Abstract
There is a strong demand from the wine industry for methodologies to reduce the alcohol content of wine without compromising wine's sensory characteristics. We assessed the potential of adaptive laboratory evolution strategies under hyperosmotic stress for generation of Saccharomyces cerevisiae wine yeast strains with enhanced glycerol and reduced ethanol yields. Experimental evolution on KCl resulted, after 200 generations, in strains that had higher glycerol and lower ethanol production than the ancestral strain. This major metabolic shift was accompanied by reduced fermentative capacities, suggesting a trade-off between high glycerol production and fermentation rate. Several evolved strains retaining good fermentation performance were selected. These strains produced more succinate and 2,3-butanediol than the ancestral strain and did not accumulate undesirable organoleptic compounds, such as acetate, acetaldehyde, or acetoin. They survived better under osmotic stress and glucose starvation conditions than the ancestral strain, suggesting that the forces that drove the redirection of carbon fluxes involved a combination of osmotic and salt stresses and carbon limitation. To further decrease the ethanol yield, a breeding strategy was used, generating intrastrain hybrids that produced more glycerol than the evolved strain. Pilot-scale fermentation on Syrah using evolved and hybrid strains produced wine with 0.6% (vol/vol) and 1.3% (vol/vol) less ethanol, more glycerol and 2,3-butanediol, and less acetate than the ancestral strain. This work demonstrates that the combination of adaptive evolution and breeding is a valuable alternative to rational design for remodeling the yeast metabolic network.
INTRODUCTION
Over the past 20 years, the alcohol content of wine has increased considerably, by about 2% (vol/vol), as a result of the high sugar content of the grapes currently used. This is mainly due to developments in winemaking practices, with the harvest of very mature grapes being favored to adapt to consumer demand for rich and ripe fruit flavor in wine. This trend poses major problems for the wine industry. The market is currently oriented toward beverages with moderate alcohol contents, in line with public prevention policies and consumer health issues and preferences. In addition, as some countries impose taxes on alcohol content, this trend raises economic issues. High levels of alcohol can alter the sensorial quality of wines by increasing the perception of hotness and, to a lesser extent, by decreasing the perception of sweetness, acidity, and aroma (1, 2). Also, high ethanol levels generated during fermentation may inhibit yeast activity and can lead to sluggish or stuck fermentations (3). Consequently, reduction of the ethanol content of wine has been a major focus of wine research at various steps of the wine-making process. Several viticulture strategies are being developed to decrease sugar accumulation in grapes. These approaches include the selection of adequate grape varieties that accumulate less sugar and the modification of culture techniques to reduce the berry sugar accumulation, such as irrigation, canopy management, or limitation of photosynthesis (4). Physical techniques for dealcoholization—for example, reverse osmosis, nanofiltration, and distillation—have also been developed and are available in the short term (5, 6). However, dealcoholization treatments are expensive to implement and may have detrimental effects on the organoleptic quality of the wine (7).
An attractive and inexpensive option would be to use yeasts that produce less alcohol from the same amount of sugar. Indeed, there have been many efforts to develop engineered wine yeast strains with reduced ethanol yield (6, 8). One of the most efficient approaches was to divert metabolism toward increased production of glycerol and thus away from ethanol (9, 10). In Saccharomyces cerevisiae, glycerol plays major roles in redox homeostasis and in osmotic stress resistance: it is the main compatible solute in yeast (11). Glycerol is usually found in wines at concentrations in the range of 5 to 9 g/liter and contributes positively to the quality of wine by providing body and sweetness (12). It may also confer viscosity at very high concentrations (above 25 g/liter), as in Botrytis wines. Rerouting carbon toward glycerol led to a substantial decrease in ethanol production (13, 14, 15, 16) and accumulation of various compounds, including acetate and acetoin, both undesirable for wine sensorial quality (15, 17). Rational engineering of key reactions at the acetaldehyde branch point allowed the accumulation of these undesirable compounds to be limited. This resulted in low-alcohol strains being obtained in which the carbon flux was redirected toward glycerol and 2,3-butanediol, a polyol with no sensorial impact in wines (9, 17).
These engineered wine yeasts have the potential to reduce the alcohol content of wine by 1 to 3% (vol/vol). However, the poor consumer acceptance of DNA recombinant technology in food is a major barrier to their commercialization. Consequently, there is a great interest in using alternative approaches not involving genetic modification to improve the properties of wine yeast strains.
One remarkable property of microorganisms is their ability to adapt rapidly to different environmental conditions. This property has been exploited in recent years by conducting adaptive laboratory evolution (ALE) experiments to study the principles and characteristics of evolution (18, 19, 20). Adaptive evolution, based on long-term adaptation of yeast under environmental or metabolic constraints, has been used to improve yeast strains for biotechnological applications, including wine making (21, 22, 23, 24, 25, 26).
However, there has not previously been any description of an evolutionary approach that successfully generated strains with substantially reduced ethanol yield. We therefore exploited adaptive evolution, using selective conditions involving hyperosmotic stress, to divert the metabolism of a commercial wine yeast strain toward increased glycerol production and lower ethanol production. Experimental evolutions using sodium chloride to generate osmotic stress have been used to study evolutionary processes (19, 20, 27) and in more applied work to increase the tolerance of baking strains to freezing (28). NaCl-resistant evolved industrial strains were obtained, but the production of glycerol and ethanol by the evolved strains was not affected.
In this study, we developed adaptive evolution methodology based on sequential increases of osmotic/saline stress to divert carbons toward glycerol and away from the production of ethanol. We subjected a commercial, diploid, heterozygous wine yeast strain to evolutionary pressure under hyperosmotic conditions for 450 generations. We then studied the fermentation and metabolic characteristics of the evolved strains in the laboratory to elucidate the metabolic basis of the phenotypes resulting from the evolution. We also generated an intrastrain hybrid from the evolved strain and performed a pilot-scale assessment of the potential of these strains for the wine market.
MATERIALS AND METHODS
Yeast strain and growth conditions.
The S. cerevisiae wine yeast strain Lalvin EC1118 was used as the ancestral strain. Strains were propagated in rich YPD medium (1% Bacto yeast extract, 2% Bacto peptone, 2% glucose) or in synthetic SD medium (0.67% Difco yeast nitrogen base without amino acids, 2% glucose) and maintained on YPD plates (2% agar) at 4°C or stored at −80°C in 20% glycerol.
KCl resistance assay.
EC1118 was grown in 60 ml YPD with KCl in concentrations ranging from 0.5 to 3 M, at 28°C, under agitation. The optical density at 600 nm (OD600) was measured each 6 h until 240 h, and growth rates were compared for the different conditions.
Adaptive evolution experiments.
Adaptive evolution was based on a long-term serial transfer procedure using KCl as stress inducer. The strain EC1118 was cultured overnight at 28°C in 5 ml of YPD, and the resulting cell suspension was used to inoculate capped tubes (13 ml), each containing 5 ml medium with 1% Bacto yeast extract, 2% Bacto peptone, 8% glucose, and 1.25 M KCl (Sigma-Aldrich). Duplicate evolution experiments and also a control experiment without stress were performed. The cultures were incubated at 28°C under agitation at 225 rpm. During the first phase (175 days), the KCl concentrations were sequentially increased from 1.25 M to 2.4 M, corresponding to an average increase of about 0.05 M at each transfer. During the second phase (475 days), the concentration of KCl was maintained at 2.4 M; after 7 days, corresponding to about 7 generations, the optical density of the culture at 600 nm (OD600) was measured, and an aliquot was used to inoculate a fresh medium such that the OD600 was 1. Such serial transfers were repeated for 450 generations. Every 50 generations, 1-ml samples of the evolving population were taken and stored at −80°C in 20% glycerol for subsequent analysis.
Wine fermentation. (i) Laboratory scale.
Batch fermentation experiments were carried out in synthetic medium (MS), which mimics a standard grape juice. MS medium was prepared as described by Bely et al. (29) with the following modifications: 260 g/liter glucose, 210 mg/liter assimilable nitrogen, 7.5 mg/liter ergosterol, 0.21 g/liter Tween, and 2.5 mg/liter oleic acid (MS210 medium). Fermentations in grape must were carried out under the same conditions, using Chardonnay-Coursan 2011 previously flash pasteurized. The fermentations were performed in 330-ml fermenters containing 300 ml medium, inoculated with 0.5 × 106 cells per ml, and incubated at 28°C with continuous stirring (350 rpm). To study the metabolic flexibility of the evolved and ancestral strains, different temperatures were used (16, 20, 24, 32, and 34°C).
Fermentation kinetics were monitored by calculation of the amount of CO2 released determined by weighing the fermenters manually. All fermentation experiments were performed in triplicate.
Extracellular metabolites and volatile compounds were assayed at the end of the fermentation.
(ii) Pilot scale.
Pilot-scale fermentations were performed in 1-hl cylindrical stainless-steel tanks with Syrah variety grape must. Syrah must contained 255 g/liter sugars and 138 mg/liter nitrogen. Grape musts were flash pasteurized and stored at 2°C before fermentation. Grape musts were inoculated at 25 g/hl with EC1118 or K300.1(b) active dry yeasts (Lallemand, Toulouse, France) and at 5.106 cells/ml with the H2 strain.
CO2 production was determined using a Brooks 5810 TR series gas flow meter (Brooks Instrument, Hatfield, PA), as described by Aguera and Sablayrolles (30). Fermentations were carried out under isothermal conditions at 28°C. Dissolved oxygen was added during fermentation to limit the risk of stuck fermentation, as described by Blateyron (31). Transfers of 3, 5, and 17 mg/liter oxygen were performed when the CO2 released reached 7.2, 13.5, and 45 g/liter, respectively. Nitrogen (72 mg/liter) was added under the form of 25 g/hl diaminopimelic acid (DAP) and 20 g/hl FermaidE at 45 g/liter of CO2 released.
Sporulation and breeding.
Sporulation of strain K300.1(b) was performed on solid medium (Bacto yeast extract, 0.1%; glucose, 0.05%; potassium acetate, 1%; adenine, 0.002%). After about 10 days, sporulation was confirmed by microscopic examination. The asci were dissected with a dissection microscope (Singer MSM300) to isolate the spores. Since EC1118 and K300.1(b) are heterozygotes for the HO gene, half of the progeny were expected to be haploid and the other half diploid. The spores were therefore tested for ploidy using mating-type PCR (32). A total of 156 haploid spores were selected and further characterized during wine fermentation at laboratory scale in synthetic must MS as described above. After 15 and 30 days of fermentation at 28°C, a sample of the supernatant was analyzed to determine the glycerol concentration. High-glycerol-producing strains were selected for further breeding. Crosses between haploids of different mating types were conducted on YPD agar plates, and diploid cells were checked by the absence of mating using Mata and Matα strains. Spores of the hybrid strain were generated as described above, and a second-generation hybrid H2 was obtained by mating spores producing the highest glycerol levels.
Drop tests.
Drop tests were carried out from a 1-day culture in synthetic defined (SD) medium inoculated at 1 OD600 unit/ml from a preculture on the same medium. Cells were washed with sterile water, suspended at 0.75 OD600 unit/ml, and then serially diluted from 10−1 to 10−3 in sterile water. Serial dilutions of cells were spotted onto SD plates as a control. For resistance assays, serial dilutions were spotted onto SD medium with either 2 or 4 M KCl, 2 or 4 M NaCl, or 2 or 4 M sorbitol. Alternatively, cells were diluted to 0.75 OD600 unit in a salt solution (either 2 or 4 M KCl or 2 or 4 M NaCl), incubated for 24 h at 28°C, washed, serially diluted, and spotted onto SD plates. Plates were incubated for 2 days at 28°C, and growth was compared to that of the parental strain.
Viability of evolved strains.
Ancestral and evolved cells were grown in 50 ml of YPGluKCl (1% Bacto yeast extract, 2% Bacto peptone, 8% glucose, 2.4 M KCl) inoculated at 0.1 OD unit/ml from an overnight preculture in YPD. The size of the cell population, extracellular metabolites, and viability were monitored for 7 days. The assays were performed in triplicate. Viability was determined using a flow cytometer (Accuri, BD Biosciences) to count 20,000 cells diluted and washed in 300 μl 1× phosphate-buffered saline (PBS) (137 mmol/liter NaCl [Sigma-Aldrich], 2.7 mmol/liter KCl, 100 mmol/liter Na2HPO4 [Sigma], 2 mmol/liter KH2PO4 [Sigma], pH 7.5) with 3 μl of propidium iodide (Calbiochem) previously diluted to 0.1 mg/ml in sterile water.
Analytical methods.
Cell densities were determined by measuring the OD600 with a Secomam UVILine 9400 or by using a Coulter ZBI cell counter linked to a C56 Channelyzer fitted with a probe with a 100-mm aperture (Beckman Coulter). Dry weight was determined gravimetrically by filtering 10 ml of sample through a Millipore filter (pore size, 0.45 μm) and drying the sample for 24 h at 100°C. Extracellular glucose, glycerol, ethanol, pyruvate, succinate, and acetate concentrations were determined by high-pressure liquid chromatography (HPLC), using an HPX-87H ion exclusion column (Bio-Rad). The ethanol concentration of the wine produced from Syrah grape must was determined by HPLC and by densitometry.
Volatile aromatic compounds (acetoin and 2,3-butanediol) were assayed by gas chromatography (GC). Acetoin and butanediol were extracted into chloroform according to the Hagenauer-Hener protocol (33), with the following modifications: 1 ml of hexanol (Sigma) as an internal standard (1:1,000 [vol/vol]) in 10% ethanol (VWR) was added to 1 ml of sample. The organic phase was dried and 1 μl was injected into a 30-m Megabore column (DB-WAX; J&W Scientific) on a GC apparatus, HP 6890. The acetaldehyde concentrations were determined enzymatically according to the Lundquist method (34).
The osmolality was measured using a Vapro 5520 device (Wescor) with a sample volume of 10 μl.
RESULTS
Adaptive evolution under hyperosmotic KCl medium and isolation of high-glycerol-producing evolved strains.
To evolve strain EC1118, we performed batch cultures in YPD–8% glucose with a gradual increase of osmotic stress. KCl stress was chosen because it generates osmotic and salt stresses, but unlike NaCl, it does not cause cation toxicity (35). We used a relatively high sugar concentration (8%) to maintain good fermentative performances of the evolved strain in rich sugar medium. In preliminary experiments, we tested the effects of various KCl concentrations on EC1118 growth and found that the addition of 1.25 M KCl on YPD–8% glucose reduced the growth of EC1118 4 times (data not shown). We thus started the adaptive laboratory evolution (ALE) experiments in YPD–8% glucose containing 1.25 M KCl. The osmolality of this medium was 2,105 mmol/kg, compared to 480 mmol/kg for YPD–8% glucose. The concentrations of KCl were progressively increased up to 2.4 M, corresponding to an osmolality of 3,730 mmol/kg, and were maintained at that level thereafter. Duplicate ALE experiments were performed for each condition, and one control ALE experiment, without osmotic stress, was done.
We analyzed samples collected each 50 generations until 400 generations to monitor the dynamics of each evolution experiment. The populations obtained were characterized during fermentation of the synthetic must MS210 at 28°C. The glycerol concentration in the growth medium was measured at the end of the fermentation as a first indicator of the success or failure of the adaptation. Adaptation on KCl medium generated evolved populations with increased glycerol production during wine fermentation (Fig. 1), whereas no increase of glycerol was observed in the control experiment (evolution of EC1118 without stress). A similar increase in glycerol production was observed in the two parallel KCl experiments a and b. In fermentations with both a and b lineages, the concentration of glycerol produced by fermentation reached 12 g/liter for evolved populations at 200 generations; the value for the ancestral EC1118 was 8.5 g/liter. The KCl ALE experiment was pursued for 450 generations (total duration of almost 2 years), but only little variation in glycerol production was observed after 200 generations.
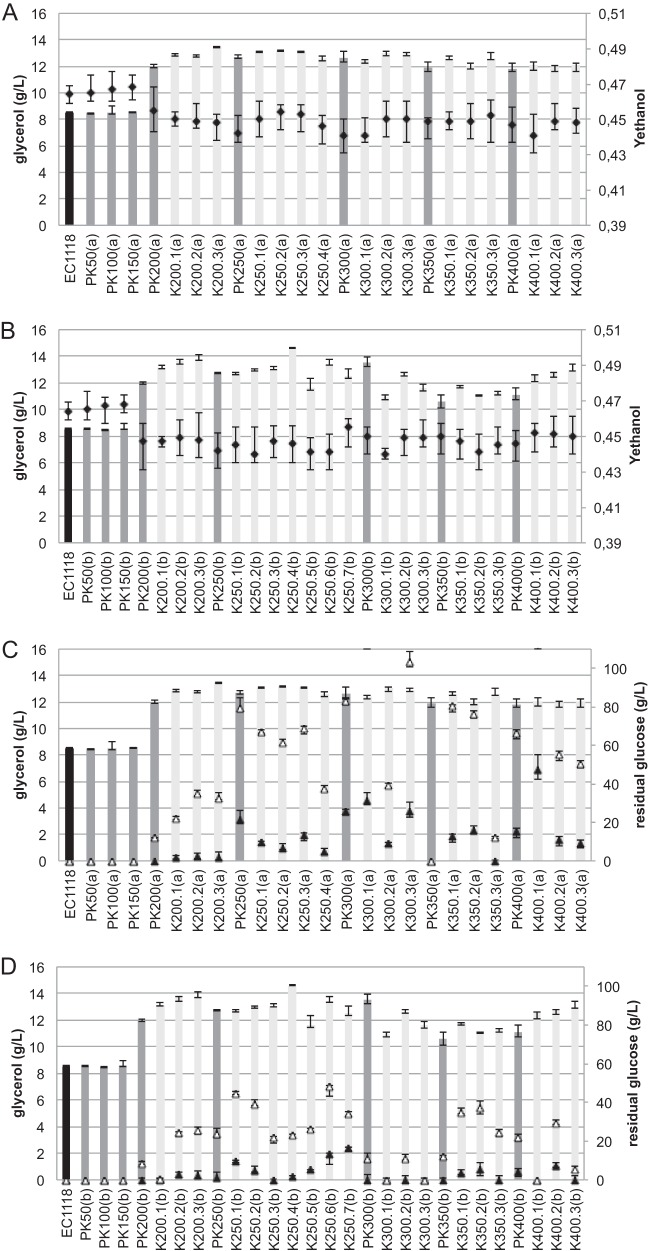
FIG 1.
Glycerol concentration (bars) and ethanol yield (Y [g/g glucose consumed]) (black diamonds) (A and B), and glycerol concentration (bars) and residual glucose after 15 (white triangles) and 30 (black triangles) days of fermentation (C and D) for evolved populations (dark gray) and isolates (evolved strains) (light gray) from the independent lineages a (A and C) and b (B and D). Fermentations were carried out in 300 ml MS210 containing 260 g/liter glucose at 28°C in triplicate.
First characterization of the evolved strains during wine fermentation.
After several generations, due to the natural accumulation of mutations, nonhomogeneous populations of yeasts should be present in samples obtained from ALE experiments. The yeast population sampled at different times during the KCl-ALE experiment was streaked on YPD plates, and isolated colonies were selected. These isolates, here called “evolved strains,” were characterized during wine fermentation on MS210 medium (Fig. 1). All of the evolved strains obtained after 200 generations produced more glycerol than the ancestral strain; glycerol production remained stable after 200 generations. Consistent with the rerouting of carbons and NADH oxidation resulting from increased glycerol production (14), all evolved strains showed reduced ethanol yield. The ethanol yield was between 0.450 and 0.440 for the evolved strains and 0.464 for the reference ancestral strain (Fig. 1A and B). The evolved mutants showed reduced sugar consumption (Fig. 1C and D). Thus, there was a correlation between high glycerol yield, reduced ethanol yield, and diminution of fermentative properties.
We studied in more detail six KCl-evolved strains producing high glycerol levels while retaining good fermentative performance and obtained from populations isolated after 200, 250, and 300 generations, including three from lineage (a)—K200.1(a), K250.1(a), and K300.2(a)—and three from lineage (b)—K200.1(b), K250.3(b), and K300.1(b). The EC1118 origin of these strains was confirmed by karyotype analysis (data not shown).
High-glycerol-producing strains survive better under conditions of osmotic stress and carbon restriction.
The resistance of the evolved strains to hyperosmotic stress was assessed by growth on KCl, NaCl, or sorbitol SD plates. Under these conditions, we did not observe any significant difference in growth between EC1118 and the evolved strains (data not shown): under the conditions of the ALE experiment (YPD, 80 g/liter glucose, 2.4 M KCl), the specific growth rate and maximal cell number reached by the evolved strains were similar to those of the ancestral strain. Therefore, yeast cells that evolved under these conditions did not display growth adaptation to osmotic stress. We therefore examined whether other components of fitness, such as viability, had been improved during the evolution experiment. Cell viability was monitored during culture involving a 7-day transfer cycle under the conditions of the evolution experiment (YPD, 80 g/liter glucose, 2.4 M KCl). After complete glucose exhaustion (about 4 days), the evolved mutants survived better than the ancestral strain. After 7 days (corresponding to the time of transfer to fresh medium during the evolution experiment), almost all EC1118 cells had died, whereas the number of viable cells of the evolved mutants was considerably higher (Fig. 2). The viability of the evolved mutants at 7 days correlated with glycerol production at the same time point (data not shown). Therefore, the main adaptation to the selective pressure imposed on yeast cells during the adaptive evolution experiment is improved survival under conditions of salt stress and carbon restriction.
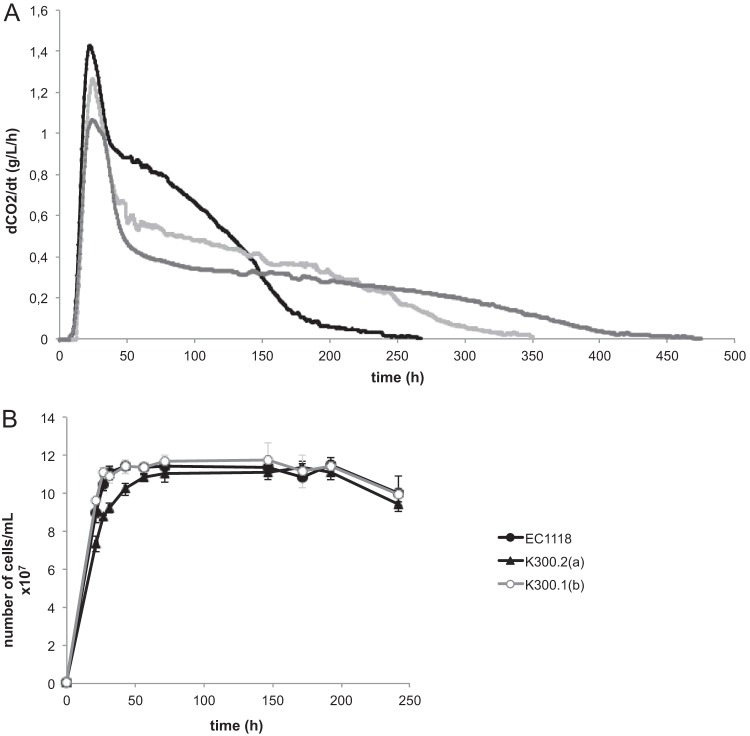
FIG 2.
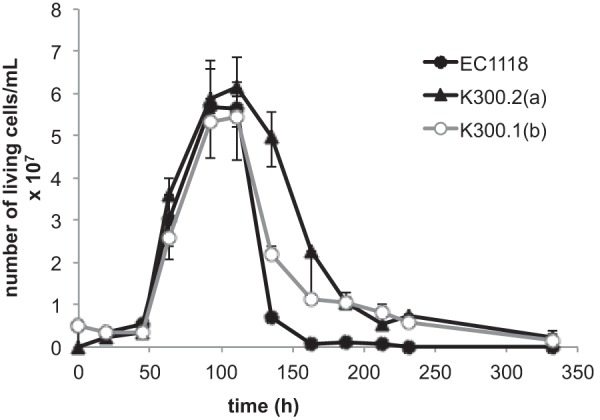
Selective advantage of the evolved strains. Viability of EC1118, K300.2(a), and K300.1(b) during culture in YPD plus 8% glucose and 2.4 M KCl at 28°C. Sugar exhaustion is observed after 100 h.
Characterization of the selected KCl-evolved strains during wine fermentation.
The characteristics of the six selected KCl-evolved strains and the ancestral strain were studied in detail during wine fermentation in anaerobic batch cultures on MS medium. All of the strains were able to complete the fermentation, although the durations of the fermentation differed between the evolved strains (Table 1). Two evolved strains, K200.1(b) and K300.1(b), consumed all of the sugar in less than 2 weeks, like the ancestral strain, whereas 1 month or more was required for the four other evolved strains. Sugar was completely exhausted only after 40 days by K250.1(a) and K300.2(a). The fermentation rates of two evolved strains having distinct fermentation capacities, K300.2(a) and K300.1(b), are shown in Fig. 3A. The evolved strains exhibited an overall decrease of fermentation performance in comparison to the ancestral strain, which is consistent with the reduced sugar consumption observed before, but were nevertheless able to complete the fermentation. The final cell populations were the same between the ancestral strain and these two evolved strains, despite the fact that K300.2(a) showed slower growth than K300.1(b) (Fig. 3B).
TABLE 1.
Metabolites measured for EC1118 and evolved strains after 30 days of fermentation on MS210 with 260 g/liter glucose at 28°C
| Parameter | Result for strain: |
||||||
|---|---|---|---|---|---|---|---|
| EC1118 | K200.1(a) | K250.1(a) | K300.2(a) | K200.1(b) | K250.3(b) | K300.1(b) | |
| Main compounds (g/liter) | |||||||
| Consumed glucose | 259.9 ± 0.1 | 258.5 ± 1.4 | 250.7 ± 4.5 | 251.3 ± 5.0 | 260.0 ± 0.1 | 258.6 ± 1.5 | 259.8 ± 0.3 |
| CO2 | 117 ± 0 | 112 ± 1 | 110 ± 1 | 109 ± 1 | 112 ± 0 | 112 ± 2 | 110 ± 1 |
| Biomass (80%)a | 4.0 ± 0.1 | 3.8 ± 0.1 | 4.0 ± 0.2 | 3.7 ± 0.2 | 3.6 ± 0.2 | 3.9 ± 0.0 | 4.0 ± 0.1 |
| Ethanol | 120.6 ± 0.6 | 116.3 ± 1.3 | 112.9 ± 1.5 | 113.2 ± 1.0 | 116.3 ± 0.7 | 115.5 ± 1.6 | 114.0 ± 0.8 |
| Glycerol | 8.5 ± 0.1 | 13.2 ± 0.1 | 12.7 ± 0.1 | 12.6 ± 0.2 | 13.3 ± 0.5 | 13.9 ± 0.2 | 14.2 ± 0.3 |
| Succinate | 0.9 ± 0.0 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.1 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.7 ± 0.1 |
| Pyruvate | 0.22 ± 0.03 | 0.20 ± 0.00 | 0.20 ± 0.01 | 0.19 ± 0.00 | 0.20 ± 0.01 | 0.19 ± 0.01 | 0.19 ± 0.02 |
| Acetate | 0.9 ± 0.2 | 0.7 ± 0.0 | 0.9 ± 0.0 | 1.1 ± 0.0 | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| Acetaldehyde | 0.011 ± 0.001 | 0.016 ± 0.001 | 0.023 ± 0.002 | 0.031 ± 0.007 | 0.025 ± 0.005 | 0.031 ± 0.010 | 0.029 ± 0.001 |
| Acetoin | NDc | ND | ND | ND | ND | ND | ND |
| 2,3-Butanediol | 0.45 ± 0.02 | 1.00 ± 0.09 | 0.89 ± 0.30 | 1.08 ± 0.14 | 0.87 ± 0.11 | 1.10 ± 0.18 | 1.60 ± 0.06 |
| Balance (%)b | |||||||
| Carbon | 97.6 ± 0.6 | 96.9 ± 0.8 | 97.2 ± 2.5 | 97.0 ± 2.7 | 96.4 ± 0.8 | 96.7 ± 0.8 | 95.9 ± 0.5 |
| Redox | 97.4 ± 0.6 | 97.3 ± 0.7 | 97.5 ± 2.1 | 97.3 ± 2.1 | 96.6 ± 0.8 | 97.1 ± 1.7 | 96.4 ± 0.6 |
| Yield | |||||||
| EtOH (g/g glucose consumed) | 0.464 ± 0.002 | 0.450 ± 0.004 | 0.450 ± 0.010 | 0.450 ± 0.010 | 0.447 ± 0.003 | 0.447 ± 0.009 | 0.440 ± 0.003 |
| Glycerol (g/g glucose consumed) | 0.033 ± 0.000 | 0.051 ± 0.000 | 0.051 ± 0.001 | 0.050 ± 0.002 | 0.051 ± 0.002 | 0.054 ± 0.001 | 0.054 ± 0.001 |
| Glycerol/EtOH ratio (%) | 7.05 ± 0.07 | 11.32 ± 0.09 | 11.27 ± 0.11 | 10.09 ± 0.23 | 11.46 ± 0.40 | 12.01 ± 0.26 | 12.38 ± 0.27 |
| Ethanol production (% [vol/vol]) | 15.29 ± 0.08 | 14.82 ± 0.13 | 14.84 ± 0.32 | 14.84 ± 0.34 | 14.74 ± 0.09 | 14.72 ± 0.29 | 14.50 ± 0.09 |
| Glucose (g) required for 1% (vol/vol) ethanol production | 17.00 ± 0.07 | 17.53 ± 0.09 | 17.52 ± 0.11 | 17.52 ± 0.23 | 17.64 ± 0.40 | 17.67 ± 0.26 | 17.97 ± 0.27 |
| Residual sugar (g/liter) at: | |||||||
| 15 days | 0.1 ± 0.1 | 20.2 ± 1.4 | 58.7 ± 4.5 | 30.4 ± 5.0 | 0.1 ± 0.1 | 22.5 ± 1.5 | 0.2 ± 0.3 |
| 30 days | 0.1 ± 0.1 | 1.5 ± 1.4 | 9.3 ± 4.5 | 8.7 ± 5.0 | 0.0 ± 0.1 | 1.4 ± 1.5 | 0.1 ± 0.3 |
Biomass measured at 80% of fermentation advancement.
Carbon balance represents the ratio between carbon moles of fermentation by-products and carbon moles of glucose. Redox balance represents the ratio between the reductance degree of fermentation by-products and the reductance degree of glucose.
ND, not detected (<10 mg/ml).
FIG 3.
Fermentation performance (A) and cell population (B) for the ancestral strain EC1118 (black) and the evolved strains K300.2(a) and K300.1(b) (dark and light gray) on MS210 medium containing 260 g/liter glucose at 28°C. dCO2/dt, first derivate of CO2 produced with respect to time (t).
We determined the concentration of the most abundant by-products after 30 days of fermentation (Table 1). Carbon and redox balances were close to 100% for all strains. All evolved strains produced glycerol at concentrations 48 to 67% higher than that produced by EC1118, and the ethanol content in the synthetic wines was reduced by 0.45 to 0.80% (vol/vol). The evolved strains also produced larger amounts of succinate, 2,3-butanediol, and acetaldehyde than the ancestral strain. Succinate production by K200.1(a) and K300.2(a) was 22% and 88.9% higher than that by EC1118; the production of acetaldehyde by K200.1(a) and K300.2(a) was 45.5 to 181.8% higher, respectively, and that of 2,3-butanediol was 93% to 255.6% higher. The concentration of these compounds was also affected in strains overexpressing GPD1, in which the carbon flux is redirected toward glycerol formation at the expense of ethanol (14, 15, 17). In contrast, unlike previously described engineered strains, we did not find significant changes in the production of acetate and acetoin by the evolved strains.
Although similar phenotypes were observed for the two replicates, lineage b was characterized by slightly greater glycerol production and better fermentative performances (faster fermentation rate and shorter duration of the fermentation). K300.1(b) was the most promising evolved strain in terms of fermentation performances and production of glycerol, succinate, 2,3-butanediol, and ethanol.
Metabolic properties of the evolved strain K300.1(b) at various temperatures on synthetic and natural grape musts.
Wine can be produced in a large range of fermentation temperatures—usually from 16°C for white wines to 28°C and higher for red wines. We therefore compared the metabolic properties of the ancestral strain and the evolved strain K300.1(b) over a wide range of temperatures (16, 20, 24, 28, 32, and 34°C) in MS210 medium containing 260 g/liter sugars. For temperatures between 16 and 28°C, both strains consumed all or most of the sugar, while for the two highest temperatures, residual sugar concentrations of 43 and 53 g/liter for EC1118 and 47 and 59 g/liter for K300.1(b) were observed at 32 and 34°C, respectively.
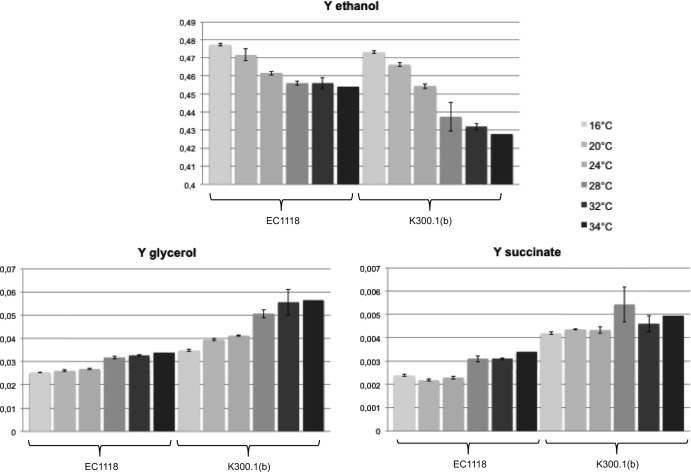
The yields of by-products were determined after 30 days of fermentation (Fig. 4). At all temperatures, K300.1(b) was clearly differentiated from EC1118 on the basis of high glycerol, high succinate, and low ethanol yields. We found that the yields of glycerol and succinate increased with increasing temperature, whereas the ethanol yield decreased. Therefore, temperature affects yeast metabolism, confirming earlier findings (36). The differences in these metabolites between K300.1(b) and EC1118 were larger for the three highest temperatures. Compared to EC1118, the ethanol yields of the evolved strain were reduced by 0.14% (vol/vol), 0.18% (vol/vol), and 0.24% (vol/vol) at 16, 20, and 24°C and by 0.61% (vol/vol), 0.80% (vol/vol), and 0.87% (vol/vol) at 28, 32, and 34°C, respectively. Therefore, a metabolic shift is observed between 24 and 28°C. To examine whether a similar behavior can be observed on natural must, fermentation was carried out in Chardonnay-Coursan, under similar conditions, at 24 and 28°C. Under these conditions, the ethanol levels were reduced by 0.12% (vol/vol) at 24°C and 0.42% (vol/vol) at 28°C, confirming the results obtained in synthetic must. These results highlight a more flexible metabolism in the evolved strain regarding temperature, with the reduction of ethanol yield maximized at a temperature of 28°C and above.
FIG 4.
By-product yields (Y [g/g glucose consumed]) for strains EC1118 and K300.1(b). Metabolites were measured after 30 days of fermentation in 300 ml of MS210 containing 260 g/liter glucose at 16, 20, 24, 28, 32, and 34°C.
Enhanced glycerol production by breeding.
To further increase the ability of the evolved strain K300.1(b) to produce glycerol and to decrease its ability to produce ethanol during an alcoholic fermentation, we further submitted strain K300.1(b) to conventional breeding. To this end, we produced about 150 haploid yeast spores from K300.1(b) and selected haploid strains of opposite mating type having the highest capacity to produce glycerol. Mating between two spores producing 20.9 and 16.2 g/liter glycerol during fermentation on MS medium generated a first-generation hybrid. After sporulation and spore mating, a second-generation hybrid, H2, which produced 16.8 g/liter glycerol, was obtained.
Pilot-scale assessment of the K300.1(b) evolved strain and of the hybrid H2.
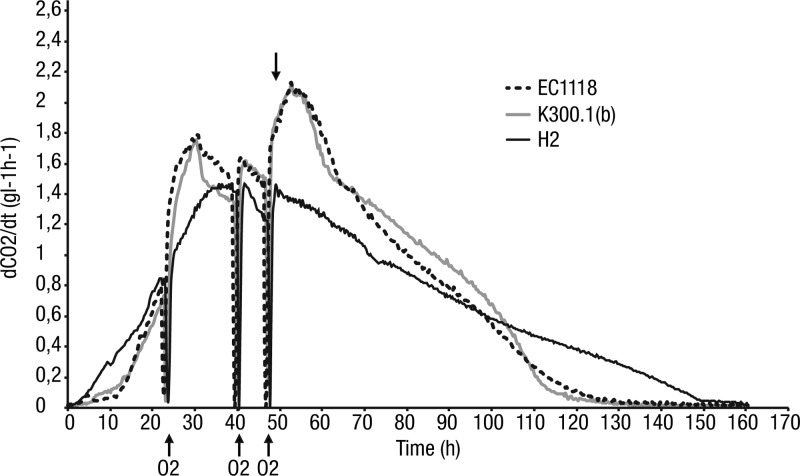
To validate the results obtained at laboratory scale, we compared the behaviors and metabolic properties of K300.1(b), H2, and EC1118 during pilot-scale fermentation, using a Syrah grape must, at 28°C (Table 2). To be as close as possible to industrial conditions, the strains K300.1(b) and EC1118 were used in the form of active dry yeast and inoculated after a standard rehydration procedure (see Materials and Methods). To avoid stuck fermentation, oxygen and nitrogen were added during fermentation (Fig. 5). H2 had a fermentation rate slightly lower than those of EC1118 and K300.1(b), but all strains were able to complete the fermentation, despite the high sugar concentration (255 g/liter). K300.1(b) and H2 produced more glycerol (14.1 g/liter and 17.9 g/liter versus 10.8 g/liter) and slightly more succinate than EC1118. The ethanol contents of the wines produced by K300.1(b) and H2 were reduced by 0.6% (vol/vol) and 1.3% (vol/vol). These results are in agreement with those obtained at laboratory scale. The production of acetic acid by the evolved and hybrid strains was greatly reduced compared to that of EC1118. The production of acetic acid for EC1118 and K300.1(b) was much lower than on synthetic must, which is in agreement with previous observations (unpublished data). In summary, the results obtained in grape must at pilot scale confirm the metabolic shift of the evolved strain and show a greater metabolic reprograming of the hybrid derived from the evolved strain. In addition, this standard analysis of the wine obtained did not reveal adverse side effects.
TABLE 2.
Metabolites measured in wines obtained by fermentation of Syrah must with EC1118, K300.1(b), and H2 at pilot scale (1 hl)a
| Parameter | Result for strain: |
||
|---|---|---|---|
| EC1118 | K300.1(b) | H2 | |
| Main compounds (g/liter) | |||
| Consumed sugar | 254.6 ± 0.1 | 254.5 ± 0.0 | 254.7 ± 0.2 |
| Ethanol | 118.4 ± 1.2 | 113.6 ± 0.9 | 107.8 ± 0.8 |
| Glycerol | 10.8 ± 0.4 | 14.1 ± 0.4 | 17.9 ± 0.8 |
| Succinate | 1.3 ± 0.1 | 1.8 ± 0.1 | 1.5 ± 0.1 |
| Pyruvate | 0.13 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 |
| Acetate | 0.5 ± 0.1 | 0.1 ± 0.0 | NDb |
| Acetaldehyde | 0.016 ± 0.008 | 0.021 ± 0.001 | 0.020 ± 0.006 |
| Acetoin | ND | ND | 0.024 ± 0.005 |
| 2,3-Butanediol | 1.11 ± 0.18 | 1.98 ± 0.38 | 3.93 ± 0.30 |
| Yield | |||
| EtOH (g/g glucose consumed) | 0.465 ± 0.005 | 0.446 ± 0.003 | 0.423 ± 0.003 |
| Glycerol (g/g glucose consumed) | 0.042 ± 0.002 | 0.055 ± 0.000 | 0.070 ± 0.003 |
| Glycerol/EtOH (%) | 9.09 ± 0.34 | 12.37 ± 0.05 | 16.57 ± 0.84 |
| Ethanol production (% [vol/vol]) | 15.01 ± 0.15 | 14.40 ± 0.11 | 13.67 ± 0.10 |
| Glucose (g) required for 1% (vol/vol) ethanol production | 16.99 ± 0.07 | 17.71 ± 0.07 | 18.66 ± 0.08 |
Metabolites were determined by HPLC, GC, or enzymatically. Ethanol was measured by HPLC and by distillation and electronic densitometry. Means and standard deviations of 3 to 4 independent measurements are shown.
ND, not detected (<10 mg/ml).
FIG 5.
Kinetics of wine fermentation on Syrah for EC1118, K300.1(b), and H2. Nitrogen (72 mg/liter) was added in the form of 25 g/hl DAP and 20 g/hl FermaidE at 45 g/liter of CO2 release (time point indicated by an arrow). dCO2/dt, first derivate of CO2 produced with respect to time (t).
DISCUSSION
In this study, we used adaptive laboratory evolution (ALE) to develop low-alcohol wine yeasts by redirecting the metabolism of strain EC1118 toward glycerol. Yeast cultures were serially transferred under hyperosmotic conditions during 450 generations using KCl as osmotic and salt stress agent. The stress imposed was severe, from osmolalities of 2,105 to 3,730 mmol/kg. These levels of stress are above those generally used under laboratory conditions to study responses to osmotic stress (20 g/liter glucose, 1.2 M NaCl, corresponding to an osmolality of 2,070 mmol/kg). The KCl stress generated strains in which the carbon flux was redirected toward glycerol. In comparison, we also used sorbitol as an osmotic agent (from 1.5 to 2.4 g/liter, corresponding to 1,480 to 2,105 mmol/kg), but these conditions failed to generate strains with increased glycerol production (data not shown). This clear difference in effect may be a consequence of the different natures of the stress agent (salt versus osmotic stress) and/or the higher level of stress in the KCl-ALE experiment than the sorbitol-ALE experiment. The evolved strains obtained from the KCl-ALE experiment were not more resistant than the ancestral strain to osmotic or salt stress but showed a gain of fitness due to better viability under conditions of salt stress and carbon starvation, the conditions under which cells were transferred to a fresh medium. No increase in glycerol production was observed in the ALE control experiment with EC1118 without KCl stress (data not shown). Therefore, it is likely that the redirection of carbon fluxes toward glycerol was driven by the combination of high KCl concentration and carbon starvation stresses.
The link between survival and glycerol is intriguing. Usually, cells die after the culture enters the stationary phase, when one or all of the nutrients are missing. However, if the only nutrient missing is the carbon source, cells survive longer (37). Under carbon limitation, nutrient sensing depends on the Sch9, Tor, and Ras proteins, which are activated and converge on the protein kinase Rim15; Rim15 regulates the transcription factors Msn4/Msn2 and Gis1, involved in cellular protection and longevity, also called “chronological life span” (CLS). Recent work indicates that glycerol production is required for CLS regulation (38), and various distinct mechanisms have been suggested. Unlike glucose and ethanol, glycerol does not inhibit the transactivation of Msn2/Msn4 and Gis1, which play important roles in general stress resistance and longevity (38). However, glycerol production may affect aging through the modulation of the intracellular redox balance, because its production contributes to the maintenance of the NAD+/NADH ratio. Overexpression of the malate-aspartate NADH shuttle was also demonstrated to extend the CLS (39). Also, high osmolarity extends the life span by activating Hog1, leading to an increase in the biosynthesis of glycerol from glycolytic intermediates (40). Links between aging and redox metabolism during wine fermentation have also been highlighted (41).
Our detailed characterization of the KCl-evolved mutants during wine fermentation revealed that the evolved strains had undergone substantial changes to their central carbon metabolism: carbons in these strains are mainly rerouted toward glycerol, 2,3-butanediol, and succinate at the expense of ethanol. The absence of a stress resistance phenotype and the improved fitness under carbon-restricted and stress conditions suggest that the primary target of evolution is not the HOG pathway. The origin of the observed phenotype might rely on indirect mutations disturbing the redox balance, causing a redirection of carbon flux. Other factors, such as a lower glucose uptake rate, might also play a role in the phenotype. Indeed, the net flux through the tricarboxylic acid (TCA) cycle increased significantly with decreasing glucose uptake (42), which is reminiscent of the increased succinate production and lower fermentation rate in the evolved strains. On the other hand, the same study found that glycerol production is less dependent on the rate of glucose uptake and more influenced by environmental conditions. Other studies using genome-wide approaches are necessary to elucidate the underlying mechanisms.
As observed previously in engineered strains overexpressing GPD1 (14, 15, 17), increased glycerol production is associated with a reduction of ethanol synthesis due to lower carbon availability and NADH shortage, and this is accompanied by perturbations at the acetaldehyde and pyruvate nodes. For example, strains overexpressing GPD1 and producing large amounts of glycerol but low levels of ethanol, accumulate succinate and 2,3-butanediol but also undesirable compounds, including acetaldehyde, acetate, and acetoin (15, 17). Our evolved strain did not accumulate high levels of the latter compounds, possibly due to the smaller increase in glycerol production than that in the engineered strains or due to a different metabolic strategy. In yeast, acetoin is reduced to 2,3-butanediol by the 2,3-butanediol dehydrogenase (43). We previously showed that the balance between acetoin and 2,3-butanediol in the engineered strains is influenced by the amounts of glycerol produced (15). In strains producing high glycerol levels, acetoin accumulated because of the limited capacity of the 2,3-butanediol dehydrogenase and the decreased availability of NADH, as this cofactor is mainly reoxidized through glycerol synthesis (9). In our previous study (14), we found that strains overproducing glycerol at moderate levels (such as W18GPD1 or W6GPD1), comparable to those produced by the evolved mutants in this study, did not accumulate acetoin. As the evolved strains, these strains also accumulated acetaldehyde at low levels, which can be explained by a limitation of the alcohol dehydrogenase. These levels remain in the range of usual concentrations in wines and are unlikely to cause a sensory problem. In contrast, the reduced accumulation of acetate by the evolved mutants is surprising because there was acetate accumulation in all cases, independent of the level of glycerol accumulated by the GPD1 strains (11). This suggests that the modifications of the metabolic network in the evolved mutants differ from those in the genetically engineered strains. In particular, these data may suggest an uncoupling of the response to the stress used as selection pressure and carbon metabolism-related glycerol production. Another major difference involves the compromised fermentation performances of the evolved strains, suggesting that the mutations responsible for the rerouting of metabolism in these strains also negatively affect the glycolytic rate. This finding contrasts with the improved fermentation performances of GPD1 strains during the stationary phase of wine fermentation (15, 17). It therefore appears that adaptive evolution resulted in the utilization of routes different from those operating in rationally engineered strains.
We provide herein a description of a non-genetically modified organism (non-GMO) strategy allowing a substantial increase in glycerol production and decrease in the alcohol yield of a commercial wine yeast strain. Importantly, we also demonstrate that the evolved strain obtained can be further improved by using a conventional breeding strategy. A greater glycerol increase and ethanol reduction were obtained in hybrids derived from the evolved strain, which may result from an optimal combination of K300.1(b) ALE selected mutated alleles and original alleles from the heterozygous background.
Similar characteristics were found for the evolved strain K300.1(b) in synthetic and natural grape musts, except that acetate production was reduced in wines obtained from grape musts. Interestingly, evolved strain K300.1(b) has a higher metabolic flexibility than the ancestral strain with respect to temperature, with metabolic differences between the two strains being greatest at temperatures higher than 24°C. Although understanding the bases for this temperature-dependent phenotype requires further studies, this indicates that the evolved strain might be particularly useful for the production of red wines, which are usually produced in a temperature range of 25 to 30°C and are most affected by very high alcohol levels (for example, above 15% [vol/vol]). Whether the hybrid H2 has retained this characteristic or not remains to be determined.
Pilot-scale trials with the evolved strain at 28°C showed ethanol level reductions of 0.45% (vol/vol) in Grenache (data not shown) and 0.61% (vol/vol) in Syrah (Table 2). A preliminary Grenache wine tasting by a panel of seven wine experts did not reveal any defect of the wines produced at pilot scale, confirming the good overall attributes of the evolved strains reported in this study. The H2 hybrid strain showed a more marked phenotype, with a reduction of the alcohol content of wine by 1.3% (vol/vol) in Syrah. For both strains, the diversion was explained by marked increased synthesis of glycerol and 2,3-butanediol, which were both used as carbon and redox sinks. Thus, compared to previous attempts to divert carbons toward the pentose phosphate pathway (26) or toward glycerol by adaptive evolution using sulfites (25), this evolutionary strategy resulted in a much higher diversion of carbons.
Our study demonstrates that a combination of adaptive evolution and breeding strategies is a valuable alternative to rational engineering for the generation of non-genetically modified, low-ethanol-producing yeasts. Further pilot-scale trials on different grape must varieties and wine making conditions, as well as sensorial analysis of the wines obtained, are required to fully evaluate the value of these strains for the wine market. Several studies highlighted the interest in non-Saccharomyces yeasts to produce wine with a lower ethanol concentration, when used in a sequential inoculation regimen with an S. cerevisiae wine strain (44). Although the use of an S. cerevisiae strain with low ethanol yield is much easier to implement and monitor than a mixed culture, combining the advantages of evolved S. cerevisiae and non-Saccharomyces strains might allow further reduction in the ethanol levels of wines. In the future, these strains could represent an essential tool in a global strategy involving a combination of integrated approaches (vine-plant variety and physical and biological methods) to fine-tune the alcohol content of wines.
ACKNOWLEDGMENTS
We thank Stéphane Guézenec for generating and analyzing haploid segregants of K300.1(b), Jessica Noble for breeding experiments, Pierre Delobel for assistance with flow cytometer assays, Evelyne Aguera for pilot-scale experiments, and Carole Camarasa for helpful discussions.
This study was supported by BIOFLAVOUR Cost Action 464 FA0907.
Footnotes
Published ahead of print 14 February 2014
REFERENCES
- 1.Escudero A, Campo E, Fariña L, Cacho J, Ferreira V. 2007. Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 55:4501–4510. 10.1021/jf0636418 [DOI] [PubMed] [Google Scholar]
- 2.Gawel R, Francis L, Waters EJ. 2007. Statistical correlations between the in-mouth textural characteristics and the chemical composition of Shiraz wines. J. Agric. Food Chem. 55:2683–2687. 10.1021/jf0633950 [DOI] [PubMed] [Google Scholar]
- 3.Buescher WA, Siler CE, Morris JR, Threlfall RT, Main GL, Cone GC. 2001. High alcohol wine production from grape juice concentrates. Am. J. Enol. Vitic. 52:345–351 [Google Scholar]
- 4.Novello V, De Palma L. 2013. Viticultural strategy to reduce alcohol levels in wine, p 3–8. In Vigne et Vin Publications Internationales (ed), Alcohol level reduction in wine. Proceedings of the 1st Oenoviti International Symposium, Bordeaux, France [Google Scholar]
- 5.Aguera E, Bes M, Roy A, Camarasa C, Sablayrolles J-M 2010. Partial removal of ethanol during fermentation to obtain reduced-alcohol wines. Am. J. Enol. Vitic. 61:53–60 [Google Scholar]
- 6.Schmidtke LM, Blackman JW, Agboola SO. 2012. Production technologies for reduced alcoholic wines. J. Food Sci. 77:R25–R41. 10.1111/j.1750-3841.2011.02448.x [DOI] [PubMed] [Google Scholar]
- 7.Liguori L, Russo P, Albanese D, Di Matteo M. 2013. Evolution of quality parameters during red wine dealcoholization by osmotic distillation. Food Chem. 140:68–75. 10.1016/j.foodchem.2013.02.059 [DOI] [PubMed] [Google Scholar]
- 8.Kutyna DR, Varela C, Henschke PA, Chambers PJ, Stanley GA. 2010. Microbiological approaches to lowering ethanol concentration in wine. Trends Food Sci. Technol. 21:293–302. 10.1016/j.tifs.2010.03.004 [DOI] [Google Scholar]
- 9.Ehsani M, Fernández MR, Biosca JA, Julien A, Dequin S. 2009. Engineering of 2,3-butanediol dehydrogenase to reduce acetoin formation by glycerol-overproducing, low-alcohol Saccharomyces cerevisiae. Appl. Environ. Microbiol. 75:3196–3205. 10.1128/AEM.02157-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varela C, Kutyna DR, Solomon MR, Black CA, Borneman A, Henschke PA, Pretorius IS, Chambers PJ. 2012. Evaluation of gene modification strategies for the development of low-alcohol-wine yeasts. Appl. Environ. Microbiol. 78:6068–6077. 10.1128/AEM.01279-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blomberg A, Adler L. 1992. Physiology of osmotolerance in fungi. Adv. Microb. Physiol. 33:145–212. 10.1016/S0065-2911(08)60217-9 [DOI] [PubMed] [Google Scholar]
- 12.Noble AC, Bursick GF. 1984. The contribution of glycerol to perceived viscosity and sweetness in white wine. Am. J. Enol. Vitic. 35:110–112 [Google Scholar]
- 13.Nevoigt E, Stahl U. 1996. Reduced pyruvate decarboxylase and increased glycerol-3-phosphate dehydrogenase [NAD+] levels enhance glycerol production in Saccharomyces cerevisiae. Yeast 12:1331–1337 [DOI] [PubMed] [Google Scholar]
- 14.Michnick S, Roustan J-L, Remize F, Barre P, Dequin S. 1997. Modulation of glycerol and ethanol yields during alcoholic fermentation in Saccharomyces cerevisiae strains overexpressed or disrupted for GPD1 encoding glycerol 3-phosphate dehydrogenase. Yeast 13:783–793 [DOI] [PubMed] [Google Scholar]
- 15.Remize F, Roustan J-L, Sablayrolles J-M, Barre P, Dequin D. 1999. Glycerol overproduction by engineered Saccharomyces cerevisiae wine yeast strains leads to substantial changes in by-product formation and to a stimulation of fermentation rate in stationary phase. Appl. Environ. Microbiol. 65:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Barros Lopes M, Rehman A, Gockowiak H, Heinrich AJ, Langridge P, Henschke PA. 2000. Fermentation properties of a wine yeast over-expressing the Saccharomyces cerevisiae glycerol 3-phosphate dehydrogenase gene (GPD2). Aust. J. Grape Wine Res. 6:208–215. 10.1111/j.1755-0238.2000.tb00181.x [DOI] [Google Scholar]
- 17.Cambon B, Monteil V, Remize F, Camarasa C, Dequin S. 2006. Effects of GPD1 overexpression in Saccharomyces cerevisiae commercial wine yeast strains lacking ALD6 genes. Appl. Environ. Microbiol. 72:4688–4694. 10.1128/AEM.02975-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kvitek DJ, Sherlock G. 2011. Reciprocal sign epistasis between frequently experimentally evolved adaptive mutations causes a rugged fitness landscape. PloS Genet. 7:e1002056. 10.1371/journal.pgen.1002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhar R, Sägesser R, Weikert C, Yuan J, Wagner A. 2011. Adaptation of Saccharomyces cerevisiae to saline stress through laboratory evolution. J. Evol. Biol. 24:1135–1153. 10.1111/j.1420-9101.2011.02249.x [DOI] [PubMed] [Google Scholar]
- 20.Gerstein AC, Cleathero LA, Mandegar MA, Otto SP. 2011. Haploids adapt faster than diploids across a range of environments. J. Evol. Biol. 24:531–540. 10.1111/j.1420-9101.2010.02188.x [DOI] [PubMed] [Google Scholar]
- 21.Cakar ZP, Seker UOS, Tamerler C, Sonderegger M, Sauer U. 2005. Evolutionary engineering of multiple-stress resistant Saccharomyces cerevisiae. FEMS Yeast Res. 5:569–578. 10.1016/j.femsyr.2004.10.010 [DOI] [PubMed] [Google Scholar]
- 22.McBryde C, Gardner JM, de Barros Lopes M, Jiranek BV. 2006. Generation of novel wine yeast strains by adaptive evolution. Am. J. Enol. Vitic. 57:423–430 [Google Scholar]
- 23.Wisselink HW, Toirkens MJ, Wu Q, Pronk JT, van Maris AJA. 2009. Novel evolutionary engineering approach for accelerated utilization of glucose, xylose, and arabinose mixtures by engineered Saccharomyces cerevisiae strains. Appl. Environ. Microbiol. 75:907–914. 10.1128/AEM.02268-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanley D, Fraser S, Chambers PJ, Rogers P, Stanley GA. 2010. Generation and characterisation of stable ethanol-tolerant mutants of Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 37:139–149. 10.1007/s10295-009-0655-3 [DOI] [PubMed] [Google Scholar]
- 25.Kutyna DR, Varela C, Stanley GA, Borneman AR, Henschke PA, Chambers PJ. 2012. Adaptive evolution of Saccharomyces cerevisiae to generate strains with enhanced glycerol production. Appl. Microbiol. Biotechnol. 93:1175–1184. 10.1007/s00253-011-3622-7 [DOI] [PubMed] [Google Scholar]
- 26.Cadière A, Ortiz-Julien A, Camarasa C, Dequin S. 2011. Evolutionary engineered Saccharomyces cerevisiae wine yeast strains with increased in vivo flux through the pentose phosphate pathway. Metab. Eng. 13:263–271. 10.1016/j.ymben.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 27.Bell G, Gonzalez A. 2011. Adaptation and evolutionary rescue in metapopulations experiencing environmental deterioration. Science 332:1327–1330. 10.1126/science.1203105 [DOI] [PubMed] [Google Scholar]
- 28.Aguilera J, Andreu P, Randez-Gil F, Prieto JA. 2010. Adaptive evolution of baker's yeast in a dough-like environment enhances freeze and salinity tolerance. Microb. Biotechnol. 3:210–221. 10.1111/j.1751-7915.2009.00136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bely M, Sablayrolles J-M, Barre P. 1990. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J. Ferment. Bioeng. 70:246–252. 10.1016/0922-338X(90)90057-4 [DOI] [Google Scholar]
- 30.Aguera E, Sablayrolles J-M. 2005. Pilot scale vinifications (100l). III. Controlled fermentations. InfoWine Internet J. Vitic. Enol. http://www.infowine.com/default.asp?scheda=2784&provenienza=15 [Google Scholar]
- 31.Blateyron L, Aguera E, Dubois C, Gerland C, Sablayrolles J-M. 1998. Control of oxygen additions during alcoholic fementations. Vitic. Enol. Sci. 53:131–135 [Google Scholar]
- 32.Huxley C, Green ED, Dunham I. 1990. Rapid assessment of S. cerevisiae mating type by PCR. Trends Genet. 6:236. 10.1016/0168-9525(90)90190-H [DOI] [PubMed] [Google Scholar]
- 33.Hagenauer-Hener U, Henn D, Fettmar F, Mosandl A, Schmitt A. 1990. 2,3 Butanediol-Direkte Bestimmung der Stereoisomeren im Wein. Dtsch. Lebensm.-Rundsch. 86:273–276 [Google Scholar]
- 34.Lundquist F. 1974. Acetaldehyd: Bestimmung mit Aldehyd-dehydrogenase, p 1509–1513 In Bergmeyer HU. (ed), Methods of enzymatic analysis, Verlag Chemie, Weinheim, Germany. [Google Scholar]
- 35.García MJ, Ríos G, Ali R, Bellés JM, Serrano R. 1997. Comparative physiology of salt tolerance in Candida tropicalis and Saccharomyces cerevisiae. Microbiology 143:1125–1131. 10.1099/00221287-143-4-1125 [DOI] [PubMed] [Google Scholar]
- 36.Torija MJ, Rozès N, Poblet M, Guillamón JM, Mas A. 2003. Effects of fermentation temperature on the strain population of Saccharomyces cerevisiae. Int. J. Food Microbiol. 80:47–53. 10.1016/S0168-1605(02)00144-7 [DOI] [PubMed] [Google Scholar]
- 37.Granot D, Snyder M. 1993. Carbon source induces growth of stationary phase yeast cells, independent of carbon source metabolism. Yeast 9:465–479. 10.1002/yea.320090503 [DOI] [PubMed] [Google Scholar]
- 38.Wei M, Fabrizio P, Madia F, Hu J, Ge H, Li LM, Longo VD. 2009. Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PloS Genet. 5:e1000467. 10.1371/journal.pgen.1000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Easlon E, Tsang F, Skinner C, Wang C, Lin S-J. 2008. The malate-aspartate NADH shuttle components are novel metabolic longevity regulators required for calorie restriction-mediated life span extension in yeast. Genes Dev. 22:931–944. 10.1101/gad.1648308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaeberlein M, Andalis AA, Fink GR, Guarente L. 2002. High osmolarity extends life span in Saccharomyces cerevisiae by a mechanism related to calorie restriction. Mol. Cell. Biol. 22:8056–8066. 10.1128/MCB.22.22.8056-8066.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orozco H, Matallana E, Aranda A. 2013. Genetic manipulation of longevity-related genes as a tool to regulate yeast life span and metabolite production during winemaking. Microb. Cell Factories 12:1. 10.1186/1475-2859-12-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heyland J, Fu J, Blank LM. 2009. Correlation between TCA cycle flux and glucose uptake rate during respiro-fermentative growth of Saccharomyces cerevisiae. Microbiology 155:3827–3837. 10.1099/mic.0.030213-0 [DOI] [PubMed] [Google Scholar]
- 43.González E, Fernández MR, Larroy C, Solà L, Pericàs MA, Parés X, Biosca JA. 2000. Characterization of a (2R,3R)-2,3-butanediol dehydrogenase as the Saccharomyces cerevisiae YAL060W gene product. Disruption and induction of the gene. J. Biol. Chem. 275:35876–35885. 10.1074/jbc.M003035200 [DOI] [PubMed] [Google Scholar]
- 44.Contreras A, Hidalgo C, Henschke PA, Chambers PJ, Curtin C, Varela C. 2013. Evaluation of non-Saccharomyces yeast for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 80:1670–1678. 10.1128/AEM.03780-13 [DOI] [PMC free article] [PubMed] [Google Scholar]