Abstract
Toll-like receptors (TLRs) of the innate immune system are known targets for enhancing vaccine efficacy. We investigated whether imiquimod, a synthetic TLR7 agonist, can expedite the immune response against influenza virus infection when combined with influenza vaccine. BALB/c mice were immunized intraperitoneally with monovalent A(H1N1)pdm09 vaccine combined with imiquimod (VCI) prior to intranasal inoculation with a lethal dose of mouse-adapted A(H1N1)pdm09 virus. For mice immunized 3 days before infection, the survival rates were significantly higher in the VCI group (60%, mean survival time[MST], 11 days) than in the vaccine-alone (30%; MST, 8.8 days), imiquimod-alone (5%; MST, 8.4 days), and phosphate-buffered saline (PBS) (0%; MST, 6.2 days) groups (P < 0.01). In the VCI group, 45 and 35% of the mice survived even when they were infected 2 days or 1 day after immunization. Virus-specific serum IgM, IgG, and neutralizing antibodies appeared earlier with higher geometric mean titers in the VCI group than in the control groups. The pulmonary viral load was significantly lower at all time points postinfection in the VCI, vaccine-alone, and imiquimod-alone groups than in the PBS control group (P < 0.05). The protection induced by VCI was specific for A(H1N1)pdm09 virus but not for A(H5N1) virus. Since imiquimod combined with RNase-treated vaccine is as protective as imiquimod combined with untreated vaccine, mechanisms other than TLR7 may operate in expediting and augmenting immune protection. Moreover, increased gamma interferon mRNA expression and IgG isotype switching, which are markers of the Th1 response induced by imiquimod, were not apparent in our mouse model. The mechanisms of imiquimod-induced immune protection deserve further study.
INTRODUCTION
Influenza vaccination is an effective strategy to prevent both seasonal and pandemic influenza virus infections and reduce the risk of influenza-related complications, including myocardial infarction and stroke (1, 2). Several preparations of influenza vaccines are currently available, including the inactivated influenza whole-virus vaccine, virion-free “split” virus or subunit vaccine, recombinant hemagglutinin (HA) vaccine, and live attenuated influenza virus vaccine (3). Some vaccines contain an adjuvant such as aluminum salt, AS04, which contains both alum and monophosphoryl lipid A and MF59 (oil-in-water emulsion) to enhance efficacy. Nevertheless, meta-analysis estimated that the overall efficacy of these vaccines is around 70% (4). Recently, strategies have been employed to improve vaccine immunogenicity, including vaccination via the intradermal route (5, 6) and administration of new vaccine adjuvants by recruiting the functions of the pattern recognition receptors (PRRs) in the innate immune system. These PRRs include the Toll-like receptors (TLRs), retinoic acid-inducible gene-I-like receptors, and NOD-like receptors (7–9). Through recognition of and binding to pathogen-associated molecular patterns (PAMPs) conserved in microbes, PRRs could detect the various invading pathogens. The engagement of PRRs with PAMPs will immediately activate innate immune responses against the invading pathogen (10). This efficient activation of the innate immune response at the initial stage is critical for subsequent induction of the more effective adaptive immune response (11–13).
There are at least 10 types of TLRs present in humans (14). The natural ligand for TLR7 is a single-stranded RNA (ssRNA) molecule present in the viral genome or produced during viral replication (15–17). Activation of TLR7 induces antigen-presenting cells such as dendritic cells through the upregulation of human leukocyte antigen and costimulatory molecules (18–20). TLR7 signaling also augments the secretion of proinflammatory cytokines (17, 21). The role of TLR7 in the induction of the adaptive immune response has also been demonstrated by enhancing antibody-producing B cell differentiation (16, 22), facilitating antibody isotype class switching (13), and increasing long-term B cell memory (7). TLR7 also plays significant roles in both influenza virus infection and vaccination (19, 21, 23). Inactivated whole-virus influenza vaccine had better immunogenicity than split virus and subunit vaccine formulations; this was attributed to the activation of TLR7 by viral genome RNA present in the vaccine preparation (16, 24). Augmentation of vaccine immunogenicity by incorporating a synthetic TLR7 agonist has also been demonstrated in human immunodeficiency virus (25), human papillomavirus (26), and malaria (27) vaccine studies. The rationale for using a TLR7 agonist as a vaccine adjuvant is to trigger the activation and maturation of dendritic cells (28, 29), which effectively bridge the innate and adaptive immune responses.
Because of the frequent antigenic changes in the influenza virus and the continuous threat of an influenza pandemic and avian influenza viruses (1, 30–32), the development of a timely, antigen-matched, effective vaccine is a challenging task. The A(H1N1)pdm09 virus spread globally within 2 months because of a lack of immunity in the general population (33, 34). An immunization strategy that could induce rapid onset of immunity against imminent infection is thus highly desirable. In this study, we investigated whether the TLR7 agonist imiquimod could accelerate the onset of a protective antibody response to influenza vaccine.
MATERIALS AND METHODS
Reagents, virus strains, and animals.
The TLR7 agonist imiquimod (InvivoGen, San Diego, CA) was prepared with endotoxin-free water at 0.5 mg/ml in small aliquots and stored at −20°C until use. The monovalent A(H1N1)pdm09 vaccine PANENZA containing split, inactivated whole virus with HA protein at 30 μg/ml equivalent to A/California/7/2009 (H1N1) virus was obtained from Sanofi Pasteur (Swiftwater, PA). To prepare inactivated H5N1 whole-virus vaccine, A/VNM/1194/2004 virus was cultured in 10-day-old specific-pathogen-free (SPF) chicken embryos. Allantoic fluid was harvested at 36 h after inoculation and inactivated with 0.1% (vol/vol) formalin at 4°C for 7 days. Inactivation efficiency was determined by plaque assay on Madin-Darby canine kidney (MDCK) cells. The inactivated virus was then purified and concentrated by sucrose gradient ultracentrifugation at 28,000 rpm. In some experiments, the vaccine was treated with 100 μg/ml RNase at 37°C for 30 min to remove the ssRNA present in the vaccine preparation. The virus strains for mouse challenge experiments include the A/HK/415742/2009 (H1N1) mouse-adapted strain (35, 36) and A/VNM/1194/2004 (H5N1). The virus was propagated in 10-day-old SPF chicken embryos. Allantoic fluid was titrated on MDCK cells for the determination of 50% tissue culture infective doses (TCID50) and PFU counts. Mouse 50% lethal doses (LD50) were determined with 6- to 8-week-old female BALB/c mice as described previously (35, 36). Female BALB/c mice were obtained from the Animal Unit of the University of Hong Kong. Virus challenge experiments were performed in a biosafety level 2 (H1N1 virus) or 3 (H5N1 virus) animal facility according to the standard operating procedures approved by the Animal Ethics Committee.
Vaccination procedure with or without imiquimod and virus challenge.
As illustrated in Fig. 1, 6- to 8-week-old female BALB/c mice were randomly divided into groups. Imiquimod (50 μg in 100 μl) and vaccine (3 μg of HA protein in 100 μl) were injected intraperitoneally alone or in combination at 1, 2, or 3 days prior to virus infection (designated day −1, −2, or −3) or immediately before virus infection (day 0). The intramuscular route was not chosen because it would be difficult to inject 200 μl into hind-leg muscles without leakage. Mice in the negative-control group were injected with the same volume of phosphate-buffered saline (PBS). On the day of virus infection, a dose of the virus equal to 10 times the LD50 (2 × 103 TCID50) in 20 μl PBS was inoculated via the intranasal route into mice under ketamine (100 mg/kg) and xylazine (10 mg/kg) anesthesia. Body weight and survival were monitored for 14 days postinfection (p.i.). For sample collection, three to five mice in each group were sacrificed on days 1, 2, 4, and 6 p.i. Blood and lung samples were collected for further studies. Vaccination and virus challenge experiments were repeated two to four times, and the survival rates presented are the averages of all of the experiments.
FIG 1.
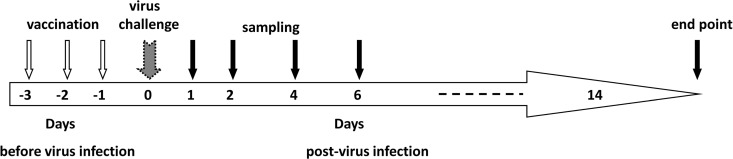
Diagram of the experimental design. PANENZA vaccine with or without imiquimod was administered by intraperitoneal injection 1, 2, or 3 days prior to virus infection. The date of vaccination was designated day −1, −2, or −3. Day 0 was the day of virus inoculation. At 1, 2, 4, and 6 days after virus infection, mice from each group were sacrificed for lung and blood sample collection. Survival and body weight after virus infection were observed for 14 days.
Real-time reverse transcriptase PCR (RT-PCR) detection of lung viral loads.
Total RNA was reverse transcribed into cDNA with Superscript RT II (Invitrogen Corp., Carlsbad, CA) and influenza virus-specific primer Uni12 (AGC AAA AGC). Real-time quantitative PCR was performed on a LightCycler 480 system with SYBR green I Master (Roche Applied Sciences, Indianapolis, IN) and a gene-specific primer pair (forward primer, GAT ACA CCA GTC CAC GAT TG; reverse primer, ACC ATC CAT CTA CCA TCC C) targeting the viral H1 gene. The pcDNA3.1 plasmid containing the H1 gene fragment was used as the standard (37).
Plaque assays.
For the detection of neutralizing antibodies in serum by plaque reduction assay, all serum samples were first treated with receptor-destroying enzyme (RDE; Denka Seiken, Tokyo, Japan) at 37°C overnight and then heat inactivated at 56°C for 30 min. Serum samples were then serially diluted 2-fold and mixed with 50 PFU of mouse-adapted A(H1N1)pdm09 virus. The serum-virus mixtures were incubated for 1 h at 37°C, added to MDCK cells in 24-well plates, and then allowed to adsorb for 1 h at 37°C. After adsorption, the cells were washed with PBS, overlaid with minimum essential medium (MEM) containing 2 μg/ml of l-1-tosylamide-2-phenylethyl chloromethyl ketone (TPCK)-treated trypsin and 2% low-melting-point agarose, and returned to 37°C for another 72 h of incubation. The cells were then fixed and stained with 0.5% crystal violet before the plaques in each dilution were counted. Uninfected mouse serum samples were used as negative controls. Percentages of plaque reduction in tested samples were calculated against the negative control, which was set at 100%. All of the data presented are the means of two experiments.
Detection of A(H1N1)pdm09-specific IgM and IgG in serum by EIA.
Vaccine antigen was used to coat 96-well immunoplates (Nunc-Immuno Modules; Nunc A/S, Roskilde, Denmark) at 100 μl per well containing 2 μg of HA/ml in 0.05 M NaHCO3 (pH 9.6) as described previously (38). The plate was incubated at 4°C overnight and then blocked with 1% normal goat serum at 37°C for 1 h. One hundred microliters of diluted serum was added, and the mixture was incubated at 37°C for 1 h. The plate was washed six times with PBS before 100 μl of horseradish peroxidase-conjugated secondary antibodies (Life Technology, Carlsbad, CA) was added and the mixture was incubated for 1 h at 37°C. The reaction was developed by adding 100 μl of diluted 3,3′,5,5′-tetramethylbenzidine solution (Life Technology, Carlsbad, CA) for incubation for 15 min at 37°C and stopped with 100 μl 1 N H2SO4. The optical density (OD) was read at 450 nm. For antibody detection, we used serial 2-fold dilutions of serum starting at 1:200 for IgM and at 1:500 for IgG, IgG2a, and IgG2b. Uninfected mouse sera were used as a negative control to set the enzyme immunoassay (EIA) OD cutoff value. The cutoff OD was set at the mean OD of uninfected serum at all dilutions plus 3 standard deviations. Serum dilutions yielding an OD above the cutoff value were calculated. The reciprocal of the average of duplicates was expressed as the titer.
MN and HAI assays.
Microneutralization (MN) and hemagglutination inhibition (HAI) assays were performed as described previously, with RDE-treated serum samples (38, 39). The HAI test was performed by incubating 25 μl of serially 2-fold-diluted (starting at 1:10) serum with 25 μl of 4 hemagglutination units of mouse-adapted A(H1N1)pdm09 virus at room temperature for 1 h. The plates were read after 40 min of incubation with 50 μl of 0.5% turkey red blood cells (Lampire Biological Laboratories, Pipersville, PA). For the MN test, diluted sera were mixed with 100 TCID50 of mouse-adapted A(H1N1)pdm09 virus and incubated at 37°C for 1 h. The serum-virus mixture was then added to MDCK cells in a 96-well plate. After adsorption for 1 h at 37°C, the cells were washed and incubated with MEM containing 1% penicillin-streptomycin and 2 μg/ml TPCK-treated trypsin at 37°C for 72 h. The cytopathic effect was recorded.
Detection of IFNs, cytokines, and chemokines by real-time RT-PCR.
Pulmonary levels of alpha interferon (IFN-α), IFN-β, IFN-γ, interleukin-1β (IL-1β), IL-6, and macrophage inflammatory protein 1α (MIP-1α) mRNA expression were determined by real-time RT-PCR. Total RNA was extracted from lung tissue homogenates with a Qiagen RNeasy Minikit (Qiagen, Germantown, MD). cDNA was synthesized by Superscript RT II (Life Technology, Carlsbad, CA) with oligo(dT) primer. Real-time quantitative PCR was performed on a 7900HT Fast Real-Time PCR System (Applied Biosystems, Life Technology, Carlsbad, CA) with SYBR green I Master (Roche Applied Sciences, Indianapolis, IN) and gene-specific primers. The expression of mouse β-actin was quantified and used for RNA normalization, and the threshold cycle (ΔΔCT) method was used to estimate the differential gene expression levels. The primers used for real-time PCR were as follows: IFN-α forward, 5′-ARSYTGTSTGATGCARCAGGT-3′; IFN-α reverse, 5′-GGWACACAGTGATCCTGTGG-3′; IFN-β forward, 5′-TGGGAGATGTCCTCAACTGC-3′; IFN-β reverse, 5′-CCTGCAACCACCACTCATTC-3′; IFN-γ forward, 5′-AAGCGTCATTGAATCACACC-3′; IFN-γ reverse, 5′-CGAATCAGCAGCGACTCCTT-3′; IL-1β forward, 5′-GCCTTGGGCCTCAAAGGAAAGAATC-3′; IL-1β reverse, 5′-GGAAGACACAGATTCCATGGTGAAG-3′; IL-6 forward, 5′-TGGAGTCACAGAAGGAGTGGCTAAG-3′; IL-6 reverse, 5′-TCTGACCACAGTGAGGAATGTCCAC-3′; MIP-1α forward, 5′-CCCAGCCAGGTGTCATTTTCC-3′; MIP-1α reverse, 5′-GCATTCAGTTCCAGGTCAGTG-3′; β-actin forward;, 5′-ACGGCCAGGTCATCACTATTG-3′; β-actin reverse, 5′-CAAGAAGGAAGGCTGGAAAAG-3′.
Statistical analysis.
Mouse survival rates after a virus challenge in different groups were analyzed by the Kaplan-Meier method and the log rank test. Serum antibody titers were compared by the Mann-Whitney U test. Pulmonary viral loads and cytokine and chemokine profiles were analyzed by Student's t test. IBM SPSS Statistics 20.0 was used for statistical computation. A P value of <0.05 was considered statistically significant.
RESULTS
Imiquimod in combination with influenza virus A(H1N1)pdm09 vaccine protected mice from an early lethal challenge with A(H1N1)pdm09 virus but not A(H5N1).
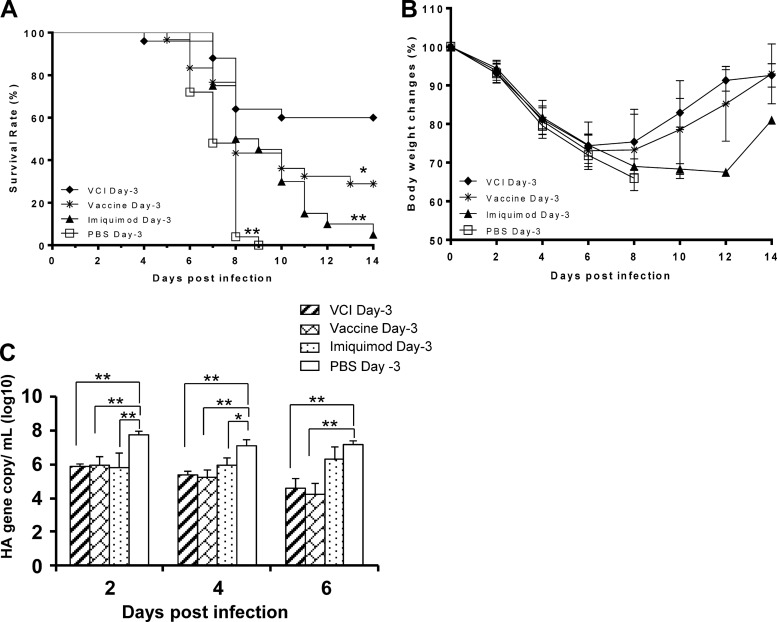
Mice that received VCI 3 days prior to A(H1N1)pdm09 virus infection (VCI day −3) showed 60% survival at 14 days p.i., with a mean survival time (MST) of 11.0 days when challenged with the A(H1N1)pdm09 virus (Fig. 2A). The survival rate of the VCI day −3 group was significantly higher than that of the groups that received the vaccine alone (vaccine day −3) (survival rate, 30%; MST, 8.8 days; P = 0.029), imiquimod alone (imiquimod day −3) (survival rate, 5%; MST, 8.4 days; P < 0.001), and the PBS control (PBS day −3) (survival rate, 0%; MST, 6.2 days; P < 0.001). There was a rebound of body weight in the VCI day −3 group from day 8 p.i. onward (Fig. 2B), indicating recovery from infection. The survival rates of the vaccine day −3 and imiquimod day −3 groups were significantly higher than that of the PBS control group (P < 0.001). The pulmonary viral titers, as determined by quantitative RT-PCR, were not significantly different among the VCI day −3 group, the vaccine day −3 group, and the imiquimod day −3 group on days 2, 4, and 6 p.i. However, the pulmonary viral titers of the VCI day −3 and vaccine day −3 groups were significantly lower than that of the PBS day −3 control group (Fig. 2C).
FIG 2.
Survival rates, body weights, and pulmonary viral titers of infected BALB/c mice. Three days before a viral challenge, mice received an intraperitoneal injection of imiquimod (50 μg) and PANENZA vaccine (3 μg of HA) in combination (VCI day −3; ⧫), vaccine alone (vaccine day −3; *), imiquimod alone (imiquimod day −3; ▲), or PBS (PBS day −3; □). On the day of infection, mouse-adapted A(H1N1)pdm09 virus (10 times the mouse LD50) was inoculated intranasally. Survival (A) and body weight (B) were monitored for 14 days. n = 20 to 30 per group. Results are mean data from five experiments. *, P < 0.05; **, P < 0.01 (compared with the VCI day −3 group). (C) Lung samples were taken at the indicated times after virus infection and homogenized for pulmonary virus titration by quantitative real-time RT-PCR. n = 9 for the PBS group, and n = 8 for all of the other groups. *, P < 0.05; **, P < 0.01. Error bars indicate standard deviations.
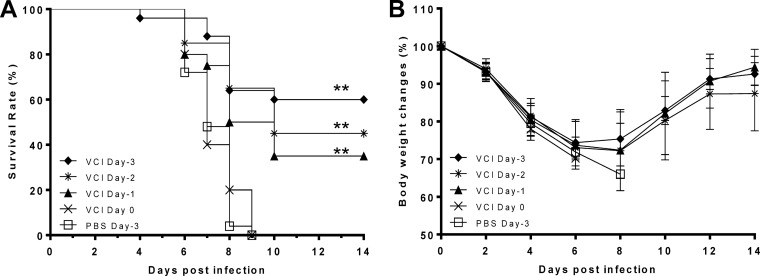
When the time interval between VCI immunization and virus challenge was shortened to 2 days (VCI day −2 group) or 1 day (VCI day −1 group), the survival rates fell to 45% (MST, 10.3 days) and 35% (MST, 9.3 days), respectively (Fig. 3). However, even the survival rate of the VCI day −1 group was significantly higher than that of the PBS control group (P < 0.001), indicating that VCI immunization could still elicit significant immunity against virus infection at 24 h before a virus challenge. However, VCI did not offer any protection when administered on the day of a virus challenge.
FIG 3.
Survival rates (A) and body weight (B) changes of mice immunized at different times prior to virus infection. Immunized mice received imiquimod (50 μg) and PANENZA vaccine (3 μg of HA) in combination (VCI) on day −3 (⧫), day −2 (*), day −1 (▲), or day 0 (×). Control mice received PBS on day −3 (□). On the day of infection (day 0), mice were infected with 10 times the mouse LD50 of mouse-adapted A(H1N1)pdm09 virus and monitored for 14 days. n = 20 to 30 per group. Results are mean data from five experiments. Error bars indicate standard deviations. **, P < 0.01 (compared with the PBS group).
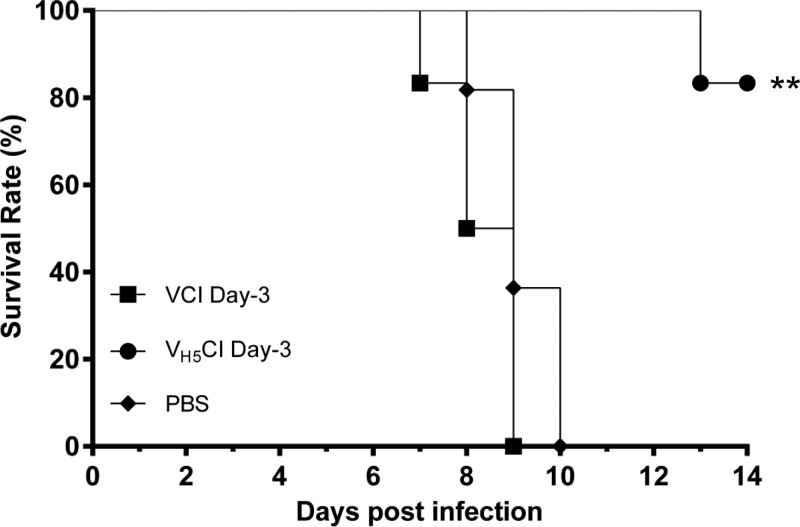
To investigate whether VCI can induce protection against a different influenza virus subtype, we challenge VCI day −3 mice with A(H5N1) virus (Fig. 4). When challenged with the A(H5N1) virus, all of the mice in the VCI day −3 group (6/6) died, while only 17% (1/6) of the mice that received inactivated A(H5N1) virus vaccine combined with imiquimod 3 days prior to infection (VH5CI day −3) died (P < 0.01).
FIG 4.
Survival rates of BALB/c mice challenged intranasally with H5N1 virus A/VNM/1194/2004. Mice received imiquimod (50 μg) and PANENZA vaccine (3 μg of HA) in combination (■) (VCI day −3), imiquimod (50 μg) and formalin-inactivated A/VNM/1194/2004 vaccine (3 μg of HA) in combination (●) (VH5CI day −3), or PBS (⧫) on day −3 before virus infection. On the day of infection, mice were inoculated intranasally with 5 times the mouse LD50 of A/VNM/1194/2004 diluted in 20 μl of PBS. Survival was observed for 14 days after virus infection. **, P < 0.01 (compared with the imiquimod-PANENZA vaccine [VCI day −3] or PBS group). n = 6 per group.
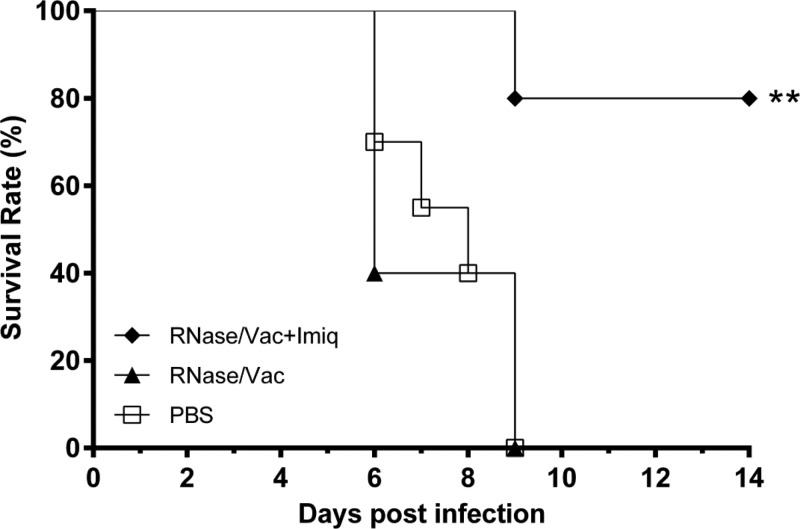
By quantitative real-time RT-PCR, we verified that a small amount of viral RNA was present in the PANENZA vaccine preparation (8 × 104 copies/ml; data not shown). To assess the effect of A(H1N1)pdm09 virus ssRNA in the A(H1N1)pdm09 vaccine in eliciting protection, we used RNase to remove the ssRNA from the vaccine preparation. All of the A(H1N1)pdm09-infected mice that received RNase-treated A(H1N1)pdm09 vaccine without imiquimod died, while only 17% (1/6) of the infected mice that received RNase-treated vaccine with imiquimod died (P < 0.001) (Fig. 5).
FIG 5.
PANENZA vaccine was treated with 100 μg/ml RNase at 37°C for 30 min. RNase-treated vaccine (3 μg of HA) was injected intraperitoneally alone (RNase/Vac; ▲) or in combination with 50 μg of imiquimod (RNase/Vac+imq; ⧫) 3 days prior to virus infection. Mice in the control group received the same volume of PBS (□). The mice were then infected intranasally with 10 times the mouse LD50 of mouse-adapted A(H1N1)pdm09 virus. Survival was observed for 14 days after virus infection. **, P < 0.01 (compared with the RNase-treated vaccine or PBS group). n = 6 per group.
Early production of virus-specific serum IgM and IgG in the VCI day −3 group.
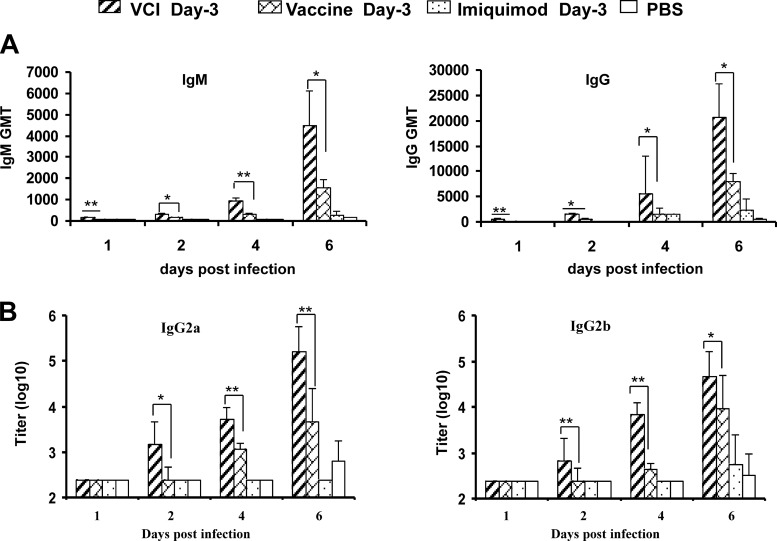
On day 1 p.i., VCI day −3 immunization induced the production of A(H1N1)pdm09-specific IgM and IgG in 60% (geometric mean titer [GMT], 151) and 100% (GMT, 500) of the mice, while none of the mice in the other groups had detectable IgM or IgG titers (Table 1; Fig. 6A). On day 6 p.i., the GMTs of both IgM and IgG in the VCI day −3 group were significantly higher than those of the vaccine day −3 group (IgM, 4,526 versus 1,600 [P < 0.05]; IgG, 20,749 versus 4,876 [P < 0.05]). VCI day −3 immunization induced significantly higher levels of IgG2a and IgG2b than in the other groups (Fig. 6B). These results suggest that VCI day −3 immunization could induce early onset of virus-specific antibody production.
TABLE 1.
GMTs of serum antibodies
| Group | GMTa at: |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 p.i. |
Day 2 p.i. |
Day 4 p.i. |
Day 6 p.i. |
|||||||||||||
| HAI | MN | IgM | IgG | HAI | MN | IgM | IgG | HAI | MN | IgM | IgG | HAI | MN | IgM | IgG | |
| VCI day −3 | <10 | <10 | 151c (3/5) | 500c (4/5) | <10 | <10 | 348d (5/5) | 1516d (5/5) | <10 | 13d (1/8) | 951c (8/8) | 5657d (8/8) | 269 (8/8) | 494c (8/8) | 4526d (8/8) | 20749d (8/8) |
| VCI day −2 | NDb | ND | <200 | <500 | <10 | <10 | 152 (3/5) | <500 (0/5) | <10 | <10 | 696c (5/5) | 1,149 (5/5) | 226 (6/6) | 202 (6/6) | 2,851 (6/6) | 8,000 (6/6) |
| VCI day −1 | ND | ND | <200 | <500 | <10 | <10 | 174 (4/5) | <500 (0/5) | <10 | <10 | 303 (5/5) | 758 (5/5) | 211 (5/5) | 160 (5/5) | 1,393 (6/6) | 4,000 (5/5) |
| Vaccine day −3 | ND | ND | <200 | <500 | <10 | <10 | 174 (4/5) | 435 (3/5) | <10 | <10 | 336 (8/8) | 1,682 (8/8) | 119 (6/7) | 54 (5/7) | 1,600 (7/7) | 4,876 (6/7) |
| Imiquimod day −3 | ND | ND | <200 | <500 | <10 | <10 | <200 | <500 | <10 | <10 | <200 | 500 (3/8) | 10 (2/8) | 11 (2/8) | 200 (5/8) | 1,834 (7/8) |
| PBS | <10 | <10 | <200 | <500 | <10 | <10 | <200 | <500 | <10 | <10 | <200 | <500 | <10 | <10 | 119 (3/9) | 354 (3/9) |
An HAI assay titer of ≥1:40 was considered positive. Vaccine day −3, vaccine alone given 3 days prior to virus infection. An MN assay titer of ≥1:40 was considered positive. The values in parentheses are the number positive/number tested.
ND, not done.
P < 0.01 compared with the vaccine-alone group (Mann-Whitney U test).
P < 0.05 compared with the vaccine-alone group (Mann-Whitney U test).
FIG 6.
GMTs of A(H1N1)pdm09 virus-specific IgM and IgG (A) and IgG subtypes (B) in mouse serum detected by EIA. Mice were given different immunization regimens on day −3. At the indicated times after virus infection, sera were collected and tested for virus-specific antibodies. n = 7 to 9 per group. Error bars indicate standard deviations. *, P < 0.05; **, P < 0.01 (compared with the vaccine day −3 group).
Early production of viral neutralizing antibody in the VCI group.
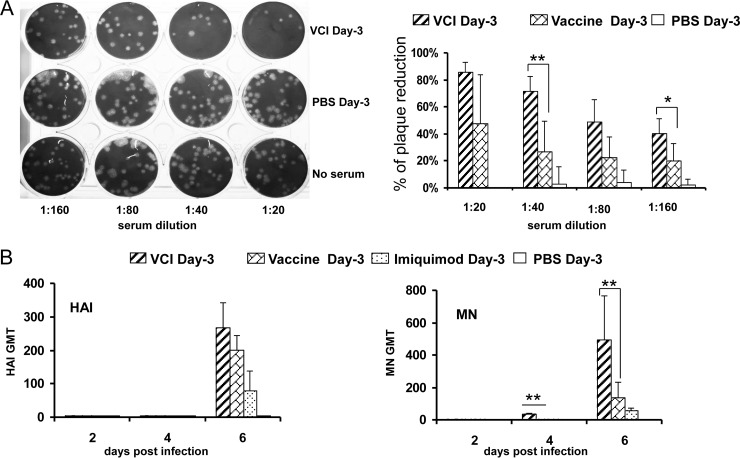
By MN assay, the earliest time point that showed detectable neutralizing antibody was day 4 p.i. in the VCI day −3 group, with 12.5% (1/8) of the samples showing an MN titer of 13 (Table 1). On the other hand, neutralizing antibody was not detected until day 6 p.i in the vaccine day −3 group. On day 6 p.i., the HAI and MN GMTs were much higher in the VCI day −3 group (GMTs, 269 and 494, respectively) than in the vaccine day −3 group, but only the difference in MN titers was statistically significant (P < 0.01). In view of the high IgG titer (GMT, 5,657) on day 4 p.i in the VCI day −3 group, the neutralizing antibody titer was also measured by plaque reduction assay. All five serum samples from the VCI day −3 group inhibited plaque formation by 50% at a 1:80 dilution, while none (0/5) of the sera from the vaccine day −3 group showed 50% reduction in plaque number even at a dilution of 1:40 (Fig. 7).
FIG 7.
Neutralizing antibody titers in mouse sera. All mice received different immunization regimens on day −3. (A) Representative image of plaque inhibition by diluted sera collected on day 4 p.i. (left). The percentage of inhibition of plaque formation was calculated against the negative control (no serum), which was taken as 100%. Data represent the means plus standard deviations (right). (B) HAI and MN assays of sera collected at various time points postinfection. GMTs of HAI (left) and MN (right) antibodies are shown. n = 7 to 9 per group. Error bars indicate standard deviations. *, P < 0.05; **, P < 0.01 (compared with the vaccine day −3 group).
IL-1β and MIP-1α mRNA levels were elevated in mouse lung tissue.
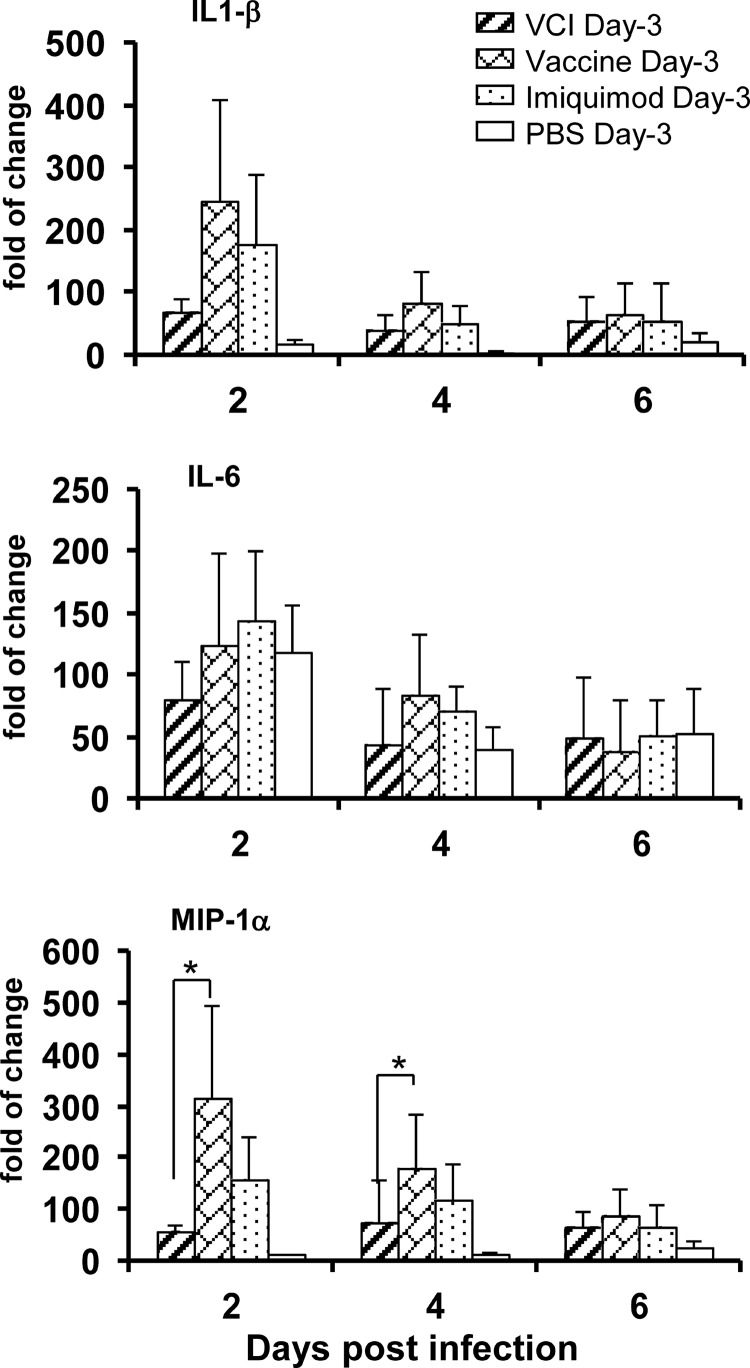
There was no difference in the IL-1β and IL-6 mRNA levels between the VCI day −3 and vaccine day −3 groups on days 2 and 4 p.i. However, the levels of MIP-1α mRNA were significantly lower in the VCI day −3 group than in the vaccine day −3 group on days 2 (P = 0.033) and 4 (P = 0.045) p.i. (Fig. 8). On the other hand, the levels of pulmonary proinflammatory cytokine IL-1β and MIP-1α mRNA expression were significantly higher in the VCI day −3 group than in the PBS control group on day 2 p.i. (P = 0.004 and P = 0.001, respectively). IL-6 production appeared to be blunted in the VCI group compared with that in the PBS group, but the difference was not significant.
FIG 8.
Pulmonary IL-1β, IL-6, and MIP-1α mRNA expression levels were determined by real-time RT-PCR. Mice that received different immunization regimens on day −3 were infected with 10 times the LD50 of mouse-adapted A(H1N1)pdm09. Lung homogenates were used for RNA extraction and detection of IL-1β, IL-6, and MIP-1α mRNAs. n = 9 for the PBS group; n = 8 for the other groups. Lung samples from uninfected mice (n = 3) were used as the baseline control to compare the changes in gene expression. Error bars indicate standard deviations. *, P < 0.05 (compared with the vaccine day −3 group).
Induction of IFN-α, IFN-β, and IFN-γ in the mouse lung.
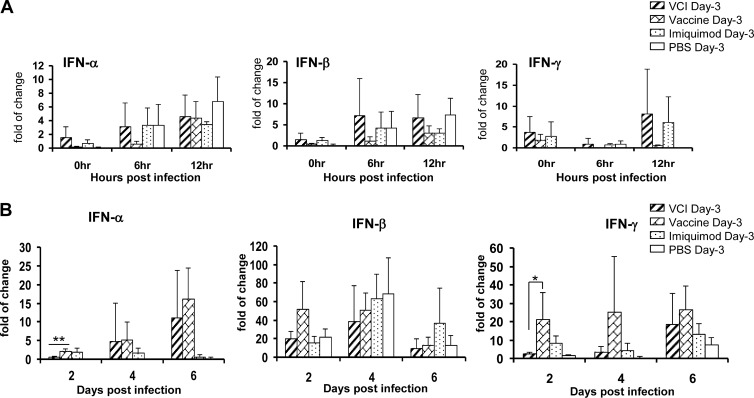
As imiquimod has been reported to induce type I IFN and IFN-γ production (40, 41), we determined the levels of IFN-α, IFN-β, and IFN-γ mRNA expression in mouse lungs at different time points. There was no significant difference in the pulmonary IFN-α, IFN-β, and IFN-γ mRNA levels of any of the groups before or after a viral challenge within the first 12 h (Fig. 9A). However, on day 2 p.i, the pulmonary IFN-α and IFN-γ mRNA levels of the VCI day −3 group were lower than those of the vaccine day −3 or imiquimod day −3 group (Fig. 9B).
FIG 9.
IFN-α, IFN-β, and IFN-γ mRNA expression levels in mouse lungs at the indicated times were determined by real-time RT-PCR. Mice that received different immunization regimens on day −3 were infected with 10 times the LD50 of mouse-adapted A(H1N1)pdm09. Relative levels of IFN-α, IFN-β, and IFN-γ mRNA expression were determined by real-time RT-PCR with gene-specific primers (A, B). Lung samples from uninfected mice (n = 3) were used as the baseline control to compare the changes in gene expression. n = 9 for the PBS group; n = 8 for other groups. Error bars indicate standard deviations. *, P < 0.05; **, P < 0.01 (compared with the vaccine day −3 group).
DISCUSSION
Rapid induction of a neutralizing antibody response by influenza vaccine is essential in the early control of influenza, especially during a novel pandemic or avian influenza outbreak in an immunologically naive population (38). The influenza vaccines currently available for human use require 14 to 21 days to achieve adequate levels of neutralizing antibody, even if an adjuvant is added to the vaccine (5, 42, 43). In this study, we sought to investigate whether the synthetic TLR7 agonist imiquimod can accelerate the protective immune response. We showed that imiquimod significantly expedited and augmented the humoral immune responses against influenza A(H1N1)pdm09 virus when administered together with influenza vaccine. VCI conferred significant protection on BALB/c mice against early lethal viral challenges, which could not be achieved by imiquimod or vaccine alone. Furthermore, the protection afforded by VCI was specific to the A(H1N1)pdm09 virus but not the A(H5N1) virus. A study using a glucopyranosyl lipid adjuvant and a stable emulsion of oil in water showed improved survival of mice if the vaccine was given at least 3 days prior to a viral challenge (44). Further experiments are needed to determine whether another adjuvant, such as AS03 or MF59, can also achieve a protective effect within such a short period. In our present study, we have shown that a protective effect could be accomplished in mice that received VCI as late as 1 day before a viral challenge.
Compared to the vaccine day −3 group, the VCI day −3 group had earlier production of A(H1N1)pdm09-specific IgM, IgG, and neutralizing antibodies. Previous studies reported that imiquimod improved the antibody response via the TLR7 pathway. TLR7 activation leads to the induction of proinflammatory cytokines such as IL-6, chemokines such as MIP-1α, and antiviral cytokines such as IFN-α, IFN-β, and IFN-γ (45). Induction of cytokines leads to the activation of antigen-presenting cells and other components of innate immunity, eventually activating the Th1 cellular immune response. In our study, TLR7 appeared to play some role in the protection of the vaccine day −3 group because the removal of ssRNA from the vaccine day −3 group with RNase abolished the protective response of the vaccine day −3 group but not that of the VCI day −3 group. Weldon et al. previously showed that imiquimod can enhance IFN-γ, leading to a Th1 cellular immune response and isotype switching, which resulted in a specific increase in the level of IgG2a (40). However, in our study, the increase in the pulmonary IFN-γ level was much smaller in the VCI day −3 group than in the vaccine day −3 group, together with the increase in the total serum IgG, IgG2a, and IgG2b levels. Our results suggest that imiquimod augmented antibody levels not solely by increasing the Th1 response. Other branches of the immune system are likely to be important in the imiquimod-mediated protective response. One possibility is the direct activation of B cells without T cell help. Imiquimod has been shown to activate B cells in the absence of T cells (46). In addition to the stimulation of T helper cells and B cells, imiquimod has also been reported to stimulate cytotoxic T cells and natural killer cells (46). Evidence also suggests that imiquimod can act via the TLR-independent adenosine receptor-signaling pathway (45) by reducing the adenylyl cyclase activity, suppressing the negative regulatory feedback mechanism, which can augment the proinflammatory activity. Adjuvants such as Freund's complete adjuvant, which were previously thought to enhance the immune response via TLR signaling, did not require TLR signaling to elicit a robust immune response (47). Further studies are needed to clarify the exact pathways that lead to the protective immune response elicited by the VCI approach.
The pulmonary viral load was lower in the VCI day −3 group than in the PBS group, but there was no significant difference among the pulmonary viral loads of the VCI day −3, vaccine day −3, and imiquimod day −3 groups. Since there was also better survival in the vaccine day −3 and imiquimod day −3 groups than in the PBS group, the results suggest that the lighter viral load may be associated with the survival benefit. However, since the survival rates of the vaccine day −3 and imiquimod day −3 groups were lower than that of the VCI day −3 group, other factors may be operating. One possibility is the difference in the induction of cytokine/chemokine responses. We have shown that the day 2 and 4 levels of MIP-1α, IL-1β, and IL-6 were lower in the VCI day −3 group than in the vaccine day −3 group, although the difference was only significant for MIP-1α. Therefore, besides protection from enhanced cytokine and chemokine production, imiquimod may have activated an additional protective immune response through other pathways. Although cytokines and chemokines are necessary for successful immune protection, this may be a double-edged sword as cytokine dysregulation may lead to an adverse outcome (48).
The imiquimod day −3 group had a significantly higher survival rate than the PBS group. There was also a lighter pulmonary viral load and earlier development and higher titers of A(H1N1)pdm09 virus-specific IgG, HI antibody, and neutralizing antibody in the imiquimod day −3 group than in the PBS group. Therefore, imiquimod can expedite and augment the immune response to an influenza virus challenge even without prior specific immune stimulation by the A(H1N1)pdm09 vaccine. Therefore, imiquimod, by enhancing the innate immune response alone, can provide some protection, but the response is markedly inferior to the augmented adaptive immune response induced by VCI.
In the present study, we have shown that imiquimod can accelerate the immune response induced by vaccine. In the future, it would be important to study the effect of imiquimod on long-term immunity. One recent study of children has shown that a single vaccination with 7.5 μg A/California/7/2009 antigen and a full dose of MF59 adjuvant was able to meet all of the U.S. and European licensure criteria for seroprotection up to 1 year after immunization (49). Similar studies of adults also demonstrated that the seroprotection rate was preserved in 70% of the MF59-adjuvanted vaccine recipients at 10 months postvaccination (50) and protective immune response was maintained for up to 6 months in the AS03(A)-adjuvanted vaccine recipients (51).
Unlike previous reports of induction of IFN-α and -β by imiquimod, such a phenomenon was not obvious in our mouse model. Our present findings suggest that multiple mechanisms of the innate immune system may be operating in the induction of an optimal adaptive immune response. Though the exact mechanism is still under investigation, we postulate that imiquimod may potentiate early vaccine-specific antigenic presentation by antigen-presenting cells and therefore early activation of the humoral immune system. Further study of more relevant routes of vaccine administration, such as intradermal or intramuscular injection, should be undertaken. The potential clinical application of this vaccination strategy undoubtedly warrants attention, and the underlying mechanisms governing the early onset of virus-specific antibody production deserve further investigation in clinical trials.
ACKNOWLEDGMENTS
This work was partly supported by the National Science and Technology Major Project of China (grant 2009ZX10004-306), the Providence Foundation Limited in memory of the late Lui Hac Minh, and the Health and Medical Research Fund of the Food and Health Bureau, Hong Kong Special Administrative Region, China.
Footnotes
Published ahead of print 12 February 2014
REFERENCES
- 1.Cheng VC, To KK, Tse H, Hung IF, Yuen KY. 2012. Two years after pandemic influenza A/2009/H1N1: what have we learned? Clin. Microbiol. Rev. 25:223–263. 10.1128/CMR.05012-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hung IF, Leung AY, Chu DW, Leung D, Cheung T, Chan CK, Lam CL, Liu SH, Chu CM, Ho PL, Chan S, Lam TH, Liang R, Yuen KY. 2010. Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clin. Infect. Dis. 51:1007–1016. 10.1086/656587 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2013. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013-2014. MMWR Recomm. Rep. 62(RR-07):1–43 (Erratum, 62:906.) [PubMed] [Google Scholar]
- 4.Osterholm MT, Kelley NS, Sommer A, Belongia EA. 2012. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 12:36–44. 10.1016/S1473-3099(11)70295-X [DOI] [PubMed] [Google Scholar]
- 5.Hung IF, Levin Y, To KK, Chan KH, Zhang AJ, Li P, Li C, Xu T, Wong TY, Yuen KY. 2012. Dose sparing intradermal trivalent influenza (2010/2011) vaccination overcomes reduced immunogenicity of the 2009 H1N1 strain. Vaccine 30:6427–6435. 10.1016/j.vaccine.2012.08.014 [DOI] [PubMed] [Google Scholar]
- 6.Hung IF, Levin Y, To KK. 2012. Quantitative and qualitative analysis of antibody response after dose sparing intradermal 2009 H1N1 vaccination. Vaccine 30:2707–2708. 10.1016/j.vaccine.2011.12.069 [DOI] [PubMed] [Google Scholar]
- 7.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. 2011. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543–547. 10.1038/nature09737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pashine A, Valiante NM, Ulmer JB. 2005. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 11:S63–S68. 10.1038/nm1210 [DOI] [PubMed] [Google Scholar]
- 9.Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Mark Saltzman W, Mellman I, Ledizet M, Fikrig E, Flavell RA, Fahmy TM. 2009. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine 27:3013–3021. 10.1016/j.vaccine.2009.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melchjorsen J. 2013. Learning from the messengers: innate sensing of viruses and cytokine regulation of immunity—clues for treatments and vaccines. Viruses 5:470–527. 10.3390/v5020470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwasaki A, Medzhitov R. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995. 10.1038/ni1112 [DOI] [PubMed] [Google Scholar]
- 12.Randhawa AK, Shey MS, Keyser A, Peixoto B, Wells RD, de Kock M, Lerumo L, Hughes J, Hussey G, Hawkridge A, Kaplan G, Hanekom WA, Hawn TR, South African Tuberculosis Vaccine Initiative T 2011. Association of human TLR1 and TLR6 deficiency with altered immune responses to BCG vaccination in South African infants. PLoS Pathog. 7:e1002174. 10.1371/journal.ppat.1002174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, Marsland BJ. 2007. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J. Immunol. 178:2182–2191 [DOI] [PubMed] [Google Scholar]
- 14.Lim KH, Staudt LM. 2013. Toll-like receptor signaling. Cold Spring Harb. Perspect. Biol. 5:a011247. 10.1101/cshperspect.a011247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A. 101:5598–5603. 10.1073/pnas.0400937101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeisy-Scott V, Kim JH, Davis WG, Cao W, Katz JM, Sambhara S. 2012. TLR7 recognition is dispensable for influenza virus A infection but important for the induction of hemagglutinin-specific antibodies in response to the 2009 pandemic split vaccine in mice. J. Virol. 86:10988–10998. 10.1128/JVI.01064-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welte T, Reagan K, Fang H, Machain-Williams C, Zheng X, Mendell N, Chang GJ, Wu P, Blair CD, Wang T. 2009. Toll-like receptor 7-induced immune response to cutaneous West Nile virus infection. J. Gen. Virol. 90:2660–2668. 10.1099/vir.0.011783-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajagopal D, Paturel C, Morel Y, Uematsu S, Akira S, Diebold SS. 2010. Plasmacytoid dendritic cell-derived type I interferon is crucial for the adjuvant activity of Toll-like receptor 7 agonists. Blood 115:1949–1957. 10.1182/blood-2009-08-238543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaminski MM, Ohnemus A, Cornitescu M, Staeheli P. 2012. Plasmacytoid dendritic cells and Toll-like receptor 7-dependent signalling promote efficient protection of mice against highly virulent influenza A virus. J. Gen. Virol. 93:555–559. 10.1099/vir.0.039065-0 [DOI] [PubMed] [Google Scholar]
- 20.Mouriès J, Moron G, Schlecht G, Escriou N, Dadaglio G, Leclerc C. 2008. Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood 112:3713–3722. 10.1182/blood-2008-03-146290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, Uematsu S, Kawai T, Akira S. 2007. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J. Immunol. 179:4711–4720 [DOI] [PubMed] [Google Scholar]
- 22.Boeglin E, Smulski CR, Brun S, Milosevic S, Schneider P, Fournel S. 2011. Toll-like receptor agonists synergize with CD40L to induce either proliferation or plasma cell differentiation of mouse B cells. PLoS One 6:e25542. 10.1371/journal.pone.0025542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz N, Beerli RR, Bauer M, Jegerlehner A, Dietmeier K, Maudrich M, Pumpens P, Saudan P, Bachmann MF. 2012. Universal vaccine against influenza virus: linking TLR signaling to anti-viral protection. Eur. J. Immunol. 42:863–869. 10.1002/eji.201041225 [DOI] [PubMed] [Google Scholar]
- 24.Geeraedts F, Goutagny N, Hornung V, Severa M, de Haan A, Pool J, Wilschut J, Fitzgerald KA, Huckriede A. 2008. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by Toll-like receptor signalling. PLoS Pathog. 4:e1000138. 10.1371/journal.ppat.1000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. 2005. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc. Natl. Acad. Sci. U. S. A. 102:15190–15194. 10.1073/pnas.0507484102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern PL, van der Burg SH, Hampson IN, Broker TR, Fiander A, Lacey CJ, Kitchener HC, Einstein MH. 2012. Therapy of human papillomavirus-related disease. Vaccine 30(Suppl 5):F71–F82. 10.1016/j.vaccine.2012.05.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Othoro C, Johnston D, Lee R, Soverow J, Bystryn JC, Nardin E. 2009. Enhanced immunogenicity of Plasmodium falciparum peptide vaccines using a topical adjuvant containing a potent synthetic Toll-like receptor 7 agonist, imiquimod. Infect. Immun. 77:739–748. 10.1128/IAI.00974-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW, Noelle RJ, Miller RL, Kedl RM, Fitzgerald-Bocarsly P, Tomai MA, Vasilakos JP. 2002. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell. Immunol. 218:74–86. 10.1016/S0008-8749(02)00517-8 [DOI] [PubMed] [Google Scholar]
- 29.Russo C, Cornella-Taracido I, Galli-Stampino L, Jain R, Harrington E, Isome Y, Tavarini S, Sammicheli C, Nuti S, Mbow ML, Valiante NM, Tallarico J, De Gregorio E, Soldaini E. 2011. Small molecule Toll-like receptor 7 agonists localize to the MHC class II loading compartment of human plasmacytoid dendritic cells. Blood 117:5683–5691. 10.1182/blood-2010-12-328138 [DOI] [PubMed] [Google Scholar]
- 30.To KK, Chan JF, Chen H, Li L, Yuen KY. 2013. The emergence of influenza A H7N9 in human beings 16 years after influenza A H5N1: a tale of two cities. Lancet Infect. Dis. 13:809–821. 10.1016/S1473-3099(13)70167-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.To KK, Ng KH, Que TL, Chan JM, Tsang KY, Tsang AK, Chen H, Yuen K. 2012. Avian influenza A H5N1 virus: a continuous threat to humans. Emerg. Microbes Infect. 1:e25. 10.1038/emi.2012.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrat F, Flahault A. 2007. Influenza vaccine: the challenge of antigenic drift. Vaccine 25:6852–6862. 10.1016/j.vaccine.2007.07.027 [DOI] [PubMed] [Google Scholar]
- 33.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, Brammer TL, Cox NJ, Tumpey TM, Katz JM. 2009. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 361:1945–1952. 10.1056/NEJMoa0906453 [DOI] [PubMed] [Google Scholar]
- 34.Zhang AJ, To KK, Tse H, Chan KH, Guo KY, Li C, Hung IF, Chan JF, Chen H, Tam S, Yuen KY. 2011. High incidence of severe influenza among individuals over 50 years of age. Clin. Vaccine Immunol. 18:1918–1924. 10.1128/CVI.05357-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan KH, Zhang AJ, To KK, Chan CC, Poon VK, Guo K, Ng F, Zhang QW, Leung VH, Cheung AN, Lau CC, Woo PC, Tse H, Wu W, Chen H, Zheng BJ, Yuen KY. 2010. Wild type and mutant 2009 pandemic influenza A (H1N1) viruses cause more severe disease and higher mortality in pregnant BALB/c mice. PLoS One 5:e13757. 10.1371/journal.pone.0013757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang AJ, To KK, Li C, Lau CC, Poon VK, Chan CC, Zheng BJ, Hung IF, Lam KS, Xu A, Yuen KY. 2013. Leptin mediates the pathogenesis of severe 2009 pandemic influenza A(H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J. Infect. Dis. 207:1270–1280. 10.1093/infdis/jit031 [DOI] [PubMed] [Google Scholar]
- 37.Zheng BJ, Chan KW, Lin YP, Zhao GY, Chan C, Zhang HJ, Chen HL, Wong SS, Lau SK, Woo PC, Chan KH, Jin DY, Yuen KY. 2008. Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc. Natl. Acad. Sci. U. S. A. 105:8091–8096. 10.1073/pnas.0711942105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.To KK, Zhang AJ, Hung IF, Xu T, Ip WC, Wong RT, Ng JC, Chan JF, Chan KH, Yuen KY. 2012. High titer and avidity of nonneutralizing antibodies against influenza vaccine antigen are associated with severe influenza. Clin. Vaccine Immunol. 19:1012–1018. 10.1128/CVI.00081-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan KH, To KK, Hung IF, Zhang AJ, Chan JF, Cheng VC, Tse H, Che XY, Chen H, Yuen KY. 2011. Differences in antibody responses of individuals with natural infection and those vaccinated against pandemic H1N1 2009 influenza. Clin. Vaccine Immunol. 18:867–873. 10.1128/CVI.00555-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weldon WC, Zarnitsyn VG, Esser ES, Taherbhai MT, Koutsonanos DG, Vassilieva EV, Skountzou I, Prausnitz MR, Compans RW. 2012. Effect of adjuvants on responses to skin immunization by microneedles coated with influenza subunit vaccine. PLoS One 7:e41501. 10.1371/journal.pone.0041501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196–200. 10.1038/ni758 [DOI] [PubMed] [Google Scholar]
- 42.Barrett PN, Berezuk G, Fritsch S, Aichinger G, Hart MK, El-Amin W, Kistner O, Ehrlich HJ. 2011. Efficacy, safety, and immunogenicity of a Vero-cell-culture-derived trivalent influenza vaccine: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 377:751–759. 10.1016/S0140-6736(10)62228-3 [DOI] [PubMed] [Google Scholar]
- 43.Cox RJ, Pedersen G, Madhun AS, Svindland S, Saevik M, Breakwell L, Hoschler K, Willemsen M, Campitelli L, Nostbakken JK, Weverling GJ, Klap J, McCullough KC, Zambon M, Kompier R, Sjursen H. 2011. Evaluation of a virosomal H5N1 vaccine formulated with Matrix M adjuvant in a phase I clinical trial. Vaccine 29:8049–8059. 10.1016/j.vaccine.2011.08.042 [DOI] [PubMed] [Google Scholar]
- 44.Clegg CH, Roque R, Van Hoeven N, Perrone L, Baldwin SL, Rininger JA, Bowen RA, Reed SG. 2012. Adjuvant solution for pandemic influenza vaccine production. Proc. Natl. Acad. Sci. U. S. A. 109:17585–17590. 10.1073/pnas.1207308109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schön MP, Schön M. 2007. Imiquimod: mode of action. Br. J. Dermatol. 157(Suppl 2):8–13. 10.1111/j.1365-2133.2007.08265.x [DOI] [PubMed] [Google Scholar]
- 46.Tomai MA, Imbertson LM, Stanczak TL, Tygrett LT, Waldschmidt TJ. 2000. The immune response modifiers imiquimod and R-848 are potent activators of B lymphocytes. Cell. Immunol. 203:55–65. 10.1006/cimm.2000.1673 [DOI] [PubMed] [Google Scholar]
- 47.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. 2006. Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science 314:1936–1938. 10.1126/science.1135299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.To KK, Hung IF, Li IW, Lee KL, Koo CK, Yan WW, Liu R, Ho KY, Chu KH, Watt CL, Luk WK, Lai KY, Chow FL, Mok T, Buckley T, Chan JF, Wong SS, Zheng B, Chen H, Lau CC, Tse H, Cheng VC, Chan KH, Yuen KY. 2010. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin. Infect. Dis. 50:850–859. 10.1086/650581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nassim C, Christensen S, Henry D, Holmes S, Hohenboken M, Kanesa-Thasan N. 2012. Identification of antigen and adjuvant doses resulting in optimal immunogenicity and antibody persistence up to 1 year after immunization with a pandemic A/H1N1 influenza vaccine in children 3 to <9 years of age. Pediatr. Infect. Dis. J. 31:e59–65. 10.1097/INF.0b013e31824b9545 [DOI] [PubMed] [Google Scholar]
- 50.Song JY, Cheong HJ, Seo YB, Kim IS, Noh JY, Heo JY, Choi WS, Lee J, Kim WJ. 2012. Comparison of the long-term immunogenicity of two pandemic influenza A/H1N1 2009 vaccines, the MF59-adjuvanted and unadjuvanted vaccines, in adults. Clin. Vaccine Immunol. 19:638–641. 10.1128/CVI.00026-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferguson M, Risi G, Davis M, Sheldon E, Baron M, Li P, Madariaga M, Fries L, Godeaux O, Vaughn D. 2012. Safety and long-term humoral immune response in adults after vaccination with an H1N1 2009 pandemic influenza vaccine with or without AS03 adjuvant. J. Infect. Dis. 205:733–744. 10.1093/infdis/jir641 [DOI] [PubMed] [Google Scholar]