Abstract
Strains of Lactobacillus plantarum were grown and stored in cherry (ChJ), pineapple (PJ), carrot (CJ), and tomato (TJ) juices to mimic the chemical composition of the respective matrices. Wheat flour hydrolysate (WFH), whey milk (W), and MRS broth were also used as representatives of other ecosystems. The growth rates and cell densities of L. plantarum strains during fermentation (24 h at 30°C) and storage (21 days at 4°C) differed only in part, being mainly influenced by the matrix. ChJ and PJ were the most stressful juices for growth and survival. Overall, the growth in juices was negatively correlated with the initial concentration of malic acid and carbohydrates. The consumption of malic acid was noticeable for all juices, but mainly during fermentation and storage of ChJ. Decreases of branched-chain amino acids (BCAA)—with the concomitant increase of their respective branched alcohols—and His and increases of Glu and gamma-aminobutyric acid (GABA) were the main traits of the catabolism of free amino acids (FAA), which were mainly evident under less acidic conditions (CJ and TJ). The increase of Tyr was found only during storage of ChJ. Some aldehydes (e.g., 3-methyl-butanal) were reduced to the corresponding alcohols (e.g., 3-methyl-1-butanol). After both fermentation and storage, acetic acid increased in all fermented juices, which implied the activation of the acetate kinase route. Diacetyl was the ketone found at the highest level, and butyric acid increased in almost all fermented juices. Data were processed through multidimensional statistical analyses. Except for CJ, the juices (mainly ChJ) seemed to induce specific metabolic traits, which differed in part among the strains. This study provided more in-depth knowledge on the metabolic mechanisms of growth and maintenance of L. plantarum in vegetable and fruit habitats, which also provided helpful information to select the most suitable starters for fermentation of targeted matrices.
INTRODUCTION
When environmental conditions are favorable, microorganisms primarily devote resources to growth, whereas under nutrient limitation, most of the energy is invested in survival (1). This dichotomy is formalized through the Pirt linear equation for substrate consumption (2), which shares metabolic energy between biosynthetic and maintenance processes. As the microbial demand for maintenance energy is usually constant, less energy is available for growth-associated processes in the presence of hostile environments, where resources are limiting (3).
More than other food ecosystems, raw fruits and some vegetables possess intrinsic chemical and physical parameters that make them particularly hostile environments for microorganisms. The extremely acid environment, buffering capacity, high concentration of carbohydrates, indigestible nutrients (e.g., fiber, inulin, and fructo-oligosaccharides), and antinutritional and inhibitory factors (e.g., tannins and polyphenol compounds) (4, 5, 6) are the main features of raw fruits and some vegetables (7).
Lactic acid bacteria are the most widely used group of bacteria in the food industry. Recently, several vegetables and fruits (6, 8, 9, 10) were successfully subjected to fermentation by lactic acid bacteria, which were selected within the autochthonous microbiota. Lactic acid fermentation of fresh vegetables and fruits is a low-cost and sustainable process that aims to retain the sensory and nutritional features of the raw matrices and extend shelf life under safe conditions. Lactobacillus plantarum is one of the species of lactic acid bacteria most frequently found or used to ferment vegetables and fruits (6, 8, 9, 10). L. plantarum is a highly heterogeneous and versatile species (6), very often encountered in plant, dairy, meat, and wheat fermentations and as a natural inhabitant of the gastrointestinal tract in humans and animals (11, 12). Its natural contamination and broad commercial application reflect its remarkable ecological adaptability to different habitats. The capacity to ferment a broad range of carbohydrates (13) and other energy sources and to metabolize several polyphenol compounds (14), the possession of a broad portfolio of enzymes (e.g., β-glucosidase, p-coumaric acid decarboxylase, and general decarboxylase) (14), and the synthesis of several antimicrobial compounds (15) are considered to be the most suitable features for niche adaptation.
Adaptation to vegetable and fruit ecosystems markedly varied within species and strains of lactic acid bacteria. This is because of the diversity of the plant environments, which, in turn, reflects on the microbial capacity to share metabolic energy between biosynthesis (e.g., use of alternative substrates) and maintenance (e.g., global stress responses) (3). The right balance between growth during fermentation, also including enzyme activities that have positive effects on the sensory, nutritional, and functional features, and survival at elevated numbers during storage is indispensable to guarantee high standards during vegetable and fruit processing by lactic acid bacteria (16). Nevertheless, the metabolic adaptation and response of lactic acid bacteria to vegetable and fruit ecosystems was poorly investigated compared to other fermented foods (e.g., dairy and cereal products). We sought more in-depth knowledge of the mechanisms of growth and survival in diverse and hostile vegetable and fruit habitats with the aim of describing specific metabolic traits, which allows better design of fermentation strategies based on selected strains of lactic acid bacteria for targeted raw matrices.
With the above-mentioned aim, this study investigated the growth and survival of several strains of L. plantarum under environmental conditions such as those characterizing vegetables and fruits. A panel of various metabolome approaches was used to describe the responses. Multidimensional statistical analyses were used to define the correlations between the chemical compositions of plant matrices and the growth and survival of L. plantarum strains, as well as the differences among bacterial strains, based on metabolic responses.
MATERIALS AND METHODS
Preparation of media.
Fruit (cherry [ChJ] and pineapple [PJ]) and vegetable (carrot [CJ] and tomato [TJ]) juice media were chosen as model systems for the study as representatives of diverse ecosystems. They were prepared as described by Di Cagno et al. (8). Fruits and vegetables were separately homogenized, centrifuged (10,000 × g; 20 min; 4°C), heat treated (121°C for 10 min), filtered onto a Whatman apparatus (Polycarp 75 SPF; Whatman International, Maidstone, England), sterilized by filtration on 0.22-μm membrane filters (Millipore), and stored at −20°C before use. Wheat flour hydrolysate (WFH) and whey milk (W) were chosen as representative media for other ecosystems where lactic acid bacteria are largely used and studied. WFH was produced as described by Di Cagno et al. (17). Commercial W (Sigma Chemical Co., Milan, Italy) was resuspended (5% [wt/vol] in tap water), filtered through a Whatman apparatus (Whatman International), sterilized by filtration on 0.22-μm membrane filters (Millipore), and stored at 4°C before use. MRS broth (Oxoid, Basingstoke, Hampshire, England) was used as the control medium for optimal growth. The main chemical compositions of the culture media are shown in Table S1 in the supplemental material.
Microorganisms and growth conditions.
L. plantarum CIL6 from cherry (6), L. plantarum 1MR20 from pineapple (10), L. plantarum C2 from carrot (8), L. plantarum POM1 from tomato (9), L. plantarum DC400 from Italian wheat sourdough (17), and L. plantarum CC3M8 from Caciocavallo Pugliese cheese (18) were used for fermentation. All bacterial strains belonged to the Culture Collection of the Department of Soil, Plant and Food Sciences, University of Bari, Bari, Italy. Except for L. plantarum DC400, which was propagated on MRS broth modified by the addition of fresh yeast extract (5% [vol/vol]) and 28 mM maltose at a final pH of 5.6 (mMRS), all the strains were propagated in MRS broth at 30°C for 24 h. The 24-h-old cells were harvested by centrifugation (10,000 × g; 10 min at 4°C), washed twice in 50 mM sterile potassium phosphate buffer (pH 7.0), resuspended in sterile distilled water to a final optical density at 620 nm (OD620) of 2.5 (final cell number corresponding to ca. 9.0 log CFU ml−1), and used to inoculate (4% [vol/vol]; initial cell number corresponding to ca. 7.0 log CFU g−1) each of the culture media. Incubation was at 30°C for 24 h, and further storage was allowed for 21 days at 4°C. Assays were performed in triplicate under a total of 42 sets of experimental conditions (126 samples). Samples were analyzed at the end of fermentation and after storage. Cell enumeration was carried out by plating onto mMRS or MRS agar.
Chemical compositions of media.
pH was measured with a Foodtrode electrode (Hamilton, Bonaduz, Switzerland). Total titratable acidity (TTA) was measured on 10 ml of medium diluted with 90 ml of distilled water (Classic Blender; PBI International) and expressed as the amount (ml) of 0.1 M NaOH needed to achieve a pH of 8.3. Soluble solids were measured using an Atago digital refractometer (Chemifarm srl, Parma, Italy). The refractive index was recorded and converted to degrees Brix. Measurements were carried out at 25 ± 0.5°C. Total polyphenol compounds were determined according to the method of Slinkard and Singleton (19). Gallic acid was the standard, and the concentration of total polyphenol compounds was calculated as gallic acid milliequivalents. The buffering capacities of the media were measured using the method of Pai et al. (20). One-hundred milliliters of each medium was titrated with 1 N HCl. The values were expressed as the amount of HCl (mmol) needed to drop 1 pH unit per unit volume (1 liter).
Kinetics of growth and acidification.
The kinetics of growth and acidification were determined and modeled according to the Gompertz equation as modified by Zwietering et al. (21): y = k + A exp{−exp[(μmax or Vmax e/A)(λ − t) + 1]}, where k is the initial level of the dependent variable to be modeled (log CFU ml−1 or pH units), A is the difference in cell density or units of pH (ΔpH) between inoculation and the stationary phase, μmax and Vmax are the maximum growth rate (expressed as Δlog CFU ml−1 h−1) and the maximum acidification rate (expressed as ΔpH h−1), respectively, λ is the length of the lag phase (expressed in hours), and t is the time.
Determination of carbohydrates, organic acids, and free amino acids.
Thirty milliliters of medium was diluted in 90 ml of 50 mM phosphate buffer, pH 7.0. The suspension was kept at 40°C for 1 h under gentle stirring (150 rpm) and centrifuged at 10,000 × g for 10 min. The supernatant was filtered through a Millex-HA 0.22-μm-pore-size filter (Millipore Co.) and used for determinations. Organic acids and carbohydrates were determined through high-performance liquid chromatography (HPLC) analysis using the Äkta Purifier System (GE Healthcare), which was equipped with an Aminex HPX-87H column (ion exclusion; Bio-Rad) and a UV detector operating at 210 nm (22) or with a Spherisorb column (Waters, Milford, MA, USA) and the PerkinElmer 200a refractive index detector (PerkinElmer, Waltham, MA, USA), respectively. Total and individual free amino acids (FAA) were analyzed with a Biochrom 30 series amino acid analyzer (Biochrom Ltd., Cambridge Science Park, England), as described by Rizzello et al. (23).
Determination of volatile components and volatile free fatty acids.
Volatile components (VOC) were analyzed through purge and trap coupled with gas chromatography-mass spectrometry (PT–GC-MS), according to the method of Di Cagno et al. (9). Volatile free fatty acids (VFFA) were extracted by solid-phase microextraction coupled with GC-MS (SPME–GC-MS). One milliliter of sample was mixed with 100 μl ultra-high-quality (UHQ) water and 100 μl 2 N H2SO4 in a 10-ml glass vial, and the sealed vial was allowed to rest for 10 min at 60°C. An SPME fiber (CAR/PDMS 75 μm; Supelco) was placed in the headspace of the vial for 30 min at 60°C. Then, it was removed and desorbed for 5 min in a splitless chromatograph injector at 240°C. The chromatograph (6890; Agilent Instruments) was equipped with a Stabilwax-DA column (Restek; 30 m long, 0.32-μm inside diameter [i.d.], and 0.5 μm thick). The oven temperature was 120°C during the first 2 min, and then it was increased to 160°C (2°C min−1) and to 250°C (10°C 3 min−1). The pressure was kept constant at 41 kPa. Quantification was carried out by external calibration, using a mixed solution of VFFA standards (Sigma). Quantification of VOC was expressed as log arbitrary units of area of an ion characteristic of the compound, and quantification of VFFA was expressed in ppm (vol/vol).
Malolactic activity assays.
Cell suspensions were harvested from the media by centrifugation (8,000 × g; 15 min) and washed twice with tartrate K2HPO4 buffer, pH 3.5. The pellet was resuspended in 5 ml of buffer. Aliquots of cell suspension (1 ml, corresponding to ca. 109 CFU ml−1) were added to 25 ml of buffer (final volume) in 50-ml Erlenmeyer flasks. The headspace was flushed with N2, and suspensions were initially equilibrated for 10 min at the reaction temperature. The assay was carried out as described by Herrero et al. (24). The results were expressed as the specific activity (μmol of l-malic acid degraded per min per mg [dry weight]).
Cell membrane integrity.
The cell membrane integrity of L. plantarum strains was estimated using the LIVE/DEAD BacLight Bacterial Viability Kit (Molecular Probes, Inc., Cambridge Bioscience, Cambridge, United Kingdom), according to the manufacturer's instructions. The stained bacterial suspensions were observed using a Leica LDMC (Leica Microsystems SpA, Milan, Italy) with a 60× objective. Cell numbers were determined from images using the Image-Pro Plus image analysis software (Media Cybernetics Inc., Silver Spring, MD) (25).
Statistical analyses.
Data (at least three replicates) were subjected to one-way analysis of variance (ANOVA), and pair comparison of treatment means was achieved by Tukey's procedure at a P value of <0.05, using the statistical software Statistica 7.0 for Windows. Data were processed and analyzed in the free statistical environment R (CRAN; http://cran.r-project.org/), according to the specific procedures described below. Distances and correlations among objects (R mode) or among descriptors (Q mode) were calculated with the dist (Euclidean method) and cor (Pearson correlation) functions of the base statistical package, respectively. Principal-coordinate analysis (PCoA) was carried out and plotted with the cmdscale function, which served to calculate the variability expressed in the two axes of the plot. The distance among bacterial strains for PCoA was obtained for each group of descriptors (carbohydrates, organic acids, free amino acids, volatile components, and volatile free fatty acids) by averaging the distance between strains in each single medium. Pseudoheatmaps were used to synthetically visualize the correlations among volatile compounds or among strains, using as inputs the concentrations of all descriptors (carbohydrates, organic acids, free amino acids, volatile compounds, and volatile free fatty acids) both at the end of fermentation and at the end of storage. The heatmap function was used, setting the color to a 12-grade rainbow scale spanning from −0.1 (red) to 1 (light yellow).
RESULTS
Kinetics of growth and acidification.
ChJ, PJ, CJ, and TJ were used as model systems to mimic the chemical compositions of the respective fruits and vegetables. Strains of L. plantarum were isolated from various foods, and the juices or other media (WFH and W) used were representative of the sources of isolation.
All L. plantarum strains grew under all the conditions, but the increases of cell density (A) depended on the medium (Table 1). W, ChJ, and PJ induced the longest λ and the lowest A. The microbial growth in WFH, TJ, and CJ was similar to the optimum, which was found in MRS medium. L. plantarum DC400 and POM1 had the highest A values and the lowest λ values in almost all juices. Due to the low initial pH values (4.38 ± 0.04 and 3.64 ± 0.03), TJ and PJ were subjected to mild lactic acidification (Table 1). No decrease of pH was found during fermentation of ChJ, which had the highest buffering capacity (45.0 ± 2.1 mmol HCl pH−1 liter−1). The greatest decreases of pH were found for WFH and CJ, which approached those found in MRS medium. The Pearson correlation matrix between the chemical compositions of juices and A was calculated (data not shown). Except for L. plantarum DC400, the A values of the strains were negatively correlated with the concentrations of malic acid and glucose (0.92 to 0.988 and 0.935 to 0.983, respectively). A positive correlation (0.975) between A and the initial pH value of each medium was found for L. plantarum DC400.
TABLE 1.
Parameters of the growth and acidification kinetics of six L. plantarum strains during fermentation of different media at 30°C for 24 h
| Mediuma | L. plantarum strain | Parameterb value |
|||||
|---|---|---|---|---|---|---|---|
| Growth |
Acidification |
||||||
| A | μmax | λ | ΔpH | Vmax | λ | ||
| MRS | CIL6 | 2.60 ± 0.07B | 0.28 ± 0.02DEFGH | 0.59 ± 0.07T | 1.98 ± 0.09B | 0.23 ± 0.03BC | 3.07 ± 0.06K |
| ChJ | 1.07 ± 0.05J | 0.21 ± 0.02KLM | 2.90 ± 0.04J | ND | ND | ND | |
| CJ | 1.84 ± 0.04E | 0.24 ± 0.02HIJKL | 1.04 ± 0.05S | 1.70 ± 0.08C | 0.15 ± 0.04D | 4.91 ± 0.06G | |
| TJ | 1.76 ± 0.06EF | 0.22 ± 0.02JKLM | 1.73 ± 0.02P | 0.58 ± 0.04G | 0.05 ± 0.05E | 3.04 ± 0.04K | |
| PJ | 1.68 ± 0.05FG | 0.20 ± 0.01 M | 5.04 ± 0.06E | 0.45 ± 0.06I | 0.02 ± 0.04F | 5.61 ± 0.08E | |
| WFH | 1.55 ± 0.04G | 0.22 ± 0.02JKLM | 0.67 ± 0.06T | 2.12 ± 0.12AB | 0.29 ± 0.04AB | 1.08 ± 0.08R | |
| W | 0.97 ± 0.03K | 0.13 ± 0.03PQ | 2.94 ± 0.04J | 0.84 ± 0.05E | 0.03 ± 0.05F | 2.39 ± 0.06O | |
| MRS | C2 | 2.68 ± 0.03B | 0.39 ± 0.03BC | 2.78 ± 0.05K | 2.22 ± 0.11A | 0.23 ± 0.02BC | 2.42 ± 0.04O |
| ChJ | 1.19 ± 0.06J | 0.16 ± 0.01OP | 6.46 ± 0.11D | ND | ND | ND | |
| CJ | 2.05 ± 0.05D | 0.27 ± 0.04DEFGHI | 2.22 ± 0.02 M | 1.54 ± 0.08C | 0.15 ± 0.03D | 7.28 ± 0.14B | |
| TJ | 1.99 ± 0.03D | 0.27 ± 0.02EFGHI | 2.30 ± 0.04L | 0.58 ± 0.05G | 0.08 ± 0.04E | 4.88 ± 0.03G | |
| PJ | 2.15 ± 0.06C | 0.30 ± 0.01DE | 7.74 ± 0.14C | 0.29 ± 0.04J | 0.02 ± 0.03F | 5.74 ± 0.06D | |
| WFH | 2.28 ± 0.07C | 0.24 ± 0.02HIJKL | 1.40 ± 0.07Q | 2.30 ± 0.12A | 0.37 ± 0.04A | 2.88 ± 0.07 M | |
| W | 0.95 ± 0.03K | 0.09 ± 0.02R | 7.51 ± 0.10C | 0.34 ± 0.05I | 0.03 ± 0.02F | 8.78 ± 0.16A | |
| MRS | POM1 | 2.75 ± 0.04A | 0.32 ± 0.04CDEF | 0.71 ± 0.04T | 2.23 ± 0.11A | 0.22 ± 0.02BC | 2.39 ± 0.03O |
| ChJ | 1.13 ± 0.06J | 0.20 ± 0.03KLM | 2.39 ± 0.05L | ND | ND | ND | |
| CJ | 2.50 ± 0.07B | 0.21 ± 0.01LM | 1.50 ± 0.05Q | 1.68 ± 0.11C | 0.14 ± 0.03D | 6.50 ± 0.11C | |
| TJ | 1.99 ± 0.05DE | 0.24 ± 0.02HIJKL | 1.29 ± 0.05Q | 0.71 ± 0.05F | 0.06 ± 0.04F | 3.48 ± 0.04J | |
| PJ | 2.20 ± 0.02C | 0.15 ± 0.02OPQ | 1.17 ± 0.09S | 0.37 ± 0.04IJ | 0.01 ± 0.05F | 2.44 ± 0.08O | |
| WFH | 1.68 ± 0.03G | 0.52 ± 0.06A | 2.26 ± 0.04 M | 2.11 ± 0.11AB | 0.31 ± 0.02A | 1.65 ± 0.13Q | |
| W | 0.81 ± 0.03L | 0.08 ± 0.02R | 9.67 ± 0.13B | 0.36 ± 0.07IJ | 0.03 ± 0.03F | 9.00 ± 0.12A | |
| MRS | 1MR20 | 2.58 ± 0.04B | 0.46 ± 0.05AB | 2.30 ± 0.02L | 2.07 ± 0.13AB | 0.21 ± 0.03BC | 2.55 ± 0.08N |
| ChJ | 0.62 ± 0.06G | 0.09 ± 0.02R | 3.28 ± 0.03I | ND | ND | ND | |
| CJ | 1.77 ± 0.07EF | 0.26 ± 0.01GHI | 3.60 ± 0.09H | 1.57 ± 0.09C | 0.11 ± 0.02E | 4.85 ± 0.13FGH | |
| TJ | 1.48 ± 0.02H | 0.29 ± 0.02DEFG | 4.71 ± 0.04F | 0.62 ± 0.02G | 0.04 ± 0.04F | 3.98 ± 0.09I | |
| PJ | 1.79 ± 0.03EF | 0.21 ± 0.03JKLM | 10.83 ± 0.12A | 0.31 ± 0.05J | 0.02 ± 0.03F | 4.79 ± 0.06H | |
| WFH | 1.44 ± 0.04H | 0.33 ± 0.05BCD | 2.83 ± 0.08J | 2.04 ± 0.14AB | 0.34 ± 0.02A | 2.12 ± 0.10P | |
| W | 1.10 ± 0.05J | 0.07 ± 0.04R | 1.04 ± 0.07S | 1.06 ± 0.03D | 0.04 ± 0.03F | 2.39 ± 0.07O | |
| MRS | DC400 | 2.72 ± 0.06AB | 0.42 ± 0.04AB | 2.07 ± 0.04N | 2.08 ± 0.10AB | 0.21 ± 0.03BC | 2.12 ± 0.12P |
| ChJ | 1.77 ± 0.05EF | 0.10 ± 0.02R | 1.77 ± 0.02P | ND | ND | ND | |
| CJ | 1.99 ± 0.03D | 0.21 ± 0.02KLM | 0.98 ± 0.05S | 1.66 ± 0.09C | 0.19 ± 0.02CD | 7.15 ± 0.13B | |
| TJ | 1.80 ± 0.03E | 0.15 ± 0.01PQ | 1.16 ± 0.09R | 0.67 ± 0.06G | 0.07 ± 0.05E | 5.91 ± 0.11D | |
| PJ | 1.43 ± 0.05H | 0.10 ± 0.01R | 7.40 ± 0.09C | ND | ND | ND | |
| WFH | 1.54 ± 0.08GH | 0.17 ± 0.01NP | 0.46 ± 0.09U | 2.16 ± 0.14AB | 0.25 ± 0.03B | 3.16 ± 0.09L | |
| W | 1.17 ± 0.02J | 0.05 ± 0.04R | 6.65 ± 0.10D | 0.36 ± 0.03IJ | 0.03 ± 0.06E | 3.25 ± 0.08L | |
| MRS | CC3M8 | 2.86 ± 0.08A | 0.27 ± 0.03DEFGHI | 1.19 ± 0.05R | 2.22 ± 0.09A | 0.24 ± 0.02B | 1.89 ± 0.11Q |
| ChJ | 1.17 ± 0.05J | 0.14 ± 0.04NPQ | 0.38 ± 0.04U | ND | ND | ND | |
| CJ | 1.63 ± 0.03G | 0.29 ± 0.03DEFGH | 1.94 ± 0.06O | 1.79 ± 0.10C | 0.18 ± 0.03CD | 6.49 ± 0.10C | |
| TJ | 1.69 ± 0.06F | 0.26 ± 0.02FGHIJ | 3.74 ± 0.08H | 0.71 ± 0.06F | 0.07 ± 0.03E | 3.37 ± 0.08J | |
| PJ | 1.84 ± 0.03E | 0.24 ± 0.01IJK | 4.76 ± 0.04F | 0.48 ± 0.04H | 0.02 ± 0.04F | 2.85 ± 0.03 M | |
| WFH | 1.45 ± 0.04H | 0.20 ± 0.03KLM | 0.38 ± 0.03U | 2.17 ± 0.11AB | 0.34 ± 0.02A | 2.13 ± 0.03P | |
| W | 1.31 ± 0.02I | 0.09 ± 0.04R | 4.48 ± 0.07G | 0.24 ± 0.04K | 0.02 ± 0.04F | 5.04 ± 0.04F | |
For the manufacture of the media, see Materials and Methods.
Growth and acidification data were modeled according to the Gompertz equation as modified by Zwietering et al. (21). Parameters for growth: A, difference in log CFU ml−1 between the initial value and the value reached after 24 h; μmax, maximum growth rate (log CFU ml−1 h−1); λ, length of the lag phase (h). Parameters for acidification: ΔpH, difference in pH (units) between the initial value (pH0) and the value reached after 24 h (pH24); Vmax, maximum acidification rate (ΔpH h−1); λ, length of the lag phase (h). Shown are mean values ± standard deviations for the three batches of each type of vegetable, analyzed in duplicate. ND, not detectable. Means within the columns followed by different letters (A to U) are significantly different (P < 0.05).
Cell viability.
The cell viability of L. plantarum strains slightly (P < 0.05) decreased in all media (ca. 0.15 to 0.8 log CFU ml−1) during 21 days of storage at 4°C. The only exception was L. plantarum DC400, which decreased ca. 1.5 log CFU ml−1 in ChJ, PJ, and CJ. The decrease of strain DC400 was limited during storage of WFH (ca. 0.5 log CFU ml−1).
The analysis with a LIVE/DEAD BacLight Bacterial Viability Kit (see Fig. S1 in the supplemental material) and the related quantification with Image-Pro Plus image software confirmed that the number of intact cells of L. plantarum DC400 significantly (P < 0.05) varied during storage and depended on the medium. The percentage of dead/damaged cells with respect to total cells varied between 7 and 8 (MRS and WFH, CJ and TJ) to 13 to 17% (PJ and ChJ). The estimated percentage of dead/damaged cells with respect to total cells of L. plantarum C2 did not exceed ca. 1% throughout storage in all the media. Apart from the medium, the above ratio for the other strains was always lower than ca. 6%.
Carbohydrates, organic acids, and free amino acids.
Independent of the medium used, the stoichiometric ratio between glucose, fructose, sucrose, maltose, lactose, galactose, and/or malic acid consumed and lactic acid synthesized was almost respected for all the strains (see Table S2 in the supplemental material). Compared to prior fermentation, the concentrations of citric acid in fermented juices did not significantly (P > 0.05) vary. As expected, the concentration of carbohydrates significantly (P < 0.05) decreased during fermentation and storage of MRS and WFH. The decrease of lactose was slight (P < 0.05) in all fermented W media. The consumption of carbohydrates did not differentiate (P > 0.05) the L. plantarum strains during fermentation and storage of MRS, WFH, and W. The concentrations of glucose and fructose of ChJ did not significantly (P > 0.05) vary during fermentation and storage. Almost the same was found for PJ. On the other hand, the concentrations of glucose and fructose markedly decreased (P < 0.05) during fermentation of CJ (ca. 15 and 10%, respectively) and TJ (ca. 11% for both carbohydrates).
Lactic acid was always the major fermentation end product. The lowest level was found in fermented W (see Table S2 in the supplemental material). Compared to prior fermentation, the concentration of malic acid in all juices significantly (P < 0.05) decreased during fermentation. The greatest decrease was found for ChJ. It ranged from 18% (strain C2) to 32% (strain DC400). After fermentation, the molar ratios between consumed malic acid and glucose/fructose were 1.77 to 1.28 (ChJ), 0.86 to 0.47 (CJ), 0.60 to 0.15 (PJ), and 0.35 to 0.30 (TJ). The highest ratios were found for juices fermented with L. plantarum DC400. A decrease of malic acid was also found during storage of ChJ, PJ, and CJ. The above ratios increased during storage of most of the fermented ChJ. L. plantarum DC400 showed the highest malolactic specific activity. Cells harvested from fermented ChJ and PJ showed higher enzyme activity than those from CJ and TJ (1.15 ± 0.11 and 1.05 ± 0.32 versus 0.05 ± 0.01 and 0.09 ± 0.02 μmol l-malic acid degraded per min per mg dry weight, respectively). Almost the same trend was found for the other strains.
The initial concentration of FAA in the juices varied between 587 ± 25 mg liter−1 (PJ) and 2,395 ± 51 mg liter−1 (TJ) (see Table S3 in the supplemental material). FAA increased during fermentation of ChJ (ca. 17 to 25%) and TJ (ca. 6 to 17%). Marked decreases were found for CJ (ca. 30 to 44%) and PJ (ca. 13 to 44%). The initial concentrations of FAA in MRS, WFH, and W were almost unchanged after fermentation. In particular, the concentration of branched-chain amino acids (BCAA) (Val, Ile, and Leu) decreased (P < 0.05) during fermentation of all juices, especially CJ and TJ. Almost the same was found for His, especially in TJ and PJ. Glu increased (P < 0.05) during fermentation of TJ only to markedly decrease during storage. The same trend was found for gamma-aminobutyric acid (GABA) (P < 0.05). FAA increased during storage of all fermented ChJ and PJ. An increase of Tyr was found only during storage of ChJ, which was fermented with L. plantarum CIL6 and C2 (see Table S3 in the supplemental material). Significant (P < 0.05) increases of FAA were also found during storage of fermented MRS and W.
WFH, W, and MRS were used only to have a comparison with the metabolic traits that characterize microbial growth and maintenance in juices. Further analyses were carried out only on ChJ, CJ, TJ, and PJ.
Volatile components and volatile free fatty acids.
One-hundred-fifty-five VOC were identified through PT–GC-MS, which belonged to the following chemical classes: aldehydes (16 compounds identified), alcohols (26 compounds identified), ketones (29 compounds identified), esters (30 compounds identified), and sulfur compounds (10 compounds identified). The profile and the level of VOC differentiated the four raw juices. For instance, TJ was more concentrated in 2- and 3-methyl-1-butanol, PJ in 2-nonanone and almost all esters, CJ in methanol and 2-propanone, and ChJ in ethanol and benzaldehyde (data not shown). Only volatile components that mainly (P < 0.05) differentiated fermented juices and were indicative of some metabolic traits were further considered (see Table S4 in the supplemental material). Except for benzeneacetaldehyde, most of the aldehydes, especially 3-methyl-butanal, 2-methyl-butanal, and 2-hexenal, significantly (P < 0.05) decreased during fermentation of almost all juices. Several branched alcohols (e.g., 3-methyl-1-butanol and 2-methyl-1-butanol) increased (P < 0.05) during both fermentation and storage. A marked increase of most of the ketones was found during fermentation. 2,3-Butanedione (diacetyl) showed the highest concentration for all the fermented juices. Its level further increased during storage of ChJ and CJ.
During fermentation and storage, all 10 VFFA (C2 to C8) significantly (P < 0.05) differentiated juices and strains. Only VFFA that mainly (P < 0.05) differentiated fermented juices and were indicative of some metabolic traits are shown (see Table S4 in the supplemental material). Acetic acid increased in almost all fermented juices, especially in TJ and PJ. L. plantarum POM1, 1MR20, and C2 showed the greatest increases. During storage, the concentrations of acetic acid increased for all fermented ChJ (ca. 26 to 293 ppm) and CJ (ca. 35 to 404 ppm) and for PJ and TJ when fermented with L. plantarum POM1 (ca. 465 and 344 ppm, respectively). Propionic, isobutyric, 3-methyl-butyric, and 2-methyl-butyric acids decreased during fermentation of CJ. The opposite was found for all fermented TJ. The concentrations of butyric acid increased in ChJ and, especially, TJ, mainly when fermented with strain C2. No significant (P > 0.05) variations were found throughout storage.
Multidimensional statistical analyses.
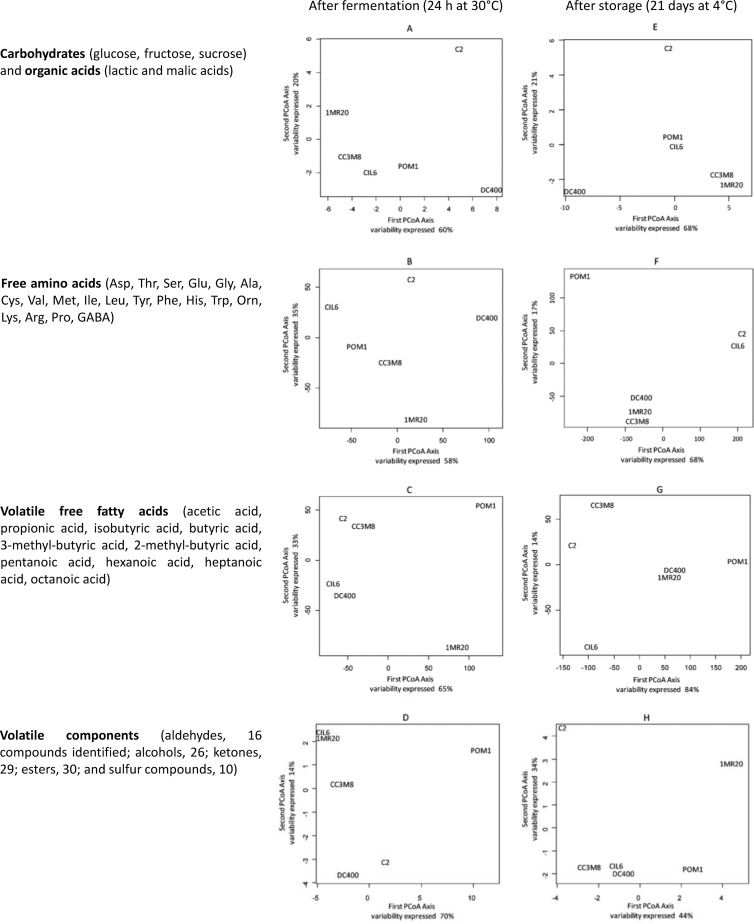
PCoA was used to differentiate the behavior of L. plantarum strains by considering all the juices. Overall, strains of L. plantarum behaved rather differently, and the distribution of the strains after fermentation was quite different from that after storage. The consumption of carbohydrates and the concentration of organic acids during fermentation and storage (Fig. 1A and E), and the concentration of FAA during fermentation (Fig. 1B), mostly differentiated L. plantarum DC400 and C2, with opposite behavior, from the other strains. This opposite behavior was mainly related to the consumption of malic acid (highest for strain DC400) and glucose and fructose during fermentation and storage of CJ and PJ (highest for strain C2) and to the different profiles of FAA. L. plantarum C2, together with strain 1MR20, was also distinguished based on the variation of VOC during storage (Fig. 1H), mainly due to the lowest levels of some alcohols and the highest levels of some esters and diacetyl, especially in PJ. The levels of VFFA (mainly the levels of acetic acid) and VOC (mainly the lowest levels of some alcohols) during fermentation (Fig. 1C and D) and the concentration of FAA, which increased after storage of TJ and PJ (Fig. 1F), mainly distinguished strain POM1. L. plantarum CIL6 mainly differed based on the lowest levels of some VFFA after storage (Fig. 1G). As shown by the analysis of Euclidean distances, FAA and VFFA allowed the maximum discrimination among strains after both fermentation and storage.
FIG 1.
PCoA based on the concentrations of carbohydrates and organic acids (A and E), free amino acids (B and F), volatile free fatty acids (C and G), and volatile components (D and H) after fermentation (24 h at 30°C) (A to D) and storage (21 days at 4°C) (E to H) of vegetable (CJ and TJ) and fruit (ChJ and PJ) juices with L. plantarum CIL6, 1MR20, C2, POM1, DC400, and CC3M8. The first two axes are graphed. The descriptors used are listed beside each plot.
After fermentation, the matrices of correlation between carbohydrates, organic acids, VOC, and FAA were elaborated (data not shown). In particular, malic acid was strongly and negatively correlated with Ser (mean correlation value, −0.74) and especially His (−0.88). Glu and GABA were strongly and positively correlated (0.88). Several aldehydes (2-pentenal, 2-hexenal, 2,4-hexadienal, and 2-heptenal) were strongly correlated with several alcohols (1-pentanol, 1-hexanol, 1-penten-3-ol, 3-hexen-1-ol, 3-methyl-2-butanol, 3-methyl-1-butanol, 2-methyl-1-butanol, and 3-methyl-1-pentanol) and ketones (3-pentanone, 4-heptanone, 2-octanone, 3-octanone, 6-methyl-5-hepten-2-one, 1-phenyl-ethanone, and 3,5,5-trimethyl-2-cyclohexenone) (see Fig. S2 in the supplemental material).
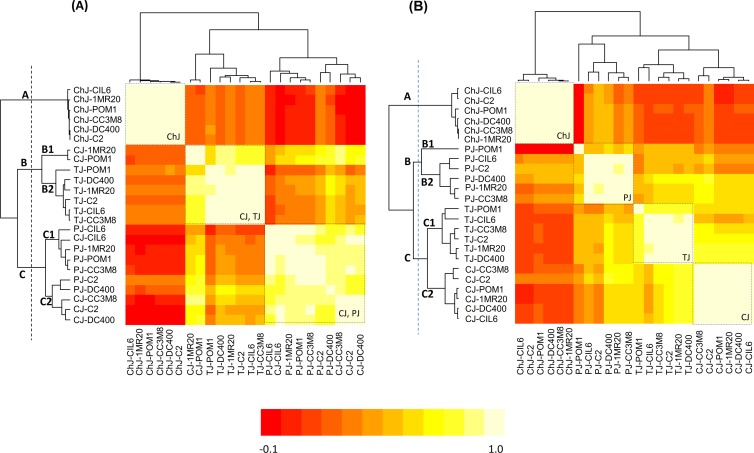
Correlation among strains based on the concentrations of all descriptors (carbohydrates, organic acids, FAA, VOC, and VFFA) after fermentation and storage was shown through pseudoheatmaps (Fig. 2A and B). This analysis mainly shows how the vegetable and fruit juices influenced the behavior of strains. After fermentation (Fig. 2A), the juices were grouped into three clusters. Cluster A grouped only strains fermenting ChJ, where strains were highly correlated with each other (light-yellow square). Clusters B and C grouped TJ and CJ, and PJ and CJ, respectively. Strains fermenting TJ or PJ grouped homogeneously, while those fermenting CJ were scattered. Although not to the same extent observed for ChJ, strains fermenting TJ or PJ were rather highly correlated with each other. After storage, nearly all bacterial strains were highly correlated within the fermenting juice and were grouped in the same cluster. Only two exceptions (TJ-POM1 and PJ-POM1) were found.
FIG 2.
Pseudoheatmap showing correlation between L. plantarum CIL6, 1MR20, C2, POM1, DC400, and CC3M8 based on the concentrations of carbohydrates (glucose, fructose, and sucrose), organic acids (lactic and malic acids), free amino acids (Asp, Thr, Ser, Glu, Gly, Ala, Cys, Val, Met, Ile, Leu, Tyr, Phe, His, Trp, Orn, Lys, Arg, Pro, and GABA), volatile free fatty acids (acetic acid, propionic acid, isobutyric acid, butyric acid, 3-methyl-butyric acid, 2-methyl-butyric acid, pentanoic acid, hexanoic acid, heptanoic acid, and octanoic acid), and volatile compounds (aldehydes, 16 compounds; alcohols, 26; ketones, 29; esters, 30; sulfur compounds, 10) after fermentation (24 h at 30°C) (A) and storage (21 days at 4°C) (B) of vegetable (CJ and TJ) and fruit (ChJ and PJ) juices. A 12-grade rainbow scale ranging from a minimum of −0.1 (red) to a maximum of 0.1 (light yellow) was used. Clusters are separated by the vertical dashed lines.
DISCUSSION
L. plantarum has a relatively simple carbon metabolism mainly devoted to lactic acid synthesis, but one of its striking features is its enormous flexibility with respect to catabolic substrates (26). This study aimed to provide new insights into how diverse the metabolic response of L. plantarum strains is with respect to well-known food habits (e.g., dairy and cereals) and depending on diverse vegetable and fruit matrices to drive safe and functional fermentations.
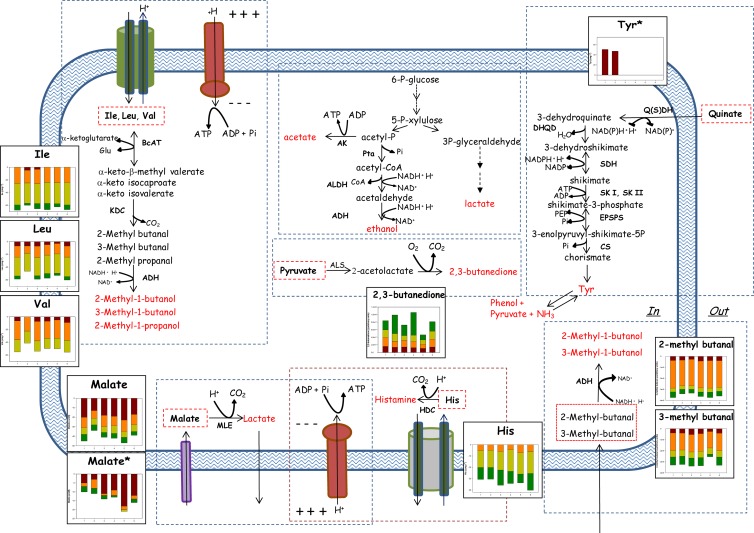
As expected, fruit juices (ChJ and PJ) were the most stressful for microbial growth. Except for strain DC400, growth was negatively correlated with the initial concentrations of malic acid and carbohydrates (glucose and fructose). Decrease of external and intracellular pH, alteration of cell membrane permeability (27), and/or reduction of proton motive force (28) are the main side effects caused by malic acid (29). The consumption of malic acid was noticeable for all juices but mainly during fermentation and storage of ChJ. This juice has a low pH and possesses other intrinsic features (e.g., the highest concentration of carbohydrates and total phenols) that determined its single clustering after both fermentation and storage (Fig. 2A and B) (6, 30). Decarboxylation of malic acid provides energy advantages due to the increased intracellular pH (31) and the synthesis of reducing power (32) (Fig. 3). L. plantarum DC400 showed the highest malolactic specific activity and cell membrane injury, especially when cells were harvested from acid juices (e.g., ChJ). Only in this strain was growth positively correlated with the initial pH. L. plantarum DC400 was isolated from wheat sourdough. Cereal matrices have pH values of 5.6 to 6.0, and acidification, which occurs gradually during fermentation, is the main environmental modification. Multidimensional PCoA based on carbohydrates and organic acids mainly distinguished L. plantarum DC400 and highlighted the opposite metabolic responses of strain C2, which was isolated from carrots (Fig. 1A and E). The strain showed a lower percentage of dead/damaged cells during storage, less intense malolactic fermentation, and stable and low molar ratios between consumed malic acid and glucose/fructose. The behaviors of the other strains were intermediate. Exposure to high levels of carbohydrates (e.g., ChJ and PJ) leads to inefficient metabolism and/or catabolic repression, and bacteria need to equilibrate the extra- and intracellular concentrations (33). Consumption of carbohydrates was consistent and similar to that in other favorable media (WFH and MRS broth) only in vegetable juices (CJ and TJ), which had lower concentrations of glucose and fructose and were less acidic than fruit juices (ChJ and PJ). None of the previous studies (6, 8, 9, 10) showed a shift of the main energy source used, depending on the vegetable and fruit matrices, and this also differentiated the behavior of L. plantarum with respect to dairy or wheat ecosystems, where carbohydrates are mostly fermented. Indeed, depending on whether the environmental conditions were favorable (CJ and TJ) or unfavorable (ChJ and PJ), the trend of L. plantarum strains seemed to turn from pathways mainly devoted to growth (fermentation of carbohydrates) to routes that mainly allowed maintenance (e.g., malolactic fermentation) (34, 35). This consideration is strengthened by the results of the pseudoheatmaps in Fig. 2A and B, which showed that in most cases the juice matrix determined a homogeneous metabolic response of the strains.
FIG 3.
Schematic representation of the presumptive metabolic pathways in L. plantarum CIL6, C2, POM1, 1MR20, DC400, and CC3M8 during fermentation (24 h at 30°C) and storage (21 days at 4°C) of vegetable (CJ and TJ) and fruit (ChJ and PJ) juices. Changes in the amounts of the substrates and products (average of three replicates) are represented by histograms in which each strain is indicated on the x axes as follows: 1, CIL6; 2, C2; 3, POM1; 4, 1MR20; 5, DC400; and 6, CC3M8. The histograms marked with asterisks refer to changes in the amounts during storage. Different colored bars represent juices: ChJ (red), CJ (orange), TJ (yellow), and PJ (green). BcAT, branched-chain aminotransferase; KDC, α-keto acid decarboxylase; ADH, alcohol dehydrogenase; MLE, malolactic enzyme; HDC, histidine decarboxylase; Q(S)DH, quinate/shikimate dehydrogenase; DHQD, 3-dehydroquinate dehydratase; SDH, shikimate dehydrogenase; SK I, shikimate kinase I; SK II, shikimate kinase II; EPSPS, EPSP synthase; CS, chorismate synthase; AK, acetate kinase; Pta, phosphotransacetylase; ALDH, acetaldehyde dehydrogenase.
The catabolism of FAA is another mechanism for microbial adaptation to surrounding environments, which was investigated in depth for lactic acid bacteria growing in cheeses and sourdoughs (36) but poorly for vegetable and fruit fermentations (16). As shown by the analysis of Euclidean distances, different responses between the strains were mainly related to the catabolism of FAA (Fig. 1B and F). Due to the high nitrogen content, fermentation of the rich media MRS broth, WFH, and W was not affected by significant variations of FAA during fermentation. On the other hand, BCAA (Val, Ile, and Leu) decreased during fermentation of juices (mainly CJ and TJ) and seemed to be converted into their respective branched alcohols (2-methyl-1-butanol, 3-methyl-1-butanol, and 2-methyl-1-propanol), whose levels concomitantly increased (Fig. 3). Consumption of BCAA into their corresponding 2-ketoacids leads to gain of ATP and allows the regeneration of Glu from α-ketoglutarate (35, 37). Conversion of Glu to GABA also enhances acid resistance (38, 39). All fermented TJ showed increased levels of Glu and GABA, and in general, the two compounds were positively correlated. A decreasing trend, mainly when TJ and PJ were fermented with strain CC3M8 (isolated from cheese), was also found for His. The decarboxylation of His into histamine provides energy through the generation of proton motive force (40) (Fig. 3). The matrices of correlation showed a negative correlation between His and malic acid, which suggested the alternative use of these sources (41). Overall, it seemed that the catabolism of FAA is a mechanism of adaptation that is more pronounced under less acidic conditions and for vegetable juices (CJ and TJ), whereas malolactic fermentation prevails in a very acidic environment (ChJ). The increase of Tyr, which was found only during storage of ChJ, mainly when fermented with strains CIL6 (isolated from cherries) and C2, may deserve specific consideration. Quinate is largely present in cherries, and it may act as a precursor of Tyr through a number of reactions (Fig. 3) (42). Tyr is a stimulatory amino acid for the growth of L. plantarum and, in general, its catabolism is also involved in the mechanism of intracellular pH regulation (43). First, this study showed that some traits of the catabolism of FAA are also indispensable for growth and adaptation of L. plantarum to vegetable and fruit matrices.
Several VOC identified during juice fermentation and storage were inherent aroma components of the vegetables and fruits used (44). Overall, alcohols, ketones, ketoacids, and terpenes are synthesized by lactic acid bacteria when subjected to environmental stresses (45–49). Some aldehydes (e.g., 3-methyl-butanal, 2-methyl-butanal, or 2-hexenal) that decreased during fermentation were statistically correlated with the corresponding and increasing alcohols (e.g., 3-methyl-1-butanol, 2-methyl-1-butanol, or 2-hexen-1-ol). The low redox potential of the juices may have directly caused the reduction of unstable aldehydes and ketones to primary and secondary alcohols (50), or as discussed previously, branched alcohols may have been derived from the catabolism of BCAA (Fig. 3). As previously shown during sourdough fermentation (51), microbial activity may also have been responsible for this reduction, which increases the capacity to recycle NADH cofactors (Fig. 3). After both fermentation and storage, acetic acid markedly increased in all fermented juices. This implies activation of the acetate kinase route of the phosphogluconate pathway by L. plantarum strains. The marked activation of this route strictly relies on the availability of external acceptors of electrons, such as aldehydes, which were reduced to the corresponding alcohols (Fig. 3). Almost the same mechanism of activation was shown during sourdough fermentation (51), but none of the previous studies (16) highlighted its occurrence during vegetable and fruit fermentations. Diacetyl was the ketone found at the highest level in all fermented juices (mainly PJ fermented with L. plantarum CIL6, 1MR20, and C2). The synthesis of neutral diacetyl is induced at the transcriptional level by acidic conditions, which presumably contributes to intracellular pH regulation by decreasing the level of pyruvate (52) (Fig. 3).
Data were processed through multidimensional statistical analyses (Fig. 1 and 2A and B) to show the effects of the matrices and to differentiate L. plantarum strains. Due to their inherent and different chemical characteristics, TJ, PJ, and especially ChJ induced specific metabolic responses in almost all the strains during fermentation. CJ did not exert the same selective pressure. Except for POM1 in TJ and PJ, the responses of all the strains during storage were determined by the type of juice. Based on the metabolic responses that were induced by juices, strains might be selected for targeted fermentations. Some examples follow. Strain CIL6 was the most suitable strain to ferment ChJ because of the high survival, the capacity to consistently activate malolactic fermentation, the highest synthesis of diacetyl and GABA, and the metabolism of Tyr, which may positively influence the microbiological and sensory features of fermented cherries. Strain POM1 could be selected to ferment TJ because of the greatest increase of cell numbers and highest concentrations of FAA and GABA, the catabolism of BCAA and His, and the capacity to consistently activate the acetate kinase route. Almost the same suitable features were shown by strain POM1 during fermentation of PJ. As shown in Fig. 2B, POM1 was the only one that did not correlate with the other strains during fermentation of TJ and PJ. Overall, CJ seemed to be the juice in which all the strains behaved similarly, and C2 was the strain that showed the highest survival during storage of all the juices.
This study provided more in-depth knowledge on the metabolic mechanisms of growth and maintenance of L. plantarum, which depended on vegetable and fruit habitats and differed in part from other well-described responses in other food ecosystems (e.g., dairy and sourdough products). The metabolic responses of the strains differed in part, which was helpful in selecting the most suitable starters for industrial-scale fermentation of targeted matrices.
Supplementary Material
Footnotes
Published ahead of print 31 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03885-13.
REFERENCES
- 1.Nyström T. 2004. Growth versus maintenance: a trade-off dictated by RNA polymerase availability and sigma factor competition? Mol. Microbiol. 54:855–862. 10.1111/j.1365-2958.2004.04342.x [DOI] [PubMed] [Google Scholar]
- 2.Pirt S. 1965. The maintenance energy of bacteria in growing cultures. Proc. R. Soc. Lond. B Biol. Sci. 163:224–231. 10.1098/rspb.1965.0069 [DOI] [PubMed] [Google Scholar]
- 3.Redon E, Loubiere P, Cocaign-Bousquet M. 2005. Transcriptome analysis of the progressive adaptation of Lactococcus lactis to carbon starvation. J. Bacteriol. 187:3589–3592. 10.1128/JB.187.10.3589-3592.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckenhüskes HJ. 1997. Fermented vegetables, p 595–609 In Doyle PD, Beuchat LR, Montville TJ. (ed), Food microbiology: fundamentals and frontiers, 2nd ed. ASM Press, Washington, DC [Google Scholar]
- 5.Rodrìguez H, Curiel JA, Landete JM, de Las Rivas B, de Felipe FL, Gòmez-Cordovés C. 2009. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 132:79–90. 10.1016/j.ijfoodmicro.2009.03.025 [DOI] [PubMed] [Google Scholar]
- 6.Di Cagno R, Surico RF, Minervini G, Rizzello CG, Lovino R, Servili M, Taticchi A, Urbani S, Gobbetti M. 2011. Exploitation of sweet cherry (Prunus avium L.) puree added by stem infusion through fermentation by selected autochthonous lactic acid bacteria. Food Microbiol. 28:900–909. 10.1016/j.fm.2010.12.008 [DOI] [PubMed] [Google Scholar]
- 7.Demir N, Bahçeci KS, Acar J. 2006. The effects of different initial Lactobacillus plantarum concentrations on some properties of fermented carrot juice. J. Food Process. Preserv. 30:352–363. 10.1111/j.1745-4549.2006.00070.x [DOI] [Google Scholar]
- 8.Di Cagno R, Surico RF, Siragusa S, De Angelis M, Paradiso A, Minervini F, De Gara L, Gobbetti M. 2008. Selection and use of autochthonous mixed starter for lactic acid fermentation of carrots, French beans or marrows. Int. J. Food Microbiol. 127:220–228. 10.1016/j.ijfoodmicro.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 9.Di Cagno R, Surico RF, Paradiso A, De Angelis M, Salmon JC, Buchin S, De Gara L, Gobbetti M. 2009. Effect of autochthonous lactic acid bacteria starters on health-promoting and sensory properties of tomato juices. Int. J. Food Microbiol. 128:473–483. 10.1016/j.ijfoodmicro.2008.10.017 [DOI] [PubMed] [Google Scholar]
- 10.Di Cagno R, Cardinali G, Minervini G, Antonielli L, Rizzello CG, Ricciuti P, Gobbetti M. 2010. Taxonomic structure of the yeasts and lactic acid bacteria microbiota of pineapple (Ananas comosus L. Merr.) and use of autochthonous starters for minimally processing. Food Microbiol. 27:381–389. 10.1016/j.fm.2009.11.012 [DOI] [PubMed] [Google Scholar]
- 11.Filya I, Sucu E, Karabulut A. 2004. The effect of Propionibacterium acidipropionici, with or without Lactobacillus plantarum, on the fermentation and aerobic stability of wheat sorghum and maize silages. J. Appl. Microbiol. 97:818–826. 10.1111/j.1365-2672.2004.02367.x [DOI] [PubMed] [Google Scholar]
- 12.Noonpakdee W, Sitthimonchai S, Panyim S, Lertsiri S. 2004. Expression of the catalase gene catA in starter culture Lactobacillus plantarum TISTR850 tolerates oxidative stress and reduces lipid oxidation in fermented meat product. Int. J. Food Microbiol. 95:127–135. 10.1016/j.ijfoodmicro.2004.01.020 [DOI] [PubMed] [Google Scholar]
- 13.Bringel F, Quenee P, Tailliez P. 2001. Polyphasic investigation of the diversity within Lactobacillus plantarum related strains revealed two L. plantarum subgroups. Syst. Appl. Microbiol. 24:561–571. 10.1078/0723-2020-00061 [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez H, Landete JM, de las Rivas B, Muñoz R. 2008. Metabolism of food phenolic acids by Lactobacillus plantarum CECT 748T. Food Chem. 107:1393–1398. 10.1016/j.foodchem.2007.09.067 [DOI] [Google Scholar]
- 15.Helander IM, von Wright A, Mattila-Sandholm TM. 1997. Potential of lactic acid bacteria and novel antimicrobials against Gram-negative bacteria. Trends Food Sci. Technol. 8:146–150. 10.1016/S0924-2244(97)01030-3 [DOI] [Google Scholar]
- 16.Di Cagno R, Coda R, De Angelis M, Gobbetti M. 2013. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 33:1–10. 10.1016/j.fm.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 17.Di Cagno R, De Angelis M, Limitone A, Minervini F, Simonetti MC, Buchin S, Gobbetti M. 2007. Cell-cell communication in sourdough lactic acid bacteria: a proteomic study in Lactobacillus sanfranciscensis CB1. Proteomics 7:2430–2446. 10.1002/pmic.200700143 [DOI] [PubMed] [Google Scholar]
- 18.Di Cagno R, De Pasquale I, De Angelis M, Gobbetti M. 2012. Accelerated ripening of Caciocavallo Pugliese cheese with attenuated adjuncts of selected nonstarter lactobacilli. J. Dairy Sci. 95:4784–4795. 10.3168/jds.2011-5283 [DOI] [PubMed] [Google Scholar]
- 19.Slinkard K, Singleton VL. 1997. Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Vitic. 28:49–55 [Google Scholar]
- 20.Pai SC, Tsa YJ, Yang TI. 2001. pH and buffering capacity problems involved in the determination of ammonia in saline water using the indophenol blue spectrophotometric method. Anal. Chim. Acta 434:209–216. 10.1016/S0003-2670(01)00851-0 [DOI] [Google Scholar]
- 21.Zwietering MH, Jongeberger I, Roumbouts FM, Van 't Riet K. 1990. Modelling of bacterial growth curve. Appl. Environ. Microbiol. 56:1875–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeppa G, Conterno L, Gerbi V. 2001. Determination of organic acids, sugars, diacetyl, and acetoin in cheese by high-performance liquid chromatography. J. Agric. Food. Chem. 49:2722–2726. 10.1021/jf0009403 [DOI] [PubMed] [Google Scholar]
- 23.Rizzello CG, Nionelli L, Coda R, De Angelis M, Gobbetti M. 2010. Effect of sourdough fermentation on stabilisation, and chemical and nutritional characteristics of wheat germ. Food Chem. 119:1079–1089. 10.1016/j.foodchem.2009.08.016 [DOI] [Google Scholar]
- 24.Herrero M, García L, Díaz M. 2003. Malolactic bioconversion using a Oenococcus oeni strain for cider production: effect of yeast extract supplementation. J. Ind. Microbiol. Biotechnol. 30:699–704. 10.1007/s10295-003-0102-9 [DOI] [PubMed] [Google Scholar]
- 25.Biggerstaff JP, Le Puil M, Weidow BL, Prater J, Glass K, Radosevich M, White DC. 2006. New methodology for viability testing in environmental samples. Mol. Cell. Probes 20:141–146. 10.1016/j.mcp.2005.11.006 [DOI] [PubMed] [Google Scholar]
- 26.Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers O, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MWEJ, Stiekema W, Klein Lankhorst RM, Bron PA, Hoffer SM, Nierop Groot MN, Kerkhoven R, de Vries M, Ursing B, de Vos WM, Siezen RJ. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. U. S. A. 100:1990–1995. 10.1073/pnas.0337704100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beuchat LR. 1998. Surface decontamination of fruits and vegetables eaten raw: a review, p 42 In WHO document FSF/FOS/98.2. World Health Organization, Geneva, Switzerland [Google Scholar]
- 28.Jay JM. 2000. Modern food microbiology, 6th ed, p 257 Chapman and Hall, New York, NY [Google Scholar]
- 29.Kubota H, Senda S, Tokuda H, Uchiyama H, Nomura N. 2009. Stress resistance of biofilm and planktonic Lactobacillus plantarum subsp. plantarum JCM 1149. Food Microbiol. 26:592–597. 10.1016/j.fm.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 30.Henick-Kling T, Cox DJ, Olsen EB. 1991. Production de l'energie durant la fermentation malolactique. Rev. Française Oenol. 132:63–66 [Google Scholar]
- 31.Henick-Kling T. 1986. Growth and metabolism of Leuconostoc oenos and Lactobacillus plantarum in wine. Ph.D. thesis, University of Adelaide, Adelaide, Australia [Google Scholar]
- 32.Kunkee RE. 1991. Some roles of malic acid in the malolactic fermentation in wine making. FEMS Microbiol. Lett. 88:55–72 [Google Scholar]
- 33.Teusink B, Wiersma A, Jacobs L, Notebaart RA, Smid EJ. 2009. Understanding the adaptive growth strategy of Lactobacillus plantarum by in silico optimisation. PLoS Comput. Biol. 5:e1000410. 10.1371/journal.pcbi.1000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Passos FV, Fleming HP, Hassan HM, McFeeters RF. 2003. Effect of malic acid on the growth kinetics of Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 63:207–211. 10.1007/s00253-003-1375-7 [DOI] [PubMed] [Google Scholar]
- 35.Goffin P, van de Bunt B, Giovane M, Leveau JHJ, Höppener-Ogawa S, Teusink B, Hugenholtz J. 2010. Understanding the physiology of Lactobacillus plantarum at zero growth. Mol. Syst. Biol. 6:413–425. 10.1038/msb.2010.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez M, Zúñiga M. 2006. Amino acid catabolic pathways of lactic acid bacteria. Crit. Rev. Microbiol. 32:155–183. 10.1080/10408410600880643 [DOI] [PubMed] [Google Scholar]
- 37.Christensen JE, Dudley EG, Pederson JA, Steele JL. 1999. Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 76:217–246. 10.1023/A:1002001919720 [DOI] [PubMed] [Google Scholar]
- 38.Di Cagno R, Mazzacane F, Rizzello CG, De Angelis M, Giuliani G, Meloni M, De Servi B, Gobbetti M. 2010. Synthesis of γ-aminobutyric acid (GABA) by Lactobacillus plantarum DSM19463: functional grape must beverage and dermatological applications. Appl. Microbiol. Biotechnol. 86:731–741. 10.1007/s00253-009-2370-4 [DOI] [PubMed] [Google Scholar]
- 39.Su MS, Schlicht S, Gänzle MG. 2011. Contribution of glutamate decarboxylase in Lactobacillus reuteri to acid resistance and persistence in sourdough fermentation. Microb. Cell Fact. 10:S8. 10.1186/1475-2859-10-S1-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molenaar D, Hagting A, Alkema H, Driessen AJM, Konings WN. 1993. Characteristics and osmoregulatory roles of uptake systems for proline and glycine betaine in Lactococcus lactis. J. Bacteriol. 175:5438–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farías ME, Manca de Nadra MC, Rollán GC, Strasser de Saad AM. 1995. Histidine decarboxilase production by Lactobacillus hilgardii: effect of organic acids. Curr. Microbiol. 31:15–18. 10.1007/BF00294627 [DOI] [Google Scholar]
- 42.Johansson L, Lidén G. 2006. Transcriptome analysis of a shikimic acid producing strain of Escherichia coli W3110 grown under carbon- and phosphate-limited conditions. J. Biotechnol. 126:528–545. 10.1016/j.jbiotec.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 43.Kumagai H, Yamada H, Matsui H, Ohkishi H, Ogata K. 1970. Tyrosine phenol lyase. J. Biol. Chem. 245:1767–1772 [PubMed] [Google Scholar]
- 44.Buttery RG, Ling LC. 1993. Volatile components of tomato fruit and plant parts: relationship and biogenesis, p 22–33 In Teranishi R, Buttery RG, Sugisawa H. (ed), Bioactive volatile compounds from plants. ASC, Washington, DC [Google Scholar]
- 45.Fernández M, Kleerebezem M, Kuipers OP, Siezen RJ, van Kranenburg R. 2002. Regulation of the metC-cysK operon, involved in sulfur metabolism in Lactococcus lactis. J. Bacteriol. 184:82–90. 10.1128/JB.184.1.82-90.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guerzoni ME, Vernocchi P, Ndagijimana M, Gianotti A, Lanciotti R. 2007. Generation of aroma compounds in sourdough: effects of stress exposure and lactobacilli-yeasts interactions. Food Microbiol. 24:139–148. 10.1016/j.fm.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 47.Vermeulen N, Gänzle MG, Vogel RF. 2007. Glutamine deamidation by cereal-associated lactic acid bacteria. J. Appl. Microbiol. 103:1197–1205. 10.1111/j.1365-2672.2007.03333.x [DOI] [PubMed] [Google Scholar]
- 48.Vernocchi P, Ndagijimana M, Serrazanetti D, Gianotti A, Vallicelli M, Guerzoni E. 2008. Influence of starch addition and dough microstructure on fermentation aroma production by yeasts and lactobacilli. Food Chem. 108:1217–1225. 10.1016/j.foodchem.2007.06.050 [DOI] [Google Scholar]
- 49.Chambellon E, Rijnen L, Lorquet F, Gitton C, van Hylckama Vlieg JE, Wouters JA, Yvon M. 2009. The D-2-hydroxyacid dehydrogenase incorrectly annotated PanE is the sole reduction system for branched-chain 2-keto acids in Lactococcus lactis. J. Bacteriol. 191:873–881. 10.1128/JB.01114-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molimard P, Spinnler HE. 1996. Review: compounds involved in the flavor of surface mold-ripened cheeses: origins and properties. J. Dairy Sci. 79:169–184. 10.3168/jds.S0022-0302(96)76348-8 [DOI] [Google Scholar]
- 51.Gänzle M, Vermeulen N, Vogel RF. 2007. Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiol. 24:128–138. 10.1016/j.fm.2006.07.006 [DOI] [PubMed] [Google Scholar]
- 52.García-Quintáns N, Repizo G, Martín M, Magni C, López P. 2008. Activation of the diacetyl/acetoin pathway in Lactococcus lactis subsp. lactis bv. diacetylactis CRL264 by acidic growth. Appl. Environ. Microbiol. 74:1988–1996. 10.1128/AEM.01851-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.