Abstract
The development of malaria vaccines is challenging, partly because the immunogenicity of recombinant malaria parasite antigens is low. We previously demonstrated that parasite antigens integrated into a tricomponent immunopotentiating complex increase antiparasitic immunity. In this study, the B domains of a group G Streptococcus (SpG) strain and Peptostreptococcus magnus (PpL) were used to evaluate whether vaccine efficacy is influenced by the type of immunoglobulin-binding domain (IBD) in the tricomponent complex. IBDs were fused to a pentameric cartilage oligomeric matrix protein (COMP) to increase the binding avidity of the complexes for their targets. The COMP-IBD fusion proteins generated (COMP-SpG and COMP-PpL and the previously constructed COMP-Z) bound a large fraction of splenic B lymphocytes but not T lymphocytes. These carrier molecules were then loaded with an ookinete surface protein of Plasmodium vivax, Pvs25, by chemical conjugation. The administration of the tricomponent complexes to mice induced more Pvs25-specific serum IgG than did the unloaded antigen. The PpL complex, which exhibited a broad Ig-binding spectrum, conferred higher vaccine efficacy than did the Z or SpG complexes when evaluated with a membrane feed assay. This study demonstrates that this tricomponent immunopotentiating system, incorporating IBDs as the B-lymphocyte-targeting ligands, is a promising technology for the delivery of malaria vaccines, particularly when combined with an aluminum salt adjuvant.
INTRODUCTION
Although tremendous efforts have been made to control malaria in recent decades, the parasite is still transmitted in 99 countries around the world, and 219 million new cases and 660,000 deaths are estimated to have occurred in 2010 (1). Unfortunately, however, even the most advanced candidate malaria vaccine, RTS,S, which is currently in phase 3 clinical trials, has shown only moderate efficacy against clinical malaria (30% to 50%) and severe malaria (25% to 45%) in young children and infants (2). Furthermore, malaria control has been primarily directed against Plasmodium falciparum, but to eliminate and eradicate malaria outside Africa, Plasmodium vivax must also be controlled, although its contribution has been largely neglected until now (3). Therefore, second- and third-generation malaria vaccines that control both P. falciparum and P. vivax are urgently required.
Because the goal of global malaria control is its elimination and eradication, not only blood-stage vaccines that prevent the disease are necessary but also vaccines that severely impair parasite transmission based on sexual- or mosquito-stage antigens. However, unlike blood-stage antigens, only a few sexual-stage candidate vaccines have been developed, including the P25 antigens (Pfs25 and Pvs25, which block the transmission of P. falciparum and P. vivax, respectively) (4–7). We have demonstrated in preclinical studies that the P25 antigens, formulated and administered with various vaccination regimens, are promising candidates for malaria transmission-blocking vaccines (TBVs) (6–12). Recent clinical trials of TBVs have also suggested that P25 antigens per se are promising candidate TBVs (13–15). However, an immune-enhancing technology that specifically focuses on increasing humoral immunity, with a long-term memory response, is a crucial prerequisite if transmission-blocking antigens are to be clinically available in the future.
Antigen delivery to professional antigen-presenting cells (APCs) facilitates the uptake and presentation of antigens to T cells to induce antigen-specific humoral immunity or cell-mediated immunity (16–18). This delivery of specific protein antigens seems to be particularly important under steady-state conditions, because protein antigens alone often do not induce an immune response to a level sufficient to defend the host. Furthermore, the development of malaria vaccines probably requires recombinantly produced protein antigens, particularly those vaccines that target blood-stage and mosquito-stage parasites, because these types of vaccines rely more on humoral immunity than on cell-mediated immunity for their effectiveness. This is largely because protein antigens, if they are sufficiently immunogenic, are good inducers of antibody production.
Dendritic cells (DCs) are professional APCs with highly efficient and specialized functions in the uptake and presentation of foreign antigens to lymphocytes, allowing them to mount an appropriate immune response (17, 18). However, B lymphocytes also take up foreign antigens, primarily via surface immunoglobulins (Igs) (B-cell receptors [BCRs]), and present the protein epitopes through the major histocompatibility complex class II–T-cell receptor interaction to induce antigen-specific antibody production (16, 19–21). Thus, B lymphocytes are unique in that they are both APCs and effector cells. Direct antigen presentation to B lymphocytes followed by T-cell activation facilitates the induction of an efficient immune response. Another unique feature of B lymphocytes that distinguishes them from DCs is that they can recognize conformational epitopes, such as those presented on the surfaces of protein antigens. It is noteworthy that in the lymph nodes, antigens captured by noncognate B lymphocytes can be presented to cognate B lymphocytes (22). Therefore, it is theoretically plausible that antigens delivered to B-lymphocyte follicles in the lymph nodes, using Ig-binding ligands, may increase the likelihood that the antigens would encounter cognate B lymphocytes residing in the lymph node follicles. By exploiting this immunological mechanism, it may be possible to augment the immune responses against otherwise weakly immunogenic recombinant antigens (23).
To address one of the primary challenges facing the development of malaria vaccines, the low immunogenicity of recombinant malaria candidate vaccines, we undertook to functionally improve the antigen delivery system that we previously designated the “tricomponent immunopotentiating system” (12). For this system to function, a ligand moiety is essential because the antigen integrated into the system without a ligand failed to augment the antigen-specific immune response (12). The ligand moiety integrated into the tricomponent complex is an immunoglobulin-binding domain (IBD), which specifically targets B lymphocytes.
In this study, three types of IBDs were chosen as ligands to be fused to a pentameric coiled-coil domain of cartilage oligomeric matrix protein (COMP). They were (i) the Z domain of Staphylococcus aureus protein A (SpA), (ii) the B domain of a group G Streptococcus (SpG), and (iii) the B domain of Peptostreptococcus magnus (PpL). Although both the Z and SpG domains bind the IgGs of various animal species, they have distinct structural characteristics: the Z domain has three short α-helical coils folded into a compact bundle (24, 25), whereas the SpG domain has a four-stranded β-sheet connected by one α-helix (24, 26). The PpL domain has an overall chain fold similar to that of the SpG domain but, unlike the Z and SpG domains, binds the light chains of all Ig isotypes, including IgG, IgM, IgA, IgE, and IgD (24, 27). Therefore, PpL has a broad Ig-binding spectrum but binds only the kappa-type light chains (24). This selection of IBDs to be used as ligands of the tricomponent complex was chosen to evaluate whether IBDs with vastly different overall chain folds and Ig-binding profiles can be efficiently used in fusion proteins with the COMP coiled-coil domain and to determine their antigen delivery function and vaccine efficacy. Homogeneous pentameric fusion proteins were produced from all the COMP-IBD fusion proteins in Escherichia coli and were loaded with a recombinant P. vivax ookinete surface protein, Pvs25. The resultant tricomponent complexes were then evaluated for their TBV efficacy with a membrane feed assay.
MATERIALS AND METHODS
Expression of COMP-IBD fusion proteins.
The COMP-Z fusion protein was expressed in E. coli BL21(DE3) and purified with nickel-nitrilotriacetic acid (Ni-NTA) affinity chromatography, as previously described (12). To construct the expression plasmid for the COMP-SpG fusion protein, two overlapping oligonucleotides (5′-GTCGACCCGGCGGTGACGACCTATAAACTGGTTATTAACGGCAAAACCCTGAAAGGTGAAACCACCACCGAAGCCGTGGATGCAGCGACCGCCGAAAAGGTGTTTAAAC-3′ and 5′-CTCGAGTTATTCCGTCACGGTAAACGTTTTGGTCGCATCGTCGTAGGTCCACTCACCATCGACGCCATTATCGTTCGCATACTGTTTAAACACCTTTTCGGCGGTCGCT-3′, containing a SalI and an XhoI site [underlined sequences], respectively) were annealed in their 3′ complementary regions and then extended by DNA polymerase in a thermal cycling process to produce double-stranded DNA. The resultant fragment was first subcloned into pCR2.1 (Invitrogen, Carlsbad, CA, USA) and then excised from the plasmid by digestion with SalI and XhoI for subsequent insertion at the unique XhoI site in the plasmid pET-22b-COMP-spacer (12) to express the COMP-SpG fusion protein containing the pelB leader peptide for secretion. Similarly, two overlapping oligonucleotides (5′-GTCGACAGCGAAGAGGAAGTGACCATTAAAGCCAACCTGATTTTTGCGAATGGTAGCACCCAGACCGCGGAATTTAAAGGCACCTTTGAAAAAGCGACCAGCGAGGCGTATGCGTAT-3′ and 5′-CTCGAGTTAACCGGCGAATTTGATATTCAGGGTATAGCCTTTATCCGCAACATCCACGGTATATTCGCCATTATCTTTTTTCAGGGTGTCCGCATACGCATACGCCTCGCTGGTCGCT-3′, containing a SalI and an XhoI site [underlined sequences], respectively) were used for the construction of the COMP-PpL expression plasmid. The expression in E. coli BL21(DE3) and purification of the COMP-SpG and COMP-PpL fusion proteins with Ni-NTA affinity chromatography were performed as described previously for the COMP-Z fusion protein (12).
Western blotting and size exclusion chromatography.
Ni-affinity-purified COMP lacking any IBD and the three COMP-IBD fusion proteins were subjected to 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie brilliant blue or transferred to a polyvinylidene difluoride membrane for Western blotting. For Western blotting, the membrane was blocked with 10% skimmed milk in phosphate-buffered saline (PBS) and then incubated with horseradish-peroxidase-conjugated human IgG (hIgG) or hIgG containing the kappa or lambda light chain (Beckman Coulter Inc., Brea, CA, USA). Chemiluminescence was detected with the Western Lightning ECL kit (Perkin-Elmer Inc., Waltham, MA, USA).
Size exclusion chromatography was used to estimate the molecular masses of COMP and the three COMP-IBD fusion proteins in PBS using a HiLoad 16/60 Superdex 75-pg column (GE Healthcare, Little Chalfont, United Kingdom) at a flow rate of 0.8 ml/min.
Chemical conjugation of Pvs25 antigen to the COMP-IBDs.
Recombinant Pvs25H-A protein, expressed in Pichia pastoris and purified as described previously (10), was chemically conjugated to each COMP-IBD fusion protein using a heterobifunctional cross-linker, N-succinimidyl-3-(2-pyridyldithio)propionate (SPDP; Thermo Scientific Inc., Rockford, IL, USA), as described previously (12). Briefly, Pvs25H-A (2 mg/ml in PBS-EDTA) was incubated with SPDP (0.6 mM final concentration) for 1 h at room temperature and buffer exchanged with PBS (Zeba Spin desalting column; Thermo Scientific Inc.). Simultaneously, each COMP-IBD (2 mg/ml in PBS-EDTA) was treated with dithiothreitol (DTT; 50 mM) for 30 min at 37°C and buffer exchanged with PBS as before. SPDP-treated Pvs25H-A and DTT-treated COMP-IBD were mixed for conjugation in a 10:1 molar ratio at room temperature overnight and then buffer exchanged with PBS as before. The endotoxin levels in the conjugated samples were determined with Detoxi-Gel endotoxin removal gel (Thermo Scientific Inc.). The loading of the Pvs25H-A antigen onto the COMP-IBD fusion proteins was confirmed with an hIgG enzyme-linked immunosorbent assay (ELISA), as previously described (12).
Evaluation of the binding affinities of COMP-IBDs for Ig isotypes.
The binding strengths of the COMP-IBD fusion proteins for the mouse and human Ig isotypes were evaluated according to the method described by Friguet et al. (28), with slight modification (12). Briefly, each Ig isotype (5 to 10 μg/well) was applied as a coating to a 96-well microtiter plate (Sumilon; Sumitomo Bakelite Co., Ltd., Tokyo, Japan) at 4°C overnight and then blocked with 1% bovine serum albumin (BSA) in PBS at 37°C for 1 h. COMP or each of the COMP-IBDs (2 μg/well) was added to the wells and incubated at 37°C for 1 h; then, hIgG (50 μg/ml, 37°C, 1 h) was applied to mask the unbound free IBDs. A mouse or rabbit anti-His tag antibody (1:4,000; GE Healthcare) was added and incubated at 37°C for 1 h; then, an anti-mouse or anti-rabbit IgG-alkaline phosphatase (AP) conjugate (1:4,000; Sigma-Aldrich, St. Louis, MO, USA) was applied and incubated at 37°C for 1 h. The AP substrate (Bio-Rad Laboratories Inc., Redmond, WA, USA) was added and incubated at room temperature for 20 min to detect the bound proteins.
Flow cytometric analysis of the COMP-IBD target cells.
COMP (1 mg, 31.25 nmol) or the COMP-IBD fusion proteins (1 mg, 13.8 nmol) were treated with DTT (50 mM) for 37°C at 30 min and buffer exchanged with PBS (Zeba Spin desalting column; Thermo Scientific Inc.). The reduced COMP or COMP-IBDs (1 mg) were incubated overnight at 4°C with a 25-fold molar excess of fluorescein-5-maleimide (Thermo Scientific Inc.) and then buffer exchanged with PBS (Zeba Spin desalting column). Splenocytes from naive BALB/c mice were suspended in RPMI medium (Gibco Inc., Grand Island, NY, USA) and collected by centrifugation (377 × g, 10 min). Red blood cells were lysed (17 mM Tris buffer [pH 7.6], 140 mM NH4Cl) at room temperature for 5 min, and the cells were washed with PBS and resuspended in PBS. The cells (5 × 105 cells/ml) in PBS with or without 2% normal (naive) mouse serum were incubated with fluorescein-labeled COMP or COMP-IBDs (3.1 pmol). Human IgG (5 μg/ml) was added to mask the unbound free IBDs, and the 2.4G2 monoclonal antibody (BD Biosciences, Sparks, MD, USA) was added to block the Fc receptors. The cells were washed and incubated with R-phycoerythrin (PE)-conjugated anti-CD19 (1D3) or anti-CD3e (145-2C11) or the isotype control antibody. Finally, the cells were washed with PBS and the volume was adjusted to 1 ml with PBS. At each step, the samples were incubated at 4°C for 20 min. The antibodies were used at the concentrations recommended by the manufacturer (BD Biosciences). Data were acquired with a FACSCalibur flow cytometer and analyzed with CellQuest software (BD Biosciences).
Immunization of mice.
Seven-week-old female BALB/c mice (Japan SLC, Shizuoka, Japan), with eight mice per group, were immunized in weeks 0, 2, and 4 via a subcutaneous (s.c.) route. For all immunization studies, the same amount of Pvs25H-A antigen (30 μg) was administered as the conjugated (total protein dose, 40.8 μg for COMP-Z:Pvs25H-A, 40.2 μg for COMP-SpG:Pvs25H-A, and 41.9 μg for COMP-PpL:Pvs25H-A) or unconjugated Pvs25H-A protein (total protein dose, 30 μg). Aluminum hydroxide (Imject alum adjuvant; Thermo Scientific Inc.) was used as the adjuvant where indicated. The animal experimental protocols were approved by the University of the Ryukyus Animal Care and Use Committee, and the experiments were conducted according to the institutional ethical guidelines for animal experiments.
Antigen-specific serum IgG titers determined by ELISA.
Antisera were collected from the immunized mice in week 6, and the serum IgG titers were determined as described previously (8–11). Briefly, a 96-well microtiter plate (Sumilon; Sumitomo Bakelite Co., Ltd.) was coated with Pvs25H-A (5 μg/ml in bicarbonate buffer at 4°C overnight) and blocked with 1% BSA in PBS at 37°C for 2 h. Twofold serial dilutions of the antisera, starting with a 50-fold dilution in PBS with 0.5% BSA, were incubated at 37°C for 2 h. An anti-mouse IgG-AP conjugate (1:4,000; Sigma-Aldrich) (37°C, 2 h) and then the AP substrate (Bio-Rad Laboratories Inc.) were added and incubated at 37°C for 20 min, before the optical density at 415 nm (OD415) was measured with a microplate reader (Bio-Rad Laboratories Inc.). The antibody titers were defined as the serum dilutions that gave an OD415 value equal to 0.1 or as the serum dilutions for which a dilution of 1 magnitude higher gave OD415 values of less than 0.1.
Membrane feed assay.
To determine the TBV efficacy of the induced mouse antisera, a mosquito membrane feed assay was performed as described previously (8–12). Single-species infection with P. vivax was confirmed with Giemsa staining of thick and thin blood smears. The experiments involving human subjects were approved by the Ethics Committee of the Faculty of Tropical Medicine of Mahidol University and the Thai Ministry of Public Health.
Statistical analysis.
The Wilcoxon-Mann-Whitney test was performed to compare the antibody titers and the numbers of oocysts per mosquito between the nonimmune control group and the indicated immunization groups or between two indicated immunization groups. The Kruskal-Wallis test was used to compare the antibody titers and the numbers of oocysts per mosquito among the indicated groups. The χ2 test was performed to analyze the differences in the proportions of parasite-free mosquitoes in the total number of mosquitoes examined between the nonimmune control group and the indicated immunization groups or between two indicated immunization groups. All statistical analyses were conducted with JMP software version 8.0 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Expression analysis of the COMP-IBD fusion proteins.
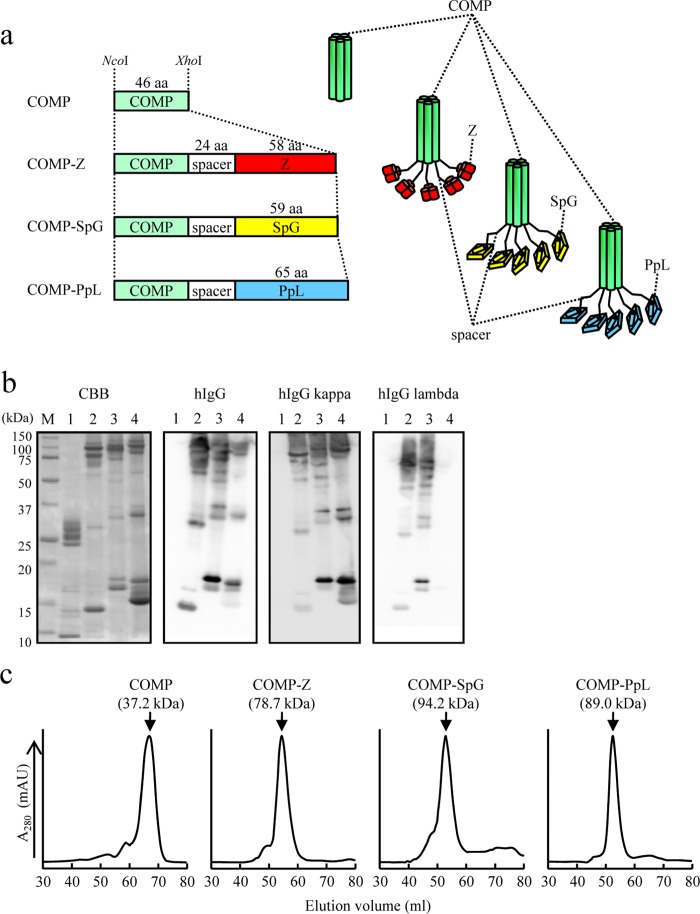
COMP devoid of any IBD and the COMP-IBD fusion proteins (Fig. 1a) were expressed as secreted proteins in E. coli BL21(DE3) and were affinity purified from the culture supernatants with Ni-NTA. Although the proteins appeared as several bands of various molecular masses on SDS-PAGE (Fig. 1b), a single major chromatographic peak was detected with size exclusion chromatography, with estimated molecular masses of 37.2 kDa for COMP, 78.7 kDa for COMP-Z, 94.2 kDa for COMP-SpG, and 89.0 kDa for COMP-PpL (Fig. 1c). These molecular masses appeared to be higher than the calculated values for their pentamers (i.e., 32.0 kDa, 72.5 kDa, 71.4 kDa, and 74.7 kDa, respectively), and the discrepancies may be attributable to the rodlike structure of the COMP coiled-coil domain (29, 30). These data indicate that the COMP-IBDs were produced in E. coli as homogeneous pentamers but were disassembled into the constituent components with various valences in the presence of a denaturing agent, because coiled coils are vulnerable to disassembly by denaturing agents such as SDS (12). Given that the COMP coiled-coil domain has interstrand disulfide bonds at its C terminus (PDB: 1VDF), the incomplete formation of the disulfide bonds should create a series of multimers in the presence of SDS. Thus, COMP and its IBD fusion proteins appeared on SDS-PAGE as ladder patterns (presumably as monomers through pentamers). The COMP-IBDs, but not COMP, bound hIgG (Fig. 1b). COMP-PpL bound only hIgG with the kappa, but not the lambda, light chain (Fig. 1b).
FIG 1.
Expression of the COMP-IBDs. (a) Schematic drawing of the coiled-coil domain of the cartilage oligomeric matrix protein (COMP) fused to immunoglobulin-binding domains (IBDs). The coiled-coil domain (green) is fused to the Z domain (red), a derivative of the B domain of Staphylococcus aureus protein A; the B domain of group G Streptococcus protein G (yellow); or the B domain of Peptostreptococcus magnus protein L (blue). The fusion proteins contain a 24-amino-acid (aa) spacer region between the coiled coil and the IBD. The coding sequences of the fusion proteins were placed between the NcoI and XhoI sites of pET-22b and thus were expressed as pelB fusion proteins for secretion from Escherichia coli BL21(DE3). The COMP and COMP-Z constructs were engineered in our previous study (12). (b) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 15%) of nickel-affinity-purified COMP (lanes 1), COMP-Z (lanes 2), COMP-SpG (lanes 3), and COMP-PpL (lanes 4). The protein bands were stained with Coomassie brilliant blue (CBB) or subjected to Western blotting and then reacted with hIgG or hIgG containing the kappa or lambda light chain. For Western blotting, horseradish-peroxidase-conjugated antibodies were directly applied to the membrane to detect the COMP-IBD fusion proteins. Lane M, molecular mass marker. (c) COMP or the COMP-IBDs were subjected to size exclusion chromatography. Their estimated molecular masses, based on the standard protein marker, are indicated in parentheses.
Binding profiles of the COMP-IBD fusion proteins to Ig isotypes and splenic lymphocytes.
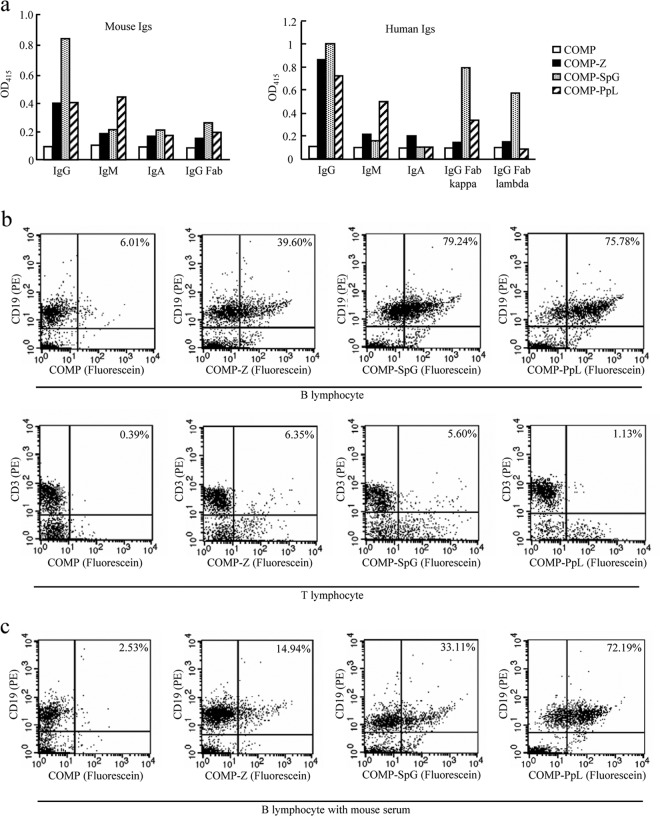
The COMP-IBD fusion proteins were first analyzed with ELISAs to assess their capacities for binding mouse or human Ig isotypes (Fig. 2a). All the COMP-IBDs bound both mouse and human IgGs, but COMP-SpG displayed relatively higher affinity than did the other two fusion proteins. In contrast to the Z and SpG complexes, COMP-PpL strongly bound both mouse and human IgMs. Furthermore, as expected, the PpL complex bound only Igs with kappa, but not lambda, light chains. In conclusion, all the fusion proteins showed similar binding patterns to mouse and human Ig isotypes.
FIG 2.
Binding of the COMP-IBD tricomponent complexes to mouse and human Ig isotypes and mouse lymphocytes in vitro. (a) Affinity of the COMP-IBD fusion proteins for mouse and human immunoglobulin (Ig) isotypes. COMP lacking any IBD was used as the negative control. The relative binding strength is expressed as OD415. (b and c) Flow cytometric analysis of the COMP-IBDs without (b) or with (c) normal mouse serum. Freshly isolated splenocytes from naive BALB/c mice were double stained with PE-conjugated anti-CD19 or anti-CD3 monoclonal antibody and fluorescein-labeled COMP or COMP-IBDs and then analyzed on a FACSCalibur flow cytometer, as described previously (12). Representative contour plots show the percentage of the fusion complex bound to CD19+ B lymphocytes or CD3+ T lymphocytes.
The COMP-IBDs were then analyzed by flow cytometry to determine whether they bound splenic CD19+ B lymphocytes or CD3+ T lymphocytes (Fig. 2b). All the COMP-IBDs, but not COMP lacking an IBD, bound substantial proportions of the B-lymphocyte population (Fig. 2b, top four panels); approximately 40%, 80%, and 75% of B lymphocytes were bound by fluorescein-conjugated COMP-Z, COMP-SpG, and COMP-PpL, respectively. In contrast, none of the COMP-IBDs bound T lymphocytes (Fig. 2b, bottom four panels), confirming that the fusion proteins specifically bind to B lymphocytes. It is reasonable to envisage that loading antigens on the COMP-IBDs would negatively affect their B-lymphocyte binding capacity. However, we think this is unlikely to occur, first, because the antigen conjugation is site specific and the sites are distantly located from the IBDs on the fusion proteins, and second, because the B-lymphocyte binding experiments were conducted using the fluorescein-labeled COMP-IBD fusion proteins which were generated by the same conjugation method used to generate the COMP-IBD:Pvs25H-A tricomponent complexes evaluated in immunization experiments described in Fig. 3.
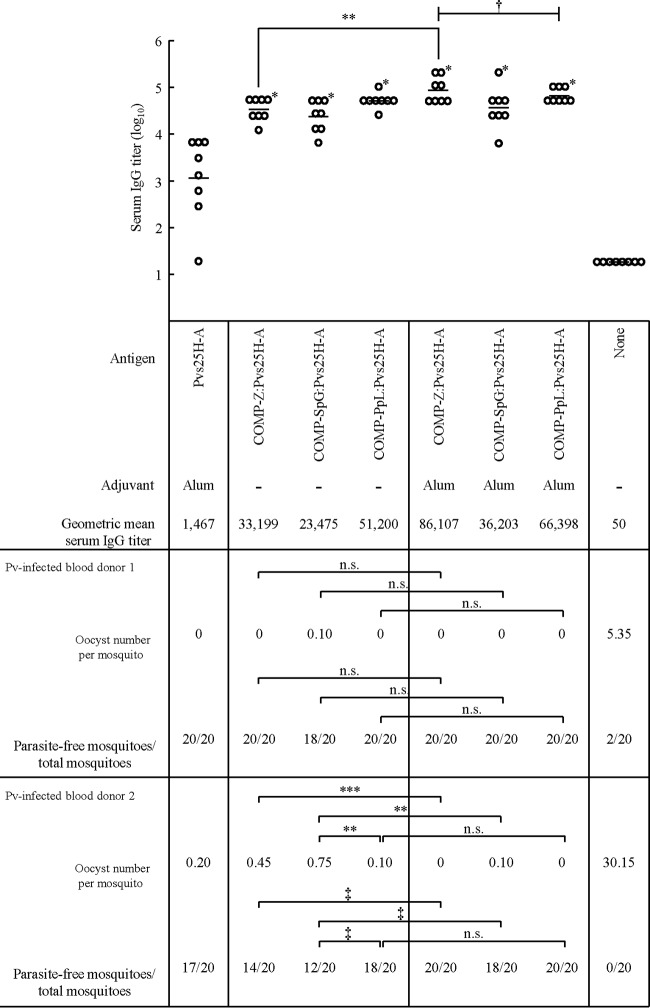
FIG 3.
Antibody responses and transmission-blocking vaccine efficacy of the COMP-IBD tricomponent complexes. Female BALB/c mice (eight mice per group) were immunized with 30 μg of the Pvs25H-A antigen as a conjugated (total protein dose: COMP-Z:Pvs25H-A, 40.8 μg; COMP-SpG:Pvs25H-A, 40.2 μg; and COMP-PpL:Pvs25H-A, 41.9 μg) or unconjugated protein. The amount of endotoxin contaminating the final immunization material was shown to be less than 15 pg/μg of the total protein. Aluminum hydroxide (alum) was used as the adjuvant where indicated. The mice were immunized three times via a subcutaneous route in weeks 0, 2, and 4, and their antisera were collected 2 weeks after the third immunization (week 6) to evaluate the Pvs25-specific IgG titers. The serum IgG titers were defined as the serum dilution that resulted in an OD415 of 0.1 or the serum dilution for which a 1-point-higher dilution (2-fold) resulted in an OD415 of less than 0.1. The titers are expressed as geometric means. *, P < 0.001 versus the Pvs25H-A/alum-immunized group on the Wilcoxon-Mann-Whitney test; **, P < 0.05 between the two indicated groups on the Wilcoxon-Mann-Whitney test; ***, P < 0.01 between the two indicated groups on the Wilcoxon-Mann-Whitney test; †, P < 0.05 among the three indicated groups on the Kruskal-Wallis test. All immunized groups, including that immunized with Pvs25H-A/alum, displayed a statistically significant serum IgG response compared with that of the nonimmunized group (P < 0.001 on the Wilcoxon-Mann-Whitney test). In the membrane feed assay, a 2-fold dilution of the pooled mouse antisera was used to evaluate the transmission-blocking efficacy. The number of oocysts per mosquito and the number of parasite-free mosquitoes compared with the total number of mosquitoes (20 mosquitoes) are shown. ‡, P < 0.05 between the two indicated groups on the χ2 test. n.s., not significant.
It was assumed that since the IBDs bind B lymphocytes via BCR, they might also bind free antibodies in vivo. To investigate this possibility, we analyzed the binding of the fusion proteins to B lymphocytes in the presence of normal mouse serum (Fig. 2c). The results showed that the mouse serum strongly inhibited the binding (by more than 50%) of the Z and SpG complexes, whereas essentially no inhibition was observed for the PpL complex. This unexpected result suggests that COMP-PpL may function in the delivery of loaded vaccine antigens to B-lymphocyte follicles even when the antigens are administered via routes where immunoglobulin molecules are abundant.
Vaccine efficacy of the tricomponent complexes.
The recombinant Pvs25H-A protein was loaded onto each COMP-IBD fusion protein by chemical conjugation, as described previously (12). The cysteine residues present within the COMP coiled-coil domain (PDB: 1VDF) were used as the specific loading sites for the antigen, and loading was confirmed with an hIgG ELISA (data not shown). The endotoxin contaminating the final immunizing materials was shown to be less than 15 pg endotoxin/μg of total protein. Mice were immunized s.c. three times, each time with 30 μg of the unloaded Pvs25H-A antigen or with one of the tricomponent complexes carrying 30 μg of the antigen. We found that loading the antigen onto the carrier molecules increased the antibody response by more than 1 order of magnitude, as determined from the mean IgG titers, and the alum adjuvant further increased these titers (Fig. 3).
Next, the TBV efficacy of the tricomponent complexes was evaluated with a mosquito membrane feed assay, as described previously (8–12). Twenty mosquitoes were fed on one or the other blood sample prepared from two patients in Thailand, who were confirmed to be singly infected with P. vivax (Pv-infected blood donors 1 and 2 in Fig. 3). The mosquitoes were then dissected to count the numbers of oocysts that had developed in their midguts. Approximately 5 and 30 oocysts were found in each mosquito, on average, when nonimmune mouse serum was incubated with the infected blood samples from donors 1 and 2, respectively (Fig. 3). This indicated that the severity of infection in the mosquitoes was much higher when they were fed the blood from donor 2 than when they were fed the blood from donor 1. Incubation of the induced mouse immune sera with the blood from donor 1 strongly inhibited oocyst formation. Except for the SpG complex administered without alum adjuvant, all the immune sera conferred a complete transmission blockade, meaning that all the mosquitoes were completely free of parasite. In contrast, most of the mosquitoes were infected with the parasite when nonimmune serum was mixed with the blood from donor 1. When we used the blood from donor 2, which displayed a relatively high infectivity level, reduced TBV effects were observed. A complete transmission blockade was observed only for the Z and PpL complexes mixed with alum adjuvant. A marked reduction in the TBV efficacy when infection severity was high was also observed in our previous studies, particularly when the alum adjuvant was omitted from the tricomponent complex or the alum was simply mixed with an unloaded antigen (11, 12). Taken together, these results suggest that when infection severity was low, as seen in donor 1, even the use of alum adjuvant alone exerted a strong TBV effect. Meanwhile, when infection severity was moderate to high, as seen in donor 2, a simple mixture of alum and an unloaded antigen was insufficient to exert an adequate TBV effect, emphasizing that a combination of alum adjuvant and the designed antigen is required to cope with any degree of parasite infection severity to ensure a complete transmission blockade. This is important because even one oocyst developing in the midgut of a mosquito can produce thousands of infectious sporozoites in its salivary glands.
DISCUSSION
Recombinant subunit vaccines, in general, require adjuvants that effectively elicit an innate immune response, or vehicles that deliver antigens to professional APCs, or any other molecular design that enhances the immunogenicity of the protein antigen. These might include such strategies as ligating the vaccine antigens to large immunogenic proteins to produce increased numbers of T-cell epitopes, transforming them into high-molecular-mass soluble aggregates for increased B-cell stimulation, or integrating them into particulate structures, such as that used for the development of the RTS,S candidate malaria vaccine. Many candidate malaria vaccines under evaluation in preclinical or clinical trials are recombinant proteins or synthetic peptides derived from the preerythrocytic, erythrocytic, or sexual stage of the parasite (15) and are therefore very likely to require adjuvants in the final formulations. For example, the most advanced candidate malaria vaccine, the RTS,S/AS01, which is currently in phase 3 clinical trials, is a recombinantly produced viruslike particle composed of the hepatitis B virus surface antigen carrying the C-terminal region of the P. falciparum circumsporozoite protein, mixed with a novel adjuvant (31).
In this study, we addressed one of the primary challenges facing TBV development, the low immunogenicity of the recombinant ookinete surface protein, Pvs25, as a candidate vaccine. A past TBV clinical trial of the Pvs25 candidate formulated with Alhydrogel was discouraging because of its low immunogenicity in vaccinated individuals (13). Therefore, the adjuvant was later changed to Montanide ISA 51, which, as expected, significantly improved the immunogenicity and efficacy of the TBV, although unacceptable reactogenicity related to the adjuvant led to the discontinuation of the clinical study (14). A safety evaluation of Pfs25 conjugated to Pseudomonas aeruginosa exoprotein A formulated with Alhydrogel is now under way, in expectation that elevated immunogenicity with robust safety will be achieved (15, 32, 33).
Alum adjuvants have a long history of safe use in humans and animals. Therefore, if the development of a TBV is based on this adjuvant, strategies to augment the immunogenicity of the vaccine antigen per se will be needed. A very-high-magnitude humoral immune response with a long-lasting memory response (of at least 1 year) is of paramount importance for TBVs. This is in clear contrast to vaccines based on preerythrocytic-stage antigens, which ideally induce humoral immunity combined with cell-mediated immunity. However, a distinct advantage of sexual-stage antigens, such as P25, over other antigens expressed in the asexual stage is their less polymorphic nature. Moreover, the membrane feed assay, as demonstrated in this and other studies, allows the fast and reliable preclinical evaluation of TBV candidates and vaccine formulations.
In this study, we have demonstrated that the integration of the Pvs25 antigen into the tricomponent complexes enhances its TBV efficacy. The IBD adopted a pentameric configuration when linked to the COMP coiled-coil domain, and this particular molecular configuration has been shown to be important in increasing the immunogenicity of the loaded antigen, compared with other pentameric structures, such as that generated by five tandemly linked IBDs (12). Therefore, not only the multimeric structure of the IBDs but their particular molecular configurations, as exhibited by the COMP-IBDs, may also contribute to the high immunopotentiating capacity of the tricomponent complex. Therefore, the type of IBD, its multimeric nature, and its particular structural configuration might all influence the effective outcome of the vaccine. It is noteworthy that the PpL complex conferred complete transmission blockade in the presence of an alum adjuvant, and its vaccine efficacy remained high, even in the absence of adjuvant. One possible reason for its high immune-enhancing capacity is the broad Ig-binding spectrum of PpL (Fig. 2a and b), so that a large repertoire of B lymphocytes are targeted in the lymphoid follicles (20). IBDs can bind free antibodies, so their ligand function may be abrogated when administered in vivo, where antibodies are abundant. In fact, the binding of the Z and SpG complexes to B lymphocytes was strongly inhibited by normal mouse serum (Fig. 2c). We also previously confirmed that the COMP-Z:Pvs25 tricomponent complex failed to induce an antigen-specific antibody response when given via an intraperitoneal or intravenous route (12). However, to our surprise, COMP-PpL sustained its high B-lymphocyte binding capacity even in the presence of serum (Fig. 2c).
We did not examine the precise immunological mechanism behind the immune-enhancing capacity of the COMP-IBD fusion proteins. One possible explanation of this mechanism is, as mentioned above, that B lymphocytes are APCs, although not as efficient as DCs, so targeting B lymphocytes may increase the chance that the antigen will encounter its cognate B lymphocyte in the lymphoid follicle. Another possible explanation is that in the lymph nodes, the antigen captured by noncognate B lymphocytes might be ultimately transferred to cognate B lymphocytes (22, 34). This may increase the chance that the antigen will encounter its cognate B lymphocytes and other professional APCs, such as resident conventional DCs, in the lymph nodes (35). Thus, IBDs may function to concentrate the loaded antigen in the B-lymphocyte follicles in the lymph nodes and prevent their removal by irrelevant cells. This would reduce any waste of the administered antigen. Furthermore, because the IBDs are designed to be multimeric, the complexes may form cross-links with BCRs to induce B-cell stimulation (16, 36).
It is reasonable to ask why a low-titer antibody response could have effective transmission-blocking activity. In other words, we may ask why Pvs25/alum conferred a relatively high transmission blockade (100% [20/20] and 85% [17/20] for blood samples from donors 1 and 2, respectively), although the antibody titer was low (1,467). Although the ELISA titer was low, the anti-Pvs25 antibodies induced by Pvs25/alum had a greater antiparasitic effect than did the antibodies induced by the COMP-IBD:Pvs25 without alum, even though the latter induced high IgG titers (20,000 to 50,000). This discrepancy between the antibody titer and the vaccine efficacy may have arisen because the alum adjuvant increases the antiparasitic effects of the induced antibodies. Thus, the alum adjuvant is very likely to affect the quality of the antibodies produced by the B cells. Indeed, aluminum hydroxide is known to activate the Nalp3 inflammasome (27). Alum is also known to induce the production of interleukin-5, which acts on B cells to facilitate their proliferation and antibody class switching. These effects on the innate and adaptive immune responses attributable to alum may increase the binding affinity of antibodies for the antigen and may thus enhance the antiparasitic function of the antigen-specific immunity induced. This is not necessarily reflected in the magnitude of the antibody titer. Delivery vehicles, such as the tricomponent complex, may simply concentrate the vaccine antigens in the draining lymph nodes, where the immune response is initiated. However, the alum, and perhaps other adjuvants, may modify the quality of the induced immune response so that it exerts additional host defense functions, which are not simply reflected in the magnitude of the antibody titer. Therefore, antigen-delivery systems and adjuvants probably have distinct immunological functions and outcomes. This is the most important reason for the combined use of these two immunopotentiating elements in the development of recombinant protein vaccines.
The P25 antigens of P. falciparum and P. vivax are by far the leading candidates for malaria TBVs. However, P25/alum has proved insufficient as a TBV in recent clinical trials (13). Moreover, the use of oil-based adjuvants may not be tolerated because their potential reactogenicity is high (14). Therefore, molecular designs that include an innovative novel antigen-delivery technology are even more necessary than previously realized for the future development of malaria TBVs.
ACKNOWLEDGMENTS
We thank Tetsuya Harakuni for his excellent technical assistance.
This work was supported by the following grants: Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI (20590425, 23590490, and 23117008) and the Japan Society for the Promotion of Science (JSPS) KAKENHI (23406007) in Japan; the Program for the Promotion of Basic Research Activities for Innovative Biosciences from the Bio-oriented Technology Research Advancement Institution of Japan; and a Cooperative Research Grant from the Institute of Tropical Medicine, Nagasaki University, Japan.
Footnotes
Published ahead of print 12 February 2014
REFERENCES
- 1.World Health Organization. 2012. World malaria report 2012. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2012/report/en/index.html [Google Scholar]
- 2.Alonso PL, Tanner M. 2013. Public health challenges and prospects for malaria control and elimination. Nat. Med. 19:150–155. 10.1038/nm.3077 [DOI] [PubMed] [Google Scholar]
- 3.Mendis K, Sina BJ, Marchesini P, Carter R. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64:97–106 [DOI] [PubMed] [Google Scholar]
- 4.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, McCutchan TF, Miller LH. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333:74–76. 10.1038/333074a0 [DOI] [PubMed] [Google Scholar]
- 5.Kaslow DC. 1990. Immunogenicity of Plasmodium falciparum sexual stage antigens: implications for the design of a transmission blocking vaccine. Immunol. Lett. 25:83–86. 10.1016/0165-2478(90)90096-9 [DOI] [PubMed] [Google Scholar]
- 6.Hisaeda H, Stowers AW, Tsuboi T, Collins WE, Sattabongkot JS, Suwanabun N, Torii M, Kaslow DC. 2000. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect. Immun. 68:6618–6623. 10.1128/IAI.68.12.6618-6623.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuboi T, Tachibana M, Kaneko O, Torii M. 2003. Transmission-blocking vaccine of vivax malaria. Parasitol. Int. 52:1–11. 10.1016/S1383-5769(02)00037-5 [DOI] [PubMed] [Google Scholar]
- 8.Arakawa T, Tsuboi T, Kishimoto A, Sattabongkot J, Suwanabun N, Rungruang T, Matsumoto Y, Tsuji N, Hisaeda H, Stowers A, Shimabukuro I, Sato Y, Torii M. 2003. Serum antibodies induced by intranasal immunization of mice with Plasmodium vivax Pvs25 co-administered with cholera toxin completely block parasite transmission to mosquitoes. Vaccine 21:3143–3148. 10.1016/S0264-410X(03)00258-5 [DOI] [PubMed] [Google Scholar]
- 9.Arakawa T, Komesu A, Otsuki H, Sattabongkot J, Udomsangpetch R, Matsumoto Y, Tsuji N, Wu Y, Torii M, Tsuboi T. 2005. Nasal immunization with a malaria transmission-blocking vaccine candidate, Pfs25, induces complete protective immunity in mice against field isolates of Plasmodium falciparum. Infect. Immun. 73:7375–7380. 10.1128/IAI.73.11.7375-7380.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyata T, Harakuni T, Tsuboi T, Sattabongkot J, Kohama H, Tachibana M, Matsuzaki G, Torii M, Arakawa T. 2010. Plasmodium vivax ookinete surface protein Pvs25 linked to cholera toxin B subunit induces potent transmission-blocking immunity by intranasal as well as subcutaneous immunization. Infect. Immun. 78:3773–3782. 10.1128/IAI.00306-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyata T, Harakuni T, Sugawa H, Sattabongkot J, Kato A, Tachibana M, Torii M, Tsuboi T, Arakawa T. 2011. Adenovirus-vectored Plasmodium vivax ookinete surface protein, Pvs25, as a potential transmission-blocking vaccine. Vaccine 29:2720–2726. 10.1016/j.vaccine.2011.01.083 [DOI] [PubMed] [Google Scholar]
- 12.Miyata T, Harakuni T, Tsuboi T, Sattabongkot J, Ikehara A, Tachibana M, Torii M, Matsuzaki G, Arakawa T. 2011. Tricomponent immunopotentiating system as a novel molecular design strategy for malaria vaccine development. Infect. Immun. 79:4260–4275. 10.1128/IAI.05214-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malkin EM, Durbin AP, Diemert DJ, Sattabongkot J, Wu Y, Miura K, Long CA, Lambert L, Miles AP, Wang J, Stowers A, Miller LH, Saul A. 2005. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine 23:3131–3138. 10.1016/j.vaccine.2004.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, Aebig J, Orcutt A, Muratova O, Song G, Lambert L, Zhu D, Miura K, Long C, Saul A, Miller LH, Durbin AP. 2008. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One 3:e2636. 10.1371/journal.pone.0002636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. 2013. Tables of malaria vaccine projects globally. The rainbow tables. World Health Organization, Geneva, Switzerland: http://www.who.int/vaccine_research/links/Rainbow/en [Google Scholar]
- 16.Kakiuchi T, Chesnut RW, Grey HM. 1983. B cells as antigen-presenting cells: the requirement for B cell activation. J. Immunol. 131:109–114 [PubMed] [Google Scholar]
- 17.Cella M, Sallusto F, Lanzavecchia A. 1997. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 9:10–16. 10.1016/S0952-7915(97)80153-7 [DOI] [PubMed] [Google Scholar]
- 18.Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252. 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- 19.Clark MR, Massenburg D, Zhang M, Siemasko K. 2003. Molecular mechanisms of B cell antigen receptor trafficking. Ann. N. Y. Acad. Sci. 987:26–37. 10.1111/j.1749-6632.2003.tb06030.x [DOI] [PubMed] [Google Scholar]
- 20.Wang LD, Clark MR. 2003. B-cell antigen-receptor signalling in lymphocyte development. Immunology 110:411–420. 10.1111/j.1365-2567.2003.01756.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scandella E, Fink K, Junt T, Senn BM, Lattmann E, Forster R, Hengartner H, Ludewig B. 2007. Dendritic cell-independent B cell activation during acute virus infection: a role for early CCR7-driven B-T helper cell collaboration. J. Immunol. 178:1468–1476 [DOI] [PubMed] [Google Scholar]
- 22.Ng PP, Jia M, Patel KG, Brody JD, Swartz JR, Levy S, Levy R. 2012. A vaccine directed to B cells and produced by cell-free protein synthesis generates potent antilymphoma immunity. Proc. Natl. Acad. Sci. U. S. A. 109:14526–14531. 10.1073/pnas.1211018109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arakawa T. 2011. Adjuvants: no longer a ‘dirty little secret', but essential key players in vaccines of the future. Expert Rev. Vaccines 10:1–5. 10.1586/erv.10.140 [DOI] [PubMed] [Google Scholar]
- 24.Tashiro M, Montelione GT. 1995. Structures of bacterial immunoglobulin-binding domains and their complexes with immunoglobulins. Curr. Opin. Struct. Biol. 5:471–481. 10.1016/0959-440X(95)80031-X [DOI] [PubMed] [Google Scholar]
- 25.Tashiro M, Tejero R, Zimmerman DE, Celda B, Nilsson B, Montelione GT. 1997. High-resolution solution NMR structure of the Z domain of staphylococcal protein A. J. Mol. Biol. 272:573–590. 10.1006/jmbi.1997.1265 [DOI] [PubMed] [Google Scholar]
- 26.Gronenborn AM, Filpula DR, Essig NZ, Achari A, Whitlow M, Wingfield PT, Clore GM. 1991. A novel, highly stable fold of the immunoglobulin binding domain of streptococcal protein G. Science 253:657–661. 10.1126/science.1871600 [DOI] [PubMed] [Google Scholar]
- 27.Wikstrom M, Sjobring U, Kastern W, Bjorck L, Drakenberg T, Forsen S. 1993. Proton nuclear magnetic resonance sequential assignments and secondary structure of an immunoglobulin light chain-binding domain of protein L. Biochemistry (Mosc.) 32:3381–3386. 10.1021/bi00064a023 [DOI] [PubMed] [Google Scholar]
- 28.Friguet B, Chaffotte AF, Djavadi-Ohaniance L, Goldberg ME. 1985. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Methods 77:305–319. 10.1016/0022-1759(85)90044-4 [DOI] [PubMed] [Google Scholar]
- 29.Lupas A. 1996. Coiled coils: new structures and new functions. Trends Biochem. Sci. 21:375–382. 10.1016/S0968-0004(96)10052-9 [DOI] [PubMed] [Google Scholar]
- 30.Lupas AN, Gruber M. 2005. The structure of alpha-helical coiled coils. Adv. Protein Chem. 70:37–78. 10.1016/S0065-3233(05)70003-6 [DOI] [PubMed] [Google Scholar]
- 31.Olotu A, Lusingu J, Leach A, Lievens M, Vekemans J, Msham S, Lang T, Gould J, Dubois MC, Jongert E, Vansadia P, Carter T, Njuguna P, Awuondo KO, Malabeja A, Abdul O, Gesase S, Mturi N, Drakeley CJ, Savarese B, Villafana T, Lapierre D, Ballou WR, Cohen J, Lemnge MM, Peshu N, Marsh K, Riley EM, von Seidlein L, Bejon P. 2011. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5–17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect. Dis. 11:102–109. 10.1016/S1473-3099(10)70262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimp RL, Jr, Rowe C, Reiter K, Chen B, Nguyen V, Aebig J, Rausch KM, Kumar K, Wu Y, Jin AJ, Jones DS, Narum DL. 2013. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 31:2954–2962. 10.1016/j.vaccine.2013.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian F, Wu Y, Muratova O, Zhou H, Dobrescu G, Duggan P, Lynn L, Song G, Zhang Y, Reiter K, MacDonald N, Narum DL, Long CA, Miller LH, Saul A, Mullen GE. 2007. Conjugating recombinant proteins to Pseudomonas aeruginosa ExoProtein A: a strategy for enhancing immunogenicity of malaria vaccine candidates. Vaccine 25:3923–3933. 10.1016/j.vaccine.2007.02.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phan TG, Grigorova I, Okada T, Cyster JG. 2007. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 8:992–1000. 10.1038/ni1494 [DOI] [PubMed] [Google Scholar]
- 35.Caminschi I, Shortman K. 2012. Boosting antibody responses by targeting antigens to dendritic cells. Trends Immunol. 33:71–77. 10.1016/j.it.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 36.Tolar P, Sohn HW, Liu W, Pierce SK. 2009. The molecular assembly and organization of signaling active B-cell receptor oligomers. Immunol. Rev. 232:34–41. 10.1111/j.1600-065X.2009.00833.x [DOI] [PubMed] [Google Scholar]