Abstract
Our previous studies showed that intranasal vaccination of vitamin A-deficient (VAD) mice failed to induce normal levels of upper respiratory tract IgA, a first line of defense against respiratory virus infection. Here we demonstrate that the impaired responses in VAD animals are corrected by a single intranasal application of retinyl palmitate with the vaccine. Results encourage the clinical testing of intranasal vitamin A supplements to improve protection against respiratory viral disease in VAD populations.
TEXT
Vitamin A deficiencies (VADs) and insufficiencies have been long-standing problems in the developing and developed worlds, particularly in situations of low socioeconomic status, prematurity, and disease (1, 2). The problem is related in part to poor diets but also to the inability of some individuals to process and transport vitamin A from the intestinal tract to target tissues. In young children, liver stores of vitamin A are low, and in cases of disease, both serum retinol and retinol binding proteins (necessary for retinol transport) exist at extremely low concentrations. Diarrhea and urinary excretion are associated with the inability of some diseased children to process vitamin A (3).
In the context of vitamin A deficiencies, mucosal IgA responses toward viruses and viral vaccines are impaired both in the gut and in the upper respiratory tract (URT) (4–6). IgG responses are relatively resistant to VAD compared to IgA responses, yielding an increase in IgG/IgA ratios at the mucosal surface. Serum antibody responses, being predominantly of the IgG isotype, are essentially unchanged in VAD mice compared to control mice (4, 5). Given that respiratory tract mucosal IgA serves as a correlate of protection against respiratory virus infections and lends to the protection conferred by respiratory virus vaccines (7–11), the lack of healthy IgA responses may render VAD individuals particularly susceptible to the diseases responsible for one-fifth of all deaths among children under the age of five years (2, 12).
Vitamin A is acquired in the diet and can be stored in the liver as retinol esters or can be transported through the circulatory system in the form of retinol bound to retinol binding protein (13). A ubiquitously distributed subfamily of enzymes, the alcohol dehydrogenases, convert retinol to retinaldehyde, but the further conversion of retinaldehyde to retinoic acid, the most important metabolite for healthy immune function, requires a subfamily of aldehyde dehydrogenases (ALDH1A) with restricted tissue and cell distribution (6).
ALDH1A expression was previously proposed to occur primarily within dendritic cells (DC) of the gut (6). However, we recently discovered that ALDH1A is constitutively expressed by epithelial cells lining the upper and lower respiratory tract airways (14). We found that respiratory tract epithelial cells were also able to produce cytokines supportive of IgA production and to upregulate IgA secretion following B cell activation in a tissue culture setting. On the basis of these results, we hypothesized that an intranasal (i.n.) application of vitamin A administered at the time of i.n. viral vaccination could correct the loss of vaccine-induced IgA in the URT. Here we show that a single dose of vitamin A in the form of retinyl palmitate or retinol administered by the i.n. route at the time of i.n. vaccination suffices to correct URT IgA responses in VAD hosts.
To establish VAD mice, pregnant female C57BL/6 (H2b) mice (day 4 to 5 estrus) from Jackson Laboratories (Bar Harbor, ME) were placed on characterized diets (Harlan Laboratories, Madison, WI) in filter-top cages in a biosafety level 2+ containment area. Animal care was as specified by the Association for Assessment and Accreditation for Laboratory Animal Care (AAALAC) guidelines and approved by the Institutional Animal Care and Use Committee (IACUC).
The VAD diet (catalog no. TD.10762; Harlan) was formulated with casein, dl-methionine, sucrose, corn starch, cotton seed oil, cellulose, mineral mix AIN-76 (catalog no. 170815; Harlan), calcium carbonate, vitamin mix (lacking vitamin A) plus choline, and food coloring. The control diet included vitamin A palmitate at 15 IU/g (catalog no. TD.10764; Harlan). Animals were sustained on the diet throughout their pregnancies, and weaned pups were on the diet until adulthood (when experiments were initiated) and throughout experimentation. Samples from VAD and control mice were tested for retinol at the Texas Veterinary Diagnostic Laboratory (Fort Collins). Mice on the control diet exhibited serum retinol levels of greater than 20 μg/dl, whereas retinol could not be detected in the sera of VAD mice (<10 μg/dl).
The first experiments were designed to test the hypothesis that administration of vitamin A by the i.n. route at the time of vaccination would suffice to correct the impaired vaccine-induced IgA in the VAD URT. To test this hypothesis, we grouped mice to receive replication-competent Sendai virus (SeV), a parainfluenza virus type 1 (PIV-1) of mice (currently being developed as a Jennerian vaccine for human PIV-1 [15]), with or without a vitamin A supplement. Mice were anesthetized with avertin and then administered 250 to 500 PFU of SeV by the i.n. route. The supplement was either retinyl palmitate (600 IU/mouse in 12 μl water-glycerin; Nutrisorb A [Interplexus Inc., Kent, WA]) or retinol (300 μg in 12 μl ethanol) (Sigma, St. Louis, MO,), delivered 15 min after infection to ensure that excipients would not interfere with infection.
Four weeks later, we tested mice for SeV-specific IgA in nasal washes (NW) and IgA antibody-forming cells (AFCs) in diffuse nasal-associated lymphoid tissues (d-NALT). Mice were first anesthetized with avertin and exsanguinated. NW samples were collected by exposing the trachea and washing the upper trachea and nasal cavity with 200 μl of phosphate-buffered saline (PBS). Mice were perfused with PBS injected through the retro-orbital sinus, after which d-NALT were collected. d-NALT were isolated by removing skin, lower jaws, palates (including the attached o-NALT), muscles, cheek bones and incisors from the heads. The remaining snouts were cut into small pieces and digested with 4 mg/ml collagenase in PBS at 37°C for 30 min. Cells were then purified on Percoll gradients as described previously (16).
Mucosal IgA was tested with an SeV-specific enzyme-linked immunosorbent assay (ELISA) (16). Briefly, purified SeV was lysed in disruption buffer (0.05% Triton X-100, 60 mM KCl, 10 mM Tris [pH 7.8]) and diluted with PBS to 10 μg/ml for the coating of 96-well ELISA plates. The plates were stored overnight at 4°C. The plates were blocked with PBS containing 1% bovine serum albumin (BSA), after which serially diluted test samples were applied for a 1-h incubation at 37°C. The plates were washed with PBS-Tween 20 (0.05%) and incubated with alkaline phosphatase-conjugated goat anti-mouse IgA (catalog no. 1040-04; Southern Biotech, Birmingham, AL). Assays were then developed with p-nitrophenyl phosphate (catalog no. N2640; Sigma-Aldrich) and read at an optical density (OD) of 405 nm. IgA titers were determined using one-site binding nonlinear regression software (GraphPad Prism; GraphPad Software, San Diego, CA).
IgA antibody-forming cells were tested by enzyme-linked immunosorbent spot assays (ELISPOTs). Briefly, ELISPOT plates (MultiScreen-IP filter plates [catalog no. MAIPS4510; Millipore, Billerica, MA]) were coated with purified SeV at 1 μg/100 μl/well overnight at 4°C (16). The wells were washed 4 times with PBS and blocked with medium containing 10% fetal calf serum (FCS). The cells were then applied to the wells (5 × 104 to 1 × 105 cells/well) and incubated for 3 h at 37°C. The plates were washed 4 times with PBS and 4 times with PBS-Tween 20. Then, alkaline phosphatase-conjugated goat anti-mouse IgA (100 μl) was added in PBS-Tween 20 with 1% BSA. After overnight incubation at 4°C, the antibodies were removed, and the plates were developed with 5-bromo-4-chloro-3-indolylphosphate (BCIP)/Nitro Blue Tetrazolium (NBT) substrate (catalog no. B5655; Sigma-Aldrich). The plates were rinsed with water, and spots were counted using a Nikon dissecting scope. Studies were focused on the IgA response, because the IgG response is relatively stable in the context of VAD (5).
Unpaired t tests were used to compare ELISA and ELISPOT data among groups (GraphPad Prism software). All experiments were repeated to ensure reproducibility.
Results are shown in Fig. 1. As demonstrated in Fig. 1A and as expected, the responses in VAD mice were significantly reduced compared to responses in control mice. In contrast, mice that had received just one dose of i.n. retinyl palmitate or retinol showed improved virus-specific IgA responses. Vitamin A delivered in the form of retinyl palmitate was superior to retinol, likely due to the unstable nature of retinol (17). For the same reason, retinyl palmitate is often the preferred product for use in clinical vitamin A supplementation programs (18, 19).
FIG 1.
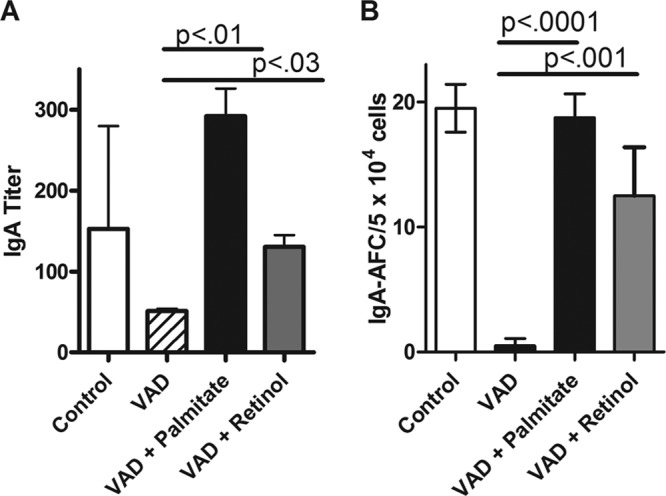
Intranasal vitamin A supplements correct impaired mucosal IgA responses in the context of VAD. Mice were reared on VAD or control diets prior to infection with SeV. SeV was administered to VAD mice with or without supplements with retinyl palmitate (600 IU/mouse) or retinol (300 μg/mouse). Immune responses were assayed approximately 4 weeks later. (A) Nasal wash titers of SeV-specific IgA are shown for each group of mice. Mice were tested individually. The numbers of mice per group were 4, 2, 4, and 5, respectively, for the control, VAD, VAD-plus-palmitate, and VAD-plus-retinol groups. A repeat experiment yielded similar results with the same numbers of mice in the respective groups. (B) SeV-specific IgA-producing AFC of the d-NALT are shown per 5 × 104 cells. The numbers of mice per group were 5, 2, 4, and 5, respectively, for the groups of control, VAD, VAD-plus-palmitate, and VAD-plus-retinol mice. The tissue samples were combined in each group and tested in quadruplicate. A repeat experiment yielded similar results with the same numbers of mice in the respective groups. Values are means plus standard errors of the means (error bars). P values are shown to demonstrate significance for comparisons of the values for VAD mice and VAD mice given a supplement.
Results shown in Fig. 1B demonstrate clearly that IgA-producing AFCs were also much reduced in VAD animals but were improved when VAD mice received retinyl palmitate or retinol by the i.n. route with the vaccine. Because SeV-specific IgA-producing cells constituted a large fraction of all IgA-producing cells in the URT 1 month after SeV infection, we found that both the SeV-specific and total IgA-producing AFC numbers were reduced in the context of VAD, and both were improved when mice were given vitamin A (data not shown).
Retinyl palmitate is recognized as a standard and relatively safe form of vitamin A, but it can be associated with toxicities at high doses. The precise human dose required to ensure efficacy without toxicity remains a point of controversy. Past studies have yielded differing results, presumably due to differences in target population ages and preexisting vitamin deficiencies and to differences in vitamin sources and doses. Hussey and Klein reported that a dose of 400,000 IU vitamin A palmitate associated with safe and significant protection from morbidity and mortality in children with measles in Cape Town, South Africa (20). Huiming et al. similarly reported that two oral doses of vitamin A (100,000 IU/dose in infants and 200,000 IU/dose in older children) reduced the risk of mortality in measles virus-infected children in the <2-year-old age group (21). When vitamin A is administered to VAD infants with immunizations, the World Health Organization (WHO) now recommends an oral dose of 100,000 IU in infants and 200,000 IU in children 12 months or older (12). In other circumstances, lower doses may be recommended to reduce the risk of transient side effects, including fontanelle bulging (22), but questions remain as to whether lower oral doses (e.g., 25,000 IU) are sufficient to provide substantial benefit to VAD children (23).
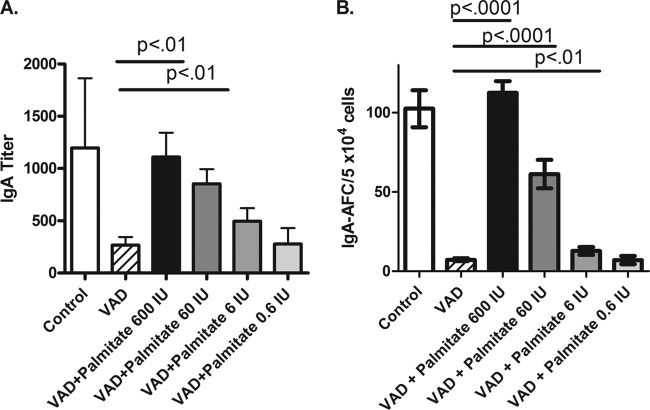
To gain a better understanding of the i.n. retinyl palmitate dose requirements in our preclinical model of VAD, we tested serial dilutions of retinyl palmitate in PBS administered 15 min after SeV vaccinations. The results in Fig. 2 show that a single i.n. dose of 600 IU was best, but a dose of only 60 IU retinyl palmitate significantly improved mucosal IgA and d-NALT IgA AFC responses in VAD animals. Future studies are planned to examine the durability of the mucosal IgA response supported by vitamin supplements and to determine whether responses at other mucosal surfaces (e.g., the gastrointestinal tract) are influenced when vitamin A supplements are delivered by the intranasal route. The locally administered dose of 60 IU per 20-g mouse (3,000 IU/kg of body weight) falls far below the high dose recommended by the WHO (100,000 IU/infant) on a dose/weight scale and is unlikely to cause the adverse events typical of high-dose, orally administered vitamin A.
FIG 2.
Vitamin A may be administered at a low dose with vaccine by the i.n. route to correct mucosal IgA responses in VAD animals. Control mice and VAD mice were infected with SeV. SeV was administered to VAD mice with or without retinyl palmitate given at the doses indicated in the figure. Mucosal IgA titers (A) and d-NALT IgA AFC responses (B) are shown. For ELISAs, mice were tested individually. The numbers of mice per group were 4, 5, 4, 5, 5, and 5, respectively, for the control group and groups given VAD, VAD plus palmitate 600 IU, VAD plus palmitate 60 IU, VAD plus palmitate 6 IU, and VAD plus palmitate 0.6 IU. A repeat experiment yielded similar results with 5, 5, 5, 5, 4, and 4 mice in the respective groups. For ELISPOTs, mouse tissue samples in each group were pooled and tested in quadruplicate. The numbers of mice per group in each experiment were the same as for the ELISAs described above. Values are means and standard errors of the means (error bars). P values are shown for comparisons of the values for VAD mice and VAD mice given a supplement.
Are VAD mice more susceptible than control mice to infections after first or second exposures to SeV? Because mice are the natural hosts of SeV, control mice lose weight following their first exposure to SeV but then mount a robust immune response that is virtually always protective against a second infection. We found that despite low levels of mucosal IgA, VAD mice experienced viral titers (on day 7 postexposure) and weight loss (from days 0 to 10 postexposure) values that were fairly similar to those of control mice after their first SeV infection (4). Studies of histopathology and tissue repair are in progress. VAD mice were also protected against a second exposure to SeV (data not shown). Future plans are to test protection of mice in the context of a Jennerian vaccine approach to mimic our human clinical studies (15). In this case, we will immunize mice with human parainfluenza virus type 1 as we have described previously (24) and determine whether vaccinated VAD mice are more susceptible than control mice to a subsequent challenge with SeV.
In conclusion, we have demonstrated that mucosal IgA responses toward an intranasal respiratory virus vaccine are impaired in VAD mice but that responses can be corrected with a single intranasal dose of vitamin A at the time of vaccination. Decades ago, the World Health Organization established policies and recommendations that vitamin A supplements should be administered orally to VAD children during vaccination campaigns. In many cases, supplementation programs have proven advantageous, but as described above, benefits may not always be evident, perhaps due to insufficient dosing and/or poor transport of vitamin A from the gut to target tissues in VAD hosts. The study described here suggests an alternative strategy. Results encourage clinical studies of the logistically feasible delivery of vitamin A supplements by the i.n. route at the time of respiratory virus vaccination. The dosing requirements for retinyl palmitate by the i.n. route may be relatively low, because vitamin is delivered directly to its target tissue and does not rely on transport from the gut. If future clinical studies exhibit the successes demonstrated in this report, coformulation of respiratory virus vaccines (e.g., SeV-based vaccines or FluMist) with vitamin A may simplify the logistics of supplementation while enhancing vaccine efficacy. An improved and robust local response to viral vaccines may ultimately enhance the protection of VAD individuals from the devastating consequences of respiratory viral disease.
ACKNOWLEDGMENTS
This study was supported in part by NIH NIAID grant R01 AI088729, NIH NCI grant P30-CA21765, and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Published ahead of print 19 February 2014
REFERENCES
- 1.Shenai JP, Chytil F, Jhaveri A, Stahlman MT. 1981. Plasma vitamin A and retinol-binding protein in premature and term neonates. J. Pediatr. 99:302–305. 10.1016/S0022-3476(81)80484-2 [DOI] [PubMed] [Google Scholar]
- 2.Sommer A, Tarwotjo I, Hussaini G, Susanto D. 1983. Increased mortality in children with mild vitamin A deficiency. Lancet ii:585–588. 10.1016/S0140-6736(83)90677-3 [DOI] [PubMed] [Google Scholar]
- 3.Stephensen CB, Alvarez JO, Kohatsu J, Hardmeier R, Kennedy JI, Jr, Gammon RB., Jr 1994. Vitamin A is excreted in the urine during acute infection. Am. J. Clin. Nutr. 60:388–392 [DOI] [PubMed] [Google Scholar]
- 4.Rudraraju R, Surman SL, Jones BG, Sealy R, Woodland DL, Hurwitz JL. 2012. Reduced frequencies and heightened CD103 expression among virus-induced CD8+ T cells in the respiratory tract airways of vitamin A-deficient mice. Clin. Vaccine Immunol. 19:757–765. 10.1128/CVI.05576-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surman SL, Rudraraju R, Sealy R, Jones B, Hurwitz JL. 2012. Vitamin A deficiency disrupts vaccine-induced antibody-forming cells and the balance of IgA/IgG isotypes in the upper and lower respiratory tract. Viral Immunol. 25:341–344. 10.1089/vim.2012.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mora JR, Iwata M, Von Andrian UH. 2008. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 8:685–698. 10.1038/nri2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills J, Van Kirk JE, Wright PF, Chanock RM. 1971. Experimental respiratory syncytial virus infection of adults. Possible mechanisms of resistance to infection and illness. J. Immunol. 107:123–130 [PubMed] [Google Scholar]
- 8.McIntosh K, Masters HB, Orr I, Chao RK, Barkin RM. 1978. The immunologic response to infection with respiratory syncytial virus in infants. J. Infect. Dis. 138:24–32. 10.1093/infdis/138.1.24 [DOI] [PubMed] [Google Scholar]
- 9.Carter NJ, Curran MP. 2011. Live attenuated influenza vaccine (FluMistR; Fluenz): a review of its use in the prevention of seasonal influenza in children and adults. Drugs 71:1591–1622. 10.2165/11206860-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 10.Clements ML, Betts RF, Tierney EL, Murphy BR. 1986. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J. Clin. Microbiol. 24:157–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambrose CS, Wu X, Jones T, Mallory RM. 2012. The role of nasal IgA in children vaccinated with live attenuated influenza vaccine. Vaccine 30:6794–6801. 10.1016/j.vaccine.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. 2013. Immunization, vaccines and biologicals, vitamin A supplementation. World Health Organization, Geneva, Switzerland: http://www.who.int/vaccines/en/vitamina.shtml [Google Scholar]
- 13.Napoli JL. 2012. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim. Biophys. Acta 1821:152–167. 10.1016/j.bbalip.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudraraju R, Jones BG, Surman SL, Sealy RE, Thomas PG, Hurwitz JL. 2014. Respiratory tract epithelial cells express retinaldehyde dehydrogenase ALDH1A and enhance IgA production by stimulated B cells in the presence of vitamin A. PLoS One 9:e86554. 10.1371/journal.pone.0086554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slobod KS, Shenep JL, Lujan-Zilbermann J, Allison K, Brown B, Scroggs RA, Portner A, Coleclough C, Hurwitz JL. 2004. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine 22:3182–3186. 10.1016/j.vaccine.2004.01.053 [DOI] [PubMed] [Google Scholar]
- 16.Rudraraju R, Surman S, Jones B, Sealy R, Woodland DL, Hurwitz JL. 2011. Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8+ T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology 410:429–436. 10.1016/j.virol.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FitzGerald O, Fennelly JJ, Hingerty DJ. 1962. Studies on the stability of vitamin A in the stomach and small intestine. Gut 3:264–266. 10.1136/gut.3.3.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahl R, Bhandari N, Kant S, Molbak K, Ostergaard E, Bhan MK. 2002. Effect of vitamin A administered at Expanded Program on Immunization contacts on antibody response to oral polio vaccine. Eur. J. Clin. Nutr. 56:321–325. 10.1038/sj.ejcn.1601325 [DOI] [PubMed] [Google Scholar]
- 19.Bahl R, Bhandari N, Wahed MA, Kumar GT, Bhan MK. 2002. Vitamin A supplementation of women postpartum and of their infants at immunization alters breast milk retinol and infant vitamin A status. J. Nutr. 132:3243–3248 [DOI] [PubMed] [Google Scholar]
- 20.Hussey GD, Klein M. 1990. A randomized, controlled trial of vitamin A in children with severe measles. N. Engl. J. Med. 323:160–164. 10.1056/NEJM199007193230304 [DOI] [PubMed] [Google Scholar]
- 21.Huiming Y, Chaomin W, Meng M. 2005. Vitamin A for treating measles in children. Cochrane Database Syst. Rev. 2005:CD001479. 10.1002/14651858.CD001479.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Francisco A, Chakraborty J, Chowdhury HR, Yunus M, Baqui AH, Siddique AK, Sack RB. 1993. Acute toxicity of vitamin A given with vaccines in infancy. Lancet 342:526–527. 10.1016/0140-6736(93)91648-6 [DOI] [PubMed] [Google Scholar]
- 23.Rahman MM, Mahalanabis D, Wahed MA, Islam MA, Habte D. 1995. Administration of 25,000 IU vitamin A doses at routine immunisation in young infants. Eur. J. Clin. Nutr. 49:439–445 [PubMed] [Google Scholar]
- 24.Sangster M, Smith FS, Coleclough C, Hurwitz JL. 1995. Human parainfluenza virus-type 1 immunization of infant mice protects from subsequent Sendai virus infection. Virology 212:13–19. 10.1006/viro.1995.1448 [DOI] [PubMed] [Google Scholar]