Abstract
The different strains of Bacillus cereus can grow at temperatures covering a very diverse range. Some B. cereus strains can grow in chilled food and consequently cause food poisoning. We have identified a new sensor/regulator mechanism involved in low-temperature B. cereus growth. Construction of a mutant of this two-component system enabled us to show that this system, called CasKR, is required for growth at the minimal temperature (Tmin). CasKR was also involved in optimal cold growth above Tmin and in cell survival below Tmin. Microscopic observation showed that CasKR plays a key role in cell shape during cold growth. Introducing the casKR genes in a ΔcasKR mutant restored its ability to grow at Tmin. Although it was first identified in the ATCC 14579 model strain, this mechanism has been conserved in most strains of the B. cereus group. We show that the role of CasKR in cold growth is similar in other B. cereus sensu lato strains with different growth temperature ranges, including psychrotolerant strains.
INTRODUCTION
The Bacillus cereus group, also known as B. cereus sensu lato, consists of ubiquitous Gram-positive spore-forming bacteria. This group comprises seven closely related species, including B. cereus sensu stricto and B. cytotoxicus, that cause emetic and/or diarrheal syndromes in food poisoning (1–3). Some rare strains of B. weihenstephanensis, another species of this bacterial group, can also be emetic (4). These bacteria cause a number of food safety issues, as they are able to produce heat-resistant spores and thus survive in food even after processes such as cooking or pasteurization. Compared to the incidence of food poisoning caused by B. cereus sensu stricto, food poisoning caused by B. cytotoxicus or B. weihenstephanensis is rare (1, 3). The risk for consumers is mostly tied to high doses (105 to 108 CFU) of cells or spores contaminating the ingested food and thus depends on the ability of the bacterium to multiply during the food's shelf life (2, 5, 6). As some strains of B. cereus have the ability to grow at temperatures found in the chill chain, identifying the mechanisms involved in low-temperature adaptation may help predict how this bacterium behaves in refrigerated food and enable more accurate risk prediction and better risk prevention.
Bacterial adaptation to low temperature is a complex and multifactorial process involving both the genetic background of the bacteria (7) and an array of mechanisms (5). Concerning the genetic background, seven phylogenetic groups (groups I to VII) were defined in B. cereus sensu lato, and B. cereus sensu stricto was broadly positioned in these groups (groups II to VI), while B. weihenstephanensis and B. cytotoxicus were merged with groups VI and VII, respectively (7). B. cereus sensu stricto is thus expected to show a broadly diverse genetic background, in contrast to B. cytotoxicus or B. weihenstephanensis. Interestingly, each phylogenetic group (groups I to VII) was assigned a specific range of growth temperatures on the basis of both genetic and phenotypic criteria (7). These seven phylogenetic groups could also be seen as seven thermotypes, running from a psychrotolerant (cold-tolerant) group to moderately psychrotolerant, mesophilic, and moderately thermotolerant (heat-tolerant) groups. B. cereus sensu stricto thus spans different thermotypes containing psychrotolerant, mesophilic, or intermediate strains, whereas B. weihenstephanensis contains only psychrotolerant strains and B. cytotoxicus contains only moderately thermotolerant strains. It was suggested that in the course of evolution, changes in temperature tolerance limits have fashioned historical patterns of global ecological diversification in B. cereus sensu lato (7).
The mechanisms involved in adaptation to low temperature are equally multifactorial, as illustrated by the various mechanisms identified so far in other model bacteria and also described in B. cereus (5). For instance, membrane fatty acid (FA) composition adjustments can increase the proportion of low-melting-point FAs (like unsaturated FAs and branched-chain FAs) (8–10). RNA helicases that enable the RNA unfolding needed for proper translation and/or RNA degradation also play a major role in B. cereus low-temperature adaptation (11).
Among the mechanisms that allow organisms to change in response to environmental conditions, two-component systems (2CSs) are signal transduction systems that are almost ubiquitous in bacteria (12). 2CSs are known to sense a wide range of environmental stressors, enabling cells to elaborate a response by regulating the expression of genes required for adaptation (13–17). 2CSs basically comprise a histidine kinase (HK) that senses an environmental stimulus (either directly or after interaction with accessory proteins) and a cognate response regulator (RR) that usually functions as a transcriptional regulator. One of the first studies on a 2CS involved in cold adaptation identified DesKR, which is able to sense an increase in membrane thickness in response to a decrease in temperature in B. subtilis (18, 19). DesKR consequently regulates the expression of a desaturase gene responsible for maintaining membrane fluidity during B. subtilis growth at low temperature. More recently, a Clostridium botulinum 2CS important for cold tolerance was discovered, and the mechanisms involved were investigated (20, 21). In a Gram-negative bacterium, the CheA/CheY 2CS mutant of Yersinia pseudotuberculosis was impaired during growth at 3°C (22). 2CSs of other Gram-negative bacteria have been described to be temperature sensors for bacterial virulence control, such as CorSR in Pseudomonas (23) and PhoPQ in Edwardsiella (24). Recent research has revealed how a 2CS contributes to the high adaptability of B. cereus strains that enables these bacteria to persist in processed foods (25). Some 2CSs were shown to play diverse roles in B. cereus adaptation (26–30), but most of the 2CSs found among B. cereus sensu lato strains have an unknown function (31), and none has yet been shown to be involved in low-temperature adaptation.
In the course of experiments to better understand the role of the numerous 2CSs with unknown function found among B. cereus sensu lato strains, we mutated one of them (BC_2216-BC_2217) in the mesophilic model strain B. cereus sensu stricto ATCC 14579. We found that this new 2CS is necessary for low-temperature adaptation not only in mesophilic strains but also in two psychrotolerant strains belonging to distinct phylogenetic groups of B. cereus sensu lato.
MATERIALS AND METHODS
Strains and growth conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. B. cereus cells were grown in Luria broth (LB) or brain heart infusion (BHI) with vigorous shaking (200 rpm) at 37°C, 30°C, 13°C, 12°C, 8°C, or 10°C. Escherichia coli cells were routinely grown in LB medium with shaking at 37°C. When required, the antibiotic concentrations used for bacterial selection were as follows: erythromycin (Em) at 10 μg ml−1, kanamycin (Km) at 150 μg ml−1, or spectinomycin (Sp) at 275 μg ml−1 for B. cereus and ampicillin at 100 μg ml−1 for E. coli. E. coli ET12567 with dam dcm mutations was used to generate unmethylated plasmid DNA for the electrotransformation of B. cereus. B. cereus and E. coli strains were transformed by electroporation as previously described (32, 33). When appropriate, the lag phase (defined as the initial optical density [OD] + 0.05 OD), the time to reach stationary phase, or the maximal OD was indicated to compare bacterial growth curves under various conditions.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Relevant genotype and characteristicsa | Reference or source |
|---|---|---|
| Strains | ||
| B. cereus | ||
| ATCC 14579 wt | ATCC 14579 wt, phylogenetic group IV | ATCC |
| ATCC 14579 ΔcasKR | ATCC 14579 wt ΔBC_2216-BC_2217 Kmr | This study |
| ATCC 14579 ΔBC_5411-12 | wt ΔBC_5412-BC_5411 Spr | This study |
| AH187 wt | AH187 = F4810/72 wt, phylogenetic group III | PHLS, UK |
| AH187 ΔcasKR | AH187 wt ΔBCAH187_A2374-A2375 Kmr | This study |
| MM3 wt | MM3 wt, phylogenetic group II | Naval Medical Research Center (U.S. Navy) |
| MM3 ΔcasKR | MM3 wt Δbcere0006_20650-20660 Kmr | This study |
| Rock 3-28 wt | Rock 3-28 wt, phylogenetic group V | Naval Medical Research Center (U.S. Navy) |
| Rock 3-28 ΔcasKR | Rock 3-28 wt Δbcere0019_20130-20120 Kmr | This study |
| E. coli | ||
| TG1 | Δ(lac-proAB) supE thi hsd-5 (F′ traD36 proA+ proB+ lacIq lacZΔM15), general-purpose cloning host | Laboratory collection |
| SCS110 | rpsL (Strr) thr leu endA thi-1 lacY galK galT ara tonA tsx dam dcm supE44 Δ(lac-proAB) [F′ traD36 proAB lacIqZΔM15], for generation of unmethylated DNA | Laboratory collection |
| Plasmids | ||
| pHT304-18 | Apr and Emr cloning vehicle | 57 |
| pHT304-Km | pHT304-18 derivative harboring an Kmr cassette | 26 |
| pMAD | Apr Emr shuttle vector, thermosensitive origin of replication | 38 |
| pMADΔcasKR | pMAD derivatives harboring constructs for the allelic replacement of casK-casR, Apr Emr Kmr | This study |
| pHT-casKR | 2,848-bp region surrounding casK and casR genes cloned in SalI and BamHI sites of pHT304-18 | This study |
| pMADΔBC_5412-11 | pMAD derivatives harboring constructs for the allelic replacement of BC_5412-BC_5411, Apr Emr Spr | This study |
Km, kanamycin; Ap, ampicillin; Em, erythromycin, Sp, spectinomycin.
To compare the growth of phylogenetic group II, III, and V mutants with that of their parental strains, an automated turbidimeter (Microbiology Bioscreen C reader; Labsystems, Uxbridge, United Kingdom) was used as previously described (8). Cultures were performed as follows. A fresh colony was used to inoculate 10 ml LB and incubated at 30°C with shaking for 9 h. Ten microliters was then used to inoculate 10 ml fresh LB, and the culture was incubated at 30°C with shaking for 17 h. This second culture was used to inoculate 1 ml fresh LB to reach a concentration of 105 CFU per ml. Three replicate microplate wells were filled with dilutions of inoculated medium to a final volume of 300 μl per well. A negative control of uninoculated LB broth was made. Cultures were incubated under vigorous constant shaking, and the OD at 600 nm (OD600) was measured at 15-min intervals at 37°C, 42°C, or 45°C or at 1-h intervals at 13°C, 12°C, 10°C, or 8°C over an incubation period of 48 h (at 37°C and above) or 10 days (for all other temperatures). At least three independent experiments were performed for each growth condition.
The same protocol was used for low-pH (pH 5.5, 5.0, and 4.7), high-pH (pH 8.5 and 8.6), and high-osmolarity (4 and 6% NaCl) conditions in the presence of oxidative agents [paraquat dichloride at 100 μM and 150 μM, 2,2′-azo-bis-(2-methyl-propionamidine) dihydrochloride (AAPH) at 100 mM and 150 mM, H2O2 at 0.020% and 0.025%]. Alternatively, growth was performed in 50 ml LB placed in 250-ml Erlenmeyer flasks in the presence of ethanol (4% and 6%) or ion chelators (EDTA at 100 μM, EGTA at 100 μM, 2,2′-bipyridyl at 100 μM and 500 μM), and the OD600 was regularly checked on a Helios Epsilon spectrophotometer (Thermo Scientific, Rockford, IL). All chemicals were purchased from Sigma-Aldrich.
Standard tests for B. cereus, such as growth on Mossel (also called mannitol-egg yolk-polymyxin [MYP]) agar or on sheep blood agar, were performed as described in Bergey's manual (34), and API 50CH strips (bioMérieux, Marcy l'Etoile, France) were used per the manufacturer's protocol.
Microscopic observations.
Bacterial cells were regularly observed under a phase-contrast microscope at a magnification of ×1,000. To visualize cell shape at a higher magnification (up to ×14,000), transmission electron microscopy (TEM) was used as previously described (11, 35). Briefly, wild-type (wt) and mutant cells grown at 12°C or 37°C were harvested in early stationary phase and transferred to glutaraldehyde at 2.5% in 0.1 M sodium cacodylate buffer (pH 7.2) containing ruthenium red (1 mg/ml). Cells were washed three times and postfixed with 2% osmium tetroxide, washed again, mixed with 30% ethanol, and then embedded in 3% agar before dehydration with increasing concentrations of ethanol. Ethanol was replaced with propylene oxide and sequentially exchanged with Araldite resin. Samples were polymerized for 48 h at 60°C before cutting. Thin 70-nm-wide sections were cut with an ultramicrotome and stained with uranyl acetate and lead citrate. Observations were done under a transmission electron microscope (TEM; FEI-Philips CM10; Philips, Eindhoven, The Netherlands).
Cell survival experiments.
Cell survival at 4°C was tested by inoculating 2.5 ml LB in 10-ml tubes with exponential-phase subcultures of wt and mutant cells and incubating at 4°C for up to 840 h (35 days). The number of survivors was determined by plating 100-μl volumes of 10-fold serial dilutions of the culture stored on triplicate LB agar plates and counting the colonies formed after 18 h of incubation at 30°C. This experiment was performed in triplicate. Strain survival at 4°C was modeled as previously described using the function log N = log N0 − (t/δ)p, where N0 is the initial population, N is the population at time t, δ is the time to the first decimal reduction, and p is the curvature index (36). To model the shape of the curve, δ and p were calculated using the Microsoft Excel 2010 solver function.
Nucleic acid manipulations.
Plasmid DNA was extracted from B. cereus and E. coli by a standard alkaline lysis procedure using a Wizard SV miniprep purification system (Promega, Charbonnières, France), with an additional incubation with lysozyme used to lyse B. cereus cells, as previously described (37). Chromosomal DNA was extracted from B. cereus cells harvested in mid-log phase as described previously (37). Restriction enzymes and T4 DNA ligase were used as recommended by the manufacturer (Promega). Oligonucleotide primers (Table 2) were synthesized by Eurogentec (Seraing, Belgium). PCR was performed in a GeneAmp PCR system 2400 thermal cycler (Perkin-Elmer, Courtaboeuf, France) using Expand high-fidelity DNA polymerase (Roche Applied Science, Meylan, France). Amplified DNA fragments were purified using a PCR purification kit (Roche) and separated on 0.7% agarose gels after digestion as previously described (37). Digested DNA fragments were extracted from agarose gels with a centrifugal filter device (Montage DNA gel extraction kit; Millipore, Molsheim, France). All constructions were confirmed by DNA sequencing (Millegen, Labege, France).
TABLE 2.
Primers used in this study
| Oligonucleotide | Sequence (5′–3′)a |
|---|---|
| 5Up-2216-17-Eco | GGCGAATTCGTAGTTGGTGATGAGAATGAAC |
| 3Up-2216-17-Pst | TGGACTGCAGCAATCCTTCAGGTCTTAATTG |
| 5Dn-2216-17-Sal | TGCAGTCGACGAAAACGAGAGAAGAATTTGA |
| 3Dn-2216-17-Bam | GCGGGATCCTTTATCCTGTAAAAACCGATTA |
| Km5out | CGGTATAATCTTACCTATCACC |
| Km3out | TACTCTGATGTTTTATATCTTTTCTAA |
| BC2215-Fw | CCATTTAAATACGTCGGTGT |
| BC2218-Rv | ATATGTTCGGCAATACTTCG |
| 5Up-5412-11 | TCGATGCATGCCATGGACCTGAAAAGGTGGTAAATC |
| 3Up-5412-11 | TTGTCTACAGATTAACTCTTGCTAACCGATTTCTT |
| 5Dn-5412-11 | TCACGGTTTACCCACAGAAACAAATCCCGATGTTA |
| 3Dn-5412-11 | GGGCGATATCGGATCCTTGCGTACGATCAGATAAAT |
| 5spcR | TTAATCTGTAGACAAATTGTGA |
| 3spcR | GTGGGTAAACCGTGAATATC |
| Spc5out | TCACAATTTGTCTACAGATTAA |
| Spc3out | GATATTCACGGTTTACCCAC |
| Cpl2216-17-Fw-Sal | GACGCGTCGACAGGTAGAGCAGACGATTCAA |
| Cpl2216-17-Rv-Bam | GCGGGATCCTTTATCCTGTAAAAACCGATTA |
| qPCR-BC2216-F | AAAAGAGATGCGTCGTTTAG |
| qPCR-BC2216-R | ACACACCAACAGTTTCCTTC |
| qPCR-16S-F | GGTAGTCCACGCCGTAAACG |
| qPCR-16S-R | GACAACCATGCACCACCTG |
Restriction enzyme sites are underlined.
RNA extraction and real-time reverse transcription (RT)-PCR were performed on a LightCycler instrument (Roche) using a QuantiFast SYBR green RT-PCR kit (Qiagen), as previously described (8), and the primer pair qPCR-BC2216-F and qPCR-BC2216-R (Table 2).
Mutant construction.
The BC_2216 and BC_2217 genes encoding a putative histidine kinase and a cognate response regulator, respectively, were interrupted in B. cereus ATCC 14579 by allelic exchange with a cassette conferring kanamycin resistance (Kmr), as previously described (38). Briefly, DNA fragments of the BC_2216 upstream region and the BC_2217 downstream region were PCR amplified using the primer pairs 5Up-2216-17-Eco/3Up-2216-17-Pst and 5Dn-2216-17-Sal/3Dn-2216-17-Bam, respectively (Table 2). PCR products were digested with EcoRI/PstI and SalI/BamHI using the primer-incorporated restriction sites (Table 2). In parallel, the Kmr cassette (a 1.5-kb fragment corresponding to the aphA3 kanamycin resistance gene with its own promoter) was digested from pHT304-Km (Table 1) with PstI/SalI. The three digested DNA fragments were purified, ligated into EcoRI/BamHI-digested pMAD (Table 1), and introduced by electroporation into E. coli TG1. Unmethylated plasmids were then prepared from E. coli ET12567, and the resulting recombinant plasmid, pMADΔBC_2216-17, was transformed into B. cereus ATCC 14579. Transformants were then subjected to allelic exchange as previously described (38). Colonies that were resistant to Km and sensitive to Em arose through a double-crossover event in which the chromosomal wt copies of the BC_2216 and BC_2217 genes were deleted and replaced by the Kmr cassette. Chromosomal allele exchange in the mutant was checked by PCR using the appropriate primer pairs (BC2215-Fw/Km5out and Km3out/BC2218-Rv; Table 2). PCR products were sequenced for confirmation.
Similarly, the BC_5412 and BC_5411 genes encoding putative paralogs of the BC_2216 and BC_2217 genes, respectively, were interrupted in B. cereus ATCC 14579. First, the SpR cassette, a 1.2-kb fragment containing the spc spectinomycin resistance gene with its own promoter (39), was PCR amplified from pDIA (obtained from I. Martin-Verstraete [40]) using primer pair 5spcR and 3spcR (Table 2). In parallel, primer couples 5Up-5412-11/3Up-5412-11 and 5Dn-5412-11/3Dn-5412-11 (Table 2) harboring a DNA sequence overlapping the SpR cassette or the pMAD vector were used to amplify upstream and downstream regions from the BC_5412 and BC_5411 genes. These two PCR fragments were purified and mixed with both the SpR cassette and BamHI/NcoI-digested pMAD to be ligated altogether using an InFusion HD cloning kit (Clontech, Mountain View, CA) according to the manufacturer's instructions. The mixture was transformed into E. coli as recommended by the manufacturer. The appropriate plasmid was isolated and checked by DNA sequencing of the insert region. It was then transformed into B. cereus ATCC 14579 and subjected to allelic exchange, as described above.
Complementation in trans of the mutant lacking BC_2216 and BC_2217 (the ΔBC_2216-17 mutant) was performed by introducing both the BC_2216 and BC_2217 genes with a large upstream region (889 bp upstream from ATG) presumably containing their own promoters on plasmid pHT304 (Table 1). This 2,848-bp DNA region was obtained by PCR amplification using the primers Cpl2216-17-Fw-Sal and Cpl2216-17-Rv-Bam (Table 2).
In addition to the ATCC 14579 model strain (group IV), we selected three other strains from distinct phylogenetic groups whose genome sequences were available, i.e., strains AH187 (group III), MM3 (group II), and Rock 3-28 (group V). Their ability to grow at low temperature, particularly the minimal temperature for growth (Tmin), was checked as described in Bergey's manual (34). The studied strains showed different degrees of temperature tolerance, from psychrotolerance with a Tmin of 7°C (MM3) to moderate psychrotolerance with a Tmin of 8°C (Rock 3-28) and mesophilic ability with Tmins of 10°C (ATCC 14579) and 12°C (AH187).
Transformation by electroporation into the AH187, MM3, and Rock 3-28 strains was performed as described above using the recombinant plasmid pMADΔBC_2216-17 (Table 1) isolated from the ATCC 14579 strain. The protocol leading to disruption of the desired genes by a double crossover was then realized as described above for the ATCC 14579 strain, using the following modifications for the two psychrotolerant strains (MM3 and Rock 3-28). Successive cultures were performed with a gradual increase (by steps of 2°C or 4°C) of the incubation temperature from 30°C up to 37°C for MM3 or up to 40°C for Rock 3-28 (these strains are unable to grow at temperatures above 40°C) in order to allow conditions in which the thermosensitive plasmid could be lost following a recombination event. When Km-resistant and Em-sensitive colonies arose through a double-crossover event, we checked for correct chromosomal allele exchange by PCR, as described above for the ATCC 14579 strain.
In silico analysis.
Affiliation to phylogenetic groups for the strains used in this study and for the 196 B. cereus genomes that were available in databases at the time of the search (November 2013) was established as previously described (3), using panC sequence similarity. The search for BC_2216 and BC_2217 orthologs was performed by use of the Integrated Microbial Genomes (IMG) interface (41). In the first step, candidate homologs were identified on the basis of BLASTp similarities with a 1e−2 E-value cutoff and with low complexity soft masking turned on. In the second step, the ortholog relationship between BC_2216 and BC_2217 genes and homolog genes in all other genomes was established through the use of bidirectional best hits. Because paralogs were present in many strains (BC_5411 and BC_5412 in ATCC 14579), we considered BC_2216-BC_2217 orthologs to be genuine when orthologs of the surrounding genes were present with conserved synteny. To achieve this, gene neighborhoods in all available genomes were compared using the Conserved Neighborhood Viewer bundled with the IMG interface (42). Protein domains in BC_2216, BC_2217, BC_5412, and BC_5411 were identified using the SMART tool (43, 44).
RESULTS
The BC_2216 and BC_2217 genes encode a 2CS and are overexpressed at low temperature.
According to their annotation, the BC_2216 and BC_2217 genes found in the B. cereus ATCC 14579 mesophilic strain encode, respectively, a putative histidine kinase and a response regulator. The predicted BC_2216 protein indeed contains the classical transmitter domains of histidine kinases: a ATP binding domain (HATPase domain, residues 288 to 372) and a dimerization and phosphoacceptor domain (Pfam:HisKA_3 domain, residues 186 to 247). BC_2216 has no predicted transmembrane domain, but its N-terminal region displays a GAF domain (Pfam 01590). BC_2217, the presumed cognate response regulator of BC_2216, displays a phosphoacceptor site (REC domain, residues 6 to 120) and a DNA-binding motif (helix-turn-helix domain, residues 151 to 207), suggesting that BC_2217 has DNA regulatory ability. The BC_2216 and BC_2217 proteins thus presumably form a 2CS.
The level of expression of the BC_2216 gene was quantified by RT-quantitative PCR during low-temperature (12°C) growth and compared to that at an optimal temperature (37°C). RNA samples were extracted from cells collected at three times during growth kinetics, in mid-exponential phase (OD600 = 0.5), end exponential phase (OD600 = 1.0), or stationary phase (OD600 = 2.5). Results showed 7.2-fold, 10.4-fold, and 9.7-fold overexpression, respectively, during growth at 12°C compared to that during growth at 37°C, suggesting an important role of the BC_2216-BC_2217 2CS under this growth condition.
Mutation of the BC_2216 and BC_2217 genes causes impaired low-temperature growth.
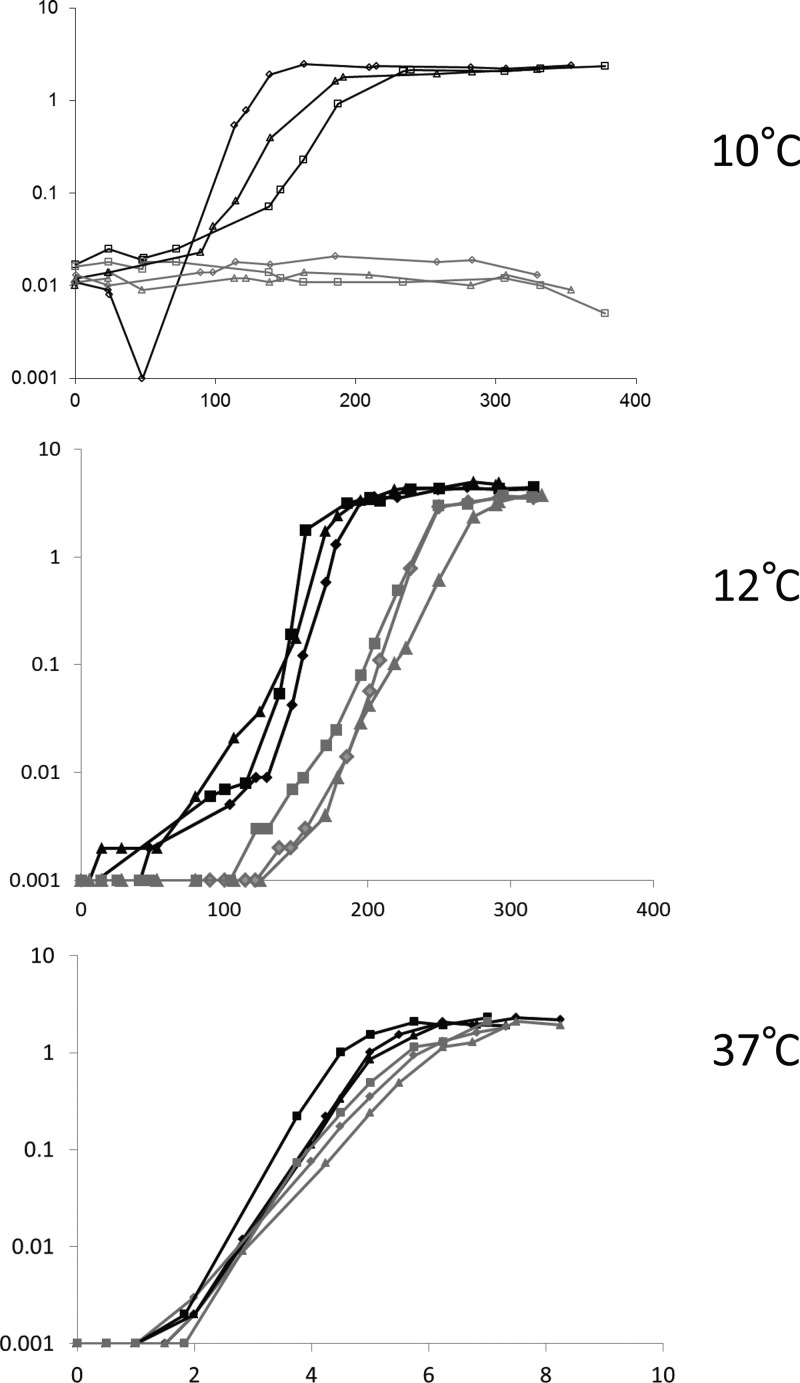
A BC_2216-BC_2217 mutant of the B. cereus ATCC 14579 strain was constructed by allelic exchange between the two 2CS-encoding genes and a kanamycin resistance cassette (Table 1), and the phenotype of this mutant was compared to that of its parental strain during growth at various temperatures (Fig. 1).
FIG 1.
Growth curves of B. cereus wt and ΔBC_2216-17 strains at various temperatures. Growth of the wt (black) and ΔBC_2216-17 (gray) strains was performed in 100 ml LB at the indicated temperature under shaking. Three biological replicates are shown for each condition (different symbols represent different replicates). During the lag phase at 10°C, cells sometimes formed temporary small aggregates which caused a decrease in the measured OD. y axes indicate the ODs at 600 nm; x axes indicate times (in hours).
At the Tmin of this strain (i.e., 10°C), the wt growth curves varied slightly between experiments. The lag phase of the 3 replicates was about 114 ± 14 h (mean ± standard error of the mean [SEM]), and they reached stationary phase in 196 ± 21 h after inoculation, with a maximal OD of 2.3 ± 0.1. Under the same conditions, the BC_2216-BC_2217 mutant did not show any growth, even after 300 h of incubation.
At 12°C, the mutant was able to grow, but the mean ± SEM lag phases of the wt strains (91.3 ± 6.9 h) and the mutant strains (189.3 ± 6.7 h) from three independent cultures were significantly different (P < 0.05, Student t test), indicating that the mutant was slower to adapt to this cold temperature than the wt strain. The maximum OD was also significantly lower for the mutant than for the wt (3.68 ± 0.10 versus 4.58 ± 0.19, respectively; P < 0.05). Thus, mutant growth was impaired at 12°C.
In contrast, at the optimal temperature (i.e., 37°C), the growth curves of the two strains were similar. The cells reached the stationary phase, with only a slight delay of 1.19 h (P < 0.05) for the mutant compared to the time to reach the stationary phase for the wt strain, but maximal ODs were not significantly different (2.01 ± 0.09 for the mutant versus 2.06 ± 0.01 for the wt; P > 0.05).
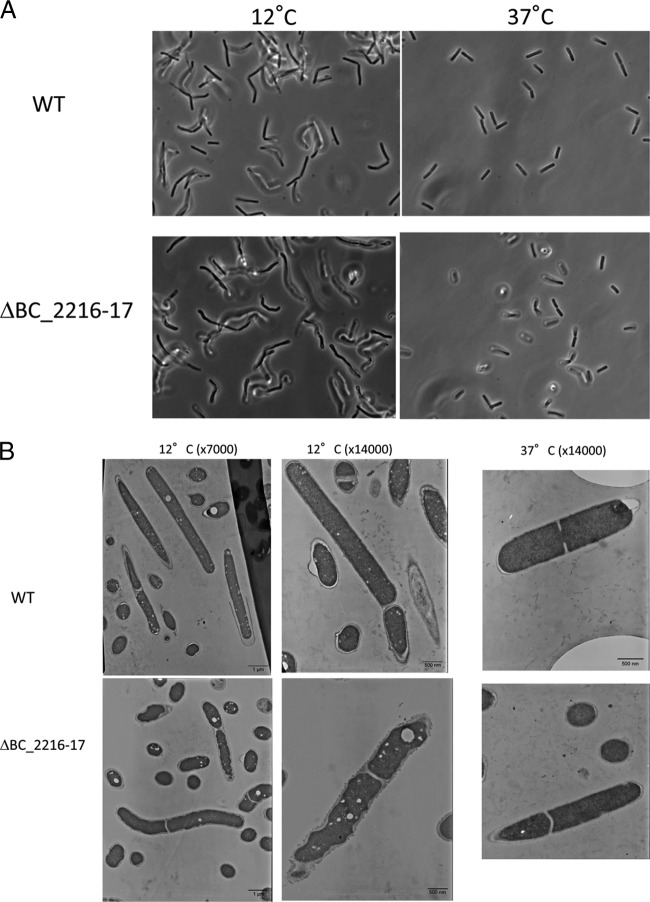
Microscopic observations were performed during growth at low and optimal temperatures (Fig. 2). Both the wt and mutant strains showed slight cell elongation during growth at 12°C compared to the cell size with growth at 37°C (Fig. 2A). There was no difference in cell size between the wt and mutant strains. Cells of the two strains checked at a higher magnification by TEM showed a similar cell structure at the optimal growth temperature (i.e., 37°C) (Fig. 2B). In contrast, when incubated at low temperature (12°C), cells of the mutant strain displayed an atypical morphology compared to that of the wt strain, as their cytoplasm displayed a wavy shape, suggesting an irregular thickness of the cell wall.
FIG 2.
Microscopic observations of B. cereus wt and ΔBC_2216-17 strains grown at 12 and 37°C until the stationary phase. Microscopic observations were made under a phase-contrast microscope at a magnification of ×1,000 (A), and under a TEM at magnifications of ×7,000 and ×14,000 (B).
Given that cold is a major stressor for bacteria, we investigated whether the growth of the BC_2216-BC_2217 mutant was impaired when it encountered other stressful conditions. Growth of both strains was measured under various conditions: under conditions of high temperature, low pH, high pH, and high osmolarity and in the presence of ethanol, ion chelators, or oxidative agents. Under all the tested conditions, the BC_2216-BC_2217 mutant strain showed growth similar to that of the wt (see Table S1 in the supplemental material).
In addition, the phenotype of the mutant was compared to that of its parental strain after growth on various media. Growth on Mossel agar and growth on sheep blood agar showed that the mutant and the wt strains displayed the same lecithinase activity and hemolytic profile, respectively. Tests run with an API 50CH kit to determine the bacterial oxidation of 49 different carbohydrates did not find any differences between the two strains.
Taken together, these data suggest that the major phenotype of this mutant compared to that of the wt was its growth impairment at low temperature. We therefore propose the name casK (where cas represents cold adaptation sensor) for the BC_2216 gene, which encodes a putative histidine kinase, and casR for the BC_2217 gene, which encodes a putative response regulator.
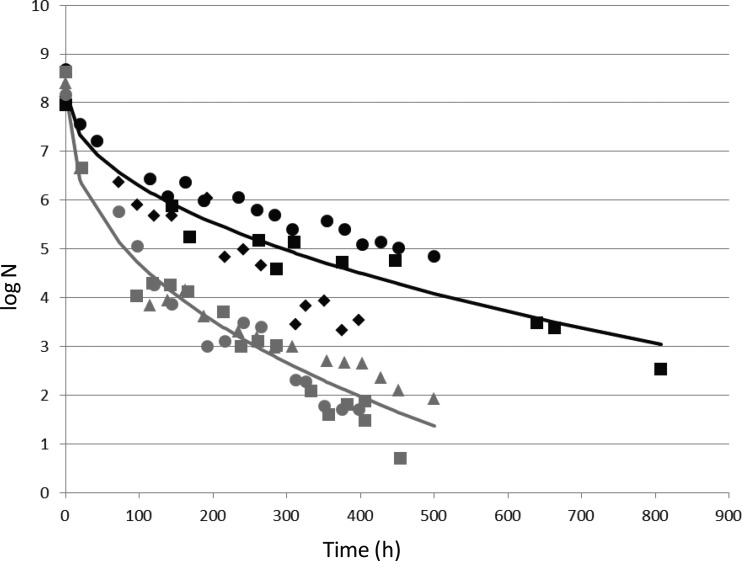
Survival at temperatures below the minimal temperature of growth.
Given the impaired growth at low temperature, we investigated the ability of the ATCC 14579 wt and ΔcasKR strains to survive at temperatures below the minimal temperature of growth (i.e., below 10°C). The two strains were incubated at 4°C in LB, and the CFU were enumerated over time. As shown in Fig. 3, viable counts of both strains decreased regularly over time during incubation at 4°C, but the viability loss was significantly faster for the casKR mutant than for the wt strain. A viability loss of 3 log CFU was reached in 103 ± 6 h (mean ± SEM) for the mutant strain, whereas a viability loss of 3 log CFU was reached in 292 ± 46 h for the wt (P < 0.05, Student t test). Thus, after 300 h of incubation, the viable counts were significantly higher for the wt (4.7 ± 0.6 log CFU, mean ± SEM) than for the casKR mutant (2.5 ± 0.3 log CFU) (P < 0.05).
FIG 3.
Survival of B. cereus wt and ΔcasKR strains at 4°C. B. cereus wt (black symbols) and ΔcasKR (gray symbols) strains in bacterial suspensions were incubated in LB at 4°C. CFU enumeration was performed every day. Different symbols represent data from 3 different replicates. The curves represent theoretical data calculated as described in Materials and Methods.
In order to determine whether long-term storage in LB medium at 37°C could also impair survival in the same way, we ran a control experiment. The results indicated that viability loss was very limited, with a 1.1-log-unit loss for the ATCC 14579 wt versus a 1.3-log-unit loss for the casKR mutant (means of duplicate experiments) after 400 h of incubation at 37°C (see Fig. S2 in the supplemental material). This result indicates that the ability to survive long-term storage in LB involves CasKR only at low temperature.
Complementation of the mutant phenotype.
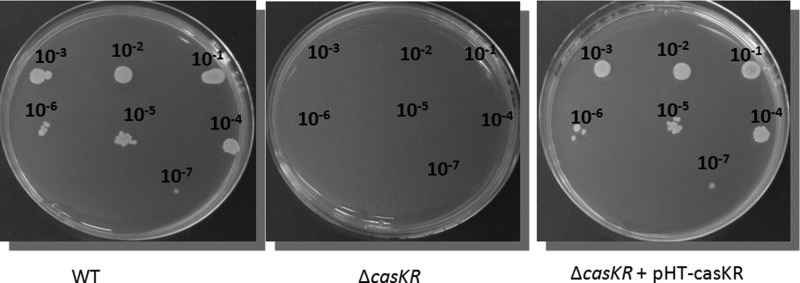
Complementation of the ΔcasKR strain was performed by introducing on a plasmid the casKR genes and a large upstream region presumably containing the casKR promoter (Table 1). While no growth was observed for the ΔcasKR strain without complementation at the Tmin (i.e., 10°C), growth ability was restored for the ΔcasKR strain complemented with pHT-casKR (Fig. 4). Growth was detected on LB agar even at a 10−7 dilution, similar to what was observed for the ATCC 14579 wt strain (Fig. 4). This result confirms that the casKR deletion is genuinely responsible for the cold growth impairment of the mutant strain.
FIG 4.
Complementation of ΔcasKR restores the ability to grow at low temperature. Five microliters of serial dilutions of the B. cereus wt, ΔcasKR, and ΔcasKR/pHT-casKR strains (grown in liquid medium at 30°C and adjusted to 108 CFU/ml) was spotted on LB agar, and the strains were incubated at 10°C for 20 days.
The CasKR paralog is not required for B. cereus low-temperature growth.
The B. cereus ATCC 14579 BC_5412 and BC_5411 genes present high sequence similarity with the casK and casR genes located elsewhere on the chromosome (49.5% and 54.0% identity, respectively). Consequently, the BC_5412-BC_5411 2CS could be considered a paralog of CasKR. We therefore investigated whether BC_5412-BC_5411 could play a role similar to that of CasKR during B. cereus growth at low temperature. A BC_5412-BC_5411 mutant was constructed, and its growth was tested at the optimal temperature and at low temperature. There was no observable difference between the BC_5412-BC_5411 mutant and its parental strain in terms of the kinetics of growth at 37°C (data not shown) or 12°C (see Fig. S3 in the supplemental material). At Tmin (i.e., 10°C), this mutant was still able to grow on LB agar similarly to the wt (see Fig. S3 in the supplemental material).
Role of CasKR among other B. cereus sensu lato strains.
To determine whether the casKR genes are present among other strains of the various phylogenetic groups of B. cereus sensu lato, we performed a search for casKR (BC_2216-BC_2217) orthologs with conserved synteny of gene neighborhoods (see Fig. S4 in the supplemental material). Sequence similarity and E values are reported for each ortholog in Table S5 in the supplemental material. Orthologs of casKR were found in strains belonging to five of the seven known phylogenetic groups of B. cereus sensu lato, i.e., groups II to VI (Table 3; see Fig. S4 and Table S5 in the supplemental material). While they were absent in group VII (a moderately thermotolerant group), casKR orthologs were present in mesophilic group I, but synteny was not conserved, particularly for downstream genes.
TABLE 3.
Search for casKR orthologs among the seven phylogenetic groups of B. cereus sensu latoa
| Phylogenetic group | Detection of orthologs of BC_2215, BC_2216, BC_2217, BC_2218 | Psychrotolerant strain | Human-pathogenic strain | Strain used for construction of casKR mutant during this study |
|---|---|---|---|---|
| I | −, +, +, − | − | − | ND |
| II | +, +, +, + | + | + | MM3 |
| III | +, +, +, − | − | + | AH187 |
| IV | +, +, +, + | − | + | ATCC 14579 |
| V | +, +, +, + | + | + | Rock 3-28 |
| VI | +, +, +, + | + | − | ND |
| VII | −, −, −, + | − | + | NA |
An in silico BLASTp search was performed to screen for orthologs of the BC_2216-BC_2217 genes among 196 B. cereus strains of the various phylogenetic groups (n = 7) for which genomic data were available. A plus sign indicates that BC_2216 and BC_2217 orthologs (in bold), in addition to the BC_2215 and BC_2218 orthologs, were found with a conserved synteny. A minus sign indicates an absence of the ortholog or that synteny was not conserved. Among each of the seven phylogenetic groups, the presence of human-pathogenic strains and psychrotolerant strains is indicated, as previously described (3, 7). During this study, CasKR mutants of strains belonging to four of the seven phylogenetic groups were constructed, as indicated. ND, not done; NA, not applicable.
Outside B. cereus sensu lato, tBLASTN analysis revealed that the best similarity was found in B. megaterium (51% identity for casK and 62% for casR), but the synteny was not conserved. In other species, similarity was lower and the synteny was still not conserved. For instance, in B. subtilis strain 168, the best similarity was obtained with yhcYZ, a 2CS of unknown function (48% identity for casK and 47% for casR). These genes, found outside B. cereus sensu lato strains, may be considered putative casKR orthologs, but their function remains to be investigated to clarify this point.
Thus, the casKR genes are widespread among B. cereus sensu lato strains. Psychrotolerant and moderately psychrotolerant strains are clustered in three out of seven phylogenetic groups of B. cereus sensu lato (7), and they all displayed orthologs of casKR genes. We thus investigated whether the role of these orthologs was similar to that observed in the mesophilic ATCC 14579 strain (group IV), despite a lower Tmin for these strains. Orthologs of the casKR genes were also found among strains belonging to phylogenetic group III, which are considered to have a higher Tmin (12°C) than the ATCC 14579 strain (10°C). We therefore investigated the role played by casKR in three strains representative of three additional phylogenetic groups by constructing a casKR mutant for each strain.
For the mesophilic AH187 strain (phylogenetic group III), the psychrotolerant MM3 strain (group II), and the psychrotolerant Rock 3-28 strain (group V) studied, the casKR mutants and their parental strains displayed a similar growth ability at 37°C (Fig. 5). In contrast, all three casKR mutants showed impaired growth at low temperature. In addition, the mutants of the two psychrotolerant strains did not show any growth at 8°C, a temperature close to their Tmins (i.e., 8°C for the Rock 3-28 strain and 7°C for the MM3 strain). These results showed that the CasKR 2CS is required for growth at low temperature among mesophilic as well as psychrotolerant B. cereus sensu lato strains, whatever the value of the Tmin. In addition, CasKR seems to be particularly efficient when the bacteria are submitted to a temperature close to their Tmin.
FIG 5.
Growth at various temperatures of wt and ΔcasKR strains from phylogenetic groups II, III, and V of B. cereus sensu lato. Growth of wt and ΔcasKR strains belonging to phylogenetic group II (strain MM3) (A, D, F), group III (strain AH187) (B, G), and group V (strain Rock 3-28) (C, E, H) was performed in an automated turbidimeter with shaking at 37°C (A to C), at a low temperature of 13°C (D) or 10°C (E), or at a temperature close to the Tmin of the strains of 8°C (F, H) or 12°C (G). y axes indicate the OD at 600 nm; x axes indicate time (in hours). Mean values ± SDs of three biological replicates are shown for each condition. Black curves, wt strains; gray curves, ΔcasKR mutants.
DISCUSSION
In this study, we identified a pair of genes that play a major role in B. cereus growth and survival at low temperature. CasKR, the newly identified 2CS, is widespread among B. cereus sensu lato strains. 2CSs have a wide array of functions, and a few 2CSs have already been shown to be involved in the low-temperature response in other bacteria (DesKR in B. subtilis, CheAY in Y. pseudotuberculosis, CBO0365-CBO0366 in C. botulinum) (18, 20–22). According to the proposed 2CS classification scheme (45, 46), CasK belongs to the class II family of histidine kinases and CasR belongs to the NarL family of cognate response regulators, which puts CasKR in the same family as DesKR. However, despite their signal transduction function, these two 2CSs have completely different genetic organizations (for instance, the desaturase-encoding genes are located on different loci in B. cereus, contrary to what is observed in B. subtilis) and the two HKs display no similarity within their N-terminal sensory domains. Among the various 2CSs present in the ATCC 14579 model strain of B. cereus, CasKR (the BC_2216-BC_2217 2CS) and its paralog, the BC_5412-BC_5411 2CS, seem to be atypical, as their kinases do not display a transmembrane domain, suggesting that they belong to those rare HKs that have a cytoplasmic location (47). Despite the sequence similarity between these two paralogs, only CasKR is overexpressed at low temperature (S. Chamot and J. Brillard, unpublished results) and only CasKR is necessary for optimal growth at low temperature. According to SMART analysis, the sensory N-terminal region of CasK contains a GAF domain (44). These domains are able to bind cyclic nucleotides and are present, for instance, in cyclic GMP-specific phosphodiesterases or in phytochromes (48). To our knowledge, a link between such a domain and sensing of low temperatures has never been described. This raises the question of the signal perceived by CasK under cold conditions. A 2CS from Staphylococcus aureus, YhcSR, presents some sequence similarity with CasKR. As YhcS lacks a transmembrane domain, this histidine kinase probably has an intracytoplasmic location, like CasK (49). This 2CS was shown to be essential for cell viability, and it controls the expression of an ABC transporter that seems to play a role under high-osmolarity conditions (50). The full viability of our CasKR mutant and its unaffected growth in the presence of high NaCl concentrations compared to the growth of the wt suggest that YhcSR and CasKR play different roles in cell physiology.
Whatever B. cereus sensu lato strains among the several phylogenetic groups that were tested, ΔcasKR mutants of those strains showed impaired growth at low temperature compared to the growth of their parental strains. In ATCC 14579, survival at 4°C was also impaired in the ΔcasKR mutant compared to that of the wt. In contrast, the wt and the mutant showed similar survival at a subfreezing temperature (−20°C) (S. Diomandé and J. Brillard, unpublished data). These results suggest a major role of CasKR when cells are physiologically active (10°C) or when metabolic activity is strongly reduced (at 4°C), but not when cells are frozen.
Another phenotype of the CasKR mutant is the modified cell shape with an irregular cell wall instead of the elongated rod shape regularly observed for the wt cells at low temperature. Strong modifications of the structures of B. cereus cells have been described before, for instance, in a cshA RNA helicase mutant with a substantially stronger phenotype (11). In another study under conditions involving both low temperature and low redox potential, elongation of the cells coupled to a default in cell separation was observed (35).
Model strains are good tools to identify new mechanisms, but they may significantly differ phenotypically from food-poisoning strains (51), which may also be true for B. cereus (29, 52, 53). B. cereus sensu lato has the singularity of displaying a wide panel of strains: some are pathogenic and mainly belong to mesophilic or thermotolerant groups III, IV, and VII, whereas others belong to psychrotolerant groups II and V, where fewer pathogenic strains have been so far described, and the most psychrotolerant strains from group VI have been only marginally associated with human pathogenicity (3, 4). Therefore, the ability of some B. cereus sensu lato strains both to grow at low temperature and to cause human infections or food poisoning makes it important to identify the mechanisms involved in cold growth of both mesophilic and psychrotolerant strains. The CasKR 2CS has been identified in various strains belonging to phylogenetic groups II, III, IV, V, and VI, and we proved its involvement in low-temperature growth in strains representative of phylogenetic groups II, III, IV, and V, two of which are classified as psychrotolerant and two of which are classified as mesophilic. It is tempting to speculate that CasKR could also play a similar role in psychrotolerant strains belonging to phylogenetic group VI (B. weihenstephanensis), but this was not investigated here because several attempts to obtain a casKR mutant in two different strains from this group failed. Such unsuccessful assays could reveal a major role of CasKR in these strains, but clarification of this point will have to be deciphered by further investigations. Excluding the AH187 emetic strain (54), the strains chosen here were not isolated from a food-poisoning outbreak. However, all these strains have the pathogenic potential to cause food-borne illness, as their genomes contain genes involved in virulence (e.g., nhe, plcR) (2, 55). Our results illustrate that psychrotolerant B. cereus sensu lato strains could require CasKR to achieve efficient growth during food storage prior to a possible food-poisoning event.
Thus, CasKR seems to be a dedicated 2CS among B. cereus sensu lato strains that could participate in the low-temperature adaptation of both mesophilic and psychrotolerant strains, despite their different ranges of growth temperature. Interestingly, casKR orthologs are absent from the genomes of strains of phylogenetic group VII, i.e., the most thermotolerant strains of B. cereus sensu lato. Through this coincidence, it could be assumed that the lack of casKR genes might be linked to the inability of strains from this group to grow at temperatures below 18°C. However, these strains also probably lack many of the tools needed for low-temperature growth, given that they have a fairly smaller genome than other B. cereus sensu lato strains (53). Although the chromosomal region of casKR seems to have been conserved (see Fig. S4 in the supplemental material), these strains are also phylogenetically remote from other B. cereus sensu lato strains and therefore constitute a distinct species (B. cytotoxicus) in this group (1).
Conclusion.
Food safety issues caused by B. cereus occur because this food-borne bacterium is able to proliferate in food prior to ingestion by the consumer (2). Despite the use of refrigeration processes to limit bacterial proliferation in food, some psychrotolerant strains of B. cereus can still grow at these low temperatures. In addition, occasional leaks in the chill chain may also create conditions that allow the growth of mesophilic strains of B. cereus. Understanding the mechanisms involved in the cold adaptation of the vegetative cells of B. cereus may help to more accurately estimate the risk of proliferation in food. Efforts to identify such mechanisms have generally been performed in model mesophilic strains (8, 11, 56). Here, we identified CasKR, a 2CS with a previously unknown function which seems to be a general mechanism among B. cereus sensu lato strains that could participate in the low-temperature adaptation of both mesophilic and psychrotolerant strains. The detailed mechanism of this 2CS in cold sensing and adaptive response will have to be deciphered by further studies.
Supplementary Material
ACKNOWLEDGMENTS
INRA and the Provence Alpes-Côte d'Azur Regional Council provided the financial support for S.D.'s Ph.D.
We thank the Naval Medical Research Center, U.S. Navy, for the gifts of strains MM3 and Rock 3-28. We thank F. Carlin for help with the survival data analysis and V. Sanchis and M. Marceau for their helpful discussions.
Footnotes
Published ahead of print 7 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00090-14.
REFERENCES
- 1.Guinebretiere MH, Auger S, Galleron N, Contzen M, De Sarrau B, De Buyser ML, Lamberet G, Fagerlund A, Granum PE, Lereclus D, De Vos P, Nguyen-The C, Sorokin A. 2013. Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus group occasionally associated with food poisoning. Int. J. Syst. Evol. Microbiol. 63:31–40. 10.1099/ijs.0.030627-0 [DOI] [PubMed] [Google Scholar]
- 2.Stenfors Arnesen LP, Fagerlund A, Granum PE. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32:579–606. 10.1111/j.1574-6976.2008.00112.x [DOI] [PubMed] [Google Scholar]
- 3.Guinebretiere MH, Velge P, Couvert O, Carlin F, Debuyser ML, Nguyen-The C. 2010. Ability of Bacillus cereus group strains to cause food poisoning varies according to phylogenetic affiliation (groups I to VII) rather than species affiliation. J. Clin. Microbiol. 48:3388–3391. 10.1128/JCM.00921-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorsen L, Hansen BM, Nielsen KF, Hendriksen NB, Phipps RK, Budde BB. 2006. Characterization of emetic Bacillus weihenstephanensis, a new cereulide-producing bacterium. Appl. Environ. Microbiol. 72:5118–5121. 10.1128/AEM.00170-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brillard J, Broussolle V. 2012. Mechanisms involved in low-temperature adaptation in Bacillus cereus, p 125–145 In Requena JM. (ed), Stress response in microbiology. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 6.Carlin F, Brillard J, Broussolle V, Clavel T, Duport C, Jobin M, Guinebretiere MH, Auger S, Sorokine A, Nguyen-The C. 2010. Adaptation of Bacillus cereus, an ubiquitous worldwide-distributed foodborne pathogen, to a changing environment. Food Res. Int. 43:1885–1894. 10.1016/j.foodres.2009.10.024 [DOI] [Google Scholar]
- 7.Guinebretiere MH, Thompson FL, Sorokin A, Normand P, Dawyndt P, Ehling-Schulz M, Svensson B, Sanchis V, Nguyen-The C, Heyndrickx M, De Vos P. 2008. Ecological diversification in the Bacillus cereus group. Environ. Microbiol. 10:851–865. 10.1111/j.1462-2920.2007.01495.x [DOI] [PubMed] [Google Scholar]
- 8.Brillard J, Jehanno I, Dargaignaratz C, Barbosa I, Ginies C, Carlin F, Fedhila S, Nguyen-the C, Broussolle V, Sanchis V. 2010. Identification of Bacillus cereus genes specifically expressed during growth at low temperatures. Appl. Environ. Microbiol. 76:2562–2573. 10.1128/AEM.02348-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Sarrau B, Clavel T, Clerte C, Carlin F, Ginies C, Nguyen-The C. 2012. Influence of anaerobiosis and low temperature on Bacillus cereus growth, metabolism, and membrane properties. Appl. Environ. Microbiol. 78:1715–1723. 10.1128/AEM.06410-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haque MA, Russell NJ. 2004. Strains of Bacillus cereus vary in the phenotypic adaptation of their membrane lipid composition in response to low water activity, reduced temperature and growth in rice starch. Microbiology 150:1397–1404. 10.1099/mic.0.26767-0 [DOI] [PubMed] [Google Scholar]
- 11.Pandiani F, Brillard J, Bornard I, Michaud C, Chamot S, Nguyen-the C, Broussolle V. 2010. Differential involvement of the five RNA helicases in adaptation of Bacillus cereus ATCC 14579 to low growth temperatures. Appl. Environ. Microbiol. 76:6692–6697. 10.1128/AEM.00782-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galperin MY. 2005. A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts. BMC Microbiol. 5:35. 10.1186/1471-2180-5-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galperin MY. 2010. Diversity of structure and function of response regulator output domains. Curr. Opin. Microbiol. 13:150–159. 10.1016/j.mib.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krell T, Lacal J, Busch A, Silva-Jimenez H, Guazzaroni ME, Ramos JL. 2010. Bacterial sensor kinases: diversity in the recognition of environmental signals. Annu. Rev. Microbiol. 64:539–559. 10.1146/annurev.micro.112408.134054 [DOI] [PubMed] [Google Scholar]
- 15.Mascher T, Helmann JD, Unden G. 2006. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 70:910–938. 10.1128/MMBR.00020-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szurmant H, White RA, Hoch JA. 2007. Sensor complexes regulating two-component signal transduction. Curr. Opin. Struct. Biol. 17:706–715. 10.1016/j.sbi.2007.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitworth DE, Cock PJ. 2009. Evolution of prokaryotic two-component systems: insights from comparative genomics. Amino Acids 37:459–466. 10.1007/s00726-009-0259-2 [DOI] [PubMed] [Google Scholar]
- 18.Aguilar PS, Hernandez-Arriaga AM, Cybulski LE, Erazo AC, de Mendoza D. 2001. Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis. EMBO J. 20:1681–1691. 10.1093/emboj/20.7.1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cybulski LE, Martin M, Mansilla MC, Fernandez A, de Mendoza D. 2010. Membrane thickness cue for cold sensing in a bacterium. Curr. Biol. 20:1539–1544. 10.1016/j.cub.2010.06.074 [DOI] [PubMed] [Google Scholar]
- 20.Dahlsten E, Zhang Z, Somervuo P, Minton NP, Lindstrom M, Korkeala H. 2014. The cold-induced two-component system CBO0366/CBO0365 regulates metabolic pathways with novel roles in group I Clostridium botulinum ATCC 3502 cold tolerance. Appl. Environ. Microbiol. 80:306–319. 10.1128/AEM.03173-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindstrom M, Dahlsten E, Soderholm H, Selby K, Somervuo P, Heap JT, Minton NP, Korkeala H. 2012. Involvement of two-component system CBO0366/CBO0365 in the cold shock response and growth of group I (proteolytic) Clostridium botulinum ATCC 3502 at low temperatures. Appl. Environ. Microbiol. 78:5466–5470. 10.1128/AEM.00555-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palonen E, Lindstrom M, Karttunen R, Somervuo P, Korkeala H. 2011. Expression of signal transduction system encoding genes of Yersinia pseudotuberculosis IP32953 at 28 degrees C and 3 degrees C. PLoS One 6:e25063. 10.1371/journal.pone.0025063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun Y, Smirnova AV, Schenk A, Weingart H, Burau C, Muskhelishvili G, Ullrich MS. 2008. Component and protein domain exchange analysis of a thermoresponsive, two-component regulatory system of Pseudomonas syringae. Microbiology 154:2700–2708. 10.1099/mic.0.2008/018820-0 [DOI] [PubMed] [Google Scholar]
- 24.Chakraborty S, Li M, Chatterjee C, Sivaraman J, Leung KY, Mok YK. 2010. Temperature and Mg2+ sensing by a novel PhoP-PhoQ two-component system for regulation of virulence in Edwardsiella tarda. J. Biol. Chem. 285:38876–38888. 10.1074/jbc.M110.179150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abee T, Wels M, de Been M, den Besten H. 2011. From transcriptional landscapes to the identification of biomarkers for robustness. Microb. Cell Fact. 10(Suppl 1):S9. 10.1186/1475-2859-10-S1-S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brillard J, Susanna K, Michaud C, Dargaignaratz C, Gohar M, Nielsen-Leroux C, Ramarao N, Kolsto AB, Nguyen-the C, Lereclus D, Broussolle V. 2008. The YvfTU two-component system is involved in plcR expression in Bacillus cereus. BMC Microbiol. 8:183. 10.1186/1471-2180-8-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Been M, Tempelaars MH, van Schaik W, Moezelaar R, Siezen RJ, Abee T. 2010. A novel hybrid kinase is essential for regulating the sigma(B)-mediated stress response of Bacillus cereus. Environ. Microbiol. 12:730–745. 10.1111/j.1462-2920.2009.02116.x [DOI] [PubMed] [Google Scholar]
- 28.Duport C, Zigha A, Rosenfeld E, Schmitt P. 2006. Control of enterotoxin gene expression in Bacillus cereus F4430/73 involves the redox-sensitive ResDE signal transduction system. J. Bacteriol. 188:6640–6651. 10.1128/JB.00702-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagerlund A, Brillard J, Furst R, Guinebretiere MH, Granum PE. 2007. Toxin production in a rare and genetically remote cluster of strains of the Bacillus cereus group. BMC Microbiol. 7:43. 10.1186/1471-2180-7-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song F, Peng Q, Brillard J, Buisson C, de Been M, Abee T, Broussolle V, Huang D, Zhang J, Lereclus D, Nielsen-LeRoux C. 2012. A multicomponent sugar phosphate sensor system specifically induced in Bacillus cereus during infection of the insect gut. FASEB J. 26:3336–3350. 10.1096/fj.11-197681 [DOI] [PubMed] [Google Scholar]
- 31.de Been M, Francke C, Moezelaar R, Abee T, Siezen RJ. 2006. Comparative analysis of two-component signal transduction systems of Bacillus cereus, Bacillus thuringiensis and Bacillus anthracis. Microbiology 152:3035–3048. 10.1099/mic.0.29137-0 [DOI] [PubMed] [Google Scholar]
- 32.Dower WJ, Miller JF, Ragsdale CW. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127–6145. 10.1093/nar/16.13.6127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lereclus D, Arantes O, Chaufaux J, Lecadet M. 1989. Transformation and expression of a cloned delta-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol. Lett. 51:211–217 [DOI] [PubMed] [Google Scholar]
- 34.Claus D, Berkeley RCW. 1986. Genus Bacillus Cohn 1872, 174AL, p 1105–1139 In Sneath PHA, Mair NS, Sharpe ME, Holt JG. (ed), Bergey's manual of systematic bacteriology, vol 2 Williams & Wilkins, Baltimore, MD [Google Scholar]
- 35.de Sarrau B, Clavel T, Bornard I, Nguyen-the C. 2013. Low temperatures and fermentative metabolism limit peptidoglycan digestion of Bacillus cereus. Impact on colony forming unit counts. Food Microbiol. 33:213–220. 10.1016/j.fm.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 36.Mafart P, Couvert O, Gaillard S, Leguerinel I. 2002. On calculating sterility in thermal preservation methods: application of the Weibull frequency distribution model. Int. J. Food Microbiol. 72:107–113. 10.1016/S0168-1605(01)00624-9 [DOI] [PubMed] [Google Scholar]
- 37.Brillard J, Lereclus D. 2007. Characterization of a small PlcR-regulated gene co-expressed with cereolysin O. BMC Microbiol. 7:52. 10.1186/1471-2180-7-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnaud M, Chastanet A, Debarbouille M. 2004. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl. Environ. Microbiol. 70:6887–6891. 10.1128/AEM.70.11.6887-6891.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy E. 1985. Nucleotide sequence of a spectinomycin adenyltransferase AAD(9) determinant from Staphylococcus aureus and its relationship to AAD(″)(9). Mol. Gen. Genet. 200:33–39. 10.1007/BF00383309 [DOI] [PubMed] [Google Scholar]
- 40.Laouami S, Messaoudi K, Alberto F, Clavel T, Duport C. 2011. Lactate dehydrogenase A promotes communication between carbohydrate catabolism and virulence in Bacillus cereus. J. Bacteriol. 193:1757–1766. 10.1128/JB.00024-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markowitz VM, Chen IM, Palaniappan K, Chu K, Szeto E, Grechkin Y, Ratner A, Jacob B, Huang J, Williams P, Huntemann M, Anderson I, Mavromatis K, Ivanova NN, Kyrpides NC. 2012. IMG: the Integrated Microbial Genomes database and comparative analysis system. Nucleic Acids Res. 40:D115–D122. 10.1093/nar/gkr1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markowitz VM, Korzeniewski F, Palaniappan K, Szeto E, Werner G, Padki A, Zhao X, Dubchak I, Hugenholtz P, Anderson I, Lykidis A, Mavromatis K, Ivanova N, Kyrpides NC. 2006. The Integrated Microbial Genomes (IMG) system. Nucleic Acids Res. 34:D344–D348. 10.1093/nar/gkj024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Letunic I, Doerks T, Bork P. 2012. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 40:D302–D305. 10.1093/nar/gkr931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultz J, Milpetz F, Bork P, Ponting CP. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. U. S. A. 95:5857–5864. 10.1073/pnas.95.11.5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fabret C, Feher VA, Hoch JA. 1999. Two-component signal transduction in Bacillus subtilis: how one organism sees its world. J. Bacteriol. 181:1975–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mizuno T. 1997. Compilation of all genes encoding two-component phosphotransfer signal transducers in the genome of Escherichia coli. DNA Res. 4:161–168. 10.1093/dnares/4.2.161 [DOI] [PubMed] [Google Scholar]
- 47.Krell T, Busch A, Lacal J, Silva-Jimenez H, Ramos JL. 2009. The enigma of cytosolic two-component systems: a hypothesis. Environ. Microbiol. Rep. 1:171–176. 10.1111/j.1758-2229.2009.00020.x [DOI] [PubMed] [Google Scholar]
- 48.Ho YS, Burden LM, Hurley JH. 2000. Structure of the GAF domain, a ubiquitous signaling motif and a new class of cyclic GMP receptor. EMBO J. 19:5288–5299. 10.1093/emboj/19.20.5288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun J, Zheng L, Landwehr C, Yang J, Ji Y. 2005. Identification of a novel essential two-component signal transduction system, YhcSR, in Staphylococcus aureus. J. Bacteriol. 187:7876–7880. 10.1128/JB.187.22.7876-7880.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan M, Hall JW, Yang J, Ji Y. 2012. The essential yhcSR two-component signal transduction system directly regulates the lac and opuCABCD operons of Staphylococcus aureus. PLoS One 7:e50608. 10.1371/journal.pone.0050608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muniesa M, Hammerl JA, Hertwig S, Appel B, Brussow H. 2012. Shiga toxin-producing Escherichia coli O104:H4: a new challenge for microbiology. Appl. Environ. Microbiol. 78:4065–4073. 10.1128/AEM.00217-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brillard J, Lereclus D. 2004. Comparison of cytotoxin cytK promoters from Bacillus cereus strain ATCC 14579 and from a B. cereus food-poisoning strain. Microbiology 150:2699–2705. 10.1099/mic.0.27069-0 [DOI] [PubMed] [Google Scholar]
- 53.Lapidus A, Goltsman E, Auger S, Galleron N, Segurens B, Dossat C, Land ML, Broussolle V, Brillard J, Guinebretiere MH, Sanchis V, Nguyen-the C, Lereclus D, Richardson P, Wincker P, Weissenbach J, Ehrlich SD, Sorokin A. 2008. Extending the Bacillus cereus group genomics to putative food-borne pathogens of different toxicity. Chem. Biol. Interact. 171:236–249. 10.1016/j.cbi.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 54.Ehling-Schulz M, Svensson B, Guinebretiere M-H, Lindback T, Andersson M, Schulz A, Fricker M, Christiansson A, Granum PE, Martlbauer E, Nguyen-The C, Salkinoja-Salonen M, Scherer S. 2005. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 151:183–197. 10.1099/mic.0.27607-0 [DOI] [PubMed] [Google Scholar]
- 55.Salamitou S, Ramisse F, Brehelin M, Bourguet D, Gilois N, Gominet M, Hernandez E, Lereclus D. 2000. The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiology 146:2825–2832 [DOI] [PubMed] [Google Scholar]
- 56.Broussolle V, Pandiani F, Haddad N, Michaud C, Carlin F, Nguyen-the C, Brillard J. 2010. Insertional mutagenesis reveals genes involved in Bacillus cereus ATCC 14579 growth at low temperature. FEMS Microbiol. Lett. 306:177–183. 10.1111/j.1574-6968.2010.01953.x [DOI] [PubMed] [Google Scholar]
- 57.Arantes O, Lereclus D. 1991. Construction of cloning vectors for Bacillus thuringiensis. Gene 108:115–119. 10.1016/0378-1119(91)90495-W [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.