Abstract
Subunit vaccines against anthrax based on recombinant protective antigen (PA) potentially offer more consistent and less reactogenic anthrax vaccines but require adjuvants to achieve optimal immunogenicity. This study sought to determine in a murine model of pulmonary anthrax infection whether the polysaccharide adjuvant Advax or the innate immune adjuvant murabutide alone or together could enhance PA immunogenicity by comparison to an alum adjuvant. A single immunization with PA plus Advax adjuvant afforded significantly greater protection against aerosolized Bacillus anthracis Sterne strain 7702 than three immunizations with PA alone. Murabutide had a weaker adjuvant effect than Advax when used alone, but when murabutide was formulated together with Advax, an additive effect on immunogenicity and protection was observed, with complete protection after just two doses. The combined adjuvant formulation stimulated a robust, long-lasting B-cell memory response that protected mice against an aerosol challenge 18 months postimmunization with acceleration of the kinetics of the anamnestic IgG response to B. anthracis as reflected by ∼4-fold-higher anti-PA IgG titers by day 2 postchallenge versus mice that received PA with Alhydrogel. In addition, the combination of Advax plus murabutide induced approximately 3-fold-less inflammation than Alhydrogel as measured by in vivo imaging of cathepsin cleavage resulting from injection of ProSense 750. Thus, the combination of Advax and murabutide provided enhanced protection against inhalational anthrax with reduced localized inflammation, making this a promising next-generation anthrax vaccine adjuvanting strategy.
INTRODUCTION
Bacillus anthracis is a spore-forming, Gram-positive bacterium that is the causative agent of anthrax. The primary infectious form of B. anthracis is spores, which are tolerant of hostile environments and survive for years, germinating into vegetative bacilli when presented with appropriate conditions (1). Three forms of the disease exist, depending on the route of exposure. Cutaneous anthrax occurs when spores are introduced through a skin wound, gastrointestinal anthrax results from ingestion of spores, and inhalation anthrax, the most severe form of the disease, occurs when spores are inhaled into the lungs (2). Inhalational anthrax is of particular concern since it represents the route of exposure following the intentional release of anthrax spores into an environment, as in the case of bioterrorism. The accidental release of anthrax spores in the Russian city of Sverdlovsk in 1979 (3) and the deliberate dissemination of anthrax spores by mail in the United States in 2001 (4) demonstrated the potential risks of future attacks and spurred the development of improved vaccines highly protective against inhalational anthrax (5).
Following inhalation into the alveolar space, spores are engulfed by pulmonary phagocytes, such as macrophages and dendritic cells (DC) (6–9). Once inside the cell, spores germinate, bacilli escape the endosome, and bacterial replication occurs in the cytosol (10–12). B. anthracis has several major virulence factors, including its toxin components lethal factor (LF), edema factor (EF), and protective antigen (PA) and the poly-γ-d-glutamic acid polymer capsule (13, 14). LF is a zinc-dependent metalloprotease capable of cleaving host cell mitogen-activated protein kinase kinases in the cell cytosol (15, 16). EF is an adenylate cyclase that increases intracellular concentrations of cyclic AMP, which also disrupts host cell responses (17–19). Both EF and LF require PA to function. PA complexes with the toxin subunits EF and LF and interacts with host cell receptors to translocate the toxins into the cytosol (14). Due to its essential role in anthrax pathogenesis, recombinant PA is the lead candidate in development of next-generation anthrax vaccines (20, 21).
Recombinant PA vaccines require formulation with an appropriate adjuvant to achieve optimal immunogenicity (22). Although, to date, human trials of PA vaccines have used alum adjuvant (23, 24), other adjuvants, including Ribi adjuvant formulation for parenteral formulations (25) and cholera toxin and poly(I·C) for intranasal formulations (26, 27), have been shown to be effective in animal studies. Hence, use of alternative adjuvant technologies (28) may allow the development of more-effective and better-tolerated PA vaccines.
Polysaccharide-based adjuvants have shown promise in improving vaccine immunogenicity (29) Advax is an adjuvant made from delta inulin that was developed through the NIH Adjuvant Development Program (30). Inulin is a plant-derived polysaccharide that can exist as a number of different isoforms (31). The delta isoform of inulin forms a semicrystalline structure that possesses immune-modulatory activity and has been shown to enhance the immunogenicity and protection of a wide variety of vaccines, including those against Japanese encephalitis virus (32), West Nile virus (33), influenza virus (34), human immunodeficiency virus (35), and hepatitis B virus (36). Advax has also been shown to safely enhance the immunogenicity of a pandemic H1N12009pdm influenza virus vaccine in human subjects (37). The ability of Advax adjuvant to induce humoral and T-cell responses to a broad range of antigens with low reactogenicity makes it an interesting candidate to test in place of alum for development of next-generation PA-based anthrax vaccines.
Murabutide, a synthetic derivative of the bacterial cell wall peptidoglycan muramyl dipeptide (MDP), is another candidate adjuvant for anthrax vaccines. MDP is the minimal necessary element of the bacterial cell wall required for immune recognition by nucleotide-binding oligomerization domain-containing protein 2 (NOD2) receptors (38–40). Murabutide has similar adjuvant capacity but lacks the pyrogenicity and toxicity of MDP (41–45). Murabutide activates NOD2 receptors, which are located on antigen-presenting cells and T cells, thereby enhancing adaptive immune responses (46–49). Previous studies showed that murabutide was able to enhance specific antibody responses to a variety of antigens (50–52). Murabutide is well tolerated by humans and is weakly pyrogenic, with minimal adverse events (45, 53).
This study examined whether Advax or murabutide alone or together could enhance the immunogenicity of PA vaccine and thereby provide more robust and longer-lasting protection against anthrax infection. As vaccine tolerability is a significant factor affecting vaccine uptake, the study also sought to confirm the low inflammatory potential of Advax and murabutide adjuvants observed in other studies.
MATERIALS AND METHODS
Mice.
Female A/J mice were purchased from the National Cancer Institute (Bethesda, MD) or Jackson Laboratory (Bar Harbor, ME). Mice were between 6 and 12 weeks of age at the time of experiment commencement. All animal procedures were performed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with protocols approved by the Center for Biologics Evaluation and Research (CBER) Animal Care and Use Committee and the principles outlined in the Guide for the Care and Use of Laboratory Animals by the Institute for Laboratory Animal Resources, National Research Council.
Vaccinations.
Mice were immunized by subcutaneous (s.c.) injection with 100 μl of each vaccine preparation. Vaccine preparations contained 50 ng of PA per dose (determined to be optimal for adjuvant experiments; data not shown). Alhydrogel adjuvant (Brenntag Biosector, Denmark) was mixed with PA and incubated on ice for 1 h before injection per manufacturer instructions and injected at 0.3 mg/ml. Murabutide (Invivogen, San Diego, CA) was mixed with PA and injected at a concentration of 1 mg/ml. Advax adjuvant (Batch Ad1; Vaxine, Australia) was mixed with PA immediately and injected at a concentration of 10 mg/ml. Mice were bled via tail bleeding at various times after vaccination, and serum was collected for measurement of antibody titers.
Measurement of serum antibody titers.
Total serum immunoglobin G (IgG) antibody titers to PA were determined using a quantitative anti-PA enzyme-linked immunosorbent assay (ELISA). Reagents were obtained from KPL (Gaithersburg, MD). Plates were coated overnight at 4°C with 1 μg/ml of PA in coating buffer. Plates were washed with phosphate-buffered saline (PBS)-Tween and then blocked for 2 h with assay diluent at room temperature (RT). Samples were added and serially diluted, and plates were incubated overnight at 4°C. Plates were washed, and horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG was added. Plates were incubated at RT for 1 h and then given a final thorough wash. Plates were developed with SureBlue TMB Microwell Peroxidase Substrate and stopped with Stop Solution. Plates were read using a VersaMax ELISA microplate reader and data analyzed using SoftMax Pro software, as previously described (54).
Challenges.
Bacillus anthracis Sterne (strain 7702) aerosol challenges were performed as previously described (55). Briefly, mice were exposed to aerosolized spores for 90 min using a nose-only exposure system (CH Technologies, Westwood, NJ) with fresh air supplied for 10 min before and after spore exposure. The spore inoculum for each challenge contained 12 ml of 5 × 109 spores/ml in distilled water with 0.01% Tween 80. The 90-min aerosol exposure results in a retained dose in the lungs of ∼4 × 106, which is approximately 20 times the 50% lethal dose (LD50) for this model (55). Mice were monitored for survival for 10 days. No deaths were observed after day 6 in any challenged mice, so data in figures are shown only for the first 7 days. For each challenge, control mice were sacrificed following challenge and lungs were homogenized, serially diluted, and plated on BHI agar plates to determine an approximate challenge dose.
Inflammation imaging.
Mice were immunized subcutaneously (s.c.) with each PA-adjuvant combination as described above. The next day, they received tail vein injections of ProSense 750 (PerkinElmer, Waltham, MA). Mice were anesthetized with isofluorane and imaged for fluorescence according to the manufacturer's instructions using a PerkinElmer In Vivo Imaging System (IVIS). Data were quantitated using ImageJ densitometry analysis.
Statistical analyses.
Mortality data were plotted into Kaplan-Meier curves and assessed for significance by the log-rank test. GraphPad Prism 5.0 for Windows (GraphPad Software, San Diego, CA) was used for drawing graphs and statistical analysis. Significant differences between proportions were assessed by Fisher's exact test and differences in means by Student's t test or by one-way analysis of variance (ANOVA) using Dunnett's posttest. Differences were considered statistically significant when P was <0.05.
RESULTS
Single-dose immunization with PA plus either Advax or Alhydrogel enhances survival following anthrax aerosol challenge.
To determine whether Advax or murabutide enhanced PA immunogenicity in a murine model, mice were immunized s.c. with a single dose of 50 ng of PA (a dose determined to provide only partial protection from aerosol anthrax spore challenge; data not shown) alone or combined with 1 mg of Advax (optimal dose determined by titration; data not shown), 100 μg of murabutide, or 35 μg of Alhydrogel as a positive control. Mice were challenged 28 days following the final immunization with 20 LD50s of aerosolized Sterne strain B. anthracis spores, as previously described, and monitored for survival for 10 days. All control unvaccinated mice died within 6 days of challenge (survival, 0/8; 0%); similarly, the majority of mice immunized with PA alone succumbed to challenge (survival, 2/8; 25%) (Fig. 1). Significantly improved survival over unimmunized mice or mice immunized with PA alone was seen in mice immunized with PA plus Advax adjuvant (survival, 7/8; 87.5%) (P = 0.001 or P = 0.04 versus unimmunized or PA-alone results, respectively) which was not significantly different from the survival results in the positive-control Alhydrogel group (survival, 8/8; 100%). The trend to increased survival in the murabutide group failed to reach significance (survival, 4/8; 50%) (P = 0.07 or P = 0.6, respectively, versus unimmunized or PA-alone results) (Fig. 1).
FIG 1.
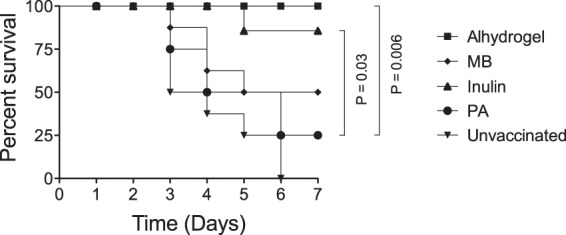
Efficacy of Advax- or murabutide-adjuvanted single-dose PA vaccine against B. anthracis aerosol challenge. Groups of mice (n = 8) were immunized once s.c. with a 50-ng dose of PA unadjuvanted or adjuvanted with Advax (1 mg), murabutide (MB) (0.1 mg), or Alhydrogel (0.035 mg) or were left unvaccinated. Mice were challenged 28 days postimmunization with 20 LD50 of aerosolized Sterne strain anthrax spores.
Boosting enhances protection by Advax- or murabutide-adjuvanted PA vaccine.
Typically, to maximize immunogenicity, anthrax vaccines are administered as priming doses with multiple boosts. To assess the ability of Advax or murabutide to maximize anti-PA IgG responses and anthrax protection across multiple immunizations, mice were immunized once, twice, or three times s.c. at 2-week intervals with PA alone or adjuvanted with Advax or murabutide. Animals immunized with a single dose of PA plus Alhydrogel served as positive controls. Mice were bled 28 days after the final immunization, and serum anti-PA IgG was measured by ELISA. Following a single immunization or two immunizations, mice immunized with PA adjuvanted with Advax or murabutide had lower anti-PA titers than mice immunized with PA with Alhydrogel, but the advantage of Alhydrogel was lost after the third immunization (Fig. 2A). There were no significant differences at each of the study time points in the anti-PA IgG titers induced by PA adjuvanted with either Advax or murabutide. Thirty days after their final vaccination, all mice were challenged with aerosolized B. anthracis. Notably, a single immunization with PA plus Advax gave significantly better protection (survival, 9/12 mice; 75%) than three doses of unadjuvanted PA alone (survival, 3/12; 25%) (P = 0.0016). Paralleling the progressive increase in anti-PA IgG titers seen with each PA vaccine boost with Advax (Fig. 2A), survival also improved with each vaccine boost: from survival of 9/12 mice (75%) after a single immunization of PA with Advax to survival of 10/12 mice (83%) after two immunizations and survival of 12/12 mice (100%) after three immunizations (Fig. 2B). Murabutide-adjuvanted PA provided only modest protection after a single immunization (survival, 5/12; 42%) which was not significantly different from the survival achieved with a single dose of unadjuvanted PA (survival, 3/12; 25%), but this improved after a second immunization (survival, 9/12; 75%) and reached 100% protection after the third immunization (survival, 12/12; 100%) (Fig. 2C), thereby comparing favorably to the significantly lower protection achieved with three doses of PA alone (survival, 3/12; 25%) (P = 0.007).
FIG 2.
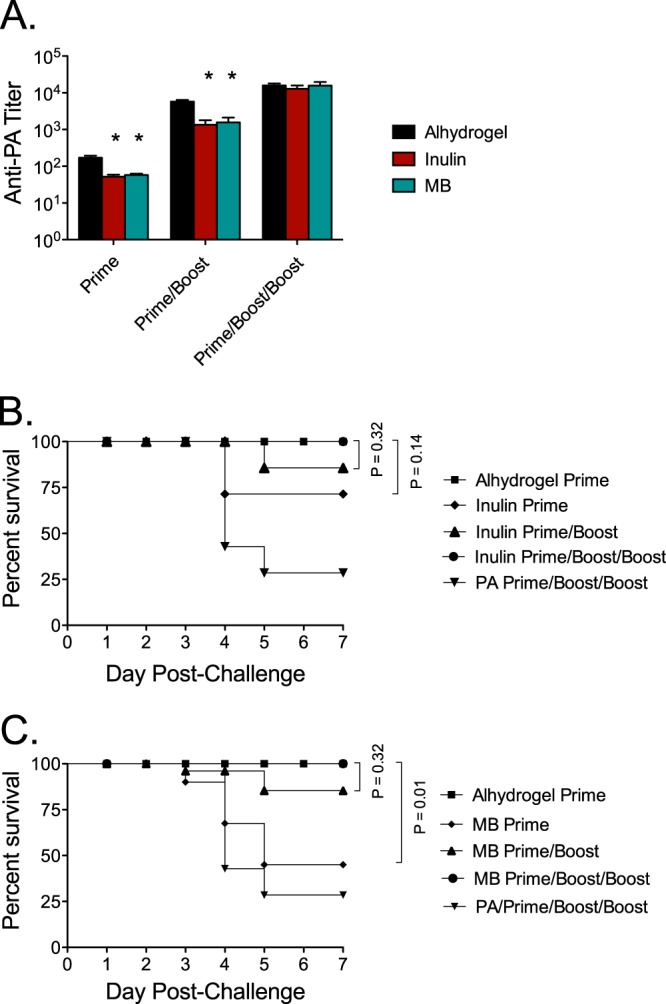
Booster vaccinations enhance anti-PA antibody response to and survival of challenge. (A) Groups of mice (n = 12) were immunized once, twice, or three times s.c. with 50 ng of PA unadjuvanted or adjuvanted with either Advax (1 mg), murabutide (MB) (0.1 mg), or Alhydrogel (0.035 mg). Mice were bled 28 days following their last immunization for measurement of anti-PA IgG titers by ELISA. Mice were then challenged with 20 LD50 of aerosolized Sterne strain anthrax spores. (B and C) Survival curves are shown for animals immunized with Advax versus Alhydrogel (B) or murabutide versus Alhydrogel (C). *, significantly different from Alhydrogel results (P ≤ 0.001).
Efficacy of adjuvant formulation of Advax and murabutide combined.
Since the initial studies demonstrated that both Advax and, to a lesser extent, murabutide individually improved anti-PA IgG responses and protection, we asked whether combining these two adjuvants might further enhance the anti-PA response and protection. Mice were immunized twice s.c. 2 weeks apart with 50 ng of PA adjuvanted with Alhydrogel, Advax, or murabutide or with an Advax plus murabutide combined formulation. Mice were bled 28 days following the final immunization, and serum was assayed for anti-PA IgG by ELISA. Consistent with the results presented in Fig. 1 and 2, after two immunizations, murabutide or Advax individually stimulated lower anti-PA IgG titers than Alhydrogel (Fig. 3A). However, when used in combination, after two PA immunizations, the Advax plus murabutide combined formulation induced anti-PA titers (129,100 ± 11,000) higher than the PA plus Alhydrogel immunization formulation (105,300 ± 7,900) (Fig. 3A). Following challenge, the mice immunized twice with the Advax plus murabutide combined formulation achieved complete protection (survival, 12/12; 100%), thereby matching the protection of mice immunized twice with PA plus Alhydrogel (survival, 12/12; 100%) (Fig. 3B). Mice immunized with two doses of PA with Advax alone also exhibited a high level of protection (survival, 10/12; 83%) (results not statistically significant [n.s.]), whereas mice immunized with two doses of PA with murabutide alone had significantly lower survival (survival, 5/12; 42%) (P = 0.009) than the Alhydrogel or Advax plus murabutide combined formulation groups (Fig. 3B).
FIG 3.
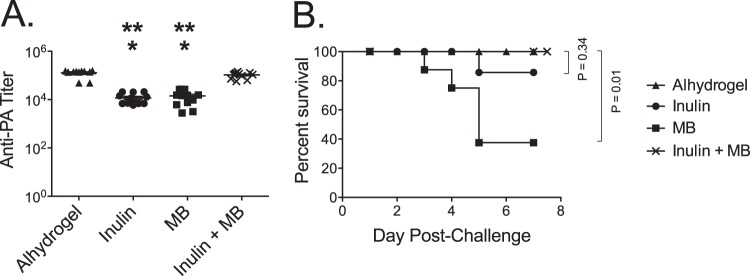
The combination of Advax and murabutide improves anti-PA antibody responses and protection. (A) Groups of mice (n = 12) were immunized twice s.c. with a 50-ng dose of PA adjuvanted with Alhydrogel (0.035 mg), Advax (1 mg), murabutide (MB) (0.1 mg), or Advax plus murabutide. Mice were bled 28 days postimmunization, and anti-PA IgG was measured by ELISA. Statistical analysis was done to compare these groups; a single asterisk (*) indicates a significant difference from Alhydrogel results (P ≤ 0.0001), and double asterisks (**) indicate a significant difference from the results obtained with Advax plus murabutide (P ≤ 0.0001). (B) Survival curves are shown for mice challenged with 20 LD50 of aerosolized Sterne strain anthrax spores.
Effects of adjuvants on long-term anti-PA antibody responses.
An important characteristic for anthrax vaccines is the ability to induce long-lasting protective immunity to anthrax exposure. To determine whether the combination of Advax plus murabutide was able to induce durable anti-PA responses, mice were immunized three times s.c. at 2-week intervals with 50 ng of PA adjuvanted with Alhydrogel or the Advax plus murabutide combined formulation. Serum samples were collected 11 months following the last immunization and assayed for anti-PA IgG by ELISA and the mice then challenged. Eleven months postimmunization, there was no statistically significant difference in anti-PA IgG titers (Fig. 4) between the Alhydrogel and the Advax plus murabutide combined formulation groups and all mice from both groups survived aerosol challenge (data not shown).
FIG 4.
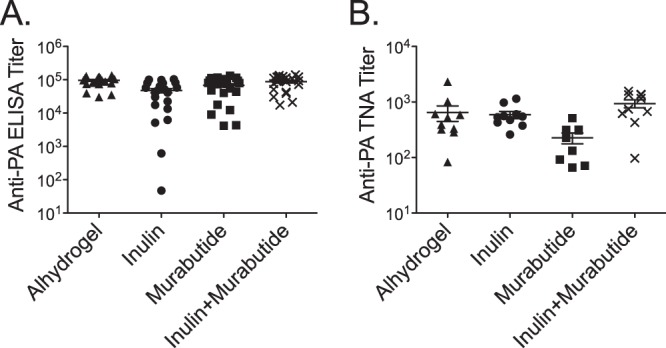
The combination of Advax and murabutide adjuvant induces a long-lasting anti-PA IgG response. (A) Groups of mice (n = 24) were immunized three times s.c. with 2 weeks between immunizations with a 50-ng dose of PA adjuvanted with either Alhydrogel (0.035 mg) or the combination of Advax (1 mg) plus murabutide (MB) (0.1 mg). (A) Mice were bled 11 months postimmunization for measurement of anti-PA IgG by ELISA. (B) Toxin neutralization antibody (TNA) titers.
Anamnestic antibody responses in immunized mice.
Mice that had been immunized 18 months earlier with three doses of PA adjuvanted with Alhydrogel or the Advax plus murabutide combined formulation were challenged with aerosolized anthrax, as described earlier. Mice were bled 2 and 5 days postchallenge, and anti-PA IgG was measured by ELISA. Anti-PA IgG titers were significantly (P = 0.02) higher 2 days postchallenge in mice immunized with PA adjuvanted with the Advax plus murabutide combined formulation (37,840 ± 4,510) than in mice immunized with PA plus Alhydrogel (15,740 ± 7,545) (Fig. 5). Anti-PA IgG titers were also higher in mice immunized with Advax plus murabutide 5 days postchallenge, although the difference was no longer statistically significant.
FIG 5.
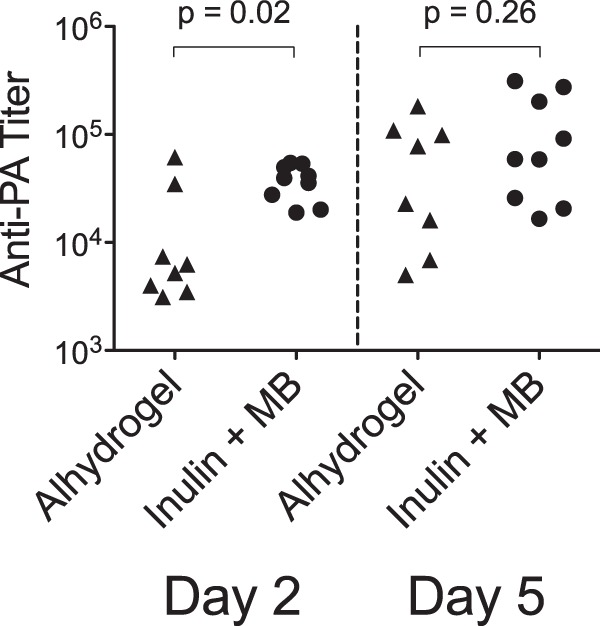
Comparison of anamnestic antibody responses to PA in immunized mice subjected to a late challenge. Groups of mice were immunized three times s.c. with 2 weeks between immunizations with a 50-ng dose of PA adjuvanted with Alhydrogel (0.035 mg) (n = 8) or with Advax (1 mg) plus murabutide (MB) (0.1 mg) (n = 9). Eighteen months postimmunization, mice were challenged with 20 LD50 of aerosolized Sterne strain anthrax spores and then bled at day 2 and day 5 postchallenge for measurement of anti-PA IgG by ELISA.
PA vaccine adjuvanted with the Advax plus murabutide combination adjuvant induces less injection site inflammation.
Advax (36) and murabutide (45) have both previously been individually shown to be nonpyrogenic and nonreactogenic, consistent with their adjuvant action being associated with little or no inflammation. To assess the level of inflammation induced by PA formulated with the Advax and murabutide combined formulation versus PA formulated with Alhydrogel, mice were immunized s.c. at the base of the tail with PA adjuvanted with either Alhydrogel or the Advax plus murabutide combined formulation. Two days postimmunization, mice were injected intravenously with Prosense 750 and then imaged. Prosense 750 is a compound that is optically silent in its unactivated state but becomes highly fluorescent when cleaved by cathepsins produced during inflammation, thereby providing an in vivo assay of vaccine-induced inflammation (56). Mice immunized with PA plus Alhydrogel (Fig. 6A) showed greater fluorescence around the injection site than mice immunized with PA plus Advax and murabutide (Fig. 6B). Quantified by densitometry, mice immunized with PA plus Alhydrogel had a significantly (∼3-fold) higher level of Prosense 750 activation (52.24 ± 2.78) (P = 0.001) than mice immunized with Advax plus murabutide (15.33 ± 5.84) (Fig. 6C), consistent with lower levels of inflammation induced by the Advax plus murabutide combination adjuvant.
FIG 6.
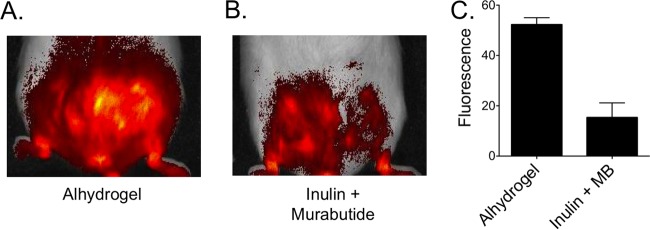
Comparison of inflammations induced by PA adjuvanted with Alhydrogel versus Advax and murabutide combination adjuvant. (A and B) Groups of mice (n = 4) were immunized s.c. with a single 50-ng dose of PA adjuvanted with Alhydrogel (0.035 mg) or Advax (1 mg) plus murabutide (MB) (0.1 mg). Two days postimmunization, mice were injected with Prosense 750 FAST and then imaged using an IVIS imaging system. Representative images are shown for mice that received PA with Alhydrogel (A) or Advax plus murabutide (B). (C) The mean total fluorescence intensities over the injection site were quantitated using ImageJ, demonstrating levels of Prosense 750 cleavage for mice receiving PA vaccine with Alhydrogel that were significantly higher than those seen with the mice receiving Advax plus murabutide (P = 0.001).
DISCUSSION
The development of an immunogenic, nonreactogenic anthrax vaccine capable of providing long-lasting protective immunity is highly desirable. As PA is the major protective antigen in the anthrax vaccine adsorbed (AVA, the only U.S.-licensed anthrax vaccine [57]), efforts to develop next-generation anthrax vaccines have been focused on strategies using purified PA (23) (24). In this murine study, we showed that the immunogenicity of PA was enhanced by formulation with Advax adjuvant and to a lesser extent with murabutide and that this translated into enhanced protection against a B. anthracis aerosol challenge compared to mice immunized with PA administered alone. After three immunizations, Advax or murabutide adjuvants were individually as effective at induction of anti-PA antibodies as the Alhydrogel adjuvant. The early advantage of Alhydrogel may potentially be explained by the fact that it provides a marked Th2 bias favoring early antibody production (58), whereas Advax (34) and murabutide (41) adjuvants both have a more balanced Th2 and Th1 action, resulting in enhanced cellular as well as antibody immunity. Although Advax, after a single dose or two doses, enhanced anti-PA IgG titers to the same extent as murabutide, it conferred significantly higher protection against anthrax challenge than murabutide, suggesting that higher anti-PA IgG titers alone do not fully explain the enhanced anthrax protection obtained with Advax. The highest dose tested in mouse aerosol challenge model was equivalent to approximately 20 LD50s. While the efficacy of these vaccinations against higher bacterial loads of anthrax is not known, these results are indicative of strong protection against inhalational anthrax. Adjuvants containing advax and murabutide, when formulated together, had an additive effect on anti-PA IgG titers and provided 100% survival to challenge after just two immunizations, thereby equaling the protection achieved with the Alhydrogel adjuvant. The duration of protection induced by both inactivated and recombinant anthrax vaccines is typically short, necessitating multiple frequent booster immunizations (57). In a rabbit immunization model, only 37.5% protection was seen 12 months after two immunizations with alum-adjuvated PA compared to 74.1% protection at 6 months, with a strong correlation being evident between anti-PA ELISA titers in individual animals at 6 months and their survival when challenged at 12 months (59). The Advax plus murabutide combination was effective in inducing long-lived B-cell memory, as demonstrated as late as 18 months postimmunization of mice by a more rapid and potent anamnestic anti-PA IgG response versus Alyhrogel-immunized mice 2 days post-anthrax challenge. This is crucial for optimal vaccine effectiveness, as the rapid kinetics of death from inhalational B. anthracis infection demands an equally fast memory immune response for protection in the event of a bioterrorist attack or other exposure to potentially lethal anthrax spores.
The additive adjuvant effects of the Advax and murabutide combination could be explained by the compounds having nonoverlapping actions, activating different aspects of the immune response. Murabutide activates the innate immune system via binding to nucleotide-binding oligomerization domain-containing protein 2 (NOD2), thereby activating monocytes and dendritic cells (DC), resulting in enhanced humoral and cellular immune responses (40, 45, 49). The mechanism of action for the Advax adjuvant has yet to be fully deciphered, but Advax particles have been shown to bind to dendritic cells and monocytes, leading to upregulation of antigen presentation and costimulatory molecules (P. Cooper and N. Petrovsky, international patent application no. WO 2006/024100 A1), leading to enhanced T- and B-cell responses to coadministered antigens (34). This additive effect of Advax and murabutide is notable, as not all adjuvant combinations result in enhanced immunogenicity and protection. For example, the addition of imiquimod (TLR7 agonist) or resiquimod (TLR8 agonist) to CpG oligonucleotide adjuvant (TLR9 agonist) did not enhance the adjuvant effect (60). As in this example, the two adjuvants in the combinations were using similar TLR pathways; utilization of adjuvants that activate different immune pathways may represent an important strategy for the development of combination adjuvants able to induce synergistic protection.
A desirable feature for all vaccines is safety and low reactogenicity. Advax and murabutide have been individually tested in multiple animal and human studies that have shown both adjuvants to be safe and with low reactogenicity and no pyrogenicity (36, 45). This is the first report of these adjuvants being used in combination, and it is notable that, despite additive effects on vaccine immunogenicity, this did not come at the expense of tolerability, as no major injection site reactions or obvious systemic illnesses were observed in groups injected with Advax plus murabutide. Through the use of the imaging agent Prosense 750, which fluoresces when cleaved by cathepsins produced by inflammatory cells (56), we confirmed the low inflammatory potential of vaccine formulated with Advax plus murabutide even compared to PA formulated with Alhydrogel, itself a well-tolerated vaccine adjuvant. As adverse reactions associated with inflammation are significant barriers to uptake of vaccines, the minimal inflammatory profile of the Advax plus murabutide combined formulation is an important asset. Studies are ongoing into the mechanism of interaction of these two adjuvants, but these data demonstrating their ability alone or particularly in combination to stimulate long-lasting protective humoral immunity to PA support their potential for anthrax vaccine development.
ACKNOWLEDGMENTS
This work was supported by internal FDA funds provided to support the research program of T.J.M. The funding agency had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The development of Advax adjuvant was supported by contracts U01AI061142 and HHSN272200800039C from the National Institutes of Health (NIH).
We all made substantial contributions to this work. B.F. contributed to the conception and design of the study, the acquisition of and analysis of data, and the drafting of the article. T.J.M. contributed to the conception and design of the study, the analysis and interpretation of data, and the revising of the article for important intellectual content. T.J.M. was responsible for final approval of the version to be submitted. N.P. contributed to the analysis and interpretation of data and revision of the article for important intellectual content. A.V. contributed to the acquisition of data and revision of the article for important intellectual content. We all approved the final article.
Footnotes
Published ahead of print 19 February 2014
REFERENCES
- 1.Mock M, Fouet A. 2001. Anthrax. Annu. Rev. Microbiol. 55:647–671. 10.1146/annurev.micro.55.1.647 [DOI] [PubMed] [Google Scholar]
- 2.Barnes JM. 1947. The development of anthrax following the administration of spores by inhalation. Br. J. Exp. Pathol. 28:385–394 [Google Scholar]
- 3.Meselson M, Guillemin J, Hugh-Jones M, Langmuir A, Popova I, Shelokov A, Yampolskaya O. 1994. The Sverdlovsk anthrax outbreak of 1979. Science 266:1202–1208. 10.1126/science.7973702 [DOI] [PubMed] [Google Scholar]
- 4.Greene CM, Reefhuis J, Tan C, Fiore AE, Goldstein S, Beach MJ, Redd SC, Valiante D, Burr G, Buehler J, Pinner RW, Bresnitz E, Bell BP. 2002. Epidemiologic investigations of bioterrorism-related anthrax, New Jersey, 2001. Emerg. Infect. Dis. 8:1048–1055. 10.3201/eid0810.020329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhalla DK, Warheit DB. 2004. Biological agents with potential for misuse: a historical perspective and defensive measures. Toxicol. Appl. Pharmacol. 199:71–84. 10.1016/j.taap.2004.03.009 [DOI] [PubMed] [Google Scholar]
- 6.Brittingham KC, Ruthel G, Panchal RG, Fuller CL, Ribot WJ, Hoover TA, Young HA, Anderson AO, Bavari S. 2005. Dendritic cells endocytose Bacillus anthracis spores: implications for anthrax pathogenesis. J. Immunol. 174:5545–5552 [DOI] [PubMed] [Google Scholar]
- 7.Cleret A, Quesnel-Hellmann A, Vallon-Eberhard A, Verrier B, Jung S, Vidal D, Mathieu J, Tournier JN. 2007. Lung dendritic cells rapidly mediate anthrax spore entry through the pulmonary route. J. Immunol. 178:7994–8001 [DOI] [PubMed] [Google Scholar]
- 8.Guidi-Rontani C, Levy M, Ohayon H, Mock M. 2001. Fate of germinated Bacillus anthracis spores in primary murine macrophages. Mol. Microbiol. 42:931–938. 10.1046/j.1365-2958.2001.02695.x [DOI] [PubMed] [Google Scholar]
- 9.Ross JM. 1957. The pathogenesis of anthrax following the administration of spores by the respiratory route. J. Pathol. Bacteriol. 73:485–494. 10.1002/path.1700730219 [DOI] [Google Scholar]
- 10.Dixon TC, Fadl AA, Koehler TM, Swanson JA, Hanna PC. 2000. Early Bacillus anthracis-macrophage interactions: intracellular survival survival and escape. Cell. Microbiol. 2:453–463. 10.1046/j.1462-5822.2000.00067.x [DOI] [PubMed] [Google Scholar]
- 11.Hu H, Sa Q, Koehler TM, Aronson AI, Zhou D. 2006. Inactivation of Bacillus anthracis spores in murine primary macrophages. Cell. Microbiol. 8:1634–1642. 10.1111/j.1462-5822.2006.00738.x [DOI] [PubMed] [Google Scholar]
- 12.Kang TJ, Fenton MJ, Weiner MA, Hibbs S, Basu S, Baillie L, Cross AS. 2005. Murine macrophages kill the vegetative form of Bacillus anthracis. Infect. Immun. 73:7495–7501. 10.1128/IAI.73.11.7495-7501.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon TC, Meselson M, Guillemin J, Hanna PC. 1999. Anthrax. N. Engl. J. Med. 341:815–826. 10.1056/NEJM199909093411107 [DOI] [PubMed] [Google Scholar]
- 14.Young JA, Collier RJ. 2007. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 76:243–265. 10.1146/annurev.biochem.75.103004.142728 [DOI] [PubMed] [Google Scholar]
- 15.Duesbery NS, Webb CP, Leppla SH, Gordon VM, Klimpel KR, Copeland TD, Ahn NG, Oskarsson MK, Fukasawa K, Paull KD, Vande Woude GF. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734–737. 10.1126/science.280.5364.734 [DOI] [PubMed] [Google Scholar]
- 16.Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C. 1998. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem. Biophys. Res. Commun. 248:706–711. 10.1006/bbrc.1998.9040 [DOI] [PubMed] [Google Scholar]
- 17.Hoover DL, Friedlander AM, Rogers LC, Yoon IK, Warren RL, Cross AS. 1994. Anthrax edema toxin differentially regulates lipopolysaccharide-induced monocyte production of tumor necrosis factor alpha and interleukin-6 by increasing intracellular cyclic AMP. Infect. Immun. 62:4432–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leppla SH. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. U. S. A. 79:3162–3166. 10.1073/pnas.79.10.3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tournier JN, Quesnel-Hellmann A, Mathieu J, Montecucco C, Tang WJ, Mock M, Vidal DR, Goossens PL. 2005. Anthrax edema toxin cooperates with lethal toxin to impair cytokine secretion during infection of dendritic cells. J. Immunol. 174:4934–4941 [DOI] [PubMed] [Google Scholar]
- 20.Iacono-Connors LC, Welkos SL, Ivins BE, Dalrymple JM. 1991. Protection against anthrax with recombinant virus-expressed protective antigen in experimental animals. Infect. Immun. 59:1961–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chichester JA, Manceva SD, Rhee A, Coffin MV, Musiychuk K, Mett V, Shamloul M, Norikane J, Streatfield SJ, Yusibov V. 16 January 2013. A plant-produced protective antigen vaccine confers protection in rabbits against a lethal aerosolized challenge with Bacillus anthracis Ames spores. Hum. Vaccin. Immunother. 10.4161/hv.23233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watkinson A, Soliakov A, Ganesan A, Hirst K, Lebutt C, Fleetwood K, Fusco PC, Fuerst TR, Lakey JH. 2013. Increasing the potency of an alhydrogel-formulated anthrax vaccine by minimizing antigen-adjuvant interactions. Clin. Vaccine Immunol. 20:1659–1668. 10.1128/CVI.00320-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorse GJ, Keitel W, Keyserling H, Taylor DN, Lock M, Alves K, Kenner J, Deans L, Gurwith M. 2006. Immunogenicity and tolerance of ascending doses of a recombinant protective antigen (rPA102) anthrax vaccine: a randomized, double-blinded, controlled, multicenter trial. Vaccine 24:5950–5959. 10.1016/j.vaccine.2006.05.044 [DOI] [PubMed] [Google Scholar]
- 24.Campbell JD, Clement KH, Wasserman SS, Donegan S, Chrisley L, Kotloff KL. 2007. Safety, reactogenicity and immunogenicity of a recombinant protective antigen anthrax vaccine given to healthy adults. Hum. Vaccin. 3:205–211. 10.4161/hv.3.5.4459 [DOI] [PubMed] [Google Scholar]
- 25.Fowler K, McBride BW, Turnbull PC, Baillie LW. 1999. Immune correlates of protection against anthrax. J. Appl. Microbiol. 87:305. 10.1046/j.1365-2672.1999.00898.x [DOI] [PubMed] [Google Scholar]
- 26.Boyaka PN, Tafaro A, Fischer R, Leppla SH, Fujihashi K, McGhee JR. 2003. Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J. Immunol. 170:5636–5643 [DOI] [PubMed] [Google Scholar]
- 27.Sloat BR, Cui Z. 2006. Nasal immunization with anthrax protective antigen protein adjuvanted with polyriboinosinic-polyribocytidylic acid induced strong mucosal and systemic immunities. Pharm. Res. 23:1217–1226. 10.1007/s11095-006-0206-9 [DOI] [PubMed] [Google Scholar]
- 28.Petrovsky N, Aguilar JC. 2004. Vaccine adjuvants: current state and future trends. Immunol. Cell Biol. 82:488–496. 10.1111/j.0818-9641.2004.01272.x [DOI] [PubMed] [Google Scholar]
- 29.Petrovsky N, Cooper PD. 2011. Carbohydrate-based immune adjuvants. Expert Rev. Vaccines 10:523–537. 10.1586/erv.11.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper PD, Petrovsky N. 2011. Delta inulin: a novel, immunologically active, stable packing structure comprising beta-D-[2 -> 1] poly(fructo-furanosyl) alpha-D-glucose polymers. Glycobiology 21:595–606. 10.1093/glycob/cwq201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper PD, Barclay TG, Ginic-Markovic M, Petrovsky N. 2013. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology 23:1164–1174. 10.1093/glycob/cwt053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lobigs M, Pavy M, Hall RA, Lobigs P, Cooper P, Komiya T, Toriniwa H, Petrovsky N. 2010. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J. Gen. Virol. 91:1407–1417. 10.1099/vir.0.019190-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrovsky N, Larena M, Siddharthan V, Prow NA, Hall RA, Lobigs M, Morrey J. 2013. An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody. J. Virol. 87:10324–10333. 10.1128/JVI.00480-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honda-Okubo Y, Saade F, Petrovsky N. 2012. Advax, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine 30:5373–5381. 10.1016/j.vaccine.2012.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cristillo AD, Ferrari MG, Hudacik L, Lewis B, Galmin L, Bowen B, Thompson D, Petrovsky N, Markham P, Pal R. 2011. Induction of mucosal and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations. J. Gen. Virol. 92:128–140. 10.1099/vir.0.023242-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saade F, Honda-Okubo Y, Trec S, Petrovsky N. 2013. A novel hepatitis B vaccine containing Advax, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine 31:1999–2007. 10.1016/j.vaccine.2012.12.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon DL, Sajkov D, Woodman RJ, Honda-Okubo Y, Cox MM, Heinzel S, Petrovsky N. 2012. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine 30:5407–5416. 10.1016/j.vaccine.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philpott DJ, Girardin SE. 2004. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol. Immunol. 41:1099–1108. 10.1016/j.molimm.2004.06.012 [DOI] [PubMed] [Google Scholar]
- 39.Strominger JL. 2007. Bacterial cell walls, innate immunity and immunoadjuvants. Nat. Immunol. 8:1269–1271. 10.1038/ni1207-1269 [DOI] [PubMed] [Google Scholar]
- 40.Vidal VF, Casteran N, Riendeau CJ, Kornfeld H, Darcissac EC, Capron A, Bahr GM. 2001. Macrophage stimulation with Murabutide, an HIV-suppressive muramyl peptide derivative, selectively activates extracellular signal-regulated kinases 1 and 2, C/EBPbeta and STAT1: role of CD14 and Toll-like receptors 2 and 4. Eur. J. Immunol. 31:1962–1971. [DOI] [PubMed] [Google Scholar]
- 41.Cho HJ, Kim JY, Lee Y, Kim JM, Kim YB, Chun T, Oh YK. 2010. Enhanced humoral and cellular immune responses after sublingual immunization against human papillomavirus 16 L1 protein with adjuvants. Vaccine 28:2598–2606. 10.1016/j.vaccine.2010.01.013 [DOI] [PubMed] [Google Scholar]
- 42.Darcissac EC, Bahr GM, Pouillart PR, Riveau GJ, Parant MA. 1996. Selective potentiation of cytokine expression in human whole blood by murabutide, a muramyl dipeptide analogue. Cytokine 8:658–666. 10.1006/cyto.1996.0088 [DOI] [PubMed] [Google Scholar]
- 43.Darcissac EC, Truong MJ, Dewulf J, Mouton Y, Capron A, Bahr GM. 2000. The synthetic immunomodulator murabutide controls human immunodeficiency virus type 1 replication at multiple levels in macrophages and dendritic cells. J. Virol. 74:7794–7802. 10.1128/JVI.74.17.7794-7802.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geddes K, Magalhaes JG, Girardin SE. 2009. Unleashing the therapeutic potential of NOD-like receptors. Nat. Rev. Drug Discov. 8:465–479. 10.1038/nrd2783 [DOI] [PubMed] [Google Scholar]
- 45.Telzak E, Wolff SM, Dinarello CA, Conlon T, el Kholy A, Bahr GM, Choay JP, Morin A, Chedid L. 1986. Clinical evaluation of the immunoadjuvant murabutide, a derivative of MDP, administered with a tetanus toxoid vaccine. J. Infect. Dis. 153:628–633. 10.1093/infdis/153.3.628 [DOI] [PubMed] [Google Scholar]
- 46.Tada H, Aiba S, Shibata K, Ohteki T, Takada H. 2005. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect. Immun. 73:7967–7976. 10.1128/IAI.73.12.7967-7976.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Traub S, von Aulock S, Hartung T, Hermann C. 2006. MDP and other muropeptides–direct and synergistic effects on the immune system. J. Endotoxin Res. 12:69–85. 10.1179/096805106X89044 [DOI] [PubMed] [Google Scholar]
- 48.Viala J, Sansonetti P, Philpott DJ. 2004. Nods and ‘intracellular' innate immunity. C. R. Biol. 327:551–555. 10.1016/j.crvi.2004.02.010 [DOI] [PubMed] [Google Scholar]
- 49.Vidal V, Dewulf J, Bahr GM. 2001. Enhanced maturation and functional capacity of monocyte-derived immature dendritic cells by the synthetic immunomodulator murabutide. Immunology 103:479–487. 10.1046/j.1365-2567.2001.01269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amiel C, De La Tribonniere X, Vidal V, Darcissac E, Mouton Y, Bahr GM. 2002. Clinical tolerance and immunologic effects after single or repeated administrations of the synthetic immunomodulator murabutide in HIV-1-infected patients. J. Acquir. Immune Defic. Syndr. 30:294–305. 10.1097/00126334-200207010-00005 [DOI] [PubMed] [Google Scholar]
- 51.Jackson EM, Herbst-Kralovetz MM. 2012. Intranasal vaccination with murabutide enhances humoral and mucosal immune responses to a virus-like particle vaccine. PLoS One 7:e41529. 10.1371/journal.pone.0041529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ali R, Kumar S, Naqvi RA, Sheikh IA, Rao DN. 2013. Multiple antigen peptide consisting of B- and T-cell epitopes of F1 antigen of Y. pestis showed enhanced humoral and mucosal immune response in different strains of mice. Int. Immunopharmacol. 15:97–105. 10.1016/j.intimp.2012.10.029 [DOI] [PubMed] [Google Scholar]
- 53.Bahr GM, Darcissac E, Bevec D, Dukor P, Chedid L. 1995. Immunopharmacological activities and clinical development of muramyl peptides with particular emphasis on murabutide. Int. J. Immunopharmacol. 17:117–131. 10.1016/0192-0561(94)00094-5 [DOI] [PubMed] [Google Scholar]
- 54.Loving CL, Khurana T, Osorio M, Lee GM, Kelly VK, Stibitz S, Merkel TJ. 2009. Role of anthrax toxins in dissemination, disease progression, and induction of protective adaptive immunity in the mouse aerosol challenge model. Infect. Immun. 77:255–265. 10.1128/IAI.00633-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loving CL, Kennett M, Lee GM, Grippe VK, Merkel TJ. 2007. Murine aerosol challenge model of anthrax. Infect. Immun. 75:2689–2698. 10.1128/IAI.01875-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr 1999. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 17:375–378. 10.1038/7933 [DOI] [PubMed] [Google Scholar]
- 57.Wright JG, Quinn CP, Shadomy S, Messonnier N. 2010. Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm. Rep. 59(rr06):1–30 [PubMed] [Google Scholar]
- 58.Grun JL, Maurer PH. 1989. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell. Immunol. 121:134–145. 10.1016/0008-8749(89)90011-7 [DOI] [PubMed] [Google Scholar]
- 59.Little SF, Ivins BE, Webster WM, Fellows PF, Pitt ML, Norris SL, Andrews GP. 2006. Duration of protection of rabbits after vaccination with Bacillus anthracis recombinant protective antigen vaccine. Vaccine 24:2530–2536. 10.1016/j.vaccine.2005.12.028 [DOI] [PubMed] [Google Scholar]
- 60.Weeratna RD, Makinen SR, McCluskie MJ, Davis HL. 2005. TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848). Vaccine 23:5263–5270. 10.1016/j.vaccine.2005.06.024 [DOI] [PubMed] [Google Scholar]


