Abstract
In Shiga toxin-producing Escherichia coli (STEC), induction of Shiga toxin-encoding bacteriophages (Stx phages) causes the release of free phages that can later be found in the environment. The ability of Stx phages to survive different inactivation conditions determines their prevalence in the environment, the risk of stx transduction, and the generation of new STEC strains. We evaluated the infectivity and genomes of two Stx phages (Φ534 and Φ557) under different conditions. Infectious Stx phages were stable at 4, 22, and 37°C and at pH 7 and 9 after 1 month of storage but were completely inactivated at pH 3. Infective Stx phages decreased moderately when treated with UV (2.2-log10 reduction for an estimated UV dose of 178.2 mJ/cm2) or after treatment at 60 and 68°C for 60 min (2.2- and 2.5-log10 reductions, respectively) and were highly inactivated (3 log10) by 10 ppm of chlorine in 1 min. Assays in a mesocosm showed lower inactivation of all microorganisms in winter than in summer. The number of Stx phage genomes did not decrease significantly in most cases, and STEC inactivation was higher than phage inactivation under all conditions. Moreover, Stx phages retained the ability to lysogenize E. coli after some of the treatments.
INTRODUCTION
Shiga toxin-producing Escherichia coli (STEC) strains are pathogenic and cause a wide range of diseases, with symptoms varying from noncomplicated diarrhea to the life-threatening hemolytic-uremic syndrome (HUS) (1, 2). STEC produces two immunologically distinct toxins known as Shiga toxin 1 (Stx1) and Shiga toxin 2 (Stx2), and both toxins present diverse variants (1).
The genes encoding Stx in E. coli are located in the genomes of inducible temperate bacteriophages (Stx phages) (3). Induction of the lytic cycle of Stx phages causes an increase in production of Shiga toxin, which is the virulence factor responsible for severe complications associated with the infection, such as HUS (2, 4). In addition to the increase in Stx expression, the lysis of the cell caused by Stx phages allows the release of Stx outside the cell and also the dissemination of Stx phages. Free Stx phages spread within the gut and are excreted with the feces (5). In terms of occurrence in the environment, Stx phages have been found in water bodies containing fecal contamination of human or animal origin (6–12). Infectious Stx phages have also been detected in food samples, and despite the abundance of Stx phages in these samples, they showed levels of bacterial indicators (aerobic colony counts and E. coli) that make them acceptable for consumption according to European regulations (13).
The widespread distribution of Stx phages in different environments indicates that they must be able to persist under diverse conditions. Previous studies suggested the persistence of environmental Stx phages (7). Newly developed molecular methods for the quantification of Stx phages (14), the development of new approaches for the optimal detection of plaques formed by infectious Stx phages (15), and optimized protocols for the generation of lysogens of Stx phages allowed us to collect more reliable and quantifiable data about the persistence of infectious Stx phages and Stx phage genomes following various inactivation and disinfection processes and to evaluate their capacity for transduction after such treatments. Our hypothesis is that if Stx phages could persist better than STEC against the different inactivation conditions assayed, they possibly can generate lysogens after some of these conditions. If our hypothesis is true, this fact, together with their wide occurrence in different environments, should lead to the conclusion that Stx phages are a potential threat for the emergence of new STEC serotypes in the environment.
MATERIALS AND METHODS
Bacteriophages, strains, and media.
Stx2 bacteriophages Φ534 and Φ557 were induced from E. coli O157:H7 stx2-positive strains A534 and A557 isolated from cattle (16). Recombinant phages Φ534Δstx::cat and Φ557Δstx::cat (17), in which a fragment of the stx was replaced by a chloramphenicol acetyltransferase (cat) gene, were used for transduction experiments. Bacteriophage SC12 was isolated from river water (18) and was used as a control virulent phage infecting E. coli strain WG5.
E. coli WG5 (ATCC 700078) was used as the host strain to evaluate the infectivity of Stx phages and the control phage and as a host for the generation of lysogens. Wild-type E. coli O157:H7 strain A557 (16) was the STEC strain used to evaluate bacterial inactivation.
Luria-Bertani (LB) broth and LB agar were used to culture bacteria. LB soft agar containing 0.7% agar was supplemented with 5% glycerol (15) to improve plaque observations. Trypticase soy agar (TSA) and ChromoCult coliform agar (Merck, Darmstadt, Germany) were used for the quantification of STEC strains. When necessary, media were supplemented with chloramphenicol (20 μg/ml). Phosphate-buffered saline (PBS) (137 mM NaCl, 8 mM Na2HPO4, 1.46 mM KH2PO4, 2.7 mM KCl [pH 7.4]) was used to make dilutions of bacteria and phages.
E. coli strain DH5α transformed with pBAD-TOPO Vector (Invitrogen Corporation, Barcelona, Spain) containing a fragment of the stx2 gene was used to prepare the standard for a real-time quantitative PCR (qPCR) (14).
Induction and isolation of Stx2 bacteriophages.
Lysogens of Stx phages Φ534 and Φ557 (16) were incubated under agitation (180 rpm) at 37°C to an optical density at 600 nm (OD600) of 0.3, as measured with a spectrophotometer (Spectronic 501; Milton Roy, Belgium). To induce Stx2 bacteriophages, mitomycin C (0.5 μg/ml) was added to the culture and incubated overnight at 37°C in the dark in a shaker. Phage lysates were prepared by filtration of bacterial cultures through low protein-binding 0.22-μm pore size membrane filters (Millex-GP; Millipore Bedford, MA) and were enumerated by plaque blot analysis as described below. Phage lysates were diluted to obtain the same phage density for both phages (106 PFU/ml) used for the experiments. To be certain that no inactivation occurred other than that due to the treatment applied, fresh phage suspensions were prepared at the beginning of each experiment and then diluted and quantified to obtain the same densities of phages.
Infectivity of bacteriophages.
The infectivity of the induced phages (Stx phages and SC12) after all treatments at different time intervals was evaluated using the double-agar layer technique (19), with E. coli WG5 grown to the exponential phase (OD600 of 0.3) as the host strain. One milliliter of the culture was mixed with 1 ml of serial decimal dilutions of phage suspensions obtained before (time zero [t0]) and after the different treatments and 2.5 ml of LB soft agar (LB broth with 0.7% agarose) containing 0.5% glycerol. The mixture was poured onto LB plates and incubated at 37°C overnight. After incubation, SC12 plaques were directly enumerated. Plaques of Stx phages were transferred to a nylon membrane and enumerated after plaque blot hybridization as described below.
Plaque blot hybridization.
Plates containing between 100 and 300 PFU were selected and the plaques were transferred to nylon membranes (Hybond N+; Amersham Pharmacia Biotech, Barcelona, Spain). Membranes were hybridized according to a standard procedure (20) at 64°C with a 369-bp fragment digoxigenin (DIG)-labeled stx2-A probe (16) or a 1,015-bp fragment digoxigenin-labeled cat probe (17), prepared as described elsewhere. Stringent hybridization was carried out using a DIG DNA labeling and detection kit (Roche Diagnostics, Barcelona, Spain) in accordance with the manufacturer's instructions.
Isolation of phage DNA.
Before (t0) and at different time intervals after each treatment, phage lysates were treated with DNase (0.2 mg/ml), proteinase K (0.5 mg/ml), and phenol-chloroform (1:1) to extract phage DNA, as described previously (20). Purified DNA was eluted in a final volume of 50 μl of sterile bidistilled water and quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Thermo Scientific, Wilmington, MA). The integrity of the DNA was confirmed by gel electrophoresis and ethidium bromide staining. Purified DNA was used to quantify the number of gene copies (GC) by real-time qPCR.
PCR procedures.
Conventional PCRs were performed using a GeneAmp 2700 PCR system (Applied Biosystems, Barcelona, Spain). The primers for amplification of a fragment of the A subunit of the stx2 gene were UP378 (GCGTTTTGACCATCTTCGT) and LP378 (ACAGGAGCAGTTTCAGACAG) (6).
Real time qPCR assays for stx2 were used to quantify stx GC in the phage DNA (14). The primers and probes (900 nM for primer and 200 nM for the TaqMan probe) were used under standard conditions in a Step One real-time PCR system (Applied Biosystems, Spain), as described previously (14). The stx real-time qPCR assay has an efficiency of 94 to 100% and a detection limit of 5.29 stx copies.
Generation of lysogens with recombinant phages.
The ability of the Stx phages to generate lysogens after inactivation treatments was evaluated using the same Stx phages, in which a fragment of the stx was replaced by a chloramphenicol acetyltransferase (cat) gene (Φ534Δstx::cat and Φ557Δstx::cat), which thereby confers resistance to chloramphenicol on those strains that have incorporated the Stx phage (17). Suspensions of the modified stx2::cat phages obtained by mitomycin C induction from their respective lysogens as described above were obtained. Infectious particles were enumerated by plaque blotting with the cat-DIG probe (17), and phage lysates were diluted to contain 106 PFU/ml. Phages in the suspension were used to lysogenize E. coli strain WG5 (the same strain used as the host when evaluating the infectivity of Stx phages) before and after inactivation with chlorination, UV radiation, and thermal treatment.
In these experiments, 1 ml of the phage suspension was mixed with 1 ml of the culture of the host strain grown to the exponential growth phase (OD600 = 0.5). The mixture was then incubated in 5 ml of LB broth for 15 h at 37°C in a shaker. Serial dilutions of this culture were plated onto LB agar with chloramphenicol (20 μg/ml).
Colonies suspected of being lysogens were confirmed via PCR using a combination of primers for Stx (rho-stx [ATATCTGCGCCGGGTCTG]) and the primer located within the cat gene (inversCm5 [AACAGTACTGCGATGAGTG]) (amplicon of 448 bp).
Influence of pH and temperature on Stx2 phages.
Stx phage lysates containing 106 PFU/ml of phage were incubated in 10-ml tubes at 4°C, 22°C, and 37°C for 1, 3, 7, 14, 21, and 28 days.
Phage lysates containing 106 PFU/ml of each phage were adjusted to different pHs (3, 7, and 9) (by adding 37% HCl for pH 3 or 1 N NaOH for pH 9) and incubated in 10-ml tubes for 1, 3, 7, 14, 21, and 28 days at 4°C. At each interval, pH was confirmed before conducting the infectivity and quantification assays.
Inactivation conditions.
For Stx phages, infectivity was evaluated by plaque blotting and the number of genomic copies of stx in phage DNA was evaluated by real-time qPCR. As controls, the virulent phage SC12 was enumerated according to plaque formation, and STEC, used as the bacterial control, was enumerated according to colony growth on MacConkey agar plates.
Aliquots of phage lysates containing approximately 105 to 106 PFU/ml of the Stx phages, 106 to 107 PFU/ml of SC12, and 105 to 106 CFU/ml of STEC were used for the inactivation experiments.
For heat inactivation, the phages and bacteria were placed in 1.5-ml tubes and incubated in an incubator (Hybex Microarray Incubation System, SciGene) at 60°C and 70°C for 30 and 60 min.
For UV treatment, samples were placed 10 cm below an 8-W, germicidal UV lamp (model G30T8; 0.099-mW/cm2 irradiance at a 253.7-nm wavelength; Sankyo Denki, Tokyo, Japan) for 1 to 30 min (21). The lamp was warmed up for 15 to 30 min before starting the experiments. The UV dose was calculated using the equation D = I × T, where D is the dose, T is the exposure time (60, 300, 600, and 1,800 s), and I is the “fluence rate” (or intensity) of the lamp.
For chlorine inactivation, phage lysates and bacteria were diluted 1:20 in double-distilled water, to avoid any interference between organic matter in the media and the chlorine. Samples were treated with 10 ppm of chlorine supplied as sodium hypochlorite and incubated for 1, 3, 5, 10, and 20 min at room temperature (21°C). Residual chlorine was neutralized by adding 3% (wt/vol) sodium thiosulfate (22) before conducting the infectivity and quantification assays.
Natural inactivation.
To simulate inactivation under natural conditions, we used an outdoor pond that was supplied with nonchlorinated water from a well and provided a habitat for some species of goldfish. Phage lysates and bacterial cultures were diluted 1:10 in the well water used for these experiments. To avoid interference with microorganisms present in the water, the well water was filter sterilized using 0.22-μm-pore-size membrane filters (Millipore, Bedford, MA). Suspensions containing each Stx phage, phage SC12, and STEC were separately placed into dialysis tubes (cutoff, 14 kDa), which were sealed and placed in the outdoor pond with a water volume of 60 m3, protected by a cage, at a depth of 20 cm. The experiments were performed in the summer (August; temperature of 19.5° to 29.5°C and solar radiation of 23 MJ/m2) and winter (February; temperature of 3.5 to 14.5°C and solar radiation of 8.0 MJ/m2). The pH (6.8) and turbidity (4.1 to 6.8 nephelometric turbidity units [NTU]) of the pond were stable at all times. The tubes containing the phage lysates and the bacterial cultures were collected at various intervals and analyzed.
Statistical analyses.
Computation of data and statistical tests were performed using the Statistical Package for Social Science software (SPSS). One- or two-way analysis of variance (ANOVA) was used to assess whether the values of each microorganism showed significant reductions with each treatment and at the times assayed. For all statistical analyses, P values below 0.05 were considered significant.
RESULTS
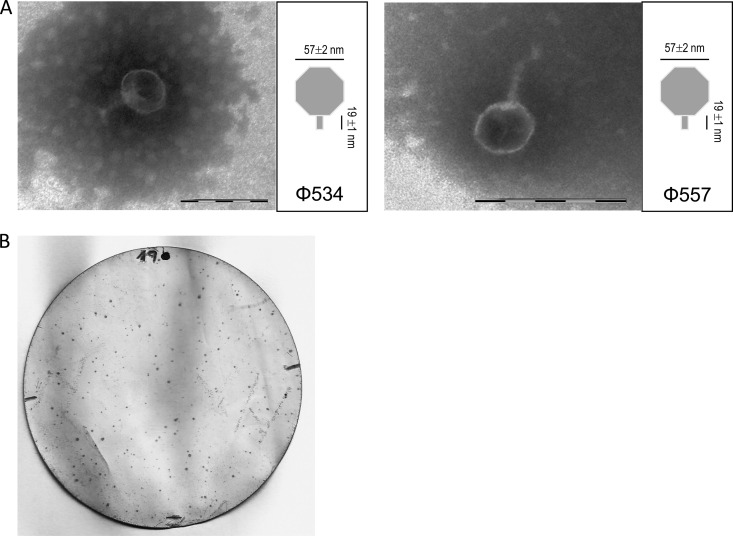
Lysogenic laboratory strains containing Stx phages Φ534 and Φ557 showed a reduction in optical density after mitomycin C treatment, indicating induction of the lytic cycle by activation of temperate Stx phages present in the strains. On the basis of morphology, hexagonal head and short tail, these phages belong to the family Podoviridae (Fig. 1A) (23), which is one of the most representative among Stx2 bacteriophages. Accordingly, plaques of lysis were obtained after plating suspensions of Stx phages obtained from the induced lysogens onto an agar monolayer containing bacterial host strain E. coli WG5. The largest and clearest plaques were obtained in the presence of 5% glycerol in the LB soft agar, and therefore, this approach was used in subsequent experiments.
FIG 1.
(A) Micrographs of Stx phages Φ534 and Φ557. Bar, 100 nm. (B) Positive signals of plaques of lysis generated by Stx phages on E. coli strain WG5 after plaque blot hybridization with a DIG-labeled stx2-A probe.
Although plaques of lysis were directly visible on the agar monolayer, many were small and difficult to visualize. For more accurate enumeration, plaque blot hybridization was performed. The data on infectious PFU presented in the following sections are based on counts of the plaque blot signals (Fig. 1B).
Stability of Stx phages.
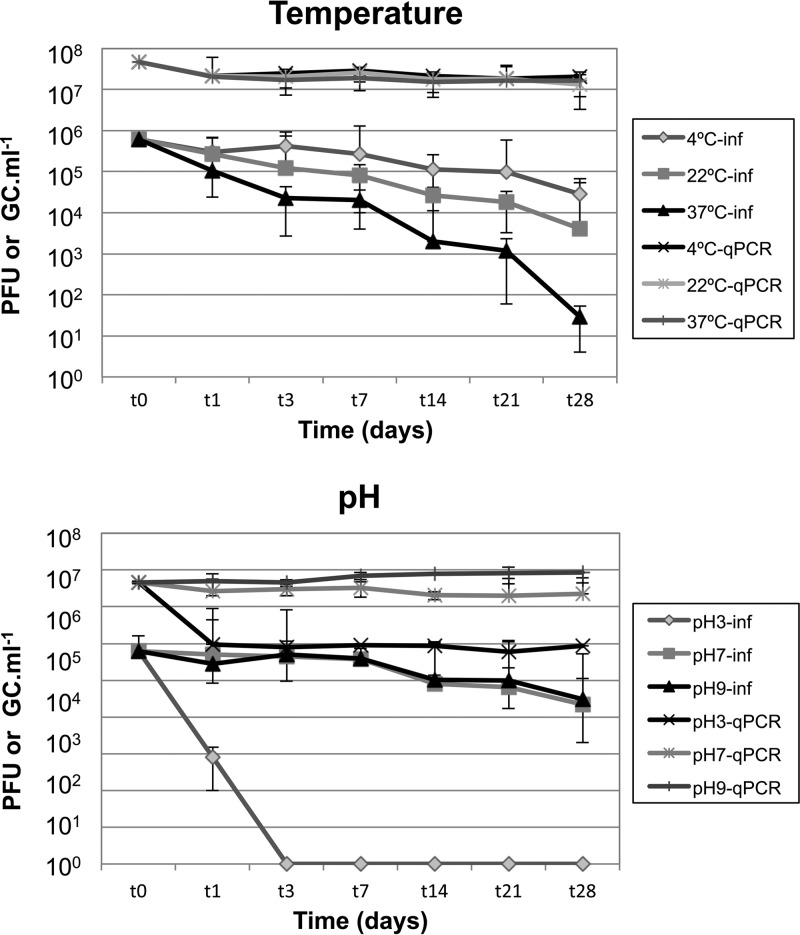
The stability and inactivation experiments were performed using two Stx phages (Φ534 and Φ557). Both phages were used at a concentration of 106 PFU/ml and showed very similar stabilities; therefore, the results are presented as the averages of the two Stx phages. Stx phages showed similar reductions in the number of PFU over time at different temperatures (Fig. 2, top). After 1 month of storage at 4°C a reduction of less than 1.5 log10 units was observed, while storage at 22°C led to a reduction of 2.2 log10 units. Phages showed less stability at 37°C, with PFU decreasing by less than 2 log10 units after the first week of storage but by more than 4 log10 units after 1 month. Reduction of infectious phages was nevertheless statistically significant (ANOVA, P < 0.05) for the three temperatures assayed.
FIG 2.
Stability of Stx phages after 1 month of storage at various temperatures (4°C, 22°C, and 37°C) and at different pHs (3, 7, and 9) after 1 month of storage at 4°C. Values for Stx phages are presented as the average of values obtained for phage Φ534 and phage Φ557. Infectious phages (inf) were evaluated by plaque blot and values expressed as PFU/ml. Phage genomes were evaluated by qPCR, and the results are expressed as GC/ml. Values for Stx phages are the averages of phage Φ534 and phage Φ557 in three independent experiments. The x axis is not presented at scale.
Since Stx phages are only known to carry one stx copy, the stx GC values in phage DNA can be extrapolated to the number of genomes of Stx phages in each sample. The results of qPCR showed a nonsignificant (P > 0.05) reduction, less than 1 log10, in the number of Stx phage genomes at the three temperatures after 1 month.
Stability at different pH values was evaluated at 4°C, since that temperature showed the lowest reduction in the number of infectious Stx phages (or PFU) and therefore the lowest influence on stability. When exposed to low pH (pH 3.0), Stx phages lost their infectivity after only 1 day, and no plaques were observed (Fig. 1B). Accordingly, a significant reduction (P < 0.0.5), 1.5 log10 units, of the Stx phage GC at pH 3.0 was also observed by qPCR; this was the only pH that caused a reduction in the number of Stx phage genomes. Stx phages showed almost identical inactivation, 1.5 and 1.3 log10 PFU/ml, respectively, at pHs 7.0 and 9.0 after 1 month of storage. The qPCR also reflected the stability at pHs 7.0 and 9.0, with reductions of less than 0.3 log10 GC/ml after 1 month. Reduction of Stx phages was statistically significant (P < 0.05) at different pH values but not statistically significant (P > 0.05) for qPCR values at pHs 7.0 and 9.0.
Inactivation treatments.
The persistence of the Stx phages following different inactivation treatments was compared with that of phage SC12, a lytic phage infecting E. coli WG5, and the STEC wild-type strain A557 (serotype O157:H7, the strain from which phage Φ557 was isolated). These were exposed to chlorination, UV treatment and high temperatures (Table 1). The inactivation of Stx phages (Φ534 and Φ557 together), SC12, and STEC by these treatments was evaluated in three independent experiments for each condition.
TABLE 1.
Log10 reductions of Stx phages (infectious and genomes), infectious phage SC12 and STEC under three disinfection processesa
| Treatment | Time (min) | Log10 reduction |
|||
|---|---|---|---|---|---|
| Stx phage |
Phage SC12, PFU/ml | STEC, CFU/ml | |||
| PFU/ml | GC/ml | ||||
| Chlorination (10 ppm) | 1 | 3.29 (0.52) | 0.13 (0.05) | 4.58 (0.59) | 4.89 (0.00) |
| 3 | 3.68 (0.53) | 0.56 (0.03) | 4.98(0.10) | >4.89b | |
| 5 | 3.73 (0.77) | 0.60 (0.24) | 4.88 (0.31) | >4.89 | |
| 10 | 4.24 (0.88) | 0.55 (0.32) | 5.25 (0.66) | >4.89 | |
| 20 | 4.79 (0.01) | 0.62 (0.24) | 5.80 (1.41) | >4.89 | |
| UV (0.099 mW/cm2) | 1 | 0.44 (0.54) | −0.08 (0.00) | 1.42 (0.68) | 5.80 (0.00) |
| 5 | 1.15 (1.48) | −0.08 (0.00) | 2.04 (0.87) | >5.80 | |
| 10 | 1.61 (0.98) | 0.13 (0.02) | 2.63 (0.13) | >5.80 | |
| 30 | 2.20 (0.69) | 0.22 (0.10) | >3.00b | >5.80 | |
| Heat, 60°C | 30 | 1.85 (0.78) | 0.58 (0.05) | 0.62 (0.05) | 5.07 (0.00) |
| 60 | 2.22 (1.26) | 0.54 (0.02) | 1.54 (0.94) | >5.07 | |
| Heat, 70°C | 30 | 2.49 (0.80) | 0.60 (0.12) | 2.54 (0.48) | >5.07 |
| 60 | 2.51 (1.02) | 0.74 (0.07) | 3.56 (1.02) | >5.07 | |
Reduction was calculated as the difference of each microorganism in log10 units between the beginning of the experiment (time zero) and a given time. Results for Stx phages are the averages of those obtained with phage Φ534 and phage Φ557. Standard deviations are in parentheses.
Below the detection limit.
The infectious Stx phages Φ534 and Φ557 showed significant (P < 0.05) inactivation, more than 3 log10 units, after the first minute of chlorination, while the number of gene copies was not significantly (P > 0.05) reduced, by less than 1 log10 unit, even after 20 min. The activity of phage SC12 was also significantly (P < 0.05) reduced after 1 min of treatment. However, the strongest inactivation was observed for STEC, which, at almost 105 CFU/ml, fell below the limit of detection after the first minute of treatment.
In terms of UV treatment, we calculated estimated UV doses of 5.94, 29.7, 59.4, and 178.2 mJ/cm2 at each time, and the approach allowed comparison between microorganisms, as shown previously (7, 21). The Stx phages Φ534 and Φ557 were not particularly sensitive to UV treatment and showed reductions in PFU of 2.2 log10 units after 30 min. Slightly higher inactivation was shown at all time points by phage SC12. Again, the STEC strain showed a reduction of more than 5 log10 units after only 5 min (29.7 mJ/cm2) of treatment. Infectious Stx phages, SC12, and STEC showed statistically significant reductions (P < 0.05) over time. Analysis of the phage genomes revealed that the Stx phage particles were not affected by UV treatment since GC values did not decline significantly at any time (Student's t test, P > 0.05) (Table 1).
Following heat treatment, the degree of inactivation of infectious particles was slightly lower after 30 than after 60 min, although the difference was not great and most of the inactivation seemed to have occurred during the first 30 min. Treatment at 60°C had an effect on inactivation similar to that of treatment at 70°C for Stx phages (around 2 to 2.5 log10 units of inactivation at both temperatures and times), whereas phage SC12 showed a significantly (P < 0.05) lower inactivation at 60°C than at 70°C. Stx phage genomes were still detectable by qPCR and showed a nonsignificant (P > 0.05) reduction in GC/ml, less than 1 log10 unit at all times intervals and temperatures, which indicates that at least the phage DNA containing the stx remained intact within the phage capsid and could be amplified. In contrast, a drastic reduction, 5 log10 units, was observed in the STEC strain after treatment at 60°C for only 30 min.
Natural inactivation.
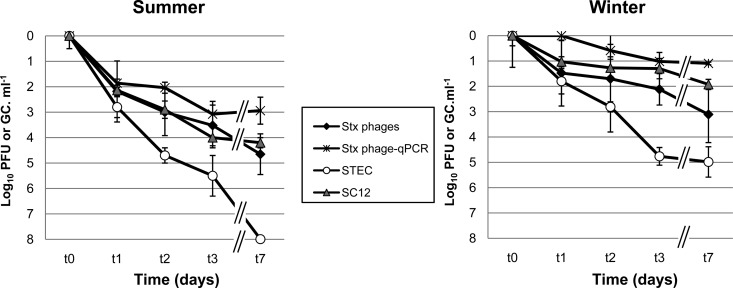
The inactivation of infectious Stx phages (Φ534 and Φ557), SC12, and STEC in the mesocosm was significantly higher (P < 0.05) for all microorganisms evaluated in summer than in winter (Fig. 3). The strongest inactivation was shown by STEC in summer, with a decay of more than 7 log10 units, meaning that this microorganism fell below the detection limit. In summer, the Stx phages and phage SC12 decayed by more than 4 log10 units in 7 days and showed similar inactivation patterns, while phage SC12 persisted better than Stx phages in winter, showing 1 log10 unit less reduction than Stx phages. Although STEC showed lower inactivation in winter than in summer, it was still the least persistent entity in winter. The Stx phages evaluated by qPCR again showed the lowest reduction in this set of experiments, and their reductions were always lower than those of infectious Stx phages. However, although in winter only 1 log10 unit of reduction was observed, there was still a reduction in the amount of Stx phage genomes in summer of 3 log10 units, indicating that some factor in the mesocosm was degrading phage DNA, even by damaging the phage capsids, which would cause DNA release and degradation and as a consequence a GC reduction, or by affecting the integrity of the phage DNA inside the capsid. Reductions showed by all microorganisms, including evaluation of Stx phages by qPCR, were statistically significant (P < 0.05) in the mesocosm experiments.
FIG 3.
Logarithmic reduction calculated for Stx phages (infectivity and genomes), SC12 (infectivity), and STEC (colony counts) in an outdoor mesocosm in summer and winter. Values for Stx phages are presented as the average of values obtained for phage Φ534 and phage Φ557. All values are the average of three independent experiments conducted during the winter or summer season.
Generation of lysogens by Stx phages after inactivation treatments.
The ability of the Stx phages to generate lysogens after the different inactivation processes was evaluated by counting chloramphenicol-resistant lysogens of E. coli strain WG5 generated after incubation with Stx phages. For these experiments, we used the same Stx phages as assayed previously but in which a fragment of stx was replaced by the cat gene, which confers chloramphenicol resistance, generating phages Φ534Δstx::cat and Φ557Δstx::cat. These phages allow chloramphenicol selection of the cells that they lysogenize. At time zero, 10% of the lysogens generated were confirmed by PCR, while all lysogens generated after inactivation experiments were confirmed by PCR.
The numbers of lysogenic colonies generated (Table 2) indicated the ability of phages to transduce stx after treatment, and although the results are presented as numerical data, it should be noted that these numbers were obtained after an incubation step (see Materials and Methods); therefore, the results should be considered qualitatively rather than quantitatively. For the same reason the results obtained for the two Stx phages are presented separately, and variations in the number of lysogens obtained for each one should therefore not be considered quantitatively (Table 2).
TABLE 2.
Ability of Stx phages to lysogenize E. coli strain WG5 after different inactivation processes
| Treatment | Time (min) | No. of E. coli colonies (CFU/ml) generateda |
|||
|---|---|---|---|---|---|
| Φ534Δstx::cat |
Φ557Δstx::cat |
||||
| 1 | 2 | 1 | 2 | ||
| None (before treatment) | 0 | 300 | 110 | 407 | 210 |
| Chlorination (10 ppm) | 1 | 1 | 1 | 1 | 1 |
| 3 | 0 | 0 | 0 | 0 | |
| UV (0.099 mW/cm2) | 1 | 37 | 40 | 20 | 50 |
| 10 | 4 | 0 | 8 | 10 | |
| Heat, 60°C | 30 | 0 | 0 | 0 | 0 |
| Heat, 70°C | 30 | 0 | 0 | 0 | 0 |
The results of two independent experiments (1 and 2) are presented.
In general, the number of infectious Stx phages that persisted after treatment correlated with the number of lysogens generated with the exception of thermal treatment. The number of lysogens obtained before any treatment was 2 log10 units (Table 2), while after UV and chlorination treatment, a few lysogens were still generated but in very low numbers in the shortest times; no lysogens were observed when longer times of treatment were applied. However, no lysogens were generated after thermal treatment under any of the conditions assayed.
DISCUSSION
Dissemination of the phages encoding the genes of Shiga toxin is the most likely mechanism accounting for the spread of these toxin genes among diverse E. coli strains and other bacterial genera. Human infections indicate that the STEC strains most commonly associated with HUS belong to particular serogroups, including O26, O45, O111, O103, O121, and O145, accounting for the non-O157 serotype, plus O157:H7 (24) and serotype O104:H4, which has recently been included. However, the transduction of stx genes could lead to the emergence of new pathogenic clones that have not yet been described. Among multiple examples of bacterial conversion by Stx phages in vivo and in vitro (16, 25–29), the STEC O104:H4 strain that caused the huge outbreak of HUS in Germany in May 2011, and that also affected France, is the most recent case (30, 31).
The German O104:H4 strain presumably acquired the Stx phage in a late evolutionary event, generating a highly virulent pathogen that, unlike other enteroaggregative E. coli strains, produced the Shiga toxin (32). The Stx phage in the O104:H4 strain was closely related to a particular Stx phage of an E. coli O111 strain (33), suggesting that the Stx phage was released by another STEC strain and that after an undetermined period of persistence in the environment, it infected and lysogenized the German strain (34). The origin of this Stx phage is unknown, and although recent reports indicate that the Stx2a phage in this strain is similar to those found in STEC isolated from cattle in Germany (35), other sources of the Stx phage that could better explain the origin and epidemiology of the strain should not be ruled out.
Stx phages have been found in various environments (5, 6, 8–14, 16), and this gives a clue to their role in the permanence and mobilization of stx in extraintestinal environments. However, the success of this spread among the bacterial population depends on their ability to retain their infectious abilities under the conditions found in a given environment. Stx phages have been shown to retain their stability and infectivity under various food-related conditions (36) and, to some extent, in the presence of certain food preservatives (37).
In the present study, the STEC was inactivated by 1.5 to 5.4 log10 units more than Stx phages following inactivation treatments and by 2 to 3 log10 units more than Stx phages under natural inactivation conditions. This is consistent with previous reports (7). Phage SC12, which showed lower persistence in laboratory experiments, persists similarly to or better than Stx phages in the mesocosm. SC12 is a phage isolated from river water (18), and it could be better adapted to natural inactivation than the temperate Stx phages. It should also be noted that the mesocosm used to evaluate natural inactivation allowed the simultaneous evaluation of the effect of a range of factors, with the likely exception of the effect of grazing by protozoa. Nevertheless, the latter has been reported to influence STEC survival, leading to greater persistence of Stx-positive bacteria than non-Stx bacteria (38) but having no effect on phages (39). Furthermore, the inactivation observed in the mesocosm could be attributable to solar radiation and/or temperature, as pH and other factors were relatively constant over time. The uncertainty regarding these factors makes it difficult to explain the reduction in genome copies observed, but in any case, solar radiation would seem to be the most critical inactivating factor, alone or as a part of a synergistic effect with other factors, affecting either the integrity of the phage capsids and/or the phage DNA. Radiation and oxidation agents have been reported to result in modifications to viral proteins (capsid protein backbone cleavage) and nucleic acids (40). Nevertheless, important variations can be observed depending on the virus assayed, and previous studies in the same mesocosm system with other phages showed similar reduction of infectious phages but almost no reduction when evaluating phage genomes by qPCR (21).
The low decay of stx copies from Stx phages after different treatments indicated that the phage DNA, at least in the fragment used to amplify the stx gene, remained unaltered despite these treatments. These results were not correlated with the decay in infectivity, however, as demonstrated previously (14) and confirmed in this study. Molecular methods provide a lot of information and are easy to perform for some viruses, like Stx phages, but they do not provide information of their infectivity. Infectious methods are also limited by the use of the right host strain (or cell culture). For Stx phages there is an additional limitation because the plaques generated are sometimes poorly visible. The comparison of infectivity and qPCR methods in the present study shows a better picture of Stx phage inactivation than the use of plaque assay alone. The differences between both approaches should be considered in those studies evaluating the abundance of Stx phages in different environments, which are mostly done by qPCR methods (11–14). Moreover, the potential danger of these phages stems from their ability to generate lysogens. An interesting finding of our study is that some Stx phages persist sufficiently to retain their capability to lysogenize an E. coli strain after some disinfection processes. This capability is shown, however, at low rates, particularly in chlorination, and with the exception of thermal treatment. Although the causes of inhibition of lysogeny after thermal treatment are unknown, the treatment should affect the mechanisms of the phage for the establishment of lysogeny while not its ability to cause lysis.
The generation of lysogens after the treatments is particularly important because of the implications that lysogenization could have for the emergence of new strains in extraintestinal environments, which can be the real threat to human health. The lysogens in our experiments were selected by antibiotics to facilitate the detection on the agar plates, and lower frequencies would probably be obtained without selection, but it should be noted that the lysogenization step in liquid culture was conducted without any antibiotic selection (see Materials and Methods). Our assays, which were conducted under laboratory conditions, may or may not be the most optimal for lysogenization, since transduction under “in vivo” conditions seems to be very effective (25, 26, 28, 29). The Stx phage induced from the Norwegian O103:H25 isolate was shown to be capable of lysogeny after storage in a dry-fermented sausage model (36).
Two Stx phages were evaluated in this study, although it is well known that Stx phages are a highly heterogeneous group (3, 9, 16). The results presented herein are therefore not necessarily applicable to all Stx phages, but the two phages in this study were selected considering their characteristics and for practical reasons. They were both induced from O157:H7 strains, and although they are genetically different (16), they present the morphology of Podoviridae phages, a morphological type commonly found in Stx2 phages (16) and to which the prototype Stx2phage 933W belongs (41). Finally, both phages generate phage suspensions after induction from their lysogens with a constantly high number of phages, which allowed the replicates presented in our study. Other studies on the survival of Stx phages have been conducted using only one Stx phage of the same morphological type (36, 37).
The previous observations of our group and other authors (16, 36, 42, 43) suggest that certain Stx phages (including phage 933W) (44) tend to lose infectivity when they are induced from their lysogenic host and stored for a few hours at 4°C. These previous observations were contradictory with the fact that a substantial number of infectious Stx phages were detectable in extraintestinal environments and that there was no plausible explanation at that time. In the light of the present results, it now seems clear that some Stx phages can remain infectious even after storage, which is consistent with their widespread occurrence in extraintestinal environments. Moreover, since Stx phages could be found in food-related environments, their persistence after storage should be considered in terms of permanence in food and the potential ingestion of Stx phages by the consumers.
Generally speaking, because of their simple structure and composition, numerous bacteriophages persist relatively successfully in the environment and are relatively resistant to natural and anthropogenic stressors (7, 18, 45). Our results are also consistent with reports on other E. coli phages after either thermal treatment (46, 47), UV treatment (48, 49), or chlorination (7, 21, 50). Chlorinating water with 10 ppm of chlorine would inactivate bacteria, which would be considered suitable for consumption according to current practices in many parts of the world. However, Stx phages may still be detected in chlorinated samples. Stx phages are not the only example of phages encoding virulence genes that persist under certain inactivation conditions more than their bacterial hosts (21). Moreover, some environmental conditions, such as sunlight, could even cause the induction of temperate bacteriophages (51). The real extent and risk of transduction of stx genes by temperate phages under these conditions, and the correspondence between these transduction events and the emergence of new virulent STEC strains, remain to be elucidated.
ACKNOWLEDGMENTS
This study was supported by the Spanish Ministry of Education and Science (AGL2012-30880) and the Generalitat de Catalunya (2009SGR1043) and by the Spanish Reference Network of Biotechnology (XeRBa). Anna Allué-Guardia has a grant from the Generalitat de Catalunya.
Footnotes
Published ahead of print 24 January 2014
REFERENCES
- 1.Erickson MC, Doyle MP. 2007. Food as a vehicle for transmission of Shiga toxin-producing Escherichia coli. J. Food Prot. 70:2426–2449 [DOI] [PubMed] [Google Scholar]
- 2.Melton-Celsa A, Mohawk K, Teel L, O'Brien A. 2012. Pathogenesis of Shiga-toxin producing Escherichia coli. Curr. Top. Microbiol. Immunol. 357:67–103. 10.1007/82_2011_176 [DOI] [PubMed] [Google Scholar]
- 3.Herold S, Karch H, Schmidt H. 2004. Shiga toxin-encoding bacteriophages—genomes in motion. Int. J. Med. Microbiol. 294:115–121. 10.1016/j.ijmm.2004.06.023 [DOI] [PubMed] [Google Scholar]
- 4.Noris M, Mescia F, Remuzzi G. 2012. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat. Rev. Nephrol. 8:622–633. 10.1038/nrneph.2012.195 [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Castillo A, Quirós P, Navarro F, Miró E, Muniesa M. 2013. Shiga toxin 2-encoding bacteriophages in human fecal samples from healthy individuals. Appl. Environ. Microbiol. 79:4862–4868. 10.1128/AEM.01158-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muniesa M, Jofre J. 1998. Abundance in sewage of bacteriophages that infect Escherichia coli O157:H7 and that carry the Shiga toxin 2 gene. Appl. Environ. Microbiol. 64:2443–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muniesa M, Lucena F, Jofre J. 1999. Comparative survival of free Shiga toxin 2-encoding phages and Escherichia coli strains outside the gut. Appl. Environ. Microbiol. 65:5615–5618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanji Y, Mizoguchi K, Akitsu T, Morita M, Hori K, Unno H. 2002. Fate of coliphage in waste water treatment process and detection of phages carrying the Shiga toxin type 2 gene. Water Sci. Technol. 46(11-12):285–289 [PubMed] [Google Scholar]
- 9.Muniesa M, Serra-Moreno R, Jofre J. 2004. Free Shiga toxin bacteriophages isolated from sewage showed diversity although the stx genes appeared conserved. Environ. Microbiol. 6:716–725. 10.1111/j.1462-2920.2004.00604.x [DOI] [PubMed] [Google Scholar]
- 10.Dumke R, Schröter-Bobsin U, Jacobs E, Röske I. 2006. Detection of phages carrying the Shiga toxin 1 and 2 genes in waste water and river water samples. Lett. Appl. Microbiol. 42:48–53. 10.1111/j.1472-765X.2005.01809.x [DOI] [PubMed] [Google Scholar]
- 11.Imamovic L, Ballesté E, Jofre J, Muniesa M. 2010. Quantification of Shiga toxin-converting bacteriophages in wastewater and in fecal samples by real-time quantitative PCR. Appl. Environ. Microbiol. 76:5693–5701. 10.1128/AEM.00107-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rooks DJ, Yan Y, McDonald JE, Woodward MJ, McCarthy AJ, Allison HE. 2010. Development and validation of a qPCR-based method for quantifying Shiga toxin-encoding and other lambdoid bacteriophages. Environ. Microbiol. 12:1194-1204. 10.1111/j.1462-2920.2010.02162.x [DOI] [PubMed] [Google Scholar]
- 13.Imamovic L, Muniesa M. 2011. Quantification and evaluation of infectivity of Shiga toxin-encoding bacteriophages in beef and salad. Appl. Environ. Microbiol. 77:3536–3540. 10.1128/AEM.02703-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imamovic L, Serra-Moreno R, Jofre J, Muniesa M. 2010. Quantification of Shiga toxin 2-encoding bacteriophages, by real-time PCR and correlation with phage infectivity. J. Appl. Microbiol. 108:1105–1114. 10.1111/j.1365-2672.2010.04664.x [DOI] [PubMed] [Google Scholar]
- 15.Santos SB, Carvalho CM, Sillankorva S, Nicolau A, Ferreira EC, Azeredo J. 2009. The use of antibiotics to improve phage detection and enumeration by the double-layer agar technique. BMC Microbiol. 9:148. 10.1186/1471-2180-9-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muniesa M, Blanco JE, De Simón M, Serra-Moreno R, Blanch AR, Jofre J. 2004. Diversity of stx2 converting bacteriophages induced from Shiga-toxin-producing Escherichia coli strains isolated from cattle. Microbiology 150:2959–2971. 10.1099/mic.0.27188-0 [DOI] [PubMed] [Google Scholar]
- 17.Serra-Moreno R, Acosta S, Hernalsteens JP, Jofre J, Muniesa M. 2006. Use of the lambda red recombinase system to produce recombinant prophages carrying antibiotic resistance genes. BMC Mol. Biol. 7:31. 10.1186/1471-2199-7-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durán AE, Muniesa M, Méndez X, Valero F, Lucena F, Jofre J. 2002. Removal and inactivation of indicator bacteriophages in fresh waters. J. Appl. Microbiol. 92:338–347. 10.1046/j.1365-2672.2002.01536.x [DOI] [PubMed] [Google Scholar]
- 19.Adams MH. 1959. Bacteriophages, p 27–30 Interscience Publishers, New York, NY [Google Scholar]
- 20.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 21.Allué-Guardia A, Jofre J, Muniesa M. 2012. Stability and infectivity of cytolethal distending toxin type V gene-carrying bacteriophages in a water mesocosm and under different inactivation conditions. Appl. Environ. Microbiol. 78:5818–5823. 10.1128/AEM.00997-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durán AE, Muniesa M, Mocé-Llivina L, Campos C, Jofre J, Lucena F. 2003. Usefulness of different groups of bacteriophages as model micro-organisms for evaluating chlorination. J. Appl. Microbiol. 95:29–37. 10.1046/j.1365-2672.2003.t01-1-01948.x [DOI] [PubMed] [Google Scholar]
- 23.Franki RIB, Fauquet CM, Knudson DL, Brown F. (ed). 1991. Classification and nomenclature of viruses. Fifth report of the International Committee on Taxonomy of Viruses. Springer-Verlag, Vienna, Austria [Google Scholar]
- 24.USDA Food Safety and Inspection Service. 2011. Risk profile for pathogenic non-O157 Shiga toxin-producing Escherichia coli (non-O157 STEC). USDA Food Safety and Inspection Service, Washington, DC [Google Scholar]
- 25.Acheson DW, Reidl J, Zhang X, Keusch GT, Mekalanos JJ, Waldor MK. 1998. In vivo transduction with Shiga toxin 1-encoding phage. Infect. Immun. 66:4496–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt H, Bielaszewska M, Karch H. 1999. Transduction of enteric Escherichia coli isolates with a derivative of Shiga toxin 2-encoding bacteriophage ϕ3538 isolated from Escherichia coli O157:H7. Appl. Environ. Microbiol. 65:3855–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James CE, Stanley KN, Allison HE, Flint HJ, Stewart CS, Sharp RJ, Saunders JR, McCarthy AJ. 2001. Lytic and lysogenic infection of diverse Escherichia coli and Shigella strains with a verocytotoxigenic bacteriophage. Appl. Environ. Microbiol. 67:4335–4337. 10.1128/AEM.67.9.4335-4337.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tóth I, Schmidt H, Dow M, Malik A, Oswald E, Nagy B. 2003. Transduction of porcine enteropathogenic Escherichia coli with a derivative of a Shiga toxin 2-encoding bacteriophage in a porcine ligated ileal loop system. Appl. Environ. Microbiol. 69:7242–7247. 10.1128/AEM.69.12.7242-7247.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solheim HT, Sekse C, Urdahl AM, Wasteson Y, Nesse LL. 2013. Biofilm as an environment for dissemination of stx genes by transduction. Appl. Environ. Microbiol. 79:896–900. 10.1128/AEM.03512-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchholz U, Bernard H, Werber D, Böhmer MM, Remschmidt C, Wilking H, Deleré Y, an der Heiden M, Adlhoch C, Dreesman J, Ehlers J, Ethelberg S, Faber M, Frank C, Fricke G, Greiner M, Höhle M, Ivarsson S, Jark U, Kirchner M, Koch J, Krause G, Luber P, Rosner B, Stark K, Kühne M. 2011. German outbreak of Escherichia coli O104:H4 associated with sprouts. N. Engl. J. Med. 365:1763–1770. 10.1056/NEJMoa1106482 [DOI] [PubMed] [Google Scholar]
- 31.King LA, Nogareda F, Weill FX, Mariani-Kurkdjian P, Loukiadis E, Gault G, Jourdan-DaSilva N, Bingen E, Macé M, Thevenot D, Ong N, Castor C, Noël H, Van Cauteren D, Charron M, Vaillant V, Aldabe B, Goulet V, Delmas G, Couturier E, Le Strat Y, Combe C, Delmas Y, Terrier F, Vendrely B, Rolland P, de Valk H. 2012. Outbreak of Shiga toxin-producing Escherichia coli O104:H4 associated with organic fenugreek sprouts, France, June 2011. Clin. Infect. Dis. 54:1588–1594. 10.1093/cid/cis255 [DOI] [PubMed] [Google Scholar]
- 32.Bielaszewska M, Mellmann A, Zhang W, Köck R, Fruth A, Bauwens A, Peters G, Karch H. 2011. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect. Dis. 11:671–676. 10.1016/S1473-3099(11)70165-7 [DOI] [PubMed] [Google Scholar]
- 33.Laing CR, Zhang Y, Gilmour MW, Allen V, Johnson R, Thomas JE, Gannon VP. 2012. A comparison of Shiga-toxin 2 bacteriophage from classical enterohemorrhagic Escherichia coli serotypes and the German E. coli O104:H4 outbreak strain. PLoS One 7:e37362. 10.1371/journal.pone.0037362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muniesa M, Hammerl JA, Hertwig S, Appel B, Brüssow H. 2012. Shiga toxin-producing Escherichia coli O104:H4: a new challenge for microbiology. Appl. Environ. Microbiol. 78:4065–4073. 10.1128/AEM.00217-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beutin L, Hammerl JA, Reetz J, Strauch E. 2013. Shiga toxin-producing Escherichia coli strains from cattle as a source of the Stx2a bacteriophages present in enteroaggregative Escherichia coli O104:H4 strains. Int. J. Med. Microbiol. 303:595–602. 10.1016/j.ijmm.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 36.Rode TM, Axelsson L, Granum PE, Heir E, Holck A, L'Abée-Lund TM. 2011. High stability of Stx2 phage in food and under food-processing conditions. Appl. Environ. Microbiol. 77:5336–5341. 10.1128/AEM.00180-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subils T, Aquili V, Ebner G, Balagué C. 2012. Effect of preservatives on Shiga toxigenic phages and Shiga toxin of Escherichia coli O157:H7. J. Food Prot. 75:959–965. 10.4315/0362-028X.JFP-11-332 [DOI] [PubMed] [Google Scholar]
- 38.Mauro SA, Opalko A, Lindsay K, Colon MP, Koudelka GB. 2013. The microcosm mediates the persistence of Shiga toxin-producing Escherichia coli in freshwater ecosystems. Appl. Environ. Microbiol. 79:4821–4828. 10.1128/AEM.01281-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez JM, Sherr EB, Sherr BF. 1990. Size-selective grazing on bacteria by natural assemblages of estuarine flagellates and ciliates. Appl. Environ. Microbiol. 56:583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wigginton KR, Pecson BM, Sigstam T, Bosshard F, Kohn T. 2012. Virus inactivation mechanisms: impact of disinfectants on virus function and structural integrity. Environ. Sci. Technol. 46:12069–12078. 10.1021/es3029473 [DOI] [PubMed] [Google Scholar]
- 41.Plunkett G, III, Rose DJ, Durfee TJ, Blattner FR. 1999. Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7: Shiga toxin as a phage late-gene product. J. Bacteriol. 181:1767–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuchs S, Mühldorfer I, Donohue-Rolfe A, Kerényi M, Emödy L, Alexiev R, Nenkov P, Hacker J. 1999. Influence of RecA on in vivo virulence and Shiga toxin 2 production in Escherichia coli pathogens. Microb. Pathog. 27:13–23. 10.1006/mpat.1999.0279 [DOI] [PubMed] [Google Scholar]
- 43.de Sablet T, Bertin Y, Vareille M, Girardeau JP, Garrivier A, Gobert AP, Martin C. 2008. Differential expression of stx2 variants in Shiga toxin-producing Escherichia coli belonging to seropathotypes A and C. Microbiology 154:176–186. 10.1099/mic.0.2007/009704-0 [DOI] [PubMed] [Google Scholar]
- 44.O'Brien AD, Newland JW, Miller SF, Holmes RK, Smith HW, Formal SB. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694–696. 10.1126/science.6387911 [DOI] [PubMed] [Google Scholar]
- 45.Ackermann HW, Tremblay D, Moineau S. 2004. Long-term bacteriophage preservation. WFCC Newsl. 38:35–40 [Google Scholar]
- 46.Mocé-Llivina L, Muniesa M, Pimenta-Vale H, Lucena F, Jofre J. 2003. Survival of bacterial indicator species and bacteriophages after thermal treatment of sludge and sewage. Appl. Environ. Microbiol. 69:1452–1456. 10.1128/AEM.69.3.1452-1456.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunault C, Pourcher AM, Burton CH. 2011. Using temperature and time criteria to control the effectiveness of continuous thermal sanitation of piggery effluent in terms of set microbial indicators. J. Appl. Microbiol. 111:1492–1504. 10.1111/j.1365-2672.2011.05144.x [DOI] [PubMed] [Google Scholar]
- 48.Tartera C, Bosch A, Jofre J. 1988. The inactivation of bacteriophages infecting Bacteroides fragilis by chlorine treatment and UV-irradiation. FEMS Microbiol. Lett. 56:313–316. 10.1111/j.1574-6968.1988.tb03198.x [DOI] [Google Scholar]
- 49.Lee HS, Sobsey MD. 2011. Survival of prototype strains of somatic coliphage families in environmental waters and when exposed to UV low-pressure monochromatic radiation or heat. Water Res. 45:3723–3734. 10.1016/j.watres.2011.04.024 [DOI] [PubMed] [Google Scholar]
- 50.Dee SW, Fogelman JC. 1992. Rates of inactivation of waterborne coliphages by monochloramine. Appl. Environ. Microbiol. 58:3136–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faruque SM, Asadulghani, Rahman MM, Waldor MK, Sack DA. 2000. Sunlight-induced propagation of the lysogenic phage encoding cholera toxin. Infect. Immun. 68:4795–4801. 10.1128/IAI.68.8.4795-4801.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]