Abstract
The tuberculin skin test for diagnosing tuberculosis (TB) in cattle lacks specificity if animals are sensitized to environmental mycobacteria, as some antigens in purified protein derivative (PPD) prepared from Mycobacterium bovis are present in nonpathogenic mycobacteria. Three immunodominant TB antigens, ESAT6, CFP10, and Rv3615c, are present in members of the pathogenic Mycobacterium tuberculosis complex but absent from the majority of environmental mycobacteria. These TB antigens have the potential to enhance skin test specificity. To increase their immunogenicity, these antigens were displayed on polyester beads by translationally fusing them to a polyhydroxyalkanoate (PHA) synthase which mediated formation of antigen-displaying inclusions in recombinant Escherichia coli. The most common form of these inclusions is poly(3-hydroxybutyric acid) (PHB). The respective fusion proteins displayed on these PHB inclusions (beads) were identified using tryptic peptide fingerprinting analysis in combination with matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). The surface exposure and accessibility of antigens were assessed by enzyme-linked immunosorbent assay (ELISA). Polyester beads displaying all three TB antigens showed greater reactivity with TB antigen-specific antibody than did beads displaying only one TB antigen. This was neither due to cross-reactivity of antibodies with the other two antigens nor due to differences in protein expression levels between beads displaying single or three TB antigens. The triple-antigen-displaying polyester beads were used for skin testing of cattle and detected all cattle experimentally infected with M. bovis with no false-positive reactions observed in those sensitized to environmental mycobacteria. The results suggested applicability of TB antigen-displaying polyester inclusions as diagnostic reagents for distinguishing TB-infected from noninfected animals.
INTRODUCTION
Bovine tuberculosis (TB) is a major animal health problem worldwide, with approximately 50 million cattle infected with Mycobacterium bovis, the causative agent of this disease (1). Control of this disease is predominantly achieved using a “test and slaughter” program, where cattle are regularly tested using the tuberculin skin test which utilizes purified protein derivatives prepared from M. bovis (bovine PPD) and Mycobacterium avium (avian PPD) or bovine PPD alone and reacting animals are slaughtered. By use of these programs together with movement control of animals, bovine TB has been eradicated from a number of countries (2). Bovine PPD is a poorly defined mix of proteins, lipids, and carbohydrates, and in certain situations, the use of PPD in the skin test lacks specificity as some of its antigenic components are present in nonpathogenic environmental mycobacteria. Recently, there has been interest in developing highly specific skin test reagents utilizing selected proteins from the Mycobacterium tuberculosis complex which are not expressed by the majority of the environmental mycobacteria or the human tuberculosis vaccine strain, bacille Calmette-Guérin (BCG) (3, 4). For widespread use of a skin test, the skin test reagents must be produced at a relatively low cost. This could be achieved by producing them as a recombinant fusion protein and increasing their immunogenicity by displaying them on nanoparticles. To achieve this, a new development has been the display of foreign proteins on polyester inclusions produced by recombinant bacteria (5).
Polyhydroxyalkanoates (PHAs) are naturally occurring biopolyesters produced by various bacteria and some archaeal species and composed of (R)-3-hydroxy fatty acids. They are synthesized during imbalanced nutrient availability in which excess carbon is available and deposited as spherical water-insoluble cytoplasmic inclusions (6, 7). PHAs, of which poly(3-hydroxybutyric acid) (PHB) is the most common form, rely on various enzymes for their synthesis. The PHB biosynthesis in Cupriavidus necator requires three key enzymes: β-ketothiolase (PhaA), acetoacetyl coenzyme A (CoA) reductase (PhaB), and the polyester synthase (PhaC). PhaA and PhaB are involved in the formation of the precursor molecule (R)-3-hydroxybutyryl-CoA, which is polymerized to PHB by PhaC (8, 9). Bacteria are able to produce 5 to 10 polyester beads on average per cell, and the size is 100 to 500 nm in diameter (10). These beads contain an amorphous hydrophobic polyester core surrounded by proteins, such as PhaC, depolymerase, and structural proteins (6, 7).
Recently, polyester beads have been considered a versatile platform for the display of foreign proteins (11). Extensive engineering of PhaC enabled the identification of variable dispensable regions which can be functionally replaced by foreign proteins without impacting polyester bead formation (12). Examples of foreign protein functions which have been successfully displayed on polyester beads using PhaC engineering are enzymes, binding domains/peptides, and antigens (13–15). Simultaneous fusions of several foreign proteins as well as coproduction of different PhaC fusion proteins enabled the codisplay of different protein functions on the same polyester bead with defined stoichiometry of the various proteins (12, 16–18). Foreign target proteins have been displayed on polyester beads for subsequent cleavage and purification (19–22). Polyester beads displaying antigens were found to be immunogenic and mediated Th1 and Th2 immune responses leading to protective immunity against TB (14, 23, 24). Hence, custom-made polyester beads displaying selected TB antigens might serve as a specific TB diagnostic reagent in skin tests.
Three specific TB antigens, the 6-kDa early secretory antigenic target (ESAT6), the 10-kDa culture filtrate protein (CFP10), and Rv3615c, are expressed by members of the pathogenic M. tuberculosis complex, which includes M. bovis, but are not expressed by the majority of nonpathogenic environmental mycobacteria and the BCG vaccine strain (25, 26). These immunodominant proteins have been evaluated in the gamma interferon (IFN-γ) test for the diagnosis of TB in cattle, and results have been very satisfactory (27–29). Recently, the skin testing of cattle using a combination of these three proteins indicated that they had a high sensitivity for detection of animals infected with M. bovis while differentiating against those vaccinated with BCG (3, 4). The current study investigated whether these three antigens could be functionally displayed on polyester beads and whether these beads would perform effectively in cattle TB skin tests. The aim is to develop a cost-effective TB skin test, which could differentiate cattle infected with M. bovis from those naturally sensitized to environmental mycobacteria.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. The E. coli strain used for cloning, XL1-Blue (Stratagene), transformed with the expression plasmids was grown in Luria broth (LB) (Difco, Detroit, MI) supplemented with tetracycline (12.5 μg/ml) and ampicillin (75 μg/ml). Medium for growth of E. coli BL21(DE3) used for production of polyester beads contained chloramphenicol (50 μg/ml) instead of tetracycline.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| E. coli strains | ||
| BL21(DE3) | F− dcm ompT hsdS(rB− mB−) gal λ(DE3) | Stratagene |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
| Plasmid name | ||
| pET-14b | Apr; T7 promoter | Novagen |
| pET-14b phaC | pET-14b derivative containing phaC gene fragment | 22 |
| pET-14b cfp10-phaC | pET-14b derivative containing NdeI fragment gene cfp10 fused to the 5′ end of phaC | This study |
| pET-14b rv3615c-phaC | pET-14b derivative containing NdeI fragment gene rv3615c fused to the 5′ end of phaC | This study |
| pET-14b esat6-phaC | pET-14b derivative containing NdeI fragment gene esat6 fused to the 5′ end of phaC | This study |
| pET-14b cfp10-linker-rv3615c-phaC | pET-14b derivative containing SpeI fragment genes cfp10-linker-rv3615c fused to the 5′ end of phaC | This study |
| pET-14b cfp10-linker-rv3615c-phaC-esat6 | pET-14b derivative containing SpeI fragment genes cfp10-linker-rv3615c fused to the 5′ end of phaC and XhoI/BamHI fragment gene esat6 fused to the 3′ end of phaC via a linker sequence | This study |
| pMCS69 | Cmr; T7 promoter, pBBR1MCS derivative containing the genes phaA and phaB from C. necator colinear to lac promoter | 31 |
| pUC57-esat6 | Cloning vector, ColE1 origin, Apr; XhoI/BamHI fragment gene esat6 | This study |
Tetr, tetracycline resistance; Apr, ampicillin resistance; Cmr, chloramphenicol resistance.
Plasmids, oligonucleotides, and generation of plasmids for production of biobeads displaying mycobacterial antigens.
Plasmids and primers used in this study are listed in Table 1 and Table S1 in the supplemental material, respectively. General molecular cloning procedures were implemented as described elsewhere (30). DNA sequences of new plasmid constructs were verified by DNA sequencing. The biosynthesis of polyester requires, in addition to the polyester synthase gene (phaC), the enzymes PhaA and PhaB for precursor synthesis, and these enzymes were encoded on the plasmid pMCS69 (31).
To display the TB genes on the surfaces of the polyester beads, the genes encoding ESAT6 with amino acid sequence MTEQQWNFAGIEAAASAIQGNVTSIHSLLDEGKQSLTKLAAAWGGSGSEAYQGVQQKWDATATELNNALQNLARTISEAGQAMASTEGNVTGMFA, CFP10 with amino acid sequence MAEMKTDAATLAQEAGNFERISGDLKTQIDQVESTAGSLQGQWRGAAGTAAQAAVVRFQEAANKQKQELDEISTNIRQAGVQYSRADEEQQQALSSQMGF, and RV3615c with amino acid sequence MTENLTVQPERLGVLASHHDNAAVDASSGVEAAAGLGESVAITHGPYCSQFNDTLNVYLTAHNALGSSLHTAGVDLAKSLRIAAKIYSEADEAWRKAIDGLFT were synthesized by DNA2.0 (CA, USA), optimized to the codon usage of E. coli. An NdeI restriction site was inserted at both the 5′ and the 3′ ends of each gene.
The respective synthesized NdeI fragments encoding CFP10, ESAT6, or Rv3615c were inserted into the plasmid pHAS-Ag85A-ESAT-6 (23) after hydrolysis with NdeI, which resulted in plasmids pET-14b cfp10-phaC, pET-14b Rv3615c-phaC, and pET-14b Esat-6-PhaC, respectively.
The plasmid construct pET-14b cfp10-linker-rv3615c-phaC-esat6, carrying the three TB antigen genes, was made as follows.
The XhoI-BamHI fragment carrying the esat6 gene was subcloned into pET-14b cfp10-linker-rv3615c-phaC-MalE hydrolyzed with XhoI-BamHI to generate the final plasmid, pET-14b cfp10-linker-rv3615c-phaC-esat6, which contained the three TB antigen genes.
A schematic representation of the plasmids used to display TB antigens on the surface of the polyester beads is shown in Fig. 1. The DNA constructs containing one TB gene, pET-14b cfp10-phaC, pET-14b rv3615c-phaC, and pET-14b esat6-phaC, and three TB genes, pET-14b cfp10-linker-rv3615c-phaC-esat6, are shown.
FIG 1.
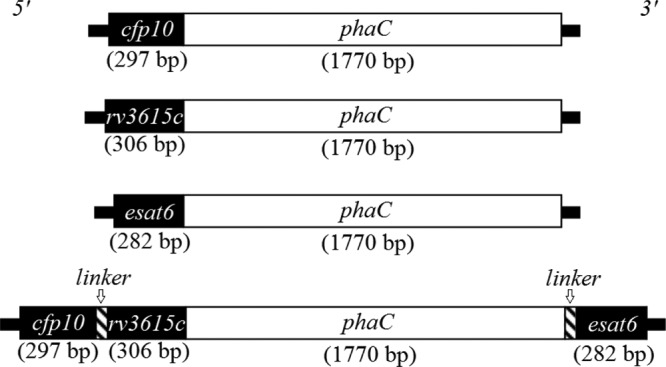
Schematic representation of hybrid genes encoding fusion proteins which mediated production of polyester beads displaying TB antigens.
Production and isolation of polyester beads.
Polyester beads displaying mycobacterial proteins or control beads alone were produced in E. coli BL21(DE3) as previously described (23).
Briefly, E. coli strains were grown at 37°C in LB, induced with 1 mM isopropyl-β-d-thiogalactopyranoside to induce protein, and cultured for a further 48 h at 25°C. The presence of polyester inclusions was observed by staining cells with the fluorescent lipophilic dye Nile Red and by using fluorescence microscopy (32). Transmission electron microscopy (TEM) was employed to assess the shape and size of polyester inclusion as well as the number per cell. Polyester granules were isolated as previously described (33). Protease inhibitors (Complete product, EDTA free; Roche, USA) were added as required. To confirm the functionality of the PhaC enzyme, the polyester content of the cells was quantitatively determined by gas chromatography-mass spectroscopy (GC-MS) (34).
Analysis of proteins attached to polyester beads.
Proteins attached to the polyester beads were separated by SDS-PAGE using 8% polyacrylamide gels and stained with Coomassie blue. Proteins of interest were excised from the gels and subjected to tryptic peptide fingerprinting using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (17).
ELISA.
Specific activity of the beads displaying the TB antigens was determined by enzyme-linked immunosorbent assay (ELISA) as previously described (23). Briefly, a high-binding-capacity microtiter plate (Greiner Bio-One) was coated overnight at 4°C with 100 μl of purified PHB beads displaying TB proteins or control beads, diluted in carbonate-bicarbonate coating buffer, pH 9.6 (Sigma-Aldrich), at protein concentrations ranging from 100 μg/ml to 0.05 μg/ml over serial dilutions. As positive controls, the microtiter plates were also coated overnight at 4°C with 100 μl of free single antigen or a mixture of the three single TB antigens, kindly provided by H. M. Vordermeier (AHVLA, United Kingdom), diluted in carbonate-bicarbonate coating buffer, at protein concentrations ranging from 1 μg/ml to 0.125 μg/ml. Plates were washed with phosphate-buffered saline (PBS) containing 0.05% (vol/vol) Tween 20 (PBST) and blocked with 3% (wt/vol) bovine serum albumin (BSA) for 1 h at room temperature. Plates were washed with PBST and incubated with mouse monoclonal antibody to CFP10 or ESAT6 (both antibodies were from Abcam, Cambridge, United Kingdom) or polyclonal rabbit sera produced against recombinant Rv3615c (AgResearch, Hamilton, New Zealand) or preimmune rabbit serum for 1 h at room temperature. The rabbit antiserum against Rv3615c was produced by immunizing a rabbit with 100 μg of Rv3615c mixed in incomplete Freund's adjuvant (Sigma) and revaccinating the rabbit twice at 3-week intervals. Following washing with PBST, plates were incubated for 1 h with anti-mouse IgG-horseradish peroxidase (HRP) conjugate or anti-rabbit HRP conjugate. After further washing, o-phenylenediamine (OPD) substrate (Abbott Diagnostics, IL, USA) was added and incubated for 15 min at room temperature. The reaction was stopped with 0.5 N H2SO4, and the absorbance was measured at 490 nm on an ELx808iu ultramicrotiter plate reader (Bio-Tek Instruments Inc., USA) (16). Results were expressed as optical density units at 490 nm.
TB skin test on cattle.
Ten 15-month-old Friesian-cross cattle were experimentally infected with a dose of approximately 5 × 103 CFU of M. bovis intratracheally as previously described (35). These animals were kept on pasture in an isolation unit which was completely separate from paddocks where the 14 control cattle of equivalent age and breed were grazing. All cattle were sourced from TB-free herds and located in TB-free regions of New Zealand. Prior to the M. bovis inoculation, the cattle tested negative in the gamma interferon test (Bovigam test; Prionics, Switzerland) for bovine TB, and the control cattle were negative in this test throughout the study using the standard interpretation as described previously (36). At 27 weeks after M. bovis infection, the infected and control cattle were tested in a comparative cervical skin test comparing responses for triple-antigen TB polyester beads and control polyester beads with those for PPD from M. bovis (PPD-B; 5,000 IU/0.1-ml injection) and M. avium (PPD-A; 2,500 IU/0.1-ml injection) (AsureQuality, Upper Hutt, New Zealand). The 0.1-ml inoculum of the TB polyester bead reagent contained 3.3 μg of fusion protein, comprising 0.9 μg of the mycobacterial proteins and 2.4 μg of PhaC protein, mixed in PBS, while the control polyester beads contained 3.3 μg of PhaC protein in PBS. The skin thickness at the site of injection was measured with calipers immediately prior to injection and 72 h later, and results were expressed as the change in skin thickness (mm). The M. bovis-infected animals were killed and necropsied at 28 weeks after infection. Tuberculous lesions typical of those found in naturally infected animals were identified in the lungs and/or pulmonary lymph nodes of the 10 animals, and M. bovis was cultured from the lesions of all these animals using the Bactec method and confirmation by AccuProbe. All animal procedures were approved by the AgResearch Grasslands animal ethics committee.
Statistical analyses.
The Kruskal-Wallis test was used to compare the in vitro ELISA responses for the antigen activity on the beads and analyses of the cross-reactivity of the antisera with heterologous antigens. Skin test responses to PPD-B and the triple-TB-antigen beads were compared by analysis of variance (ANOVA). The correlation between the skin test responses for bovine PPB and the triple-antigen bead in the experimentally infected cattle was undertaken using Spearman's rank correlation test. Statistical analyses were conducted using the Agricolae package in R.3.0.1, and statistical significance was defined when P was <0.05.
RESULTS
Production and characterization of polyester beads displaying mycobacterial antigens.
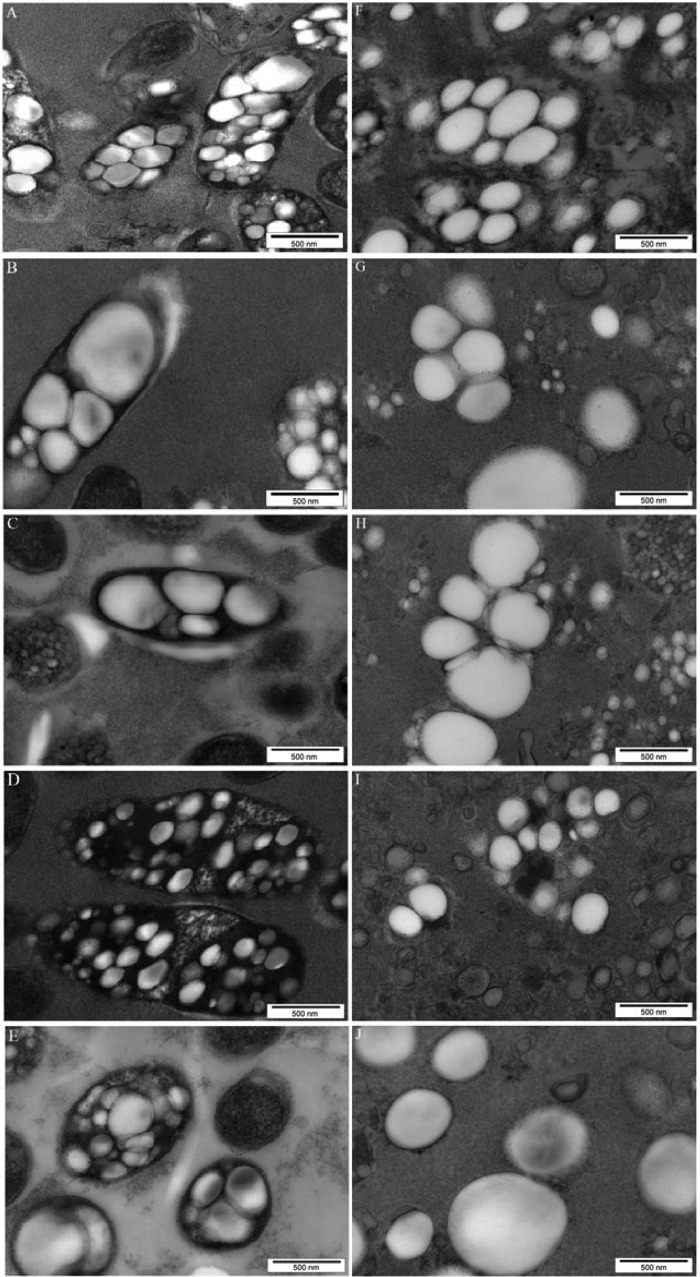
Plasmids encoding PhaC and containing either esat6, cfp10, or rv3615c or all three mycobacterial genes were constructed as described in Materials and Methods. The modular compositions of the various hybrid genes and encoded fusion proteins are outlined in Fig. 1. Hybrid genes were constructed to encode fusion proteins which mediate intracellular production of polyester beads displaying either single TB antigens or all three TB antigens. Respective plasmids were introduced into E. coli cells harboring the plasmid pMCS69, which mediates provision of precursors for polyester synthesis. E. coli cells harboring the various plasmids were cultured to produce polyester beads displaying one or three TB antigens. The presence of intracellular polyester beads in the E. coli cells was indicated by fluorescence microscopy using Nile Red staining (see Fig. S1 in the supplemental material). Transmission electron microscopy showed formation of polyester beads mediated by the respective fusion protein inside recombinant E. coli (Fig. 2). GC-MS analysis showed that cells were accumulating the polyester polyhydroxybutyrate, contributing to 31.5%, 10%, 30%, 14.3%, and 40% of cellular dry weight when genes encoding PhaC, CFP10-PhaC, Rv3615c-PhaC, ESAT6-PhaC, and CFP10-Rv3615c-PhaC-ESAT6 were present, respectively.
FIG 2.
TEM analysis of recombinant E. coli harboring various plasmids (A to E) and of isolated polyester beads displaying mycobacterial antigens (F to J). (A and F) pET-14b phaC; (B and G) pET-14b cfp10-phaC; (C and H) pET-14b rv3615c-phaC; (D and I) pET-14b esat6-phaC; (E and J) pET-14b cfp10-rv3615c-phaC-esat6.
Display of recombinant PhaC-TB antigen fusion protein on polyester beads.
To test whether recombinant fusion proteins were immobilized to polyester beads and whether proteins were susceptible to proteolytic degradation, SDS-PAGE was performed to analyze the polyester bead protein profile. Dominant protein bands were observed, the apparent molecular weights of which corresponded to proteins with theoretical molecular masses of 63 kDa for PhaC, 74.3 kDa for the ESAT6-PhaC fusion, 75.2 kDa for CFP10-PhaC, 75.2 kDa for PhaC-Rv3615c, and 98.1 kDa for CFP10-Rv3615c-PhaC-ESAT6 (Fig. 3). Moreover, these four fusion proteins (CFP10-PhaC, Rv3615c-PhaC, ESAT6-PhaC, and CFP10-Rv3615c-PhaC-ESAT6) were identified by tryptic peptide fingerprinting using MALDI-TOF MS (17) (see Table S2 in the supplemental material). Densitometry analysis of the SDS-PAGE gel showed that PhaC accounted for 20% of the total protein in the PhaC bead fraction; CFP10-PhaC, Rv3615c-PhaC, ESAT6-PhaC, and CFP10-Rv3615c-PhaC-ESAT6 accounted for about 26% of the total protein in their corresponding bead fraction.
FIG 3.
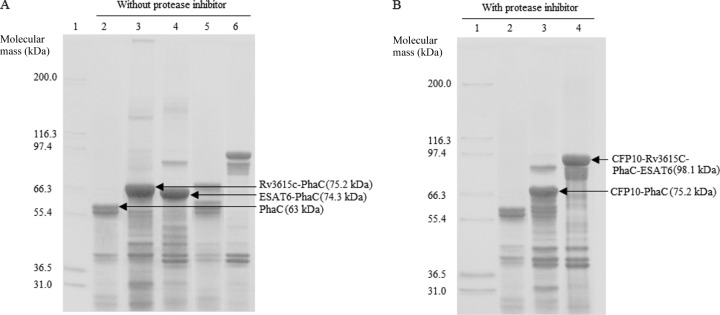
Protein profiles of polyester beads isolated from recombinant E. coli harboring various plasmids without (A) and with (B) a protease inhibitor treatment. (A) Samples without protease inhibitor treatment during bead extraction. Lane 1, molecular weight marker (Mark 12; Invitrogen); lane 2, PhaC (wild type, 63 kDa); lane 3, Rv3615c-PhaC (75.2 kDa); lane 4, ESAT6-PhaC (74.3 kDa); lane 5, CFP10-PhaC (75.2 kDa); lane 6, CFP10-Rv3615c-PhaC-ESAT6 (98.1 kDa). (B) Samples with protease inhibitor treatment during bead isolation. Lane 1, molecular weight marker; lane 2, PhaC; lane 3, CFP10-PhaC; lane 4, CFP10-Rv3615c-PhaC-ESAT6. The presence of PhaC-TB antigen fusion proteins was confirmed by tryptic peptide fingerprinting using MALDI-TOF MS (see Table S2 in the supplemental material).
Protein degradation was also assessed. As shown in Fig. 3, the samples in Fig. 3A were not treated with a protease inhibitor during bead isolation. The Rv3615c-PhaC and ESAT6-PhaC fusion proteins were stable and did not show degradation (Fig. 3A, lanes 3 and 4). However, there was an increased level of protein degradation in the CFP10-PhaC and CFP10-Rv3615c-PhaC-ESAT6 fusion proteins (Fig. 3A, lanes 5 and 6). The samples in Fig. 3B were treated with protease inhibitor during bead extraction. The degradation of these two recombinant fusion proteins containing CFP10 was significantly inhibited by protease inhibitor (Fig. 3B, lanes 3 and 4). This suggested that the recombinant fusion proteins containing CFP10 were sensitive to protease digestion during bead isolation and purification (Fig. 3). A schematic view of the triple-antigen-displaying polyester beads is provided in Fig. 4.
FIG 4.
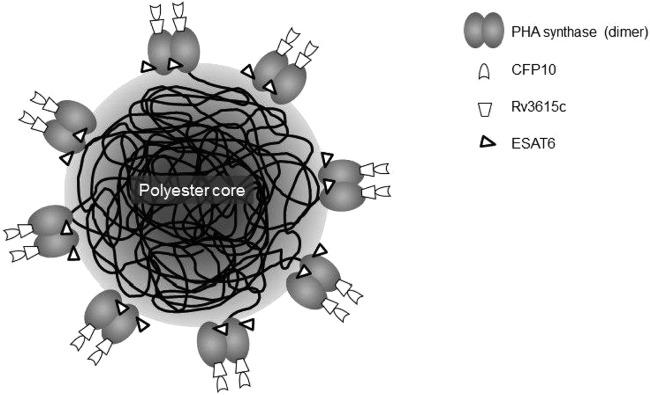
Model of a triple-TB-antigen-displaying polyester bead produced by recombinant E. coli.
Assessment of TB antigen reactivity in vitro.
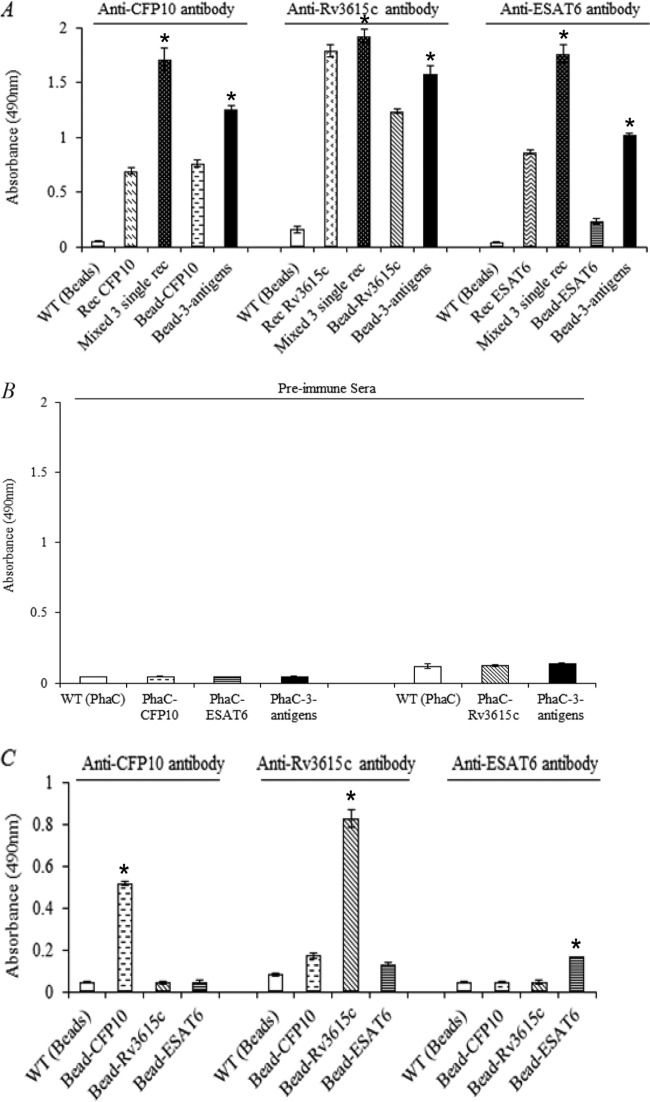
The applicability of polyester beads displaying one or more TB antigens as TB diagnostic reagents was assessed. ELISA was used to probe the accessibility and structural integrity of TB antigens displayed on the surface of polyester beads in vitro (Fig. 5).
FIG 5.
Assessment of TB antigen reactivity in vitro. The ELISA is described in Materials and Methods. All measurements were conducted in triplicate. The mean of TB antigen reactivity to a specific antibody is reported ± the standard deviation. (A) Reactivity of TB antigens on the surface of polyester beads. Wild type (WT) (beads) is the negative control. Recombinant TB antigens (CFP10, Rv3615c, ESAT6, and the mixture of CFP10, Rv3615c, and ESAT6) were used as positive controls. The testing samples are immobilized TB antigens (bead-CFP10, bead-Rv3615c, bead-ESAT6, and bead-CFP10-Rv3615c-ESAT6). The anti-CFP10 and anti-ESAT6 were mouse monoclonal antibodies, and the anti-RV3615C polyclonal antibody was produced in a rabbit. *, significantly higher than single antigen (P < 0.05) (B) Control experiment; the rabbit preimmune serum was used to replace the three specific antibodies as indicated in panel A. (C) Cross-reactivities of specific antibodies with the other two TB antigens. The total protein concentrations of control beads, TB antigen beads, and free recombinant antigens in this figure were 1 μg/ml, 1 μg/ml, and 0.125 μg/ml, respectively. *, significantly higher than all other beads (P < 0.05).
In the reactivity test for the immobilized TB antigens (Fig. 5A), the negative control was the polyester beads carrying only PhaC. The positive controls were free single antigen and a mixture of the three antigens (CFP10, Rv3615c, and ESAT6). Beads carrying the various TB antigens were analyzed. A very low antibody binding of the negative control was observed, which indicated that the polyester beads with no TB antigen displayed did not have binding sites for the three antibodies (anti-CFP10, anti-Rv3615c, and anti-ESAT6). In contrast, there was a dramatic increase in the antibody binding of the positive controls. Particularly, the antibody binding of the immobilized triple TB antigens was significantly higher than that for the displayed single antigens; this was also observed with the soluble triple and soluble single TB antigens (Fig. 5A, P < 0.05).
Moreover, the negative-control experiment using rabbit preimmune sera instead of the three specific antibodies showed that all the samples have very low antibody binding to the negative-control serum (Fig. 5B). Furthermore, the cross-reactivity test showed that the antibody binding of TB antigens was specific and that the antibody binding of TB antigens was significantly higher when the corresponding antibody was applied compared to that for the heterologous antigens (Fig. 5C, P < 0.05).
Assessment of TB antigen reactivity in vivo.
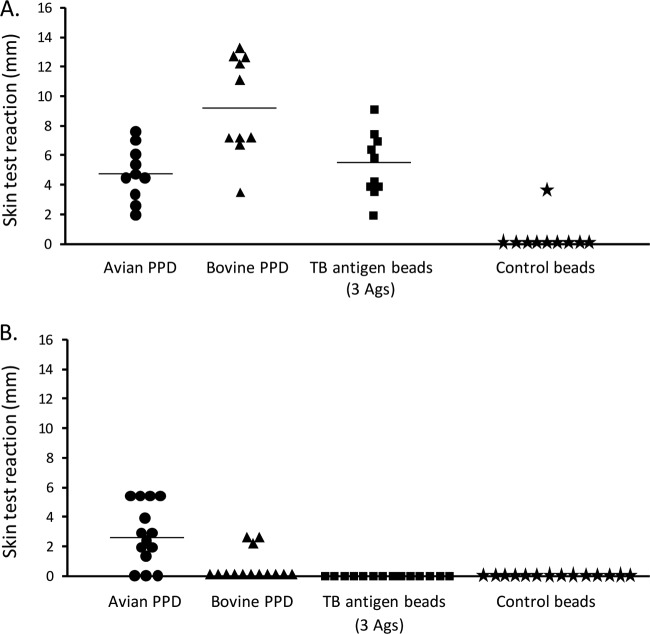
To test the immunogenicity and specificity of the triple-TB-antigen-displaying beads in vivo, the respective beads were intradermally injected into cattle. Four reagents, beads displaying the triple TB antigens, control beads, PPD-B, and PPD-A, were administered for the TB skin tests (Fig. 6). All of the experimentally infected animals responded positively in the skin test (≥1-mm increase in skin test thickness) to PPD-B alone, and in addition, all PPD-B responses were greater than those for PPD-A. Responses to a 0.9-μg injection of TB antigens displayed on polyester beads were also positive for all animals, and there were no significant differences in the responses between PPD-B and TB polyester beads (P > 0.05). Despite all of the infected animals responding to both of these skin test reagents, there was no significant linear correlation between the sizes of the reactions for the two reagents in individual animals (Spearman's rank correlation, rho = 0.133, P = 0.714). Two animals produced a weak response (2.5-mm increase in skin thickness) to the control polyester beads. In contrast, when testing these skin test reagents in 14 equivalent-aged cattle, naturally sensitized to environmental mycobacteria, none responded positively to the TB polyester bead reagents, while 11 responded positively to PPD-A and three to PPD-B. Eleven of the 14 noninfected animals were retested with avian and bovine PPD and the TB polyester beads 7 months later. The two animals with the strongest responses to avian PPD both responded to bovine PPD, although these responses were smaller than those for avian PPD. However, both animals did not produce a positive response to the TB polyester beads, indicating the specificity of the TB polyester beads.
FIG 6.
TB skin test response. Four diagnostic reagents, avian PPD, bovine PPD, beads displaying three TB antigens (Ags), and control beads, were used to skin test cattle. The result was positive if the increase in skin thickness was ≥1 mm. (A) Cattle experimentally infected by M. bovis. (B) Noninfected cattle naturally exposed to environmental mycobacteria. The bar indicates the median.
DISCUSSION
Previous studies had shown that polyester inclusions can be engineered to display desired antigens (37). These polyester beads showed immunogenicity in vivo leading to specific Th1 and Th2 immune responses (14, 23, 24). The aim of this study was to genetically engineer E. coli to produce fusion proteins which mediate formation of polyester beads displaying selected TB antigens (CFP10, ESAT6, and Rv3615c) suitable for TB skin test applications. The display of selected TB antigens at the surface of bioengineered immunogenic polyester beads is likely to be important for the sensitivity and specificity of the TB skin test. As outlined in Fig. 1, various hybrid genes were constructed and mediated polyester bead production in recombinant E. coli (see Fig. S1 in the supplemental material and Fig. 2). These data suggested that the PhaC domain remained active in all of the fusion proteins, i.e., catalyzed synthesis of the polyester and mediated bead formation (38, 39). Fusion proteins attached to the polyester bead surface which contained the antigen CFP10 were found to be susceptible to protease degradation, which could be avoided by the addition of protease inhibitors during bead isolation (Fig. 3). Previous studies showed that CFP10 existed in at least as four different forms, possibly due to posttranslational modifications (40). It is possible that the respective posttranslational modifications were not achieved in recombinant E. coli, hence leading to partial unfolding of this antigen and susceptibility to protease degradation. Therefore, the degradation of the fusion proteins containing CFP10 after bead isolation and purification might be due to the exposure of these unstable forms of CFP10 to protease, which in turn led to protein degradation.
Polyester bead surface display of the respective TB antigen fused to PhaC was investigated by using specific anti-TB antigen antibodies in combination with ELISA (Fig. 5A). Assessment of cross-reactivity of the specific anti-TB antigen antibodies as well as assessment of possible nonspecific antibody binding using preimmune sera suggested that the anti-TB antigen antibodies were highly specific and that the antigen-displaying beads did not nonspecifically bind to antibodies (Fig. 5B and C). The various beads were found to display the respective TB antigen(s). Interestingly, the triple-TB-antigen-displaying beads showed greater reactivity with the specific antibodies than did the respective single-TB-antigen-displaying beads. This was not due to varying expression levels, as similar amounts of fusion protein were detected per bead mass according to densitometry analysis (Fig. 3). The high reactivity of the reagent containing the three immobilized TB antigens is also not caused by cross-reactivity of antibodies. Each antibody specifically interacted only with the corresponding TB antigen (Fig. 5C). A possible explanation for the greater reactivity for the antigens when displayed on the triple-antigen beads could relate to greater accessibility of the antibodies to the proteins. This could result from differences in the secondary or tertiary structure of the proteins when displayed on the triple-antigen beads, or the PhaC fusion proteins may be displaced further apart at the surface on the triple-antigen beads.
The polyester bead containing the triple TB antigens was selected to investigate its applicability as a TB skin test reagent due to its high reactivity with specific antibodies. In addition, the manufacture of only one multiple-antigen polyester bead type should be more cost-effective than producing several single-antigen beads. There is also a demand for a specific diagnostic reagent which distinguishes TB-infected from environmental strain-sensitized or BCG-vaccinated individuals. A comparative cervical skin test is used in many countries for the diagnosis of bovine TB, as both PPD-A and PPD-B contain antigens common to many environmental mycobacteria and a stronger response to PPD-B than to PPD-A is indicative of infection with M. bovis (41). TB antigens CFP10, Rv3615c, and ESAT6 (25) are found in members of the pathogenic M. tuberculosis complex which includes M. bovis, and therefore, responses to these antigens would indicate a more specific response than that for PPD-B, containing a crude mix of antigens. In this TB skin test study (Fig. 6), all three reagents (TB antigens), PPD-A, PPD-B, and the triple-TB-antigen bead reagent, were able to identify TB-infected cattle with a high sensitivity (Fig. 6A). However, only the bead reagent containing the triple TB antigens could accurately identify noninfected cattle, naturally exposed to environmental mycobacteria, while PPD-A and PPD-B gave false-positive results (Fig. 6B).
Previous studies have shown that a protein cocktail containing purified soluble CFP10, ESAT6, and Rv3615c could be used as a skin test reagent to distinguish TB-infected from noninfected and BCG-vaccinated cattle (3, 4). Moreover, the immunodominant antigen Rv3615c had been found to identify ESAT6- and/or CFP10-unresponsive TB-infected cattle (29). The addition of Rv3615c to the reagent containing ESAT6 and CFP10 significantly improves the sensitivity of skin tests in the naturally infected cattle and does not cause responses in vaccinated or healthy cattle (3), indicating that the use of a protein cocktail of CFP10, ESAT6, and Rv3615c would be beneficial for TB skin testing. Furthermore, antigens from pathogenic mycobacteria, such as antigen 85A and ESAT6, had been shown to stimulate stronger cellular immune responses when they were displayed on the surface of polyester beads than when they were free antigens (24). Accordingly, CFP10-Rv3615c-ESAT6 immobilized on the surface of a polyester bead might act as a more sensitive and cost-effectively producible diagnostic reagent than the respective free antigens.
One note of caution is that although the genes for esat6 and cfp10 are absent in most environmental mycobacteria, these genes are present in Mycobacterium kansasii, which can cause infections in both humans and cattle (42, 43). Indeed, humans with clinical disease resulting from M. kansasii infection have detectable IFN-γ responses to recombinant and peptide mixes of ESAT6 and CFP10 (42). Waters et al. (43) observed that cattle experimentally infected with M. kansasii produced IFN-γ responses in blood cultures to recombinant ESAT6-CFP10 but less than those infected with M. bovis, although no serum antibody responses to ESAT6 or CFP10 were detected in the M. kansasii-infected cattle. Second, the polyester beads may contain lipopolysaccharide or heat shock proteins which potentially could affect specificity. One experimentally infected animal in the current study produced a positive response to the control beads which may have been a response to heat shock proteins contained in the beads, while none of the control cattle responded to the control beads.
No correlation was found between the size of the skin test responses to PPD-B and the triple-TB-antigen polyester beads in the experimentally infected cattle. This is not surprising, as the strength of the skin test responses to different mycobacterial proteins may vary during the course of the disease and the disease may progress at different rates in individual animals. Recently, it has been found that the three most immunodominant proteins in PPD for inducing skin test responses to TB (GroES, GroEL2, and DnaK) are highly conserved chaperone proteins (44). As the disease progresses, responses to the highly conserved chaperone proteins may increase, while it is recognized that immune responses to proteins such as ESAT6 are first observed at an early stage of M. bovis infection in cattle (45).
The reagent of triple-TB-antigen-displaying polyester beads might also have a potential use in the diagnosis of human TB, mainly caused by M. tuberculosis. Indeed, CFP10, ESAT6, and Rv3615c from M. tuberculosis strongly cross-react with the orthologous proteins in M. bovis, a causative agent of bovine TB (25, 26), and these antigens are immunodominant in both active and latent human TB infection (25, 29).
This novel polyester bead TB diagnostic reagent containing the immobilized triple antigens has high specificity and sensitivity, allowing for quick and accurate screening of the cattle infected by TB. In addition, the polyester bead-based TB skin test has the potential to differentiate between infected and BCG-vaccinated animals as the chosen antigens are not produced by BCG strains. The flexibility of this polyester bead technology would also allow adaptation of this new skin test reagent to newly developed vaccine by implementing differentiating antigens. Overall, it was demonstrated in this study that bacterial polyester beads can be engineered to simultaneously display three TB-specific antigens and that these recombinant beads show specific and sensitive performance as TB skin test reagents. This improved skin test showed fewer false-positive results in cattle than did the commercial skin test reagents, which in turn reduces a significant economic loss for the end user.
Supplementary Material
ACKNOWLEDGMENTS
We thank Gary Yates and Maree Joyce for the M. bovis challenge inoculum and the culture of M. bovis from the animals and H. M. Vordermeier for kindly providing the recombinant mycobacterial antigens for in vitro confirmatory studies.
The research was funded by Massey University and PolyBatics Ltd. and a grant from the New Zealand Ministry of Business, Innovation and Employment.
Footnotes
Published ahead of print 14 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.04168-13.
REFERENCES
- 1.Waters WR, Palmer MV, Buddle BM, Vordermeier HM. 2012. Bovine tuberculosis vaccine research: historical perspectives and recent advances. Vaccine 30:2611–2622. 10.1016/j.vaccine.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 2.Cousins DV. 2001. Mycobacterium bovis infection and control in domestic livestock. Rev. Sci. Tech. 20:71–85 [DOI] [PubMed] [Google Scholar]
- 3.Whelan AO, Clifford D, Upadhyay B, Breadon EL, McNair J, Hewinson GR, Vordermeier MH. 2010. Development of a skin test for bovine tuberculosis for differentiating infected from vaccinated animals. J. Clin. Microbiol. 48:3176–3181. 10.1128/JCM.00420-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casal C, Bezos J, Diez-Guerrier A, Alvarez J, Romero B, de Juan L, Rodriguez-Campos S, Vordermeier M, Whelan A, Hewinson RG, Mateos A, Dominguez L, Aranaz A. 2012. Evaluation of two cocktails containing ESAT-6, CFP-10 and Rv-3615c in the intradermal test and the interferon-gamma assay for diagnosis of bovine tuberculosis. Prev. Vet. Med. 105:149–154. 10.1016/j.prevetmed.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 5.Rehm BHA. 2010. Bacterial polymers: biosynthesis, modifications and applications. Nat. Rev. Microbiol. 8:578–592. 10.1038/nrmicro2354 [DOI] [PubMed] [Google Scholar]
- 6.Grage K, Jahns AC, Parlane N, Palanisamy R, Rasiah IA, Atwood JA, Rehm BHA. 2009. Bacterial polyhydroxyalkanoate granules: biogenesis, structure, and potential use as nano-/micro-beads in biotechnological and biomedical applications. Biomacromolecules 10:660–669. 10.1021/bm801394s [DOI] [PubMed] [Google Scholar]
- 7.Rehm BHA. 2003. Polyester synthases: natural catalysts for plastics. Biochem. J. 376:15–33. 10.1042/BJ20031254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peoples OP, Sinskey AJ. 1989. Poly-β-hydroxybutyrate(PHB) biosynthesis in Alcaligenes eutrophus H16. Identification and characterization of the PHB polymerase gene (phbC). J. Biol. Chem. 264:15298–15303 [PubMed] [Google Scholar]
- 9.Peoples OP, Sinskey AJ. 1989. Poly-β-hydroxybutyrate biosynthesis in Alcaligenes eutrophus H16. Characterization of the genes encoding β-ketothiolase and acetoacetyl-CoA reductase. J. Biol. Chem. 264:15293–15297 [PubMed] [Google Scholar]
- 10.Steinmann B, Christmann A, Heiseler T, Fritz J, Kolmar H. 2010. In vivo enzyme immobilization by inclusion body display. Appl. Environ. Microbiol. 76:5563–5569. 10.1128/AEM.00612-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Draper JL, Rehm BH. 2012. Engineering bacteria to manufacture functionalized polyester beads. Bioengineered 3:203–208. 10.4161/bioe.19567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atwood JA, Rehm BHA. 2009. Protein engineering towards biotechnological production of bifunctional polyester beads. Biotechnol. Lett. 31:131–137. 10.1007/s10529-008-9836-9 [DOI] [PubMed] [Google Scholar]
- 13.Brockelbank JA, Peters V, Rehm BHA. 2006. Recombinant Escherichia coli strain produces a ZZ domain displaying biopolyester granules suitable for immunoglobulin G purification. Appl. Environ. Microbiol. 72:7394–7397. 10.1128/AEM.01014-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parlane NA, Grage K, Lee JW, Buddle BM, Denis M, Rehm BHA. 2011. Production of a particulate hepatitis C vaccine candidate by an engineered Lactococcus lactis strain. Appl. Environ. Microbiol. 77:8516–8522. 10.1128/AEM.06420-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blatchford PA, Scott C, French N, Rehm BHA. 2012. Immobilization of organophosphohydrolase OpdA from Agrobacterium radiobacter by overproduction at the surface of polyester inclusions inside engineered Escherichia coli. Biotechnol. Bioeng. 109:1101–1108. 10.1002/bit.24402 [DOI] [PubMed] [Google Scholar]
- 16.Jahns AC, Haverkamp RG, Rehm BHA. 2008. Multifunctional inorganic-binding beads self-assembled inside engineered bacteria. Bioconjug. Chem. 19:2072–2080. 10.1021/bc8001979 [DOI] [PubMed] [Google Scholar]
- 17.Jahns AC, Rehm BHA. 2009. Tolerance of the Ralstonia eutropha class I polyhydroxyalkanoate synthase for translational fusions to its C terminus reveals a new mode of functional display. Appl. Environ. Microbiol. 75:5461–5466. 10.1128/AEM.01072-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullaney JA, Rehm BHA. 2010. Design of a single-chain multi-enzyme fusion protein establishing the polyhydroxybutyrate biosynthesis pathway. J. Biotechnol. 147:31–36. 10.1016/j.jbiotec.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 19.Backstrom BT, Brockelbank JA, Rehm BHA. 2007. Recombinant Escherichia coli produces tailor-made biopolyester granules for applications in fluorescence activated cell sorting: functional display of the mouse interleukin-2 and myelin oligodendrocyte glycoprotein. BMC Biotechnol. 7:3. 10.1186/1472-6750-7-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grage K, Peters V, Rehm BHA. 2011. Recombinant protein production by in vivo polymer inclusion display. Appl. Environ. Microbiol. 77:6706–6709. 10.1128/AEM.05953-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooks DO, Blatchford PA, Rehm BHA. 2013. Bioengineering of bacterial polymer inclusions catalyzing the synthesis of N-acetyl neuraminic acid. Appl. Environ. Microbiol. 79:3116–3121. 10.1128/AEM.03947-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters V, Rehm BHA. 2005. In vivo monitoring of PHA granule formation using GFP-labeled PHA synthases. FEMS Microbiol. Lett. 248:93–100. 10.1016/j.femsle.2005.05.027 [DOI] [PubMed] [Google Scholar]
- 23.Parlane NA, Wedlock DN, Buddle BM, Rehm BHA. 2009. Bacterial polyester inclusions engineered to display vaccine candidate antigens for use as a novel class of safe and efficient vaccine delivery agents. Appl. Environ. Microbiol. 75:7739–7744. 10.1128/AEM.01965-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parlane NA, Grage K, Mifune J, Basaraba RJ, Wedlock DN, Rehm BHA, Buddle BM. 2012. Vaccines displaying mycobacterial proteins on biopolyester beads stimulate cellular immunity and induce protection against tuberculosis. Clin. Vaccine Immunol. 19:37–44. 10.1128/CVI.05505-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millington KA, Fortune SM, Low J, Garces A, Hingley-Wilson SM, Wickremasinghe M, Kon OM, Lalvani A. 2011. Rv3615c is a highly immunodominant RD1 (region of difference 1)-dependent secreted antigen specific for Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. U. S. A. 108:5730–5735. 10.1073/pnas.1015153108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waters WR, Nonnecke BJ, Palmer MV, Robbe-Austermann S, Bannantine JP, Stabel JR, Whipple DL, Payeur JB, Estes DM, Pitzer JE, Minion FC. 2004. Use of recombinant ESAT-6: CFP-10 fusion protein for differentiation of infections of cattle by Mycobacterium bovis and by M. avium subsp avium and M. avium subsp paratuberculosis. Clin. Diagn. Lab. Immunol. 11:729–735. 10.1128/CDLI.11.4.729-735.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buddle BM, Parlane NA, Keen DL, Aldwell FE, Pollock JM, Lightbody K, Andersen P. 1999. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin. Diagn. Lab. Immunol. 6:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vordermeier HM, Whelan A, Cockle PJ, Farrant L, Palmer N, Hewinson RG. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571–578. 10.1128/CDLI.8.3.571-578.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidders B, Pirson C, Hogarth PJ, Hewinson RG, Stoker NG, Vordermeier HM, Ewer K. 2008. Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex. Infect. Immun. 76:3932–3939. 10.1128/IAI.00150-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 31.Amara AA, Rehm BHA. 2003. Replacement of the catalytic nucleophile cysteine-296 by serine in class II polyhydroxyalkanoate synthase from Pseudomonas aeruginosa-mediated synthesis of a new polyester: identification of catalytic residues. Biochem. J. 374:413–421. 10.1042/BJ20030431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiekermann P, Rehm BHA, Kalscheuer R, Baumeister D, Steinbuchel A. 1999. A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch. Microbiol. 171:73–80. 10.1007/s002030050681 [DOI] [PubMed] [Google Scholar]
- 33.Peters V, Rehm BHA. 2008. Protein engineering of streptavidin for in vivo assembly of streptavidin beads. J. Biotechnol. 134:266–274. 10.1016/j.jbiotec.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 34.Brandl H, Gross RA, Lenz RW, Fuller RC. 1988. Pseudomonas oleovorans as a source of poly(beta-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl. Environ. Microbiol. 54:1977–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buddle BM, Keen D, Thomson A, Jowett G, McCarthy AR, Heslop J, Delisle GW, Stanford JL, Aldwell FE. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10–16. 10.1016/0034-5288(95)90023-3 [DOI] [PubMed] [Google Scholar]
- 36.Buddle BM, Livingstone PG, de Lisle GW. 2009. Advances in ante-mortem diagnosis of tuberculosis in cattle. N. Z. Vet. J. 57:173–180. 10.1080/00480169.2009.36899 [DOI] [PubMed] [Google Scholar]
- 37.Jahns AC, Rehm BHA. 2012. Relevant uses of surface proteins—display on self-organized biological structures. Microb. Biotechnol. 5:188–202. 10.1111/j.1751-7915.2011.00293.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehm BHA. 2006. Genetics and biochemistry of polyhydroxyalkanoate granule self-assembly: the key role of polyester synthases. Biotechnol. Lett. 28:207–213. 10.1007/s10529-005-5521-4 [DOI] [PubMed] [Google Scholar]
- 39.Rehm BHA. 2007. Biogenesis of microbial polyhydroxyalkanoate granules: a platform technology for the production of tailor-made bioparticles. Curr. Issues Mol. Biol. 9:41–62 www.horizonpress.com/cimb/v/v9/03.pdf [PubMed] [Google Scholar]
- 40.MacGurn JA, Raghavan S, Stanley SA, Cox JS. 2005. A non-RD1 gene cluster is required for Snm secretion in Mycobacterium tuberculosis. Mol. Microbiol. 57:1653–1663. 10.1111/j.1365-2958.2005.04800.x [DOI] [PubMed] [Google Scholar]
- 41.Monaghan ML, Doherty ML, Collins JD, Kazda JF, Quinn PJ. 1994. The tuberculin test. Vet. Microbiol. 40:111–124. 10.1016/0378-1135(94)90050-7 [DOI] [PubMed] [Google Scholar]
- 42.Arend SM, de Haas P, Leyten E, Rosenkrands I, Rigouts L, Andersen P, Mijs W, van Dissel JT, van Soolingen D. 2005. ESAT-6 and CFP-10 in clinical versus environmental isolates of Mycobacterium kansasii. J. Infect. Dis. 191:1301–1310. 10.1086/428950 [DOI] [PubMed] [Google Scholar]
- 43.Waters WR, Palmer MV, Thacker TC, Payeur JB, Harris NB, Minion FC, Greenwald R, Esfandiari J, Andersen P, McNair J, Pollock JM, Lyashchenko KP. 2006. Immune responses to defined antigens of Mycobacterium bovis in cattle experimentally infected with Mycobacterium kansasii. Clin. Vaccine Immunol. 13:611–619. 10.1128/CVI.00054-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang H, Troudt J, Grover A, Arnett K, Lucas M, Cho YS, Bielefeldt-Ohmann H, Taylor J, Izzo A, Dobos KM. 2011. Three protein cocktails mediate delayed-type hypersensitivity responses indistinguishable from that elicited by purified protein derivative in the guinea pig model of Mycobacterium tuberculosis infection. Infect. Immun. 79:716–723. 10.1128/IAI.00486-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollock JM, Andersen P. 1997. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovis in cattle. Infect. Immun. 65:2587–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.