Abstract
The aim of this study is to provide understanding of microgravity effects on important food-borne bacteria, Escherichia coli O157:H7 ATCC 35150, ATCC 43889, and ATCC 43895, cultured in nutrient-rich or minimal medium. Physiological characteristics, such as growth (measured by optical density and plating), cell morphology, and pH, were monitored under low-shear modeled microgravity (LSMMG; space conditions) and normal gravity (NG; Earth conditions). In nutrient-rich medium, all strains except ATCC 35150 showed significantly higher optical density after 6 h of culture under LSMMG conditions than under NG conditions (P < 0.05). LSMMG-cultured cells were approximately 1.8 times larger than NG-cultured cells at 24 h; therefore, it was assumed that the increase in optical density was due to the size of individual cells rather than an increase in the cell population. The higher pH of the NG cultures relative to that of the LSMMG cultures suggests that nitrogen metabolism was slower in the latter. After 24 h of culturing in minimal media, LSMMG-cultured cells had an optical density 1.3 times higher than that of NG-cultured cells; thus, the higher optical density in the LSMMG cultures may be due to an increase in both cell size and number. Since bacteria actively grew under LSMMG conditions in minimal medium despite the lower pH, it is of some concern that LSMMG-cultured E. coli O157:H7 may be able to adapt well to acidic environments. These changes may be caused by changes in nutrient metabolism under LSMMG conditions, although this needs to be demonstrated in future studies.
INTRODUCTION
Microgravity, a major factor that represents the environmental conditions in space, induces various changes in organisms (1). The human immune system becomes weaker in space, and microgravity is thought to induce changes by affecting the distribution of body fluids as well as through other as-yet-unidentified mechanisms (2–6). A classic theoretical study of microorganisms in a “space environment” concluded that microgravity conditions had no discernible effect on cells less than 10 μm in diameter, including bacteria (7). However, recent studies show that microgravity conditions do have an effect on bacterial growth kinetics and stress resistance, both of which are increased under such conditions (8–12). Therefore, these robust pathogens could pose a threat to human health in space, particularly if they contaminate foods and infect immunocompromised individuals.
Opportunistic pathogens have been isolated despite efforts to control microorganisms within spacecraft (13–16). Previous studies show that microorganisms are ubiquitous in spacecraft, with crew members or ground-supplied materials being the predominant sources of bacterial contamination (13, 17, 18). Because such pathogens could multiply and contaminate other materials within a closed environment in which the air, water, and food supplies are recycled, they may pose a severe health risk. Subsequent studies of microorganisms in a space environment focused on Staphylococcus, Bacillus, and Pseudomonas; these bacteria are ubiquitous in the natural environment and may contaminate devices/surfaces and cause health-related problems (14, 19–21). However, few studies have examined food-borne pathogens under microgravity conditions, and none have studied Escherichia coli O157:H7, a food-borne pathogen that can cause serious illness and even death.
E. coli O157:H7 can contaminate food and water and cause hemorrhagic colitis and hemolytic-uremic syndrome (22–24). Several outbreaks of E. coli O157:H7 in acidic foods, such as apple cider and mayonnaise, have raised concerns about the acid tolerance of this pathogen (25–28). Several studies examined the survival of E. coli O157:H7 in acidic foods and identified acid-resistant and acid-adaptable strains (26, 29–32). The pathogen can survive under harsh conditions, and as few as 10 microorganisms have the potential to contaminate food/water consumed by crew members and cause gastrointestinal disease (22). Infectious diseases are difficult to deal with in a space environment; therefore, comprehensive studies on the behavior of this pathogen under microgravity conditions are warranted.
During space flight, bacteria may be exposed to different forms of environmental stress. One example is nutrient change. Microbiotas are often subjected to different nutrient conditions in the natural environment (33); therefore, examining the behavior of bacteria under those conditions in a space environment would be useful. Here, we examined changes in the physiological properties of three strains of E. coli O157:H7 (two acid-resistant strains and one acid-adaptable strain) cultured in rich Luria-Bertani (LB) medium or in nutrient-poor M9 minimal medium under simulated microgravity conditions. Low-shear modeled microgravity (LSMMG) and normal gravity (NG) conditions were simulated in the laboratory using devices specially designed by NASA: a high-aspect ratio vessel (HARV) rotary cell culture system. We then examined (i) bacterial growth, (ii) morphology, and (iii) the pH of the cell cultures under LSMMG and NG conditions.
MATERIALS AND METHODS
Preparation of bacterial strains.
All studies were performed using the E. coli O157:H7 strains ATCC 35150 (acid resistant; human feces isolate), ATCC 43889 (acid adaptable; human feces isolate), and ATCC 43895 (acid resistant; ground beef isolate), considering the various acid resistance and isolation source of the bacteria (32). All strains were obtained from the Food Microbiology Culture Collection at Korea University (Seoul, Republic of Korea) and stored at −20°C in LB medium (Difco, Sparks, MD) containing 20% glycerol. For the experiments, each strain was grown overnight in LB medium or in M9 minimal medium (Sigma-Aldrich, St. Louis, MO) containing 0.4% glucose (Sigma-Aldrich) at 37°C on a shaking incubator at 225 rpm (VS-8480S; Vision Scientific, Seoul, Republic of Korea). The turbidity of the culture was measured in a SmartSpec Plus spectrophotometer (Bio-Rad, Hercules, CA).
Simulation of low-shear modeled microgravity and normal gravity.
Static overnight cultures in LB medium or in M9 minimal medium containing 0.4% glucose were diluted 1:500 and 1:150, respectively, in fresh medium to achieve an optical density at 600 nm (OD600) of 0.01. To produce LSMMG and NG conditions, the diluted cell cultures were introduced into a 50-ml HARV apparatus (Synthecon, Houston, TX). The HARV supplies a gentle “orbit” of fluid to the bacterial cells such that they can be maintained under low-shear and low-turbulence conditions (34). The filled HARV (with zero headspace) was rotated at 25 rpm around a horizontal axis to generate LSMMG conditions (Fig. 1A), of which gravitational force was reduced by centrifugal and other force and around a vertical axis to generate NG conditions (Fig. 1B). All incubations were performed at 37°C. A gas-permeable membrane of the HARV allowed constant air exchange during the bacterial growth.
FIG 1.
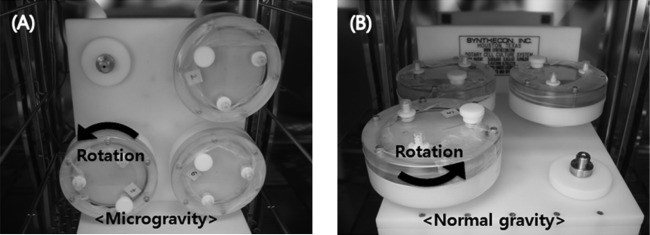
Operating orientations of the HARV apparatus. (A) Under the low-shear modeled microgravity (LSMMG) condition, the axis of rotation of the HARV is vertical to the gravity force vector. (B) Under the normal gravity (NG) condition, the axis of rotation is horizontal to the gravity force vector.
Growth measurements. (i) Optical density measurements.
A sterile 5-ml syringe was used to remove 1 ml of cell culture through a port on the front plate of the HARV after 0, 3, 6, 12, 24, and 48 h of culture. Zero headspace within the HARV was maintained by adding an equal amount of M9 minimal medium with no glucose. Bacterial growth was monitored by measuring the OD600 (Bio-Rad). Each experiment was repeated six times.
(ii) Plate counting method.
The plate counting method was used to determine the cell populations under LSMMG and NG conditions. Briefly, 1 ml of cell culture was removed from each vessel after 6 and 24 h of cultivation, which represent the mid-exponential and stationary growth phases, respectively. The samples were serially diluted 10-fold in M9 minimal medium with no glucose. The diluted samples (100 μl) were spread plated on duplicate LB agar plates and incubated for 24 h at 37°C. Colonies were counted and bacterial counts were expressed as log CFU/ml. Each experiment was repeated six times.
Scanning electron microscopy.
Morphological changes in E. coli O157:H7 cells cultured under LSMMG and NG conditions were observed by scanning electron microscopy (SEM). Cells were cultured in LB or M9 minimal medium for 24 h, removed from the vessel, and then fixed by overnight immersion in modified Karnovsky's fixative (2% paraformaldehyde and 2% glutaraldehyde in 0.05 M sodium cacodylate buffer) (35). After fixation, bacterial cells were collected on polycarbonate membranes (Whatman Nuclepore Track-Etch, 25 mm, 0.2-μm pore size; Dassel, Germany), which were placed in filter funnels and then washed three times in 0.05 M sodium cacodylate buffer. The samples were postfixed in 2% osmium tetroxide in 0.1 M cacodylate buffer for 2 h and then dehydrated through a graded series of ethanol solutions (30, 50, 70, 80, 90, and 100%; three times for 10 min in each solution). Bacterial cells on the polycarbonate membranes were immersed twice in 100% hexamethyldisilazane (each for 10 min) and dried for 2 h. The dried membranes were then mounted on stubs, coated with gold, and examined by SEM (JSM 5410LV; JEOL, Tokyo, Japan). SEM image analysis of each bacterial cell (n = 100 per group; 10 bacterial cells were randomly selected at 10 spots) was performed using ImageJ software (U.S. National Institutes of Health, Bethesda, MD; http://rsbweb.nih.gov/ij/) and normalized according to diameter (μm) and area (μm2).
pH measurements.
Changes in the pH of the cell cultures were measured with an S20 SevenEasy pH meter (Mettler Toledo, Schwerzenbach, Switzerland). The pH meter was sequentially calibrated with commercially available two-point standard buffer solutions at pH 7.00 and pH 4.01 (Mettler Toledo). Samples were removed from the cultures over the course of 48 h (0, 3, 6, 12, 24, and 48 h), and the pH was measured at room temperature.
Statistical analysis.
SAS software version 9.1 (SAS Institute, Inc., Cary, NC) was used for all data analysis. Data were evaluated using a general linear model for variance analysis. Tukey's test was used to determine the significance of the differences (P < 0.05, P < 0.01, and P < 0.001) in bacterial growth and pH in the tested E. coli O157:H7 cultures under LSMMG and NG conditions.
RESULTS
Growth kinetics of E. coli O157:H7 in nutrient-rich medium.
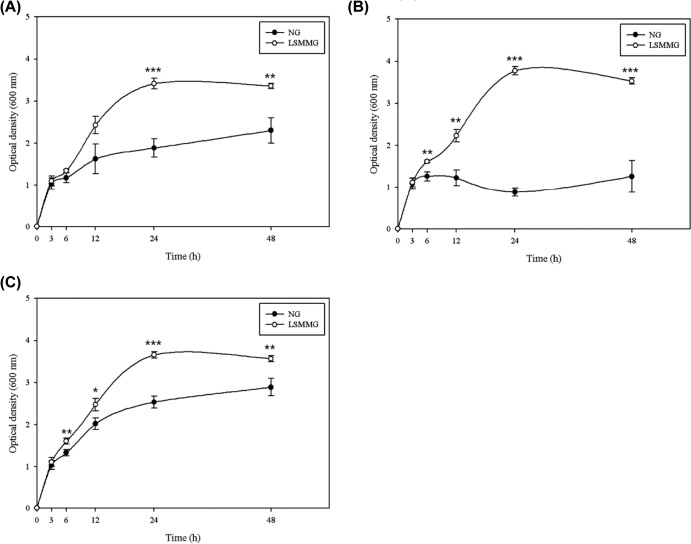
The growth curves of bacteria cultured in nutrient-rich LB medium are plotted based on the optical density at 600 nm (Fig. 2). In general, the growth patterns of E. coli O157:H7 cultured under different gravity conditions were not significantly different for the first 3 h of cultivation (P > 0.05); however, there was a marked difference at later times. While there was no significant difference in growth between E. coli O157:H7 ATCC 35150 cultured under LSMMG and NG conditions at 6 to 12 h, the growth of strains ATCC 43889 and ATCC 43895 was significantly greater under LSMMG conditions at 6 (both P < 0.01) and at 12 h (P < 0.01 for ATCC 43889 and P < 0.05 for ATCC 43895). After 24 h, the optical density remained constant, indicating that the cells had reached the stationary phase (the exception was ATCC 43889 cultured under NG conditions, which reached the stationary phase after 6 h). Of the three strains of E. coli O157:H7 tested, ATCC 43889 showed the greatest difference in growth under LSMMG and NG conditions, which appeared to be due to the very low optical density values when cultured under NG conditions (Fig. 1B). All strains achieved high optical densities after 24 h of culture under LSMMG conditions (LSMMG, 3.42 to 3.77; NG, 0.88 to 2.54; P < 0.001). At the end of the culture period (48 h), there were marked differences in the growth of bacteria cultured under LSMMG and NG conditions (LSMMG, 3.36 to 3.57; NG, 1.26 to 2.89; P < 0.01 for ATCC 35150 and 43895 and P < 0.001 for ATCC 43889). The viability of the cells cultured in LB medium was examined using a conventional plate counting method (Table 1). Unlike the results obtained using optical density measurements, there was no significant difference of the viable population between LSMMG and NG conditions at any time point (P > 0.05). However, the number of bacterial cells under LSMMG conditions tended to be greater than that under NG conditions. After 6 h of culture, the cell populations under LSMMG and NG conditions were 9.29 to 9.51 and 9.18 to 9.27 log CFU/ml, respectively. After 24 h, these increased to 9.78 to 10.0 log CFU/ml under LSMMG conditions and to 9.65 to 9.90 log CFU/ml under NG conditions.
FIG 2.
Growth of E. coli O157:H7 ATCC 35150 (A), ATCC 43889 (B), and ATCC 43895 (C) strains cultured under LSMMG and NG conditions in LB medium. Data represent the means ± standard errors (SE) from six independent experiments. Optical density measurements under LSSMG conditions were significantly higher than those under NG conditions (*, P < 0.05; **, P < 0.01; and ***, P < 0.001).
TABLE 1.
Log cell numbers of E. coli O157:H7 ATCC 35150, ATCC 43889, and ATCC 43895 cultured under LSMMG and NG conditions for 6 and 24 h in LB medium or M9 minimal medium containing 0.4% glucose
| Medium | Time (h) | Log CFU/mla |
|||||
|---|---|---|---|---|---|---|---|
|
E. coli O157:H7 ATCC 35150 |
E. coli O157:H7 ATCC 43889 |
E. coli O157:H7 ATCC 43895 |
|||||
| LSMMG | NG | LSMMG | NG | LSMMG | NG | ||
| LB | 0 | 6.87 ± 0.11 A | 6.87 ± 0.11 A | 6.43 ± 0.14 A | 6.43 ± 0.14 A | 6.93 ± 0.24 A | 6.93 ± 0.24 A |
| 6 | 9.51 ± 0.14 B | 9.27 ± 0.07 B | 9.34 ± 0.10 B | 9.19 ± 0.05 B | 9.29 ± 0.08 B | 9.18 ± 0.07 B | |
| 24 | 9.78 ± 0.06 B | 9.76 ± 0.11 C | 9.93 ± 0.31 B | 9.65 ± 0.33 B | 10.00 ± 0.12 C | 9.90 ± 0.15 C | |
| M9 and 0.4% glucose | 0 | 5.62 ± 0.30 A | 5.62 ± 0.30 A | 5.64 ± 0.23 A | 5.64 ± 0.23 A | 5.69 ± 0.27 A | 5.69 ± 0.27 A |
| 6 | 8.76 ± 0.05 B | 8.72 ± 0.05 B | 8.76 ± 0.04 B | 8.65 ± 0.07 B | 8.57 ± 0.10 B | 8.47 ± 0.11 B | |
| 24 | 9.09 ± 0.04 BD | 8.78 ± 0.07 BE | 9.12 ± 0.04 CD | 8.95 ± 0.07 BE | 9.13 ± 0.02 CD | 8.89 ± 0.04 CE | |
Means ± SE; n = 6 samples for each gravity condition. A to C, values in the same column for each medium are significantly different (P < 0.05); D and E, values in the same row for each strain are significantly different (P < 0.05).
Growth kinetics of E. coli O157:H7 cultured in minimal medium.
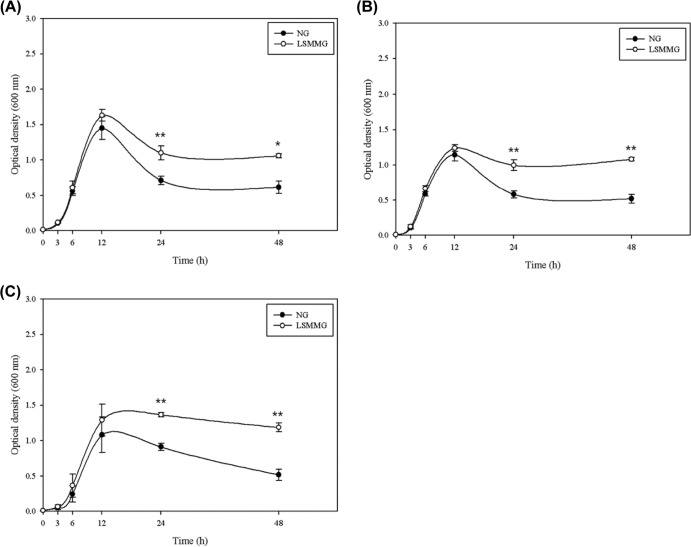
The growth curves for bacteria cultured in M9 minimal medium containing 0.4% glucose are shown in Fig. 3. There were no significant differences in the exponential growth of E. coli O157:H7 strains cultured under different gravity conditions (from 0 to 12 h) (P > 0.05). However, differences between LSMMG and NG conditions were observed after 12 h (stationary phase). At 24 h, cells cultured under LSMMG conditions reached a higher optical density than cells cultured under NG conditions (LSMMG, 1.00 to 1.37; NG, 0.58 to 0.91; P < 0.01). A similar pattern was seen after 48 h (LSMMG, 1.06 to 1.19; NG, 0.52 to 0.62; P < 0.05 for ATCC 35150 and P < 0.01 for all others).
FIG 3.
Growth of E. coli O157:H7 ATCC 35150 (A), ATCC 43889 (B), and ATCC 43895 (C) strains under LSMMG and NG conditions in M9 minimal medium containing 0.4% glucose. Data represent the means ± standard errors (SE) from six independent experiments. Optical density measurements under LSSMG conditions were significantly higher than those under NG conditions (*, P < 0.05; **, P < 0.01).
The viability of the cells cultured in M9 minimal medium was also examined using a conventional plate-counting method (Table 1). There was no difference in the number of viable bacteria at 6 h between LSMMG and NG conditions; however, the numbers of viable cells after culture for 24 h under LSMMG conditions were significantly greater than those after culture under NG conditions (LSMMG, 9.09 to 9.13 log CFU/ml; NG, 8.47 to 8.95 log CFU/ml; P < 0.05). The difference between LSMMG- and NG-cultured cells at 24 h was 0.17 to 0.31 log CFU/ml, which means that the amount of bacterial cells under LSMMG conditions was twice that under NG conditions.
Bacterial morphology visualized by scanning electron microscopy.
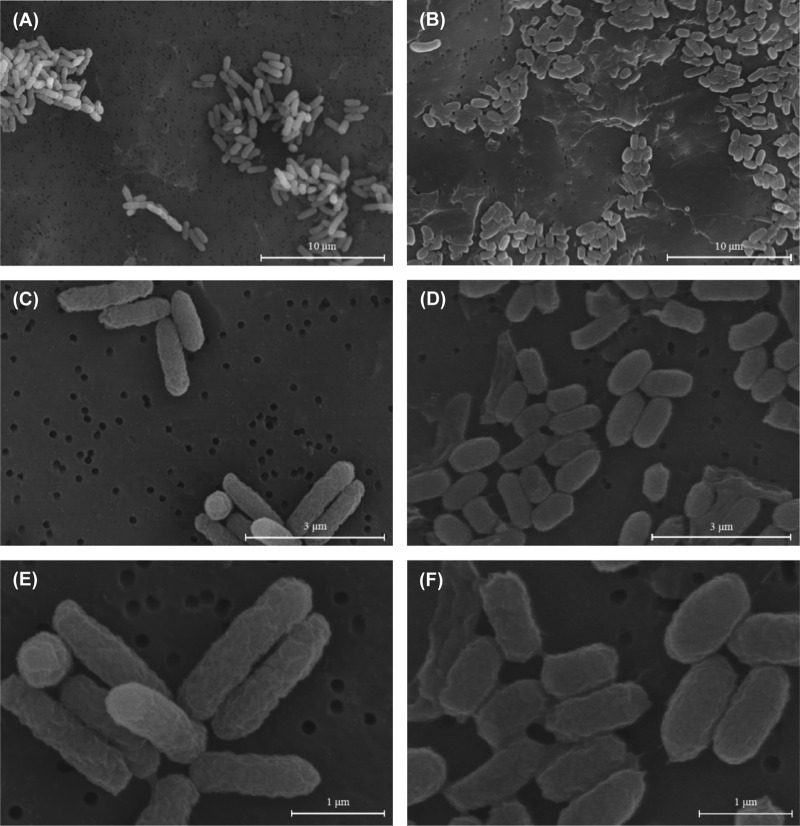
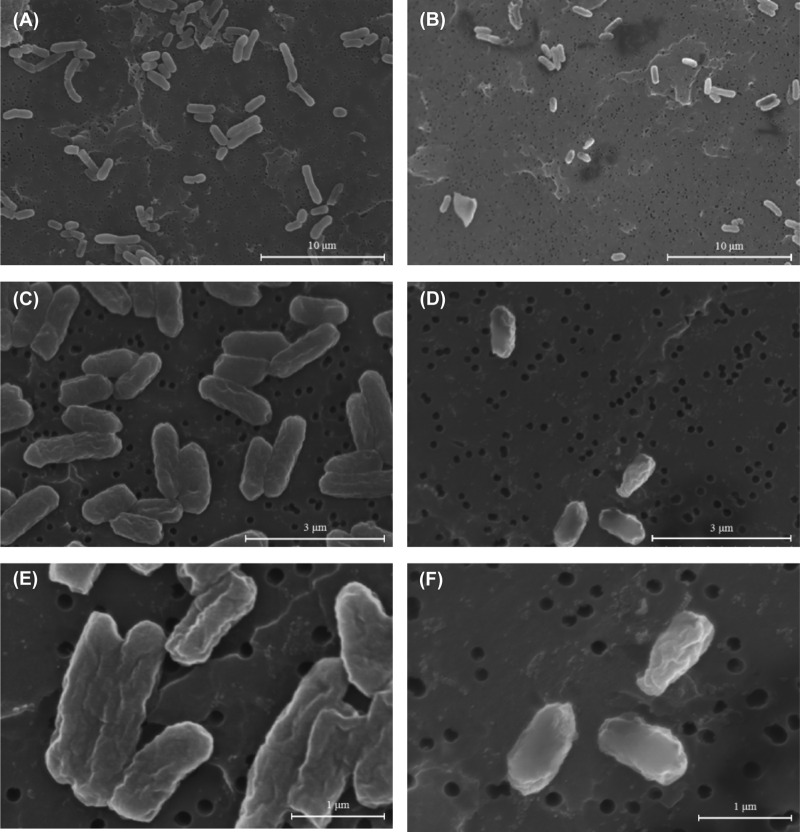
We performed SEM analysis to examine changes in the morphology of bacteria cultured under LSMMG and NG conditions. Bacterial cells were cultured in LB medium or in M9 minimal medium under LSMMG and NG conditions for 24 h (regarded as the initial stationary phase). The SEM images of E. coli O157:H7 cells under LSMMG conditions revealed a notable difference in morphology compared with that of NG-cultured cells (Fig. 4 and 5). Bacterial cells cultured in LB medium under LSMMG conditions adopted a longish rod shape, whereas their NG counterparts were smaller and oval shaped. Cells cultured in M9 minimal medium showed shrunken cell membranes due to the lower pH (this was not observed in cells cultured in LB medium). In addition, cells cultured under LSMMG conditions tended to be larger than those cultured under NG conditions. To obtain a more meaningful comparison of cell sizes, we randomly selected bacterial cells at 10 different spots (n = 100 per group) and analyzed them using the ImageJ program (Table 2). Bacterial cells cultured in LB medium under LSMMG conditions were on average 1.84 μm (1.41 to 2.30 μm) in diameter, with an area of 0.77 μm2 (0.59 to 1.06 μm2). Cells cultured under NG conditions were on average 0.97 μm (0.76 to 1.20 μm) in diameter, with an area of 0.43 μm2 (0.33 to 0.54 μm2). Cells cultured in M9 minimal medium under LSMMG conditions had an average diameter of 1.49 μm (1.22 to 1.75 μm) and an area of 0.84 μm2 (0.57 to 1.10 μm2), whereas those cultured under NG conditions had an average diameter of 1.18 μm (0.93 to 1.51 μm) and an area of 0.63 μm2 (0.47 to 1.10 μm2).
FIG 4.
Scanning electron micrograph of E. coli O157:H7 ATCC 35150 cultured under LSMMG conditions (magnification, ×4,000 [A], ×15,000 [C], and ×30,000 [E]) and under NG conditions (magnification, ×4,000 [B], ×15,000 [D], and ×30,000 [F]) for 24 h in LB medium. Bars in panels A and B, 10 μm; panels C and D, 3 μm; and panels E and F, 1 μm.
FIG 5.
Scanning electron micrograph of E. coli O157:H7 ATCC 35150 cultured under LSMMG conditions (magnification, ×4,000 [A], ×15,000 [C], and ×30,000 [E]) and under NG conditions (magnification, ×4,000 [B], ×15,000 [D], and ×30,000 [F]) for 24 h in M9 minimal medium containing 0.4% glucose. Bars in panels A and B, 10 μm; panels C and D, 3 μm; and panels E and F, 1 μm.
TABLE 2.
Cell size of E. coli O157:H7 ATCC 35150 cultured under LSMMG and NG conditions in LB medium or M9 minimal medium containing 0.4% glucosea
| Medium | Condition | Diameter (μm) |
Area (μm2) |
||||
|---|---|---|---|---|---|---|---|
| Min | Max | Avg | Min | Max | Avg | ||
| LB | LSMMG | 1.41 | 2.30 | 1.84 ± 0.28 A | 0.59 | 1.06 | 0.77 ± 0.15 A |
| NG | 0.76 | 1.20 | 0.97 ± 0.15 B | 0.33 | 0.54 | 0.43 ± 0.07 B | |
| M9 and 0.4% glucose | LSMMG | 1.22 | 1.75 | 1.49 ± 0.18 A | 0.57 | 1.10 | 0.84 ± 0.18 A |
| NG | 0.93 | 1.51 | 1.18 ± 0.19 B | 0.47 | 1.10 | 0.63 ± 0.21 B | |
Means ± SD; n = 100 samples for each gravity condition. A and B, values in the same column for each medium are significantly different (P < 0.05).
pH measurements.
Changes in pH of the cultures were measured over the time of cultivation in the HARV bioreactors (Table 3). The initial pH of the cell cultures in LB medium was 7.06 to 7.09, indicating neutral conditions. The pH of the culture medium decreased during the first 3 h of culture (LSMMG, 6.29 to 6.54; NG, 6.35 to 6.67). The only significant difference in pH between LSMMG and NG conditions was noted for ATCC 43895 (P < 0.05). After 3 h, the pH of the cultures gradually increased, with the gap between the LSMMG and NG cultures widening up until 6 h. The pH under LSMMG conditions was significantly lower than that under NG conditions at 6 and 12 h (P < 0.05). However, this trend was reversed after 24 h, i.e., the pH of the LSMMG cultures was higher at the end of the culture period (LSMMG, 8.25 to 8.35; NG, 8.04 to 8.19; P < 0.05).
TABLE 3.
Changes in pH during the growth of E. coli O157:H7 ATCC 35150, ATCC 43889, and ATCC 43895 under LSMMG and NG in LB medium
| Time (h) | pHa |
|||||
|---|---|---|---|---|---|---|
| ATCC 35150 |
ATCC 43889 |
ATCC 43895 |
||||
| LSMMG | NG | LSMMG | NG | LSMMG | NG | |
| 0 | 7.08 ± 0.01 D | 7.08 ± 0.01 C | 7.09 ± 0.01 D | 7.09 ± 0.01 D | 7.06 ± 0.04 D | 7.06 ± 0.04 C |
| 3 | 6.29 ± 0.11 F | 6.35 ± 0.10 D | 6.37 ± 0.03 F | 6.42 ± 0.04 E | 6.54 ± 0.03 EG | 6.67 ± 0.04 DH |
| 6 | 6.86 ± 0.07 EG | 7.12 ± 0.07 CH | 6.86 ± 0.01 EG | 7.15 ± 0.05 DH | 6.98 ± 0.10 DG | 7.24 ± 0.10 CH |
| 12 | 7.44 ± 0.03 CG | 7.75 ± 0.06 BH | 7.44 ± 0.02 CG | 7.68 ± 0.12 CH | 7.50 ± 0.06 CG | 7.67 ± 0.07 BH |
| 24 | 8.01 ± 0.01 BG | 7.98 ± 0.01 AH | 7.97 ± 0.06 B | 7.95 ± 0.09 B | 8.08 ± 0.04 BG | 7.95 ± 0.06 AH |
| 48 | 8.25 ± 0.04 AG | 8.04 ± 0.12 AH | 8.31 ± 0.01 AG | 8.19 ± 0.08 AH | 8.35 ± 0.01 AG | 8.11 ± 0.14 AH |
Means ± SD; n = 3 samples for each gravity condition. A to F, values in the same column are significantly different (P < 0.05); G and H, values in the same row for each strain are significantly different (P < 0.05).
Changes in the pH of the cultures grown in M9 minimal medium are shown in Table 4. The initial pH was 7.01 to 7.06, indicating neutral conditions. The pH of the LSMMG cultures decreased up until 24 h (pH 5.11 to 5.50) before increasing again up until 48 h; the exception was ATCC 35150 cultured under LSMMG conditions, in which the pH was maintained after 24 h. The pH of the NG cultures decreased up until 12 h (pH 5.45 to 5.94) and then remained at this level throughout the experiment. There were significant differences between the pH values of the LSMMG and NG cultures at 24 h (P < 0.05); LSMMG cultures showed significantly lower pH values than NG cultures. Although the pH of the LSMMG cultures at 48 h was not significantly different from that of the NG cultures (LSMMG, 5.47 ± 0.01; NG, 5.70 ± 0.15), it tended to be lower in the LSMMG cultures.
TABLE 4.
Changes in pH during the growth of E. coli O157:H7 ATCC 35150, ATCC 43889, and ATCC 43895 under LSMMG and NG conditions in M9 minimal medium containing 0.4% glucose
| Time (h) | pHa |
|||||
|---|---|---|---|---|---|---|
| ATCC 35150 |
ATCC 43889 |
ATCC 43895 |
||||
| LSMMG | NG | LSMMG | NG | LSMMG | NG | |
| 0 | 7.06 ± 0.01 A | 7.06 ± 0.01 A | 7.04 ± 0.01 A | 7.04 ± 0.01 A | 7.01 ± 0 A | 7.01 ± 0 A |
| 3 | 7.07 ± 0.01 A | 7.06 ± 0.01 A | 7.02 ± 0.01 A | 7.02 ± 0.02 A | 7.02 ± 0.02 A | 7.00 ± 0.03 A |
| 6 | 6.90 ± 0.07 A | 6.90 ± 0.02 A | 6.83 ± 0.01 B | 6.84 ± 0.03 A | 6.97 ± 0.01 AE | 7.02 ± 0.02 AF |
| 12 | 5.52 ± 0.32 B | 5.45 ± 0.17 B | 5.49 ± 0.11 C | 5.60 ± 0.11 B | 5.88 ± 0.13 B | 5.94 ± 0.33 B |
| 24 | 5.11 ± 0.03 CE | 5.38 ± 0.11 BF | 5.22 ± 0.02 DE | 5.54 ± 0.12 BF | 5.50 ± 0.03 CE | 5.67 ± 0.06 BF |
| 48 | 5.30 ± 0.03 BC | 5.45 ± 0.11 B | 5.47 ± 0.01 CE | 5.70 ± 0.15 BF | 5.72 ± 0.06 B | 5.81 ± 0.11 B |
Means ± SD; n = 3 samples for each gravity condition. A to D, values in the same column are significantly different (P < 0.05); E and F, values in the same row for each strain are significantly different (P < 0.05).
DISCUSSION
Several studies have examined bacterial growth under microgravity conditions (20, 36–42). The early study of microbes in a space environment reported that the growth of E. coli was no different in space than on Earth (36). Gasset et al. showed that the generation time of E. coli grown under microgravity conditions was slightly reduced compared with that of their NG counterparts, but there were no changes in bacterial cell size (37). Subsequent investigations of E. coli under LSMMG conditions reported a shortened lag phase, a longer exponential phase, and a doubling of the final cell population (38–41). Bacillus subtilis showed properties similar to those of E. coli, i.e., bacterial growth started earlier and was faster than that of the control (20). Wilson et al. also reported that LSMMG conditions reduced the generation time of Salmonella cultured in M9 minimal medium (42).
Here, we showed that three strains of E. coli O157:H7 grew better under LSMMG conditions than under NG conditions. The growth rates of bacterial cells cultured in LB and M9 minimal media under LSMMG and NG conditions differed after 3 and 12 h, respectively, with the optical density of LSMMG cultures being much higher than that of NG cultures. These results suggest that E. coli O157:H7 might grow more vigorously under microgravity conditions regardless of the nutrient conditions. Of the three strains of E. coli O157:H7 tested, E. coli O157:H7 ATCC 43889 produced a different growth curve in LB medium under NG conditions. The bacteria reached the stationary state much earlier under NG conditions, showing much lower optical densities than cells cultured under LSMMG conditions. However, this result was not confirmed by the plate counts.
Optical density is a measure of (i) bacterial population and (ii) the size of individual cells. Under LSMMG conditions, the cell plating experiments did not confirm the large increase in optical density when cultured in LB medium. We assume that this must be due to an increase in the size of individual cells. The optical density of E. coli O157:H7 ATCC 35150 cultured under LSMMG conditions for 24 h was twice that of cells cultured under NG conditions; however, the plate counts were not significantly different (P > 0.05). As shown in Table 2, cells cultured under LSMMG conditions were approximately 1.8 times larger than those cultured under NG conditions; thus, the higher optical density of the LSMMG cultures may be due to an increase in cell size. Similar morphological changes observed in other strains (data not shown) and higher LSMMG/NG ratios of optical density support the assumption.
A second assumption for the effects of microgravity on E. coli O157:H7 concerns active bacterial growth under microgravity in the minimal media. Both the optical density and number of CFU/ml of E. coli O157:H7 were significantly higher under LSMMG conditions than under NG conditions. In the case of ATCC 35150, the optical density under LSMMG conditions was 1.5 times that under NG conditions, and the plate counts were twice as high. Cells cultured under LSMMG conditions were 1.3 times larger than those cultured under NG conditions. Thus, we believe that the size of the cell population and the morphological characteristics of the bacteria themselves might have resulted in the high optical density when cultured in M9 medium under LSMMG conditions. The reasons for these changes may be related to the different metabolic responses of E. coli O157:H7 under different culture conditions. LB medium is a nutrient-rich medium, and the main ingredients are tryptone, yeast extract, and NaCl. M9 is a minimal medium often used for direct comparison with LB medium.
In the present study, changes in pH due to bacterial metabolism were observed under different nutrient conditions. When cultured in LB medium, bacteria metabolize carbohydrates first; thus, the pH of the culture falls due to the production of acidic by-products, such as acetate (43). Since LB medium has a low carbohydrate concentration, cells metabolize amino acids, which makes the medium more alkaline (44). During the first 3 h of culture in LB medium, E. coli O157:H7 metabolized carbohydrate and the pH of the cultures decreased. There was no difference between the pH of the medium under LSMMG and NG conditions during this period. We assume that the similarity in pHs may be due to the cells not being exposed to microgravity for a sufficient length of time for it to affect carbohydrate metabolism. The higher pH of the NG cultures after 3 to 6 h suggests that nitrogen metabolism by cells during the exponential phase of growth under LSMMG conditions was slower than that of cells cultured under NG conditions (Table 3). As the trend of pH was reversed after 24 h, it is assumed that the initial slow nitrogen metabolism of LSMMG cultures caused there to be a lot of nitrogen left in the cultures, and then E. coli O157:H7 may have metabolized the nutrients actively to maintain its large size.
When E. coli O157:H7 cells were cultured in M9 minimal medium, the bacteria metabolized glucose and produced acidic by-products; therefore, the pH of the cultures fell (Table 4). There was no difference in the pH of cultures under NG or LSMMG conditions. These results suggest that microgravity has little effect on sugar metabolism by E. coli O157:H7. At the end of the exponential phase, the bacteria encounter a stressful environment due to a lack of nutrients, the accumulation of waste materials, and increased cell mass (45). In M9 minimal medium, the acidic conditions resulting from glucose metabolism may act as a stress factor that inhibits bacterial growth, even if nutrients are still present in the medium. Despite the lower pH of the LSMMG cultures, the optical density and CFU/ml were much higher than those of cultures grown under NG conditions. This may support the hypothesis that cells cultured under LSMMG conditions may be more resistant to acidic conditions and that bacterial cells could survive by utilizing the remaining nutrients. However, the stress resistance of E. coli O157:H7 under LSMMG conditions needs further investigation if we are to obtain clear evidence to support this hypothesis.
To summarize, the present study found that bacterial cells cultured under microgravity conditions increased in size. To the best of our knowledge, no studies have reported changes in the size and shape of pathogenic bacteria cultured under LSMMG conditions. These findings further strengthen the notion that LSMMG enhances the growth of bacterial cells. It is thought that microgravity affects the metabolism of nutrients by E. coli O157:H7. However, the changes in pH observed in the present study are not sufficient to fully reflect changes in bacterial metabolism, and further studies are needed to identify the specific mechanism(s) underlying nutrient metabolism under these conditions. Such studies may provide scientific clues that will contribute to further understanding of the physiology of E. coli O157:H7, particularly under conditions that mimic a space environment.
ACKNOWLEDGMENTS
This study was supported by the Korea Research Foundation (NRF-2011-0012712).
We thank the Institute of Biomedical Science and Food Safety at Korea University for providing the equipment and facilities.
Footnotes
Published ahead of print 31 January 2014
REFERENCES
- 1.Guéguinou N, Huin-Schohn C, Bascove M, Bueb J-L, Tschirhart E, Legrand-Frossi C, Frippiat J-P. 2009. Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth's orbit? J. Leukoc. Biol. 86:1027–1038. 10.1189/jlb.0309167 [DOI] [PubMed] [Google Scholar]
- 2.Criswell-Hudak BS. 1991. Immune response during space flight. Exp. Gerontol. 26:289–296. 10.1016/0531-5565(91)90022-E [DOI] [PubMed] [Google Scholar]
- 3.Sonnenfeld G. 2005. The immune system in space, including Earth-based benefits of space-based research. Curr. Pharm. Biotechnol. 6:343–349. 10.2174/1389201054553699 [DOI] [PubMed] [Google Scholar]
- 4.Rykova M, Antropova E, Larina I, Morukov B. 2008. Humoral and cellular immunity in cosmonauts after the ISS missions. Acta Astronautica 63:697–705. 10.1016/j.actaastro.2008.03.016 [DOI] [Google Scholar]
- 5.Sonnenfeld G, Shearer WT. 2002. Immune function during space flight. Nutrition 18:899–903. 10.1016/S0899-9007(02)00903-6 [DOI] [PubMed] [Google Scholar]
- 6.Borchers AT, Keen CL, Gershwin ME. 2002. Microgravity and immune responsiveness: implications for space travel. Nutrition 18:889–898. 10.1016/S0899-9007(02)00913-9 [DOI] [PubMed] [Google Scholar]
- 7.Pollard EC. 1965. Theoretical studies on living systems in the absence of mechanical stress. J. Theor. Biol. 8:113–123. 10.1016/0022-5193(65)90097-4 [DOI] [PubMed] [Google Scholar]
- 8.Leys N, Hendrickx L, De Boever P, Baatout S, Mergeay M. 2004. Space flight effects on bacterial physiology. J. Biol. Regul. Homeost. Agents 18:193–199 [PubMed] [Google Scholar]
- 9.Nickerson CA, Ott CM, Mister SJ, Morrow BJ, Burns-Keliher L, Pierson DL. 2000. Microgravity as a novel environmental signal affecting Salmonella enterica serovar Typhimurium virulence. Infect. Immun. 68:3147–3152. 10.1128/IAI.68.6.3147-3152.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenzweig JA, Abogunde O, Thomas K, Lawal A, Nguyen Y-U, Sodipe A, Jejelowo O. 2010. Spaceflight and modeled microgravity effects on microbial growth and virulence. Appl. Microbiol. Biotechnol. 85:885–891. 10.1007/s00253-009-2237-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nickerson CA, Ott CM, Wilson JW, Ramamurthy R, LeBlanc CL, Höner zu Bentrup K, Hammond T, Pierson DL. 2003. Low-shear modeled microgravity: a global environmental regulatory signal affecting bacterial gene expression, physiology, and pathogenesis. J. Microbiol. Methods 54:1–11. 10.1016/S0167-7012(03)00018-6 [DOI] [PubMed] [Google Scholar]
- 12.Horneck G, Klaus DM, Mancinelli RL. 2010. Space microbiology. Microbiol. Mol. Biol. Rev. 74:121–156. 10.1128/MMBR.00016-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierson DL. 2007. Microbial contamination of spacecraft. Gravit. Space Biol. Bull. 14:1–6 [PubMed] [Google Scholar]
- 14.Novikova N. 2004. Review of the knowledge of microbial contamination of the Russian manned spacecraft. Microb. Ecol. 47:127–132. 10.1007/s00248-003-1055-2 [DOI] [PubMed] [Google Scholar]
- 15.La Duc M, Kern R, Venkateswaran K. 2004. Microbial monitoring of spacecraft and associated environments. Microb. Ecol. 47:150–158. 10.1007/s00248-003-1012-0 [DOI] [PubMed] [Google Scholar]
- 16.Novikova N, De Boever P, Poddubko S, Deshevaya E, Polikarpov N, Rakova N, Coninx I, Mergeay M. 2006. Survey of environmental biocontamination on board the International Space Station. Res. Microbiol. 157:5–12. 10.1016/j.resmic.2005.07.010 [DOI] [PubMed] [Google Scholar]
- 17.Puleo J, Oxborrow G, Fields N, Herring C, Smith L. 1973. Microbiological profiles of four Apollo spacecraft. Appl. Microbiol. 26:838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry CA. 1970. Summary of medical experience in the Apollo 7 through 11 manned spaceflights. Aerospace Med. 41:500. [PubMed] [Google Scholar]
- 19.Rosado H, Doyle M, Hinds J, Taylor PW. 2010. Low-shear modelled microgravity alters expression of virulence determinants of Staphylococcus aureus. Acta Astronautica 66:408–413. 10.1016/j.actaastro.2009.06.007 [DOI] [Google Scholar]
- 20.Mennigmann H, Lange M. 1986. Growth and differentiation of Bacillus subtilis under microgravity. Naturwissenschaften 73:415–417. 10.1007/BF00367283 [DOI] [PubMed] [Google Scholar]
- 21.England L, Gorzelak M, Trevors J. 2003. Growth and membrane polarization in Pseudomonas aeruginosa UG2 grown in randomized microgravity in a high aspect ratio vessel. Biochim. Biophys. Acta 1624:76–80. 10.1016/j.bbagen.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 22.Tarr PI. 1995. Escherichia coli O157:H7: clinical, diagnostic, and epidemiological aspects of human infection. Clin. Infect. Dis. 20:1–8. 10.1093/clinids/20.1.1 [DOI] [PubMed] [Google Scholar]
- 23.Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee GY, Jang HI, Hwang IG, Rhee MS. 2009. Prevalence and classification of pathogenic Escherichia coli isolated from fresh beef, poultry, and pork in Korea. Int. J. Food Microbiol. 134:196–200. 10.1016/j.ijfoodmicro.2009.06.013 [DOI] [PubMed] [Google Scholar]
- 25.Besser RE, Lett Weber SMJT, Doyle MP, Barrett TJ, Wells JG, Griffin PM. 1993. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA 269:2217–2220. 10.1001/jama.1993.03500170047032 [DOI] [PubMed] [Google Scholar]
- 26.Weagant SD, Bryant JL, Bark DH. 1994. Survival of Escherichia coli O157:H7 in mayonnaise and mayonnaise-based sauces at room and refrigerated temperatures. J. Food Prot. 57:629–631 [DOI] [PubMed] [Google Scholar]
- 27.Arnold K, Kaspar C. 1995. Starvation-and stationary-phase-induced acid tolerance in Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:2037–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhee M-S, Lee S-Y, Dougherty RH, Kang D-H. 2003. Antimicrobial effects of mustard flour and acetic acid against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 69:2959–2963. 10.1128/AEM.69.5.2959-2963.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller LG, Kaspar CW. 1994. Escherichia coli O157:H7 acid tolerance and survival in apple cider. J. Food Prot. 57:460–464 [DOI] [PubMed] [Google Scholar]
- 30.Abdul-Raouf U, Beuchat L, Ammar M. 1993. Survival and growth of Escherichia coli O157:H7 in ground, roasted beef as affected by pH, acidulants, and temperature. Appl. Environ. Microbiol. 59:2364–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reitsma CJ, Henning DR. 1996. Survival of enterohemorrhagic Escherichia coli O157:H7 during the manufacture and curing of cheddar cheese. J. Food Prot. 59:460–464 [DOI] [PubMed] [Google Scholar]
- 32.Berry ED, Cutter CN. 2000. Effects of acid adaptation of Escherichia coli O157:H7 on efficacy of acetic acid spray washes to decontaminate beef carcass tissue. Appl. Environ. Microbiol. 66:1493–1498. 10.1128/AEM.66.4.1493-1498.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groat RG, Schultz J, Zychlinsky E, Bockman A, Matin A. 1986. Starvation proteins in Escherichia coli: kinetics of synthesis and role in starvation survival. J. Bacteriol. 168:486–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammond T, Hammond J. 2001. Optimized suspension culture: the rotating-wall vessel. Am. J. Physiol. Renal Physiol. 281:F12–F25 [DOI] [PubMed] [Google Scholar]
- 35.Kwak TY, Kim NH, Rhee MS. 2011. Response surface methodology-based optimization of decontamination conditions for Escherichia coli O157:H7 and Salmonella Typhimurium on fresh-cut celery using thermoultrasound and calcium propionate. Int. J. Food Microbiol. 150:128–135. 10.1016/j.ijfoodmicro.2011.07.025 [DOI] [PubMed] [Google Scholar]
- 36.Bouloc P, D'Ari R. 1991. Escherichia coli metabolism in space. J. Gen. Microbiol. 137:2839–2843. 10.1099/00221287-137-12-2839 [DOI] [PubMed] [Google Scholar]
- 37.Gasset G, Tixador R, Eche B, Lapchine L, Moatti N, Toorop P, Woldringh C. 1994. Growth and division of Escherichia coli under microgravity conditions. Res. Microbiol. 145:111–120. 10.1016/0923-2508(94)90004-3 [DOI] [PubMed] [Google Scholar]
- 38.Klaus D, Simske S, Todd P, Stodieck L. 1997. Investigation of space flight effects on Escherichia coli and a proposed model of underlying physical mechanisms. Microbiology 143:449–455. 10.1099/00221287-143-2-449 [DOI] [PubMed] [Google Scholar]
- 39.Vukanti R, Mintz E, Leff L. 2008. Changes in gene expression of E. coli under conditions of modeled reduced gravity. Microgravity Sci. Technol. 20:41–57. 10.1007/s12217-008-9012-9 [DOI] [Google Scholar]
- 40.Baker PW, Leff L. 2004. The effect of simulated microgravity on bacteria from the Mir space station. Microgravity Sci. Technol. 15:35–41. 10.1007/BF02870950 [DOI] [PubMed] [Google Scholar]
- 41.Kacena M, Merrell G, Manfredi B, Smith E, Klaus D, Todd P. 1999. Bacterial growth in space flight: logistic growth curve parameters for Escherichia coli and Bacillus subtilis. Appl. Microbiol. Biotechnol. 51:229–234. 10.1007/s002530051386 [DOI] [PubMed] [Google Scholar]
- 42.Wilson JW, Ott CM, Ramamurthy R, Porwollik S, McClelland M, Pierson DL, Nickerson CA. 2002. Low-shear modeled microgravity alters the Salmonella enterica serovar Typhimurium stress response in an RpoS-independent manner. Appl. Environ. Microbiol. 68:5408–5416. 10.1128/AEM.68.11.5408-5416.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luli GW, Strohl WR. 1990. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl. Environ. Microbiol. 56:1004–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sezonov G, Joseleau-Petit D, D'Ari R. 2007. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 189:8746–8749. 10.1128/JB.01368-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wheelis M. 2011. Principles of modern microbiology, p 198–199 Jones & Bartlett Publishers, Sudbury, MA [Google Scholar]