Abstract
Monitoring of antigen-specific T-cell responses is valuable in numerous conditions that include infectious diseases, vaccinations, and opportunistic infections associated with acquired or congenital immune defects. A variety of assays that make use of peripheral lymphocytes to test activation markers, T-cell receptor expression, or functional responses are currently available. The last group of assays calls for large numbers of functional lymphocytes. The number of cells increases with the number of antigens to be tested. Consequently, cells may be the limiting factor, particularly in lymphopenic subjects and in children, the groups that more often require immune monitoring. We have developed immunochemical assays that measure secreted cytokines in the same wells in which peripheral blood mononuclear cells (PBMC) are cultured. This procedure lent itself to miniaturization and automation. Lymphoproliferation and the enzyme-linked immunosorbent spot (ELISPOT) assay have been adapted to a miniaturized format. Here we provide examples of immune profiles and describe a comparison between miniaturized assays based on cytokine secretion or proliferation. We also demonstrate that these assays are convenient for use in testing antigen specificity in established T-cell lines, in addition to analysis of PBMC. In summary, the applicabilities of miniaturization to save cells and reagents and of automation to save time and increase accuracy were demonstrated in this study using different methodological approaches valuable in the clinical immunology laboratory.
INTRODUCTION
Several conditions lead to defective cellular immunity. In particular, conditioning and immune ablation induced for hemopoietic stem cell transplantation (HSCT) in different hematological malignant and nonmalignant diseases result in persistent loss of T cells. Therefore, control of opportunistic infections sustained by viruses, fungi, and bacteria is lost and several months may elapse before cellular immune competence reconstitutes (1). Due to the fact that HSCT is more broadly applied, monitoring of T-cell responses specific for relevant opportunistic pathogens has become a relevant issue in the clinical immunology laboratory. Numerous tests are currently available (2) and efforts are being made to standardize and validate assays in interlaboratory cooperative studies (3). A limitation often encountered with these assays is that the number of available peripheral blood mononuclear cells (PBMC) needed to test antigens from different pathogens is insufficient. This is particularly the case with pediatric patients, due to limited blood volumes, and with lymphopenic patients. In both cases, miniaturization of assay formats results in a remarkable advantage, with reagent and cost reductions as additional benefits. Furthermore, automation that can or must associate with assay format miniaturization may contribute to assay standardization and robustness.
Since different T-cell assays can be used to characterize different T-cell functions, such as cytokine synthesis and proliferation and effector cytolytic activity (2), our goal is to miniaturize most of these assays to gain more information on the functions and specificities of responding T cells. We have been engaged in this effort since we reported on a novel assay performed in 384-well plates in which antigen-induced cytokine secretion was measured in the very same culture wells (4). This assay, termed cell enzyme-linked immunosorbent assay (cell-ELISA), was validated and further miniaturized in 1,536-well plates (5, 6). More recently, we also adapted lymphoproliferation to 384- and 1,536-well plates (7). Here we describe miniaturization of the enzyme-linked immunosorbent spot (ELISPOT) assay and comparative studies between different types of miniaturized assays.
MATERIALS AND METHODS
Media and reagents.
RPMI 1640 (BioWhittaker, Verviers, Belgium) supplemented with 10 mM l-glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin, and 5% autologous heparinized plasma collected after density gradient separation on lymphocyte separation medium (LSM) (BioWhittaker) was used for cell cultures. Fetal calf serum (FCS) was used at 5% to supplement media for maintenance of antigen-specific T-cell lines. Recombinant human interleukin 2 (IL-2) (Chiron, Emeryville, CA) was used at 30 U/ml for expansion of HIV- and cytomegalovirus (CMV)-specific T-cell lines. Phytohemagglutinin (PHA) (leukoagglutinin; Sigma-Aldrich, St. Louis, MO) was used at 5 μg/ml. Tritiated thymidine (specific activity 6.7 Ci/mmol; Amersham, United Kingdom) was used for pulsing of PBMC on day 4 for 16 h and for pulsing of T-cell lines on day 2 for 8 to 12 h. Tritiated thymidine was used at a 5-μCi/ml final concentration in medium.
Antigens.
Tetanus toxoid (TT) and purified protein derivative (PPD) were purchased from Statens Serum Institut, Copenhagen, Denmark. Cytomegalovirus (CMV) lysate antigen was purchased from Microbix, Toronto, Canada. Peptides were synthesized by InBios (Naples, Italy) or by JPT (Berlin, Germany) and used as selected peptide libraries containing immunodominant CD4 and CD8 peptides (CD4 and CD8 peptide pools of CMV pp65 antigen) produced as described previously (8, 9, 10). The HLA-A2 restricted NV9 peptide from CMV pp65 was also used to test CD8 responses (10). Final antigen concentrations were 10 μg/ml for proteins and 1 μg/ml for each peptide. Antigens were predispensed in the culture wells as 10× solutions, corresponding to 5 and 1 μl for the 384- and 1,536-well formats, and stored at −20°C for up to 12 months. Using the different assays, 200, 50, and 10 × 103 PBMC were seeded in each of 96-, 384-, 1,536-well plates, respectively. For fair comparison between the different formats, ELISPOT assay and lymphoproliferation results in 96- and 384-well plates were divided by 20 and by 5 in order to refer to 10 × 103 PBMC, the cell number seeded in 1,536-well plates.
Materials and instrumentation for miniaturized and automated processing.
Cultures were performed in round-bottom 384- and 1,536-well plates (number 3671 and number 3937; Corning, Lowell, MA) or in flat-bottom plates (number 16468 and number 153613; Nunc, Roskilde, Denmark). The 96-channel Hydra II liquid handler (Matrix Technologies, Hudson, NH) was used for resuspending [3H]thymidine-pulsed cells at the end of the culture period and transferring from 384- and 1,536-well plates to round-bottom 96-well plates that were eventually harvested. The 8-channel dispensers MultiWell (Matrix) and MultiDrop Combi (Thermo OY, Helsinki, Finland) were used to distribute reagents and cells in plates.
Donors and PBMC preparation.
Healthy donors recruited among laboratory workers gave their informed consent for this study. The G. Gaslini Paediatric Institute, where the blood samples were collected, does not require specific Institutional Review Board approval if an informed consent for the in vitro study is obtained from the healthy adult donors. Peripheral blood mononuclear cells (PBMC) were obtained from heparinized venous blood separated on a conventional density gradient on LSM. After washing and counting, PBMC were suspended in complete medium containing 5% autologous heparinized plasma at 1 × 106/ml.
Cytokine secretion assay (cell-ELISA).
The assay was set up and run as previously described in detail (4). Briefly, plates were coated with the capture anti-gamma interferon (IFN-γ) antibody, blocked, and stored at −20°C. Plates were thawed for use and received antigens and cells in duplicate. After incubation, the plates were frozen again. Before development, the thawed plates were washed and developed to reveal captured IFN-γ with a second biotinylated antibody, followed by alkaline phosphatase-conjugated streptavidin. The cell-ELISA reagents were purchased from Mabtech, Stockholm, Sweden. After washing, the PNPP phosphatase substrate (Sigma, St. Louis, MO) was added and the enzymatic reaction was carried out at room temperature for 30 min. The 384-well plate reader ELx800 (BioTek, Winooski, VE) and the 1,536-well plate reader Victor3 (PerkinElmer, Waltham, MA) were used for plate readout. The plates were read at 405 and the results were plotted against a titration curve included in each plate to convert optical density (OD) into IFN-γ concentrations expressed as ng/ml.
Miniaturized ELISPOT assay.
A conventional ELISPOT assay run in 96-well plates (8, 9) was adapted to miniaturized formats. Briefly, PBMC were cultured and stimulated in 384- and 1,536-well plates with round bottoms using 50 × 103 and 10 × 103 PBMC per well and antigen concentrations as described for cell-ELISA. Therefore, also in this case the PBMC numbers were reduced by 4-fold and 20-fold, respectively, in the two miniaturized formats, compared to assays run in 96-well plates with 200 × 103 PBMC. Following a 20-h incubation (activation phase), cells in the wells were resuspended either manually with a multichannel pipette or automatically with a 96-channel liquid handler and transferred to a 96-well PVDF plate (Millipore, Bedford, MA) precoated with the first antibody and containing 200 μl complete medium (cytokine release and capture phase). ELISPOT reagents were purchased from Mabtech. Plates were incubated for an additional 8 h and eventually washed and processed as for a conventional ELISPOT assay with a second biotinylated antibody, followed by alkaline phosphate-conjugated streptavidin and ELISPOT substrate. After development for 1 h at room temperature, spot-forming units (SFU) were blindly enumerated with a stereomicroscope. Results of duplicate wells are given as SFU per 10 × 103 PBMC for all formats for immediate comparison with ELISPOT assay in 1,536-well plates that use this cell number for each well.
Miniaturized lymphoproliferation assay.
The two-step procedure for harvesting of pulsed cells in 384- and 1,536-well plates was previously described in detail (7). Briefly, the plates were pulsed using either a Hamilton syringe to dispense 5 or 1 μl [3H]thymidine solution at 50 μCi/ml in phosphate-buffered saline (PBS) per well or the automatic dispenser. After overnight incubation, the cells in the plates were resuspended and transferred to round-bottom 96-well plates either manually with a multichannel pipette or automatically with the 96-channel liquid handler. At this stage, the transferred cells were collected from the 96-well plates using a 96-channel harvester. The dry glass fiber filters were counted with a Matrix 9600 detector (Canberra Packard, Cambridge, MA) in a mixture of 5% argon in nitrogen gas. Results of duplicate wells are shown as counts per minute (cpm).
Generation, maintenance, and use of HIV-specific and CMV-specific T-cell lines.
HIV-specific CD4 T-cell lines were obtained from two healthy, seronegative subjects (11–13). Line FM was specific for HIV envelope glycoprotein gp120 and recognized the immunodominant peptide 191-205 (PAGFAILKCNNTFNY). Line GLP, specific for HIV reverse transcriptase p66, recognized the immunodominant peptide 248-262 (KDSSTVNDIQKLVGK). Briefly, PBMC at 10 × 106/ml were pulsed with the relevant peptides at 1 μg/ml for 4 h at 37°C, diluted 1:5 with medium, and dispensed in 24-well plates at 2 × 106/ml at 2 ml per well. IL-2 was added 4 days later. The cells were split 1:4 when the concentration exceeded 106/ml. Restimulation cycles were performed every 3 weeks using 3 × 105 T cells cocultured with 106 autologous, 30-Gy-irradiated PBMC plus antigen in 2 ml medium. IL-2-expanded T cells were used 3 weeks after the last stimulation cycle, following a 24-hour resting phase in the absence of IL-2 to minimize residual proliferative activity. CMV-specific CD4 T-cell lines were generated with a similar protocol, based on repeated stimulation cycles with antigen-pulsed irradiated autologous PBMC, as previously reported in detail (9).
Statistics.
Results of all assays are shown as mean values of duplicates. Student's t test with differences defined as significant at P values of <0.01 was used for pairwise comparison of data obtained with different plate formats.
RESULTS
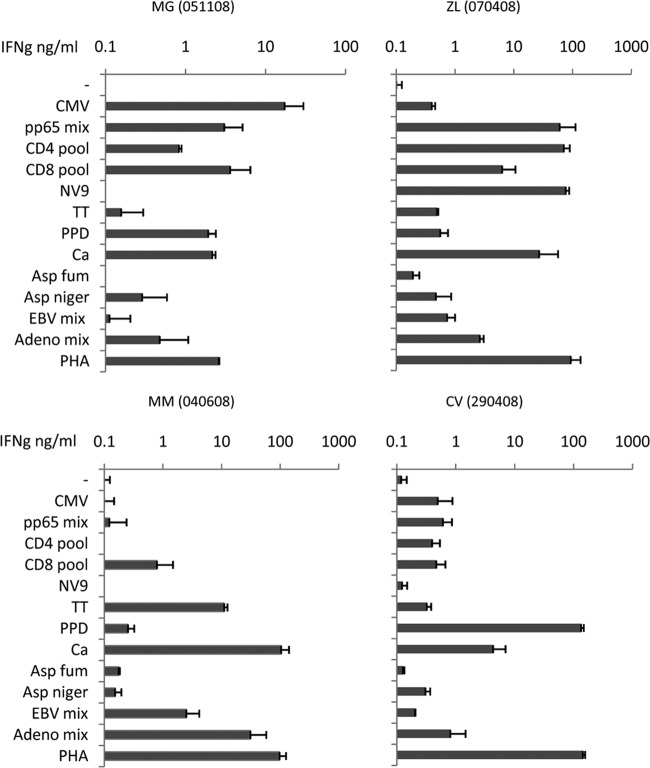
Four representative examples of specificity profiles obtained by cell-ELISA are shown in the panels of Fig. 1. Healthy donors exhibited a variety of specific responses, which included recognition of recall antigens such as TT and PPD; viral antigens from CMV, Epstein-Barr virus (EBV), and adenovirus; and fungal antigens such as Candida albicans, Aspergillus niger, and Aspergillus fumigatus.
FIG 1.
Representative examples of cell-ELISA. The panels show cell-ELISAs performed on healthy controls. Different individual profiles can be observed using the assay performed in 384-well plates. Tested antigens included CMV (CMV lysate, pp65 peptide mix, and immunodominant CD4 and CD8 peptides from pp65), recall antigens (TT and PPD), adenovirus hexon 5 and EBV LMP2 peptide mixes, fungal antigens (Candida albicans [Ca], Aspergillus niger [Asp niger], and Aspergillus fumigatus [Asp fum]). Adeno, adenovirus.
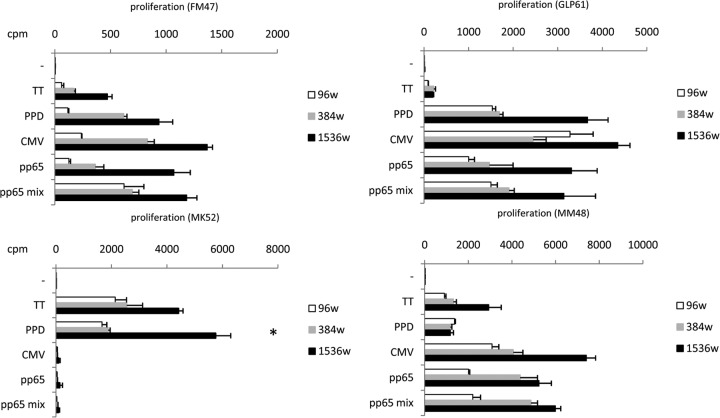
Results from four additional donors were selected as examples of different specificity profiles obtained by lymphoproliferation in miniaturized well plates, as shown in Fig. 2. It can be noted that proliferative responses in 384- and 1,536-well plates yielded greater cpm than those in 96-well plates. For easier comparison, results of 96-well plates were divided by 20 and results of 384-well plates were divided by 5 to refer to cpm of 10 × 103 PBMC, as for those cultured in 1,536-well plates. This trend was occasionally observed in previous experiments (7), in which proliferation in 384-well plates was compared to proliferation in 1,536-well plates. It should be noted, though, that a statistically significant difference was observed in few instances, as indicated in Fig. 2, 3, 4, 5. It is reasonable to anticipate that differences between formats may acquire statistical significance by improving the quality of replicates. Refined automation can certainly help attain this goal.
FIG 2.
Representative examples of microproliferation assay. The panels show microproliferation tested on four healthy subjects using the 96- well (96w) format and the miniaturized formats in 384- and 1,536-well plates. In all cases cpm are referred to as 10 × 103 PBMC. Similar reactivity profiles were obtained with the three formats. Significantly high (P < 0.01) cpm values in the smaller format are indicated by an asterisk.
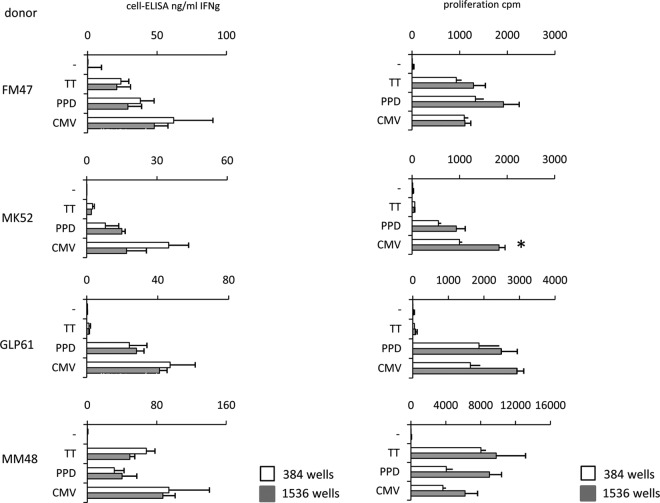
FIG 3.
Comparison of cell-ELISA and microproliferation. Four healthy donors were tested with the two assays in miniaturized formats (384- well plates, open bars, and 1,536-well plates, gray bars). Cell-ELISA results are comparable in the two formats since they refer to IFN-γ concentrations in culture conditions in which PBMC are at the same density. On the contrary, microproliferation results shown for 384-well plates were divided by 5 to show proliferation of 10 × 103 PBMC for comparison with results obtained in 1,536-well plates. This correction showed that the two formats provide similar results, with a consistent, but not significant, trend for higher efficiency of the 1,536-well format than of the 384-well format.
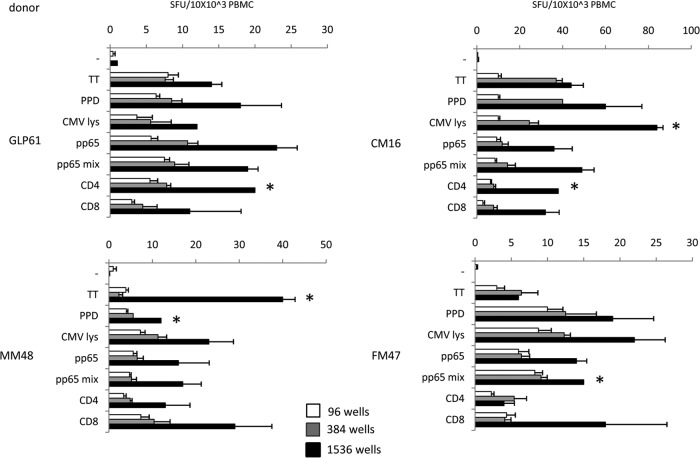
FIG 4.
ELISPOT assay in miniaturized format. Healthy donors were challenged with protein antigens (TT, PPD, CMV lysate, pp65) and with peptide antigens (pp65 peptide mix, CMV CD4 T-helper peptide pool, and CMV CD8 CTL [cytotoxic T lymphocyte] peptide pool). SFU were referred to as 10 × 103 PBMC for the three 96-, 384-, and 1,536-well formats. A consistent increase in SFU counts is evident with the smaller formats, indicating an increased efficiency of the miniaturized cultures. Significantly high (P < 0.01) SFU counts in the smaller formats are indicated by asterisks.
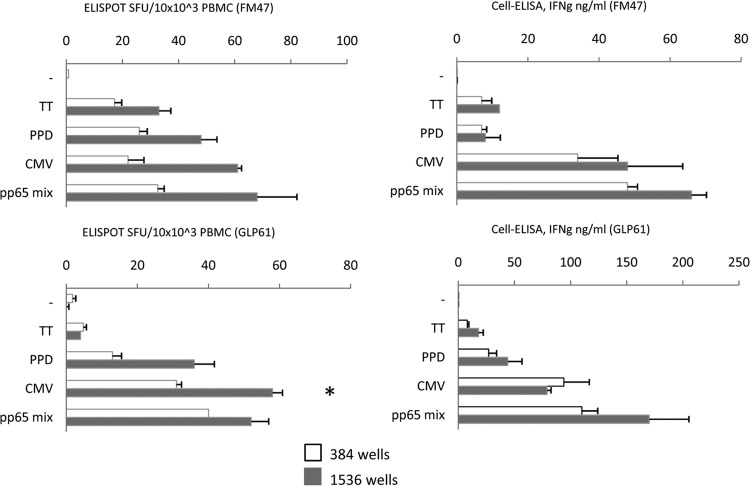
FIG 5.
Comparison of miniaturized ELISPOT assay and cell-ELISA. Two healthy donors were tested with a restricted antigen panel to include proteins (TT, PPD, CMV lysate) and peptides (CMV pp65 peptide mix). The ELISPOT assay SFU counts in the left panels are referred to as 10 × 103 PBMC for the two 384- and 1,536-well formats in order to make results of the two formats comparable. Significantly high (P < 0.01) SFU counts in the smaller format indicated by an asterisk were seen in one case only, but a consistent trend for higher efficiency of the 1,536-well format was evident. Cell-ELISA results, given as ng/ml IFN-γ concentration, were not corrected for plate format, as described in legend to Fig. 3.
The same four donors were tested in parallel using the two assays, as shown in Fig. 3. The comparison was performed in 384- and 1,536-well plates, which were seeded with 50 and 10 μl PBMC suspension per well. Even though the results of the two assays are shown using two different units (IFN-γ as ng/ml and [3H]thymidine incorporation as cpm), the specificity profiles are similar, suggesting that both assays detect specific T cells with similar efficiency. In fact, negligible responses to TT were detected in two donors, while responses to the other tested antigens were consistently positive with both assays. Results for cell-ELISA in the two formats are similar, since IFN-γ concentrations are expected not to differ in the culture supernatants. In contrast, in the case of lymphoproliferation, total [3H]thymidine incorporation as cpm depends on the number of harvested PBMC. For an easier comparison between the two formats, cpm for 384-well plates were divided by 5 and thus referred to as 10 × 103 PBMC, as seeded in the 1,536-well plates. Sensitivity thresholds (detection of low frequencies of specific T cells) were not analyzed using PBMC but, rather, using established T-cell lines, as described later.
Four healthy donors responsive to CMV were tested with recall antigens and different CMV antigens by ELISPOT assay performed in standard 96-well plates and in miniaturized plates. Figure 4 shows the SFU counts referred to as 10 × 103 PBMC for easier comparison. Overlapping response profiles were obtained in all subjects responding to different antigenic challenges. It should be noted that higher SFU counts were consistently observed in the smaller formats than SFU in 96-well plates, with significant differences between 1,536- and 384-well plates in a few cases, as indicated in the Fig. 4 legend.
We have previously described a remarkable assay correlation using cell-ELISA in 384-well plates and ELISPOT assay in 96-well plates (G. Li Pira, F. Ivaldi, N. Starc, F. Landi, S. Rutella, F. Locatelli, N. Sacchi, G. Tripodi, F. Manca, submitted for publication). Here we provide a more extensive comparison of the two assays in which cultures were performed in the miniaturized formats of 384- and 1,536-well plates (Fig. 5). PBMC were stimulated with recall and CMV antigens to include proteins and peptides. Two CMV responders were tested. The SFU counts referred to as 10 × 103 PBMC in the two formats are shown in the left panels. Similar profiles were obtained, with a higher efficiency for the smaller formats consistently confirmed, even though a significant difference was not observed. The ELISPOT assay profiles were similar to those of the cell-ELISA profiles depicted in the right panels.
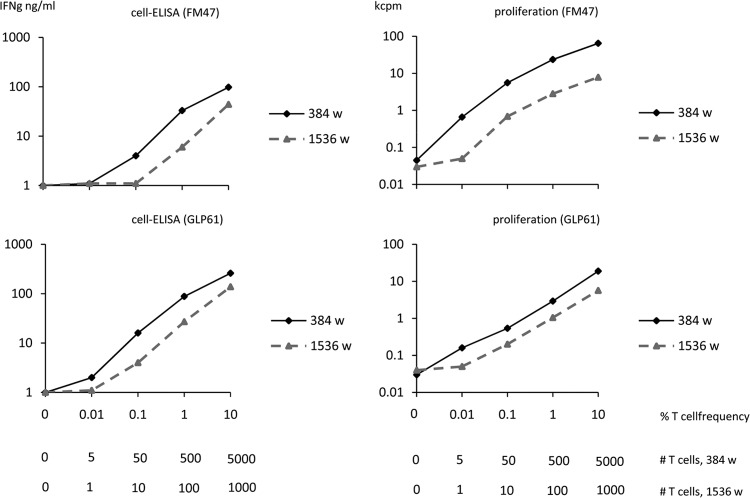
Established T-cell lines were available in the laboratory from different donors and with different specificities. From this panel, we chose two T-cell lines specific for two HIV antigens that had been generated from seronegative, nonimmune subjects. This proved particularly convenient, as the frequency of HIV-specific T cells in PBMC from these donors, used as a source of antigen-presenting cells for T-cell stimulation, was below the detection threshold. Figure 6 shows IFN-γ production and proliferative responses of T-cell lines that were measured in parallel assays in 384- and 1,536-well plates. In the first case, each well contained 50 × 103 irradiated autologous PBMC plus 5,000, 500, 50, 5, and 0 specific T cells from the established lines in a 50-μl volume. In the second case, each well contained one-fifth of the same cellular reagents, corresponding to 10 × 103 irradiated autologous PBMC plus 1,000, 100, 10, 1, and 0 T cells from the established lines in 10 μl. The results of the two parallel assays were in agreement, independently of the format. With respect to sensitivity, it can be seen that in most cases using cell-ELISA or lymphoproliferation, between 5 and 10 specific T cells can be detected using the 384- and the 1,536-well plates. These figures correspond to actual frequencies of specific T cells of 5/50,000 (0.01%) and 10/10,000 (0.1), that are in the lower range of expected frequencies of antigen-specific T cells among PBMC.
FIG 6.
Titration of HIV-specific T-cell lines spiked in autologous PBMC. The left panels indicate cell-ELISA results and the right panels indicate lymphoproliferation results. Upper graphs refer to an HIV gp120-specific T-cell line generated from donor FM47, while lower graphs refer to an HIV reverse transcriptase-p66-specific T-cell line generated from donor GLP61. Graded numbers of specific T cells were titrated in autologous irradiated PBMC as a source of antigen-presenting cells. Titrations were performed in 384- and 1,536-well plates. The horizontal axes indicate the percentage of T cells versus total PBMC, and the absolute numbers of specific T cells per well at the different dilutions for the two formats.
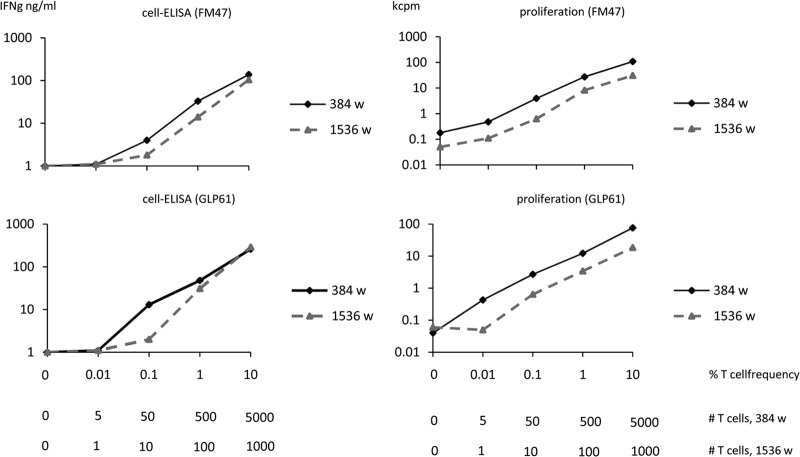
Experiments using CMV-specific CD4 T-cell lines are shown in Fig. 7. In this case, the lines generated with the whole-CMV viral lysate were responsive to a pool of overlapping peptides corresponding to the immunodominant pp65 protein. Therefore, the assays were run using the pp65 peptide mix. Similar results were obtained using HIV-specific and CMV-specific T-cell lines, with similar detection thresholds (0.01% to 0.1%) for the lowest cell concentrations.
FIG 7.
Titration of CMV-specific T-cell lines spiked in autologous PBMC. The left panels indicate cell-ELISA results and the right panels indicate lymphoproliferation results. Graded numbers of specific T cells obtained from CMV-specific T-cell lines generated from donors FM47 and GLP61 were titrated in autologous irradiated PBMC as a source of antigen-presenting cells. Titrations were performed in 384- and 1,536-well plates. The horizontal axes indicate the percentage of T cells versus total PBMC, and the absolute numbers of specific T cells per well at the different dilutions for the two formats.
DISCUSSION
The reasons for running functional T-cell-specificity assays in miniaturized and automated formats have already been outlined in the introduction and discussed in depth in previous papers (2–4). The experiments we reported here address additional points and lead to several conclusions.
First of all, informative specificity profiles were obtained using different assays in miniaturized (384-well plates) and ultraminiaturized (1,536-well plates) formats. This confirms that very few cells can be used, thus making it possible to test a single antigen with 50 × 103 or 10 × 103 PBMC with the two formats, respectively, instead of 200 × 103 to 400 × 103 PBMC using conventional 96-well plates.
Second, there was a good agreement between cell-ELISA and lymphoproliferation both in 384- and 1,536-well plates. This observation has already been reported with 384-well plates (7). The extension to the 1,536-well format is encouraging, since this should be the ultimate plate format to save cells and reagents, if some manipulation problems are worked out. These plates, in fact, require automated processing, as manual handling can be inaccurate and confusing, resulting in antigen mix up or poor replicates. Automated liquid dispensers, washers, and readers that can handle 384- and 1,536-well plates are not often present in the standard clinical immunology laboratory. Nevertheless, since their use can be extended to other miniaturized cellular immunology assays, as shown here, in addition to immunochemical and molecular biology assays, their availability should be considered in future perspectives.
It should be noted here that the assays remarkably differ in duration (1 day for cell-ELISA or ELISPOT assay, 5 days for lymphoproliferation). Therefore, a preliminary profile can be obtained by the next day, but confirmation that specific T cells carry a proliferative potential is available on day 5. The similarities between the two assays in the healthy donors we tested suggest that the lymphoproliferation assay may be dispensable, with antigen-driven production of IFN-γ reliably anticipating the proliferative response. Unfortunately, we do not know yet if this conclusion can be extended to patients with compromised cellular immunity. Particularly in patients receiving immunosuppressive drugs, cytokine secretion does not grant clonal expansion potential, and a functionally relevant discrepancy may exist between effector function and proliferative potential of memory T cells. Therefore, specific studies aimed at solving these questions need to be undertaken.
Third, the sensitivity issue was considered in light of assay miniaturization. In fact, volume reduction is simply a technicality, which can be addressed using accurate instrumentation in molecular assays that measure antibodies, other analytes, or nucleic acids. On the contrary, assay volume reduction in cellular assays must take into account the frequency of specific cells, which may be under limiting dilution conditions if reaction volumes are reduced beyond a given threshold. In fact, two parameters should be kept in mind. One is assay sensitivity, i.e., the number of specific T cells responding to a given antigenic stimulus that the can test reveal as a positive signal; the other one is the frequency of specific T cells among PBMC. In previous studies (4, 6), we have ascertained that cell-ELISA can detect IFN-γ secreted by as few as 2 to 5 specific T cells. Using limiting dilutions, we also showed that miniaturized proliferation can detect similar numbers of specific T cells (7). Here, we ran a comparative sensitivity assessment on the same T-cell samples, using established T-cell lines. This permits an accurate knowledge of the number of input-specific T cells per well, the same information being hard to gain using PBMC. As illustrated in the Results section, assuming a reasonable frequency of 0.1% T cells specific for a pathogen like CMV, one specific cell out of 1,000 PBMC can be detected. If the assay threshold is 5 specific T cells, at least 5,000 PBMC need to be cultured to detect a positive signal. To stay on the safe side, 10,000 PBMC should be seeded in the 1,536-well plate. This figure cannot be further reduced, unless higher frequencies of specific T cells are expected. A more detailed discussion on the issue of precursor frequency and liminal detection has been previously presented (14).
Fourth, a comment should be made regarding the higher efficiency of cultures performed in microplates than in standard 96-well plates. In fact we observed a consistent trend for higher responses in the smaller formats (384- versus 96- and 1,536- versus 384-well plates), as if responding T cells “appreciated” the smaller culture wells. A possible explanation is that, even if PBMC concentrations were identical and thus cell densities at the bottom of flat wells would have been the same, the smaller radius in the smaller round-bottom wells increased cell density. This likely resulted in a microenvironment in which contacts between specific T cells and antigen-presenting cells (APC) were facilitated. This condition, in fact, is closer to the three-dimensional (3D) structure of a lymphoid organ than the 2D condition of a flat-bottom well. This hypothesis demands more targeted experiments, but, if proven, it may provide additional support to the fact that the principle “the smaller the better” also applies to T-cell assays as long as miniaturization does not dilute out specific T cells to undetectable thresholds. It is also likely that PBMC resuspension allows a more efficient collection of all PBMC in the proliferation assay. Likewise, thorough dispersion of PBMC after collection of miniaturized ELISPOT assay plates before transferring to 96-well plates for cytokine capture and immunoenzymatic development may disrupt clusters of specific T cells surrounding a single APC (15) and thus reveal individual spot-forming cells rather than spot-forming units. This case, as an artifact, may underestimate the actual frequency of specific T cells in standard ELISPOT assays and thus this may be an additional advantage for the two-step procedure in 384- and 1,536-well plates.
In conclusion, miniaturization and automation of assays that monitor antigen-specific T-cell immunity is feasible and can be further improved in order to save cells and reagents, to increase assay reliability and robustness, and to broaden clinical applications. These improvements result in remarkable benefits for the monitoring of specific cellular immunity (16–18) in cancer immunotherapy, immune deficiencies, and immune reconstitution associated with various conditions. In the more specific field of adoptive immune reconstitution (19), these assays may help to identify patients requiring adoptive immunotherapy before severe infections break out, select the most appropriate donor for T-cell immune reconstitution, and define successful immune restoration.
ACKNOWLEDGMENTS
This work was supported by the Italian Ministry of Health, Rome (Finalized Project 2008-2011, Establishment of a GMP-Validated Biobank), AIDS Project 2009-2013 (40H.87), and the European Union, Brussels (grant LSHB-CT-2005-018680). G.L.P. is supported by the European Union FP7 program GA (number 242146). F.M. is a consultant for grant 40H.87. Paolo Moretti contributed to artwork.
Footnotes
Published ahead of print 29 January 2014
REFERENCES
- 1.Van Burik JA, Weisdorf D. 2005. Infections in recipients of hematopoietic stem cell transplantation, p 3486–350 In Mandell GL, Bennett JE, Dolin R. (ed), Principles and practice of infectious diseases. Churchill Livingstone, London, United Kingdom [Google Scholar]
- 2.Kern F, Li Pira G, Gratama J, Manca F, Roederer M. 2005. Measuring antigen specific immune responses: understanding immunopathogenesis and improving diagnostics in infectious diseases, autoimmunity and cancer. Trends Immunol. 9:477–484. 10.1016/j.it.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 3.Britten CM, Janetzki S, Butterfield LH, Ferrari G, Gouttefangeas C, Huber C, Kalos M, Levitsky HI, Maecker HT, Melief CJM, O'Donnel-Tormey J, Odunsi K, Old LJ, Ottenhoff THM, Ottensmeier C, Pawlec G, 4 Roederer M, Roep BO, Romero P, van der Burg SH, Walter S, Hoos A, Davis MM. 2012. T-cell assays and MIATA: the essential minimum for maximum impact. Immunity 37:1–2. 10.1016/j.immuni.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 4.Li Pira G, Ivaldi F, Bottone L, Manca F. 2007. High throughput functional microdissection of pathogen-specific T-cell immunity using antigen and lymphocyte arrays. J. Immunol. Methods 326:22–32. 10.1016/j.jim.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 5.Li Pira G, Ivaldi F, Dentone C, Righi E, Del Bono V, Viscoli C, Koopman G, Manca F. 2008. Evaluation of antigen specific T-cell responses with a miniaturized and automated method. Clin. Vaccine Immunol. 15:1811–1818. 10.1128/CVI.00322-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Pira G, Ivaldi F, Moretti P, Tripodi G, Manca F. 2010. Validation of a miniaturized method for assessment of specific T cell immunity. J. Immunol. Methods 355:68–75. 10.1016/j.jim.2010.02.010 [DOI] [PubMed] [Google Scholar]
- 7.Li Pira G, Starc N, Conforti A, Bertaina A, Rutella S, Locatelli F, Manca F. 2012. Lymphocyte proliferation specific for recall, CMV and HIV antigens in miniaturized and automated format. J. Immunol. Methods 384:135–142. 10.1016/j.jim.2012.07.022 [DOI] [PubMed] [Google Scholar]
- 8.Li Pira G, Bottone L, Ivaldi F, Pelizzoli R, Bracci L, Lozzi L, Del Galdo F, Loregian A, Palù G, Einsele H, Manca F. 2004. Identification of new Th peptides from the cytomegalovirus immunodominant protein pp65 and their use as a peptide library for generation of T cell lines for cellular immunoreconstitution. Int. Immunol. 16:635–642. 10.1093/intimm/dxh065 [DOI] [PubMed] [Google Scholar]
- 9.Li Pira G, Bottone L, Ivaldi F, Tagliamacco A, Fiordoro S, Ricciardi A, Barbano G, Manca F. 2005. Generation of cytomegalovirus (CMV)-specific CD4 T cell lines devoid of alloreactivity by use of a mixture of CMV-phosphoprotein 65 peptides for reconstitution of the T helper repertoire. J. Infect. Dis. 191:215–226. 10.1086/427040 [DOI] [PubMed] [Google Scholar]
- 10.Li Pira G, Ivaldi F, Bottone L, Koopman G, Manca F. 2007. Helper function of cytolytic lymphocytes: switching roles in the immune response. Eur. J. Immunol. 37:66–77. 10.1002/eji.200636337 [DOI] [PubMed] [Google Scholar]
- 11.Manca F, Habeshaw J, Dalgleish A. 1991. The naive repertoire of human T helper cells specific for gp120, the envelope glycoprotein of HIV. J. Immunol. 146:1964–1971 [PubMed] [Google Scholar]
- 12.Manca F. 1992. Galactose receptors and presentation of HIV envelope glycoprotein to specific human T cells. J. Immunol. 148:2278–2282 [PubMed] [Google Scholar]
- 13.Li Pira G, Bottone L, Oppezzi L, Seri M, Westby M, Caroli F, Fenoglio D, Lancia F, Ferraris A, Bottone L, Valle MT, Kunkl AL, Dalgleish A, Romeo G, Manca F. 1998. Repertoire breadth of human CD4+ T cells specific for HIV gp120 and p66 (primary antigens) and for PPD and tetanus toxoid (secondary antigens). Hum. Immunol. 59:137–148. 10.1016/S0198-8859(98)00004-4 [DOI] [PubMed] [Google Scholar]
- 14.Li Pira G, Ivaldi F, Moretti P, Manca F. 2010. High throughput T epitope mapping and vaccine development. J. Biomed. Biotechnol. 2010:325720. 10.1155/2010/325720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Pira G, Ivaldi F, Manca F. 2012. Selective binding of CD4 and CD8 T-cells to antigen-presenting cells for enrichment of CMV and HIV specific T-lymphocytes. J. Immunol. Methods 376:125–131. 10.1016/j.jim.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 16.Davis MM. 2008. A prescription for human immunology Immunity. 29:835–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoos A, Britten CM, Huber C, O'Donnell-Tormey J. 2011. A methodological framework to enhance the clinical success of cancer immunotherapy. Nat. Biotechnol. 29:867–870. 10.1038/nbt.2000 [DOI] [PubMed] [Google Scholar]
- 18.Roep BO, Buckner J, Sawcer S, Toes R, Zipp F. 2012. The problems and promises of research into human immunology and autoimmune disease. Nat. Med. 18:48–53. 10.1038/nm.2626 [DOI] [PubMed] [Google Scholar]
- 19.Li Pira G, Kapp M, Manca F, Einsele H. 2009. Pathogen specific T-lymphocytes for reconstitution of the immunocompromised host. Curr. Opin. Immunol. 21:549–556. 10.1016/j.coi.2009.08.006 [DOI] [PubMed] [Google Scholar]