Abstract
Human papillomavirus (HPV) is the etiological agent for all cervical cancers, a significant number of other anogenital cancers, and a growing number of head and neck cancers. Two licensed vaccines offer protection against the most prevalent oncogenic types, 16 and 18, responsible for approximately 70% of cervical cancer cases worldwide and one of these also offers protection against types 6 and 11, responsible for 90% of genital warts. The vaccines are comprised of recombinantly expressed major capsid proteins that self-assemble into virus-like particles (VLPs) and prevent infection by eliciting neutralizing antibodies. Adding the other frequently identified oncogenic types 31, 33, 45, 52, and 58 to a vaccine would increase the coverage against HPV-induced cancers to approximately 90%. We describe the generation and characterization of panels of monoclonal antibodies to these five additional oncogenic HPV types, and the selection of antibody pairs that were high affinity and type specific and recognized conformation-dependent neutralizing epitopes. Such characteristics make these antibodies useful tools for monitoring the production and potency of a prototype vaccine as well as monitoring vaccine-induced immune responses in the clinic.
INTRODUCTION
The two currently licensed human papillomavirus (HPV) vaccines have played an important role in reducing HPV disease since their introduction in 2006 and 2007 (1). The vaccines protect against infection by eliciting neutralizing antibodies to the L1 capsid protein. This major capsid protein self-assembles into virus-like particles (VLPs) that mimic the structure of the native virion (2, 3). The two currently licensed vaccines offer protection against HPV types 16 and 18, which account for 70% of cervical cancer cases (4); the quadrivalent vaccine also offers protection against HPV types 6 and 11, which account for 90% of genital warts (5). A second-generation HPV vaccine was developed by Merck to provide extended coverage for the next five most prevalent oncogenic HPV types: 31, 33, 45, 52, and 58. Inclusion of VLPs to the additional five oncogenic HPV types will potentially increase the coverage to approximately 90% (4).
Monitoring HPV type-specific neutralizing epitopes is an essential aspect of the development of this vaccine. A meaningful and rapid evaluation of serological responses facilitates decisions regarding the optimum vaccine and assists the collection of data for regulatory approval. Potency assays conducted in vivo (typically mouse potency assays) and functional in vitro assays such as pseudovirus and plaque assays can be laborious and difficult to standardize. Assays that utilize antibody binding and competition can act as valuable surrogates and can be readily qualified and adapted for high-throughput and automated platforms (6, 7). A critical requirement is that the antibodies that are chosen for the assays reflect the biological properties of the original potency and neutralization assays (8).
Neutralizing antibodies to HPV L1 VLPs are primarily type specific (9) and bind to surface-exposed conformational loops (10). Despite high degrees of L1 sequence homology among members of the same family, neutralizing antibodies to cross-reactive epitopes have rarely been identified (11). Panels of monoclonal antibodies (MAbs) have been generated and characterized for HPV types 6, 11, 16, and 18; they are currently used to monitor production processing and to measure vaccine-induced clinical responses (12–14). Relatively few MAbs exist for the rarer types. This report describes the characterization and selection of MAbs to these less common oncogenic types 31, 33, 45, 52, and 58.
MATERIALS AND METHODS
Generation of monoclonal antibodies.
Mouse hybridomas were developed following traditional methods as previously described (12). Briefly, BALB/c mice (Taconic, Germantown, NY) received two intraperitoneal injections of 20 μg of highly purified HPV 31, 33, 45, 52, or 58 VLPs adsorbed on Merck aluminum adjuvant. A final boost of 20 μg VLP was administered intravenously 3 days prior to fusion. A separate fusion was performed for each VLP type. Spleens were removed from sacrificed mice, and lymphocytes were fused with mouse myeloma partner SP2/0-Ag14 (ATCC 1581) by polyethylene glycol 1500 (Roche) at a ratio of 3:1. Fused hybridomas were isolated through hypoxanthine-aminopterin-thymidine medium (Sigma, Atlanta, GA) selection, and supernatants were screened by a direct enzyme-linked immunosorbent assay (ELISA) for reactivity. Positive wells were cloned by limiting dilution, grown in ascites or cell culture, purified on protein A, and quantified with isotype-specific ELISAs.
Screening ELISA for HPV type-specific and conformation dependent binding. (i) OD450 and ELISA titrations.
As a first screen, MAbs were tested at several dilutions from 10 μg/ml to 0.4 μg/ml as either purified MAbs or tissue culture supernatants. Immulon 4B microtiter plates were coated with VLP antigens of different HPV types under intact or disrupted conditions. Intact VLPs for types 6, 11, 16, 18, 31, 33, 45, 52, and 58 were solubilized in 50 mM histidine, 0.5 M NaCl, pH 6.2, and incubated overnight at 2 to 8°C. VLPs were disrupted by incubation in 0.2 M sodium carbonate buffer, pH 10.6, with 10 mM dithiothreitol (DTT) and dried on the plates overnight at 37°C. The diluted antibodies were incubated on blocked plates for 2 h at room temperature. The plates were washed, and horseradish peroxidase-conjugated goat anti-mouse IgG (heavy plus light chain [H+L]) (Invitrogen) (1:10,000 in assay diluent) was added and incubated for 1 h at room temperature. Plates were washed and developed with TMB (tetramethyl benzidine; Pierce, Rockland, IL). The reaction was stopped with 2.0 N H2SO4, and the optical density at 450 nm (OD450) was read. Background wells contained only the conjugate and no MAb.
Type-specific MAbs were identified as antibodies that reacted to only one VLP type. The following antibodies developed by Neil Christensen (Penn State University) were used to confirm specific reactivity: H16.V5, H18.J4, H6.M48, H31.A6, H33.B6, and H45.N5. K11.B2, developed at Merck Research Laboratories, was used as a control for type 11. H16.J4 (from Neil Christensen) was used as a positive control for types 52 and 58. The data for the heat maps were generated at a MAb concentration of 0.1 μg/ml. HPV VLP conformation-recognizing antibodies were identified as those with a signal to intact antigen and not to disrupted antigen.
(ii) HPV pseudovirion-based neutralization assay.
Neutralization experiments were performed by some of us at Deutsches Krebsforschungszentrum (DKFZ) using a well-characterized assay (15). Prior to receipt at DKFZ, samples were dialyzed against phosphate-buffered saline (PBS) to remove sodium azide, concentrations were confirmed by absorbance at 280 nm, and vials were recoded to blind the experimenters to the expected type specificity of the MAbs. Two neutralization experiments were performed. The first experiment determined the specificity of the MAbs against a panel of pseudovirions. All MAbs were diluted to a nominal concentration of 0.56 mg/ml. In the first assay, each MAb was further diluted 1:300 and 1:1,500. These two dilutions were tested for neutralization activity against a panel of pseudovirions (HPV types 31, 33, 45, 18, 52, 58, 35, 59, and 16). A positive control MAb, K18L220–38 (reactive to HPV L2), was included on each plate.
The second experiment was a full titration of each MAb against its reactive HPV type using a nonreactive HPV type as a control for each assay. Each MAb was titrated in duplicate over 8 points with 3-fold dilutions at a starting concentration of either 2,800 ng/ml, 280 ng/ml, or 28 ng/ml. A 50% effective concentration (EC50) was determined for each MAb using Prism 5.04 (GraphPad Software).
Dilutions of monoclonal antibodies were prepared in supplemented Dulbecco's modified Eagle medium (DMEM), and 50 μl of the dilutions was added to each well of a 96-well plate (in triplicate). The outside wells of the plate were excluded and filled with 150 μl medium to avoid potential plate effects. Next, 50 μl of the pseudovirus (16) (diluted in DMEM) was added to each sample well. The antibody-pseudovirus mixture was incubated for 20 min at room temperature before the addition of 50 μl HeLaT K4 cells (15) to each well (2.5 × 105 cells/ml). Plates were incubated for 48 h at 37°C (5% CO2). The amount of secreted Gaussia luciferase was determined in 10 μl of cell culture medium using the coelenterazine substrate and Gaussia glow juice (PJK, Germany) according to the manufacturer's instructions. A microplate luminometer (Victor3; PerkinElmer) was used to measure culture medium-associated luminescence 15 min after substrate addition.
(iii) Sequence alignment.
Sequences were translated from nucleotides to amino acids, if necessary. Amino acid sequences were multiply aligned using the default parameters in ClustalX 2.1 or VectorNTI Advance 11.5.1 AlignX. Sequence homologies were calculated from the resultant alignments.
RESULTS
Panels of MAbs were successfully prepared against VLPs of each HPV type: 31, 33, 45, 52, and 58. The MAbs were generated and developed from mouse hybridomas according to standard techniques, as detailed in Materials and Methods. We took additional steps during screening to confirm clonality and determine the antibody isotypes for each at an early stage. Once MAb clones were identified, the MAbs were characterized for four quality criteria. First, MAbs were checked for specificity to bind only to the cognate HPV VLP type against which they were generated. Second, the specificities of MAbs to intact or disrupted VLPs were determined. Third, the EC50 for binding of each MAb to its VLP (or cross-reactive VLP) was measured. Fourth, the type-specific neutralization pattern of each MAb was determined, and the neutralization EC50s were quantified. Ultimately, MAbs that best satisfied these criteria were selected for further development and sequenced to determine variable heavy and light regions.
Type-specific MAb binding to L1 VLPs.
To understand the binding specificity of each MAb, we performed a series of ELISAs. Each set of MAbs raised against a specific HPV VLP type was tested against that same VLP type and other VLP types found in currently marketed HPV vaccines and vaccine candidates. These included the oncogenic types HPV16 and HPV18 (included in Gardasil and Cervarix), HPV31, HPV33, HPV45, HPV52, HPV58, and two types implicated in anogenital warts, HPV6 and HPV11 (included in Gardasil). For each type, we tested intact VLPs and disrupted VLPs to determine if the MAbs could discriminate between the two. This is an important criterion for product release and clinical assays, as intact VLPs have been shown to and are expected to be stable and closely model the HPV virions (17, 18).
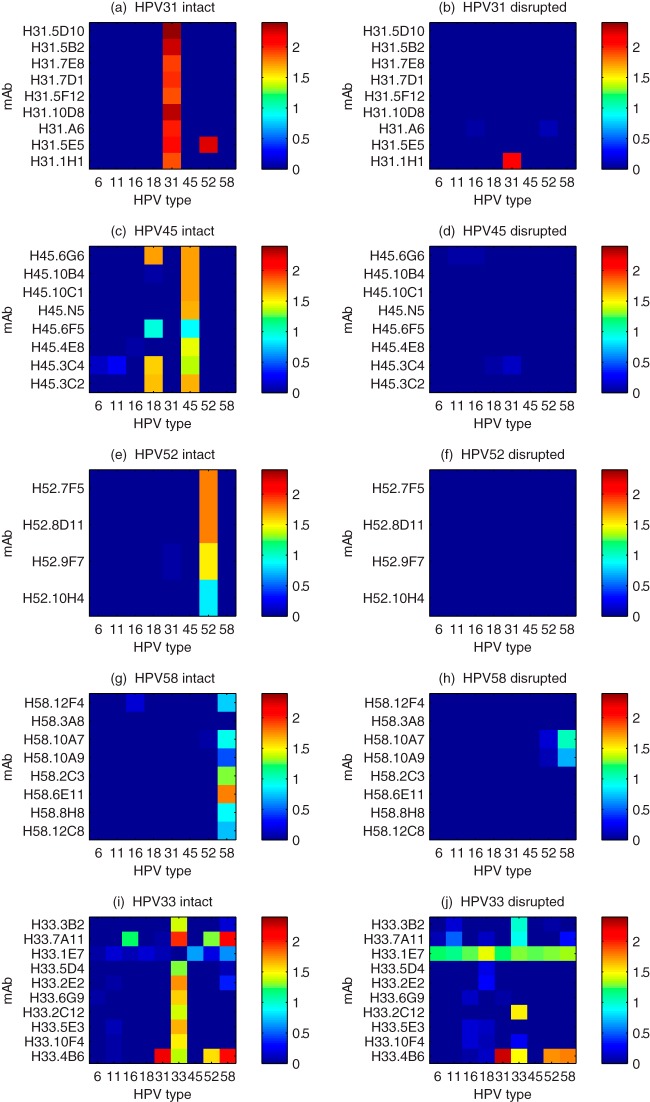
In Fig. 1, the ELISA binding of MAbs to VLPs is plotted as heatmaps, where OD450 values are represented in a false-color scale. VLPs from different HPV types were coated onto microtiter plates either as intact or disrupted particles. Intact VLPs were evaluated by electron microscopy and biophysical analysis to ensure no or low frequencies of partially formed VLPs. We review each of the HPV VLP types in turn. Binding of MAbs to HPV31 VLPs is shown in Fig. 1a and b. MAb H31.1H1 binds to both disrupted (Fig. 1b) and intact particles (Fig. 1a) and therefore recognizes a linear epitope. MAb H31.5E5 binds to a conformational epitope on both HPV31 and HPV52 intact VLPs. The remaining HPV31 MAbs are strictly type specific and recognize conformational epitopes. From this set, MAb H31.5F12 (IgG1) and MAb H31.5D10 (IgG2b) were chosen for further evaluation. These MAbs recognize only intact particles and are type specific.
FIG 1.
ELISA reactivity of MAbs generated against specific HPV types. HPV VLPs from five types were used to coat microtiter plates as either intact VLPs (left panels) or disrupted VLPs (right panels). (a) Intact HPV31 VLPs, (b) disrupted HPV31 VLPs, (c) intact HPV45 VLPs, (d) disrupted HPV45 VLPs, (e) intact HPV52 VLPs, (f) disrupted HPV52 VLPs, (g) intact HPV58 VLPs, (h) disrupted HPV58 VLPs, (i) intact HPV33 VLPs, and (j) disrupted HPV33 VLPs. In the heatmaps, the OD450s for binding of individual MAbs (y axis) versus HPV VLP types (x axis) are displayed on a false-color scale. Vertical bars of color indicate that many MAbs are type specific; horizontal bars indicate MAbs that recognize conserved epitopes on multiple tested VLPs.
Binding of MAbs to HPV45 VLPs is shown in Fig. 1c and d. All of the anti-HPV45 MAbs recognize conformation-dependent epitopes, as shown by the binding to intact HPV45 VLPs (Fig. 1c) and lack of binding to disrupted VLPs (Fig. 1d). Four of the MAbs also recognize intact HPV18 VLPs, a type closely related to HPV45. HPV45 and HPV18 are in the same α7 phylogenetic group and have a high amino acid sequence homology (88%). It is therefore not surprising that four of the eight MAbs recognize HPV18 as well as HPV45. All of the MAbs recognize only intact HPV45 VLPs and are posited to map to conformational epitopes. MAbs H45.6G6 (IgG2b)and H45.10B4 (IgG1) were chosen for subsequent study. MAb H45.6G6 is cross-reactive with HPV18; the implications of this for assays are discussed below.
In Fig. 1e and f, the binding of four HPV52 MAbs to HPV52 VLPs ELISA is graphed. Despite there being only four MAbs, they are all type specific and conformational. We chose H52.8D11 (IgG2b) and H52.9F7 (IgG2a) for further testing. All but one MAb (H58.3A8) generated against HPV58 recognize intact VLPs, as shown in Fig. 1g and h. MAb H58.12F4 slightly recognize intact VLPs, and two different MAbs (H58.10A7 and H58.10A9) recognize disrupted HPV58 and, to a lesser extent, HPV52. H58.2C3 (IgG1) and H58.6E11 (IgG2b) were selected for assay development.
Hybridomas were subsequently generated and screened against HPV33 VLPs at a later phase but following the same methods as for the previous types. All but one MAb (H33.1E7) recognize intact VLPs. H33.1E7 recognizes a well-conserved continuous epitope on all 9 HPV types (Fig. 1j). H33.3B2, H33.7A11, H33.2C12, and H33.4B6 recognize both continuous and conformational epitopes of HPV31 VLPs. H33.4B6 also recognizes continuous and conformational epitopes on VLPs from HPV31, HPV52, and HPV58. While all but one MAb bound to intact HPV33 VLPs, a few MAbs exhibited binding to other types. H33.7A11 recognized types 16, 52, and 58. H33.4B6 recognized types 31, 52, and 58 and had higher binding to 31 and 58 than to its cognate type, HPV33. Interestingly, MAb H33.1E7 recognized all nine VLPs when disrupted (Fig. 1j) and had little or no binding to any of the intact VLPs (Fig. 1i), including HPV33. From these data, we hypothesize that MAb H33.1E7 recognizes a continuous epitope that is normally buried or occluded in intact VLPs. We chose MAb H33.5D4 (IgG1) and MAb H33.6G9 (IgG2b) for subsequent characterization. Both of these MAbs recognize conformational epitopes and are type specific. Finally, the MAb pairs (two for each HPV type) selected from prior hybridomas (Fig. 1) were tested for cross-reactivity to HPV33 and shown to be negative. The selected MAb pairs are listed in Table 1.
TABLE 1.
Characteristics of anti-HPV type-specific monoclonal antibodies
| MAb | HPV type | Isotype | Function in assaysa |
|---|---|---|---|
| H31.5F12 | 31 | IgG1, κ | Capture |
| H31.5D10 | 31 | IgG2b, κ | Detection |
| H33.5D4 | 33 | IgG1, κ | Capture |
| H33.6G9 | 33 | IgG2b, κ | Detection |
| H45.6G6 | 45 | IgG2b, κ | Capture |
| H45.10B4 | 45 | IgG1, κ | Detection |
| H52.8D11 | 52 | IgG2b, κ | Capture |
| H52.9F7 | 52 | IgG2a, κ | Detection |
| H58.2C3 | 58 | IgG1, κ | Capture |
| H58.6E11 | 58 | IgG2b, κ | Detection |
Antibodies are used either to capture virus-like particles on the ELISA plate or to detect the bound VLP on the plate or bead.
Affinity of MAbs to HPV VLPs.
To quantify the binding affinities of the MAbs to the specific HPV VLPs, we conducted an extensive series of ELISAs, detailed in Materials and Methods. Briefly, HPV VLPs were used to coat plates, and MAbs were run in dilution series and detected with an anti-mouse secondary antibody. Data were analyzed by the typical four-parameter logistic equation. All of the VLP-MAb ELISA data are plotted in Fig. S1 in the supplemental material, with overlays of fits to the analytical function. The EC50 binding parameters determined from fits are listed in Table 2. All type-specific MAb EC50s were excellent, ranging from 1.7 to 4.9 ng/ml. These are more than sufficient for a robust and high-quality ELISA. The one cross-reactive MAb, H45.6G6, had an EC50 of 13 ng/ml to HPV18 VLP, a 7-fold increase from its EC50 of 1.7 ng/ml to HPV45.
TABLE 2.
Binding and neutralization EC50 concentrations for anti-HPV type-specific MAbs
| MAb | HPV type(s) and epitopea | HPV type(s) neutralized | EC50 (ng/ml) |
|
|---|---|---|---|---|
| Binding | Neutralizing | |||
| H31.5F12 | 31 | 31 | 4.1 | 5.2 |
| H31.5D10 | 31 | 31 | 3.6 | 9.1 |
| H33.5D4 | 33 | 33 | 2.3 | 0.39 |
| H33.6G9 | 33 | 33 | 2.3 | 0.38 |
| H45.6G6 | 45, 18 | 45, 18 | 1.7 (HPV45), 13 (HPV18) | 0.35 (HPV45), 270 (HPV18) |
| H45.10B4 | 45 | 45 | 4.9 | 12 |
| H52.8D11 | 52 | 52 | 4.0 | 0.49 |
| H52.9F7 | 52 | 52 | 3.4 | 0.41 |
| H58.2C3 | 58 | 58 | 2.2 | 1.1 |
| H58.6E11 | 58 | 58 | 2.1 | 1.7 |
All are conformational.
HPV type-specific pseudovirus neutralization by MAbs.
The monoclonal antibody pairs (Table 1) for HPV31, HPV33, HPV45, HPV52, and HPV58 were tested for neutralization activity in a well-characterized in vitro pseudovirus neutralization assay (15). Two experiments were performed. The first experiment examined the specificity of the MAbs against a broad panel of HPV pseudovirions, and the second experiment quantitatively determined the EC50 of each monoclonal antibody (MAb) against the HPV type or types against which it had neutralizing activity. These experiments were conducted in a blinded fashion; the experimenters did not know the HPV type against which the MAbs were generated or the specificity according to binding assays (Fig. 1; also, see Fig. S2 in the supplemental material).
For vaccine development, measuring neutralization ensures that the assays are monitoring relevant epitopes. Each MAb was tested for neutralization against all five HPV types upon which they were derived (HPV31, HPV33, HPV45, HPV52, and HPV58), the types found in the quadrivalent HPV vaccine Gardasil (HPV6, HPV11, HPV16, and HPV18), and an additional type, HPV59 (in the same phylogenetic group α7 as HPV18 and HPV45). Each MAb was tested against the panel of HPV pseudoviruses at two concentrations, 1.8 μg/ml and 0.37 μg/ml (see Materials and Methods). Each MAb that bound to HPV VLPs in a type-specific manner also completely neutralized its respective HPV pseudovirion at both concentrations tested (Fig. 2). In addition, the cross-reactive binder H45.6G6 could also neutralize HPV18 pseudovirions. As might be expected, H45.6G6 neutralized HPV45 more efficiently than HPV18. With this exception, none of the MAbs exhibited significant cross-neutralization of other HPV pseudovirion types.
FIG 2.
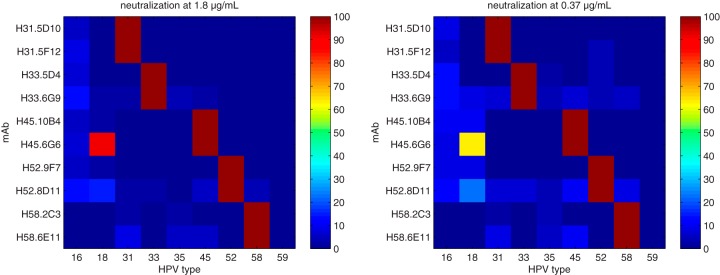
Neutralization by MAbs is specific to HPV type. All 10 of the MAbs listed in Table 1 had type-specific neutralizing activity against their respective HPV types. In addition, H45.6G6.A8 had diminished but detectable neutralizing activity for HPV18, a closely related type.
With the establishment of the specificity of neutralization, the neutralization activity of each MAb was further quantified against its cognate antigen. A complete titration series of each MAb was performed in the neutralization assay against the pseudoviruses. The neutralization data were fitted with the four-parameter logistic equation and summarized by the EC50. The neutralization curves are plotted along with nonlinear least-square fits in Fig. S2 in the supplemental material. The cross-reactive HPV45.6G6 was tested against both HPV45 and HPV18. Consistent with the neutralization map (Fig. 2) and the differential in binding affinities (Table 2), the neutralization of HPV45 pseudovirion was considerably more potent than that of HPV18, approximately 750-fold. All neutralization EC50s are listed in the last column of Table 2. Type-specific neutralizations ranged from 0.35 to 12 ng/ml. These data demonstrate that the MAbs are specifically and potentially neutralizing in the in vitro assays and indicate that the MAbs recognize epitopes that are neutralization sensitive.
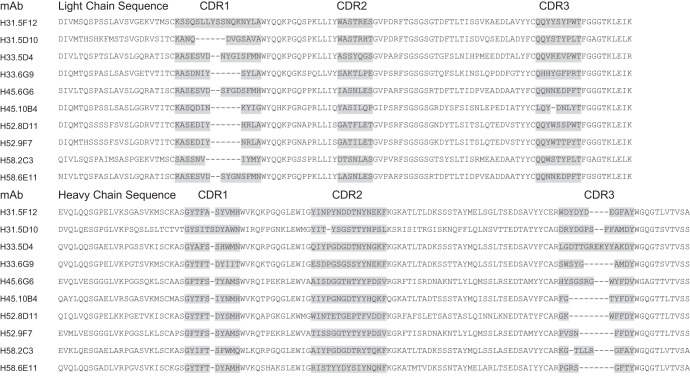
To identify the MAbs, ensure long-term availability, and facilitate sharing with the wider scientific community, the variable regions of the 10 MAbs were sequenced. Alignments of the amino acid sequences for the light chains and heavy chains are shown in Fig. 3. In each alignment, the complementarity-determining regions (CDRs) are highlighted. Although it is difficult to draw inferences from a limited set of sequences, it is interesting that pairs of MAbs with the same type specificity tend to have higher similarity than pairs of different types. For example, the light chains for HPV52 MAbs are identical with the exception of one amino acid in CDR1, one amino acid in CDR2, and three amino acids in CDR3. These cross-specific and type-specific patterns may indicate which CDRs are essential for binding to the HPV L1 epitopes and which regions determine the type specificity.
FIG 3.
Alignment of amino acid sequences of the light- and heavy-chain regions of MAbs shown in Tables 1 and 2. Complementarity-determining regions (CDRs) are shaded.
DISCUSSION
We have developed and characterized panels of monoclonal antibodies against HPV L1 VLPs of oncogenic types HPV31, HPV33, HPV45, HPV52, and HPV58. From each panel, two MAbs were selected for type specificity, sensitivity to VLP conformation, potent neutralization, and differing isotypes. Binding affinities of intact VLPs were confirmed by a surface plasmon resonance (SPR) method that we published previously (14). The MAbs were selected for use in two assays, one assay for vaccine production and one assay for vaccine trial clinical serology. The use of the same MAbs in both assays ensures that we detect the same epitopes and characteristics (correlates of neutralization and correct VLP conformation) in both phases.
The in vitro release potency (IVRP) assay (19) is used to monitor both product quality and consistency for release; the in vitro nature of the assay is in contrast to the historical in vivo mouse potency assays. Within each pair, one MAb is used to capture the VLP on the ELISA plate, and the second MAb is used for detection in the sandwich format. The role of each MAb is listed in the final column of Table 1. The final readout is through a conjugated anti-mouse reagent specific to one mouse isotype. Instead of having to generate and qualify labeled conjugates for each type-specific detection MAb, the unlabeled MAbs can be used with a secondary detection MAb. For instance, MAb H31.5D10 can be detected through anti-IgG2b MAbs that do not bind to the capture MAb, MAb H33.5D4 (IgG1). In this way, only three reagents must be labeled and qualified (anti-IgG1, anti-IgG2a, and anti-IgG2b) instead of custom modification of five HPV-specific MAbs. These three reagents are available from many commercial suppliers, which also simplifies assay development.
The second important assay for a vaccine program is a clinical assay to monitor serological responses in vaccinees. Similarly to the serological assay for Gardasil (6, 7), a multiplexed competitive Luminex immunoassay (cLIA) has been developed for the nonavalent vaccine. The same requirements for MAbs that recognize neutralizing epitopes apply. Due to the competitive nature of the assay, it is even more important that the MAbs be strictly type specific. The cLIA requires one MAb specific to each VLP type. For each type, we chose the “detection” MAb (Table 1) from the pair used in IVRP, and this MAb had to be strictly type specific to maintain the type specificity of the assay results. This is important because the potential for vaccine-induced cross-reactivity has been established. Sera from women immunized with Cervarix (HPV16/HPV18) can neutralize HPV31 and HPV45 in vitro (20, 21), presumably due to HPV16 and HPV18 cross-reactivity, respectively. Unlike same-type neutralization, the cross-reactive neutralization is apparent only after three vaccinations at months 0, 1, and 6, and the neutralization activity is reduced by more than a factor of 100. Similarly, sera from women immunized with Gardasil (HPV6, HPV11, HPV16, and HPV18) can neutralize HPV45 in vitro (22), presumably due to HPV18 cross-reactivity. The clinical examples demonstrate the possibility for cross-reactive neutralizing MAbs but also reinforce the need for additional new HPV types in a vaccine to reach high levels of type-specific neutralization of new oncogenic HPV types.
In general, when developing and selecting MAbs for vaccine release and clinical assays, we seek to satisfy four criteria. First, each MAb must be specific to one of the genotypes in the vaccine. Second, two or more MAbs that will be used in the same assay must not mutually compete or interfere. Third, the MAbs must have sufficient affinity to bind to the antigen and not rapidly dissociate. Fourth, the MAbs must represent critical quality attributes of the vaccine. These may include specificity to conformational epitopes (e.g., to discriminate between intact VLPs or properly folded antigens), recognition of neutralizing epitopes (to correlate with functional assays), and sensitivity to thermal or chemical stress upon the vaccine.
In the specific case of the IVRP assay, an intact sandwich of both MAbs in the pair binding to the VLP is required, so only one MAb must be type specific for the assay itself to be type specific. Furthermore, the second requirement for a lack of competition is not crucial due to the highly multivalent character of VLPs. The same epitope is expressed many times across the VLP surface; binding of a MAb to a single site does not significantly limit the binding of a second MAb.
The pattern of cross-reactivity of the monoclonal antibody panels (Fig. 1) is consistent with the amino acid homology (see Table S1 in the supplemental material) between the HPV L1 proteins and phylogenetic grouping (23, 24). HPV18 and HPV45 are closely related (88% homologous, group α7) compared to their homology of only 62% to 65% with the other L1 proteins considered here. HPV6 and HPV11, which are responsible for anogenital warts (92% homologous, group α10), had lower homology (64% to 68%) to the other L1 sequences. Accordingly, none of the 39 antibodies raised to the five new VLP types bind to HPV6 or HPV11 VLPs, with the exception of H33.1E7, which binds to all disrupted VLPs. The remaining types (HPV16, HPV31, HPV33, HPV52, and HPV58) all belong to group α9, with intragroup L1 homology of 77% to 90%. The exhibited cross-reactivity of some MAbs within group α9, i.e., that between 31 and 52 and that between 33 and 16, 31, 52, and 58, is consistent with their relatively high homology (see Table S1 in the supplemental material).
In summary, high-affinity and strongly neutralizing antibodies were generated, characterized, and used to develop sandwich immunoassays that identify and quantify type-specific VLP antigens. These MAbs have utility in research assays (14), vaccine product release assays (19), and clinical serological assays (6, 7) to broaden the coverage to new oncogenic HPV strains.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for experimental assistance from Roseann T. Kowalski and helpful discussions with Christine C. Roberts and Jan ter Meulen. We thank Neil Christensen for sharing several antibodies for testing and assay controls.
M.J.B., V.T., and A.C.F. are current or former employees of Merck & Co., Inc. Gardasil is a registered trademark of Merck & Co., Inc. Cervarix is a registered trademark of GlaxoSmithKline.
Footnotes
Published ahead of print 26 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00773-13.
REFERENCES
- 1.Dunne EF, Datta SD, Markowitz EL. 2008. A review of prophylactic human papillomavirus vaccines: recommendations and monitoring in the US. Cancer 113:2995–3003. 10.1002/cncr.23763 [DOI] [PubMed] [Google Scholar]
- 2.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. U. S. A. 89:12180–12184. 10.1073/pnas.89.24.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirnbauer R, Taub J, Greenstone H, Roden R, Dürst M, Gissmann L, Lowy DR, Schiller JT. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 67:6929–6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muñoz N, Méndez F, Posso H, Molano M, van den Brule AJ, Ronderos M, Meijer C, Muñoz Á 2004. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J. Infect. Dis. 190:2077–2087. 10.1086/425907 [DOI] [PubMed] [Google Scholar]
- 5.Wiley DJ, Douglas J, Beutner K, Cox T, Fife K, Moscicki A-B, Fukumoto L. 2002. External genital warts: diagnosis, treatment, and prevention. Clin. Infect. Dis. 35(Suppl 2):S210–S224. 10.1086/342109 [DOI] [PubMed] [Google Scholar]
- 6.Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, Esser MT. 2003. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed Luminex assay. Clin. Diagn. Lab. Immunol. 10:108–115. 10.1128/CDLI.10.1.108-115.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dias D, Doren JV, Schlottmann S, Kelly S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green T, Brown M, Smith J, Chirmule N, Barr E, Jansen KU, Esser MT. 2005. Optimization and validation of a multiplexed Luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin. Diagn. Lab. Immunol. 12:959–969. 10.1128/CDLI.12.8.959-969.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastrana DV, Buck CB, Pang Y-YS, Thompson CD, Castle PE, FitzGerald PC, Krüger Kjaer S, Lowy DR, Schiller JT. 2004. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321:205–216. 10.1016/j.virol.2003.12.027 [DOI] [PubMed] [Google Scholar]
- 9.Christensen ND, Kreider JW, Cladel NM, Patrick SD, Welsh PA. 1990. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J. Virol. 64:5678–5681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop B, Dasgupta J, Klein M, Garcea RL, Christensen ND, Zhao R, Chen XS. 2007. Crystal structures of four types of human papillomavirus L1 capsid proteins. J. Biol. Chem. 282:31803–31811. 10.1074/jbc.M706380200 [DOI] [PubMed] [Google Scholar]
- 11.Rizk RZ, Christensen ND, Michael KM, Müller M, Sehr P, Waterboer T, Pawlita M. 2008. Reactivity pattern of 92 monoclonal antibodies with 15 human papillomavirus types. J. Gen. Virol. 89:117–129. 10.1099/vir.0.83145-0 [DOI] [PubMed] [Google Scholar]
- 12.Smith JF, Kowalski R, Esser MT, Brown MJ, Bryan JT. 2008. Evolution of type-specific immunoassays to evaluate the functional immune response to Gardasil, a vaccine for human papillomavirus types 16, 18, 6, and 11. Hum. Vaccin. 4:134–142. 10.4161/hv.4.2.5261 (Erratum, Hum. Vaccin. Immunother. 9:938, 2013, ) [DOI] [PubMed] [Google Scholar]
- 13.Zhao Q, Modis Y, High K, Towne V, Meng Y, Wang Y, Alexandroff J, Brown M, Carragher B, Potter CS, Abraham D, Wohlpart D, Kosinski M, Washabaugh MW, Sitrin RD. 2012. Disassembly and reassembly of human papillomavirus virus-like particles produces more virion-like antibody reactivity. Virol. J. 9:52. 10.1186/1743-422X-9-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Towne V, Zhao Q, Brown M, Finnefrock AC. 2013. Pairwise antibody footprinting using surface plasmon resonance technology to characterize human papillomavirus type 16 virus-like particles with direct anti-HPV antibody immobilization. J. Immunol. Methods 388:1–7. 10.1016/j.jim.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 15.Sehr P, Rubio I, Seitz H, Putzker K, Ribeiro-Müller L, Pawlita M, Müller M. 2013. High-throughput pseudovirion-based neutralization assay for analysis of natural and vaccine-induced antibodies against human papillomaviruses. PLoS One 8:e75677. 10.1371/journal.pone.0075677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buck CB, Pastrana DV, Lowy DR, Schiller JT. 2005. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 119:445–462. 10.1385/1-59259-982-6:445 [DOI] [PubMed] [Google Scholar]
- 17.Mach H, Volkin DB, Troutman RD, Wang B, Luo Z, Jansen KU, Shi L. 2006. Disassembly and reassembly of yeast-derived recombinant human papillomavirus virus-like particles (HPV VLPs). J. Pharm. Sci. 95:2195–2206. 10.1002/jps.20696 [DOI] [PubMed] [Google Scholar]
- 18.Shi L, Sings HL, Bryan JT, Wang B, Wang Y, Mach H, Kosinski M, Washabaugh MW, Sitrin R, Barr E. 2007. GARDASIL: prophylactic human papillomavirus vaccine development—from bench top to bed-side. Clin. Pharmacol. Ther. 81:259–264. 10.1038/sj.clpt.6100055 [DOI] [PubMed] [Google Scholar]
- 19.Shank-Retzlaff M, Wang F, Morley T, Anderson C, Hamm M, Brown M, Rowland K, Pancari G, Zorman J, Lowe R, Schultz L, Teyral J, Capen R, Oswald CB, Wang Y, Washabaugh M, Jansen K, Sitrin R. 2005. Correlation between mouse potency and in vitro relative potency for human papillomavirus type 16 virus-like particles and Gardasil vaccine samples. Hum. Vaccin. 1:191–197. 10.4161/hv.1.5.2126 [DOI] [PubMed] [Google Scholar]
- 20.Kemp TJ, Hildesheim A, Safaeian M, Dauner JG, Pan Y, Porras C, Schiller JT, Lowy DR, Herrero R, Pinto LA. 2011. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine 29:2011–2014. 10.1016/j.vaccine.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemp TJ, Safaeian M, Hildesheim A, Pan Y, Penrose KJ, Porras C, Schiller JT, Lowy DR, Herrero R, Pinto LA. 2012. Kinetic and HPV infection effects on cross-type neutralizing antibody and avidity responses induced by Cervarix®. Vaccine 31:165–170. 10.1016/j.vaccine.2012.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JF, Brownlow M, Brown M, Kowalski R, Esser MT, Ruiz W, Barr E, Brown DR, Bryan JT. 2007. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum. Vaccin. 3:109–115. 10.4161/hv.3.4.4058 [DOI] [PubMed] [Google Scholar]
- 23.de Villiers E-M, Fauquet C, Broker TR, Bernard H-U, zur Hausen H. 2004. Classification of papillomaviruses. Virology 324:17–27. 10.1016/j.virol.2004.03.033 [DOI] [PubMed] [Google Scholar]
- 24.de Villiers E-M. 2013. Cross-roads in the classification of papillomaviruses. Virology 445:2–10. 10.1016/j.virol.2013.04.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.