-lactamase-producing bacterial species; however, this has not been proven (480, 481).
Penicillin resistance occurs in the closely related species Streptococcus pneumoniae, where resistance is mediated by mutations that result in conformational changes in PBPs, the targets of β-lactam antibiotics (482). Similar first-step mutations leading to penicillin nonsusceptibility have been demonstrated in group B Streptococcus (483). While GAS strains express PBPs (484, 485), they have not been shown to naturally accumulate mutations in response to penicillin exposure. In the 1950s, experimental penicillin-tolerant strains of GAS were isolated following multiple passages through medium containing sublethal concentrations of penicillin. These penicillin-tolerant GAS bacteria displayed reduced M protein production and alterations of other virulence characteristics (486, 487). Ethyl methane sulfonate mutagenesis of GAS also allowed selection of penicillin-tolerant strains, with comparatively poor growth rates (488). Thus, the lack of naturally occurring penicillin-resistant GAS isolates may be due to a fitness cost associated with such mutations (489). As β-lactamase enzymes are often plasmid encoded (490), resistance can potentially be readily transmitted between bacteria. While no streptococcal species has been found to express β-lactamase activity, continued ongoing surveillance measures are recommended (489).
Resistance to Macrolides
Macrolides (erythromycin, azithromycin, and clarithromycin) are common alternative antibiotics used for patients who are allergic to penicillin, and the lincosamide antibiotic clindamycin is commonly used for the treatment of patients with life-threatening soft tissue infections, as it may reduce exotoxin production (459, 491, 492). Macrolide resistance in GAS occurs via two mechanisms: target site modification or target drug efflux. In target site modification, erm (erythromycin ribosomal methylase) genes encode an enzyme that methylates a single adenine in 23S rRNA. This results in a conformational change in the ribosome, leading to reduced binding of macrolide-lincosamide-streptogramin B-type antibiotics (493). In target drug efflux, mefA (macrolide efflux pump) genes encode an efflux pump of 14- and 15-carbon-ring macrolides (494), conferring resistance to erythromycin only. A worldwide surveillance program conducted from 1999 to 2000 revealed geographic heterogeneity in macrolide resistance. The prevalence of macrolide-resistant isolates was high in Poland (42%), Hong Kong (28%), Italy (25%), Portugal (24%), and Spain (21%); low in the United States (6.9%), Australia (4%), Turkey (1.9%), Sweden (4%), and Switzerland (2.6%); and absent in Indonesia, Austria, Belgium, the Netherlands, and the United Kingdom (443, 495). Some countries have a high proportion of mefA-positive isolates, whereas in other countries, erm genes dominate, leading to higher levels of clindamycin resistance. There is also temporal variation in the distribution of the mefA-encoded and erm-encoded phenotypes within the same geographic region (496–500). Studies in Finland, Spain, and Italy have shown a strong correlation between macrolide usage and resistance of GAS (501–503). A rapid increase in the percentage of macrolide-resistant strains may also be due to the clonal expansion of a single resistant strain (442, 498, 504). Many countries have instituted a more restrictive use of macrolides in response to alarming reports of high rates of macrolide resistance among GAS strains. For instance, in Germany, the rate of macrolide resistance in pediatric isolates fell from 13.6% (1999 to 2003) to 2.6% (505); in Belgium, the prevalence fell from 13.5% to 3.3% during 1999 to 2006 and remained low from 2006 onward (506); and in Spain, the rate fell from 34.2% in 2001 to 2004 down to 7.4% in 2007 to 2008 (507). In South Korea, the prevalence of macrolide-resistant GAS strains fell from 44.8% in 2002 to just 4.6% in 2009; however, in this case, the decrease was more likely due to a reduction in the prevalence of a resistant emm 12 GAS clone rather than a decrease in macrolide usage (496). Despite these observed declines in some countries, many other countries still have exceptionally high levels of macrolide-resistant GAS strains; for example, >96% of GAS strains isolated from Beijing, China, in 2011 were erythromycin resistant (111). However, in this case, high rates of macrolide resistance were due to the emergence of a resistant emm 12 clone. Variations in clonal prevalence can lead to significant temporal variations in the rates of macrolide resistance among GAS strains (111, 496, 508).
Tetracycline Resistance
In GAS, tetracycline resistance is conferred by ribosome protection genes such as tet(M) or tet(O) (509). These proteins share homology with elongation factors EF-G and EF-Tu and possess GTPase activity that is important for the displacement of tetracycline from the ribosome (510, 511). Efflux pumps for tetracycline encoded by the tet(K) or tet(L) gene also confer tetracycline resistance; however, in contrast to macrolide resistance, these efflux pumps are relatively uncommon in streptococci (512, 513). In a cohort of GAS strains sampled globally between 1990 and 2000, 38% and 25% displayed resistance to tetracycline and erythromycin, respectively, and 15% of isolates were resistant to both classes of drugs (514). The erm and mef genes are often colocated with tet genes on mobile elements, and a high proportion of GAS strains are resistant to both macrolides and tetracycline (515–519); as such, tetracycline usage may also drive macrolide resistance and vice versa.
Resistance to Fluoroquinolones
GAS isolates with low-level resistance to fluoroquinolones have been reported by several groups (506, 520–524). GAS strains with high-level fluoroquinolone resistance occur far less frequently (499, 521, 524–526). Low-level fluoroquinolone resistance in GAS is brought about by point mutations within the quinolone resistance-determining region (QRDR) located within topoisomerase IV (parC and parE) (499, 521, 524–526). High-level resistance results from additional point mutations in the DNA gyrase (gyrA and gyrB) genes (521). These point mutations may be acquired by spontaneous mutation within the QRDR or via horizontal gene transfer (527). The subsequent clonal expansion of resistant strains has contributed to rapid increases in the prevalences of fluoroquinolone-resistant GAS isolates in recent years (521). The rate of occurrence of fluoroquinolone resistance is increasing. For example, in Belgium in 2008, only 4.3% of isolated GAS strains were fluoroquinolone nonsusceptible, but this figure rose to 21.6% in 2010 (506). Likewise, in Spain in 2005, a 1.9% rate of fluoroquinolone nonsusceptibility among isolated GAS strains was recorded, but this rate rose to 30.8% in 2007 (521). In both of these studies, the majority of fluoroquinolone-nonsusceptible strains were emm 6 and emm 75 GAS strains, indicating the importance of clonal spread in the dissemination of fluoroquinolone nonsusceptibility.
VACCINE CANDIDATES
A safe and effective human GAS vaccine is not yet available. A number of obstacles hinder the development of a GAS vaccine, including serotype diversity (528), antigenic variation (529), pronounced differences in the geographical distribution of serotypes (4), and, critically, the potential for GAS antigens to produce immunity against host tissue that triggers autoimmune sequelae (400). A diverse array of GAS proteins and molecules have been assessed as vaccine candidates, including cell wall-anchored proteins, membrane-associated proteins, secreted proteins, anchorless proteins, and the group A carbohydrate molecule (2, 31).
M-Protein-Based Vaccine Preparations
GAS vaccinology has been focused largely on the cell wall-anchored M protein. Historical studies administered crude whole M protein preparations to human volunteers, which was followed by challenge with live GAS bacteria in the pharynx. While these preparations decreased GAS colonization (530–532), an increased incidence of ARF was reported for some of the immunized subjects (533). Several recent M-protein-based studies have investigated the protective efficacy of the N-terminal hypervariable region (338, 444, 534–539), as this region may not be involved in autoimmune disease. Two N-terminal M protein vaccine preparations have reached human clinical trials to date: a hexavalent preparation (444, 539, 540) and a 26-valent preparation (541, 542). These preparations were well tolerated in adult volunteers. More recently, a similar multivalent vaccine was developed, which includes N-terminal peptides representing 30 GAS serotypes (543). Sera raised against this experimental vaccine exhibited bactericidal activity against all 30 vaccine serotypes as well as 24 (of 40 tested) nonvaccine serotypes. While such preparations have been shown to have the capacity to protect against select nonvaccine serotypes, they do not protect against all globally circulating serotypes (4, 81, 544–546). The 30 serotypes included are based on those prevalent in North America and Europe (543), which often differ from the diverse serotypes isolated in regions of endemicity. An alternative approach is to target the highly conserved C-repeat region of the M protein, as this eliminates concerns regarding serotype-specific antibody responses. Protection has been observed following heterologous expression of the C-repeat region by vaccinia virus (547) and Lactococcus lactis (548). Conformationally restricted C-repeat-region peptides (J8 and J14 and derivatives thereof and StreptInCor, a 55-mer peptide containing B- and T-cell epitopes from the conserved region of the M5 protein) have been extensively studied and were observed to induce bactericidal and/or protective antibodies in animal-based trials (549–560).
Other Vaccine Targets
Group A carbohydrate.
The group A carbohydrate (GAC), composed of a polyrhamnose backbone with an immunodominant GlcNAc side chain, is present on the surface of strains of all GAS serotypes irrespective of M type and has been examined as a GAS vaccine candidate. Affinity-purified anti-GAC antibodies successfully opsonized three tested GAS serotypes (561), and mice immunized with GAC were protected against both intraperitoneal and intranasal GAS challenge (562). However, immunological cross-reactivity between anti-GAC antibodies and host heart valve proteins (563) and cytoskeletal proteins, such as actin, keratin, myosin, and vimentin (392), raises important potential safety concerns regarding the use of GAC as a GAS vaccine constituent.
Other protein antigens.
A number of non-M-protein antigens have also been characterized as GAS vaccine candidates, although none of these have reached human clinical trials. Non-M-protein antigens that have been tested in animal models include fibronectin binding protein A (FbaA) (564), protein F1/SfbI (240), serum opacity factor (SOF/SfbII) (565), fibronectin binding protein 54 (FBP54) (566), R28 protein (567, 568), streptococcal protective antigen (Spa) (569), C5a peptidase (ScpA) (570–572), streptococcal hemoprotein receptor (Shr) (573), streptococcal pyrogenic exotoxin B (SpeB) (574, 575), streptococcal secreted esterase (Sse) (576), streptococcal immunoglobulin protein 35 (Sib35) (577), Streptococcus pyogenes cell envelope protease (SpyCEP) (578), arginine deiminase (ADI) (579), and trigger factor (TF) (579). While each of these antigens exhibited protection against GAS infection in selected animal models, there are concerns regarding the incorporation of a number of these antigens into GAS vaccine preparations. Expression of FbaA, protein F1/SfbI, SOF/SfbII, and Shr is limited to a subset of serotypes, and their sequences can vary among the serotypes that do express them. For example, SOF/SfbII exhibits up to 57.1% sequence divergence (529). In addition, some antigens may exhibit protection in one animal model but not another. For example, protein F1/SfbI was observed to protect against GAS intranasal challenge (240) but did not protect against subcutaneous challenge (580). Finally, a number of these antigens exhibit toxic effects in the host or possess enzymatic activity, including SOF, ScpA, SpeB, Sse, Sib35, SpyCEP, SLO, ADI, and TF. The incorporation of the active forms of these antigens into GAS vaccine preparations may raise safety concerns, and consequently, inactive forms of these antigens have been engineered by employing site-directed mutagenesis for use in vaccine studies, including SOF (581), ScpA (571), SpeB (575), SpyCEP (578, 582), SLO (582, 583), and ADI (584).
GAS Reverse Vaccinology
Reverse vaccinology is the identification of vaccine targets through in silico analysis of the genome of the target pathogen, enabling the rapid discovery and identification of all possible antigens, putative virulence factors, and surface-associated proteins (585). This technology, underpinned by proteomics, whole-genome sequencing, immunoblotting, bioinformatics, and DNA microarray analysis, has already resulted in the successful identification of a number of novel GAS antigens. For instance, one study coupled high-throughput multigenome data mining with proteomics to identify 52 potentially universal GAS vaccine candidates (586), which are yet to be tested in in vivo studies.
Demonstrating the robustness of this technology, SpyCEP has been identified as a protective GAS antigen in multiple studies employing GAS reverse vaccinology (582, 587, 588). One such study used trypsin to “shave” surface-exposed proteins, which were subsequently identified by proteomics (588). In this study, immunization with SpyCEP protected mice against subsequent GAS mucosal challenge. Another group produced genomic surface display libraries that were screened by using human serum samples from donors infected by GAS. This approach yielded 6 conserved antigens, including SpyCEP, each of which elicited protection against intranasal and intravenous challenge (587). Another approach used proteomics, immunoprotein array experiments, and flow cytometry to identify conserved, surface-associated, and/or secreted proteins of GAS. A combination of proteins (SpyCEP, Spy0269, and Spy0167) elicited protection against 4 GAS serotypes in intranasal and intraperitoneal challenge models (582).
In summary, these new approaches, in combination with more traditional vaccine research, hold promise for the development of a future GAS vaccine for use in humans.
CONCLUSIONS
The last 20 years have heralded significant advances in our understanding of the molecular mechanisms by which GAS causes human disease, as exemplified by a clinical association of covRS mutations with invasive GAS infections that was recapitulated in experimental studies of global gene expression, targeted mutagenesis, and animal infection. The crucial role of multiple components of the innate immune system in controlling GAS infection and preventing serious disease outcomes, including epithelial barrier function, antimicrobial peptides, the complement system, and phagocytic cells, including neutrophils and macrophages, is being revealed. The ability of GAS to produce serious invasive infections in previously healthy individuals defines a robust capacity of this pathogen to counteract these multifaceted host defenses. An emerging theme is the functional redundancy of GAS virulence determinants, with individual GAS factors cooperating to resist a key innate immune clearance mechanism or with a single GAS virulence determinant (e.g., M protein) harboring distinct functional domains that interfere simultaneously with multiple host defense components. Despite intensive efforts and considerable new information, the molecular mechanisms of GAS immune sequelae such as ARF and poststreptococcal glomerulonephritis remain to be conclusively defined.
The resistance of GAS to antibiotics other than penicillins and cephalosporins is an increasing concern. Apart from requiring vigilant global molecular epidemiology studies and surveillance, the spread of antibiotic resistance determinants in the GAS population should provide a fresh impetus for the development of a safe and effective commercial human vaccine against this pathogen, which ranks among the top 10 infectious disease killers worldwide. Several GAS antigens elicit protective immunity in animal models and show promise as vaccine components. A number of factors need careful consideration when developing GAS vaccines, including the conservation and serotype coverage of antigens, the geographical distribution of serotypes, the use of antigens devoid of autoimmune epitopes, the selection of human-approved adjuvants, and the design and validity of experimental animal models. Future global eradication of GAS disease may be achieved by preparations that optimally address each of these factors.
Biographies

Mark J. Walker is the Director of the Australian Infectious Disease Research Centre, a multidisciplinary network based at the University of Queensland and the QIMR Berghofer Medical Research Centre. He was awarded an Alexander Von Humboldt Fellowship in 1998 and a Fulbright Senior Scholar Fellowship in 2005. Professor Walker was elected a Fellow of the American Academy for Microbiology in 2013. He works with a collaborative network of Australian and international researchers to understand the emergence and evolution of group A Streptococcus, investigate host-pathogen interactions, and translate these research findings into new therapeutics and vaccines.

Timothy C. Barnett is currently a Senior Research Fellow at the Australian Infectious Diseases Research Centre, University of Queensland. Following his Ph.D. studies at the University of Tasmania, he completed postdoctoral training at Emory University on group A streptococcal gene regulation. His current research interests include the investigation of bacterial interactions with, and evasion of, the host innate immune system, with a particular focus on evasion of autophagy by pathogenic group A Streptococcus strains.

Jason D. McArthur is a Senior Lecturer in the School of Biological Sciences at the University of Wollongong. He received his Ph.D. from the University of Wollongong in 2003. His research interests include understanding the molecular mechanisms of plasminogen acquisition/activation that contribute to the pathogenesis of Streptococcus pyogenes and development of topical antimicrobial strategies for the treatment of biofilm-associated skin infections.

Jason N. Cole received his biotechnology degree and Ph.D. in microbiology at the University of Wollongong, Australia. In 2008, he was awarded a NHMRC Overseas Biomedical Training Fellowship to conduct microbial pathogenesis research at the University of California, San Diego. In 2012, he was awarded a NHMRC Project Grant to continue his research at the University of Queensland, Australia. Dr. Cole is currently a visiting research scientist at the University of California, San Diego. His research focuses on unraveling the invasive disease mechanism of group A Streptococcus, a human pathogen responsible for potentially fatal infections.

Christine M. Gillen completed her Ph.D. at the University of Wollongong, Australia, in 2008 studying surface proteins and potential vaccine candidates of group A Streptococcus. Following her Ph.D., Dr. Gillen was the recipient of a Helmholtz fellowship working at the Helmholtz Centre for Infection Research in Braunschweig, Germany, from 2008 to 2010. Her work in Germany focused on the pathogenesis of invasive disease of Streptococcus pneumoniae and the pathogenesis of rheumatic fever caused by zoonotic streptococcal species of group C and group G Streptococcus. In 2010, Dr. Gillen took up her current postdoctoral position at the University of Queensland, Australia, investigating the pathogenic mechanisms of group A streptococcal invasive disease.

Anna Henningham has been researching group A Streptococcus since 2004 and received her Ph.D. in Microbiology at the University of Wollongong, Australia. Her thesis characterized novel anchorless cell wall proteins and evaluated their potential as universal safe GAS vaccine targets. Her current postdoctoral research at the University of California, San Diego, explores the virulence mechanisms of group A Streptococcus, which play a role in invasive infection. She is also a trainee in the UCSD Program in Excellence in Glycosciences.

K. S. Sriprakash is the leader of the Bacterial Pathogenesis and Scabies Group at the QIMR Berghofer Medical Research Centre, Brisbane, Australia. His work covers a wide range of bacterial pathogens that cause sexually transmitted diseases (Chlamydia trachomatis and Calymmatobacterium granulomatis), melioidosis (Burkholderia pseudomallei), and streptococcal diseases. He has also previously worked on the molecular genetics of yeasts, industrial microbiology, recombination in phage lambda, and Mycobacterium.

Martina L. Sanderson-Smith obtained her Ph.D. in Molecular Microbiology from the University of Wollongong (UOW), Australia. Subsequently, she was awarded an Alexander von Humboldt Fellowship to undertake research at the Helmholtz Centre for Infection Research, Germany. Currently, she is a Senior Lecturer and NHMRC Career Development Fellow at UOW, leading a research team in the Illawarra Health and Medical Research Institute. Her research is focused on virulence mechanisms of the human pathogen Streptococcus pyogenes, with specific interests in interactions between S. pyogenes and the host fibrinolytic system and mechanisms of innate immune resistance.

Victor Nizet, M.D., is a pediatric infectious diseases physician and microbiologist who received his education and training at Reed College, Stanford University School of Medicine, Harvard University/Boston Children's Hospital, and the University of Washington in Seattle. In 1997, he joined the faculty of the University of California, San Diego, where he is now Professor of Pediatrics and Pharmacy, Chief of the Division of Pediatric Pharmacology and Drug Discovery, and Director of the institution's new Center for Immunity, Infection & Inflammation. His laboratory studies the molecular pathogenesis of leading human Gram-positive bacterial pathogens, mechanisms of host innate immune defense, and novel approaches to infectious disease therapy.
REFERENCES
- 1.Meakins JC. 1909. Phagocytic immunity in Streptococcus infections. J. Exp. Med. 11:815–824. 10.1084/jem.11.6.815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henningham A, Gillen CM, Walker MJ. 2013. Group A streptococcal vaccine candidates: potential for the development of a human vaccine. Curr. Top. Microbiol. Immunol. 368:207–242. 10.1007/82_2012_284 [DOI] [PubMed] [Google Scholar]
- 3.Smeesters PR, Mardulyn P, Vergison A, Leplae R, Van Melderen L. 2008. Genetic diversity of group A Streptococcus M protein: implications for typing and vaccine development. Vaccine 26:5835–5842. 10.1016/j.vaccine.2008.08.037 [DOI] [PubMed] [Google Scholar]
- 4.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. 2009. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect. Dis. 9:611–616. 10.1016/S1473-3099(09)70178-1 [DOI] [PubMed] [Google Scholar]
- 5.Carapetis JR, Steer AC, Mulholland EK, Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5:685–694. 10.1016/S1473-3099(05)70267-X [DOI] [PubMed] [Google Scholar]
- 6.Cunningham MW. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470–511. 10.1128/CMR.13.3.470-511.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamagni TL, Darenberg J, Luca-Harari B, Siljander T, Efstratiou A, Henriques-Normark B, Vuopio-Varkila J, Bouvet A, Creti R, Ekelund K, Koliou M, Reinert RR, Stathi A, Strakova L, Ungureanu V, Schalén C, Strep-EURO Study Group. Jasir A. 2008. Epidemiology of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol. 46:2359–2367. 10.1128/JCM.00422-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien KL, Beall B, Barrett NL, Cieslak PR, Reingold A, Farley MM, Danila R, Zell ER, Facklam R, Schwartz B, Schuchat A. 2002. Epidemiology of invasive group A Streptococcus disease in the United States, 1995-1999. Clin. Infect. Dis. 35:268–276. 10.1086/341409 [DOI] [PubMed] [Google Scholar]
- 9.Stevens DL. 1992. Invasive group A Streptococcus infections. Clin. Infect. Dis. 14:2–11. 10.1093/clinids/14.1.2 [DOI] [PubMed] [Google Scholar]
- 10.WHO. 2005. The current evidence for the burden of group A streptococcal diseases. WHO report. WHO, Geneva, Switzerland [Google Scholar]
- 11.Kaplan EL, Markowitz M. 1988. The fall and rise of rheumatic fever in the United States: a commentary. Int. J. Cardiol. 21:3–10. 10.1016/0167-5273(88)90003-4 [DOI] [PubMed] [Google Scholar]
- 12.Land MA, Bisno AL. 1983. Acute rheumatic fever. A vanishing disease in suburbia. JAMA 249:895–898 [PubMed] [Google Scholar]
- 13.Rotta J, Tikhomirov E. 1987. Streptococcal diseases worldwide: present status and prospects. Bull. World Health Organ. 65:769–777 [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens DL, Tanner MH, Winship J, Swarts R, Ries KM, Schlievert PM, Kaplan E. 1989. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N. Engl. J. Med. 321:1–7. 10.1056/NEJM198907063210101 [DOI] [PubMed] [Google Scholar]
- 15.Veasy LG, Wiedmeier SE, Orsmond GS, Ruttenberg HD, Boucek MM, Roth SJ, Tait VF, Thompson JA, Daly JA, Kaplan EL, Hill HR. 1987. Resurgence of acute rheumatic fever in the intermountain area of the United States. N. Engl. J. Med. 316:421–427. 10.1056/NEJM198702193160801 [DOI] [PubMed] [Google Scholar]
- 16.Widdowson JP, Maxted WR, Newrick CW, Parkin D. 1974. An outbreak of streptococcal sore throat and rheumatic fever in a Royal Air Force training camp; significance of serum antibody to M-associated protein. J. Hyg. (Lond.) 72:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuyama T, Ishii E, Muraoka K, Honjo S, Yamaguchi H, Hara T, Shimazaki K, Koga T, Moriya K, Ide M, Miyazaki S. 1996. Outbreak of acute glomerulonephritis in children: observed association with the T1 subtype of group A streptococcal infection in northern Kyushu, Japan. Acta Paediatr. Jpn. 38:128–131. 10.1111/j.1442-200X.1996.tb03454.x [DOI] [PubMed] [Google Scholar]
- 18.Zheng MH, Jiao ZQ, Zhang LJ, Yu SJ, Tang GP, Yan XM, He LH, Meng FL, Zhao F, Zhang MJ, Xiao D, Yang YH, Nie W, Zhang JZ, Wang ZJ. 2009. Genetic analysis of group A Streptococcus isolates recovered during acute glomerulonephritis outbreaks in Guizhou province of China. J. Clin. Microbiol. 47:715–720. 10.1128/JCM.00747-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleary PP, Kaplan EL, Handley JP, Wlazlo A, Kim ML, Hauser AR, Schlievert PM. 1992. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet 339:518–521. 10.1016/0140-6736(92)90339-5 [DOI] [PubMed] [Google Scholar]
- 20.Cole JN, Barnett TC, Nizet V, Walker MJ. 2011. Molecular insight into invasive group A streptococcal disease. Nat. Rev. Microbiol. 9:724–736. 10.1038/nrmicro2648 [DOI] [PubMed] [Google Scholar]
- 21.Tyrrell GJ, Lovgren M, St Jean T, Hoang L, Patrick DM, Horsman G, Van Caseele P, Sieswerda LE, McGeer A, Lawrence RA, Bourgault AM, Low DE. 2010. Epidemic of group A Streptococcus M/emm59 causing invasive disease in Canada. Clin. Infect. Dis. 51:1290–1297. 10.1086/657068 [DOI] [PubMed] [Google Scholar]
- 22.Ben Zakour NL, Venturini C, Beatson SA, Walker MJ. 2012. Analysis of a Streptococcus pyogenes puerperal sepsis cluster by use of whole-genome sequencing. J. Clin. Microbiol. 50:2224–2228. 10.1128/JCM.00675-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raymond J, Schlegel L, Garnier F, Bouvet A. 2005. Molecular characterization of Streptococcus pyogenes isolates to investigate an outbreak of puerperal sepsis. Infect. Control Hosp. Epidemiol. 26:455–461. 10.1086/502567 [DOI] [PubMed] [Google Scholar]
- 24.Turner CE, Dryden M, Holden MT, Davies FJ, Lawrenson RA, Farzaneh L, Bentley SD, Efstratiou A, Sriskandan S. 2013. Molecular analysis of an outbreak of lethal postpartum sepsis caused by Streptococcus pyogenes. J. Clin. Microbiol. 51:2089–2095. 10.1128/JCM.00679-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Yao W, Wang X, Li Y, Chen M, Wang G, Zhang X, Pan H, Hu J, Zeng M. 2012. Outbreak of scarlet fever associated with emm12 type group A Streptococcus in 2011 in Shanghai, China. Pediatr. Infect. Dis. J. 31:e158–e162. 10.1097/INF.0b013e31825874f3 [DOI] [PubMed] [Google Scholar]
- 26.Tse H, Bao JY, Davies MR, Maamary P, Tsoi HW, Tong AH, Ho TC, Lin CH, Gillen CM, Barnett TC, Chen JH, Lee M, Yam WC, Wong CK, Ong CL, Chan YW, Wu CW, Ng T, Lim WW, Tsang TH, Tse CW, Dougan G, Walker MJ, Lok S, Yuen KY. 2012. Molecular characterization of the 2011 Hong Kong scarlet fever outbreak. J. Infect. Dis. 206:341–351. 10.1093/infdis/jis362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasenbein ME, Warner JE, Lambert KG, Cole SE, Onderdonk AB, McAdam AJ. 2004. Detection of multiple macrolide- and lincosamide-resistant strains of Streptococcus pyogenes from patients in the Boston area. J. Clin. Microbiol. 42:1559–1563. 10.1128/JCM.42.4.1559-1563.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seppala H, Nissinen A, Jarvinen H, Huovinen S, Henriksson T, Herva E, Holm SE, Jahkola M, Katila ML, Klaukka T, Kontiainen S, Liimatainen O, Oinonen S, Passi-Metsomaa L, Huovinen P. 1992. Resistance to erythromycin in group A streptococci. N. Engl. J. Med. 326:292–297. 10.1056/NEJM199201303260503 [DOI] [PubMed] [Google Scholar]
- 29.Zimbelman J, Palmer A, Todd J. 1999. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr. Infect. Dis. J. 18:1096–1100. 10.1097/00006454-199912000-00014 [DOI] [PubMed] [Google Scholar]
- 30.Varaldo PE, Debbia EA, Nicoletti G, Pavesio D, Ripa S, Schito GC, Tempera G. 1999. Nationwide survey in Italy of treatment of Streptococcus pyogenes pharyngitis in children: influence of macrolide resistance on clinical and microbiological outcomes. Artemis-Italy Study Group. Clin. Infect. Dis. 29:869–873 [DOI] [PubMed] [Google Scholar]
- 31.Cole JN, Henningham A, Gillen CM, Ramachandran V, Walker MJ. 2008. Human pathogenic streptococcal proteomics and vaccine development. Proteomics Clin. Appl. 2:387–410. 10.1002/prca.200780048 [DOI] [PubMed] [Google Scholar]
- 32.Smeesters PR, McMillan DJ, Sriprakash KS, Georgousakis MM. 2009. Differences among group A Streptococcus epidemiological landscapes: consequences for M protein-based vaccines? Expert Rev. Vaccines 8:1705–1720. 10.1586/erv.09.133 [DOI] [PubMed] [Google Scholar]
- 33.Banks DJ, Porcella SF, Barbian KD, Beres SB, Philips LE, Voyich JM, DeLeo FR, Martin JM, Somerville GA, Musser JM. 2004. Progress toward characterization of the group A Streptococcus metagenome: complete genome sequence of a macrolide-resistant serotype M6 strain. J. Infect. Dis. 190:727–738. 10.1086/422697 [DOI] [PubMed] [Google Scholar]
- 34.Beres SB, Sylva GL, Barbian KD, Lei B, Hoff JS, Mammarella ND, Liu MY, Smoot JC, Porcella SF, Parkins LD, Campbell DS, Smith TM, McCormick JK, Leung DY, Schlievert PM, Musser JM. 2002. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. U. S. A. 99:10078–10083. 10.1073/pnas.152298499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferretti JJ, McShan WM, Ajdic D, Savic DJ, Savic G, Lyon K, Primeaux C, Sezate S, Suvorov AN, Kenton S, Lai HS, Lin SP, Qian Y, Jia HG, Najar FZ, Ren Q, Zhu H, Song L, White J, Yuan X, Clifton SW, Roe BA, McLaughlin R. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. U. S. A. 98:4658–4663. 10.1073/pnas.071559398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green NM, Zhang S, Porcella SF, Nagiec MJ, Barbian KD, Beres SB, LeFebvre RB, Musser JM. 2005. Genome sequence of a serotype M28 strain of group A Streptococcus: potential new insights into puerperal sepsis and bacterial disease specificity. J. Infect. Dis. 192:760–770. 10.1086/430618 [DOI] [PubMed] [Google Scholar]
- 37.McShan WM, Ferretti JJ, Karasawa T, Suvorov AN, Lin S, Qin B, Jia H, Kenton S, Najar F, Wu H, Scott J, Roe BA, Savic DJ. 2008. Genome sequence of a nephritogenic and highly transformable M49 strain of Streptococcus pyogenes. J. Bacteriol. 190:7773–7785. 10.1128/JB.00672-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakagawa I, Kurokawa K, Yamashita A, Nakata M, Tomiyasu Y, Okahashi N, Kawabata S, Yamazaki K, Shiba T, Yasunaga T, Hayashi H, Hattori M, Hamada S. 2003. Genome sequence of an M3 strain of Streptococcus pyogenes reveals a large-scale genomic rearrangement in invasive strains and new insights into phage evolution. Genome Res. 13:1042–1055. 10.1101/gr.1096703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smoot JC, Barbian KD, Van Gompel JJ, Smoot LM, Chaussee MS, Sylva GL, Sturdevant DE, Ricklefs SM, Porcella SF, Parkins LD, Beres SB, Campbell DS, Smith TM, Zhang Q, Kapur V, Daly JA, Veasy LG, Musser JM. 2002. Genome sequence and comparative microarray analysis of serotype M18 group A Streptococcus strains associated with acute rheumatic fever outbreaks. Proc. Natl. Acad. Sci. U. S. A. 99:4668–4673. 10.1073/pnas.062526099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumby P, Porcella SF, Madrigal AG, Barbian KD, Ricklefs SM, Sturdevant DE, Graham MR, Vuopio-Varkila J, Hoe NP, Musser JM. 2005. Evolutionary origin and emergence of a highly successful clone of serotype M1 group A Streptococcus involved multiple horizontal gene transfer events. J. Infect. Dis. 192:771–782. 10.1086/432514 [DOI] [PubMed] [Google Scholar]
- 41.Tsatsaronis JA, Hollands A, Cole JN, Maamary PG, Gillen CM, Zakour NL, Kotb M, Nizet V, Beatson SA, Walker MJ, Sanderson-Smith ML. 2013. Streptococcal collagen-like protein A and general stress protein 24 are immunomodulating virulence factors of group A Streptococcus. FASEB J. 27:2633–2643. 10.1096/fj.12-226662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bessen DE, Carapetis JR, Beall B, Katz R, Hibble M, Currie BJ, Collingridge T, Izzo MW, Scaramuzzino DA, Sriprakash KS. 2000. Contrasting molecular epidemiology of group A streptococci causing tropical and nontropical infections of the skin and throat. J. Infect. Dis. 182:1109–1116. 10.1086/315842 [DOI] [PubMed] [Google Scholar]
- 43.Ekelund K, Darenberg J, Norrby-Teglund A, Hoffmann S, Bang D, Skinhoj P, Konradsen HB. 2005. Variations in emm type among group A streptococcal isolates causing invasive or noninvasive infections in a nationwide study. J. Clin. Microbiol. 43:3101–3109. 10.1128/JCM.43.7.3101-3109.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espinosa LE, Li Z, Gomez Barreto D, Calderon Jaimes E, Rodriguez RS, Sakota V, Facklam RR, Beall B. 2003. M protein gene type distribution among group A streptococcal clinical isolates recovered in Mexico city, Mexico, from 1991 to 2000, and Durango, Mexico, from 1998 to 1999: overlap with type distribution within the United States. J. Clin. Microbiol. 41:373–378. 10.1128/JCM.41.1.373-378.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang Y, Liu X, Chang H, Ji L, Huang G, Fu Z, Zheng Y, Wang L, Li C, Shen Y, Yu S, Yao K, Ma L, Shen X, Yang Y. 2012. Epidemiological and molecular characteristics of clinical isolates of Streptococcus pyogenes collected between 2005 and 2008 from Chinese children. J. Med. Microbiol. 61:975–983. 10.1099/jmm.0.042309-0 [DOI] [PubMed] [Google Scholar]
- 46.Shea PR, Ewbank AL, Gonzalez-Lugo JH, Martagon-Rosado AJ, Martinez-Gutierrez JC, Rehman HA, Serrano-Gonzalez M, Fittipaldi N, Beres SB, Flores AR, Low DE, Willey BM, Musser JM. 2011. Group A Streptococcus emm gene types in pharyngeal isolates, Ontario, Canada, 2002-2010. Emerg. Infect. Dis. 17:2010–2017. 10.3201/eid1711.110159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wozniak A, Rojas P, Rodriguez C, Undabarrena A, Garate C, Riedel I, Roman JC, Kalergis AM, Garcia P. 2012. M-protein gene-type distribution and hyaluronic acid capsule in group A Streptococcus clinical isolates in Chile: association of emm gene markers with csrR alleles. Epidemiol. Infect. 140:1286–1295. 10.1017/S0950268811001889 [DOI] [PubMed] [Google Scholar]
- 48.Dhanda V, Vohra H, Kumar R. 2011. Group A Streptococcus virulence factors genes in north India & their association with emm type in pharyngitis. Indian J. Med. Res. 133:110–115 http://www.ijmr.org.in/article.asp?issn=0971-5916;year=2011;volume=133;issue=1;spage=110;epage=115;aulast=Dhanda [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar R, Chakraborti A, Aggarwal AK, Vohra H, Sagar V, Dhanda V, Sharma YP, Majumdar S, Hoe N, Krause RM. 2012. Streptococcus pyogenes pharyngitis & impetigo in a rural area of Panchkula district in Haryana, India. Indian J. Med. Res. 135:133–136. 10.4103/0971-5916.93437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar R, Vohra H, Chakraborty A, Sharma YP, Bandhopadhya S, Dhanda V, Sagar V, Sharma M, Shah B, Ganguly NK. 2009. Epidemiology of group A streptococcal pharyngitis & impetigo: a cross-sectional & follow up study in a rural community of northern India. Indian J. Med. Res. 130:765–771 http://icmr.nic.in/ijmr/2009/december/1215.pdf [PubMed] [Google Scholar]
- 51.Chiou CS, Wang YW, Chen PL, Wang WL, Wu PF, Wei HL. 2009. Association of the shuffling of Streptococcus pyogenes clones and the fluctuation of scarlet fever cases between 2000 and 2006 in central Taiwan. BMC Microbiol. 9:115. 10.1186/1471-2180-9-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colman G, Tanna A, Efstratiou A, Gaworzewska ET. 1993. The serotypes of Streptococcus pyogenes present in Britain during 1980-1990 and their association with disease. J. Med. Microbiol. 39:165–178. 10.1099/00222615-39-3-165 [DOI] [PubMed] [Google Scholar]
- 53.Shulman ST, Tanz RR, Dale JB, Beall B, Kabat W, Kabat K, Cederlund E, Patel D, Rippe J, Li Z, Sakota V, North American Streptococcal Pharyngitis Surveillance Group 2009. Seven-year surveillance of North American pediatric group A streptococcal pharyngitis isolates. Clin. Infect. Dis. 49:78–84. 10.1086/599344 [DOI] [PubMed] [Google Scholar]
- 54.Tyrrell GJ, Lovgren M, Forwick B, Hoe NP, Musser JM, Talbot JA. 2002. M types of group A streptococcal isolates submitted to the National Centre for Streptococcus (Canada) from 1993 to 1999. J. Clin. Microbiol. 40:4466–4471. 10.1128/JCM.40.12.4466-4471.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoe NP, Vuopio-Varkila J, Vaara M, Grigsby D, De Lorenzo D, Fu YX, Dou SJ, Pan X, Nakashima K, Musser JM. 2001. Distribution of streptococcal inhibitor of complement variants in pharyngitis and invasive isolates in an epidemic of serotype M1 group A Streptococcus infection. J. Infect. Dis. 183:633–639. 10.1086/318543 [DOI] [PubMed] [Google Scholar]
- 56.Beres SB, Carroll RK, Shea PR, Sitkiewicz I, Martinez-Gutierrez JC, Low DE, McGeer A, Willey BM, Green K, Tyrrell GJ, Goldman TD, Feldgarden M, Birren BW, Fofanov Y, Boos J, Wheaton WD, Honisch C, Musser JM. 2010. Molecular complexity of successive bacterial epidemics deconvoluted by comparative pathogenomics. Proc. Natl. Acad. Sci. U. S. A. 107:4371–4376. 10.1073/pnas.0911295107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogers S, Commons R, Danchin MH, Selvaraj G, Kelpie L, Curtis N, Robins-Browne R, Carapetis JR. 2007. Strain prevalence, rather than innate virulence potential, is the major factor responsible for an increase in serious group A Streptococcus infections. J. Infect. Dis. 195:1625–1633. 10.1086/513875 [DOI] [PubMed] [Google Scholar]
- 58.Johnson DR, Stevens DL, Kaplan EL. 1992. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J. Infect. Dis. 166:374–382. 10.1093/infdis/166.2.374 [DOI] [PubMed] [Google Scholar]
- 59.Vlaminckx B, van Pelt W, Schouls L, van Silfhout A, Elzenaar C, Mascini E, Verhoef J, Schellekens J. 2004. Epidemiological features of invasive and noninvasive group A streptococcal disease in the Netherlands, 1992-1996. Eur. J. Clin. Microbiol. Infect. Dis. 23:434–444. 10.1007/s10096-004-1147-z [DOI] [PubMed] [Google Scholar]
- 60.Metzgar D, Zampolli A. 2011. The M protein of group A Streptococcus is a key virulence factor and a clinically relevant strain identification marker. Virulence 2:402–412. 10.4161/viru.2.5.16342 [DOI] [PubMed] [Google Scholar]
- 61.Henningham A, Barnett TC, Maamary PG, Walker MJ. 2012. Pathogenesis of group A streptococcal infections. Discov. Med. 13:329–342 http://www.discoverymedicine.com/Anna-Henningham/2012/05/16/pathogenesis-of-group-a-streptococcal-infections/ [PubMed] [Google Scholar]
- 62.Choby BA. 2009. Diagnosis and treatment of streptococcal pharyngitis. Am. Fam. Physician 79:383–390 http://www.aafp.org/afp/2009/0301/p383.pdf [PubMed] [Google Scholar]
- 63.Wessels MR. 2011. Streptococcal pharyngitis. N. Engl. J. Med. 364:648–655. 10.1056/NEJMcp1009126 [DOI] [PubMed] [Google Scholar]
- 64.Bisno AL, Stevens DL. 2010. Streptococcus pyogenes, p 2593–2610 In Mandel GL, Bennett JE, Dolan R. (ed), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 7th ed. Livingstone Elsevier, Philadelphia, PA [Google Scholar]
- 65.Katz AR, Morens DM. 1992. Severe streptococcal infections in historical perspective. Clin. Infect. Dis. 14:298–307. 10.1093/clinids/14.1.298 [DOI] [PubMed] [Google Scholar]
- 66.Shulman ST, Tanz RR. 2010. Group A streptococcal pharyngitis and immune-mediated complications: from diagnosis to management. Expert Rev. Anti Infect. Ther. 8:137–150. 10.1586/eri.09.134 [DOI] [PubMed] [Google Scholar]
- 67.Ralph AP, Carapetis JR. 2013. Group A streptococcal diseases and their global burden. Curr. Top. Microbiol. Immunol. 368:1–27. 10.1007/82_2012_280 [DOI] [PubMed] [Google Scholar]
- 68.Quinn RW. 1989. Comprehensive review of morbidity and mortality trends for rheumatic fever, streptococcal disease, and scarlet fever: the decline of rheumatic fever. Rev. Infect. Dis. 11:928–953. 10.1093/clinids/11.6.928 [DOI] [PubMed] [Google Scholar]
- 69.Cole C, Gazewood J. 2007. Diagnosis and treatment of impetigo. Am. Fam. Physician 75:859–864 http://www.aafp.org/afp/2007/0315/p859.pdf [PubMed] [Google Scholar]
- 70.Valery PC, Wenitong M, Clements V, Sheel M, McMillan D, Stirling J, Sriprakash KS, Batzloff M, Vohra R, McCarthy JS. 2008. Skin infections among indigenous Australians in an urban setting in far north Queensland. Epidemiol. Infect. 136:1103–1108. 10.1017/S0950268807009740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cunningham M. 2008. Pathogenesis of group A streptococcal infections and their sequelae. Adv. Exp. Med. Biol. 609:29–42. 10.1007/978-0-387-73960-1_3 [DOI] [PubMed] [Google Scholar]
- 72.Dajani AS, Ayoub E, Bierman FZ, Bisno AL, Denny FW, Durack DT, Ferrieri P, Freed M, Gerber M, Kaplan EL, Karchmer AW, Markowitz M, Rahimtoola SH, Shulman ST, Stollerman G, Takahashi M, Taranta A, Taubert KA, Wilson W. 1992. Guidelines for the diagnosis of rheumatic-fever—Jones criteria, 1992 update. JAMA 268:2069–20731404745 [Google Scholar]
- 73.Lee JL, Naguwa SM, Cheema GS, Gershwin ME. 2009. Acute rheumatic fever and its consequences: a persistent threat to developing nations in the 21st century. Autoimmun. Rev. 9:117–123. 10.1016/j.autrev.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 74.Parks T, Smeesters PR, Steer AC. 2012. Streptococcal skin infection and rheumatic heart disease. Curr. Opin. Infect. Dis. 25:145–153. 10.1097/QCO.0b013e3283511d27 [DOI] [PubMed] [Google Scholar]
- 75.Bisno AL. 2010. Nonsuppurative poststreptococcal sequelae: rheumatic fever and glomerulonephritis, p 2611–2622 In Mandel GL, Bennett JE, Dolan R. (ed), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 7th ed. Livingstone Elsevier, Philadelphia, PA [Google Scholar]
- 76.Marshall CS, Cheng AC, Markey PG, Towers RJ, Richardson LJ, Fagan PK, Scott L, Krause VL, Currie BJ. 2011. Acute post-streptococcal glomerulonephritis in the Northern Territory of Australia: a review of 16 years data and comparison with the literature. Am. J. Trop. Med. Hyg. 85:703–710. 10.4269/ajtmh.2011.11-0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steer AC, Danchin MH, Carapetis JR. 2007. Group A streptococcal infections in children. J. Paediatr. Child Health 43:203–213. 10.1111/j.1440-1754.2007.01051.x [DOI] [PubMed] [Google Scholar]
- 78.Leckman JF, King RA, Gilbert DL, Coffey BJ, Singer HS, Dure LS, IV, Grantz H, Katsovich L, Lin H, Lombroso PJ, Kawikova I, Johnson DR, Kurlan RM, Kaplan EL. 2011. Streptococcal upper respiratory tract infections and exacerbations of tic and obsessive-compulsive symptoms: a prospective longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry 50:108–118. 10.1016/j.jaac.2010.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kurlan R, Johnson D, Kaplan EL. 2008. Streptococcal infection and exacerbations of childhood tics and obsessive-compulsive symptoms: a prospective blinded cohort study. Pediatrics 121:1188–1197. 10.1542/peds.2007-2657 [DOI] [PubMed] [Google Scholar]
- 80.O'Grady KA, Kelpie L, Andrews RM, Curtis N, Nolan TM, Selvaraj G, Passmore JW, Oppendisano F, Carnie JA, Carapetis JR. 2007. The epidemiology of invasive group A streptococcal disease in Victoria, Australia. Med. J. Aust. 186:565–569 https://www.mja.com.au/journal/2007/186/11/epidemiology-invasive-group-streptococcal-disease-victoria-australia [DOI] [PubMed] [Google Scholar]
- 81.O'Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, Albanese BA, Farley MM, Barrett NL, Spina NL, Beall B, Harrison LH, Reingold A, Van Beneden C, Active Bacterial Core Surveillance Team 2007. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin. Infect. Dis. 45:853–862. 10.1086/521264 [DOI] [PubMed] [Google Scholar]
- 82.Davies HD, McGeer A, Schwartz B, Green K, Cann D, Simor AE, Low DE. 1996. Invasive group A streptococcal infections in Ontario, Canada. Ontario Group A Streptococcal Study Group. N. Engl. J. Med. 335:547–554 [DOI] [PubMed] [Google Scholar]
- 83.Low DE, Schwartz B, McGeer A. 1998. The reemergence of severe group A streptococcal disease: an evolutionary perspective, p 93–123 In Scheld WM, Armstrong D, Hughes JM. (ed), Emerging infections 1. ASM Press, Washington, DC [Google Scholar]
- 84.Stevens DL. 1996. Invasive group A streptococcal disease. Infect. Agents Dis. 5:157–166 [PubMed] [Google Scholar]
- 85.Olsen RJ, Shelburne SA, Musser JM. 2009. Molecular mechanisms underlying group A streptococcal pathogenesis. Cell. Microbiol. 11:1–12. 10.1111/j.1462-5822.2008.01225.x [DOI] [PubMed] [Google Scholar]
- 86.Sharkawy A, Low DE, Saginur R, Gregson D, Schwartz B, Jessamine P, Green K, McGeer A, Ontario Group A Streptococcal Study Group 2002. Severe group A streptococcal soft-tissue infections in Ontario: 1992-1996. Clin. Infect. Dis. 34:454–460. 10.1086/338466 [DOI] [PubMed] [Google Scholar]
- 87.Carapetis JR, Walker AM, Hibble M, Sriprakash KS, Currie BJ. 1999. Clinical and epidemiological features of group A streptococcal bacteraemia in a region with hyperendemic superficial streptococcal infection. Epidemiol. Infect. 122:59–65. 10.1017/S0950268898001952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Norton R, Smith HV, Wood N, Siegbrecht E, Ross A, Ketheesan N. 2004. Invasive group A streptococcal disease in north Queensland (1996-2001). Indian J. Med. Res. 119(Suppl):148–151 http://icmr.nic.in/ijmr/ijmr_supp/31.pdf [PubMed] [Google Scholar]
- 89.Whitehead BD, Smith HV, Nourse C. 2011. Invasive group A streptococcal disease in children in Queensland. Epidemiol. Infect. 139:623–628. 10.1017/S0950268810001378 [DOI] [PubMed] [Google Scholar]
- 90.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Ngetsa C, Slack MP, Njenga S, Hart CA, Maitland K, English M, Marsh K, Scott JA. 2005. Bacteremia among children admitted to a rural hospital in Kenya. N. Engl. J. Med. 352:39–47. 10.1056/NEJMoa040275 [DOI] [PubMed] [Google Scholar]
- 91.Steer AC, Jenney A, Kado J, Good MF, Batzloff M, Waqatakirewa L, Mullholland EK, Carapetis JR. 2009. Prospective surveillance of invasive group A streptococcal disease, Fiji, 2005-2007. Emerg. Infect. Dis. 15:216–222. 10.3201/eid15/2.080558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steer AC, Jenney AJ, Oppedisano F, Batzloff MR, Hartas J, Passmore J, Russell FM, Kado JH, Carapetis JR. 2008. High burden of invasive beta-haemolytic streptococcal infections in Fiji. Epidemiol. Infect. 136:621–627. 10.1017/S095026880700917X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramos M, Valle R, Reaves EJ, Loayza L, Gonzalez S, Bernal M, Soto G, Hawksworth AW, Kasper MR, Tilley DH, De Mattos CA, Brown JR, Bausch DG. 2013. Outbreak of group A beta hemolytic Streptococcus pharyngitis in a Peruvian military facility, April 2012. MSMR 20:14–17 [PubMed] [Google Scholar]
- 94.Wasserzug O, Valinsky L, Klement E, Bar-Zeev Y, Davidovitch N, Orr N, Korenman Z, Kayouf R, Sela T, Ambar R, Derazne E, Dagan R, Zarka S. 2009. A cluster of ecthyma outbreaks caused by a single clone of invasive and highly infective Streptococcus pyogenes. Clin. Infect. Dis. 48:1213–1219. 10.1086/597770 [DOI] [PubMed] [Google Scholar]
- 95.Yang P, Peng X, Yang J, Dong X, Zhang M, Wang Q. 2013. A probable food-borne outbreak of pharyngitis after a massive rainstorm in Beijing, caused by emm89 group A Streptococcus rarely found in China. Int. J. Infect. Dis. 17:e471. 10.1016/j.ijid.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 96.Ertugrul BM, Erol N, Emek M, Ozturk B, Saylak OM, Cetin K, Sakarya S. 2012. Food-borne tonsillopharyngitis outbreak in a hospital cafeteria. Infection 40:49–55. 10.1007/s15010-011-0166-9 [DOI] [PubMed] [Google Scholar]
- 97.Asteberg I, Andersson Y, Dotevall L, Ericsson M, Darenberg J, Henriques-Nordmark B, Soderstrom A. 2006. A food-borne streptococcal sore throat outbreak in a small community. Scand. J. Infect. Dis. 38:988–994. 10.1080/00365540600868370 [DOI] [PubMed] [Google Scholar]
- 98.Gallo G, Berzero R, Cattai N, Recchia S, Orefici G. 1992. An outbreak of group A food-borne streptococcal pharyngitis. Eur. J. Epidemiol. 8:292–297 [DOI] [PubMed] [Google Scholar]
- 99.Kaluski DN, Barak E, Kaufman Z, Valinsky L, Marva E, Korenman Z, Gorodnitzki Z, Yishai R, Koltai D, Leventhal A, Levine S, Havkin O, Green MS. 2006. A large food-borne outbreak of group A streptococcal pharyngitis in an industrial plant: potential for deliberate contamination. Isr. Med. Assoc. J. 8:618–621 http://www.ima.org.il/FilesUpload/IMAJ/0/49/24664.pdf [PubMed] [Google Scholar]
- 100.Levy M, Johnson CG, Kraa E. 2003. Tonsillopharyngitis caused by foodborne group A Streptococcus: a prison-based outbreak. Clin. Infect. Dis. 36:175–182. 10.1086/345670 [DOI] [PubMed] [Google Scholar]
- 101.Matsumoto M, Miwa Y, Matsui H, Saito M, Ohta M, Miyazaki Y. 1999. An outbreak of pharyngitis caused by food-borne group A Streptococcus. Jpn. J. Infect. Dis. 52:127–128 [PubMed] [Google Scholar]
- 102.Sarvghad MR, Naderi HR, Naderi-Nassab M, Majdzadeh R, Javanian M, Faramarzi H, Fatehmanesh P. 2005. An outbreak of food-borne group A Streptococcus (GAS) tonsillopharyngitis among residents of a dormitory. Scand. J. Infect. Dis. 37:647–650. 10.1080/00365540510044085 [DOI] [PubMed] [Google Scholar]
- 103.Aziz RK, Kotb M. 2008. Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg. Infect. Dis. 14:1511–1517. 10.3201/eid1410.071660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fittipaldi N, Beres SB, Olsen RJ, Kapur V, Shea PR, Watkins ME, Cantu CC, Laucirica DR, Jenkins L, Flores AR, Lovgren M, Ardanuy C, Linares J, Low DE, Tyrrell GJ, Musser JM. 2012. Full-genome dissection of an epidemic of severe invasive disease caused by a hypervirulent, recently emerged clone of group A Streptococcus. Am. J. Pathol. 180:1522–1534. 10.1016/j.ajpath.2011.12.037 [DOI] [PubMed] [Google Scholar]
- 105.Beres SB, Sylva GL, Sturdevant DE, Granville CN, Liu M, Ricklefs SM, Whitney AR, Parkins LD, Hoe NP, Adams GJ, Low DE, DeLeo FR, McGeer A, Musser JM. 2004. Genome-wide molecular dissection of serotype M3 group A Streptococcus strains causing two epidemics of invasive infections. Proc. Natl. Acad. Sci. U. S. A. 101:11833–11838. 10.1073/pnas.0404163101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beres SB, Richter EW, Nagiec MJ, Sumby P, Porcella SF, DeLeo FR, Musser JM. 2006. Molecular genetic anatomy of inter- and intraserotype variation in the human bacterial pathogen group A Streptococcus. Proc. Natl. Acad. Sci. U. S. A. 103:7059–7064. 10.1073/pnas.0510279103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sitkiewicz I, Nagiec MJ, Sumby P, Butler SD, Cywes-Bentley C, Musser JM. 2006. Emergence of a bacterial clone with enhanced virulence by acquisition of a phage encoding a secreted phospholipase A2. Proc. Natl. Acad. Sci. U. S. A. 103:16009–16014. 10.1073/pnas.0607669103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fittipaldi N, Olsen RJ, Beres SB, Van Beneden C, Musser JM. 2012. Genomic analysis of emm59 group A Streptococcus invasive strains, United States. Emerg. Infect. Dis. 18:650–652. 10.3201/eid1804.111803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hsieh YC, Huang YC. 2011. Scarlet fever outbreak in Hong Kong, 2011. J. Microbiol. Immunol. Infect. 44:409–411. 10.1016/j.jmii.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 110.Lau EH, Nishiura H, Cowling BJ, Ip DK, Wu JT. 2012. Scarlet fever outbreak, Hong Kong, 2011. Emerg. Infect. Dis. 18:1700–1702. 10.3201/eid1810.120062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang P, Peng X, Zhang D, Wu S, Liu Y, Cui S, Lu G, Duan W, Shi W, Liu S, Li J, Wang Q. 2013. Characteristics of group A Streptococcus strains circulating during scarlet fever epidemic, Beijing, China, 2011. Emerg. Infect. Dis. 19:909–915. 10.3201/eid1906.121020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shelburne SA, III, Granville C, Tokuyama M, Sitkiewicz I, Patel P, Musser JM. 2005. Growth characteristics of and virulence factor production by group A Streptococcus during cultivation in human saliva. Infect. Immun. 73:4723–4731. 10.1128/IAI.73.8.4723-4731.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nobbs AH, Lamont RJ, Jenkinson HF. 2009. Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev. 73:407–450. 10.1128/MMBR.00014-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hasty DL, Ofek I, Courtney HS, Doyle RJ. 1992. Multiple adhesins of streptococci. Infect. Immun. 60:2147–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hamada S, Yamamoto T, Koga T, McGhee JR, Michalek SM, Yamamoto S. 1985. Chemical properties and immunobiological activities of streptococcal lipoteichoic acids. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 259:228–243 [DOI] [PubMed] [Google Scholar]
- 116.Schneewind O, Mihaylova-Petkov D, Model P. 1993. Cell wall sorting signals in surface proteins of Gram-positive bacteria. EMBO J. 12:4803–4811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barnett TC, Scott JR. 2002. Differential recognition of surface proteins in Streptococcus pyogenes by two sortase gene homologs. J. Bacteriol. 184:2181–2191. 10.1128/JB.184.8.2181-2191.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scott JR, Barnett TC. 2006. Surface proteins of Gram-positive bacteria and how they get there. Annu. Rev. Microbiol. 60:397–423. 10.1146/annurev.micro.60.080805.142256 [DOI] [PubMed] [Google Scholar]
- 119.Mora M, Bensi G, Capo S, Falugi F, Zingaretti C, Manetti AG, Maggi T, Taddei AR, Grandi G, Telford JL. 2005. Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proc. Natl. Acad. Sci. U. S. A. 102:15641–15646. 10.1073/pnas.0507808102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barnett TC, Patel AR, Scott JR. 2004. A novel sortase, srtC2, from Streptococcus pyogenes anchors a surface protein containing a QVPTGV motif to the cell wall. J. Bacteriol. 186:5865–5875. 10.1128/JB.186.17.5865-5875.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kang HJ, Coulibaly F, Proft T, Baker EN. 2011. Crystal structure of Spy0129, a Streptococcus pyogenes class B sortase involved in pilus assembly. PLoS One 6:e15969. 10.1371/journal.pone.0015969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Quigley BR, Zahner D, Hatkoff M, Thanassi DG, Scott JR. 2009. Linkage of T3 and Cpa pilins in the Streptococcus pyogenes M3 pilus. Mol. Microbiol. 72:1379–1394. 10.1111/j.1365-2958.2009.06727.x [DOI] [PubMed] [Google Scholar]
- 123.Smith WD, Pointon JA, Abbot E, Kang HJ, Baker EN, Hirst BH, Wilson JA, Banfield MJ, Kehoe MA. 2010. Roles of minor pilin subunits Spy0125 and Spy0130 in the serotype M1 Streptococcus pyogenes strain SF370. J. Bacteriol. 192:4651–4659. 10.1128/JB.00071-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nakata M, Kimura KR, Sumitomo T, Wada S, Sugauchi A, Oiki E, Higashino M, Kreikemeyer B, Podbielski A, Okahashi N, Hamada S, Isoda R, Terao Y, Kawabata S. 2011. Assembly mechanism of FCT region type 1 pili in serotype M6 Streptococcus pyogenes. J. Biol. Chem. 286:37566–37577. 10.1074/jbc.M111.239780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bessen DE, Kalia A. 2002. Genomic localization of a T serotype locus to a recombinatorial zone encoding extracellular matrix-binding proteins in Streptococcus pyogenes. Infect. Immun. 70:1159–1167. 10.1128/IAI.70.3.1159-1167.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Falugi F, Zingaretti C, Pinto V, Mariani M, Amodeo L, Manetti AG, Capo S, Musser JM, Orefici G, Margarit I, Telford JL, Grandi G, Mora M. 2008. Sequence variation in group A Streptococcus pili and association of pilus backbone types with Lancefield T serotypes. J. Infect. Dis. 198:1834–1841. 10.1086/593176 [DOI] [PubMed] [Google Scholar]
- 127.Kratovac Z, Manoharan A, Luo F, Lizano S, Bessen DE. 2007. Population genetics and linkage analysis of loci within the FCT region of Streptococcus pyogenes. J. Bacteriol. 189:1299–1310. 10.1128/JB.01301-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abbot EL, Smith WD, Siou GP, Chiriboga C, Smith RJ, Wilson JA, Hirst BH, Kehoe MA. 2007. Pili mediate specific adhesion of Streptococcus pyogenes to human tonsil and skin. Cell. Microbiol. 9:1822–1833. 10.1111/j.1462-5822.2007.00918.x [DOI] [PubMed] [Google Scholar]
- 129.Manetti AG, Zingaretti C, Falugi F, Capo S, Bombaci M, Bagnoli F, Gambellini G, Bensi G, Mora M, Edwards AM, Musser JM, Graviss EA, Telford JL, Grandi G, Margarit I. 2007. Streptococcus pyogenes pili promote pharyngeal cell adhesion and biofilm formation. Mol. Microbiol. 64:968–983. 10.1111/j.1365-2958.2007.05704.x [DOI] [PubMed] [Google Scholar]
- 130.Edwards AM, Manetti AG, Falugi F, Zingaretti C, Capo S, Buccato S, Bensi G, Telford JL, Margarit I, Grandi G. 2008. Scavenger receptor gp340 aggregates group A streptococci by binding pili. Mol. Microbiol. 68:1378–1394. 10.1111/j.1365-2958.2008.06220.x [DOI] [PubMed] [Google Scholar]
- 131.Fischetti VA, Pancholi V, Schneewind O. 1990. Conservation of a hexapeptide sequence in the anchor region of surface proteins from Gram-positive cocci. Mol. Microbiol. 4:1603–1605. 10.1111/j.1365-2958.1990.tb02072.x [DOI] [PubMed] [Google Scholar]
- 132.Phillips GN, Jr, Flicker PF, Cohen C, Manjula BN, Fischetti VA. 1981. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc. Natl. Acad. Sci. U. S. A. 78:4689–4693. 10.1073/pnas.78.8.4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fischetti VA. 1989. Streptococcal M protein: molecular design and biological behavior. Clin. Microbiol. Rev. 2:285–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Courtney HS, von Hunolstein C, Dale JB, Bronze MS, Beachey EH, Hasty DL. 1992. Lipoteichoic acid and M protein: dual adhesins of group A streptococci. Microb. Pathog. 12:199–208. 10.1016/0882-4010(92)90054-R [DOI] [PubMed] [Google Scholar]
- 135.Cue D, Dombek PE, Lam H, Cleary PP. 1998. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect. Immun. 66:4593–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ellen RP, Gibbons RJ. 1972. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect. Immun. 5:826–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Siemens N, Patenge N, Otto J, Fiedler T, Kreikemeyer B. 2011. Streptococcus pyogenes M49 plasminogen/plasmin binding facilitates keratinocyte invasion via integrin-integrin-linked kinase (ILK) pathways and protects from macrophage killing. J. Biol. Chem. 286:21612–21622. 10.1074/jbc.M110.202671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang JR, Stinson MW. 1994. M protein mediates streptococcal adhesion to HEp-2 cells. Infect. Immun. 62:442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rezcallah MS, Hodges K, Gill DB, Atkinson JP, Wang B, Cleary PP. 2005. Engagement of CD46 and alpha5beta1 integrin by group A streptococci is required for efficient invasion of epithelial cells. Cell. Microbiol. 7:645–653. 10.1111/j.1462-5822.2004.00497.x [DOI] [PubMed] [Google Scholar]
- 140.Cue D, Lam H, Cleary PP. 2001. Genetic dissection of the Streptococcus pyogenes M1 protein: regions involved in fibronectin binding and intracellular invasion. Microb. Pathog. 31:231–242. 10.1006/mpat.2001.0467 [DOI] [PubMed] [Google Scholar]
- 141.Schmidt KH, Mann K, Cooney J, Kohler W. 1993. Multiple binding of type 3 streptococcal M protein to human fibrinogen, albumin and fibronectin. FEMS Immunol. Med. Microbiol. 7:135–143. 10.1111/j.1574-695X.1993.tb00392.x [DOI] [PubMed] [Google Scholar]
- 142.Talay SR, Zock A, Rohde M, Molinari G, Oggioni M, Pozzi G, Guzman CA, Chhatwal GS. 2000. Co-operative binding of human fibronectin to Sfbl protein triggers streptococcal invasion into respiratory epithelial cells. Cell. Microbiol. 2:521–535. 10.1046/j.1462-5822.2000.00076.x [DOI] [PubMed] [Google Scholar]
- 143.Terao Y, Kawabata S, Kunitomo E, Murakami J, Nakagawa I, Hamada S. 2001. Fba, a novel fibronectin-binding protein from Streptococcus pyogenes, promotes bacterial entry into epithelial cells, and the fba gene is positively transcribed under the Mga regulator. Mol. Microbiol. 42:75–86. 10.1046/j.1365-2958.2001.02579.x [DOI] [PubMed] [Google Scholar]
- 144.Wang B, Yurecko RS, Dedhar S, Cleary PP. 2006. Integrin-linked kinase is an essential link between integrins and uptake of bacterial pathogens by epithelial cells. Cell. Microbiol. 8:257–266. 10.1111/j.1462-5822.2005.00618.x [DOI] [PubMed] [Google Scholar]
- 145.Okada N, Liszewski MK, Atkinson JP, Caparon M. 1995. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A Streptococcus. Proc. Natl. Acad. Sci. U. S. A. 92:2489–2493. 10.1073/pnas.92.7.2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Frick IM, Schmidtchen A, Sjobring U. 2003. Interactions between M proteins of Streptococcus pyogenes and glycosaminoglycans promote bacterial adhesion to host cells. Eur. J. Biochem. 270:2303–2311. 10.1046/j.1432-1033.2003.03600.x [DOI] [PubMed] [Google Scholar]
- 147.Frick IM, Morgelin M, Bjorck L. 2000. Virulent aggregates of Streptococcus pyogenes are generated by homophilic protein-protein interactions. Mol. Microbiol. 37:1232–1247. 10.1046/j.1365-2958.2000.02084.x [DOI] [PubMed] [Google Scholar]
- 148.Patti JM, Allen BL, McGavin MJ, Hook M. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585–617. 10.1146/annurev.mi.48.100194.003101 [DOI] [PubMed] [Google Scholar]
- 149.Yamaguchi M, Terao Y, Kawabata S. 2013. Pleiotropic virulence factor—Streptococcus pyogenes fibronectin-binding proteins. Cell. Microbiol. 15:503–511. 10.1111/cmi.12083 [DOI] [PubMed] [Google Scholar]
- 150.Schwarz-Linek U, Werner JM, Pickford AR, Gurusiddappa S, Kim JH, Pilka ES, Briggs JA, Gough TS, Hook M, Campbell ID, Potts JR. 2003. Pathogenic bacteria attach to human fibronectin through a tandem beta-zipper. Nature 423:177–181. 10.1038/nature01589 [DOI] [PubMed] [Google Scholar]
- 151.Frick IM, Crossin KL, Edelman GM, Bjorck L. 1995. Protein H—a bacterial surface protein with affinity for both immunoglobulin and fibronectin type III domains. EMBO J. 14:1674–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pancholi V, Fischetti VA. 1992. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J. Exp. Med. 176:415–426. 10.1084/jem.176.2.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Caswell CC, Oliver-Kozup H, Han R, Lukomska E, Lukomski S. 2010. Scl1, the multifunctional adhesin of group A Streptococcus, selectively binds cellular fibronectin and laminin, and mediates pathogen internalization by human cells. FEMS Microbiol. Lett. 303:61–68. 10.1111/j.1574-6968.2009.01864.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fisher M, Huang YS, Li X, McIver KS, Toukoki C, Eichenbaum Z. 2008. Shr is a broad-spectrum surface receptor that contributes to adherence and virulence in group A Streptococcus. Infect. Immun. 76:5006–5015. 10.1128/IAI.00300-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Terao Y, Kawabata S, Kunitomo E, Nakagawa I, Hamada S. 2002. Novel laminin-binding protein of Streptococcus pyogenes, Lbp, is involved in adhesion to epithelial cells. Infect. Immun. 70:993–997. 10.1128/IAI.70.2.993-997.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ozeri V, Rosenshine I, Ben-Ze'Ev A, Bokoch GM, Jou TS, Hanski E. 2001. De novo formation of focal complex-like structures in host cells by invading streptococci. Mol. Microbiol. 41:561–573. 10.1046/j.1365-2958.2001.02535.x [DOI] [PubMed] [Google Scholar]
- 157.Dombek PE, Cue D, Sedgewick J, Lam H, Ruschkowski S, Finlay BB, Cleary PP. 1999. High-frequency intracellular invasion of epithelial cells by serotype M1 group A streptococci: M1 protein-mediated invasion and cytoskeletal rearrangements. Mol. Microbiol. 31:859–870. 10.1046/j.1365-2958.1999.01223.x [DOI] [PubMed] [Google Scholar]
- 158.Ozeri V, Rosenshine I, Mosher DF, Fassler R, Hanski E. 1998. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol. Microbiol. 30:625–637. 10.1046/j.1365-2958.1998.01097.x [DOI] [PubMed] [Google Scholar]
- 159.Caswell CC, Barczyk M, Keene DR, Lukomska E, Gullberg DE, Lukomski S. 2008. Identification of the first prokaryotic collagen sequence motif that mediates binding to human collagen receptors, integrins alpha2beta1 and alpha11beta1. J. Biol. Chem. 283:36168–36175. 10.1074/jbc.M806865200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Caswell CC, Lukomska E, Seo NS, Hook M, Lukomski S. 2007. Scl1-dependent internalization of group A Streptococcus via direct interactions with the alpha2beta(1) integrin enhances pathogen survival and re-emergence. Mol. Microbiol. 64:1319–1331. 10.1111/j.1365-2958.2007.05741.x [DOI] [PubMed] [Google Scholar]
- 161.Barnett TC, Liebl D, Seymour LM, Gillen CM, Lim JY, LaRock CN, Davies MR, Schulz BL, Nizet V, Teasdale RD, Walker MJ. 2013. The globally disseminated M1T1 clone of group A Streptococcus evades autophagy for intracellular replication. Cell Host Microbe 14:675–682. 10.1016/j.chom.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, Hamada S, Yoshimori T. 2004. Autophagy defends cells against invading group A Streptococcus. Science 306:1037–1040. 10.1126/science.1103966 [DOI] [PubMed] [Google Scholar]
- 163.Kreikemeyer B, McIver KS, Podbielski A. 2003. Virulence factor regulation and regulatory networks in Streptococcus pyogenes and their impact on pathogen-host interactions. Trends Microbiol. 11:224–232. 10.1016/S0966-842X(03)00098-2 [DOI] [PubMed] [Google Scholar]
- 164.Bessen DE, Lizano S. 2010. Tissue tropisms in group A streptococcal infections. Future Microbiol. 5:623–638. 10.2217/fmb.10.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bessen DE, Sotir CM, Readdy TL, Hollingshead SK. 1996. Genetic correlates of throat and skin isolates of group A streptococci. J. Infect. Dis. 173:896–900. 10.1093/infdis/173.4.896 [DOI] [PubMed] [Google Scholar]
- 166.Bessen DE. 2009. Population biology of the human restricted pathogen, Streptococcus pyogenes. Infect. Genet. Evol. 9:581–593. 10.1016/j.meegid.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bessen DE, Manoharan A, Luo F, Wertz JE, Robinson DA. 2005. Evolution of transcription regulatory genes is linked to niche specialization in the bacterial pathogen Streptococcus pyogenes. J. Bacteriol. 187:4163–4172. 10.1128/JB.187.12.4163-4172.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kreikemeyer B, Nakata M, Oehmcke S, Gschwendtner C, Normann J, Podbielski A. 2005. Streptococcus pyogenes collagen type I-binding Cpa surface protein. Expression profile, binding characteristics, biological functions, and potential clinical impact. J. Biol. Chem. 280:33228–33239. 10.1074/jbc.M502896200 [DOI] [PubMed] [Google Scholar]
- 169.Hanski E, Caparon M. 1992. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc. Natl. Acad. Sci. U. S. A. 89:6172–6176. 10.1073/pnas.89.13.6172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kreikemeyer B, Talay SR, Chhatwal GS. 1995. Characterization of a novel fibronectin-binding surface protein in group A streptococci. Mol. Microbiol. 17:137–145. 10.1111/j.1365-2958.1995.mmi_17010137.x [DOI] [PubMed] [Google Scholar]
- 171.Rakonjac JV, Robbins JC, Fischetti VA. 1995. DNA sequence of the serum opacity factor of group A streptococci: identification of a fibronectin-binding repeat domain. Infect. Immun. 63:622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Delvecchio A, Currie BJ, McArthur JD, Walker MJ, Sriprakash KS. 2002. Streptococcus pyogenes PrtfII, but not SfbI, SfbII or Fbp54, is represented more frequently among invasive-disease isolates of tropical Australia. Epidemiol. Infect. 128:391–396 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2869834/pdf/12113482.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hondorp ER, McIver KS. 2007. The Mga virulence regulon: infection where the grass is greener. Mol. Microbiol. 66:1056–1065. 10.1111/j.1365-2958.2007.06006.x [DOI] [PubMed] [Google Scholar]
- 174.Kalia A, Bessen DE. 2004. Natural selection and evolution of streptococcal virulence genes involved in tissue-specific adaptations. J. Bacteriol. 186:110–121. 10.1128/JB.186.1.110-121.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McArthur JD, McKay FC, Ramachandran V, Shyam P, Cork AJ, Sanderson-Smith ML, Cole JN, Ringdahl U, Sjobring U, Ranson M, Walker MJ. 2008. Allelic variants of streptokinase from Streptococcus pyogenes display functional differences in plasminogen activation. FASEB J. 22:3146–3153. 10.1096/fj.08-109348 [DOI] [PubMed] [Google Scholar]
- 176.Courtney HS, Liu S, Dale JB, Hasty DL. 1997. Conversion of M serotype 24 of Streptococcus pyogenes to M serotypes 5 and 18: effect on resistance to phagocytosis and adhesion to host cells. Infect. Immun. 65:2472–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Thern A, Stenberg L, Dahlback B, Lindahl G. 1995. Ig-binding surface proteins of Streptococcus pyogenes also bind human C4b-binding protein (C4BP), a regulatory component of the complement system. J. Immunol. 154:375–386 [PubMed] [Google Scholar]
- 178.Morfeldt E, Berggard K, Persson J, Drakenberg T, Johnsson E, Lindahl E, Linse S, Lindahl G. 2001. Isolated hypervariable regions derived from streptococcal M proteins specifically bind human C4b-binding protein: implications for antigenic variation. J. Immunol. 167:3870–3877 http://www.jimmunol.org/content/167/7/3870.long [DOI] [PubMed] [Google Scholar]
- 179.Berggard K, Johnsson E, Morfeldt E, Persson J, Stalhammar-Carlemalm M, Lindahl G. 2001. Binding of human C4BP to the hypervariable region of M protein: a molecular mechanism of phagocytosis resistance in Streptococcus pyogenes. Mol. Microbiol. 42:539–551. 10.1046/j.1365-2958.2001.02664.x [DOI] [PubMed] [Google Scholar]
- 180.Horstmann RD, Sievertsen HJ, Knobloch J, Fischetti VA. 1988. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. U. S. A. 85:1657–1661. 10.1073/pnas.85.5.1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Johnsson E, Berggard K, Kotarsky H, Hellwage J, Zipfel PF, Sjobring U, Lindahl G. 1998. Role of the hypervariable region in streptococcal M proteins: binding of a human complement inhibitor. J. Immunol. 161:4894–4901 [PubMed] [Google Scholar]
- 182.Courtney HS, Hasty DL, Dale JB. 2006. Anti-phagocytic mechanisms of Streptococcus pyogenes: binding of fibrinogen to M-related protein. Mol. Microbiol. 59:936–947. 10.1111/j.1365-2958.2005.04977.x [DOI] [PubMed] [Google Scholar]
- 183.Horstmann RD, Sievertsen HJ, Leippe M, Fischetti VA. 1992. Role of fibrinogen in complement inhibition by streptococcal M protein. Infect. Immun. 60:5036–5041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sandin C, Carlsson F, Lindahl G. 2006. Binding of human plasma proteins to Streptococcus pyogenes M protein determines the location of opsonic and non-opsonic epitopes. Mol. Microbiol. 59:20–30. 10.1111/j.1365-2958.2005.04913.x [DOI] [PubMed] [Google Scholar]
- 185.Carlsson F, Sandin C, Lindahl G. 2005. Human fibrinogen bound to Streptococcus pyogenes M protein inhibits complement deposition via the classical pathway. Mol. Microbiol. 56:28–39. 10.1111/j.1365-2958.2005.04527.x [DOI] [PubMed] [Google Scholar]
- 186.Ly D, Taylor JM, Tsatsaronis JA, Monteleone MM, Skora AS, Donald CA, Maddocks T, Nizet V, West NP, Ranson M, Walker MJ, McArthur JD, Sanderson-Smith ML. 2014. Plasmin(ogen) acquisition by group A Streptococcus protects against C3b-mediated neutrophil killing. J. Innate Immun. 6:240–250. 10.1159/000353754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Staali L, Bauer S, Morgelin M, Bjorck L, Tapper H. 2006. Streptococcus pyogenes bacteria modulate membrane traffic in human neutrophils and selectively inhibit azurophilic granule fusion with phagosomes. Cell. Microbiol. 8:690–703. 10.1111/j.1462-5822.2005.00662.x [DOI] [PubMed] [Google Scholar]
- 188.Dale JB, Washburn RG, Marques MB, Wessels MR. 1996. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect. Immun. 64:1495–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Foley MJ, Wood WB., Jr 1959. Studies on the pathogenicity of group A streptococci. II. The antiphagocytic effects of the M protein and the capsular gel. J. Exp. Med. 110:617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gryllos I, Cywes C, Shearer MH, Cary M, Kennedy RC, Wessels MR. 2001. Regulation of capsule gene expression by group A Streptococcus during pharyngeal colonization and invasive infection. Mol. Microbiol. 42:61–74. 10.1046/j.1365-2958.2001.02635.x [DOI] [PubMed] [Google Scholar]
- 191.Ravins M, Jaffe J, Hanski E, Shetzigovski I, Natanson-Yaron S, Moses AE. 2000. Characterization of a mouse-passaged, highly encapsulated variant of group A Streptococcus in in vitro and in vivo studies. J. Infect. Dis. 182:1702–1711. 10.1086/317635 [DOI] [PubMed] [Google Scholar]
- 192.Stollerman GH, Dale JB. 2008. The importance of the group A Streptococcus capsule in the pathogenesis of human infections: a historical perspective. Clin. Infect. Dis. 46:1038–1045. 10.1086/529194 [DOI] [PubMed] [Google Scholar]
- 193.Crater DL, van de Rijn I. 1995. Hyaluronic acid synthesis operon (has) expression in group A streptococci. J. Biol. Chem. 270:18452–18458. 10.1074/jbc.270.31.18452 [DOI] [PubMed] [Google Scholar]
- 194.Dougherty BA, van de Rijn I. 1992. Molecular characterization of a locus required for hyaluronic acid capsule production in group A streptococci. J. Exp. Med. 175:1291–1299. 10.1084/jem.175.5.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kendall FE, Heidelberger M, Dawson MH. 1937. A serologically inactive polysaccharide elaborated by mucoid strains of group A hemolytic Streptococcus. J. Biol. Chem. 118:61–69 [Google Scholar]
- 196.DeAngelis PL, Papaconstantinou J, Weigel PH. 1993. Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. J. Biol. Chem. 268:19181–19184 [PubMed] [Google Scholar]
- 197.Ashbaugh CD, Alberti S, Wessels MR. 1998. Molecular analysis of the capsule gene region of group A Streptococcus: the hasAB genes are sufficient for capsule expression. J. Bacteriol. 180:4955–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dougherty BA, van de Rijn I. 1994. Molecular characterization of hasA from an operon required for hyaluronic acid synthesis in group A streptococci. J. Biol. Chem. 269:169–175 [PubMed] [Google Scholar]
- 199.Cole JN, Aziz RK, Kuipers K, Timmer AM, Nizet V, van Sorge NM. 2012. A conserved UDP-glucose dehydrogenase encoded outside the hasABC operon contributes to capsule biogenesis in group A Streptococcus. J. Bacteriol. 194:6154–6161. 10.1128/JB.01317-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dinkla K, Sastalla I, Godehardt AW, Janze N, Chhatwal GS, Rohde M, Medina E. 2007. Upregulation of capsule enables Streptococcus pyogenes to evade immune recognition by antigen-specific antibodies directed to the G-related alpha2-macroglobulin-binding protein GRAB located on the bacterial surface. Microbes Infect. 9:922–931. 10.1016/j.micinf.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 201.Cole JN, Pence MA, von Kockritz-Blickwede M, Hollands A, Gallo RL, Walker MJ, Nizet V. 2010. M protein and hyaluronic acid capsule are essential for in vivo selection of covRS mutations characteristic of invasive serotype M1T1 group A Streptococcus. mBio 1(4):e00191-10. 10.1128/mBio.00191-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Moses AE, Wessels MR, Zalcman K, Alberti S, Natanson-Yaron S, Menes T, Hanski E. 1997. Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A Streptococcus. Infect. Immun. 65:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wessels MR, Bronze MS. 1994. Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proc. Natl. Acad. Sci. U. S. A. 91:12238–12242. 10.1073/pnas.91.25.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wessels MR, Goldberg JB, Moses AE, DiCesare TJ. 1994. Effects on virulence of mutations in a locus essential for hyaluronic acid capsule expression in group A streptococci. Infect. Immun. 62:433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wessels MR, Moses AE, Goldberg JB, DiCesare TJ. 1991. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc. Natl. Acad. Sci. U. S. A. 88:8317–8321. 10.1073/pnas.88.19.8317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ashbaugh CD, Moser TJ, Shearer MH, White GL, Kennedy RC, Wessels MR. 2000. Bacterial determinants of persistent throat colonization and the associated immune response in a primate model of human group A streptococcal pharyngeal infection. Cell. Microbiol. 2:283–292. 10.1046/j.1462-5822.2000.00050.x [DOI] [PubMed] [Google Scholar]
- 207.Flores AR, Jewell BE, Fittipaldi N, Beres SB, Musser JM. 2012. Human disease isolates of serotype M4 and M22 group A Streptococcus lack genes required for hyaluronic acid capsule biosynthesis. mBio 3(6):e00413-12. 10.1128/mBio.00413-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hynes WL, Dixon AR, Walton SL, Aridgides LJ. 2000. The extracellular hyaluronidase gene (hylA) of Streptococcus pyogenes. FEMS Microbiol. Lett. 184:109–112. 10.1111/j.1574-6968.2000.tb08999.x [DOI] [PubMed] [Google Scholar]
- 209.Akesson P, Sjoholm AG, Bjorck L. 1996. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 271:1081–1088. 10.1074/jbc.271.2.1081 [DOI] [PubMed] [Google Scholar]
- 210.Fernie-King BA, Seilly DJ, Willers C, Wurzner R, Davies A, Lachmann PJ. 2001. Streptococcal inhibitor of complement (SIC) inhibits the membrane attack complex by preventing uptake of C567 onto cell membranes. Immunology 103:390–398. 10.1046/j.1365-2567.2001.01249.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hoe NP, Ireland RM, DeLeo FR, Gowen BB, Dorward DW, Voyich JM, Liu M, Burns EH, Jr, Culnan DM, Bretscher A, Musser JM. 2002. Insight into the molecular basis of pathogen abundance: group A Streptococcus inhibitor of complement inhibits bacterial adherence and internalization into human cells. Proc. Natl. Acad. Sci. U. S. A. 99:7646–7651. 10.1073/pnas.112039899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lukomski S, Hoe NP, Abdi I, Rurangirwa J, Kordari P, Liu M, Dou SJ, Adams GG, Musser JM. 2000. Nonpolar inactivation of the hypervariable streptococcal inhibitor of complement gene (sic) in serotype M1 Streptococcus pyogenes significantly decreases mouse mucosal colonization. Infect. Immun. 68:535–542. 10.1128/IAI.68.2.535-542.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Frick IM, Akesson P, Rasmussen M, Schmidtchen A, Bjorck L. 2003. SIC, a secreted protein of Streptococcus pyogenes that inactivates antibacterial peptides. J. Biol. Chem. 278:16561–16566. 10.1074/jbc.M301995200 [DOI] [PubMed] [Google Scholar]
- 214.Fernie-King BA, Seilly DJ, Davies A, Lachmann PJ. 2002. Streptococcal inhibitor of complement inhibits two additional components of the mucosal innate immune system: secretory leukocyte proteinase inhibitor and lysozyme. Infect. Immun. 70:4908–4916. 10.1128/IAI.70.9.4908-4916.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fernie-King BA, Seilly DJ, Lachmann PJ. 2004. The interaction of streptococcal inhibitor of complement (SIC) and its proteolytic fragments with the human beta defensins. Immunology 111:444–452. 10.1111/j.0019-2805.2004.01837.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Binks M, Sriprakash KS. 2004. Characterization of a complement-binding protein, DRS, from strains of Streptococcus pyogenes containing the emm12 and emm55 genes. Infect. Immun. 72:3981–3986. 10.1128/IAI.72.7.3981-3986.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Binks M, McMillan D, Sriprakash KS. 2003. Genomic location and variation of the gene for CRS, a complement binding protein in the M57 strains of Streptococcus pyogenes. Infect. Immun. 71:6701–6706. 10.1128/IAI.71.12.6701-6706.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pandiripally V, Gregory E, Cue D. 2002. Acquisition of regulators of complement activation by Streptococcus pyogenes serotype M1. Infect. Immun. 70:6206–6214. 10.1128/IAI.70.11.6206-6214.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Molloy EM, Cotter PD, Hill C, Mitchell DA, Ross RP. 2011. Streptolysin S-like virulence factors: the continuing sagA. Nat. Rev. Microbiol. 9:670–681. 10.1038/nrmicro2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bhakdi S, Tranum-Jensen J, Sziegoleit A. 1985. Mechanism of membrane damage by streptolysin-O. Infect. Immun. 47:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Timmer AM, Timmer JC, Pence MA, Hsu LC, Ghochani M, Frey TG, Karin M, Salvesen GS, Nizet V. 2009. Streptolysin O promotes group A Streptococcus immune evasion by accelerated macrophage apoptosis. J. Biol. Chem. 284:862–871. 10.1074/jbc.M804632200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bricker AL, Cywes C, Ashbaugh CD, Wessels MR. 2002. Nad+-glycohydrolase acts as an intracellular toxin to enhance the extracellular survival of group A streptococci. Mol. Microbiol. 44:257–269. 10.1046/j.1365-2958.2002.02876.x [DOI] [PubMed] [Google Scholar]
- 223.Hakansson A, Bentley CC, Shakhnovic EA, Wessels MR. 2005. Cytolysin-dependent evasion of lysosomal killing. Proc. Natl. Acad. Sci. U. S. A. 102:5192–5197. 10.1073/pnas.0408721102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Madden JC, Ruiz N, Caparon M. 2001. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in Gram-positive bacteria. Cell 104:143–152. 10.1016/S0092-8674(01)00198-2 [DOI] [PubMed] [Google Scholar]
- 225.Brosnahan AJ, Mantz MJ, Squier CA, Peterson ML, Schlievert PM. 2009. Cytolysins augment superantigen penetration of stratified mucosa. J. Immunol. 182:2364–2373. 10.4049/jimmunol.0803283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bryant AE, Bayer CR, Chen RY, Guth PH, Wallace RJ, Stevens DL. 2005. Vascular dysfunction and ischemic destruction of tissue in Streptococcus pyogenes infection: the role of streptolysin O-induced platelet/neutrophil complexes. J. Infect. Dis. 192:1014–1022. 10.1086/432729 [DOI] [PubMed] [Google Scholar]
- 227.Limbago B, Penumalli V, Weinrick B, Scott JR. 2000. Role of streptolysin O in a mouse model of invasive group A streptococcal disease. Infect. Immun. 68:6384–6390. 10.1128/IAI.68.11.6384-6390.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ato M, Ikebe T, Kawabata H, Takemori T, Watanabe H. 2008. Incompetence of neutrophils to invasive group A Streptococcus is attributed to induction of plural virulence factors by dysfunction of a regulator. PLoS One 3:e3455. 10.1371/journal.pone.0003455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nizet V, Beall B, Bast DJ, Datta V, Kilburn L, Low DE, De Azavedo JC. 2000. Genetic locus for streptolysin S production by group A Streptococcus. Infect. Immun. 68:4245–4254. 10.1128/IAI.68.7.4245-4254.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Betschel SD, Borgia SM, Barg NL, Low DE, De Azavedo JC. 1998. Reduced virulence of group A streptococcal Tn916 mutants that do not produce streptolysin S. Infect. Immun. 66:1671–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Miyoshi-Akiyama T, Takamatsu D, Koyanagi M, Zhao J, Imanishi K, Uchiyama T. 2005. Cytocidal effect of Streptococcus pyogenes on mouse neutrophils in vivo and the critical role of streptolysin S. J. Infect. Dis. 192:107–116. 10.1086/430617 [DOI] [PubMed] [Google Scholar]
- 232.Ofek I, Bergner-Rabinowitz S, Ginsburg I. 1970. Oxygen-stable hemolysins of group A streptococci. VII. The relation of the leukotoxic factor to streptolysin S. J. Infect. Dis. 122:517–522 [DOI] [PubMed] [Google Scholar]
- 233.Heath A, DiRita VJ, Barg NL, Engleberg NC. 1999. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect. Immun. 67:5298–5305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ginsburg I. 1999. Is streptolysin S of group A streptococci a virulence factor? APMIS 107:1051–1059. 10.1111/j.1699-0463.1999.tb01509.x [DOI] [PubMed] [Google Scholar]
- 235.Sierig G, Cywes C, Wessels MR, Ashbaugh CD. 2003. Cytotoxic effects of streptolysin O and streptolysin S enhance the virulence of poorly encapsulated group A streptococci. Infect. Immun. 71:446–455. 10.1128/IAI.71.1.446-455.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Fagan PK, Reinscheid D, Gottschalk B, Chhatwal GS. 2001. Identification and characterization of a novel secreted immunoglobulin binding protein from group A Streptococcus. Infect. Immun. 69:4851–4857. 10.1128/IAI.69.8.4851-4857.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Heath DG, Cleary PP. 1989. Fc-receptor and M-protein genes of group A streptococci are products of gene duplication. Proc. Natl. Acad. Sci. U. S. A. 86:4741–4745. 10.1073/pnas.86.12.4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Stenberg L, O'Toole P, Lindahl G. 1992. Many group A streptococcal strains express two different immunoglobulin-binding proteins, encoded by closely linked genes: characterization of the proteins expressed by four strains of different M-type. Mol. Microbiol. 6:1185–1194. 10.1111/j.1365-2958.1992.tb01557.x [DOI] [PubMed] [Google Scholar]
- 239.Carlsson F, Berggard K, Stalhammar-Carlemalm M, Lindahl G. 2003. Evasion of phagocytosis through cooperation between two ligand-binding regions in Streptococcus pyogenes M protein. J. Exp. Med. 198:1057–1068. 10.1084/jem.20030543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Guzmán CA, Talay SR, Molinari G, Medina E, Chhatwal GS. 1999. Protective immune response against Streptococcus pyogenes in mice after intranasal vaccination with fibronectin-binding protein SfbI. J. Infect. Dis. 179:901–906. 10.1086/314655 [DOI] [PubMed] [Google Scholar]
- 241.Medina E, Schulze K, Chhatwal GS, Guzman CA. 2000. Nonimmune interaction of the SfbI protein of Streptococcus pyogenes with the immunoglobulin G F(ab′)(2) fragment. Infect. Immun. 68:4786–4788. 10.1128/IAI.68.8.4786-4788.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kawabata S, Tamura Y, Murakami J, Terao Y, Nakagawa I, Hamada S. 2002. A novel, anchorless streptococcal surface protein that binds to human immunoglobulins. Biochem. Biophys. Res. Commun. 296:1329–1333. 10.1016/S0006-291X(02)02078-8 [DOI] [PubMed] [Google Scholar]
- 243.Lei B, DeLeo FR, Hoe NP, Graham MR, Mackie SM, Cole RL, Liu M, Hill HR, Low DE, Federle MJ, Scott JR, Musser JM. 2001. Evasion of human innate and acquired immunity by a bacterial homolog of CD11b that inhibits opsonophagocytosis. Nat. Med. 7:1298–1305. 10.1038/nm1201-1298 [DOI] [PubMed] [Google Scholar]
- 244.Akesson P, Moritz L, Truedsson M, Christensson B, von Pawel-Rammingen U. 2006. IdeS, a highly specific immunoglobulin G (IgG)-cleaving enzyme from Streptococcus pyogenes, is inhibited by specific IgG antibodies generated during infection. Infect. Immun. 74:497–503. 10.1128/IAI.74.1.497-503.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Okumura CY, Anderson EL, Dohrmann S, Tran DN, Olson J, von Pawel-Rammingen U, Nizet V. 2013. IgG protease Mac/IdeS is not essential for phagocyte resistance or mouse virulence of M1T1 group A Streptococcus. mBio 4(4):e00499-13. 10.1128/mBio.00499-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Agniswamy J, Lei B, Musser JM, Sun PD. 2004. Insight of host immune evasion mediated by two variants of group A Streptococcus Mac protein. J. Biol. Chem. 279:52789–52796. 10.1074/jbc.M410698200 [DOI] [PubMed] [Google Scholar]
- 247.Collin M, Olsen A. 2001. Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins. Infect. Immun. 69:7187–7189. 10.1128/IAI.69.11.7187-7189.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Collin M, Svensson MD, Sjoholm AG, Jensenius JC, Sjobring U, Olsen A. 2002. EndoS and SpeB from Streptococcus pyogenes inhibit immunoglobulin-mediated opsonophagocytosis. Infect. Immun. 70:6646–6651. 10.1128/IAI.70.12.6646-6651.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sjogren J, Okumura CY, Collin M, Nizet V, Hollands A. 2011. Study of the IgG endoglycosidase EndoS in group A streptococcal phagocyte resistance and virulence. BMC Microbiol. 11:120. 10.1186/1471-2180-11-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sjogren J, Struwe WB, Cosgrave E, Rudd PM, Stervander M, Allhorn M, Hollands A, Nizet V, Collin M. 2013. EndoS2 is a unique and conserved enzyme of serotype M49 group A Streptococcus that hydrolyzes N-linked glycans on IgG and alpha1-acid glycoprotein. Biochem. J. 455:107–118. 10.1042/BJ20130126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hytonen J, Haataja S, Gerlach D, Podbielski A, Finne J. 2001. The SpeB virulence factor of Streptococcus pyogenes, a multifunctional secreted and cell surface molecule with strepadhesin, laminin-binding and cysteine protease activity. Mol. Microbiol. 39:512–519. 10.1046/j.1365-2958.2001.02269.x [DOI] [PubMed] [Google Scholar]
- 252.Chaussee MS, Phillips ER, Ferretti JJ. 1997. Temporal production of streptococcal erythrogenic toxin B (streptococcal cysteine proteinase) in response to nutrient depletion. Infect. Immun. 65:1956–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Musser JM, Stockbauer K, Kapur V, Rudgers GW. 1996. Substitution of cysteine 192 in a highly conserved Streptococcus pyogenes extracellular cysteine protease (interleukin 1beta convertase) alters proteolytic activity and ablates zymogen processing. Infect. Immun. 64:1913–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Svensson MD, Scaramuzzino DA, Sjobring U, Olsen A, Frank C, Bessen DE. 2000. Role for a secreted cysteine proteinase in the establishment of host tissue tropism by group A streptococci. Mol. Microbiol. 38:242–253. 10.1046/j.1365-2958.2000.02144.x [DOI] [PubMed] [Google Scholar]
- 255.Cole JN, McArthur JD, McKay FC, Sanderson-Smith ML, Cork AJ, Ranson M, Rohde M, Itzek A, Sun H, Ginsburg D, Kotb M, Nizet V, Chhatwal GS, Walker MJ. 2006. Trigger for group A streptococcal M1T1 invasive disease. FASEB J. 20:1745–1747. 10.1096/fj.06-5804fje [DOI] [PubMed] [Google Scholar]
- 256.Nyberg P, Rasmussen M, Bjorck L. 2004. Alpha2-macroglobulin-proteinase complexes protect Streptococcus pyogenes from killing by the antimicrobial peptide LL-37. J. Biol. Chem. 279:52820–52823. 10.1074/jbc.C400485200 [DOI] [PubMed] [Google Scholar]
- 257.Epand RM, Vogel HJ. 1999. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1462:11–28. 10.1016/S0005-2736(99)00198-4 [DOI] [PubMed] [Google Scholar]
- 258.Sun H, Ringdahl U, Homeister JW, Fay WP, Engleberg NC, Yang AY, Rozek LS, Wang X, Sjobring U, Ginsburg D. 2004. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science 305:1283–1286. 10.1126/science.1101245 [DOI] [PubMed] [Google Scholar]
- 259.Hollands A, Gonzalez D, Leire E, Donald C, Gallo RL, Sanderson-Smith M, Dorrestein PC, Nizet V. 2012. A bacterial pathogen co-opts host plasmin to resist killing by cathelicidin antimicrobial peptides. J. Biol. Chem. 287:40891–40897. 10.1074/jbc.M112.404582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chevion M, Panos C, Linzer R, Neuhaus FC. 1974. Incorporation of D-alanine into the membrane of Streptococcus pyogenes and its stabilized L-form. J. Bacteriol. 120:1026–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Beachey EH, Courtney HS. 1989. Bacterial adherence of group A streptococci to mucosal surfaces. Respiration 55(Suppl 1):33–40 [DOI] [PubMed] [Google Scholar]
- 262.Sela S, Marouni MJ, Perry R, Barzilai A. 2000. Effect of lipoteichoic acid on the uptake of Streptococcus pyogenes by HEp-2 cells. FEMS Microbiol. Lett. 193:187–193. 10.1111/j.1574-6968.2000.tb09422.x [DOI] [PubMed] [Google Scholar]
- 263.Perego M, Glaser P, Minutello A, Strauch MA, Leopold K, Fischer W. 1995. Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J. Biol. Chem. 270:15598–15606 [DOI] [PubMed] [Google Scholar]
- 264.Kristian SA, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo RL, Nizet V. 2005. D-alanylation of teichoic acids promotes group A Streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J. Bacteriol. 187:6719–6725. 10.1128/JB.187.19.6719-6725.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cox KH, Ruiz-Bustos E, Courtney HS, Dale JB, Pence MA, Nizet V, Aziz RK, Gerling I, Price SM, Hasty DL. 2009. Inactivation of DltA modulates virulence factor expression in Streptococcus pyogenes. PLoS One 4:e5366. 10.1371/journal.pone.0005366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Edwards RJ, Taylor GW, Ferguson M, Murray S, Rendell N, Wrigley A, Bai Z, Boyle J, Finney SJ, Jones A, Russell HH, Turner C, Cohen J, Faulkner L, Sriskandan S. 2005. Specific C-terminal cleavage and inactivation of interleukin-8 by invasive disease isolates of Streptococcus pyogenes. J. Infect. Dis. 192:783–790. 10.1086/432485 [DOI] [PubMed] [Google Scholar]
- 267.Zinkernagel AS, Timmer AM, Pence MA, Locke JB, Buchanan JT, Turner CE, Mishalian I, Sriskandan S, Hanski E, Nizet V. 2008. The IL-8 protease SpyCEP/ScpC of group A Streptococcus promotes resistance to neutrophil killing. Cell Host Microbe 4:170–178. 10.1016/j.chom.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kurupati P, Turner CE, Tziona I, Lawrenson RA, Alam FM, Nohadani M, Stamp GW, Zinkernagel AS, Nizet V, Edwards RJ, Sriskandan S. 2010. Chemokine-cleaving Streptococcus pyogenes protease SpyCEP is necessary and sufficient for bacterial dissemination within soft tissues and the respiratory tract. Mol. Microbiol. 76:1387–1397. 10.1111/j.1365-2958.2010.07065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Chen CC, Cleary PP. 1990. Complete nucleotide sequence of the streptococcal C5a peptidase gene of Streptococcus pyogenes. J. Biol. Chem. 265:3161–3167 [PubMed] [Google Scholar]
- 270.Shet A, Kaplan EL, Johnson DR, Cleary PP. 2003. Immune response to group A streptococcal C5a peptidase in children: implications for vaccine development. J. Infect. Dis. 188:809–817. 10.1086/377700 [DOI] [PubMed] [Google Scholar]
- 271.O'Connor SP, Cleary PP. 1986. Localization of the streptococcal C5a peptidase to the surface of group A streptococci. Infect. Immun. 53:432–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Cleary PP, Prahbu U, Dale JB, Wexler DE, Handley J. 1992. Streptococcal C5a peptidase is a highly specific endopeptidase. Infect. Immun. 60:5219–5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.DeMaster E, Schnitzler N, Cheng Q, Cleary P. 2002. M(+) group A streptococci are phagocytized and killed in whole blood by C5a-activated polymorphonuclear leukocytes. Infect. Immun. 70:350–359. 10.1128/IAI.70.1.350-359.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Terao Y, Yamaguchi M, Hamada S, Kawabata S. 2006. Multifunctional glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pyogenes is essential for evasion from neutrophils. J. Biol. Chem. 281:14215–14223. 10.1074/jbc.M513408200 [DOI] [PubMed] [Google Scholar]
- 275.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 276.Sumby P, Barbian KD, Gardner DJ, Whitney AR, Welty DM, Long RD, Bailey JR, Parnell MJ, Hoe NP, Adams GG, Deleo FR, Musser JM. 2005. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc. Natl. Acad. Sci. U. S. A. 102:1679–1684. 10.1073/pnas.0406641102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Walker MJ, Hollands A, Sanderson-Smith ML, Cole JN, Kirk JK, Henningham A, McArthur JD, Dinkla K, Aziz RK, Kansal RG, Simpson AJ, Buchanan JT, Chhatwal GS, Kotb M, Nizet V. 2007. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat. Med. 13:981–985. 10.1038/nm1612 [DOI] [PubMed] [Google Scholar]
- 278.Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. 2006. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 16:396–400. 10.1016/j.cub.2005.12.039 [DOI] [PubMed] [Google Scholar]
- 279.Uchiyama S, Andreoni F, Schuepbach RA, Nizet V, Zinkernagel AS. 2012. DNase Sda1 allows invasive M1T1 group A Streptococcus to prevent TLR9-dependent recognition. PLoS Pathog. 8:e1002736. 10.1371/journal.ppat.1002736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Chang A, Khemlani A, Kang H, Proft T. 2011. Functional analysis of Streptococcus pyogenes nuclease A (SpnA), a novel group A streptococcal virulence factor. Mol. Microbiol. 79:1629–1642. 10.1111/j.1365-2958.2011.07550.x [DOI] [PubMed] [Google Scholar]
- 281.Bernard P. 2008. Management of common bacterial infections of the skin. Curr. Opin. Infect. Dis. 21:122–128. 10.1097/QCO.0b013e3282f44c63 [DOI] [PubMed] [Google Scholar]
- 282.Johansson L, Thulin P, Low DE, Norrby-Teglund A. 2010. Getting under the skin: the immunopathogenesis of Streptococcus pyogenes deep tissue infections. Clin. Infect. Dis. 51:58–65. 10.1086/653116 [DOI] [PubMed] [Google Scholar]
- 283.Steer AC, Lamagni T, Curtis N, Carapetis JR. 2012. Invasive group A streptococcal disease: epidemiology, pathogenesis and management. Drugs 72:1213–1227. 10.2165/11634180-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lin JN, Chang LL, Lai CH, Lin HH, Chen YH. 2013. Group A streptococcal necrotizing fasciitis in the emergency department. J. Emerg. Med. 45:781–788. 10.1016/j.jemermed.2013.05.046 [DOI] [PubMed] [Google Scholar]
- 285.Olsen RJ, Musser JM. 2010. Molecular pathogenesis of necrotizing fasciitis. Annu. Rev. Pathol. 5:1–31. 10.1146/annurev-pathol-121808-102135 [DOI] [PubMed] [Google Scholar]
- 286.Walker MJ, McArthur JD, McKay F, Ranson M. 2005. Is plasminogen deployed as a Streptococcus pyogenes virulence factor? Trends Microbiol. 13:308–313. 10.1016/j.tim.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 287.Hamilton SM, Bayer CR, Stevens DL, Lieber RL, Bryant AE. 2008. Muscle injury, vimentin expression, and nonsteroidal anti-inflammatory drugs predispose to cryptic group A streptococcal necrotizing infection. J. Infect. Dis. 198:1692–1698. 10.1086/593016 [DOI] [PubMed] [Google Scholar]
- 288.Nuwayhid ZB, Aronoff DM, Mulla ZD. 2007. Blunt trauma as a risk factor for group A streptococcal necrotizing fasciitis. Ann. Epidemiol. 17:878–881. 10.1016/j.annepidem.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kotb M, Norrby-Teglund A, McGeer A, El-Sherbini H, Dorak MT, Khurshid A, Green K, Peeples J, Wade J, Thomson G, Schwartz B, Low DE. 2002. An immunogenetic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nat. Med. 8:1398–1404. 10.1038/nm1202-800 [DOI] [PubMed] [Google Scholar]
- 290.Nooh MM, El-Genghi N, Kansal R, David CS, Kotb M. 2007. HLA transgenic mice provide evidence for a direct and dominant role of HLA class II variation in modulating the severity of streptococcal sepsis. J. Immunol. 178:3076–3083 http://www.jimmunol.org/content/178/5/3076.long [DOI] [PubMed] [Google Scholar]
- 291.Lappin E, Ferguson AJ. 2009. Gram-positive toxic shock syndromes. Lancet Infect. Dis. 9:281–290. 10.1016/S1473-3099(09)70066-0 [DOI] [PubMed] [Google Scholar]
- 292.Kasper KJ, Xi W, Rahman AKMN, Nooh MM, Kotb M, Sundberg EJ, Madrenas J, McCormick JK. 2008. Molecular requirements for MHC class II alpha-chain engagement and allelic discrimination by the bacterial superantigen streptococcal pyrogenic exotoxin C. J. Immunol. 181:3384–3392 http://www.jimmunol.org/content/181/5/3384.long [DOI] [PubMed] [Google Scholar]
- 293.Musser JM, Shelburne SA., III 2009. A decade of molecular pathogenomic analysis of group A Streptococcus. J. Clin. Invest. 119:2455–2463. 10.1172/JCI38095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. 2006. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2:e5. 10.1371/journal.ppat.0020005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Graham MR, Smoot LM, Migliaccio CA, Virtaneva K, Sturdevant DE, Porcella SF, Federle MJ, Adams GJ, Scott JR, Musser JM. 2002. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. U. S. A. 99:13855–13860. 10.1073/pnas.202353699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Federle MJ, McIver KS, Scott JR. 1999. A response regulator that represses transcription of several virulence operons in the group A Streptococcus. J. Bacteriol. 181:3649–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Voyich JM, Sturdevant DE, Braughton KR, Kobayashi SD, Lei B, Virtaneva K, Dorward DW, Musser JM, DeLeo FR. 2003. Genome-wide protective response used by group A Streptococcus to evade destruction by human polymorphonuclear leukocytes. Proc. Natl. Acad. Sci. U. S. A. 100:1996–2001. 10.1073/pnas.0337370100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Voyich JM, Braughton KR, Sturdevant DE, Vuong C, Kobayashi SD, Porcella SF, Otto M, Musser JM, DeLeo FR. 2004. Engagement of the pathogen survival response used by group A Streptococcus to avert destruction by innate host defense. J. Immunol. 173:1194–1201 http://www.jimmunol.org/content/173/2/1194.long [DOI] [PubMed] [Google Scholar]
- 299.Okada N, Geist RT, Caparon MG. 1993. Positive transcriptional control of mry regulates virulence in the group A Streptococcus. Mol. Microbiol. 7:893–903. 10.1111/j.1365-2958.1993.tb01180.x [DOI] [PubMed] [Google Scholar]
- 300.Caparon MG, Scott JR. 1987. Identification of a gene that regulates expression of M protein, the major virulence determinant of group A streptococci. Proc. Natl. Acad. Sci. U. S. A. 84:8677–8681. 10.1073/pnas.84.23.8677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Podbielski A, Flosdorff A, Weber-Heynemann J. 1995. The group A streptococcal virR49 gene controls expression of four structural vir regulon genes. Infect. Immun. 63:9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Rasmussen M, Eden A, Bjorck L. 2000. SclA, a novel collagen-like surface protein of Streptococcus pyogenes. Infect. Immun. 68:6370–6377. 10.1128/IAI.68.11.6370-6377.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ribardo DA, McIver KS. 2006. Defining the Mga regulon: comparative transcriptome analysis reveals both direct and indirect regulation by Mga in the group A Streptococcus. Mol. Microbiol. 62:491–508. 10.1111/j.1365-2958.2006.05381.x [DOI] [PubMed] [Google Scholar]
- 304.Simpson WJ, LaPenta D, Chen C, Cleary PP. 1990. Coregulation of type 12 M protein and streptococcal C5a peptidase genes in group A streptococci: evidence for a virulence regulon controlled by the virR locus. J. Bacteriol. 172:696–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kihlberg BM, Cooney J, Caparon MG, Olsen A, Bjorck L. 1995. Biological properties of a Streptococcus pyogenes mutant generated by Tn916 insertion in mga. Microb. Pathog. 19:299–315 [DOI] [PubMed] [Google Scholar]
- 306.Hondorp ER, Hou SC, Hause LL, Gera K, Lee CE, McIver KS. 2013. PTS phosphorylation of Mga modulates regulon expression and virulence in the group A Streptococcus. Mol. Microbiol. 88:1176–1193. 10.1111/mmi.12250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bernish B, van de Rijn I. 1999. Characterization of a two-component system in Streptococcus pyogenes which is involved in regulation of hyaluronic acid production. J. Biol. Chem. 274:4786–4793. 10.1074/jbc.274.8.4786 [DOI] [PubMed] [Google Scholar]
- 308.Levin JC, Wessels MR. 1998. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol. Microbiol. 30:209–219. 10.1046/j.1365-2958.1998.01057.x [DOI] [PubMed] [Google Scholar]
- 309.Dalton TL, Scott JR. 2004. CovS inactivates CovR and is required for growth under conditions of general stress in Streptococcus pyogenes. J. Bacteriol. 186:3928–3937. 10.1128/JB.186.12.3928-3937.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Churchward G. 2007. The two faces of Janus: virulence gene regulation by CovR/S in group A streptococci. Mol. Microbiol. 64:34–41. 10.1111/j.1365-2958.2007.05649.x [DOI] [PubMed] [Google Scholar]
- 311.Gryllos I, Levin JC, Wessels MR. 2003. The CsrR/CsrS two-component system of group A Streptococcus responds to environmental Mg2+. Proc. Natl. Acad. Sci. U. S. A. 100:4227–4232. 10.1073/pnas.0636231100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Trevino J, Perez N, Ramirez-Pena E, Liu Z, Shelburne SA, III, Musser JM, Sumby P. 2009. CovC simultaneously activates and inhibits the CovR-mediated repression of distinct subsets of group A Streptococcus virulence factor-encoding genes. Infect. Immun. 77:3141–3149. 10.1128/IAI.01560-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Dalton TL, Hobb RI, Scott JR. 2006. Analysis of the role of CovR and CovS in the dissemination of Streptococcus pyogenes in invasive skin disease. Microb. Pathog. 40:221–227. 10.1016/j.micpath.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 314.Maamary PG, Sanderson-Smith ML, Aziz RK, Hollands A, Cole JN, McKay FC, McArthur JD, Kirk JK, Cork AJ, Keefe RJ, Kansal RG, Sun H, Taylor WL, Chhatwal GS, Ginsburg D, Nizet V, Kotb M, Walker MJ. 2010. Parameters governing invasive disease propensity of non-M1 serotype group A streptococci. J. Innate Immun. 2:596–606. 10.1159/000317640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Engleberg NC, Heath A, Miller A, Rivera C, DiRita VJ. 2001. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J. Infect. Dis. 183:1043–1054. 10.1086/319291 [DOI] [PubMed] [Google Scholar]
- 316.Hollands A, Aziz RK, Kansal R, Kotb M, Nizet V, Walker MJ. 2008. A naturally occurring mutation in ropB suppresses SpeB expression and reduces M1T1 group A streptococcal systemic virulence. PLoS One 3:e4102. 10.1371/journal.pone.0004102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ikebe T, Ato M, Matsumura T, Hasegawa H, Sata T, Kobayashi K, Watanabe H. 2010. Highly frequent mutations in negative regulators of multiple virulence genes in group A streptococcal toxic shock syndrome isolates. PLoS Pathog. 6:e1000832. 10.1371/journal.ppat.1000832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Carroll RK, Beres SB, Sitkiewicz I, Peterson L, Matsunami RK, Engler DA, Flores AR, Sumby P, Musser JM. 2011. Evolution of diversity in epidemics revealed by analysis of the human bacterial pathogen group A Streptococcus. Epidemics 3:159–170. 10.1016/j.epidem.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 319.McKay FC, McArthur JD, Sanderson-Smith ML, Gardam S, Currie BJ, Sriprakash KS, Fagan PK, Towers RJ, Batzloff MR, Chhatwal GS, Ranson M, Walker MJ. 2004. Plasminogen binding by group A streptococcal isolates from a region of hyperendemicity for streptococcal skin infection and a high incidence of invasive infection. Infect. Immun. 72:364–370. 10.1128/IAI.72.1.364-370.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Hassell M, Fagan P, Carson P, Currie BJ. 2004. Streptococcal necrotising fasciitis from diverse strains of Streptococcus pyogenes in tropical northern Australia: case series and comparison with the literature. BMC Infect. Dis. 4:60. 10.1186/1471-2334-4-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kuo CF, Wu JJ, Lin KY, Tsai PJ, Lee SC, Jin YT, Lei HY, Lin YS. 1998. Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect. Immun. 66:3931–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Lukomski S, Burns EH, Jr, Wyde PR, Podbielski A, Rurangirwa J, Moore-Poveda DK, Musser JM. 1998. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect. Immun. 66:771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Hollands A, Pence MA, Timmer AM, Osvath SR, Turnbull L, Whitchurch CB, Walker MJ, Nizet V. 2010. Genetic switch to hypervirulence reduces colonization phenotypes of the globally disseminated group A Streptococcus M1T1 clone. J. Infect. Dis. 202:11–19. 10.1086/653124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Alam FM, Turner CE, Smith K, Wiles S, Sriskandan S. 2013. Inactivation of the CovR/S virulence regulator impairs infection in an improved murine model of Streptococcus pyogenes naso-pharyngeal infection. PLoS One 8:e61655. 10.1371/journal.pone.0061655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Sanderson-Smith ML, Dinkla K, Cole JN, Cork AJ, Maamary PG, McArthur JD, Chhatwal GS, Walker MJ. 2008. M protein-mediated plasminogen binding is essential for the virulence of an invasive Streptococcus pyogenes isolate. FASEB J. 22:2715–2722. 10.1096/fj.07-105643 [DOI] [PubMed] [Google Scholar]
- 326.Plow EF, Herren T, Redlitz A, Miles LA, Hoover-Plow JL. 1995. The cell biology of the plasminogen system. FASEB J. 9:939–945 [DOI] [PubMed] [Google Scholar]
- 327.Wiman B, Collen D. 1978. On the kinetics of the reaction between human antiplasmin and plasmin. Eur. J. Biochem. 84:573–578. 10.1111/j.1432-1033.1978.tb12200.x [DOI] [PubMed] [Google Scholar]
- 328.Wang H, Lottenberg R, Boyle MD. 1995. Analysis of the interaction of group A streptococci with fibrinogen, streptokinase and plasminogen. Microb. Pathog. 18:153–166. 10.1016/S0882-4010(95)90013-6 [DOI] [PubMed] [Google Scholar]
- 329.Coleman JL, Benach JL. 1999. Use of the plasminogen activation system by microorganisms. J. Lab. Clin. Med. 134:567–576. 10.1016/S0022-2143(99)90095-1 [DOI] [PubMed] [Google Scholar]
- 330.Dano K, Andreasen PA, Grondahl-Hansen J, Kristensen P, Nielsen LS, Skriver L. 1985. Plasminogen activators, tissue degradation, and cancer. Adv. Cancer Res. 44:139–266. 10.1016/S0065-230X(08)60028-7 [DOI] [PubMed] [Google Scholar]
- 331.Castellino FJ, Ploplis VA. 2005. Structure and function of the plasminogen/plasmin system. Thromb. Haemost. 93:647–654. 10.1160/TH04-12-0842 [DOI] [PubMed] [Google Scholar]
- 332.Marcum JA, Kline DL. 1983. Species specificity of streptokinase. Comp. Biochem. Physiol. B 75:389–394 [DOI] [PubMed] [Google Scholar]
- 333.Wang X, Lin X, Loy JA, Tang J, Zhang XC. 1998. Crystal structure of the catalytic domain of human plasmin complexed with streptokinase. Science 281:1662–1665. 10.1126/science.281.5383.1662 [DOI] [PubMed] [Google Scholar]
- 334.Yakovlev SA, Rublenko MV, Izdepsky VI, Makogonenko EM. 1995. Activating effect of the plasminogen activators on plasminogens of different mammalia species. Thromb. Res. 79:423–428. 10.1016/0049-3848(95)00131-A [DOI] [PubMed] [Google Scholar]
- 335.Sanderson-Smith ML, Zhang Y, Ly D, Donahue D, Hollands A, Nizet V, Ranson M, Ploplis VA, Walker MJ, Castellino FJ. 2013. A key role for the urokinase plasminogen activator (uPA) in invasive group A streptococcal infection. PLoS Pathog. 9:e1003469. 10.1371/journal.ppat.1003469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ben Nasr A, Wistedt A, Ringdahl U, Sjobring U. 1994. Streptokinase activates plasminogen bound to human group C and G streptococci through M-like proteins. Eur. J. Biochem. 222:267–276. 10.1111/j.1432-1033.1994.tb18865.x [DOI] [PubMed] [Google Scholar]
- 337.Berge A, Sjobring U. 1993. PAM, a novel plasminogen-binding protein from Streptococcus pyogenes. J. Biol. Chem. 268:25417–25424 [PubMed] [Google Scholar]
- 338.Sanderson-Smith M, Batzloff MR, Sriprakash KS, Dowton M, Ranson M, Walker MJ. 2006. Divergence in the plasminogen-binding group A streptococcal M protein family: functional conservation of binding site and potential role for immune selection of variants. J. Biol. Chem. 281:3217–3226. 10.1074/jbc.M508758200 [DOI] [PubMed] [Google Scholar]
- 339.Sanderson-Smith ML, Dowton M, Ranson M, Walker MJ. 2007. The plasminogen-binding group A streptococcal M protein-related protein Prp binds plasminogen via arginine and histidine residues. J. Bacteriol. 189:1435–1440. 10.1128/JB.01218-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Sanderson-Smith ML, Walker MJ, Ranson M. 2006. The maintenance of high affinity plasminogen binding by group A streptococcal plasminogen-binding M-like protein is mediated by arginine and histidine residues within the a1 and a2 repeat domains. J. Biol. Chem. 281:25965–25971. 10.1074/jbc.M603846200 [DOI] [PubMed] [Google Scholar]
- 341.Rios-Steiner JL, Schenone M, Mochalkin I, Tulinsky A, Castellino FJ. 2001. Structure and binding determinants of the recombinant kringle-2 domain of human plasminogen to an internal peptide from a group A streptococcal surface protein. J. Mol. Biol. 308:705–719. 10.1006/jmbi.2001.4646 [DOI] [PubMed] [Google Scholar]
- 342.Svensson MD, Sjobring U, Luo F, Bessen DE. 2002. Roles of the plasminogen activator streptokinase and the plasminogen-associated M protein in an experimental model for streptococcal impetigo. Microbiology 148:3933–3945 http://mic.sgmjournals.org/content/148/12/3933.long [DOI] [PubMed] [Google Scholar]
- 343.Pancholi V, Fischetti VA. 1997. A novel plasminogen/plasmin binding protein on the surface of group A streptococci. Adv. Exp. Med. Biol. 418:597–599. 10.1007/978-1-4899-1825-3_138 [DOI] [PubMed] [Google Scholar]
- 344.Knaust A, Weber MV, Hammerschmidt S, Bergmann S, Frosch M, Kurzai O. 2007. Cytosolic proteins contribute to surface plasminogen recruitment of Neisseria meningitidis. J. Bacteriol. 189:3246–3255. 10.1128/JB.01966-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Boone TJ, Burnham CA, Tyrrell GJ. 2011. Binding of group B streptococcal phosphoglycerate kinase to plasminogen and actin. Microb. Pathog. 51:255–261. 10.1016/j.micpath.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 346.Matta SK, Agarwal S, Bhatnagar R. 2010. Surface localized and extracellular glyceraldehyde-3-phosphate dehydrogenase of Bacillus anthracis is a plasminogen binding protein. Biochim. Biophys. Acta 1804:2111–2120. 10.1016/j.bbapap.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 347.D'Costa SS, Boyle MD. 1998. Interaction of a group A Streptococcus within human plasma results in assembly of a surface plasminogen activator that contributes to occupancy of surface plasmin-binding structures. Microb. Pathog. 24:341–349. 10.1006/mpat.1998.0207 [DOI] [PubMed] [Google Scholar]
- 348.Wang H, Lottenberg R, Boyle MD. 1995. A role for fibrinogen in the streptokinase-dependent acquisition of plasmin(ogen) by group A streptococci. J. Infect. Dis. 171:85–92. 10.1093/infdis/171.1.85 [DOI] [PubMed] [Google Scholar]
- 349.Katerov V, Andreev A, Schalen C, Totolian AA. 1998. Protein F, a fibronectin-binding protein of Streptococcus pyogenes, also binds human fibrinogen: isolation of the protein and mapping of the binding region. Microbiology 144:119–126. 10.1099/00221287-144-1-119 [DOI] [PubMed] [Google Scholar]
- 350.Margarit I, Bonacci S, Pietrocola G, Rindi S, Ghezzo C, Bombaci M, Nardi-Dei V, Grifantini R, Speziale P, Grandi G. 2009. Capturing host-pathogen interactions by protein microarrays: identification of novel streptococcal proteins binding to human fibronectin, fibrinogen, and C4BP. FASEB J. 23:3100–3112. 10.1096/fj.09-131458 [DOI] [PubMed] [Google Scholar]
- 351.Berge A, Bjorck L. 1995. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J. Biol. Chem. 270:9862–9867. 10.1074/jbc.270.17.9862 [DOI] [PubMed] [Google Scholar]
- 352.Herwald H, Cramer H, Morgelin M, Russell W, Sollenberg U, Norrby-Teglund A, Flodgaard H, Lindbom L, Bjorck L. 2004. M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell 116:367–379. 10.1016/S0092-8674(04)00057-1 [DOI] [PubMed] [Google Scholar]
- 353.Norrby-Teglund A, Thulin P, Gan BS, Kotb M, McGeer A, Andersson J, Low DE. 2001. Evidence for superantigen involvement in severe group A streptococcal tissue infections. J. Infect. Dis. 184:853–860. 10.1086/323443 [DOI] [PubMed] [Google Scholar]
- 354.Norrby-Teglund A, Chatellier S, Low DE, McGeer A, Green K, Kotb M. 2000. Host variation in cytokine responses to superantigens determine the severity of invasive group A streptococcal infection. Eur. J. Immunol. 30:3247–3255. [DOI] [PubMed] [Google Scholar]
- 355.Commons RJ, Smeesters PR, Proft T, Fraser JD, Robins-Browne R, Curtis N. 2014. Streptococcal superantigens: categorization and clinical associations. Trends Mol. Med. 20:48–62. 10.1016/j.molmed.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 356.Fraser JD, Proft T. 2008. The bacterial superantigen and superantigen-like proteins. Immunol. Rev. 225:226–243. 10.1111/j.1600-065X.2008.00681.x [DOI] [PubMed] [Google Scholar]
- 357.Norrby-Teglund A, Johansson L. 2013. Beyond the traditional immune response: bacterial interaction with phagocytic cells. Int. J. Antimicrob. Agents 42:S13–S16. 10.1016/j.ijantimicag.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 358.Reglinski M, Sriskandan S. 2014. The contribution of group A streptococcal virulence determinants to the pathogenesis of sepsis. Virulence 5:127–136 http://www.landesbioscience.com/journals/virulence/2012VIRULENCE0178R.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Ganem MB, De Marzi MC, Fernandez-Lynch MJ, Jancic C, Vermeulen M, Geffner J, Mariuzza RA, Fernandez MM, Malchiodi EL. 2013. Uptake and intracellular trafficking of superantigens in dendritic cells. PLoS One 8:e66244. 10.1371/journal.pone.0066244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Baker M, Gutman DM, Papageorgiou AC, Collins CM, Acharya KR. 2001. Structural features of a zinc binding site in the superantigen strepococcal pyrogenic exotoxin A (SpeA1): implications for MHC class II recognition. Protein Sci. 10:1268–1273. 10.1110/ps.330101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Earhart CA, Vath GM, Roggiani M, Schlievert PM, Ohlendorf DH. 2000. Structure of streptococcal pyrogenic exotoxin A reveals a novel metal cluster. Protein Sci. 9:1847–1851. 10.1110/ps.9.9.1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Papageorgiou AC, Collins CM, Gutman DM, Kline JB, O'Brien SM, Tranter HS, Acharya KR. 1999. Structural basis for the recognition of superantigen streptococcal pyrogenic exotoxin A (SpeA1) by MHC class II molecules and T-cell receptors. EMBO J. 18:9–21. 10.1093/emboj/18.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Arad G, Levy R, Hillman D, Kaempfer R. 2000. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat. Med. 6:414–421. 10.1038/74672 [DOI] [PubMed] [Google Scholar]
- 364.Arad G, Levy R, Nasie I, Hillman D, Rotfogel Z, Barash U, Supper E, Shpilka T, Minis A, Kaempfer R. 2011. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol. 9:e1001149. 10.1371/journal.pbio.1001149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Talkington DF, Schwartz B, Black CM, Todd JK, Elliott J, Breiman RF, Facklam RR. 1993. Association of phenotypic and genotypic characteristics of invasive Streptococcus pyogenes isolates with clinical components of streptococcal toxic shock syndrome. Infect. Immun. 61:3369–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Vlaminckx BJ, Mascini EM, Schellekens J, Schouls LM, Paauw A, Fluit AC, Novak R, Verhoef J, Schmitz FJ. 2003. Site-specific manifestations of invasive group A streptococcal disease: type distribution and corresponding patterns of virulence determinants. J. Clin. Microbiol. 41:4941–4949. 10.1128/JCM.41.11.4941-4949.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Luca-Harari B, Darenberg J, Neal S, Siljander T, Strakova L, Tanna A, Creti R, Ekelund K, Koliou M, Tassios PT, van der Linden M, Straut M, Vuopio-Varkila J, Bouvet A, Efstratiou A, Schalen C, Henriques-Normark B, Strep-EURO Study Group. Jasir A. 2009. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol. 47:1155–1165. 10.1128/JCM.02155-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Sriskandan S, Unnikrishnan M, Krausz T, Cohen J. 1999. Molecular analysis of the role of streptococcal pyrogenic exotoxin A (SpeA) in invasive soft-tissue infection resulting from Streptococcus pyogenes. Mol. Microbiol. 33:778–790. 10.1046/j.1365-2958.1999.01525.x [DOI] [PubMed] [Google Scholar]
- 369.Michaelsen TE, Andreasson IK, Langerud BK, Caugant DA. 2011. Similar superantigen gene profiles and superantigen activity in Norwegian isolates of invasive and non-invasive group A streptococci. Scand. J. Immunol. 74:423–429. 10.1111/j.1365-3083.2011.02594.x [DOI] [PubMed] [Google Scholar]
- 370.Rantala S, Vahakuopus S, Siljander T, Vuopio J, Huhtala H, Vuento R, Syrjanen J. 2012. Streptococcus pyogenes bacteraemia, emm types and superantigen profiles. Eur. J. Clin. Microbiol. Infect. Dis. 31:859–865. 10.1007/s10096-011-1385-9 [DOI] [PubMed] [Google Scholar]
- 371.Bruun T, Kittang BR, de Hoog BJ, Aardal S, Flaatten HK, Langeland N, Mylvaganam H, Vindenes HA, Skrede S. 2013. Necrotizing soft tissue infections caused by Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis of groups C and G in western Norway. Clin. Microbiol. Infect. 19:E545–E550. 10.1111/1469-0691.12276 [DOI] [PubMed] [Google Scholar]
- 372.Sun H, Wang X, Degen JL, Ginsburg D. 2009. Reduced thrombin generation increases host susceptibility to group A streptococcal infection. Blood 113:1358–1364. 10.1182/blood-2008-07-170506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Bryant AE, Hayes-Schroer SM, Stevens DL. 2003. M type 1 and 3 group A streptococci stimulate tissue factor-mediated procoagulant activity in human monocytes and endothelial cells. Infect. Immun. 71:1903–1910. 10.1128/IAI.71.4.1903-1910.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ben Nasr AB, Herwald H, Muller-Esterl W, Bjorck L. 1995. Human kininogens interact with M protein, a bacterial surface protein and virulence determinant. Biochem. J. 305:173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Ben Nasr A, Herwald H, Sjobring U, Renne T, Muller-Esterl W, Bjorck L. 1997. Absorption of kininogen from human plasma by Streptococcus pyogenes is followed by the release of bradykinin. Biochem. J. 326:657–660 [PMC free article] [PubMed] [Google Scholar]
- 376.Sriskandan S, Cohen J. 2000. Kallikrein-kinin system activation in streptococcal toxic shock syndrome. Clin. Infect. Dis. 30:961–962. 10.1086/313827 [DOI] [PubMed] [Google Scholar]
- 377.Oehmcke S, Shannon O, von Kockritz-Blickwede M, Morgelin M, Linder A, Olin AI, Bjorck L, Herwald H. 2009. Treatment of invasive streptococcal infection with a peptide derived from human high-molecular weight kininogen. Blood 114:444–451. 10.1182/blood-2008-10-182527 [DOI] [PubMed] [Google Scholar]
- 378.Linder A, Johansson L, Thulin P, Hertzén E, Morgelin M, Christensson B, Bjorck L, Norrby-Teglund A, Akesson P. 2010. Erysipelas caused by group A Streptococcus activates the contact system and induces the release of heparin-binding protein. J. Investig. Dermatol. 130:1365–1372. 10.1038/jid.2009.437 [DOI] [PubMed] [Google Scholar]
- 379.Pahlman LI, Malmstrom E, Morgelin M, Herwald H. 2007. M protein from Streptococcus pyogenes induces tissue factor expression and pro-coagulant activity in human monocytes. Microbiology 153:2458–2464. 10.1099/mic.0.2006/003285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Pahlman LI, Marx PF, Morgelin M, Lukomski S, Meijers JC, Herwald H. 2007. Thrombin-activatable fibrinolysis inhibitor binds to Streptococcus pyogenes by interacting with collagen-like proteins A and B. J. Biol. Chem. 282:24873–24881. 10.1074/jbc.M610015200 [DOI] [PubMed] [Google Scholar]
- 381.Oehmcke S, Shannon O, Morgelin M, Herwald H. 2010. Streptococcal M proteins and their role as virulence determinants. Clin. Chim. Acta 411:1172–1180. 10.1016/j.cca.2010.04.032 [DOI] [PubMed] [Google Scholar]
- 382.Pahlman LI, Morgelin M, Eckert J, Johansson L, Russell W, Riesbeck K, Soehnlein O, Lindbom L, Norrby-Teglund A, Schumann RR, Bjorck L, Herwald H. 2006. Streptococcal M protein: a multipotent and powerful inducer of inflammation. J. Immunol. 177:1221–1228 http://www.jimmunol.org/content/177/2/1221.long [DOI] [PubMed] [Google Scholar]
- 383.Shannon O, Hertzen E, Norrby-Teglund A, Morgelin M, Sjobring U, Bjorck L. 2007. Severe streptococcal infection is associated with M protein-induced platelet activation and thrombus formation. Mol. Microbiol. 65:1147–1157. 10.1111/j.1365-2958.2007.05841.x [DOI] [PubMed] [Google Scholar]
- 384.Kahn F, Hurley S, Shannon O. 2013. Platelets promote bacterial dissemination in a mouse model of streptococcal sepsis. Microbes Infect. 15:669–676. 10.1016/j.micinf.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 385.Oehmcke S, Morgelin M, Malmstrom J, Linder A, Chew M, Thorlacius H, Herwald H. 2012. Stimulation of blood mononuclear cells with bacterial virulence factors leads to the release of pro-coagulant and pro-inflammatory microparticles. Cell. Microbiol. 14:107–119. 10.1111/j.1462-5822.2011.01705.x [DOI] [PubMed] [Google Scholar]
- 386.Oehmcke S, Westman J, Malmstrom J, Morgelin M, Olin AI, Kreikemeyer B, Herwald H. 2013. A novel role for pro-coagulant microvesicles in the early host defense against Streptococcus pyogenes. PLoS Pathog. 9:e1003529. 10.1371/journal.ppat.1003529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Guilherme L, Kalil J. 2010. Rheumatic fever and rheumatic heart disease: cellular mechanisms leading autoimmune reactivity and disease. J. Clin. Immunol. 30:17–23. 10.1007/s10875-009-9332-6 [DOI] [PubMed] [Google Scholar]
- 388.Guilherme L, Kohler KF, Kalil J. 2011. Rheumatic heart disease: mediation by complex immune events. Adv. Clin. Chem. 53:31–50. 10.1016/B978-0-12-385855-9.00002-3 [DOI] [PubMed] [Google Scholar]
- 389.Guilherme L, Kalil J, Cunningham M. 2006. Molecular mimicry in the autoimmune pathogenesis of rheumatic heart disease. Autoimmunity 39:31–39. 10.1080/08916930500484674 [DOI] [PubMed] [Google Scholar]
- 390.Cunningham MW, McCormack JM, Fenderson PG, Ho MK, Beachey EH, Dale JB. 1989. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence Gln-Lys-Ser-Lys-Gln in M protein. J. Immunol. 143:2677–2683 [PubMed] [Google Scholar]
- 391.Cunningham MW, McCormack JM, Talaber LR, Harley JB, Ayoub EM, Muneer RS, Chun LT, Reddy DV. 1988. Human monoclonal antibodies reactive with antigens of the group A Streptococcus and human heart. J. Immunol. 141:2760–2766 [PubMed] [Google Scholar]
- 392.Shikhman AR, Greenspan NS, Cunningham MW. 1993. A subset of mouse monoclonal antibodies cross-reactive with cytoskeletal proteins and group A streptococcal M proteins recognizes N-acetyl-beta-D-glucosamine. J. Immunol. 151:3902–3913 [PubMed] [Google Scholar]
- 393.Fae KC, da Silva DD, Oshiro SE, Tanaka AC, Pomerantzeff PM, Douay C, Charron D, Toubert A, Cunningham MW, Kalil J, Guilherme L. 2006. Mimicry in recognition of cardiac myosin peptides by heart-intralesional T cell clones from rheumatic heart disease. J. Immunol. 176:5662–5670 http://www.jimmunol.org/content/176/9/5662.long [DOI] [PubMed] [Google Scholar]
- 394.Fae KC, Palacios SA, Nogueira LG, Oshiro SE, Demarchi LM, Bilate AM, Pomerantzeff PM, Brandao C, Thomaz PG, dos Reis M, Sampaio R, Tanaka AC, Cunha-Neto E, Kalil J, Guilherme L. 2013. Cxcl9/Mig mediates T cells recruitment to valvular tissue lesions of chronic rheumatic heart disease patients. Inflammation 36:800–811. 10.1007/s10753-013-9606-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Cunningham MW. 2012. Streptococcus and rheumatic fever. Curr. Opin. Rheumatol. 24:408–416. 10.1097/BOR.0b013e32835461d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Dinkla K, Rohde M, Jansen WT, Carapetis JR, Chhatwal GS, Talay SR. 2003. Streptococcus pyogenes recruits collagen via surface-bound fibronectin: a novel colonization and immune evasion mechanism. Mol. Microbiol. 47:861–869. 10.1046/j.1365-2958.2003.03352.x [DOI] [PubMed] [Google Scholar]
- 397.Dinkla K, Rohde M, Jansen WT, Kaplan EL, Chhatwal GS, Talay SR. 2003. Rheumatic fever-associated Streptococcus pyogenes isolates aggregate collagen. J. Clin. Invest. 111:1905–1912 http://www.jci.org/articles/view/17247/pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Dinkla K, Nitsche-Schmitz DP, Barroso V, Reissmann S, Johansson HM, Frick I-M, Rohde M, Chhatwal GS. 2007. Identification of a streptococcal octapeptide motif involved in acute rheumatic fever. J. Biol. Chem. 282:18686–18693. 10.1074/jbc.M701047200 [DOI] [PubMed] [Google Scholar]
- 399.Tandon R, Sharma M, Chandrashekhar Y, Kotb M, Yacoub MH, Narula J. 2013. Revisiting the pathogenesis of rheumatic fever and carditis. Nat. Rev. Cardiol. 10:171–177. 10.1038/nrcardio.2012.197 [DOI] [PubMed] [Google Scholar]
- 400.Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. 2003. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat. Med. 9:914–920. 10.1038/nm892 [DOI] [PubMed] [Google Scholar]
- 401.Murphy TK, Kurlan R, Leckman J. 2010. The immunobiology of Tourette's disorder, pediatric autoimmune neuropsychiatric disorders associated with Streptococcus, and related disorders: a way forward. J. Child Adolesc. Psychopharmacol. 20:317–331. 10.1089/cap.2010.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Kurlan R, Kaplan EL. 2004. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) etiology for tics and obsessive-compulsive symptoms: hypothesis or entity? Practical considerations for the clinician. Pediatrics 113:883–886 http://pediatrics.aappublications.org/content/113/4/883.full.pdf [DOI] [PubMed] [Google Scholar]
- 403.Swedo SE, Leonard HL, Rapoport JL. 2004. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) subgroup: separating fact from fiction. Pediatrics 113:907–911. 10.1542/peds.113.4.907 [DOI] [PubMed] [Google Scholar]
- 404.Kirvan CA, Swedo SE, Kurahara D, Cunningham MW. 2006. Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham's chorea. Autoimmunity 39:21–29. 10.1080/08916930500484757 [DOI] [PubMed] [Google Scholar]
- 405.de Oliveira SK. 2007. PANDAS: a new disease? J. Pediatr. (Rio J) 83:201–208. 10.2223/JPED.1615 [DOI] [PubMed] [Google Scholar]
- 406.Snider LA, Swedo SE. 2003. Post-streptococcal autoimmune disorders of the central nervous system. Curr. Opin. Neurol. 16:359–365. 10.1097/00019052-200306000-00017 [DOI] [PubMed] [Google Scholar]
- 407.Snider LA, Swedo SE. 2004. PANDAS: current status and directions for research. Mol. Psychiatry 9:900–907. 10.1038/sj.mp.4001542 [DOI] [PubMed] [Google Scholar]
- 408.van Toorn R, Weyers HH, Schoeman JF. 2004. Distinguishing PANDAS from Sydenham's chorea: case report and review of the literature. Eur. J. Paediatr. Neurol. 8:211–216. 10.1016/j.ejpn.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 409.Gibofsky A, Zabriskie JB. 1995. Rheumatic fever and poststreptococcal reactive arthritis. Curr. Opin. Rheumatol. 7:299–305. 10.1097/00002281-199507000-00006 [DOI] [PubMed] [Google Scholar]
- 410.Jansen TL, Janssen M, de Jong AJ, Jeurissen ME. 1999. Post-streptococcal reactive arthritis: a clinical and serological description, revealing its distinction from acute rheumatic fever. J. Intern. Med. 245:261–267. 10.1046/j.1365-2796.1999.0438e.x [DOI] [PubMed] [Google Scholar]
- 411.van der Helm-van Mil AH. 2010. Acute rheumatic fever and poststreptococcal reactive arthritis reconsidered. Curr. Opin. Rheumatol. 22:437–442. 10.1097/BOR.0b013e328337ba26 [DOI] [PubMed] [Google Scholar]
- 412.Iglesias-Gamarra A, Mendez EA, Cuellar ML, Ponce de Leon JH, Jimenez C, Canas C, Restrepo J, Pena M, Valle R, Espinoza LR. 2001. Poststreptococcal reactive arthritis in adults: long-term follow-up. Am. J. Med. Sci. 321:173–177. 10.1097/00000441-200103000-00003 [DOI] [PubMed] [Google Scholar]
- 413.Tokura T, Morita Y, Yorimitsu D, Horike H, Sasaki T, Kashihara N. 2008. Co-occurrence of poststreptococcal reactive arthritis and acute glomerulonephritis. Mod. Rheumatol. 18:526–528. 10.1007/s10165-008-0095-3 [DOI] [PubMed] [Google Scholar]
- 414.Jansen TL, Janssen M, de Jong AJ. 1998. Reactive arthritis associated with group C and group G beta-hemolytic streptococci. J. Rheumatol. 25:1126–1130 [PubMed] [Google Scholar]
- 415.Baird RW, Bronze MS, Kraus W, Hill HR, Veasey LG, Dale JB. 1991. Epitopes of group A streptococcal M protein shared with antigens of articular cartilage and synovium. J. Immunol. 146:3132–3137 [PubMed] [Google Scholar]
- 416.Yoshizawa N. 2000. Acute glomerulonephritis. Intern. Med. 39:687–694. 10.2169/internalmedicine.39.687 [DOI] [PubMed] [Google Scholar]
- 417.Stollerman GH. 1969. Nephritogenic and rheumatogenic group A streptococci. J. Infect. Dis. 120:258–263. 10.1093/infdis/120.2.258 [DOI] [PubMed] [Google Scholar]
- 418.Batsford SR, Mezzano S, Mihatsch M, Schiltz E, Rodriguez-Iturbe B. 2005. Is the nephritogenic antigen in post-streptococcal glomerulonephritis pyrogenic exotoxin B (Spe B) or GAPDH? Kidney Int. 68:1120–1129. 10.1111/j.1523-1755.2005.00504.x [DOI] [PubMed] [Google Scholar]
- 419.Ohkuni H, Todome Y, Yoshimura K, Yamamoto T, Suzuki H, Yokomuro K, Johnston KH, Zabriskie JB. 1991. Detection of nephritis strain-associated streptokinase by monoclonal antibodies. J. Med. Microbiol. 35:60–63. 10.1099/00222615-35-1-60 [DOI] [PubMed] [Google Scholar]
- 420.Yoshizawa N, Yamakami K, Fujino M, Oda T, Tamura K, Matsumoto K, Sugisaki T, Boyle MD. 2004. Nephritis-associated plasmin receptor and acute poststreptococcal glomerulonephritis: characterization of the antigen and associated immune response. J. Am. Soc. Nephrol. 15:1785–1793. 10.1097/01.ASN.0000130624.94920.6B [DOI] [PubMed] [Google Scholar]
- 421.Rodriguez-Iturbe B, Musser JM. 2008. The current state of poststreptococcal glomerulonephritis. J. Am. Soc. Nephrol. 19:1855–1864. 10.1681/ASN.2008010092 [DOI] [PubMed] [Google Scholar]
- 422.Rodriguez-Iturbe B. 2004. Nephritis-associated streptococcal antigens: where are we now? J. Am. Soc. Nephrol. 15:1961–1962. 10.1097/01.ASN.0000133012.53669.1C [DOI] [PubMed] [Google Scholar]
- 423.Rodriguez-Iturbe B, Batsford S. 2007. Pathogenesis of poststreptococcal glomerulonephritis a century after Clemens von Pirquet. Kidney Int. 71:1094–1104. 10.1038/sj.ki.5002169 [DOI] [PubMed] [Google Scholar]
- 424.Baldwin DS, Gluck MC, Schacht RG, Gallo G. 1974. The long-term course of poststreptococcal glomerulonephritis. Ann. Intern. Med. 80:342–358. 10.7326/0003-4819-80-3-342 [DOI] [PubMed] [Google Scholar]
- 425.Hoy WE, White AV, Dowling A, Sharma SK, Bloomfield H, Tipiloura BT, Swanson CE, Mathews JD, McCredie DA. 2012. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 81:1026–1032. 10.1038/ki.2011.478 [DOI] [PubMed] [Google Scholar]
- 426.Lopes AA, Silveira MA, Martinelli RP, Rocha H. 2001. Association between race and incidence of end-stage renal disease secondary to glomerulonephritis: influence of the histologic type and presence of arterial hypertension. Rev. Assoc. Med. Bras. 47:78–84. 10.1590/S0104-42302001000100034 [DOI] [PubMed] [Google Scholar]
- 427.White AV, Hoy WE, McCredie DA. 2001. Childhood post-streptococcal glomerulonephritis as a risk factor for chronic renal disease in later life. Med. J. Aust. 174:492–496 https://www.mja.com.au/journal/2001/174/10/childhood-post-streptococcal-glomerulonephritis-risk-factor-chronic-renal [DOI] [PubMed] [Google Scholar]
- 428.Goodfellow AM, Hoy WE, Sriprakash KS, Daly MJ, Reeve MP, Mathews JD. 1999. Proteinuria is associated with persistence of antibody to streptococcal M protein in aboriginal Australians. Epidemiol. Infect. 122:67–75. 10.1017/S0950268898001812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Hartas J, Sriprakash KS. 1999. Streptococcus pyogenes strains containing emm12 and emm55 possess a novel gene coding for distantly related sic protein. Microb. Pathog. 26:25–33. 10.1006/mpat.1998.0244 [DOI] [PubMed] [Google Scholar]
- 430.Hoe NP, Kordari P, Cole R, Liu M, Palzkill T, Huang W, McLellan D, Adams GJ, Hu M, Vuopio-Varkila J, Cate TR, Pichichero ME, Edwards KM, Eskola J, Low DE, Musser JM. 2000. Human immune response to streptococcal inhibitor of complement, a serotype M1 group A Streptococcus extracellular protein involved in epidemics. J. Infect. Dis. 182:1425–1436. 10.1086/315882 [DOI] [PubMed] [Google Scholar]
- 431.Sriprakash KS, Hartas J, White A. 2002. Antibodies to streptococcal inhibitor of complement function and M peptides in a post-streptococcal glomerulonephritis endemic region of Australia. J. Med. Microbiol. 51:589–594 http://jmm.sgmjournals.org/content/51/7/589.long [DOI] [PubMed] [Google Scholar]
- 432.Skattum L, Akesson P, Truedsson L, Sjoholm AG. 2006. Antibodies against four proteins from a Streptococcus pyogenes serotype M1 strain and levels of circulating mannan-binding lectin in acute poststreptococcal glomerulonephritis. Int. Arch. Allergy Immunol. 140:9–19. 10.1159/000091745 [DOI] [PubMed] [Google Scholar]
- 433.Karmarkar MG, Hule GP, Hase NK, Mehta PR, Walter SR, Sriprakash KS. 2013. Seroprevalence of streptococcal inhibitor of complement (SIC) suggests association of streptococcal infection with chronic kidney disease. BMC Nephrol. 14:101. 10.1186/1471-2369-14-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, Martin JM, Van Beneden C. 2012. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 55:e86–e102. 10.1093/cid/cis629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Randolph MF, Gerber MA, DeMeo KK, Wright L. 1985. Effect of antibiotic therapy on the clinical course of streptococcal pharyngitis. J. Pediatr. 106:870–875. 10.1016/S0022-3476(85)80228-6 [DOI] [PubMed] [Google Scholar]
- 436.Brink WR, Rammelkamp CH, Denny FW, Wannamaker LW. 1951. Effect in penicillin and aureomycin on the natural course of streptococcal tonsillitis and pharyngitis. Am. J. Med. 10:300–308. 10.1016/0002-9343(51)90274-4 [DOI] [PubMed] [Google Scholar]
- 437.Catanzaro FJ, Stetson CA, Morris AJ, Chamovitz R, Rammelkamp CH, Stolzer BL, Perry WD. 1954. The role of Streptococcus in the pathogenesis of rheumatic fever. Am. J. Med. 17:749–756. 10.1016/0002-9343(54)90219-3 [DOI] [PubMed] [Google Scholar]
- 438.Dajani A, Taubert K, Ferrieri P, Peter G, Shulman S. 1995. Treatment of acute streptococcal pharyngitis and prevention of rheumatic fever: a statement for health professionals. Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, the American Heart Association. Pediatrics 96:758–764 [PubMed] [Google Scholar]
- 439.Kaplan EL, Johnson DR, Del Rosario MC, Horn DL. 1999. Susceptibility of group A beta-hemolytic streptococci to thirteen antibiotics: examination of 301 strains isolated in the United States between 1994 and 1997. Pediatr. Infect. Dis. J. 18:1069–1072. 10.1097/00006454-199912000-00008 [DOI] [PubMed] [Google Scholar]
- 440.Gerber MA. 1996. Antibiotic resistance: relationship to persistence of group A streptococci in the upper respiratory tract. Pediatrics 97:971–975 [PubMed] [Google Scholar]
- 441.York MK, Gibbs L, Perdreau-Remington F, Brooks GF. 1999. Characterization of antimicrobial resistance in Streptococcus pyogenes isolates from the San Francisco Bay area of northern California. J. Clin. Microbiol. 37:1727–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Martin JM, Green M, Barbadora KA, Wald ER. 2002. Erythromycin-resistant group A streptococci in schoolchildren in Pittsburgh. N. Engl. J. Med. 346:1200–1206. 10.1056/NEJMoa013169 [DOI] [PubMed] [Google Scholar]
- 443.Canton R, Loza E, Morosini MI, Baquero F. 2002. Antimicrobial resistance amongst isolates of Streptococcus pyogenes and Staphylococcus aureus in the PROTEKT antimicrobial surveillance programme during 1999–2000. J. Antimicrob. Chemother. 50(Suppl 2):9–24. 10.1093/jac/dkf811 [DOI] [PubMed] [Google Scholar]
- 444.Dale JB. 1999. Multivalent group A streptococcal vaccine designed to optimize the immunogenicity of six tandem M protein fragments. Vaccine 17:193–200. 10.1016/S0264-410X(98)00150-9 [DOI] [PubMed] [Google Scholar]
- 445.Gerber MA, Randolph MF, DeMeo K, Feder HM, Jr, Kaplan EL. 1989. Failure of once-daily penicillin V therapy for streptococcal pharyngitis. Am. J. Dis. Child. 143:153–155 [DOI] [PubMed] [Google Scholar]
- 446.Pichichero ME, Casey JR. 2007. Bacterial eradication rates with shortened courses of 2nd- and 3rd-generation cephalosporins versus 10 days of penicillin for treatment of group A streptococcal tonsillopharyngitis in adults. Diagn. Microbiol. Infect. Dis. 59:127–130. 10.1016/j.diagmicrobio.2007.04.010 [DOI] [PubMed] [Google Scholar]
- 447.Syrogiannopoulos GA, Bozdogan B, Grivea IN, Ednie LM, Kritikou DI, Katopodis GD, Beratis NG, Applebaum PC. 2004. Two dosages of clarithromycin for five days, amoxicillin/clavulanate for five days or penicillin V for ten days in acute group A streptococcal tonsillopharyngitis. Pediatr. Infect. Dis. J. 23:857–865. 10.1097/01.inf.0000138080.74674.a2 [DOI] [PubMed] [Google Scholar]
- 448.Curtin CD, Casey JR, Murray PC, Cleary CT, Hoeger WJ, Marsocci SM, Murphy ML, Francis AB, Pichichero ME. 2003. Efficacy of cephalexin two vs. three times daily vs. cefadroxil once daily for streptococcal tonsillopharyngitis. Clin. Pediatr. 42:519–526. 10.1177/000992280304200606 [DOI] [PubMed] [Google Scholar]
- 449.Lennon DR, Farrell E, Martin DR, Stewart JM. 2008. Once-daily amoxicillin versus twice-daily penicillin V in group A beta-haemolytic streptococcal pharyngitis. Arch. Dis. Child. 93:474–478. 10.1136/adc.2006.113506 [DOI] [PubMed] [Google Scholar]
- 450.Gerber MA, Tanz RR. 2001. New approaches to the treatment of group A streptococcal pharyngitis. Curr. Opin. Pediatr. 13:51–55. 10.1097/00008480-200102000-00009 [DOI] [PubMed] [Google Scholar]
- 451.Kaplan EL, Couser R, Huwe BB, McKay C, Wannamaker LW. 1979. Significance of quantitative salivary cultures for group A and non-group A and non-group A beta-hemolytic streptococci in patients with pharyngitis and in their family contacts. Pediatrics 64:904–912 [PubMed] [Google Scholar]
- 452.Martin JM, Green M, Barbadora KA, Wald ER. 2004. Group A streptococci among school-aged children: clinical characteristics and the carrier state. Pediatrics 114:1212–1219. 10.1542/peds.2004-0133 [DOI] [PubMed] [Google Scholar]
- 453.Prevention of Invasive Group A Streptococcal Infections Workshop Participants. 2002. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin. Infect. Dis. 35:950–959. 10.1086/342692 [DOI] [PubMed] [Google Scholar]
- 454.American Academy of Pediatrics. 2012. Group A streptococcal infections, p 668–680 In Pickering LK, Baker CJ, Kimberlin DW, Long SS. (ed), Red book: 2012 report of the Committee on Infectious Diseases. American Academy of Pediatrics, Elk Grove Village, IL [Google Scholar]
- 455.Wong CJ, Stevens DL. 2013. Serious group A streptococcal infections. Med. Clin. North Am. 97:721–736. 10.1016/j.mcna.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 456.Young MH, Engleberg NC, Mulla ZD, Aronoff DM. 2006. Therapies for necrotising fasciitis. Expert Opin. Biol. Ther. 6:155–165. 10.1517/14712598.6.2.155 [DOI] [PubMed] [Google Scholar]
- 457.Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan EL, Montoya JG, Wade JC, Infectious Diseases Society of America 2005. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin. Infect. Dis. 41:1373–1406. 10.1086/497143 [DOI] [PubMed] [Google Scholar]
- 458.Allen U, Moore D. 2010. Invasive group A streptococcal disease: management and chemoprophylaxis. Can. J. Infect. Dis. Med. Microbiol. 21:115–118 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2951800/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Stevens DL, Gibbons AE, Bergstrom R, Winn V. 1988. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J. Infect. Dis. 158:23–28. 10.1093/infdis/158.1.23 [DOI] [PubMed] [Google Scholar]
- 460.Kaul R, McGeer A, Norrby-Teglund A, Kotb M, Schwartz B, O'Rourke K, Talbot J, Low DE. 1999. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—a comparative observational study. The Canadian Streptococcal Study Group. Clin. Infect. Dis. 28:800–807 [DOI] [PubMed] [Google Scholar]
- 461.Darenberg J, Ihendyane N, Sjolin J, Aufwerber E, Haidl S, Follin P, Andersson J, Norrby-Teglund A. 2003. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 37:333–340. 10.1086/376630 [DOI] [PubMed] [Google Scholar]
- 462.Cilliers A, Manyemba J, Adler AJ, Saloojee H. 2012. Anti-inflammatory treatment for carditis in acute rheumatic fever. Cochrane Database Syst. Rev. 6:CD003176. 10.1002/14651858.CD003176.pub2 [DOI] [PubMed] [Google Scholar]
- 463.Marijon E, Mirabel M, Celermajer DS, Jouven X. 2012. Rheumatic heart disease. Lancet 379:953–964. 10.1016/S0140-6736(11)61171-9 [DOI] [PubMed] [Google Scholar]
- 464.Gerber MA, Baltimore RS, Eaton CB, Gewitz M, Rowley AH, Shulman ST, Taubert KA. 2009. Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research. Endorsed by the American Academy of Pediatrics. Circulation 119:1541–1551. 10.1161/CIRCULATIONAHA.109.191959 [DOI] [PubMed] [Google Scholar]
- 465.Carapetis JR, McDonald M, Wilson NJ. 2005. Acute rheumatic fever. Lancet 366:155–168. 10.1016/S0140-6736(05)66874-2 [DOI] [PubMed] [Google Scholar]
- 466.Potter EV, Lipschultz SA, Abidh S, Poon-King T, Earle DP. 1982. Twelve to seventeen-year follow-up of patients with poststreptococcal acute glomerulonephritis in Trinidad. N. Engl. J. Med. 307:725–729. 10.1056/NEJM198209163071205 [DOI] [PubMed] [Google Scholar]
- 467.Macris MH, Hartman N, Murray B, Klein RF, Roberts RB, Kaplan EL, Horn D, Zabriskie JB. 1998. Studies of the continuing susceptibility of group A streptococcal strains to penicillin during eight decades. Pediatr. Infect. Dis. J. 17:377–381. 10.1097/00006454-199805000-00006 [DOI] [PubMed] [Google Scholar]
- 468.de Melo MC, Sa Figueiredo AM, Ferreira-Carvalho BT. 2003. Antimicrobial susceptibility patterns and genomic diversity in strains of Streptococcus pyogenes isolated in 1978-1997 in different Brazilian cities. J. Med. Microbiol. 52:251–258. 10.1099/jmm.0.04938-0 [DOI] [PubMed] [Google Scholar]
- 469.Inoue M, Kohno S, Kaku M, Yamaguchi K, Igari J, Yamanaka K. 2005. Protekt 1999-2000: a multicentre study of the antimicrobial susceptibility of respiratory tract pathogens in Japan. Int. J. Infect. Dis. 9:27–36. 10.1016/j.ijid.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 470.Melo-Cristino J, Fernandes ML. 1999. Streptococcus pyogenes isolated in Portugal: macrolide resistance phenotypes and correlation with T types. Portuguese Surveillance Group for the Study of Respiratory Pathogens. Microb. Drug Resist. 5:219–225 [DOI] [PubMed] [Google Scholar]
- 471.Ndiaye AG, Boye CS, Hounkponou E, Gueye FB, Badiane A. 2009. Antimicrobial susceptibility of select respiratory tract pathogens in Dakar, Senegal. J. Infect. Dev. Ctries. 3:660–666. 10.3855/jidc.20 [DOI] [PubMed] [Google Scholar]
- 472.Ogawa T, Terao Y, Sakata H, Okuni H, Ninomiya K, Ikebe K, Maeda Y, Kawabata S. 2011. Epidemiological characterization of Streptococcus pyogenes isolated from patients with multiple onsets of pharyngitis. FEMS Microbiol. Lett. 318:143–151. 10.1111/j.1574-6968.2011.02252.x [DOI] [PubMed] [Google Scholar]
- 473.Gastanaduy AS, Kaplan EL, Huwe BB, McKay C, Wannamaker LW. 1980. Failure of penicillin to eradicate group A streptococci during an outbreak of pharyngitis. Lancet ii:498–502 [DOI] [PubMed] [Google Scholar]
- 474.Kaplan EL, Johnson DR. 2001. Unexplained reduced microbiological efficacy of intramuscular benzathine penicillin G and of oral penicillin V in eradication of group A streptococci from children with acute pharyngitis. Pediatrics 108:1180–1186. 10.1542/peds.108.5.1180 [DOI] [PubMed] [Google Scholar]
- 475.Kaplan EL, Chhatwal GS, Rohde M. 2006. Reduced ability of penicillin to eradicate ingested group A streptococci from epithelial cells: clinical and pathogenetic implications. Clin. Infect. Dis. 43:1398–1406. 10.1086/508773 [DOI] [PubMed] [Google Scholar]
- 476.Neeman R, Keller N, Barzilai A, Korenman Z, Sela S. 1998. Prevalence of internalisation-associated gene, prtf1, among persisting group-A Streptococcus strains isolated from asymptomatic carriers. Lancet 352:1974–1977. 10.1016/S0140-6736(97)12452-7 [DOI] [PubMed] [Google Scholar]
- 477.Stjernquist-Desatnik A, Samuelsson P, Walder M. 1993. Penetration of penicillin V to tonsillar surface fluid in healthy individuals and in patients with acute tonsillitis. J. Laryngol. Otol. 107:309–312. 10.1017/S0022215100122893 [DOI] [PubMed] [Google Scholar]
- 478.Baldassarri L, Creti R, Recchia S, Imperi M, Facinelli B, Giovanetti E, Pataracchia M, Alfarone G, Orefici G. 2006. Therapeutic failures of antibiotics used to treat macrolide-susceptible Streptococcus pyogenes infections may be due to biofilm formation. J. Clin. Microbiol. 44:2721–2727. 10.1128/JCM.00512-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Ogawa T, Terao Y, Okuni H, Ninomiya K, Sakata H, Ikebe K, Maeda Y, Kawabata S. 2011. Biofilm formation or internalization into epithelial cells enable Streptococcus pyogenes to evade antibiotic eradication in patients with pharyngitis. Microb. Pathog. 51:58–68. 10.1016/j.micpath.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 480.Brook I. 1984. The role of beta-lactamase-producing bacteria in the persistence of streptococcal tonsillar infection. Rev. Infect. Dis. 6:601–607. 10.1093/clinids/6.5.601 [DOI] [PubMed] [Google Scholar]
- 481.Brook I, Gober AE. 2008. Failure to eradicate streptococci and beta-lactamase producing bacteria. Acta Paediatr. 97:193–195. 10.1111/j.1651-2227.2007.00610.x [DOI] [PubMed] [Google Scholar]
- 482.Chambers HF. 1999. Penicillin-binding protein-mediated resistance in pneumococci and staphylococci. J. Infect. Dis. 179:S353–S359. 10.1086/513854 [DOI] [PubMed] [Google Scholar]
- 483.Dahesh S, Hensler ME, Van Sorge NM, Gertz RE, Jr, Schrag S, Nizet V, Beall BW. 2008. Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to beta-lactam antibiotics. Antimicrob. Agents Chemother. 52:2915–2918. 10.1128/AAC.00461-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Leon O, Panos C. 1988. Differences in penicillin-binding proteins of Streptococcus pyogenes and two derived, stabilized L forms. J. Bacteriol. 170:4775–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Gutmann L, Williamson R, Tomasz A. 1981. Physiological properties of penicillin-binding proteins in group A streptococci. Antimicrob. Agents Chemother. 19:872–880. 10.1128/AAC.19.5.872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Rosendal K. 1957. Investigation of penicillin resistant streptococci belonging to group A. Acta Pathol. Microbiol. Scand. 39:181–188 [DOI] [PubMed] [Google Scholar]
- 487.Michael JG, Massell BF, Perkins RE. 1963. Effect of sublethal concentrations of penicillin on the virulence and antigenic composition of group A streptococci. J. Bacteriol. 85:1280–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Gutmann L, Tomasz A. 1982. Penicillin-resistant and penicillin-tolerant mutants of group A streptococci. Antimicrob. Agents Chemother. 22:128–136. 10.1128/AAC.22.1.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Horn DL, Zabriskie JB, Austrian R, Cleary PP, Ferretti JJ, Fischetti VA, Gotschlich E, Kaplan EL, McCarty M, Opal SM, Roberts RB, Tomasz A, Wachtfogel Y. 1998. Why have group A streptococci remained susceptible to penicillin? Report on a symposium. Clin. Infect. Dis. 26:1341–1345 [DOI] [PubMed] [Google Scholar]
- 490.Pantosti A, Sanchini A, Monaco M. 2007. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol. 2:323–334. 10.2217/17460913.2.3.323 [DOI] [PubMed] [Google Scholar]
- 491.Carapetis JR. 2004. Current issues in managing group A streptococcal infections. Adv. Exp. Med. Biol. 549:185–190. 10.1007/978-1-4419-8993-2_25 [DOI] [PubMed] [Google Scholar]
- 492.Stevens DL, Bryant AE, Hackett SP. 1995. Antibiotic effects on bacterial viability, toxin production, and host response. Clin. Infect. Dis. 20:S154–S157. 10.1093/clinids/20.Supplement_2.S154 [DOI] [PubMed] [Google Scholar]
- 493.Seppala H, Skurnik M, Soini H, Roberts MC, Huovinen P. 1998. A novel erythromycin resistance methylase gene (ermTR) in Streptococcus pyogenes. Antimicrob. Agents Chemother. 42:257–262. 10.1093/jac/42.2.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Clancy J, Petitpas J, Dib-Hajj F, Yuan W, Cronan M, Kamath AV, Bergeron J, Retsema JA. 1996. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol. Microbiol. 22:867–879. 10.1046/j.1365-2958.1996.01521.x [DOI] [PubMed] [Google Scholar]
- 495.Richter SS, Heilmann KP, Beekmann SE, Miller NJ, Miller AL, Rice CL, Doern CD, Reid SD, Doern GV. 2005. Macrolide-resistant Streptococcus pyogenes in the United States, 2002-2003. Clin. Infect. Dis. 41:599–608. 10.1086/432473 [DOI] [PubMed] [Google Scholar]
- 496.Koh E, Kim S. 2010. Decline in erythromycin resistance in group A streptococci from acute pharyngitis due to changes in the emm genotypes rather than restriction of antibiotic use. Korean J. Lab. Med. 30:485–490. 10.3343/kjlm.2010.30.5.485 [DOI] [PubMed] [Google Scholar]
- 497.Liu X, Shen X, Chang H, Huang G, Fu Z, Zheng Y, Wang L, Li C, Liu L, Shen Y, Yang Y. 2009. High macrolide resistance in Streptococcus pyogenes strains isolated from children with pharyngitis in China. Pediatr. Pulmonol. 44:436–441. 10.1002/ppul.20976 [DOI] [PubMed] [Google Scholar]
- 498.Perez-Trallero E, Montes M, Orden B, Tamayo E, Garcia-Arenzana JM, Marimon JM. 2007. Phenotypic and genotypic characterization of Streptococcus pyogenes isolates displaying the MLSB phenotype of macrolide resistance in Spain, 1999 to 2005. Antimicrob. Agents Chemother. 51:1228–1233. 10.1128/AAC.01054-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Perez-Trallero E, Tamayo E, Montes M, Garcia-Arenzana JM, Iriarte V. 2011. In vitro activities of retapamulin and 16 other antimicrobial agents against recently obtained Streptococcus pyogenes isolates. Antimicrob. Agents Chemother. 55:2406–2408. 10.1128/AAC.01665-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Pires R, Rolo D, Gama-Norton L, Morais A, Lito L, Salgado MJ, Johansson C, Mollerberg G, Henriques-Normark B, Goncalo-Marques J, Santos-Sanches I. 2005. Group A streptococci from carriage and disease in Portugal: evolution of antimicrobial resistance and T antigenic types during 2000-2002. Microb. Drug Resist. 11:360–370. 10.1089/mdr.2005.11.360 [DOI] [PubMed] [Google Scholar]
- 501.Gagliotti C, Nobilio L, Milandri M, Moro ML. 2006. Macrolide prescriptions and erythromycin resistance of Streptococcus pyogenes. Clin. Infect. Dis. 42:1153–1156. 10.1086/501460 [DOI] [PubMed] [Google Scholar]
- 502.Garcia-Rey C, Aguilar L, Baquero F, Casal J, Dal-Re R. 2002. Importance of local variations in antibiotic consumption and geographical differences of erythromycin and penicillin resistance in Streptococcus pneumoniae. J. Clin. Microbiol. 40:159–164. 10.1128/JCM.40.1.159-164.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Seppala H, Klaukka T, Vuopio-Varkila J, Muotiala A, Helenius H, Lager K, Huovinen P. 1997. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N. Engl. J. Med. 337:441–446 [DOI] [PubMed] [Google Scholar]
- 504.Bingen E, Bidet P, Mihaila-Amrouche L, Doit C, Forcet S, Brahimi N, Bouvet A, Cohen R. 2004. Emergence of macrolide-resistant Streptococcus pyogenes strains in French children. Antimicrob. Agents Chemother. 48:3559-3562. 10.1128/AAC.48.9.3559-3562.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Farmand S, Henneke P, Hufnagel M, Berner R. 2012. Significant decline in the erythromycin resistance of group A Streptococcus isolates at a German paediatric tertiary care centre. Eur. J. Clin. Microbiol. Infect. Dis. 31:707–710. 10.1007/s10096-011-1362-3 [DOI] [PubMed] [Google Scholar]
- 506.Van Heirstraeten L, Leten G, Lammens C, Goossens H, Malhotra-Kumar S. 2012. Increase in fluoroquinolone non-susceptibility among clinical Streptococcus pyogenes in Belgium during 2007-10. J. Antimicrob. Chemother. 67:2602–2605. 10.1093/jac/dks281 [DOI] [PubMed] [Google Scholar]
- 507.Ardanuy C, Domenech A, Rolo D, Calatayud L, Tubau F, Ayats J, Martin R, Linares J. 2010. Molecular characterization of macrolide- and multidrug-resistant Streptococcus pyogenes isolated from adult patients in Barcelona, Spain (1993-2008). J. Antimicrob. Chemother. 65:634–643. 10.1093/jac/dkq006 [DOI] [PubMed] [Google Scholar]
- 508.Silva-Costa C, Ramirez M, Melo-Cristino J, Portuguese Surveillance Group for the Study of Respiratory Pathogens 2005. Rapid inversion of the prevalences of macrolide resistance phenotypes paralleled by a diversification of T and emm types among Streptococcus pyogenes in Portugal. Antimicrob. Agents Chemother. 49:2109–2111. 10.1128/AAC.49.5.2109-2111.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Chopra I, Roberts M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232–260. 10.1128/MMBR.65.2.232-260.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Burdett V. 1996. Tet(M)-promoted release of tetracycline from ribosomes is GTP dependent. J. Bacteriol. 178:3246–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Li W, Atkinson GC, Thakor NS, Allas U, Lu CC, Chan KY, Tenson T, Schulten K, Wilson KS, Hauryliuk V, Frank J. 2013. Mechanism of tetracycline resistance by ribosomal protection protein Tet(O). Nat. Commun. 4:1477. 10.1038/ncomms2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Malhotra-Kumar S, Lammens C, Piessens J, Goossens H. 2005. Multiplex PCR for simultaneous detection of macrolide and tetracycline resistance determinants in streptococci. Antimicrob. Agents Chemother. 49:4798–4800. 10.1128/AAC.49.11.4798-4800.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Rodriguez-Avial I, Rodriguez-Avial C, Culebras E, Picazo JJ. 2003. Distribution of tetracycline resistance genes tet(M), tet(O), tet(L) and tet(K) in blood isolates of viridans group streptococci harbouring erm(B) and mef(A) genes. Susceptibility to quinupristin/dalfopristin and linezolid. Int. J. Antimicrob. Agents 21:536–541. 10.1016/S0924-8579(03)00062-1 [DOI] [PubMed] [Google Scholar]
- 514.Ayer V, Tewodros W, Manoharan A, Skariah S, Luo F, Bessen DE. 2007. Tetracycline resistance in group A streptococci: emergence on a global scale and influence on multiple-drug resistance. Antimicrob. Agents Chemother. 51:1865–1868. 10.1128/AAC.01341-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Brenciani A, Bacciaglia A, Vecchi M, Vitali LA, Varaldo PE, Giovanetti E. 2007. Genetic elements carrying erm(B) in Streptococcus pyogenes and association with tet(M) tetracycline resistance gene. Antimicrob. Agents Chemother. 51:1209–1216. 10.1128/AAC.01484-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Brenciani A, Bacciaglia A, Vignaroli C, Pugnaloni A, Varaldo PE, Giovanetti E. 2010. Phim46.1, the main Streptococcus pyogenes element carrying mef(A) and tet(O) genes. Antimicrob. Agents Chemother. 54:221–229. 10.1128/AAC.00499-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Clewell DB, Flannagan SE, Jaworski DD. 1995. Unconstrained bacterial promiscuity: the Tn916-Tn1545 family of conjugative transposons. Trends Microbiol. 3:229–236. 10.1016/S0966-842X(00)88930-1 [DOI] [PubMed] [Google Scholar]
- 518.Giovanetti E, Brenciani A, Lupidi R, Roberts MC, Varaldo PE. 2003. Presence of the I gene in erythromycin- and tetracycline-resistant strains of Streptococcus pyogenes and linkage with either the mef(A) or the erm(A) gene. Antimicrob. Agents Chemother. 47:2844–2849. 10.1128/AAC.47.9.2844-2849.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Giovanetti E, Brenciani A, Tiberi E, Bacciaglia A, Varaldo PE. 2012. ICESp2905, the erm(TR)-tet(O) element of Streptococcus pyogenes, is formed by two independent integrative and conjugative elements. Antimicrob. Agents Chemother. 56:591–594. 10.1128/AAC.05352-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Ikebe T, Hirasawa K, Suzuki R, Isobe J, Tanaka D, Katsukawa C, Kawahara R, Tomita M, Ogata K, Endoh M, Okuno R, Watanabe H. 2005. Antimicrobial susceptibility survey of Streptococcus pyogenes isolated in Japan from patients with severe invasive group A streptococcal infections. Antimicrob. Agents Chemother. 49:788–790. 10.1128/AAC.49.2.788-790.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Montes M, Tamayo E, Orden B, Larruskain J, Perez-Trallero E. 2010. Prevalence and clonal characterization of Streptococcus pyogenes clinical isolates with reduced fluoroquinolone susceptibility in Spain. Antimicrob. Agents Chemother. 54:93–97. 10.1128/AAC.00780-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Perez-Trallero E, Fernandez-Mazarrasa C, Garcia-Rey C, Bouza E, Aguilar L, Garcia-de-Lomas J, Baquero F. 2001. Antimicrobial susceptibilities of 1,684 Streptococcus pneumoniae and 2,039 Streptococcus pyogenes isolates and their ecological relationships: results of a 1-year (1998-1999) multicenter surveillance study in Spain. Antimicrob. Agents Chemother. 45:3334–3340. 10.1128/AAC.45.12.3334-3340.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Wajima T, Murayama SY, Sunaoshi K, Nakayama E, Sunakawa K, Ubukata K. 2008. Distribution of emm type and antibiotic susceptibility of group A streptococci causing invasive and noninvasive disease. J. Med. Microbiol. 57:1383–1388. 10.1099/jmm.0.2008/002642-0 [DOI] [PubMed] [Google Scholar]
- 524.Yan SS, Schreckenberger PC, Zheng X, Nelson NA, Harrington SM, Tjhio J, Fedorko DP. 2008. An intrinsic pattern of reduced susceptibility to fluoroquinolones in pediatric isolates of Streptococcus pyogenes. Diagn. Microbiol. Infect. Dis. 62:205–209. 10.1016/j.diagmicrobio.2008.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Alonso R, Mateo E, Cisterna R. 2007. Detection of parC mutations in Streptococcus pneumoniae by real-time PCR and Taqman-MGB probes. J. Microbiol. Methods 69:214–217. 10.1016/j.mimet.2006.12.016 [DOI] [PubMed] [Google Scholar]
- 526.Richter SS, Diekema DJ, Heilmann KP, Almer LS, Shortridge VD, Zeitler R, Flamm RK, Doern GV. 2003. Fluoroquinolone resistance in Streptococcus pyogenes. Clin. Infect. Dis. 36:380–383. 10.1086/345904 [DOI] [PubMed] [Google Scholar]
- 527.Pletz MW, McGee L, Van Beneden CA, Petit S, Bardsley M, Barlow M, Klugman KP. 2006. Fluoroquinolone resistance in invasive Streptococcus pyogenes isolates due to spontaneous mutation and horizontal gene transfer. Antimicrob. Agents Chemother. 50:943–948. 10.1128/AAC.50.3.943-948.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.McMillan DJ, Dreze PA, Vu T, Bessen DE, Guglielmini J, Steer AC, Carapetis JR, Van Melderen L, Sriprakash KS, Smeesters PR. 2013. Updated model of group A Streptococcus M proteins based on a comprehensive worldwide study. Clin. Microbiol. Infect. 19:E222–E229. 10.1111/1469-0691.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Gillen CM, Towers RJ, McMillan DJ, Delvecchio A, Sriprakash KS, Currie B, Kreikemeyer B, Chhatwal GS, Walker MJ. 2002. Immunological response mounted by aboriginal Australians living in the Northern Territory of Australia against Streptococcus pyogenes serum opacity factor. Microbiology 148:169–178 http://mic.sgmjournals.org/content/148/1/169.long [DOI] [PubMed] [Google Scholar]
- 530.D'Alessandri R, Plotkin G, Kluge RM, Wittner MK, Fox EN, Dorfman A, Waldman RH. 1978. Protective studies with group A streptococcal M protein vaccine. III. Challenge of volunteers after systemic or intranasal immunization with type 3 or type 12 group A Streptococcus. J. Infect. Dis. 138:712–718 [DOI] [PubMed] [Google Scholar]
- 531.Fox EN, Waldman RH, Wittner MK, Mauceri AA, Dorfman A. 1973. Protective study with a group A streptococcal M protein vaccine. Infectivity challenge of human volunteers. J. Clin. Invest. 52:1885–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Polly SM, Waldman RH, High P, Wittner MK, Dorfman A. 1975. Protective studies with a group A streptococcal M protein vaccine. II. Challenge of volunteers after local immunization in the upper respiratory tract. J. Infect. Dis. 131:217–224 [DOI] [PubMed] [Google Scholar]
- 533.Massell BF, Honikman LH, Amezcua J. 1969. Rheumatic fever following streptococcal vaccination. Report of three cases. JAMA 207:1115–1119 [PubMed] [Google Scholar]
- 534.Beachey EH, Gras-Masse H, Tarter A, Jolivet M, Audibert F, Chedid L, Seyer JM. 1986. Opsonic antibodies evoked by hybrid peptide copies of types 5 and 24 streptococcal M proteins synthesized in tandem. J. Exp. Med. 163:1451–1458. 10.1084/jem.163.6.1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Beachey EH, Seyer JM, Dale JB, Simpson WA, Kang AH. 1981. Type-specific protective immunity evoked by synthetic peptide of Streptococcus pyogenes M protein. Nature 292:457–459. 10.1038/292457a0 [DOI] [PubMed] [Google Scholar]
- 536.Dale JB, Chiang EY, Lederer JW. 1993. Recombinant tetravalent group A streptococcal M protein vaccine. J. Immunol. 151:2188–2194 [PubMed] [Google Scholar]
- 537.Dale JB, Seyer JM, Beachey EH. 1983. Type-specific immunogenicity of a chemically synthesized peptide fragment of type 5 streptococcal M protein. J. Exp. Med. 158:1727–1732. 10.1084/jem.158.5.1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Dale JB, Simmons M, Chiang EC, Chiang EY. 1996. Recombinant, octavalent group A streptococcal M protein vaccine. Vaccine 14:944–948. 10.1016/0264-410X(96)00050-3 [DOI] [PubMed] [Google Scholar]
- 539.Kotloff KL, Corretti M, Palmer K, Campbell JD, Reddish MA, Hu MC, Wasserman SS, Dale JB. 2004. Safety and immunogenicity of a recombinant multivalent group A streptococcal vaccine in healthy adults: phase I trial. JAMA 292:709–715. 10.1001/jama.292.6.709 [DOI] [PubMed] [Google Scholar]
- 540.Hall MA, Stroop SD, Hu MC, Walls MA, Reddish MA, Burt DS, Lowell GH, Dale JB. 2004. Intranasal immunization with multivalent group A streptococcal vaccines protects mice against intranasal challenge infections. Infect. Immun. 72:2507–2512. 10.1128/IAI.72.5.2507-2512.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Hu MC, Walls MA, Stroop SD, Reddish MA, Beall B, Dale JB. 2002. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect. Immun. 70:2171–2177. 10.1128/IAI.70.4.2171-2177.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.McNeil SA, Halperin SA, Langley JM, Smith B, Warren A, Sharratt GP, Baxendale DM, Reddish MA, Hu MC, Stroop SD, Linden J, Fries LF, Vink PE, Dale JB. 2005. Safety and immunogenicity of 26-valent group A Streptococcus vaccine in healthy adult volunteers. Clin. Infect. Dis. 41:1114–1122. 10.1086/444458 [DOI] [PubMed] [Google Scholar]
- 543.Dale JB, Penfound TA, Chiang EY, Walton WJ. 2011. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine 29:8175–8178. 10.1016/j.vaccine.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Abdissa A, Asrat D, Kronvall G, Shittu B, Achiko D, Zeidan M, Yamuah LK, Aseffa A. 2006. High diversity of group A streptococcal emm types among healthy schoolchildren in Ethiopia. Clin. Infect. Dis. 42:1362–1367. 10.1086/503422 [DOI] [PubMed] [Google Scholar]
- 545.Ikebe T, Hirasawa K, Suzuki R, Ohya H, Isobe J, Tanaka D, Katsukawa C, Kawahara R, Tomita M, Ogata K, Endoh M, Okuno R, Tada Y, Okabe N, Watanabe H, Working Group for Beta-Haemolytic Streptococci in Japan 2007. Distribution of emm genotypes among group A Streptococcus isolates from patients with severe invasive streptococcal infections in Japan, 2001-2005. Epidemiol. Infect. 135:1227–1229. 10.1017/S0950268807007984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Nir-Paz R, Korenman Z, Ron M, Michael-Gayego A, Cohen-Poradosu R, Valinsky L, Beall B, Moses AE. 2010. Streptococcus pyogenes emm and T types within a decade, 1996-2005: implications for epidemiology and future vaccines. Epidemiol. Infect. 138:53–60. 10.1017/S0950268809002805 [DOI] [PubMed] [Google Scholar]
- 547.Fischetti VA, Hodges WM, Hruby DE. 1989. Protection against streptococcal pharyngeal colonization with a vaccinia:M protein recombinant. Science 244:1487–1490. 10.1126/science.2660266 [DOI] [PubMed] [Google Scholar]
- 548.Mannam P, Jones KF, Geller BL. 2004. Mucosal vaccine made from live, recombinant Lactococcus lactis protects mice against pharyngeal infection with Streptococcus pyogenes. Infect. Immun. 72:3444–3450. 10.1128/IAI.72.6.3444-3450.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Batzloff MR, Hayman WA, Davies MR, Zeng M, Pruksakorn S, Brandt ER, Good MF. 2003. Protection against group A Streptococcus by immunisation with J8-diphtheria toxoid: contribution of J8- and diphtheria toxoid-specific antibodies to protection. J. Infect. Dis. 187:1598–1608. 10.1086/374800 [DOI] [PubMed] [Google Scholar]
- 550.Bauer MJ, Georgousakis MM, Vu T, Henningham A, Hofmann A, Rettel M, Hafner LM, Sriprakash KS, McMillan DJ. 2012. Evaluation of novel Streptococcus pyogenes vaccine candidates incorporating multiple conserved sequences from the C-repeat region of the M-protein. Vaccine 30:2197–2205. 10.1016/j.vaccine.2011.12.115 [DOI] [PubMed] [Google Scholar]
- 551.Bessen D, Fischetti VA. 1988. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect. Immun. 56:2666–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Brandt ER, Sriprakash KS, Hobb RI, Hayman WA, Zeng W, Batzloff MR, Jackson DC, Good MF. 2000. New multi-determinant strategy for a group A streptococcal vaccine designed for the Australian aboriginal population. Nat. Med. 6:455–459. 10.1038/74719 [DOI] [PubMed] [Google Scholar]
- 553.Bronze MS, Courtney HS, Dale JB. 1992. Epitopes of group A streptococcal M protein that evoke cross-protective local immune responses. J. Immunol. 148:888–893 [PubMed] [Google Scholar]
- 554.Georgousakis MM, Hofmann A, Batzloff MR, McMillan DJ, Sriprakash KS. 2009. Structural optimisation of a conformational epitope improves antigenicity when expressed as a recombinant fusion protein. Vaccine 27:6799–6806. 10.1016/j.vaccine.2009.08.049 [DOI] [PubMed] [Google Scholar]
- 555.Guilherme L, Faé KC, Higa F, Chaves L, Oshiro SE, Freschi de Barros S, Puschel C, Juliano MA, Tanaka AC, Spina G, Kalil J. 2006. Towards a vaccine against rheumatic fever. Clin. Dev. Immunol. 13:125–132. 10.1080/17402520600877026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Hayman WA, Brandt ER, Relf WA, Cooper J, Saul A, Good MF. 1997. Mapping the minimal murine T cell and B cell epitopes within a peptide vaccine candidate from the conserved region of the M protein of group A Streptococcus. Int. Immunol. 9:1723–1733. 10.1093/intimm/9.11.1723 [DOI] [PubMed] [Google Scholar]
- 557.Middelberg AP, Rivera-Hernadez T, Wibowo N, Lua LH, Fan Y, Magor G, Chang C, Chuan YP, Good MF, Batzloff MR. 2011. A microbial platform for rapid and low-cost virus-like particle and capsomere vaccines. Vaccine 29:7154–7162. 10.1016/j.vaccine.2011.05.075 [DOI] [PubMed] [Google Scholar]
- 558.Pandey M, Wykes MN, Hartas J, Good MF, Batzloff MR. 2013. Long-term antibody memory induced by synthetic peptide vaccination is protective against Streptococcus pyogenes infection and is independent of memory T cell help. J. Immunol. 190:2692–2701. 10.4049/jimmunol.1202333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Postol E, Alencar R, Higa FT, Freschi de Barros S, Demarchi LMF, Kalil J, Guilherme L. 2013. Streptincor: a candidate vaccine epitope against S. pyogenes infections induces protection in outbred mice. PLoS One 8:e60969. 10.1371/journal.pone.0060969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Shaila MS, Nayak R, Prakash SS, Georgousakis M, Brandt E, McMillan DJ, Batzloff MR, Pruksakorn S, Good MF, Sriprakash KS. 2007. Comparative in silico analysis of two vaccine candidates for group A Streptococcus predicts that they both may have similar safety profiles. Vaccine 25:3567–3573. 10.1016/j.vaccine.2007.01.079 [DOI] [PubMed] [Google Scholar]
- 561.Salvadori LG, Blake MS, McCarty M, Tai JY, Zabriskie JB. 1995. Group A Streptococcus-liposome ELISA antibody titers to group A polysaccharide and opsonophagocytic capabilities of the antibodies. J. Infect. Dis. 171:593–600. 10.1093/infdis/171.3.593 [DOI] [PubMed] [Google Scholar]
- 562.Sabharwal H, Michon F, Nelson D, Dong W, Fuchs K, Manjarrez RC, Sarkar A, Uitz C, Viteri-Jackson A, Suarez RS, Blake M, Zabriskie JB. 2006. Group A Streptococcus (GAS) carbohydrate as an immunogen for protection against GAS infection. J. Infect. Dis. 193:129–135. 10.1086/498618 [DOI] [PubMed] [Google Scholar]
- 563.Goldstein I, Halpern B, Robert L. 1967. Immunological relationship between Streptococcus A polysaccharide and the structural glycoproteins of heart valve. Nature 213:44–47. 10.1038/213044a0 [DOI] [Google Scholar]
- 564.Ma CQ, Li CH, Wang XR, Zeng RH, Yin XL, Feng HD, Wei L. 2009. Similar ability of FbaA with M protein to elicit protective immunity against group A Streptococcus challenge in mice. Cell. Mol. Immunol. 6:73–77. 10.1038/cmi.2009.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Courtney HS, Hasty DL, Dale JB. 2003. Serum opacity factor (SOF) of Streptococcus pyogenes evokes antibodies that opsonize homologous and heterologous SOF-positive serotypes of group A streptococci. Infect. Immun. 71:5097–5103. 10.1128/IAI.71.9.5097-5103.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Kawabata S, Kunitomo E, Terao Y, Nakagawa I, Kikuchi H, Totsuka K-I, Hamada S. 2001. Systemic and mucosal immunisations with fibronectin-binding protein Fbp54 induce protective immune response against Streptococcus pyogenes challenge in mice. Infect. Immun. 69:924–930. 10.1128/IAI.69.2.924-930.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Stalhammar-Carlemalm M, Areschoug T, Larsson C, Lindahl G. 1999. The R28 protein of Streptococcus pyogenes is related to several group B streptococcal surface proteins, confers protective immunity and promotes binding to human epithelial cells. Mol. Microbiol. 33:208–219. 10.1046/j.1365-2958.1999.01470.x [DOI] [PubMed] [Google Scholar]
- 568.Stalhammar-Carlemalm M, Areschoug T, Larsson C, Lindahl G. 2000. Cross-protection between group A and group B streptococci due to cross-reacting surface proteins. J. Infect. Dis. 182:142–149. 10.1086/315693 [DOI] [PubMed] [Google Scholar]
- 569.Dale JB, Chiang EY, Liu S, Courtney HS, Hasty DL. 1999. New protective antigen of group A streptococci. J. Clin. Invest. 103:1261–1268. 10.1172/JCI5118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Cleary PP, Matsuka YV, Hunyh T, Lam H, Olmsted SB. 2004. Immunisation with C5a peptidase from either group A or B streptococci enhances clearance of group A streptococci from intranasally infected mice. Vaccine 22:4332–4341. 10.1016/j.vaccine.2004.04.030 [DOI] [PubMed] [Google Scholar]
- 571.Ji Y, Carlson B, Kondagunta A, Cleary PP. 1997. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A Streptococcus. Infect. Immun. 65:2080–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Park HS, Cleary PP. 2005. Active and passive intranasal immunizations with streptococcal surface protein C5a peptidase prevent infection of murine nasal mucosa-associated lymphoid tissue, a functional homologue of human tonsils. Infect. Immun. 73:7878–7886. 10.1128/IAI.73.12.7878-7886.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Huang YS, Fisher M, Nasrawi Z, Eichenbaum Z. 2011. Defense from the group A Streptococcus by active and passive vaccination with the streptococcal hemoprotein receptor. J. Infect. Dis. 203:1595–1601. 10.1093/infdis/jir149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Kapur V, Maffei JT, Greer RS, Li LL, Adams GJ, Musser JM. 1994. Vaccination with streptococcal extracellular cysteine protease (interleukin-1 beta convertase) protects mice against challenge with heterologous group A streptococci. Microb. Pathog. 16:443–450. 10.1006/mpat.1994.1044 [DOI] [PubMed] [Google Scholar]
- 575.Ulrich RG. 2008. Vaccine based on a ubiquitous cysteinyl protease and streptococcal pyrogenic exotoxin A protects against Streptococcus pyogenes sepsis and toxic shock. J. Immune Based Ther. Vaccines 6:8. 10.1186/1476-8518-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Liu M, Zhu H, Zhang J, Lei B. 2007. Active and passive immunizations with the streptococcal esterase Sse protect mice against subcutaneous infection with group A streptococci. Infect. Immun. 75:3651–3657. 10.1128/IAI.00038-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Okamoto S, Tamura Y, Terao Y, Hamada S, Kawabata S. 2005. Systemic immunization with streptococcal immunoglobulin-binding protein Sib 35 induces protective immunity against group A Streptococcus challenge in mice. Vaccine 23:4852–4859. 10.1016/j.vaccine.2005.02.035 [DOI] [PubMed] [Google Scholar]
- 578.Turner CE, Kurupatia P, Wilesa S, Edwards RJ, Sriskandan S. 2009. Impact of immunization against SpyCEP during invasive disease with two streptococcal species: Streptococcus pyogenes and Streptococcus equi. Vaccine 27:4923–4929. 10.1016/j.vaccine.2009.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Henningham A, Chiarot E, Gillen CM, Cole JN, Rohde M, Fulde M, Ramachandran V, Cork AJ, Hartas J, Magor G, Djordjevic SP, Cordwell SJ, Kobe B, Sriprakash KS, Nizet V, Chhatwal GS, Margarit IYR, Batzloff MR, Walker MJ. 2012. Conserved anchorless surface proteins as group A streptococcal vaccine candidates. J. Mol. Med. 90:1197–1207. 10.1007/s00109-012-0897-9 [DOI] [PubMed] [Google Scholar]
- 580.McArthur J, Medina E, Mueller A, Chin J, Currie BJ, Sriprakash KS, Talay SR, Chhatwal GS, Walker MJ. 2004. Intranasal vaccination with streptococcal fibronectin binding protein Sfb1 fails to prevent growth and dissemination of Streptococcus pyogenes in a murine skin infection model. Infect. Immun. 72:7342–7345. 10.1128/IAI.72.12.7342-7345.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Gillen CM, Courtney HS, Schulze K, Rohde M, Wilson MR, Timmer AM, Guzman CA, Nizet V, Chhatwal GS, Walker MJ. 2008. Opacity factor activity and epithelial cell binding by the serum opacity factor protein of Streptococcus pyogenes are functionally discrete. J. Biol. Chem. 283:6359–6366. 10.1074/jbc.M706739200 [DOI] [PubMed] [Google Scholar]
- 582.Bensi G, Mora M, Tuscano G, Biagini M, Chiarot E, Bombaci M, Capo S, Falugi F, Manetti AG, Donato P, Swennen E, Gallotta M, Garibaldi M, Pinto V, Chiappini N, Musser JM, Janulczyk R, Mariani M, Scarselli M, Telford JL, Grifantini R, Norais N, Margarit I, Grandi G. 2012. Multi high-throughput approach for highly selective identification of vaccine candidates: the group A Streptococcus case. Mol. Cell. Proteomics 11:M111.015693. 10.1074/mcp.M111.015693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Chiarot E, Faralla C, Chiappini N, Tuscano G, Falugi F, Gambellini G, Taddei A, Capo S, Cartocci E, Veggi D, Corrado A, Mangiavacchi S, Tavarini S, Scarselli M, Janulczyk R, Grandi G, Margarit I, Bensi G. 2013. Targeted amino acid substitutions impair streptolysin O toxicity and group A Streptococcus virulence. mBio 4(1):e00387-12. 10.1128/mBio.00387-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Henningham A, Ericsson DJ, Langer K, Casey LW, Jovcevski B, Chhatwal GS, Aquilina JA, Batzloff MR, Kobe B, Walker MJ. 2013. Structure-informed design of an enzymatically inactive vaccine component for group A Streptococcus. mBio 4(4):e00509-13. 10.1128/mBio.00509-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Rappuoli R. 2000. Reverse vaccinology. Curr. Opin. Microbiol. 3:445–450. 10.1016/S1369-5274(00)00119-3 [DOI] [PubMed] [Google Scholar]
- 586.Sharma A, Arya DK, Sagar V, Bergmann R, Chhatwal GS, Johri AK. 2013. Identification of potential universal vaccine candidates against group A Streptococcus by using high throughput in silico and proteomics approach. J. Proteome Res. 12:336–346. 10.1021/pr3005265 [DOI] [PubMed] [Google Scholar]
- 587.Fritzer A, Senn BM, Minh DB, Hanner M, Gelbmann D, Noiges B, Henics T, Schulze K, Guzman CA, Goodacre J, von Gabain A, Nagy E, Meinke AL. 2010. Novel conserved group A streptococcal proteins identified by the antigenome technology as vaccine candidates for a non-M protein-based vaccine. Infect. Immun. 78:4051–4067. 10.1128/IAI.00295-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Rodriguez-Ortega MJ, Norais N, Bensi G, Liberatori S, Capo S, Mora M, Scarselli M, Doro F, Ferrari G, Garaguso I, Maggi T, Neumann A, Covre A, Telford JL, Grandi G. 2006. Characterization and identification of vaccine candidate proteins through analysis of the group A Streptococcus surface proteome. Nat. Biotechnol. 24:191–197. 10.1038/nbt1179 [DOI] [PubMed] [Google Scholar]
- 589.Bessen D, Jones KF, Fischetti VA. 1989. Evidence for two distinct classes of streptococcal M protein and their relationship to rheumatic fever. J. Exp. Med. 169:269–283. 10.1084/jem.169.1.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Shulman ST, Stollerman G, Beall B, Dale JB, Tanz RR. 2006. Temporal changes in streptococcal M protein types and the near-disappearance of acute rheumatic fever in the United States. Clin. Infect. Dis. 42:441–447. 10.1086/499812 [DOI] [PubMed] [Google Scholar]
- 591.Bisno AL. 1991. Group A streptococcal infections and acute rheumatic fever. N. Engl. J. Med. 325:783–793. 10.1056/NEJM199109123251106 [DOI] [PubMed] [Google Scholar]
- 592.Sriskandan S, Altmann DM. 2008. The immunology of sepsis. J. Pathol. 214:211–223. 10.1002/path.2274 [DOI] [PubMed] [Google Scholar]
- 593.Hamilton SM, Stevens DL, Bryant AE. 2013. Pregnancy-related group A streptococcal infections: temporal relationships between bacterial acquisition, infection onset, clinical findings, and outcome. Clin. Infect. Dis. 57:870–876. 10.1093/cid/cit282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Phoenix G, Das S, Joshi M. 2012. Diagnosis and management of cellulitis. BMJ 345:e4955. 10.1136/bmj.e4955 [DOI] [PubMed] [Google Scholar]
- 595.Franklin L, Nobbs AH, Bricio-Moreno L, Wright CJ, Maddocks SE, Sahota JS, Ralph J, O'Connor M, Jenkinson HF, Kadioglu A. 2013. The Agi/II family adhesin AspA is required for respiratory infection by Streptococcus pyogenes. PLoS One 8:e62433. 10.1371/journal.pone.0062433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Maddocks SE, Wright CJ, Nobbs AH, Brittan JL, Franklin L, Stromberg N, Kadioglu A, Jepson MA, Jenkinson HF. 2011. Streptococcus pyogenes antigen I/II-family polypeptide AspA shows differential ligand-binding properties and mediates biofilm formation. Mol. Microbiol. 81:1034–1049. 10.1111/j.1365-2958.2011.07749.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Talay SR, Valentin-Weigand P, Timmis KN, Chhatwal GS. 1994. Domain structure and conserved epitopes of Sfb protein, the fibronectin-binding adhesin of Streptococcus pyogenes. Mol. Microbiol. 13:531–539. 10.1111/j.1365-2958.1994.tb00448.x [DOI] [PubMed] [Google Scholar]
- 598.Jaffe J, Natanson-Yaron S, Caparon MG, Hanski E. 1996. Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two binding domains. Mol. Microbiol. 21:373–384. 10.1046/j.1365-2958.1996.6331356.x [DOI] [PubMed] [Google Scholar]
- 599.Rocha CL, Fischetti VA. 1999. Identification and characterization of a novel fibronectin-binding protein on the surface of group A streptococci. Infect. Immun. 67:2720–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Terao Y, Kawabata S, Nakata M, Nakagawa I, Hamada S. 2002. Molecular characterization of a novel fibronectin-binding protein of Streptococcus pyogenes strains isolated from toxic shock-like syndrome patients. J. Biol. Chem. 277:47428–47435. 10.1074/jbc.M209133200 [DOI] [PubMed] [Google Scholar]
- 601.Jeng A, Sakota V, Li Z, Datta V, Beall B, Nizet V. 2003. Molecular genetic analysis of a group A Streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX. J. Bacteriol. 185:1208–1217. 10.1128/JB.185.4.1208-1217.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Courtney HS, Li Y, Dale JB, Hasty DL. 1994. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect. Immun. 62:3937–3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Lukomski S, Nakashima K, Abdi I, Cipriano VJ, Ireland RM, Reid SD, Adams GG, Musser JM. 2000. Identification and characterization of the scl gene encoding a group A Streptococcus extracellular protein virulence factor with similarity to human collagen. Infect. Immun. 68:6542–6553. 10.1128/IAI.68.12.6542-6553.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Whatmore AM. 2001. Streptococcus pyogenes SclB encodes a putative hypervariable surface protein with a collagen-like repetitive structure. Microbiology 147:419–429 http://mic.sgmjournals.org/content/147/2/419.long [DOI] [PubMed] [Google Scholar]
- 605.Bates CS, Montanez GE, Woods CR, Vincent RM, Eichenbaum Z. 2003. Identification and characterization of a Streptococcus pyogenes operon involved in binding of hemoproteins and acquisition of iron. Infect. Immun. 71:1042–1055. 10.1128/IAI.71.3.1042-1055.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Lottenberg R, Broder CC, Boyle MD, Kain SJ, Schroeder BL, Curtiss R., III 1992. Cloning, sequence analysis, and expression in Escherichia coli of a streptococcal plasmin receptor. J. Bacteriol. 174:5204–5210 [DOI] [PMC free article] [PubMed] [Google Scholar]