Abstract
The Leptospira interrogans vaccines currently available are serovar specific and require regular booster immunizations to maintain protection of the host. In addition, a hamster challenge batch potency test is necessary to evaluate these vaccines prior to market release, requiring the use of a large number of animals, which is ethically and financially undesirable. Our previous work showed that the N terminus of the outer membrane protein LipL32 was altered in Leptospira interrogans serovar Canicola vaccines that fail the hamster challenge test, suggesting that it may be involved in the protective immune response. The aim of this study was to determine if vaccination with LipL32 protein alone could provide a protective response against challenge with L. interrogans serovar Canicola to hamsters. Recombinant LipL32, purified from an Escherichia coli expression system, was assessed for protective immunity in five groups of hamsters (n = 5) following a challenge with the virulent L. interrogans serovar Canicola strain Kito as a challenge strain. However, no significant survival against the L. interrogans serovar Canicola challenge was observed compared to that of unvaccinated negative controls. Subsequent histological analysis revealed reduced amounts of L. interrogans in the kidneys from the hamsters vaccinated with recombinant LipL32 protein prior to challenge; however, no significant survival against the L. interrogans serovar Canicola challenge was observed compared to that of unvaccinated negative controls. This finding corresponded to a noticeably reduced severity of renal lesions. This study provides evidence that LipL32 is involved in the protective response against L. interrogans serovar Canicola in hamsters and is the first reported link to LipL32-induced protection against kidney invasion.
INTRODUCTION
Leptospira interrogans is a flagellated waterborne zoonotic bacterium which predominantly infects mammals (1). The primary route of infection is generally either through direct contact with infected subjects or by exposure to water contaminated with their urine. Routes of entry into the host include ingestion, direct entry into the bloodstream via cuts, and inhalation of aerosols (2). The typical hosts of Leptospira include rodents, cattle, dogs, pigs, and sheep. More than 230 pathogenic serovars of Leptospira have been identified to date (3), and higher prevalences of some serovars in particular species, such as Leptospira interrogans serovar Canicola in dogs and Leptospira interrogans serovar Hardjo in sheep and cattle have been observed (4). The initial presentation of symptoms can include (5) fever, headache, myalgia, nausea, coughing, diarrhea, vomiting, and tubulointerstitial nephritis (1); left untreated, infections can result in hepatic or renal failure and death (6). Other symptoms, such as cessation of milk production and fetal loss, have also been observed in cattle (7), making leptospirosis particularly economically damaging to the farming community. Vaccination is used to protect against infection; however, the vaccines currently available are serovar specific (8), and regular booster immunizations are required to maintain immunity (9).
The proteins LipL32 (Hap-1), LipL41, LipL45, and OmpL1 have been previously identified as possible vaccine candidates (10–12) and, in some cases, have undergone in vivo trials (13, 14). LipL32 has also been shown to bind to the extracellular membrane proteins, collagen type IV and plasma fibronectin, in a calcium-independent manner (15). A LipL32 DNA vaccine was shown to confer protective immunity against L. interrogans serovar Canicola in the gerbil model (16); however, the protective effect of LipL32 against L. interrogans serovar Canicola in the hamster model has yet to be determined. The exact component(s) of the L. interrogans serovar Canicola vaccines that provide protective immunity are currently defined. However, in our previous study (17), we demonstrated that the concentration of an N-terminal LipL32 region was reduced in failed batches of L. interrogans serovar Canicola vaccines, suggesting that it may be involved in the protective immune response. The aim of the present study was to determine if LipL32 could confer protective immunity against L. interrogans serovar Canicola in hamsters. This would be an important step in establishing whether the N-terminal alteration of LipL32, observed in failed vaccine batches (17), was responsible for the inability of the failed vaccines to confer immunological protection following challenge with L. interrogans serovar Canicola.
MATERIALS AND METHODS
Ethical approval.
All animal procedures in this study were covered under the Animals (Scientific Procedures) Act 1986 by Home Office Project License No. PPL 70/7249 and were approved by the Animal Ethics Committee at the Animal Health and Veterinary Laboratories Agency (AHVLA) where all of this work was performed.
Vaccine and bacteria selection and growth conditions.
A randomly chosen bivalent vaccine, giving protection against L. interrogans serovars Canicola and Icterohaemorrhagiae, which had passed the in vivo vaccine batch potency test and had been released for commercial sale, was purchased from the manufacturer for analysis. This was designated vaccine A and used to vaccinate the positive-control group. The commercial sensitivity relating to this product did not allow precise formulations to be released by the manufacturer at the time of conducting this study; however, subsequently released formulation data have confirmed the adjuvant in vaccine A to be 1% (wt/vol) ethylene-maleic anhydride (EMA) and 3% (vol/vol) NeoCryl A-640.
L. interrogans serovar Canicola (strain Kito), for use as the challenge strain, was prepared by inoculation of 20 ml of Ellinghausen-McCullough-Johnson-Harris (EMJH) medium (Becton, Dickinson, USA) with 1 ml of pure culture (1 × 108 cell/ml) and incubated for 7 days at 30°C with orbital agitation at 50 rpm.
Expression and purification of recombinant LipL32 protein.
Cloning of the lipL32 gene into an expression vector, downstream from a polyhistidine tag, was performed using a method modified from that of Haake et al. (18). In this process, genomic DNA from Leptospira kirschneri was substituted with genomic DNA from L. interrogans serovar Canicola strain Kito, which also necessitated that the restriction enzymes XhoI and SmaI be substituted with KpnI and EcoRI (and associated primers changed accordingly); in addition, the expression strain BLR(DE3)/pLysS was substituted for BL21(DE3)/pLysS as it was more readily available. PCR was used to amplify the portion of the LipL32 gene encoding the mature protein beginning with the first residue after the amino-terminal cysteine.
The PCR amplicon product size was assessed using gel electrophoresis, the product was cleaned using a QIAquick PCR purification kit (Qiagen, USA) as per the manufacturer's instructions, and the DNA concentration was estimated using a NanoDrop ND-1000 instrument (Thermo Scientific, United Kingdom) at 260 nm. The ligation mixture was then transformed into chemically competent Escherichia coli BL21(DE3) pLysS cells (Promega, United Kingdom) as per the manufacturer's instruction, and transformants were selected on Luria-Bertani (LB) agar plates (35 μg/ml chloramphenicol and 50 μg/ml ampicillin) at 37°C overnight. The plasmid diagram of the LipL32-pRSET C construct (Fig. 1) was generated using SECentral (Sci-Ed, USA).
FIG 1.
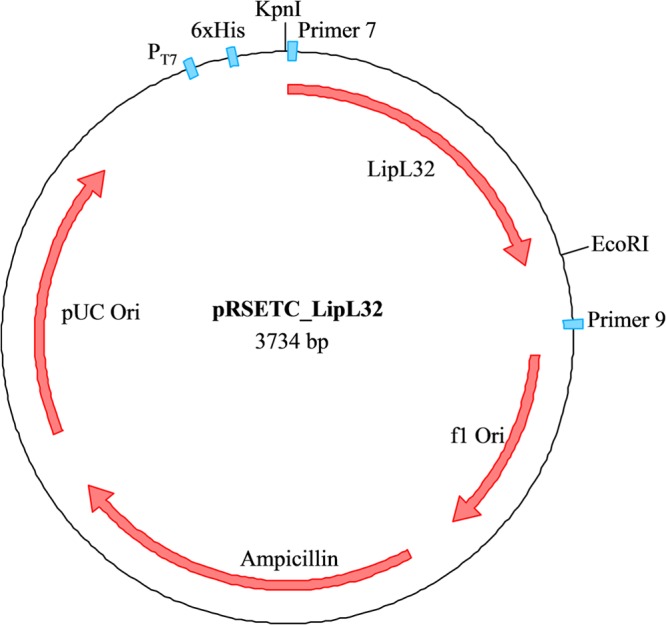
Plasmid schematic diagram of pRSET C following insertion of the LipL32 gene. Promoter (T7), the polyhistidine tag (6×His), and primers used to confirm insertion are shown in blue. The KpnI and EcoRI restriction sites used to insert the LipL32 gene are also shown. The plasmid diagram of the LipL32-pRSET C construct was generated using SECentral (Sci-Ed, USA).
LipL32 protein was expressed from the recombinant E. coli strain (500 ml) as per the manufacturer's instruction (Invitrogen, United Kingdom), using a final concentration of 2 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) to induce lipL32 expression. Cells were centrifuged (6000 × g) for 15 min at 4°C and resuspended in 10 ml lysis buffer (100 mM sodium phosphate, 6 M guanidine hydrochloride [pH 8]). Cells were lysed by 6-s pulses of probe sonication (amplitude 60) using a Vibra-Cell ultrasonic processor (Sonics and Materials, USA) for 6 min on ice and centrifuged (4000 × g) for 10 min at 4°C to remove cellular debris. The polyhistidine-tagged LipL32 protein was then purified from the retained supernatant using PureProteome nickel magnetic beads (Millipore, United Kingdom), a wash buffer (50 mM sodium phosphate, 300 mM sodium chloride, 10 mM imidazole [pH 8]), and an elution buffer (50 mM sodium phosphate, 300 mM sodium chloride, 300 mM imidazole [pH 8]) according to the manufacturer's instructions. The eluted protein was washed once with 2 ml of 1 M phosphate-buffered saline (PBS) (pH 7.2) using a 5-kDa MWCO filter (Sartorius Stedim, France) and resuspended to a final volume of 200 μl in PBS. Total protein, lipopolysaccharide (LPS), and LipL32 concentrations of the purified recombinant LipL32 protein, and vaccine A were determined (Table 1) using the Bradford, Limulus amebocyte lysate (LAL), and multiple reaction monitoring (MRM) assays as previously described (17). The purified LipL32 protein (10 μg) was assessed on a 4 to 12% NuPAGE gel stained with Coomassie blue (Fig. 2) to evaluate protein elution from the nickel magnetic beads, which showed one band at the predicted size of mature length LipL32 (27.8 kDa). An enzyme-linked immunosorbent assay (ELISA) using a LipL32-specific antibody was used to confirm the immunogenicity of the recombinant LipL32 protein prior to use in the hamster model (data not shown).
TABLE 1.
Concentrations of LipL32 (N and C termini using MRM), total protein, and LPS in recombinant LipL32 protein and vaccine A
| Substance | Concentration (mean ± 1 SD) of: |
|||
|---|---|---|---|---|
| Protein (mg/ml) | LPS (μg/ml) | LipL32 (fmol/μg) |
||
| C terminus | N terminus | |||
| Vaccine A | 9.69 ± 0.40 | 0.01 ± 0.00 | 1.07 ± 0.22 | 2.65 ± 1.09 |
| LipL32 | 21.75 ± 0.49 | 0.47 ± 0.01 | 3,096.17 ± 1,449.91 | 7,130.33 ± 2,649.79 |
FIG 2.
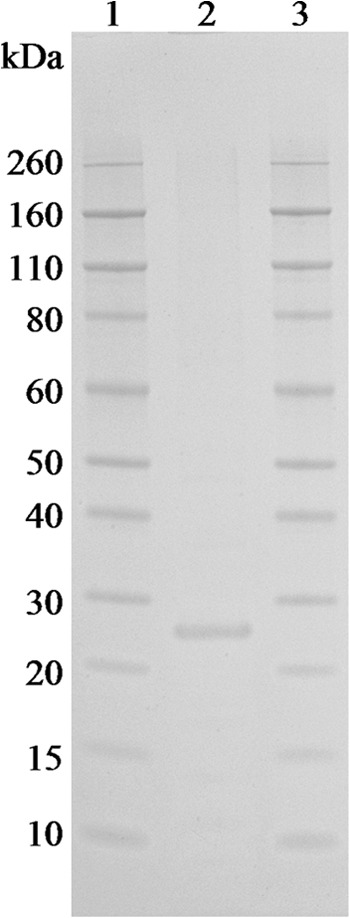
Purified LipL32 protein (lane 2; 10 μg) run on a 4 to 12% NuPAGE gel stained with Coomassie blue to detect protein. Lanes 1 and 3 contain a 3.5- to 260-kDa protein ladder.
Immunization of hamsters with test products.
Four groups of five female hamsters (≤120 g; Charles River, Germany) were vaccinated subcutaneously with either purified LipL32 protein (868 pmol N-terminal LipL32) (group 1), purified LipL32 protein (868 pmol N-terminal LipL32) with Imject alum adjuvant (0.25 ml; Thermo Scientific, USA) (group 2), vaccine A (diluted 1/40) (group 3), or 0.9% (wt/vol) physiological saline (group 4). All test products were prepared in 0.9% (wt/vol) physiological saline to a final volume of 0.5 ml. Two additional control groups of hamsters were also used to confirm that the challenge strain was appropriately virulent (group 5; n = 5) and to provide an unchallenged control (group 6; n = 3) for histological comparison.
Fifteen days following vaccination, groups 1 to 5 were challenged by intraperitoneal inoculation with 1 ml of virulent Leptospira interrogans serovar Canicola (∼1 × 108 cells/ml) strain Kito. The hamsters were routinely monitored, and their conditions were assessed using a clinical score sheet developed at the AHVLA (Table S1 in the supplemental material). Hamsters with a score of 3 or higher were judged to be in distress, likely to end in death, and were therefore humanely euthanized using halothane; all surviving hamsters were humanely euthanized on day 24 (which was 14 days after the fourth hamster in the negative control [group 5] succumbed to infection). All euthanized hamsters were observed for 5 min after halothane was administered to confirm cessation of life prior to performing any procedures.
Kidneys were excised from all hamsters at postmortem examination and dissected for assessment of infection. Half were retained for histological processing, and half were disrupted with a 10-ml syringe and cultured in EMJH medium. The presence of Leptospira was assessed in 10 fields of view using a dark-field microscope (×400 magnification).
Histology.
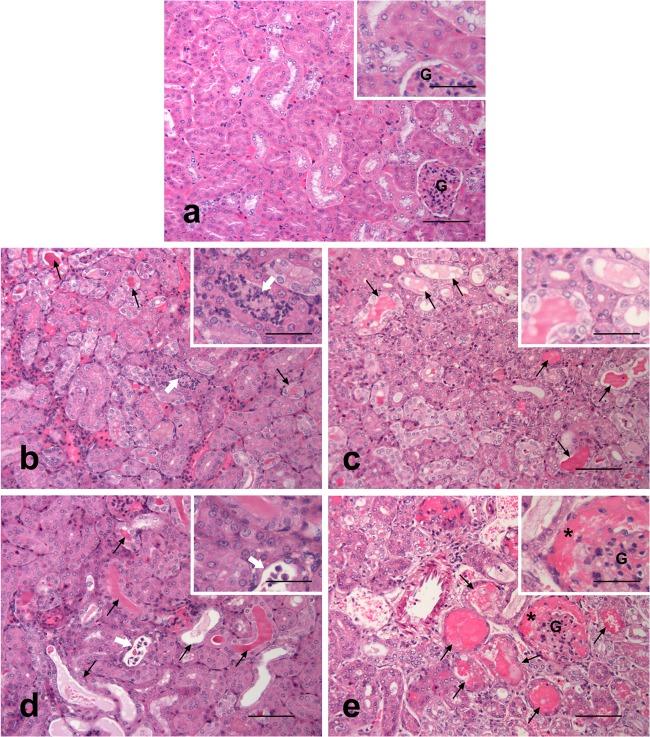
Samples from liver, spleen, and kidney were collected from all hamsters at postmortem examination and fixed in 10% (vol/vol) buffered formalin. The tissue samples were routinely processed and embedded in paraffin wax using a Hypercenter XP tissue processor (Thermo Shandon, United Kingdom). Consecutive 4-μm-thick sections were cut using a Leica RM2025 (Leica, Germany) rotary microtome. Sections were stained with hematoxylin and eosin (H&E) for microscopic examination and with Warthin-Starry silver impregnation for the visualization of leptospires in the tissues (19). Renal lesions indicative of infection, if present, were graded according to their severity (0 as normal [0% coverage], 1 as minimal [≤10% coverage], 2 as mild [11 to 25% coverage], 3 as moderate [26 to 50% coverage], and 4 as severe [≥50% coverage]) (Fig. 3) using a semiquantitative scoring system modified from that of Palaniappan et al. (20); one slide, containing approximately 100 nephrons, was assessed per animal. The number of leptospires present on the various tissues were also graded with 0 as absent, 1 as rare, 2 as few, 3 as numerous, and 4 as profuse. Slides were examined in a Leica DM4000B microscope (Leica, Germany). Pictures were taken using a Leica DFC480 digital camera and Leica Application Suite software. Adobe Photoshop Elements 4.0 (Adobe, USA) was used to adapt images for publication.
FIG 3.
Microphotographs of kidneys representative of the scoring system used to assess the extension and severity of histopathological changes in hamsters following infection with L. interrogans serovar Canicola. Infected animals displayed variable amounts of tubules containing eosinophilic protein casts (arrows) with different degrees of distension and attenuation, intratubular inflammatory infiltration (white arrows), and eosinophilic material in the uriniferous spaces of Bowman's capsules (*). (a) Control, normal structure of the cortex with the presence of a glomerulus (G) among the tubules, (b) score 1; (c) score 2; (d) score 3; (e) score 4. Hematoxylin and eosin (H&E). Scale bars, 100 μm and 50 μm (insets).
Statistical analysis.
Where appropriate, the data are presented as means and standard deviations of the means. Comparisons of the severity of the renal lesions and the invasion of Leptospira in different tissues between hamster groups 1 to 6 were performed using Student's t test; a P value of ≤0.05 was taken to be statistically significant. Kidney (∼100 nephrons), liver (2 cm2), and spleen (1 cm2) tissues from each animal were examined. Comparisons of the survivals of hamster groups 1 to 6 were performed using Fisher's exact test; a P value of ≤0.05 was taken to be statistically significant.
RESULTS
Assessment of the protective effect of recombinant LipL32 in the hamster vaccine batch potency test model.
Following challenge with virulent L. interrogans serovar Canicola, groups 1 and 2 failed the vaccine potency test on days 10 and 12, respectively, with 4/5 hamsters either succumbing to infection or having to be euthanized. One hamster from each group survived until the end of the test (day 24); however, this increased survival was not statistically significant (Table 2). Group 3 (vaccine A) passed the vaccine potency test with 4/5 hamsters surviving until day 24 (P ≤ 0.05). As expected, the negative-control groups 4 and 5 failed the test at days 11 and 10, respectively, with all hamsters either succumbing to infection or being humanely euthanized at a clinical score of 3 or higher. The hamsters in group 6 survived to day 24, confirming that the stock hamsters used were free of disease (P ≤ 0.05).
TABLE 2.
Treatments protocols applied to hamster groups 1 to 6 (n = 5) and numbers of survivorsa
| Group | Treatment | No. of survivors/no. immunized | P value |
|---|---|---|---|
| 1 | LipL32 (no adjuvant) + challenge | 1/5 | 1.000 |
| 2 | LipL32 + adjuvant + challenge | 1/5 | 1.000 |
| 3 | Vaccine A + challenge | 4/5 | 0.048 |
| 4 | Saline + challenge | 0/5 | NDb |
| 5 | No treatment + challenge | 0/5 | 1.000 |
| 6 | No treatment + no challenge | 3/3 | 0.018 |
Group 6 comprised 3 hamsters. P values are 2-sided and were obtained through comparisons with the negative control (group 4) using Fisher's exact test.
ND, not determined.
Histopathological analysis of hamsters immunized with LipL32.
Variable degrees of diffuse tubulointerstitial nephritis, consistent with Leptospira infection, were observed in the hamsters that succumbed to infection (or had to be euthanized) following challenge with L. interrogans serovar Canicola. The microscopic changes consisted of minimal infiltration of the interstitial spaces with lymphocytic cells and the frequent presence of strongly eosinophilic hyaline casts in the lumen of tubules, often associated with degeneration, necrosis, and attenuation of tubular epithelial cells. Occasional tubules also displayed a mixture of sloughed cells and leukocytes in their lumen.
Lower scores for renal lesion severity were observed in groups 1, 3, and 6 (Table 3) than in groups 2, 4, and 5 (Table 3); treatment group 1, which comprised LipL32 without adjuvant, had a significantly lower score (P ≤ 0.01) than the negative control (group 4). Only one survivor from group 3 (euthanized on day 24) showed evidence of renal pathology as a minute focal lesion; no lesions were observed in group 6 (Table 3).
TABLE 3.
Severity of renal lesions and invasion of Leptospira in hamster tissues determined through staining with hematoxylin and eosin and Warthin-Starry stains, respectivelya
| Treatment group | Severity of renal lesion score |
Invasion of Leptospira score in: |
||||||
|---|---|---|---|---|---|---|---|---|
| Kidney |
Liver |
Spleen |
||||||
| Mean ± 1 SD | P value | Mean ± 1 SD | P value | Mean ± 1 SD | P value | Mean ± 1 SD | P value | |
| 1 | 1.8 ± 1.1 | 0.004 | 1.2 ± 0.8 | 0.009 | 2.4 ± 1.3 | 0.070 | 1.0 ± 1.0 | 0.621 |
| 2 | 2.6 ± 1.5 | 0.374 | 2.0 ± 1.4 | 0.189 | 1.6 ± 1.8 | 0.034 | 1.0 ± 1.0 | 0.621 |
| 3 | 0.6 ± 0.9 | 0.001 | 0.0 ± 0.0 | NDb | 0.0 ± 0.0 | ND | 0.0 ± 0.0 | ND |
| 4 | 3.0 ± 0.7 | ND | 3.0 ± 0.0 | ND | 3.6 ± 0.5 | ND | 1.2 ± 0.45 | ND |
| 5 | 2.6 ± 0.5 | 0.178 | 2.8 ± 0.4 | 0.374 | 2.8 ± 1.6 | 0.242 | 1.2 ± 0.84 | 1.000 |
| 6 | 0.0 ± 0.0 | ND | 0.0 ± 0.0 | ND | 0.0 ± 0.0 | ND | 0.0 ± 0.0 | ND |
Scores were obtained using a semiquantitative scoring system modified from Palaniappan et al. (20). Means and standard deviations of the means for the observed scores are shown. P values were obtained through comparison with the negative control (group 4) using a Student's t test.
ND, not determined.
Leptospires, when present in the kidneys, could be observed in the interstitial spaces and tubular lumina in the renal cortex and occasionally in the lumen of blood vessels of the cortex or medulla, with no preference for any particular vascular structure. A significantly lower (P ≤ 0.01) score for Leptospira kidney invasion was observed (Table 3) in group 1 than in the negative control (group 4); no leptospires were observed in group 3 or 6.
In addition to microscopic analysis of hamster kidneys at postmortem examination, culturing was also performed to determine if Leptospira were still viable after infection. Leptospires were not observed in the kidney cultures of the animals euthanized at day 24 (groups 1 to 3 and 6), which is in agreement with the histological findings. No leptospires were observed by histological staining in the kidneys of a hamster from group 3 that died at day 17 (see Fig. S2 in the supplemental material); however, confirmatory data could not be obtained for this animal using kidney culturing due to the detection of bacterial contamination during processing.
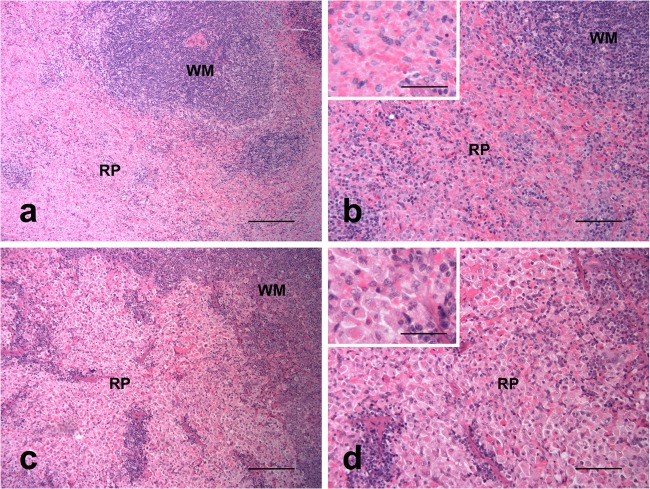
All hamsters that died (or had to be euthanized) following challenge with Leptospira displayed liver plate disarray, with loss of the normal hepatic sinusoid architecture, increased volumes of hepatocyte cytoplasm with eccentric nuclei and frequent multinucleation (Fig. 4), and multifocal infiltrations by lymphohistiocytic cells. A few random multifocal areas of necrotic hepatocytes were observed in two animals from group 4; no hepatic lesions were observed in the hamster from group 3 that died at day 17. Groups 1 and 2 showed reduced liver invasion scores (2.4 ± 1.3 and 1.6 ± 1.8, respectively) (Table 3) compared to those for group 4 (3.6 ± 0.5); however, only group 2 showed a significant (P ≤ 0.05) difference. An example of the histopathological effects of Leptospira on hamster livers is shown in Fig. 4, where loss of the normal hepatic sinusoid architecture, an increased volume of hepatocyte cytoplasm with eccentric nuclei, and frequent multinucleation can be observed. The spleens of animals that succumbed to infection (or had to be euthanized) following challenge with Leptospira showed marked hypertrophy and hyperplasia of macrophages of splenic cords in red pulp in animals dying of the disease (Fig. 5). Very few leptospires could be observed in the red pulp of hamsters showing splenic pathology, and no significant difference in splenic invasion was observed between groups (Table 3).
FIG 4.
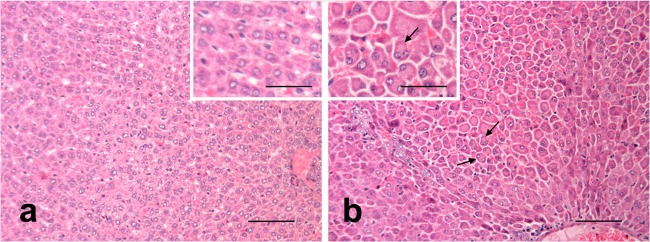
Liver. (a) Control animal, normal structure of hepatic sinusoids; (b) challenged hamster, loss of the normal hepatic sinusoid architecture and increased volume of hepatocyte cytoplasm with eccentric nuclei and frequent multinucleation. Hematoxylin and eosin. Scale bars, 100 μm and 50 μm (insets).
FIG 5.
Spleen. (a and b) Control animal, normal structure of white matter (WM) and red pulp (RP); (c and d) challenged hamster, hypertrophy and hyperplasia of the macrophages of the splenic pulp. Hematoxylin and eosin. Scale bars, 200 μm (a and c), 100 μm (b and d), and 50 μm (insets).
DISCUSSION
The recombinant LipL32 generated in this study did not result in a statistically increased survival against challenge with L. interrogans serovar Canicola (Table 2). However, a decreased score of kidney invasion (P ≤ 0.01) was observed in the group treated with recombinant LipL32 (group 1) prior to challenge with L. interrogans serovar Canicola (Table 3), which corresponded to decreased scores of kidney lesions (P ≤ 0.01), indicating that while recombinant LipL32 was unable to provide complete protection, it was able to reduce the severity of infection in the hamsters.
Interestingly, group 2, which received aluminum hydroxide adjuvant in conjunction with LipL32, did not show significantly decreased kidney invasion or lesions compared with those for the negative control (group 4). Further work is required to elucidate the mechanism behind this finding; however, it may be suggested that aluminum hydroxide is either not suitable for presentation of a single protein against L. interrogans serovar Canicola or requires an alternate dosage to elicit an effective response. As the N-terminal concentration of LipL32 in group 1 was in excess (868 pmol) of that used in group 3 (641 fmol), the results may suggest that vaccine A either contains additional components required to initiate protective immunity or possesses an increased immunostimulatory effect (either through the use of an adjuvant or other naturally occurring bacterial components). A recent study (21) demonstrated that LipL32 could provide protective immunity against L. interrogans serovar Copenhageni in hamsters when coadministered with the B subunit of E. coli heat-labile enterotoxin (LTB) as an adjuvant. It is conceivable, therefore, that the immunogenic effect of LipL32 against L. interrogans serovar Canicola will also be increased through use of LTB as an adjuvant. The initial results reported here support the need for larger studies using a range of LipL32 concentrations, in conjunction with a range of adjuvants, to fully elucidate the role of LipL32 in the vaccines. Further, it has been observed previously that adsorption with aluminum hydroxide can reduce antigen immunogenicity (22), which could explain the results seen in group 2; however, no known mechanism of action for the aluminum hydroxide interference is currently known.
It should also be noted that although aluminum hydroxide was assessed as a proxy for the adjuvant used in vaccine A, subsequent release of commercially sensitive data relating to the precise formulation of vaccine A confirmed that the adjuvants are EMA and NeoCryl. Clearly, in retrospect, an analysis of the precise formulation with LipL32 may produce effects unreported herein, and this requires further investigation. However, the results reported herein advance our understanding of the role in LipL32 in Leptospira vaccinology.
Multiple studies on the usage of LipL32 as a vaccine candidate have been reported previously and have shown that LipL32 provides protection against L. interrogans serovars Canicola (16) and Copenhageni (21, 23) but not against L. interrogans serovars Pomona (24) and Manilae (25). The results presented herein provide further confirmatory evidence that LipL32 is involved in the protective immune response against L. interrogans serovar Canicola, either on its own or in combination with other as yet undefined bacterial components. More importantly, this study is the first to directly link vaccination with a predetermined concentration of LipL32 with reduced kidney invasion of L. interrogans serovar Canicola in hamsters.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mathieu Picardeau at the Pasteur Institute (Paris, France) for the donation of L. interrogans serovar Canicola strain Kito and Jarlath Nally at the University College of Dublin (Dublin, Ireland) for the donation of an anti-LipL32 antibody.
This work was supported by a grant (G0700633) from The National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs).
Footnotes
Published ahead of print 12 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00719-13.
REFERENCES
- 1.Adler B, de la Pena Moctezuma A. 2010. Leptospira and leptospirosis. Vet. Microbiol. 140:287–296. 10.1016/j.vetmic.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 2.Woodward MJ. 2001. Leptospira, p 2137–2158 In Sussman M. (ed), Molecular medical microbiology. Academic Press, San Diego, CA [Google Scholar]
- 3.Adler B, Lo M, Seemann T, Murray GL. 2011. Pathogenesis of leptospirosis: the influence of genomics. Vet. Microbiol. 153:73–81. 10.1016/j.vetmic.2011.02.055 [DOI] [PubMed] [Google Scholar]
- 4.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM, Peru-United States Leptospirosis Consortium 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3:757–771. 10.1016/S1473-3099(03)00830-2 [DOI] [PubMed] [Google Scholar]
- 5.Collins RA. 2006. Leptospirosis. Biomed. Sci. 50:116–117, 119–121 [Google Scholar]
- 6.Levett PN. 2001. Leptospirosis. Clin. Microbiol. Rev. 14:296–326. 10.1128/CMR.14.2.296-326.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Department for Environment, Food and Rural Affairs. 2008. Zoonoses report. Defra Publications, London, United Kingdom [Google Scholar]
- 8.Koizumi N, Watanabe H. 2005. Leptospirosis vaccines: past, present, and future. J. Postgrad. Med. 51:210–214 [PubMed] [Google Scholar]
- 9.Klaasen HL, Molkenboer MJ, Vrijenhoek MP, Kaashoek MJ. 2003. Duration of immunity in dogs vaccinated against leptospirosis with a bivalent inactivated vaccine. Vet. Microbiol. 95:121–132. 10.1016/S0378-1135(03)00152-4 [DOI] [PubMed] [Google Scholar]
- 10.Dong H, Hu Y, Xue F, Sun D, Ojcius DM, Mao Y, Yan J. 2008. Characterization of the ompL1 gene of pathogenic Leptospira species in China and cross-immunogenicity of the OmpL1 protein. BMC Microbiol. 8:223. 10.1186/1471-2180-8-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullen PA, Xu X, Matsunaga J, Sanchez Y, Ko AI, Haake DA, Adler B. 2005. Surfaceome of Leptospira spp. Infect. Immun. 73:4853–4863. 10.1128/IAI.73.8.4853-4863.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Jin L, Wegrzyn A. 2007. Leptospirosis vaccines. Microb. Cell Fact. 6:39. 10.1186/1475-2859-6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seixas FK, Fernandes CH, Hartwig DD, Conceicao FR, Aleixo JA, Dellagostin OA. 2007. Evaluation of different ways of presenting LipL32 to the immune system with the aim of developing a recombinant vaccine against leptospirosis. Can. J. Microbiol. 53:472–479. 10.1139/w06-138 [DOI] [PubMed] [Google Scholar]
- 14.Haake DA, Mazel MK, McCoy AM, Milward F, Chao G, Matsunaga J, Wagar EA. 1999. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect. Immun. 67:6572–6582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauk P, Barbosa AS, Ho PL, Farah CS. 2012. Calcium binding to leptospira outer membrane antigen LipL32 is not necessary for its interaction with plasma fibronectin, collagen type IV, and plasminogen. J. Biol. Chem. 287:4826–4834. 10.1074/jbc.M111.277210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Branger C, Chatrenet B, Gauvrit A, Aviat F, Aubert A, Bach JM, Andre-Fontaine G. 2005. Protection against Leptospira interrogans sensu lato challenge by DNA immunization with the gene encoding hemolysin-associated protein 1. Infect. Immun. 73:4062–4069. 10.1128/IAI.73.7.4062-4069.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humphryes PC, Weeks ME, Gielbert A, Thomson G, Coldham NG. 2012. Analysis of multiple Leptospira interrogans serovar Canicola vaccine proteomes and identification of LipL32 as a biomarker for potency. Clin. Vaccine Immunol. 19:587–593. 10.1128/CVI.05622-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haake DA, Chao G, Zuerner RL, Barnett JK, Barnett D, Mazel M, Matsunaga J, Levett PN, Bolin CA. 2000. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect. Immun. 68:2276–2285. 10.1128/IAI.68.4.2276-2285.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bancroft JD, Stevens AL. 1996. Theory and practice of histological techniques, 4th ed. Churchill Livingstone, Edinburgh, Scotland [Google Scholar]
- 20.Palaniappan RU, McDonough SP, Divers TJ, Chen CS, Pan MJ, Matsumoto M, Chang YF. 2006. Immunoprotection of recombinant leptospiral immunoglobulin-like protein A against Leptospira interrogans serovar Pomona infection. Infect. Immun. 74:1745–1750. 10.1128/IAI.74.3.1745-1750.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grassmann AA, Felix SR, Ximendes Dos Santos C, Amaral MG, Seixas Neto AC, Fagundes MQ, Seixas FK, Da Silva EF, Conceicao FR, Dellagostin OA. 2012. Protection against lethal leptospirosis after vaccination with LipL32 coupled or coadministered with the B subunit of Escherichia coli heat-labile enterotoxin. Clin. Vaccine Immunol. 19:740–745. 10.1128/CVI.05720-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eskola J, Olander RM, Hovi T, Litmanen L, Peltola S, Kayhty H. 1996. Randomised trial of the effect of co-administration with acellular pertussis DTP vaccine on immunogenicity of Haemophilus influenzae type b conjugate vaccine. Lancet 348:1688–1692. 10.1016/S0140-6736(96)04356-5 [DOI] [PubMed] [Google Scholar]
- 23.Seixas FK, Da Silva EF, Hartwig DD, Cerqueira GM, Amaral M, Fagundes MQ, Dossa RG, Dellagostin OA. 2007. Recombinant Mycobacterium bovis BCG expressing the LipL32 antigen of Leptospira interrogans protects hamsters from challenge. Vaccine 26:88–95. 10.1016/j.vaccine.2007.10.052 [DOI] [PubMed] [Google Scholar]
- 24.Cao Y, Faisal SM, Yan W, Chang YC, McDonough SP, Zhang N, Akey BL, Chang YF. 2011. Evaluation of novel fusion proteins derived from extracellular matrix binding domains of LigB as vaccine candidates against leptospirosis in a hamster model. Vaccine 29:7379–7386. 10.1016/j.vaccine.2011.07.070 [DOI] [PubMed] [Google Scholar]
- 25.Lucas DS, Cullen PA, Lo M, Srikram A, Sermswan RW, Adler B. 2011. Recombinant LipL32 and LigA from Leptospira are unable to stimulate protective immunity against leptospirosis in the hamster model. Vaccine 29:3413–3418. 10.1016/j.vaccine.2011.02.084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.