Abstract
Identification of a pathogen is a critical first step in the epidemiology and subsequent management of a disease. A limited number of pathogens have been identified for diseases contributing to the global decline of coral populations. Here we describe Vibrio coralliilyticus strain OCN008, which induces acute Montipora white syndrome (aMWS), a tissue loss disease responsible for substantial mortality of the coral Montipora capitata in Kāne‘ohe Bay, Hawai‘i. OCN008 was grown in pure culture, recreated signs of disease in experimentally infected corals, and could be recovered after infection. In addition, strains similar to OCN008 were isolated from diseased coral from the field but not from healthy M. capitata. OCN008 repeatedly induced the loss of healthy M. capitata tissue from fragments under laboratory conditions with a minimum infectious dose of between 107 and 108 CFU/ml of water. In contrast, Porites compressa was not infected by OCN008, indicating the host specificity of the pathogen. A decrease in water temperature from 27 to 23°C affected the time to disease onset, but the risk of infection was not significantly reduced. Temperature-dependent bleaching, which has been observed with the V. coralliilyticus type strain BAA-450, was not observed during infection with OCN008. A comparison of the OCN008 genome to the genomes of pathogenic V. coralliilyticus strains BAA-450 and P1 revealed similar virulence-associated genes and quorum-sensing systems. Despite this genetic similarity, infections of M. capitata by OCN008 do not follow the paradigm for V. coralliilyticus infections established by the type strain.
INTRODUCTION
Bacteria have been implicated as etiological agents in many coral diseases; however, a causal relationship between a pathogen and disease has been established in only a limited number (1–11). Disease presentation is frequently characterized by one or both of two physical processes. Bleaching is the loss of photosynthetic symbiotic zooxanthellae from coral cells (12), and tissue loss is necrosis and eventual sloughing of infected tissue that exposes the calcium carbonate skeleton (13). Both of these responses can lead to the death of entire coral colonies. Most corals appear to have a limited number of gross morphological responses to disease; tissue loss or bleaching can also result from environmental stressors, so pathogen identification and precise disease description are critical first steps toward understanding disease processes (14).
Koch's postulates of disease causation have been utilized in the field of coral disease research as guidelines for identifying a microorganism, rather than an environmental stressor, as the cause of a specific disease (15). In general, a causal relationship between disease and pathogen is inferred after isolation of the pathogen from diseased but not healthy hosts, isolation of the microorganism in pure culture, experimental induction of disease in healthy hosts by the microorganism, and reisolation of the identical microorganism from experimentally infected hosts. Five coral pathogens have fulfilled Koch's postulates: Aurantimonas coralicida (16, 17), Serratia marcescens (3, 18), Vibrio shiloi (19), Vibrio owensii (11), and Vibrio coralliilyticus (8, 20).
V. coralliilyticus is a pathogen of particular concern because it has broad host and geographic ranges. Strains of V. coralliilyticus have been isolated from diseased corals in the Indian Ocean and Red Sea (4), the Caribbean (21), and the Great Barrier Reef and Micronesia (8), and strains have been shown to infect coral of the genera Pocillopora (20) and Pachyseris, Acropora, and Montipora (8). V. coralliilyticus has also been shown to infect noncoral hosts that include the mussel (Perna canaliculus) larva (22) and the rainbow trout (Oncorhynchus mykiss) (23).
Although various strains of V. coralliilyticus infect a broad range of hosts, pathogenesis has been studied predominantly in the strains BAA-450 and P1, which infect the corals Pocillopora damicornis and Montipora aequituberculata, respectively (4, 8, 20). Water temperature plays a prominent role in the infection of P. damicornis by BAA-450 (24). Infections at temperatures from 24 to 25°C cause bleaching of the coral host, whereas infections at 27 to 29°C cause tissue loss, and BAA-450 is avirulent at temperatures below 22°C. Increased concentrations of proteins that contribute to the virulence of other bacterial pathogens are present in or secreted by V. coralliilyticus strains BAA-450 and P1 at the higher temperature range, perhaps explaining the temperature-dependent change in disease presentation (25, 26). The level of one protein, FlhA, increased 10-fold when the temperature was shifted from 24 to 27°C (26), and an intact flhA gene was required for chemotaxis, attachment, and virulence with the coral P. damicornis (27).
In Kāne‘ohe Bay, Hawai‘i, Montipora capitata, one of the major reef-building corals, is affected by a tissue loss disease called Montipora white syndrome (MWS) (28). MWS has two disease presentations: a progressive infection designated chronic MWS (cMWS) that is caused by Vibrio owensii strain OCN002 and is characterized by subacute tissue loss and a comparatively faster acute infection designated acute MWS (aMWS) (11, 28). The infectious potential of aMWS was observed during outbreaks of rapid, widespread tissue loss in 2010 and 2011, with transmission observed between neighboring coral colonies in contact with one another (G. S. Aeby, unpublished data). Tissue loss from corals with aMWS was hypothesized to be caused by an infectious agent like that which causes cMWS and not an environmental stressor.
This study focused on the pathogenesis of V. coralliilyticus strain OCN008, which contrasts with the temperature-dependent infections of BAA-450. Koch's postulates were fulfilled for strain OCN008, establishing it as an etiological agent of acute Montipora white syndrome. OCN008 induced tissue loss on M. capitata fragments but not fragments of Porites compressa, suggesting host specificity for OCN008 infections. Comparative genomics showed that OCN008 possesses many of the putative virulence factors upregulated in strains BAA-450 and P1 in response to increases in temperature, despite the reduced influence of temperature on virulence. OCN008 is the first pathogenic V. coralliilyticus strain isolated from Hawai‘i.
MATERIALS AND METHODS
Coral collection, bacterial strains, and growth conditions.
Samples of Montipora capitata and Porites compressa for infection trials measured approximately 3 by 3 by 1 cm and were collected from a fringing reef surrounding the island Moku o Lo‘e in Kāne‘ohe Bay, Hawai‘i, which is dominated by these two coral species (28). All collected fragments were allowed to recover for 3 days in flowthrough seawater tables prior to the start of the experiments (11).
V. coralliilyticus strain OCN008 was originally isolated from a fragment of P. compressa as described previously (29). Strains OCN018 and OCN019 were isolated from diseased M. capitata displaying signs of cMWS in the same manner as OCN008. Control bacterium Alteromonas sp. strain OCN004 was isolated from healthy M. capitata as previously described (11).
Marine bacteria were grown in glycerol artificial seawater (GASW) medium (11, 30) and incubated at 25°C with aeration unless otherwise stated. Thiosulfate citrate bile salts sucrose (TCBS) agar (Sigma-Aldrich) was prepared according to the manufacturer's instructions and incubated at 25°C for the growth of Vibrio strains. Escherichia coli strains used for conjugation were maintained on Luria-Bertani (LB) medium (Sigma-Aldrich). Conjugations were conducted as previously described (11) on LB medium supplemented with 2.0% NaCl. Concentrations of antibiotics were 50 μg/ml for kanamycin, 100 μg/ml for spectinomycin, and 100 μg/ml for ampicillin.
Identification of bacterial strains.
The 16S rRNA gene of strains OCN008, OCN018, and OCN019 was amplified and sequenced using primers 8F and 1513R (31) (see Table S2 in the supplemental material). Multilocus sequence analysis (MLSA) employed concatemerized partial sequences of the genes pyrH, gapA, mreB, ftsZ, gyrB, and topA. PCR, sequencing, and MLSA were performed with the primers and by the protocols described previously (11).
Phenotypic characterization.
Extracellular enzyme activity potentially related to virulence was assessed at 23 or 27°C in triplicate using skim milk agar (Becton, Dickinson and Company) for protease activity, methyl green DNase agar (Becton, Dickinson and Company) for DNase activity, and 5% sheep blood Trypticase soy agar (Becton, Dickinson and Company) for hemolysin activity. The results from the indicator plates were evaluated according to the manufacturer's instructions. Salt tolerance was tested in triplicate by spectrophotometric assessment of growth in LB broth with final NaCl concentrations ranging from 0% to 1.0% in 0.1% increments and ranging from 1% to 10% in 0.5% increments. Growth rate was determined with aerated liquid GASW cultures inoculated 1:10,000 from an overnight culture at 23 or 27°C in triplicate. Every hour for 36 h, the optical density at 600 nm (OD600) was recorded, and dilutions were plated on solid GASW to calculate the numbers of CFU/ml.
Laboratory infection trials.
Coral fragments were acclimatized to infection trial temperatures over the course of a day prior to inoculation. Seawater passed through a 0.2-μm-pore-size filter (filtered seawater [FSW]) was used for all laboratory trials. Infection trials utilized a block design with fragments from the same colony exposed to FSW, control bacterium OCN004, or strain OCN008, as previously described (11), with the following minor modifications. Each replicate in each infection trial was collected from a unique coral colony. Control bacterium OCN004 is a strain of Alteromonas previously isolated from healthy M. capitata (11). Each fragment was tested separately in its own primary 4-liter container filled with 3 liters of FSW. Each set of primary containers was maintained in a larger, freshwater-filled secondary container that was fitted with water pumps and heaters for temperature control. Water was heated to 23 or 27°C, the average low and high sea surface temperatures of Kāne‘ohe Bay, respectively (32). For infection trials testing the effect of temperature, fragments at 27°C had corresponding fragments from the same colony at 23°C to account for genotypic differences between coral colonies.
To prepare inocula for the infection trials, an overnight culture of the desired strain was diluted to an OD600 of approximately 0.03 in GASW and incubated at either 23 or 27°C, depending on the infection temperature. Cultures grown to an OD600 of 1.6 were washed and diluted with FSW to an OD600 of 0.8 for inoculation. Each tank was inoculated to a final concentration of 108 CFU/ml of water unless indicated otherwise. For determination of the minimal infectious dose, serial 10-fold dilutions of the standard inoculum were used to generate final concentrations of 107, 106, and 105 CFU/ml of seawater. Cultures were diluted in FSW immediately preceding exposure to corals during trials and plated to determine the numbers of CFU/ml. Corals were monitored daily, and individual experiments were run for a maximum of 28 days.
Reisolation and identification of OCN008.
Prior to inoculation of corals, the RSF1010-derived vector pRL1383a was introduced into OCN008 through conjugation as previously described (11, 33) to genetically tag the bacterium. Fragments infected by tagged OCN008 were crushed in FSW, and serial dilutions from 1:10 to 1:106 were plated on solid GASW supplemented with spectinomycin, resistance to which was carried by the plasmid. From each crushed fragment, 30 colonies were screened for the plasmid by PCR using the primers pRL1383a-MCS-F and pRL1383a-MCS-R (11) (see Table S2 in the supplemental material). From each set of 30 colonies, five of the PCR products were sequenced as previously described to verify the identity of the plasmid (11).
The primers OCN008-42310-F and OCN008-43080-R were designed to amplify 772 bp of an intergenic region of the OCN008 genome (see Table S2 in the supplemental material). The intergenic region was located between two divergent genes encoding a putative carboxypeptidase (GenBank accession no. ERB67101) and a pyridoxal phosphate-dependent aspartate aminotransferase (GenBank accession no. ERB66117). The intergenic region did not share significant nucleotide sequence similarity with any other sequence in NCBI, as determined by BLAST analysis. All isolates from the four corals infected with the tagged OCN008 were also screened by PCR using the primers for the specified intergenic region. Six corals infected with the nontagged OCN008 were crushed and plated on TCBS, and 10 bacterial colonies from each coral were screened with the primers for the intergenic region and primers for the 16S rRNA gene. The resulting PCR products for the 16S rRNA gene were sequenced using the same primers used for PCR.
Analysis and comparison of the V. coralliilyticus genomes.
The genome used in this analysis was previously uploaded to GenBank under the accession number AVOO00000000 and annotated using the NCBI prokaryotic genome annotation pipeline (29). The OCN008 genome was prepared for secondary analysis and genome comparisons using the Rapid Annotation using Subsystem Technology (RAST) server and SEED viewer, version 2.0 (34, 35). Gene-to-gene comparisons were done with predicted amino acid sequences and conducted using RAST and the BLASTp program. Whole-genome in silico comparisons were done using the genome-to-genome distance calculator (GGDC), version 2.0, with the MUMmer alignment method (36–39).
Nucleotide sequence accession numbers.
Newly determined sequence data were deposited in GenBank under accession numbers KF042020, KF042021, and KF042022.
RESULTS
Isolation and identification of a strain of V. coralliilyticus from healthy P. compressa.
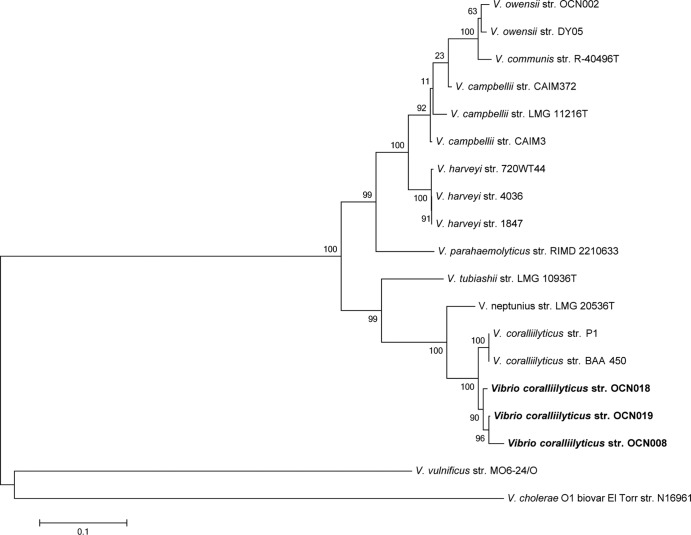
Strain OCN008 was isolated on solid GASW medium from the coral P. compressa (29). It was initially chosen for further study on the basis of an apparent zone of growth inhibition of neighboring bacteria surrounding the colony. Details of the inhibitory activity will be presented elsewhere. OCN008 was identified as a strain of V. coralliilyticus on the basis of the sequence of the gene encoding the 16S rRNA (KF042020) and MLSA (Fig. 1). Although not identical, OCN008 clustered with the previously described pathogenic V. coralliilyticus strains P1 and BAA-450.
FIG 1.
Phylogenetic tree of MLSA. Evolutionary history was inferred using the maximum likelihood method. The tree with the highest log likelihood is shown. The percentage of trees in which the associated taxa clustered together is shown next to each branch. The scale bar represents 0.1 nucleotide substitution per site.
V. coralliilyticus strains isolated from diseased Montipora capitata.
Two strains of bacteria that produced zones of growth inhibition similar to that of OCN008 were cultured from an M. capitata fragment displaying signs of chronic Montipora white syndrome (cMWS). These strains, designated OCN018 and OCN019, shared 99% and 100% sequence identity with the 16S rRNA gene of OCN008, respectively (GenBank accession no. KF042021 and KF042022, respectively). Both strains clustered with strain OCN008 in MLSA, indicating that they are strains of V. coralliilyticus and are more similar to OCN008 than to other strains of V. coralliilyticus that have been described (Fig. 1). Screening of bacteria from healthy M. capitata did not yield any V. coralliilyticus strains that were similar to OCN008.
Infection trials with M. capitata and P. compressa.
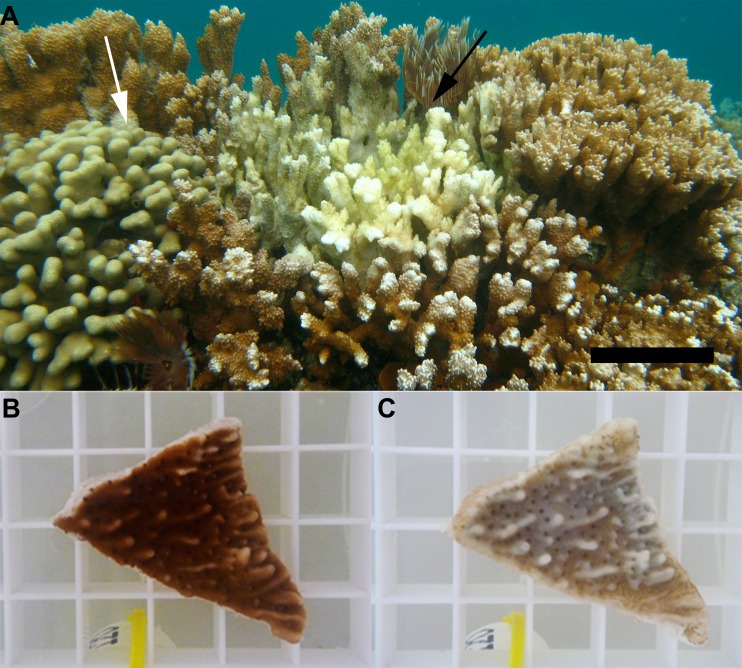
In the field, colonies of M. capitata with aMWS are often observed adjacent to healthy colonies of P. compressa (Fig. 2A), suggesting host specificity of the causative agent of aMWS. To determine if strain OCN008 could infect M. capitata or P. compressa, healthy coral was exposed to 108 CFU of OCN008 per ml of water at 27°C. All M. capitata fragments displayed acute tissue loss between 12 and 96 h postexposure, indicative of a cause-and-effect relationship between exposure to OCN008 and aMWS (McNemar's test, P = 0.001, n = 12) (Fig. 2B and C; Table 1). In contrast, none of the P. compressa fragments (n = 6) developed tissue loss under the same conditions. None of the control M. capitata fragments maintained in FSW without added bacteria (n = 12) or fragments exposed to 108 CFU/ml of the bacterial control OCN004, a strain of Alteromonas (n = 12), displayed signs of tissue loss during the experiment. When healthy M. capitata was exposed to strain OCN019, 90% of the fragments displayed tissue loss between 12 and 96 h postexposure, supporting a relationship between exposure to a strain of V. coralliilyticus and the initiation of tissue loss (McNemar's test, P = 0.007, n = 10). We conclude that OCN008 and similar strains are capable of infecting healthy M. capitata but not P. compressa.
FIG 2.
M. capitata with aMWS. (A) A colony of M. capitata with acute tissue loss (black arrow) shown adjacent to a healthy P. compressa colony (white arrow). Bar, 10 cm. (B and C) Coral fragments from an infection trial at the time of inoculation (B) and 48 h after inoculation (C). The spacing of the plastic supports of the grids under the coral fragments is 1 cm by 1 cm.
TABLE 1.
Summary of infection trials
| Virulence of OCN008 |
Infectious dose of OCN008 |
Effect of temp on OCN008 infection |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coral species | Treatment | No. of CFU/ml | Temp (°C) | No. of infected fragments/total no. testeda | Coral species | Treatment | No. of CFU/ml | Temp (°C) | No. of infected fragments/total no. tested | Coral species | Treatment | No. of CFU/ml | Temp (°C) | No. of infected fragments/total no. tested |
| M. capitata | Seawater | 27 | 0/12 | M. capitata | Seawater | 27 | 0/12 | M. capitata | Seawater | 27 | 0/6 | |||
| M. capitata | OCN004 | 108 | 27 | 0/12 | M. capitata | OCN004 | 108 | 27 | 0/12 | M. capitata | OCN004 | 108 | 27 | 0/6 |
| M. capitata | OCN008 | 108 | 27 | 12/12 | M. capitata | OCN008 | 108 | 27 | 12/12 | M. capitata | OCN008 | 108 | 27 | 6/6 |
| P. compressa | Seawater | 27 | 0/6 | M. capitata | OCN008 | 107 | 27 | 2/12 | M. capitata | Seawater | 23 | 0/6 | ||
| P. compressa | OCN004 | 108 | 27 | 0/6 | M. capitata | OCN008 | 106 | 27 | 0/12 | M. capitata | OCN004 | 108 | 23 | 0/6 |
| P. compressa | OCN008 | 108 | 27 | 0/6 | M. capitata | OCN008 | 105 | 27 | 0/12 | M. capitata | OCN008 | 108 | 23 | 5/6 |
Infected fragments represent the proportion of fragments infected under the conditions described.
Minimum infectious dose.
To determine the minimum concentration of OCN008 required for infection, M. capitata fragments were exposed to different concentrations of OCN008. All M. capitata fragments exposed to 108 CFU/ml of OCN008 (n = 12) developed tissue loss, 17% developed tissue loss with exposure to 107 CFU/ml (n = 12), and no tissue loss was observed in fragments exposed to 106 or 105 CFU/ml (n = 12 for each concentration; Table 1). Therefore, the 50% infectious dose (ID50) of strain OCN008 was estimated to be between 107 and 108 CFU/ml of seawater. All of the control fragments exposed to FSW alone (n = 12) and all fragments exposed to 108 CFU/ml of OCN004 (n = 12) remained healthy for the duration of the experiment.
Influence of water temperature on OCN008 infection.
Paired infection trials maintained at 23 and 27°C were used to determine if OCN008 infections were temperature dependent in a manner similar to BAA-450 infections. Consistent with previous trials, all of the coral fragments exposed to OCN008 at 27°C were infected between 12 and 96 h postinoculation (n = 6; Fig. 3; Table 1). Of the corresponding fragments kept at 23°C, 83% became infected between 24 and 192 h, and no additional infections were observed for the duration of the 28-day trial (n = 6). Analysis of the Kaplan-Meier survival curves for infections at 23 and 27°C found a significant difference between the incubation periods of the disease at the two temperatures (log-rank test, P = 0.0002, n = 6). However, there was no significant difference for the risk of infection, defined as the likelihood of infection under certain conditions at 23 or 27°C (McNemar's test, P = 1.0, n = 6). This suggests that at elevated temperatures, M. capitata was infected earlier after exposure to OCN008 or the incubation time was shorter, but the risk of infection was unaffected.
FIG 3.
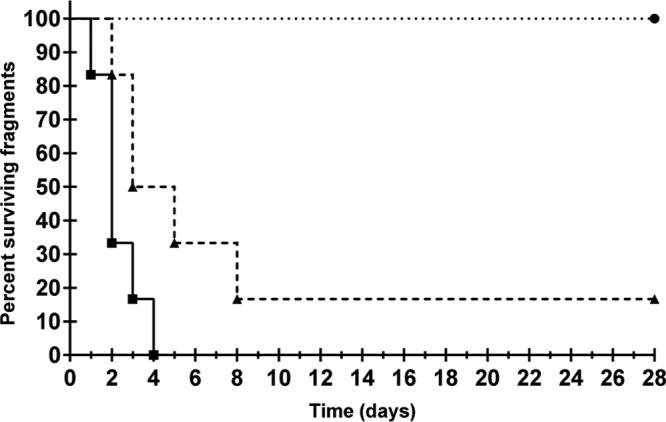
Kaplan-Meier survival curve of M. capitata in infection trials at 23 and 27°C. The percentage of surviving corals over time is plotted. Solid line, 27°C (n = 6); dashed line, 23°C (n = 6); dotted line, 23°C or 27°C with the control bacterium or FSW alone (results for each were identical). The concentration of bacteria was 108 CFU/ml of seawater.
Reisolation of OCN008 from laboratory-infected coral.
To facilitate reisolation of OCN008 and differentiate it from similar bacteria that may be naturally present on corals, plasmid pRL1383a was used to genetically tag strain OCN008 (33). Tagged OCN008 was reisolated from all four corals infected by this strain, as evidenced by identical sequences of PCR products derived from the plasmid and a unique chromosomal region of the OCN008 genome.
In addition to recovery of the plasmid-tagged strain of OCN008, nontagged OCN008 was recovered from all six of the corals tested from the infection trials described in the section above. For each of the corals, all five bacterial isolates tested yielded a PCR product with primers specific to a unique chromosomal region of the OCN008 genome. Subsequent PCR amplification and sequencing of the 16S rRNA genes from reisolated OCN008 confirmed that they were identical to the 16S rRNA gene of the OCN008 laboratory stock. No isolates identical to OCN008 were isolated from control fragments used in the infection trials.
Phenotypic characterization of OCN008.
To better describe V. coralliilyticus strain OCN008, some basic properties that may relate to its pathogenicity were evaluated. After 15 h of incubation at 27°C, OCN008 formed circular, opaque, mucoid, nonluminescent colonies on GASW agar, and colonies on TCBS agar were yellow, indicative of sucrose fermentation. Swarming motility was not observed, but at both 23°C and 27°C, beta-hemolytic, protease, lipase, and DNase activities were observed. OCN008 is naturally resistant to ampicillin (200 μg/ml) and kanamycin (25 μg/ml). The maximum doubling time was calculated to be 14 min at 23°C and 11.5 min at 27°C. Growth of OCN008 was observed at NaCl concentrations ranging from 0.7% to 6.0% (wt/vol) after 15 h of incubation, with no growth outside this range of NaCl concentrations.
Comparison of the genomes of OCN008, BAA-450, and P1.
In silico comparison of the BAA-450 genome, the draft P1 genome, and the recently released draft genome of OCN008 (29) revealed 84.30% ± 2.75% similarity between OCN008 and BAA-450, 89.40% ± 2.32% similarity between OCN008 and P1, and 83.20% ± 2.82% similarity between BAA-450 and P1 using the genome-to-genome distance calculator (35–38) (see Table S1 in the supplemental material). Proteomic analysis of BAA-450 by Kimes et al. (2011) identified genes encoding proteins upregulated after a temperature shift from 24 to 27°C (26), and a majority of these genes were also present in the OCN008 genome (see Table S3 in the supplemental material). Sequence identity between proteins from BAA-450 upregulated at least 4-fold by temperature and the corresponding proteins from OCN008 was 97 to 100%. Additionally, RAST analysis identified 31 genes present in the OCN008 genome that were absent from the BAA-450 genome (see Table S4 in the supplemental material). Predicted amino acid sequences from virulence-associated genes annotated as encoding hemolysins, proteases, lipases, type III secretion systems, prophage-related proteins, type VI secretion systems, toxins, and toxin-antitoxin systems in OCN008 were compared to those from the BAA-450 and P1 genomes (see Table S5 in the supplemental material). A homolog of the zinc-metalloprotease vcpA (GenBank accession no. AFK08686), a proposed virulence factor of P1 (25), was found in OCN008 (GenBank accession no. ERB62335) and BAA-450 (GenBank accession no. EEX33179). Two genes (GenBank accession no. ERB62950 and ERB62951) in OCN008 encode products similar to the V. cholerae RTX-like proteins, which have been suggested to be virulence factors for BAA-450 and P1 (25, 26). The genes for four potential quorum-sensing (QS) systems were identified in the OCN008 genome by comparison to characterized genes in the well-studied models for QS, Vibrio campbellii strain BAA-1116 (formally V. harveyi strain BB120) and Vibrio cholerae O1 biovar El Tor strain N16961 (40, 41) (see Table S6 in the supplemental material). All putative QS proteins encoded in the OCN008 genome (GenBank accession no. ERB66061, ERB66059, ERB62645, ERB62643, ERB62642, ERB62963, ERB62962, ERB63170, ERB63172, ERB62567, ERB66350, ERB64458, ERB62341, and ERB62638) shared 98 to 100% identity with the corresponding proteins in BAA-450 and P1. Despite differences in regulation of pathogenesis, the three V. coralliilyticus strains share similar QS and virulence-associated genes.
DISCUSSION
This report describes OCN008, a virulent strain of V. coralliilyticus that infects the coral M. capitata, which induces the tissue loss disease aMWS. Strains very similar to OCN0008 were isolated in pure culture from diseased M. capitata, but none were isolated from healthy M. capitata. Healthy fragments of M. capitata were infected by OCN008 during controlled laboratory experiments, and OCN008 could be reisolated from infected fragments. The disease lesions observed in laboratory fragments appeared to be identical to the tissue loss lesions observed during aMWS outbreaks (11). No tissue loss was observed from any of the fragments exposed to seawater alone or control bacterium OCN004, indicating that tissue loss was associated with exposure to OCN008 and not due to captivity or general exposure to a bacterial culture. Taken together, these results fulfill Koch's postulates of disease causation and identify OCN008 as a pathogen of M. capitata responsible for the tissue loss disease aMWS.
The apparent resistance of P. compressa to concentrations of OCN008 that were lethal to M. capitata indicates host specificity for this pathogen. Field observations of healthy P. compressa colonies in contact with M. capitata colonies with aMWS are consistent with the observed host specificity in infection trials. Host specificity has been observed before with V. coralliilyticus. Strain P1, the etiological agent of white syndrome in M. aequituberculata, did not infect the coral Acropora millepora in infection trials, whereas a pathogenic strain of Alteromonas was infectious under the same conditions (42). Clearly, differences in corals affect the susceptibility of each to infection by a given strain of V. coralliilyticus. V. coralliilyticus as a species is regarded as having a broad host range; however, individual strains, such as P1 and OCN008, may be more limited in their ability to cause disease in multiple host genera.
A large infectious dose of 107 to 108 CFU/ml of OCN008 was required for laboratory infection of M. capitata, yet inoculation with the same concentration of the avirulent, coral-associated Alteromonas strain OCN004 did not result in infection. Studies with the other strains of V. coralliilyticus, BAA-450 and P1, also report the use of comparatively high inocula in infection trials (8, 24). Why might such a high infectious dose be required? Corals have a large suite of defensive strategies for protection from settling organisms (43, 44). One such mechanism is thought to be mucous production and sloughing. Recent work suggests that the sloughing of mucus is effective at controlling the levels of the pathogen BAA-450 on the surface of coral, but this shedding mechanism can be overcome at levels of BAA-450 similar to the ID50 found for OCN008 (45). At concentrations at or below 5 × 106 cells/ml seawater, the levels of V. coralliilyticus on the surface layer of corals appeared to be controlled by shedding, but after exposure to 5 × 107 cells/ml, the levels of BAA-450 remained consistently at 106 cells/cm3 on the coral surface. Therefore, a large infectious dose may be required for persistence of V. coralliilyticus in the presence of mucous sloughing and other host defenses. In response to bacterial challenge by P1, Acropora millepora upregulated components of the innate immune response, suggesting that secondary defenses also protect coral from opportunistic pathogens (42). An additional level of protection against pathogens is thought to be conferred by antimicrobial compounds produced by the normal bacterial flora associated with corals. For instance, growth of the pathogens V. shiloi and V. coralliilyticus was inhibited by substances produced by bacteria isolated from the corals Oculina patagonica, Montastraea annularis, and Pseudopterogorgia americana (21, 46, 47). The combined defensive potential of coral and its associated flora suggests that corals are not easily infected by any given bacterium. It is unknown what concentration of V. coralliilyticus is required for infection in the field or if a concurrent environmental stressor is a prerequisite for infection. Factors such as physical injury have been shown to be required for disease initiation in other coral diseases (48). It is likely that OCN008 is persistent in the environment but specific conditions are required for disease to take place, a scenario consistent with sporadic outbreaks of aMWS in Kāne‘ohe Bay.
Strains similar to OCN008 were isolated from M. capitata fragments displaying signs of a chronic MWS infection, whereas no such strains were found in healthy M. capitata. This suggests that OCN008 may readily associate with compromised hosts but apparently not with healthy coral. Studies with coral from the field have shown that bacterial communities of diseased corals are enriched for Vibrios compared to communities on healthy coral (8). V. coralliilyticus strains similar to OCN008 may readily colonize preexisting cMWS lesions that have reduced host defenses, consistent with the observation that infected corals switch between cMWS and aMWS in the field (G. S. Aeby and A. Smith, unpublished data). We have shown that OCN008 can initiate acute tissue loss in M. capitata. The next step will be to determine the complex relationship between coral host, multiple bacterial pathogens, and the environmental conditions that allow infections to occur.
All infections of M. capitata by OCN008 resulted in tissue loss, regardless of temperature, and no significant difference in the numbers of coral infected by OCN008 at 23 or 27°C was observed. This is in stark contrast to the association of temperature and disease state in strain BAA-450; at 27°C BAA-450 causes tissue loss in P. damicornis, whereas bleaching is observed at temperatures of 24 to 25°C (24). It has been suggested that BAA-450 invasion of P. damicornis cells occurs only at elevated water temperatures, perhaps accounting for tissue lysis only at temperatures above 27°C (49). Despite the differences in infection by the two strains, numerous genes encoding proteins that were upregulated in BAA-450 at elevated temperature (26) were also present in OCN008. Although sets of shared genes do not necessarily suggest a conserved mechanism of infection, the different effects of temperature on disease states may be indicative of differences in the regulation of virulence factors in the two strains or of differences in the host corals. While specific environmental factors promoting OCN008 infections are not yet understood, water temperature is not a primary factor in the infection of M. capitata by OCN008, indicative of strain variation and different host responses to V. coralliilyticus.
Supplementary Material
ACKNOWLEDGMENTS
We thank Loralyn Cozy for intellectual discussion of the manuscript and staff of the Advanced Studies of Genomics, Proteomics and Bioinformatics center for technical support with genome alignment.
Coral was collected under special activity permits 2011-67 and 2011-68 from the Hawai‘i Department of Land and Natural Resources.
This work was supported by grant OCE-0961814 to G.S.A. from the National Science Foundation.
Footnotes
Published ahead of print 24 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03463-13.
REFERENCES
- 1.Rützler K, Santavy DL. 1983. The black band disease of Atlantic reef corals. Mar. Ecol. 4:301–319. 10.1111/j.1439-0485.1983.tb00116.x [DOI] [Google Scholar]
- 2.Kushmaro A, Banin E, Loya Y, Stackebrandt E, Rosenberg E. 2001. Vibrio shiloi sp. nov., the causative agent of bleaching of the coral Oculina patagonica. Int. J. Syst. Evol. Microbiol. 51:1383–1388. 10.1099/00207713-51-4-1383 [DOI] [PubMed] [Google Scholar]
- 3.Patterson KL, Porter JW, Ritchie KB, Polson SW, Mueller E, Peters EC, Santavy DL, Smith GW. 2002. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc. Natl. Acad. Sci. U. S. A. 99:8725–8730. 10.1073/pnas.092260099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Haim Y, Thompson FL, Thompson CC, Cnockaert MC, Hoste B, Swings J, Rosenberg E. 2003. Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int. J. Syst. Evol. Microbiol. 53:309–315. 10.1099/ijs.0.02402-0 [DOI] [PubMed] [Google Scholar]
- 5.Denner EB, Smith GW, Busse H, Schumann P, Narzt T, Polson SW, Lubitz W, Richardson LL. 2003. Aurantimonas coralicida gen. nov., sp. nov., the causative agent of white plague type II on Caribbean scleractinian corals. Int. J. Syst. Evol. Microbiol. 53:1115–1122. 10.1099/ijs.0.02359-0 [DOI] [PubMed] [Google Scholar]
- 6.Thompson F, Barash Y, Sawabe T, Sharon G, Swings J, Rosenberg E. 2006. Thalassomonas loyana sp. nov., a causative agent of the white plague-like disease of corals on the Eilat coral reef. Int. J. Syst. Evol. Microbiol. 56:365–368. 10.1099/ijs.0.63800-0 [DOI] [PubMed] [Google Scholar]
- 7.Cervino JM, Thompson FL, Gomez-Gil B, Lorence EA, Goreau TJ, Hayes RL, Winiarski-Cervino KB, Smith GW, Hughen K, Bartels E. 2008. The Vibrio core group induces yellow band disease in Caribbean and Indo-Pacific reef-building corals. J. Appl. Microbiol. 105:1658–1671. 10.1111/j.1365-2672.2008.03871.x [DOI] [PubMed] [Google Scholar]
- 8.Sussman M, Willis BL, Victor S, Bourne DG, Ahmed N. 2008. Coral pathogens identified for white syndrome (WS) epizootics in the Indo-Pacific. PLoS One 3:e2393. 10.1371/journal.pone.0002393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vezzulli L, Previati M, Pruzzo C, Marchese A, Bourne DG, Cerrano C, the VibrioSea Consortium 2010. Vibrio infections triggering mass mortality events in a warming Mediterranean Sea. Environ. Microbiol. 12:2007–2019. 10.1111/j.1462-2920.2010.02209.x [DOI] [PubMed] [Google Scholar]
- 10.Casamatta D, Stanić D, Gantar M, Richardson LL. 2012. Characterization of Roseofilum reptotaenium (Oscillatoriales, Cyanobacteria) gen. et sp. nov. isolated from Caribbean black band disease. Phycologia 51:489–499. 10.2216/11-10.1 [DOI] [Google Scholar]
- 11.Ushijima B, Smith A, Aeby GS, Callahan SM. 2012. Vibrio owensii induces the tissue loss disease Montipora white syndrome in the Hawaiian reef coral Montipora capitata. PLoS One 7:e46717. 10.1371/journal.pone.0046717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falkowski PG, Dubinsky Z, Muscatine L, Porter JW. 1984. Light and the bioenergetics of a symbiotic coral. Bioscience 34:705–709 [Google Scholar]
- 13.Work TM, Aeby GS. 2006. Systematically describing gross lesions in corals. Dis. Aquat. Org. 70:155–160. 10.3354/dao070155 [DOI] [PubMed] [Google Scholar]
- 14.Richardson LL. 1998. Coral diseases: what is really known? Trends. Ecol. Evol. 13:438–443. 10.1016/S0169-5347(98)01460-8 [DOI] [PubMed] [Google Scholar]
- 15.Koch R. 1876. Die Ätiologie der Milzbrand-Krankheit, begründet auf die Entwicklungsgeschichte des Bacillus Anthracis. Klassiker der Medizin, Wollstein, Grossherzogtum Posen [Google Scholar]
- 16.Richardson LL, Goldberg WM, Carlton RG, Halas JC. 1998. Coral disease outbreak in the Florida Keys: plague type II. Int. J. Trop. Biol. Conserv. 46:187–198 [Google Scholar]
- 17.Richardson LL, Goldberg WM, Kuta KG, Aronson RB, Smith GW, Ritchie KB, Halas JC, Feingold JS, Miller SL. 1998. Florida's mystery coral-killer identified. Nature 392:557–558. 10.1038/33302 [DOI] [Google Scholar]
- 18.Sutherland KP, Shaban S, Joyner JL, Porter JW, Lipp EK. 2011. Human pathogen shown to cause disease in the threatened eklhorn coral Acropora palmata. PLoS One 6:e23468. 10.1371/journal.pone.0023468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kushmaro A, Rosenberg E, Fine M, Loya Y. 1997. Bleaching of the coral Oculina patagonica by Vibrio AK-1. Mar. Ecol. Prog. Ser. 147:159–165. 10.3354/meps147159 [DOI] [Google Scholar]
- 20.Ben-Haim Y, Rosenberg E. 2002. A novel Vibrio sp. pathogen of the coral Pocillopora damicornis. Mar. Biol. 141:47–55. 10.1007/s00227-002-0797-6 [DOI] [Google Scholar]
- 21.Vizcaino MI, Johnson WR, Kimes NE, Williams K, Torralba M, Nelson KE, Smith GW, Weil E, Moeller PDR, Morris PJ. 2010. Antimicrobial resistance of the coral pathogen Vibrio coralliilyticus and Caribbean sister phylotypes isolated from a diseased octocoral. Microb. Ecol. 59:646–657. 10.1007/s00248-010-9644-3 [DOI] [PubMed] [Google Scholar]
- 22.Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L. 2009. Two pathogens of GreenshellTM mussel larvae, Perna canaliculus: Vibrio splendidus and a V. coralliilyticus/neptunius-like isolate. J. Fish Dis. 32:499–507. 10.1111/j.1365-2761.2009.01006.x [DOI] [PubMed] [Google Scholar]
- 23.Austin B, Austin D, Sutherland R, Thompson F, Swings J. 2005. Pathogenicity of vibrios to rainbow trout (Oncorhynchus mykiss, Walbaum) and Artemia nauplii. Environ. Microbiol. 7:1488–1495. 10.1111/j.1462-2920.2005.00847.x [DOI] [PubMed] [Google Scholar]
- 24.Ben-Haim Y, Zicherman-Keren M, Rosenberg E. 2003. Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl. Environ. Microbiol. 69:4236–4242. 10.1128/AEM.69.7.4236-4242.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De O Santos E, Alves N, Dias GM, Mazotto AM, Vermelho A, Vora GJ, Wilson B, Beltran VH, Bourne DG, Le Roux F, Thompson FL. 2011. Genomic and proteomic analyses of the coral pathogen Vibrio coralliilyticus reveal a diverse virulence repertoire. ISME J. 5:1471–1483. 10.1038/ismej.2011.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimes NE, Grim CJ, Johnson WR, Hasan NA, Tall BD, Kothary MH, Kiss H, Munk AC, Tapia R, Green L, Detter C, Bruce DC, Brettin TS, Colwell RR, Morris PJ. 2011. Temperature regulation of virulence factors in the pathogen Vibrio coralliilyticus. ISME J. 154:835–846. 10.1038/ismej.2011.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meron D, Efrony R, Johnson WR, Schaefer AL, Morris PJ, Rosenberg E, Greenberg EP, Banin E. 2009. Role of flagella in virulence of the coral pathogen Vibrio coralliilyticus. Appl. Environ. Microbiol. 75:5704–5707. 10.1128/AEM.00198-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aeby GS, Ross M, Williams G, Lewis T, Work T. 2010. Disease dynamics of Montipora white syndrome within Kāne‘ohe Bay, Oahu, Hawai‘i: distribution, seasonality, virulence, and transmissibility. Dis. Aquat. Organ. 91:1–8. 10.3354/dao02247 [DOI] [PubMed] [Google Scholar]
- 29.Ushijima B, Videau P, Aeby GS, Callahan SM. 2013. Draft genome sequence of Vibrio coralliilyticus strain OCN008, isolated from Kāne‘ohe Bay, Hawai‘i. Genome Announc. 1(5):e00786–13. 10.1128/genomeA.00786-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith GW, Hayasaka SS. 1982. Nitrogenase activity associated with Halodule wrightii roots. Appl. Environ. Microbiol. 43:1244–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aebischer T, Fischer A, Walduck A, Schlötelburg C, Lindig M, Schreiber S, Meyer TF, Bereswill S, Göbel UB. 2006. Vaccination prevents Helicobacter pylori-induced alterations of the gastric flora in mice. FEMS Immunol. Med. Microbiol. 46:221–229. 10.1111/rp10.1016-j.femsim.2004.05.008 [DOI] [PubMed] [Google Scholar]
- 32.Bathen KH. 1968. A descriptive study of the physical oceanography of Kāne‘ohe Bay, O‘ahu, Hawai‘i. Hawai‘i Institute of Marine Biology technical report no. 14. Hawai‘i Institute of Marine Biology, Kāne‘ohe, HI [Google Scholar]
- 33.Wolk CP, Fan Q, Zhou R, Huang G, Lechno-Yossef S, Kuritz T, Wojciuch E. 2007. Paired cloning vectors for complementation of mutations in the cyanobacterium Anabaena sp. strain PCC 7120. Arch. Microbiol. 188:551–563. 10.1007/s00203-007-0276-z [DOI] [PubMed] [Google Scholar]
- 34.Overbeek R, Begley T, Butler RM, Choudhuri JV, Chuang H-Y, Cohoon M, de Crécy-Lagard V, Diaz N, Disz T, Edwards R, Fonstein M, Frank ED, Gerdes S, Glass EM, Goesmann A, Hanson A, Iwata-Reuyl D, Jensen R, Jamshidi N, Krause L, Kubal M, Larsen N, Linke B, McHardy AC, Meyer F, Neuweger H, Olsen G, Olson R, Osterman A, Portnoy V, Pusch GD, Rodionov DA, Rückert CR, Steiner J, Stevens R, Thiele I, Vassieva O, Ye Y, Zagnitko O, Vonstein V. 2005. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 33:5691–5702. 10.1093/nar/gki866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. 10.1186/1471-2164-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. 2004. Versatile and open software for comparing large genomes. Genome Biol. 5:R12. 10.1186/gb-2004-5-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auch AF, von Jan M, Klenk H-P, Göker M. 2010. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand. Genomic Sci. 2:117–134. 10.4056/sigs.531120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auch AF, Klenk H-P, Göker M. 2010. Standard operating procedure for calculating genome-to-genome distances based on high-scoring segment pairs. Stand. Genomic Sci. 2:142–148. 10.4056/sigs.541628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meier-Kolthoff JP, Auch AF, Klenk H-P, Göker M. 2013. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:1–14. 10.1186/1471-2105-14-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bassler BL, Greenberg EP, Stevens AM. 1997. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 179:4043–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303–314. 10.1016/S0092-8674(02)00829-2 [DOI] [PubMed] [Google Scholar]
- 42.Brown T, Bourne D, Rodriguez-Lanetty M. 2013. Transcriptional activation of c3 and hsp70 as part of the immune response of Acropora millepora to bacterial challenges. PLoS One 8:e67246. 10.1371/journal.pone.0067246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ducklow HW, Mitchell R. 1979. Bacterial populations and adaptations in the mucus layers on living corals. Limnol. Oceanogr. 24:715–725. 10.4319/lo.1979.24.4.0715 [DOI] [Google Scholar]
- 44.Rublee PA, Lasker HR, Gottfried M, Roman MR. 1980. Production and bacterial colonization of mucus from the soft coral Briarium asbestinum B. Mar. Sci. 30:888–893 [Google Scholar]
- 45.Garren M, Azam F. 2012. Corals shed bacteria as a potential mechanism of resilience to organic matter enrichment. ISME J. 6:1159–1165. 10.1038/ismej.2011.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nissimov J, Rosenberg E, Munn CB. 2009. Antimicrobial properties of resident coral mucus bacteria of Oculina patagonica. FEMS Microbiol. Lett. 292:210–215. 10.1111/j.1574-6968.2009.01490.x [DOI] [PubMed] [Google Scholar]
- 47.Rypien KL, Ward JR, Azam F. 2010. Antagonistic interactions among coral-associated bacteria. Environ. Microbiol. 12:28–39. 10.1111/j.1462-2920.2009.02027.x [DOI] [PubMed] [Google Scholar]
- 48.Aeby GS, Santavy DL. 2006. Factors affecting susceptibility of the coral Montastraea faveolata to black-band disease. Mar. Ecol. Prog. Ser. 318:103–110. 10.3354/meps318103 [DOI] [Google Scholar]
- 49.Vidal-Dupiol J, Ladrière O, Meistertzheim AL, Fouré L, Adjeroud M, Mitta G. 2011. Physiological responses of the scleractinian coral Pocillopora damicornis to bacterial stress from Vibrio coralliilyticus. J. Exp. Biol. 214:1533. 10.1242/jeb.053165 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.