Abstract
Duck plague (DP) is a severe disease caused by DP virus (DPV). Control of the disease is recognized as one of the biggest challenges in avian medicine. Vaccination is an efficient way to control DPV, and an attenuated vaccine is the main routine vaccine. The attenuated DPV vaccine strain CHa is a modified live vaccine, but the systemic and mucosal immune responses induced by this vaccine have been poorly understood. In this study, the immunogenicity and efficacy of the vaccine were evaluated after subcutaneous immunization of ducks. CD4+ and CD8+ T cells were counted by flow cytometry, and humoral and mucosal Ig antibodies were analyzed by enzyme-linked immunosorbent assay (ELISA). The results showed that high levels of T cells and Ig antibodies were present postimmunization and that there were more CD4+ T cells than CD8+ T cells. Titers of humoral IgG were higher than those of humoral IgA. Local IgA was found in each sample, whereas local IgG was found only in the spleen, thymus, bursa of Fabricius, harderian gland, liver, bile, and lung. In a protection assay, the attenuated DPV vaccine completely protected ducks against 1,000 50% lethal doses (LD50) of the lethal DPV strain CHv via oral infection. These data suggest that this subcutaneous vaccine elicits sufficient systemic and mucosal immune responses against lethal DPV challenge to be protective in ducks. This study provides broad insights into understanding the immune responses to the attenuated DPV vaccine strain CHa through subcutaneous immunization in ducks.
INTRODUCTION
Duck plague (DP), also known as duck viral enteritis, is a worldwide disease caused by duck plague virus (DPV), a virus of the Herpesviridae family. DPV induces an acute disease with high mortality rates in flocks of ducks, geese, and swans (1–5). The virion of DPV is composed of an envelope and a spherical nucleocapsid which contains a double-stranded DNA. DPV can be transmitted among birds by direct contact with infected birds or indirect contact with a contaminated environment (2, 5–7). Ducks infected with DPV may die without any detectable symptoms or be observed with signs of photophobia, ataxia, and watery diarrhea and a marked reduction in egg production. The morbidity and mortality in domestic ducks can reach up to 100% (4).
Vaccination is a desirable method to prevent DPV infection (8). At present, two kinds of vaccines against DPV have been introduced to the market: attenuated and inactive vaccines (9, 10). In all cases, reports from field trials suggest that commercially available DPV vaccines significantly reduce mortality rates (9, 11, 12). An attenuated DPV vaccine was first studied in 1963, by Jansen et al., who lowered the virulence of DPV by passing it through numerous chicken embryos. Their study found that inoculated ducks could be protected against DPV challenge (13). Since then, this type of vaccine has been used extensively worldwide. Lam and Lin suggested that a humoral immune mechanism might play a role in protecting ducks in vivo and in vitro by using an attenuated DPV Sheridan-83 strain as an immunogen (14). Additionally, efficient protection induced by an attenuated DPV vaccine is dependent on the strain and concentration of DPV (10).
The route of vaccine administration is an important factor for vaccination efficiency (15, 16). The kinetics of the attenuated DPV vaccine strain CHa determined that subcutaneous administration had a larger effect on the vaccine virus distribution in tissue than the oral and nasal routes of administration did (17). The growth kinetics, back passages, residual virulence, excretion, and seroconversion of this strain have been investigated thoroughly (17–19). Briefly, the attenuated virus has a broad tissue tropism. Levels of DPV in organs peak at 90 min and then decline steadily after subcutaneous immunization with the attenuated DPV vaccine strain CHa. However, the vaccine virus can be detected by indirect immunohistochemistry until 18 weeks after immunization. Vaccinated ducks excrete virus, which can revaccinate the flock.
Systemic and mucosal immune responses are important in resisting and clearing viral infections (20, 21). Prior to this study, the immune responses after subcutaneous immunization with the DPV attenuated vaccine strain CHa had not been elucidated, and no conclusions about its protective effect could be established, since no challenge studies had been performed. The current study aimed to assess the immunogenicity of the attenuated DPV vaccine strain CHa after subcutaneous immunization and its protective effects against the lethal DPV strain CHv via oral challenge.
MATERIALS AND METHODS
Ducks.
Eighty 7-day-old Tianfu ducks were purchased from a farm. Sera were evaluated by PCR and enzyme-linked immunosorbent assay (ELISA) to verify that the ducks were free of DPV and negative for antibodies against DPV, respectively. The ducks were handled in accordance with the animal protection law of the People's Republic of China (draft dated 18 September 2009).
Vaccine and virus.
DPV strain CHv (GenBank accession no. JQ647509) was obtained by A. Cheng in Sichuan Province, China (22). Currently, the virus is stored in the Avian Disease Research Center of the Sichuan Agricultural University of China (21). The DPV attenuated vaccine strain CHa is a modified live commercial vaccine (China Animal Husbandry Industry Co. Ltd.) that was the product of a DPV CHv isolate cultured in chicken embryo fibroblasts (CEF) for 80 serial passages (17).
Grouping, vaccination, and sampling.
When the ducklings were 14 days old, they were randomly divided into groups A and B (40 ducks per group). The ducks from group A were subcutaneously immunized with a single dose of attenuated DPV vaccine (100 50% egg infective doses [EID50]), while group B ducks were injected with 0.2 ml phosphate-buffered saline (PBS) and served as negative controls. All ducks from groups A and B were injected at weeks 1, 3, and 5. At weeks 1, 2, 3, 4, 5, 6, and 8 after the first immunization, three ducks from each group were randomly selected and sacrificed under anesthesia for the collection of the blood, spleen, bursa of Fabricius, thymus, harderian gland, lung, trachea, liver, bile, duodenum, jejunum, ileum, cecum, and rectum, in compliance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Treatment of samples.
After the mesentery was removed, 1-cm sections of intestinal tract and trachea were cut and processed as tissue homogenates in 1 ml T-PBS (100 μg/ml of trypsin inhibitor [Sigma-Aldrich, St. Louis, MO] in PBS). Each of the supernatants was collected after centrifugation at 10,000 × g for 20 min at 4°C (21). Meanwhile, 0.5-g portions of the spleen, bursa of Fabricius, thymus, harderian gland, lung, and liver were cut and processed as tissue homogenates in 0.5 ml T-PBS, and the supernatants were collected as described above. Bile and blood were centrifuged, and the supernatants were collected. The fluid supernatant samples were stored at −20°C until use.
Flow cytometry for analysis of CD4+ and CD8+ T lymphocytes in peripheral blood.
The lymphocytes in peripheral blood were collected by use of lymphocyte separation medium (Huajing, Shanghai, China) according to the manufacturer's instructions. Anti-duck CD4 monoclonal antibody (AbD Serotec Ltd., United Kingdom) or anti-duck CD8 monoclonal antibody (AbD Serotec Ltd., United Kingdom) was added to the separated lymphocytes (5 × 105) and incubated at 4°C for 30 min. Fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (AbD Serotec Ltd., United Kingdom) was then added for incubation at 4°C for 30 min. The cells were washed and resuspended with PBS and then subjected to flow cytometric analysis. Viable lymphocytes were gated on the basis of forward and side scatter characteristics, and 10,000 events were analyzed for positive staining with FITC-IgG. Data analysis was carried out using BD FACSAria software (23, 24).
Detection of DPV-specific IgA and IgG.
Ninety-six-well plates were coated with 100 μl of 0.25-μg/ml purified DPV antigen and incubated overnight at 4°C. After washing 3 times with PBS plus Tween (PBST), the plates were blocked with 1% bovine serum albumin (BSA)-PBST for 1 h at 37°C. The plates were then washed, and 100 μl of each diluted fluid (prepared from sera and tissues) was added in triplicate. All wells were incubated for 1 h at 37°C. Horseradish peroxidase (HRP)-conjugated rabbit anti-duck IgG or IgA antibody (Sigma-Aldrich, St. Louis, MO) was used as the secondary antibody, at a 1:4,000 or 1:1,000 dilution, respectively, and incubated for 1 h at 37°C. After washing three times, 100 μl tetramethylbenzidine (TMB) was added. After incubation for 15 min, the reaction was terminated by addition of 100 μl 2 M H2SO4. The optical density at 450 nm (OD450) was measured in each well.
Protection assay with the attenuated DPV vaccine.
The protective ability of the attenuated DPV vaccine strain CHa against the virulent DPV strain CHv was assessed in ducks 2 weeks after the third inoculation. Ten ducks from each group were orally challenged with 1,000 50% lethal doses (LD50) of DPV strain CHv. The survival of these ducks was monitored daily for 10 days postchallenge.
Statistical analysis.
All results are expressed as means ± standard errors of the means. Student's t test was used to analyze the statistical differences in T lymphocyte and Ig antibody responses between vaccine and control groups. SPSS 16.0 was applied to analyze the protection assay, and statistical analysis of mortality was analyzed by the log rank and chi-square tests. Results were considered statistically significant if P values were <0.05 or <0.01 for comparisons with the nonimmunized group.
RESULTS
Analysis of CD4+ and CD8+ T lymphocytes in peripheral blood.
The peripheral blood lymphocytes collected and processed at 1, 2, 3, 4, 6, and 8 weeks were analyzed by flow cytometry. The results showed that the percentages of CD4+ and CD8+ T lymphocytes in the immunized group were significantly higher than those in the negative-control group (Fig. 1) (P < 0.01). The percentage of CD4+ T lymphocytes increased from the 1st week (13.7%) to the 6th week (43.44%) but then dropped to a lower level (18.77%) at the 8th week (Fig. 1A). The levels of CD4+ T lymphocytes in the negative-control group were maintained at a lower level and did not show significant alterations with time. The numbers of CD4+ T lymphocytes in the immunization group were 3 to 7 times higher than those in the negative-control group (Fig. 1A). In contrast, the values for CD8+ T lymphocytes did not show an increase but were maintained at a higher level after the first inoculation. The highest level of CD8+ T lymphocytes was presented at the 6th week, and it was almost 6 times that for the negative-control group (Fig. 1B). The results indicated that both CD4+ and CD8+ T lymphocytes from the vaccinated group reached higher levels than those in the negative-control group (P < 0.05), while the numbers of CD4+ T lymphocytes were larger than those of CD8+ T lymphocytes at the same time points.
FIG 1.
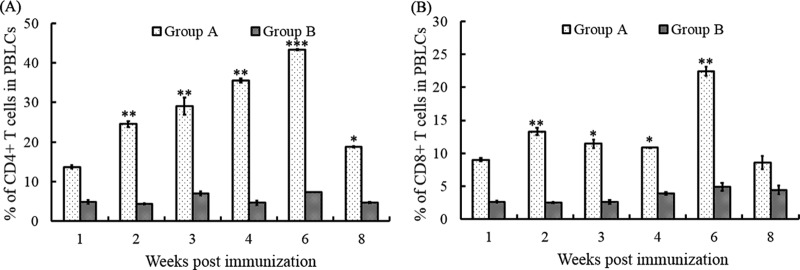
T lymphocytes in peripheral blood following subcutaneous immunization with the attenuated DPV vaccine strain CHa. (A) CD4+ T lymphocytes in peripheral blood were collected at 1, 2, 3, 4, 6, and 8 weeks and analyzed by flow cytometry. (B) CD8+ T lymphocytes in peripheral blood were collected at 1, 2, 3, 4, 6, and 8 weeks and analyzed by flow cytometry. The data presented are the mean values for three samples at each time point. *, P < 0.05 compared to control group; **, P < 0.01 compared to control group; ***, P < 0.001 compared to control group. PBLCs, peripheral blood lymphocytes.
Detection of IgA and IgG specific to DPV in serum.
There were no clinical signs of disease or death after immunization during the entire experimental period. To determine the antibody pattern, the specific IgA and IgG antibodies against DPV were detected by ELISA (Fig. 2). The results showed that IgA and IgG levels were significantly higher in the experimental group than in the negative-control group (P < 0.05). The quantity of IgA was maintained at a high level after the first inoculation and did not change significantly until the third inoculation. The level of IgA reached a peak OD450 of 0.346 at the 5th week and then decreased, but it was still higher than that of the control group at the 8th week after the first inoculation (P < 0.05) (Fig. 2A). The levels of specific anti-DPV IgG in sera increased along with the vaccination times, and they reached a peak at the 5th week, with an OD450 of 1.904. Although the IgG level decreased starting at the 6th week, it was still higher than that of the negative-control group in the 8th week (P < 0.01) (Fig. 2B).
FIG 2.
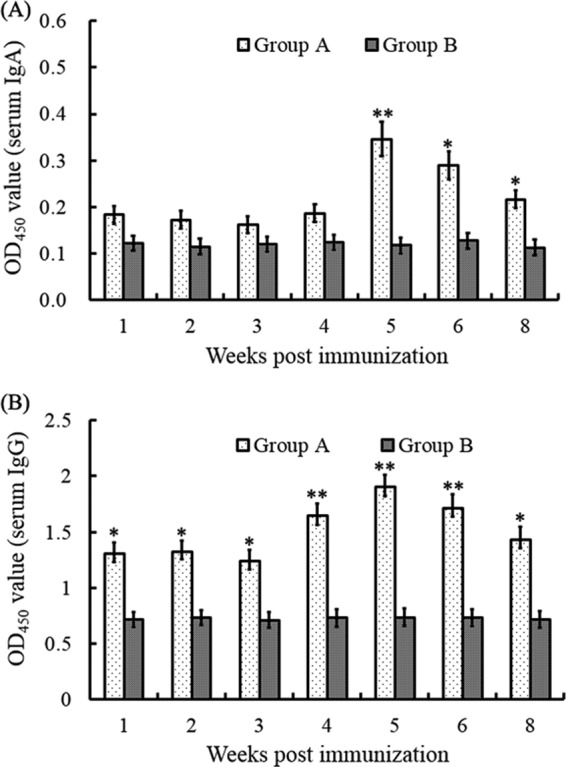
DPV-specific IgA and IgG in sera were detected by antigen-capture ELISA. Sera were collected at 1, 2, 3, 4, 5, 6, and 8 weeks, and the level of IgA (A) or IgG (B) in each sample was detected and compared with that for the negative-control group. *, P < 0.05 compared to control group; **, P < 0.01 compared to control group.
Detection of specific IgA and IgG in organs.
To assess the specific local antibodies against DPV, the DPV-specific IgA and IgG antibodies in different organs were evaluated by ELISA. The organs tested included the spleen, bursa of Fabricius, thymus, harderian gland, lung, trachea, liver, bile, duodenum, jejunum, ileum, cecum, and rectum.
Results showed that the local IgA antibody titers generated in the immunized group increased gradually in all organs from the 1st to the 5th weeks, with the highest IgA titers present in bile (Fig. 3). Among immune organs, the thymus showed the highest IgA levels from the 1st to the 5th weeks, with a peak at week 5, while IgA titers in the spleen, harderian gland, and bursa of Fabricius peaked at the 6th week (Fig. 3A). Local mucosal IgA antibody titers in digestive organs showed an upward trend from the 1st to the 6th weeks and reached the highest levels in the bile, duodenum, liver, jejunum, ileum, cecum, and rectum, with OD450 values of 0.457, 0.281, 0.267, 0.213, 0.206, 0.212, and 0.221, respectively, at the 6th week (Fig. 3B). In respiratory organs, levels of lung IgA rose sharply from the 1st to the 6th weeks and peaked, with an OD450 value of 0.369, at the 6th week. However, the levels of trachea IgA increased dramatically from the 1st to the 5th weeks and peaked, with an OD450 value of 0.319, at the 5th week (Fig. 3C). As expected, samples from the nonvaccinated control group showed no specific local IgA responses in the spleen, bursa of Fabricius, thymus, harderian gland, lung, trachea, liver, duodenum, jejunum, ileum, cecum, and rectum (data not shown).
FIG 3.
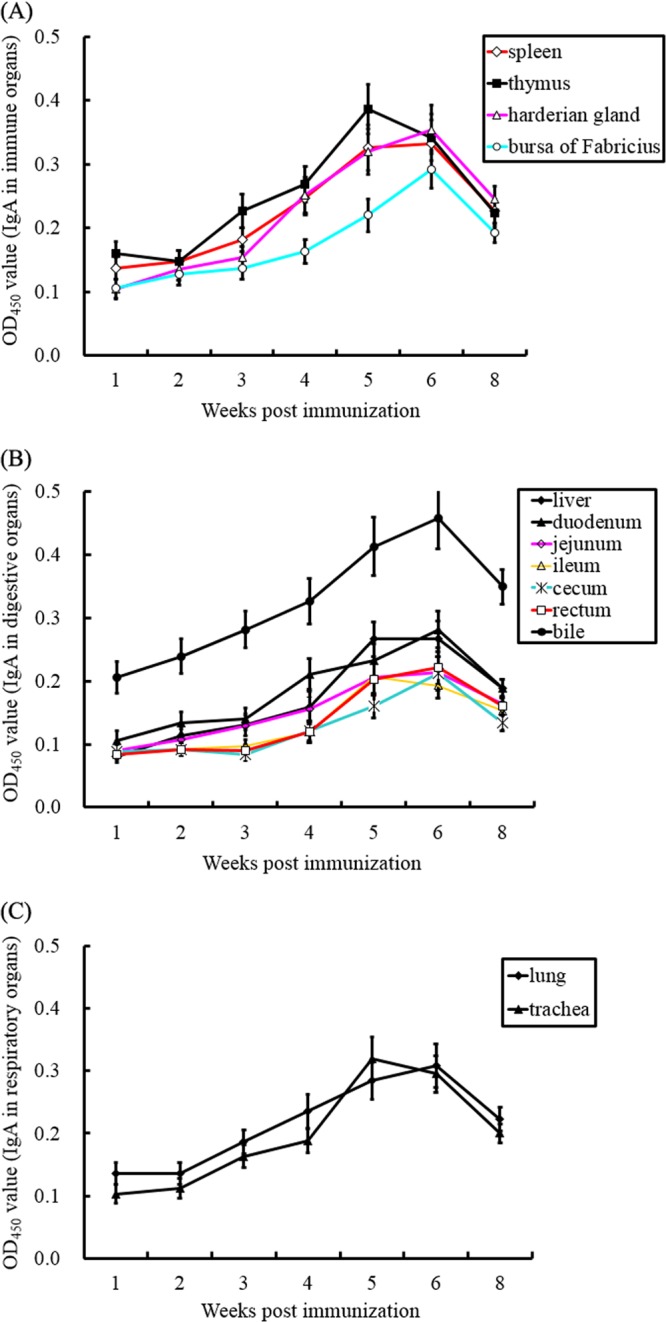
Mucosal IgA responses were assessed by ELISA. Spleens, bursas of Fabricius, thymuses, harderian glands, lungs, tracheas, livers, bile, duodena, jejuna, ilea, ceca, and rectums were obtained from the vaccinated group. IgA responses in immune (A), digestive (B), and respiratory (C) organs were tested by DPV antigen-capture ELISA.
In a parallel experiment, the levels of specific IgG in organs were detected. As shown in Fig. 4, the local IgG antibody titers reflected similar trends in the spleen, thymus, harderian gland, and bursa of Fabricius. There was a similar pattern of local IgG responses in the liver, bile, and lung. Peak levels in immune organs were observed at the 6th week. However, there was an increasing trend of antibody levels in both the harderian gland and bursa of Fabricius during the experiment. Notably, in digestive organs, the specific IgG levels in liver and bile were significantly higher than those in the intestinal tract in the experimental vaccinated group (P < 0.01), and they reached a peak value at the 6th week, with OD450 values of 0.616 and 0.518, respectively. Specific local anti-DPV IgG titers were barely detected in the duodenum, jejunum, ileum, cecum, and rectum (data not shown). In the respiratory system, local IgG responded in the lungs but not the trachea, and the titers of lung IgG reached their peak, with an OD450 of 0.691, at the 6th week. IgG responses induced by the attenuated DPV vaccine were different in different organs, while the nonvaccinated group showed no specific local IgG responses (data not shown).
FIG 4.
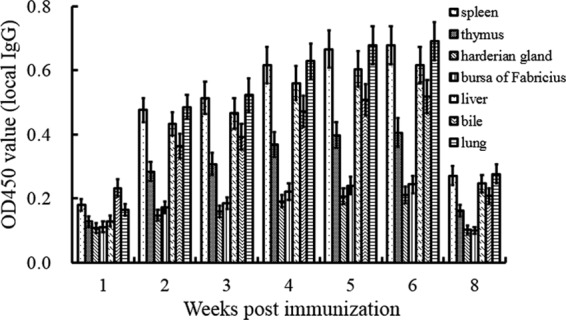
DPV-specific mucosal IgG responses were tested by ELISA. Spleens, thymuses, harderian glands, bursas of Fabricius, livers, bile, and lungs were obtained from the vaccinated group at 1, 2, 3, 4, 5, 6, and 8 weeks. The specific IgG in different organs was detected by DPV antigen-capture ELISA.
Protection studies.
To assess the protective ability of the attenuated DPV vaccine, ducks from the vaccinated and unvaccinated groups were challenged with the virulent DPV strain CHv. The results indicated that unvaccinated ducks died quickly at day 3, with high fever, severe erosions in the digestive tract, and head and/or neck swelling. These are typical clinical manifestations of DP. As shown in Fig. 5, the mortality rate for nonvaccinated ducks was significantly higher than that for vaccinated ducks (P < 0.01), and no vaccinated ducks died.
FIG 5.
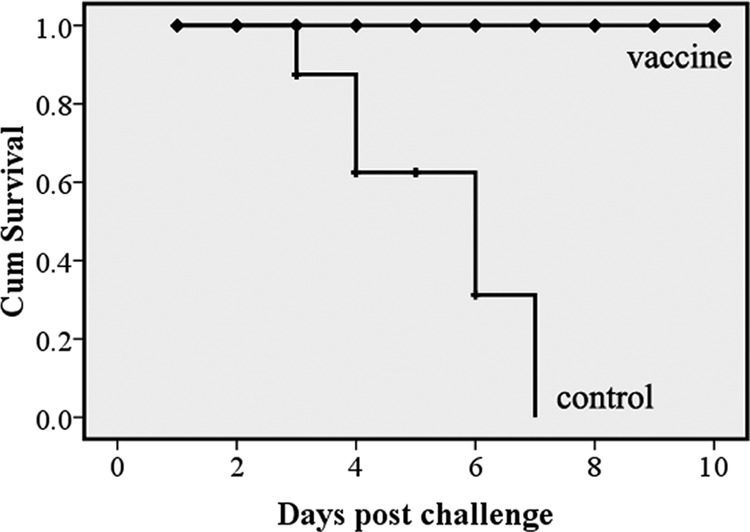
Survival curve after challenge with lethal DPV strain CHv. Ten ducks from the vaccinated group or negative-control group were orally challenged with 1,000 LD50 of DPV strain CHv on the 6th week after the first immunization. Mortality was monitored daily for 10 days after challenge. The figure was drawn as a Kaplan-Meier survival curve. The y axis shows the cumulative (Cum) survival calculated by the Kaplan-Meier method (P < 0.01).
DISCUSSION
This study was the first to investigate the systemic and mucosal immune responses to the attenuated DPV vaccine strain CHa administered subcutaneously. To evaluate the immunogenicity of the vaccine, we measured cellular, humoral, and mucosal immunity in systemic organs. After assessing systemic organizational immune responses to the attenuated DPV vaccine, we concluded that the vaccine may be an efficient immunization for prevention of DP. In this study, the attenuated DPV vaccine was found to be effective at generating T cell, IgA, and IgG responses and could protect ducks completely against virulent DPV infection.
CD4+ T lymphocytes play an important role in assisting CD8+ T lymphocytes, and both have a relationship with viral clearance responses after infection with herpesviruses (25–27). Additionally, CD4+ T cells have positive feedback with humoral immunity (28, 29). From our observations, the levels of both CD4+ and CD8+ T lymphocytes increased significantly after immunization with the attenuated vaccine. This demonstrated that the attenuated DPV vaccine, as an exogenous substance, could activate T lymphocytes and stimulate ducks to produce cell-mediated immune responses after vaccination. The reason for the number of CD4+ T lymphocytes being higher than that of CD8+ T lymphocytes may be the fact that CD4+ T lymphocytes are the predominant factor in cellular immune responses (30–32).
Serum IgG and IgA play critical roles in humoral immunity against specific antigens (21, 33). In this study, the DPV attenuated vaccine enhanced production of both IgA and IgG antibodies. The results indicated that the DPV attenuated vaccine induced specific anti-DPV humoral immune responses. In addition, the levels of serum IgG were significantly higher than those of serum IgA. This suggested that IgG is the main antibody against DPV, which coincided with previous studies (8, 21).
Mucous membranes are an important barrier and react against most pathogenic microorganisms during the early infective stage (34). Thus, the mucosal immune response is considered the first line of defense, and its elicitation is highly desired for efficacy of a vaccine (35, 36). IgA is one kind of mucosal antibody that plays a key role in mucosal defense (37). In this study, the DPV attenuated vaccine strain CHa could induce systemic mucosal IgA and IgG responses.
This study found that not only local IgA but also local IgG might contribute to protecting ducks against DPV. Local IgA was present to mediate protection in all organs sampled, which was consistent with previous studies showing that IgA is an effector molecule of the mucosal immune system (38–40). The production and distribution of local IgA in various organs in the present study are consistent with DPV having extensive tropism in all organs and local cells which can be stimulated to secrete IgA (19, 41). On the other hand, local specific IgG antibodies could mediate protection against DPV as well. Our results indicated that high levels of DPV-specific mucosal IgG appeared in the spleen, bursa of Fabricius, thymus, harderian gland, lung, liver, and bile post-primary immunization. This demonstrated that local IgG antibodies could mediate protection, which was supported by the work of Mbawuike et al., who found mucosal IgG antibodies present in a protection assay with IgA−/− mice (42). There were high levels of antigen-specific IgG in humoral immune responses, but local IgG did not appear in the duodenum, jejunum, ileum, cecum, and rectum in the current study. The reason for the negative result for intestinal IgG antibody might be that antibodies of the IgG isotype alone failed to transfer from the serum to the mucosal lumen (43).
Systemic and mucosal immune responses might contribute to protecting ducks against DPV infection. This was supported in the present challenge study and coincides with the study of Yu et al. (21). Safety is a prerequisite when using live antigens as vaccines. Neither deaths nor side effects were found in ducks after immunization with the attenuated DPV vaccine strain CHa. After challenge with the lethal DPV strain CHv, the symptoms of DP appeared only in ducks from the unvaccinated group. In the vaccine group, ducks resisted the lethal DPV strain completely.
In the present study, the comprehensive systemic and mucosal immunity against DPV in immune, digestive, and respiratory system organs was described for the first time. In summary, the present study demonstrated that subcutaneous administration of the attenuated DPV vaccine strain CHa could elicit efficient humoral, cellular, and mucosal immune responses against virulent virus challenge. The study also found that not only humoral immunity but also cell-mediated and mucosal immunity is involved in protection against lethal DPV challenge. The data presented here provide new insight into the immune mechanism of the attenuated DPV vaccine, which could confer protective immunity against lethal DPV challenge in Tianfu ducks.
ACKNOWLEDGMENTS
This research was supported by the China Agricultural Research System (grant CARS-43-8), a Ministry of Education program (grant 20125103110013), Sichuan Province research programs (grant 2013HH0042, 2013TD0015, 11ZA084, 12TD005, 2011ZO0034, and 2011JO0040), and the China 973 program (grant 2011CB111606).
Footnotes
Published ahead of print 22 January 2014
REFERENCES
- 1.Spieker JO, Yuill TM, Burgess EC. 1996. Virulence of six strains of duck plague virus in eight waterfowl species. J. Wildl. Dis. 32:453–460. 10.7589/0090-3558-32.3.453 [DOI] [PubMed] [Google Scholar]
- 2.Baudet A. 1923. Mortality in ducks in the Netherlands caused by a filterable virus; fowl plague. Tijdschr. Diergeneeskd. 50:455–459 [Google Scholar]
- 3.Converse KA, Kidd GA. 2001. Duck plague epizootics in the United States, 1967–1995. J. Wildl. Dis. 37:347–357. 10.7589/0090-3558-37.2.347 [DOI] [PubMed] [Google Scholar]
- 4.Campagnolo ER, Banerjee M, Panigrahy B, Jones RL. 2001. An outbreak of duck viral enteritis (duck plague) in domestic Muscovy ducks (Cairina moschata domesticus) in Illinois. Avian Dis. 45:522–528. 10.2307/1592999 [DOI] [PubMed] [Google Scholar]
- 5.Cheng AC, Wang MS, Liu F. 2004. The preliminary application of PCR in research of clinical diagnosis and mechanisms of immunity and pathology of duck plague virus. Chin. J. Virol. 20:364–370 [Google Scholar]
- 6.Kaleta EF, Kuczka A, Kuhnhold A, Bunzenthal C, Bonner BM, Hanka K, Redmann T, Yilmaz A. 2007. Outbreak of duck plague (duck herpesvirus enteritis) in numerous species of captive ducks and geese in temporal conjunction with enforced biosecurity (in-house keeping) due to the threat of avian influenza A virus of the subtype Asia H5N1. Dtsch. Tierarztl. Wochenschr. 114:3–11 [PubMed] [Google Scholar]
- 7.Yang X, Qi X, Cheng A, Wang M, Zhu D, Jia R, Chen X. 2010. Intestinal mucosal immune response in ducklings following oral immunisation with an attenuated duck enteritis virus vaccine. Vet. J. 185:199–203. 10.1016/j.tvjl.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 8.Lian B, Cheng A, Wang M, Zhu D, Luo Q, Jia R, Liu F, Han X, Chen X. 2011. Induction of immune responses in ducks with a DNA vaccine encoding duck plague virus glycoprotein C. Virol. J. 8:214. 10.1186/1743-422X-8-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shawky SA, Sandhu TS. 1997. Inactivated vaccine for protection against duck virus enteritis. Avian Dis. 41:461–468. 10.2307/1592206 [DOI] [PubMed] [Google Scholar]
- 10.Kulkarni DD, James PC, Sulochana S. 1998. Assessment of the immune response to duck plague vaccinations. Res. Vet. Sci. 64:199–204. 10.1016/S0034-5288(98)90125-4 [DOI] [PubMed] [Google Scholar]
- 11.Toth TE. 1970. Active immunization of White Pekin ducks against duck virus enteritis (duck plague) with modified-live-virus vaccine: immunization of ducklings. Am. J. Vet. Res. 31:1275–1281 [PubMed] [Google Scholar]
- 12.Butterfield WK, Dardiri AH. 1969. Serologic and immunologic response of ducks to inactivated and attenuated duck plague virus. Avian Dis. 13:876–887. 10.2307/1588595 [DOI] [PubMed] [Google Scholar]
- 13.Jansen J, Sr, Kurst H, Wemmenhove R. 1963. The active immunization of duck plaque. Tijdschr. Diergeneskd. 88:927–932 [Google Scholar]
- 14.Lam KM, Lin WQ. 1986. Antibody-mediated resistance against duck enteritis virus infection. Can. J. Vet. Res. 50:380–383 [PMC free article] [PubMed] [Google Scholar]
- 15.Sakamoto K, Asanuma H, Nakamura T, Kanno T, Sata T, Katano H. 2010. Immune response to intranasal and intraperitoneal immunization with Kaposi's sarcoma-associated herpesvirus in mice. Vaccine 28:3325–3332. 10.1016/j.vaccine.2010.02.091 [DOI] [PubMed] [Google Scholar]
- 16.Gimeno IM, Cortes AL, Guy JS, Turpin E, Williams C. 2011. Replication of recombinant herpesvirus of turkey expressing genes of infectious laryngotracheitis virus in specific pathogen free and broiler chickens following in ovo and subcutaneous vaccination. Avian Pathol. 40:395–403. 10.1080/03079457.2011.588196 [DOI] [PubMed] [Google Scholar]
- 17.Qi X, Yang X, Cheng A, Wang M, Guo Y, Jia R. 2009. Replication kinetics of duck virus enteritis vaccine virus in ducklings immunized by the mucosal or systemic route using real-time quantitative PCR. Res. Vet. Sci. 86:63–67. 10.1016/j.rvsc.2008.05.001 [DOI] [PubMed] [Google Scholar]
- 18.Shen FX, Ma GP, Cheng AC, Wang MS, Li CF, Sun KF, Chang H, Zhu DK, Jia RY, Chen XY, Sun T. 2010. Development and application of an indirect immunohistochemical method for the detection of duck plague virus vaccine antigens in paraffin sections and localization in the vaccinated duckling tissues. Poult. Sci. 89:1915–1923. 10.3382/ps.2010-00848 [DOI] [PubMed] [Google Scholar]
- 19.Qi X, Yang X, Cheng A, Wang M, Zhu D, Jia R. 2008. Quantitative analysis of virulent duck enteritis virus loads in experimentally infected ducklings. Avian Dis. 52:338–344. 10.1637/8120-100207-ResNote.1 [DOI] [PubMed] [Google Scholar]
- 20.Freeman ML, Burkum CE, Woodland DL, Sun R, Wu TT, Blackman MA. 2012. Importance of antibody in virus infection and vaccine-mediated protection by a latency-deficient recombinant murine gamma-herpesvirus-68. J. Immunol. 188:1049–1056. 10.4049/jimmunol.1102621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X, Jia R, Huang J, Shu B, Zhu D, Liu Q, Gao X, Lin M, Yin Z, Wang M, Chen S, Wang Y, Chen X, Cheng A. 2012. Attenuated Salmonella typhimurium delivering DNA vaccine encoding duck enteritis virus UL24 induced systemic and mucosal immune responses and conferred good protection against challenge. Vet. Res. 43:56. 10.1186/1297-9716-43-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Cheng A, Wang M, Yang Q, Zhu D, Jia R, Chen S, Zhou Y, Wang X, Chen X. 2012. Complete genomic sequence of Chinese virulent duck enteritis virus. J. Virol. 86:5965. 10.1128/JVI.00529-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, Hofer T, Radbruch A, Zinkernagel RM, Hengartner H. 2008. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J. Exp. Med. 205:53–61. 10.1084/jem.20071855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beinke S, Phee H, Clingan JM, Schlessinger J, Matloubian M, Weiss A. 2010. Proline-rich tyrosine kinase-2 is critical for CD8 T-cell short-lived effector fate. Proc. Natl. Acad. Sci. U. S. A. 107:16234–16239. 10.1073/pnas.1011556107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doymaz MZ, Rouse BT. 1992. Immunopathology of herpes simplex virus infections. Curr. Top. Microbiol. Immunol. 179:121–136 [DOI] [PubMed] [Google Scholar]
- 26.Ataera H, Simkins HM, Hyde E, Yang J, Hermans IF, Petersen TR, Ronchese F. 2013. The control of CD8+ T cell responses is preserved in perforin-deficient mice and released by depletion of CD4+CD25+ regulatory T cells. J. Leukoc. Biol. 94:825–833. 10.1189/jlb.0413200 [DOI] [PubMed] [Google Scholar]
- 27.Freeman ML, Burkum CE, Jensen MK, Woodland DL, Blackman MA. 2012. Gamma-herpesvirus reactivation differentially stimulates epitope-specific CD8 T cell responses. J. Immunol. 188:3812–3819. 10.4049/jimmunol.1102787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F. 2013. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 38:596–605. 10.1016/j.immuni.2012.11.020 [DOI] [PubMed] [Google Scholar]
- 29.Lischke T, Hegemann A, Gurka S, Vu Van D, Burmeister Y, Lam KP, Kershaw O, Mollenkopf HJ, Mages HW, Hutloff A, Kroczek RA. 2012. Comprehensive analysis of CD4+ T cells in the decision between tolerance and immunity in vivo reveals a pivotal role for ICOS. J. Immunol. 189:234–244. 10.4049/jimmunol.1102034 [DOI] [PubMed] [Google Scholar]
- 30.Phares TW, Stohlman SA, Hwang M, Min B, Hinton DR, Bergmann CC. 2012. CD4 T cells promote CD8 T cell immunity at the priming and effector site during viral encephalitis. J. Virol. 86:2416–2427. 10.1128/JVI.06797-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumamoto Y, Mattei LM, Sellers S, Payne GW, Iwasaki A. 2011. CD4+ T cells support cytotoxic T lymphocyte priming by controlling lymph node input. Proc. Natl. Acad. Sci. U. S. A. 108:8749–8754. 10.1073/pnas.1100567108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabarth N, Chamberlain L, Brett S, Tite J, Craigen J. 2010. Induction of homologous rather than heterologous antigen-specific CD4 T cell responses is critical for functional CD8 T cell responses in mice transgenic for a foreign antigen. J. Immunol. 185:4590–4601. 10.4049/jimmunol.0803994 [DOI] [PubMed] [Google Scholar]
- 33.Hendrikx LH, Ozturk K, de Rond LG, de Greeff SC, Sanders EA, Berbers GA, Buisman AM. 2011. Serum IgA responses against pertussis proteins in infected and Dutch wP or aP vaccinated children: an additional role in pertussis diagnostics. PLoS One 6:e27681. 10.1371/journal.pone.0027681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, Devillier G, Cong Z, Wei Q, Gao H, Qin C, Yang GB, Zurbriggen R, Lopalco L, Fleury S. 2011. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 34:269–280. 10.1016/j.immuni.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Koroleva EP, Kruglov AA, Kuprash DV, Nedospasov SA, Fu YX, Tumanov AV. 2010. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity 32:403–413. 10.1016/j.immuni.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. 2011. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. U. S. A. 108:5354–5359. 10.1073/pnas.1019378108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novak J, Moldoveanu Z, Julian BA, Raska M, Wyatt RJ, Suzuki Y, Tomino Y, Gharavi AG, Mestecky J, Suzuki H. 2011. Aberrant glycosylation of IgA1 and anti-glycan antibodies in IgA nephropathy: role of mucosal immune system. Adv. Otorhinolaryngol. 72:60–63. 10.1159/000324607 [DOI] [PubMed] [Google Scholar]
- 38.Tokuhara D, Yuki Y, Nochi T, Kodama T, Mejima M, Kurokawa S, Takahashi Y, Nanno M, Nakanishi U, Takaiwa F, Honda T, Kiyono H. 2010. Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. Proc. Natl. Acad. Sci. U. S. A. 107:8794–8799. 10.1073/pnas.0914121107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mestecky J, Raska M, Novak J, Alexander RC, Moldoveanu Z. 2010. Antibody-mediated protection and the mucosal immune system of the genital tract: relevance to vaccine design. J. Reprod. Immunol. 85:81–85. 10.1016/j.jri.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boullier S, Tanguy M, Kadaoui KA, Caubet C, Sansonetti P, Corthesy B, Phalipon A. 2009. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J. Immunol. 183:5879–5885. 10.4049/jimmunol.0901838 [DOI] [PubMed] [Google Scholar]
- 41.Xuefeng Q, Xiaoyan Y, Anchun C, Mingshu W, Dekang Z, Renyong J. 2008. The pathogenesis of duck virus enteritis in experimentally infected ducks: a quantitative time-course study using TaqMan polymerase chain reaction. Avian Pathol. 37:307–310. 10.1080/03079450802043775 [DOI] [PubMed] [Google Scholar]
- 42.Mbawuike IN, Pacheco S, Acuna CL, Switzer KC, Zhang Y, Harriman GR. 1999. Mucosal immunity to influenza without IgA: an IgA knockout mouse model. J. Immunol. 162:2530–2537 [PubMed] [Google Scholar]
- 43.Thompson EC. 2012. Focus issue: structure and function of lymphoid tissues. Trends Immunol. 33:255. 10.1016/j.it.2012.05.001 [DOI] [PubMed] [Google Scholar]


