Abstract
Entry and exit from dormancy are essential survival mechanisms utilized by microorganisms to cope with harsh environments. Many bacteria, including the opportunistic human pathogen Vibrio vulnificus, enter a form of dormancy known as the viable but nonculturable (VBNC) state. VBNC cells can resuscitate when suitable conditions arise, yet the molecular mechanisms facilitating resuscitation in most bacteria are not well understood. We discovered that bacterial cell-free supernatants (CFS) can awaken preexisting dormant vibrio populations within oysters and seawater, while CFS from a quorum sensing mutant was unable to produce the same resuscitative effect. Furthermore, the quorum sensing autoinducer AI-2 could induce resuscitation of VBNC V. vulnificus in vitro, and VBNC cells of a mutant unable to produce AI-2 were unable to resuscitate unless the cultures were supplemented with exogenous AI-2. The quorum sensing inhibitor cinnamaldehyde delayed the resuscitation of wild-type VBNC cells, confirming the importance of quorum sensing in resuscitation. By monitoring AI-2 production by VBNC cultures over time, we found quorum sensing signaling to be critical for the natural resuscitation process. This study provides new insights into the molecular mechanisms stimulating VBNC cell exit from dormancy, which has significant implications for microbial ecology and public health.
INTRODUCTION
At least 68 species of bacteria have been shown to enter a state of dormancy in which cells are viable but nonculturable (VBNC) (1), allowing them to evade harmful environmental stresses while maintaining the ability to return to an active state when the stress is alleviated (2). These cells are viable, as evidenced by their intact cell membranes and continued gene expression; however, they fail to replicate on routine laboratory media (2, 3). While in the VBNC state, these cells are able to resist a variety of normally fatal stresses, including antibiotic treatment (4), and they possess the ability to resuscitate within a host, potentially leading to disease initiation (5). Since these cells are not detectable by standard bacterial culture methods, the VBNC phenomenon is of significant public health and food safety concern. While there has been considerable growth in our knowledge of this dormancy state (1), very little is known regarding the molecular mechanisms for the resuscitation of VBNC cells.
Pathogenic vibrios exhibit seasonal fluctuations in abundance in water and in shellfish (6, 7), with their highest levels of culturability and incidences of infection observed during warm summer months (8, 9). The VBNC state is believed to be associated with this seasonal abundance in the environment, with culturability decreasing during winter months, when some cells enter the VBNC state, and increasing during warmer months, when these cells begin to resuscitate (10).
The opportunistic human pathogen Vibrio vulnificus is part of the normal microflora of the estuarine environment and is found concentrated within bivalves and other organisms that inhabit these waters (11, 12). V. vulnificus classically expresses its virulence after the ingestion of raw or undercooked shellfish (11), or by entry into open wounds (13), severely harming individuals with liver disease or immunodeficiency (14–16). With a case fatality rate of up to 50% (15), the majority (95%) of deaths associated with seafood in the United States are caused by this pathogen (7, 17). The VBNC state of V. vulnificus is easily studied in vitro by induction of dormancy using low-temperature incubation and of resuscitation by a simple temperature upshift (18), making it an ideal model organism for the study of this physiological state.
We previously found that upon addition of exogenous V. vulnificus or Escherichia coli cells to oysters, the culturability of the normal flora of Vibrio spp. within these oysters dramatically increased and remained high even after the added bacterial populations had been purged by the oysters (19). This indicated that preexisting and naturally present VBNC populations within these oysters resuscitated in response to the added metabolically active bacteria. In a related study, environmental water samples were enriched with the quorum sensing molecules CAI-1 and AI-2 to enhance the culturability of Vibrio cholerae (20). These findings led us to test the hypothesis that the increase in the culturability of Vibrio spp. upon the addition of exogenous metabolically active cells to oysters is due to quorum sensing signaling.
The phenomenon known as quorum sensing is the production of and response to signals (autoinducers) generated by bacteria of the same or other species in an effort to gauge the population density in a certain environment or to evaluate the cells' environmental location (21). This leads to global changes in gene expression (22) and has been shown to orchestrate virulence (23) and fitness factor production (24). For a more in-depth explanation of quorum sensing, the reader is directed to a recent review (25). Interestingly, some environmental factors that have been shown to affect quorum sensing, such as temperature, salinity, and pH, have independently been shown to play a role in VBNC dynamics (2, 26). Given these findings, we asked whether quorum sensing autoinducers mediate the resuscitation of VBNC V. vulnificus.
Here we demonstrate that the interspecific quorum sensing molecule AI-2 can resuscitate VBNC cells both in vivo and in vitro. Most importantly, we report for the first time that AI-2 is required by VBNC cells to resuscitate and that this quorum sensing-mediated process occurs naturally within VBNC cultures when environmental conditions are permissive, leading to resuscitation. Furthermore, we propose a molecular mechanism for the resuscitation of VBNC cells and show that it can be targeted to manipulate resuscitation.
MATERIALS AND METHODS
Strains and growth conditions.
Strains used in this study were V. vulnificus CMCP6, C7184, JDO1, and AH1, V. parahaemolyticus Sak-11, V. harveyi BB170, and E. coli K-12. All strains were stored at −80°C in Bacto Luria broth (LB) (BD, NJ) containing 20% glycerol. All strains were grown in Bacto heart infusion (HI) broth (BD, NJ) for 24 h at 30°C with aeration. Strains JDO1 and AH1 are luxS and rpoS mutants, respectively, and were derived from the parent strain C7184. JDO1 is a luxS mutant that is unable to produce 4,5-dihydroxy-2,3-pentanedione, an essential precursor to the autoinducer AI-2 (27), and AH1 is unable to produce the stress-related sigma factor RpoS (28). JDO1 and AH1 were grown with 2 and 15 μg/ml chloramphenicol, respectively. All bioluminescence-based quorum sensing assays were performed using autoinducer bioassay (AB) medium (29).
Resuscitation of dormant cells in oysters.
Crassostrea virginica oysters were collected from Hoop Pole Creek, Atlantic Beach, NC, during September 2012. Oysters were maintained in 30-liter aerated aquaria containing 20‰ artificial seawater (ASW) (Instant Ocean; Aquarium Systems, Mentor, OH) and were fed Phyto-Feast daily (Reed Mariculture Inc., Campbell, CA).
Cell-free supernatants (CFS) were obtained from overnight cultures of V. vulnificus C7184 and JDO1 by filter sterilization (0.22-μm syringe-driven filter; Millipore, Tullagreen, Ireland). To confirm the sterility of CFS, 100-μl aliquots of CFS were spread plated onto HI agar. Oysters were placed in 3-liter tanks (4 oysters per treatment condition) of 20‰ ASW with aeration. CFS was added to oyster tanks at a 1:100 (vol/vol) ratio. After 24 h, oysters were shucked using a sterile knife and homogenized in 20‰ ASW (1:1 [wt/vol]), using sterile blender cups (Waring, Torrington, CT). Individual oyster homogenates were serially diluted in phosphate-buffered saline (PBS) and spread plated onto ChromAgar Vibrio (ChromAgar, Paris, France) to selectively quantify Vibrio species. This medium was employed to quantify total vibrios, as it has been demonstrated previously to indiscriminately allow for growth of all Vibrio species tested (30). Oysters which were incubated with CFS were compared to control oysters that were supplemented only with ASW. As an additional control, some oysters were supplemented with HI broth to ensure that nutrient carryover had no effect on vibrio levels.
Resuscitation of dormant cells in seawater.
Natural seawater (NSW) was collected from Core Creek, Beaufort, NC, and stored at 20°C in sterile 50-ml conical tubes. Resuscitation experiments were performed within 24 h of collection. To eliminate nutrient carryover, 1 ml of overnight bacterial culture was washed twice with 15‰ ASW and incubated at 30°C for 24 h prior to collection of CFS by filter sterilization as described above. This nutrient-free CFS was added to the NSW at a 1:100 (vol/vol) ratio. The mixture was incubated at 20°C for 24 h before serially diluting and plating of the mixture on ChromAgar Vibrio. The fold change in CFU after 24 h in NSW supplemented with CFS was compared to that for controls that received only sterile 15‰ ASW. Experiments were performed in triplicate.
Production and resuscitation of in vitro VBNC cells.
VBNC cultures were produced as previously described (18). Briefly, V. vulnificus was grown overnight in HI broth, added to fresh HI broth at a 1:100 (vol/vol) ratio, and grown to logarithmic phase (optical density at 610 nm [OD610], 0.15 to 0.25). Cells were subsequently washed twice with 15‰ ASW to remove any nutrients, resuspended in 15‰ ASW at a 1:100 (vol/vol) ratio, and stored statically in a 4°C incubator. Cultures were quantified daily until V. vulnificus was no longer culturable on HI agar (<10 CFU/ml detectable).
To resuscitate VBNC cells, cultures were transferred from the 4°C incubator to room temperature (20°C). Cultures were immediately supplemented with nutrient-free CFS (collected as described above) from either V. vulnificus, V. parahaemolyticus, or E. coli, which are known to produce AI-2, at a 1:100 (vol/vol) ratio, or they were supplemented with 15‰ ASW at a 1:100 (vol/vol) ratio as a control. Additionally, we utilized nutrient-free CFS from a V. vulnificus luxS mutant strain (JDO1) unable to produce AI-2. These mixtures were serially diluted in PBS and plated at regular intervals onto HI agar to monitor resuscitation. Resuscitation curves were compared to those for control resuscitation cultures that received ASW. Three biological replicates were used for each treatment.
Use of synthetic autoinducer to induce resuscitation.
VBNC cultures were prepared as described above. The synthetic autoinducer (s)-4,5-dihydroxy-2,3-pentanedione (DPD) (OMM Scientific, Dallas, TX) spontaneously forms the quorum sensing molecule AI-2 in liquid suspension (31). AI-2 was prepared by adding a 10-fold molar excess of boric acid to DPD, making a 50 μM DPD stock solution. To resuscitate VBNC cells, dormant cultures of C7184, JDO1, and AH1 were moved to 20°C, and AI-2 was immediately added to each culture, to a final concentration of 250 nM. Cultures were serially diluted and plated onto HI agar at regular intervals to monitor resuscitation. Resuscitation times were compared to those for control resuscitation cultures that received only ASW. Experiments were performed in triplicate.
Use of quorum sensing inhibitor to prevent resuscitation.
Cinnamaldehyde is a quorum sensing inhibitor that has been shown to prevent binding of LuxR, the master regulator of quorum sensing, to DNA (32). We used a cinnamaldehyde concentration of 150 μM, which is noninhibitory to growth, as previously reported (32). Cinnamaldehyde-supplemented VBNC cultures (prepared as described above) were allowed to resuscitate by incubation at 20°C. Cultures were serially diluted and plated onto HI agar at regular intervals to monitor resuscitation. Resuscitation curves were compared to those for control resuscitation cultures that received an equal volume of ASW. Experiments were performed in triplicate.
Bioluminescence-based quorum sensing assay.
The bioluminescence-based quorum sensing assay was performed as previously described (33), with slight modifications. Briefly, Vibrio harveyi strain BB170, which is bioluminescent only in response to AI-2 signals, was grown overnight on AB agar at 30°C. Cells were then removed using a sterile swab, diluted in AB broth to an OD610 of 0.7 to 0.8, and then incubated with shaking at 30°C until the OD610 reached 1.1. A 1:5,000 dilution of this culture in fresh AB broth was prepared to create the working solution. Nutrient-free CFS was added to the working solution at a 1:10 (vol/vol) ratio for a total of 1.5 ml and was incubated at 30°C with shaking for 4 h. After incubation, 200 μl of sample was transferred to a flat-bottom 96-well plate and read using a GloMax 96 microplate luminometer (Promega, Madison, WI). Background luminescence was assayed by quantifying control samples that contained 15‰ ASW and the working solution of BB170 at a 1:10 (vol/vol) ratio for a total of 1.5 ml. Data are presented as sample luminescence levels (arbitrary units) with the average background luminescence subtracted. Experiments were performed in triplicate.
Validation of resuscitation.
To validate that during in vitro resuscitation experiments cells were truly resuscitating rather than growing from a small, undetectable population, theoretical generation times for resuscitating cells were calculated using equation 1:
| (1) |
where Ci and Cf are the starting and ending CFU values, respectively, for the 1-h time interval being analyzed. This method of validating resuscitation has been utilized by others (18, 20, 34) to demonstrate that the observed increase in culturability is indeed due to resuscitation rather than regrowth of a small culturable population.
Statistical analysis.
Data were analyzed using unpaired Student's t test, or one-way analysis of variance (ANOVA) followed by Tukey's post hoc test for multiple comparisons. Statistical significance was determined using an alpha level of 0.05. Error bars in all figures represent standard deviations (SD). All data were analyzed using GraphPad Prism (version 6.0; GraphPad Software Inc.).
RESULTS AND DISCUSSION
Addition of CFS increases culturability of vibrios in oysters and seawater.
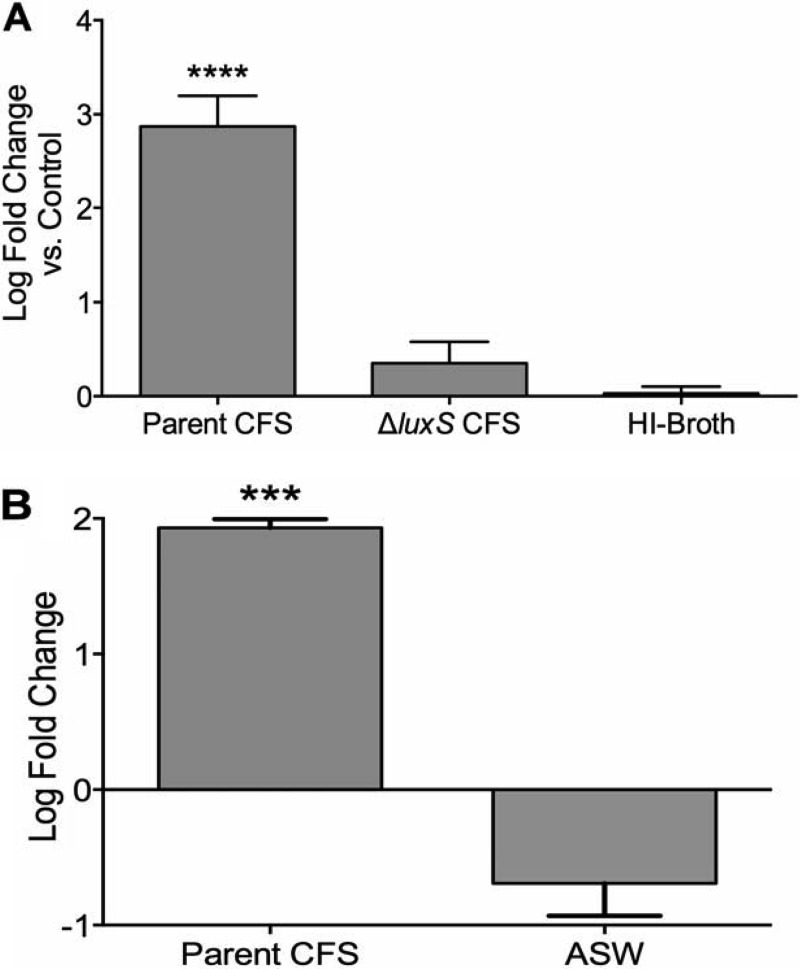
We showed previously that the addition of exogenous bacteria to oysters dramatically increased the culturability of the resident Vibrio spp. in oyster tissues, likely through the resuscitation of a dormant population (19). To evaluate whether this increase was in response to extracellular factors, CFS collected from V. vulnificus (C7184) was added to oysters, and the culturability of Vibrio spp. present among the members of the oyster microflora was monitored. We found that upon addition of CFS from AI-2-producing V. vulnificus cells to live oysters, the culturability of Vibrio spp. within these oysters increased significantly (P < 0.0001), indicating that an extracellular factor promoted in vivo resuscitation of Vibrio spp. (Fig. 1A). In contrast, the addition of nutrients (HI broth) to oysters did not result in a considerable increase in culturability (Fig. 1A). We also observed that a quorum sensing mutant lacking the ability to produce AI-2 was unable to produce the same effect (Fig. 1A), suggesting that the extracellular resuscitation-promoting factor was AI-2. A similar increase in culturability was observed when CFS was added to natural seawater (NSW) (Fig. 1B). These results provide evidence for the hypothesis that resuscitation of V. vulnificus from the VBNC state is a quorum sensing-related phenomenon.
FIG 1.
Increase in the culturability of Vibrio spp. in oysters and seawater upon addition of CFS. (A) Log fold change in culturability of Vibrio spp. in oyster tissues after 24 h of treatment with CFS from V. vulnificus C7184 (parent) or JDO1 (luxS mutant) relative to that of control oysters that received artificial seawater (ASW). Wild-type CFS significantly increased the culturability of Vibrio spp. in oysters (one-way ANOVA; P < 0.0001), whereas CFS from the luxS mutant did not. Since HI broth had little effect on culturability, nutrient addition did not affect the culturability of Vibrio spp. Error bars represent SD for 4 oysters. (B) Log fold change in levels of Vibrio spp. in NSW after 24 h (relative to that at 0 h) of treatment with either CFS from V. vulnificus C7184 or the ASW control (unpaired t test; P = 0.0004). Error bars represent SD for 3 NSW samples.
Addition of CFS or AI-2 induces early resuscitation of in vitro VBNC cultures.
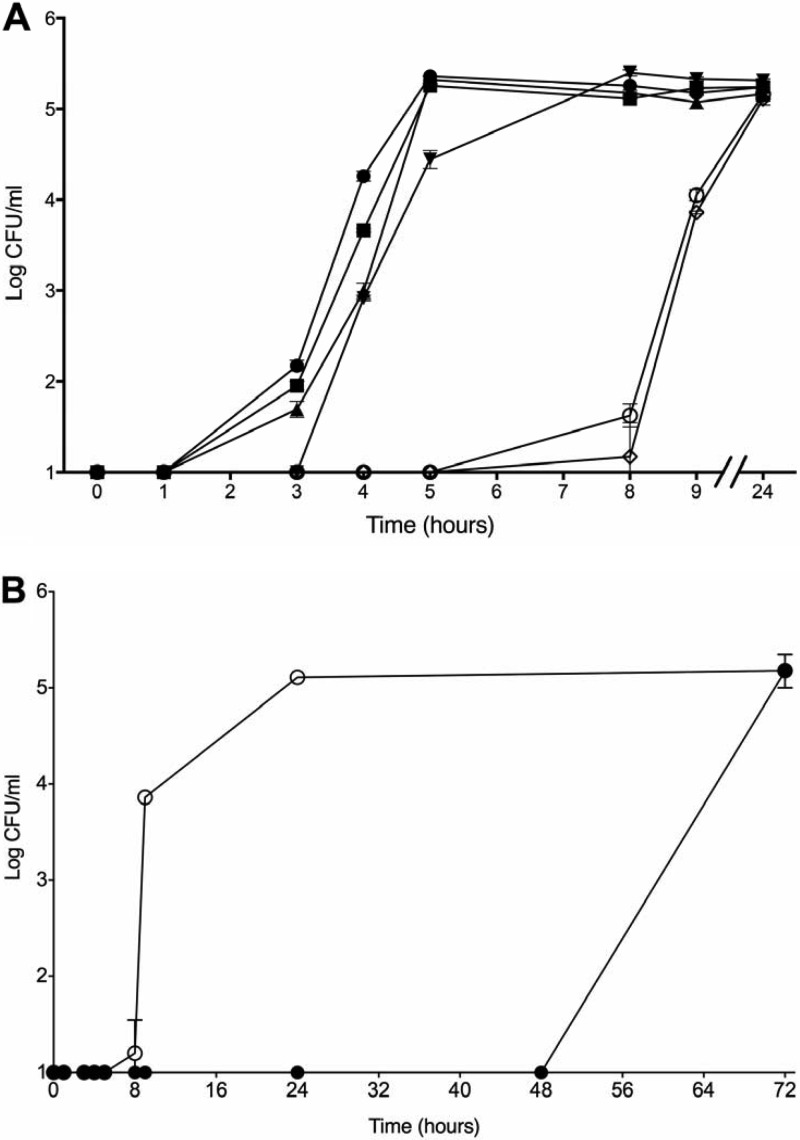
If AI-2 mediates the resuscitation of VBNC cells present within oysters and seawater, then the addition of AI-2 to in vitro VBNC cultures would also be expected to induce early resuscitation. V. vulnificus can normally be resuscitated within 7 to 9 h after a temperature upshift from 4°C to 20°C (18). Therefore, we hypothesized that the addition of AI-2 to VBNC cultures immediately after a temperature upshift would cause resuscitation to occur much earlier. The addition of CFS from wild-type AI-2-producing V. vulnificus, V. parahaemolyticus, and E. coli bacteria induced resuscitation of VBNC cells in as little as 3 h, whereas CFS from a V. vulnificus luxS mutant deficient in AI-2 production was unable to induce a faster resuscitation than that of the control (∼8 h) (Fig. 2A). Since CFS is composed of various components aside from AI-2, we tested the ability of synthetic AI-2 to induce resuscitation. Synthetic AI-2 was also able to induce early resuscitation of VBNC cells (Fig. 2A), indicating that AI-2 alone is able to induce the reactivation of dormant V. vulnificus cells during a temperature upshift.
FIG 2.
Resuscitation of VBNC V. vulnificus supplemented with CFS, synthetic AI-2, or cinnamaldehyde. (A) Culturability of V. vulnificus after temperature upshift and supplementation with CFS from V. vulnificus C7184 (parent; closed circles), JDO1 (luxS mutant; open circles), V. parahaemolyticus (closed squares), or E. coli (closed triangles) or with synthetic AI-2 (closed downward-facing triangles). Control cultures (open diamonds) received ASW. All wild-type CFS and synthetic AI-2 induced early resuscitation of VBNC cells, while CFS from the luxS mutant did not. Error bars represent SD for 3 replicates. (B) Culturability of V. vulnificus after a temperature upshift, with (closed circles) and without (open circles) 150 μM cinnamaldehyde. Error bars represent SD for 3 replicates.
Quorum sensing inhibitor delays resuscitation of in vitro VBNC cultures.
To test whether the action of AI-2-mediated resuscitation was through the master quorum sensing regulator, SmcR (a LuxR homolog) (35), we employed an inhibitor of LuxR, hypothesizing that this would inhibit the resuscitation of VBNC cells. The LuxR inhibitor, cinnamaldehyde, was able to delay resuscitation by at least 40 h (Fig. 2B). This indicates that the resuscitation-promoting ability of autoinducers is effective, at least partially, through the quorum sensing regulatory pathways and that the inhibition of quorum sensing can hinder the resuscitation process.
Validation of resuscitation.
To validate that the observed increase in culturability was in fact resuscitation rather than a regrowth of a small population of undetectable cells, we calculated the theoretical generation times required to yield such an increase if the cells were actually growing rather than resuscitating. In all cases, the generation times required to produce the observed increases in culturability were 13.9 min or less (Table 1). We determined V. vulnificus to have a generation time of 30.7 ± 0.17 min during logarithmic growth under nutrient-rich conditions (HI broth); thus, it is nearly impossible for this species to divide at a higher rate under the nutrient-depleted (ASW), nonaerated, room temperature conditions employed in our resuscitation studies. These resuscitation rates are much higher than normal rates of division would allow and therefore support our claim that these cells were truly resuscitating rather than growing from a small number of culturable cells.
TABLE 1.
Theoretical generation times required for the observed increase in culturability during Vibrio vulnificus resuscitation
| Treatment | Theoretical generation time (min) |
|---|---|
| Vibrio vulnificus CFS | 8.66 |
| Vibrio parahaemolyticus CFS | 10.57 |
| Escherichia coli CFS | 13.88 |
| Vibrio vulnificus JDO1 CFS | 7.48 |
| Synthetic AI-2 | 11.77 |
| Controla (no CFS) | 7.01 |
Untreated VBNC Vibrio vulnificus resuscitation.
Resuscitation occurs in response to increased AI-2.
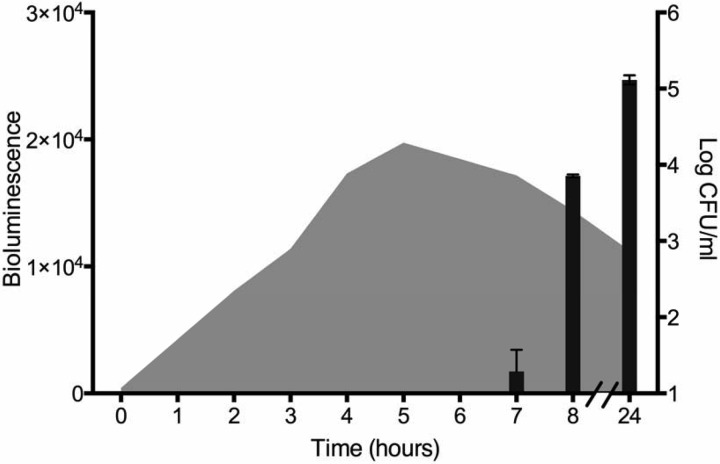
Since VBNC cultures artificially supplemented with autoinducers promoted resuscitation, it was speculated that this AI-2-mediated process might also occur naturally during a temperature upshift. To test this, the production of AI-2 and the culturability of cells during resuscitation from the VBNC state following a temperature upshift were monitored simultaneously. During resuscitation, there was a rapid increase in AI-2 levels, reaching a maximum value 5 h after temperature upshift, even though initiation of culturability was not detected until ca. 7 h (Fig. 3). Interestingly, in repeated studies, resuscitation was detected only after AI-2 reached such a maximum, independent of the temporal occurrence of the maximum, suggesting that AI-2 levels must reach a threshold value before resuscitation can begin. It is interesting that AI-2 levels were able to increase before culturability could be detected following the temperature upshift. We postulate that either VBNC cells are able to produce AI-2 before they become culturable or a small, undetectable population signals to the dormant population to initiate resuscitation. A recent study by Buerger et al. (36) provided evidence for Epstein's (37) microbial scout hypothesis, which proposes that a small portion of a dormant population resuscitates stochastically (becomes “scouts”), independent of environmental signals. If environmental conditions are not permissive, these scouts are thought to die. However, if acceptable environmental conditions are sensed, then these scouts may signal to their dormant counterparts that growth-permissive conditions have been met (36). We propose that, at least for V. vulnificus, and likely other bacteria, quorum sensing autoinducers are the signals employed by the scouts to rouse the rest of the dormant population. This hypothesis is substantiated by our observed initial increase in AI-2 production without detectable culturability, indicating that scout cells, present at levels below our detection limits, had stochastically resuscitated and were signaling to the rest of the population, via quorum sensing, that acquiescent conditions had been reached. Since our studies have shown that resuscitation in response to autoinducers is not species specific, active cells may function as scouts for a dormant population of a different genus or species through AI-2 signaling. To confirm that resuscitation during a temperature upshift occurs in response to AI-2, the ability of VBNC cultures of the V. vulnificus luxS mutant to resuscitate was examined. The mutant was unable to resuscitate from the VBNC state during a temperature upshift unless the culture was supplemented with exogenous AI-2, again indicating that AI-2 is required for resuscitation to occur in V. vulnificus (Fig. 4A).
FIG 3.
Production of AI-2 during temperature upshift-induced resuscitation from the VBNC state. The data depicted show the culturability of V. vulnificus cells (black bars) as they resuscitated from the VBNC state, with corresponding AI-2 levels (gray curve) in these cultures as measured by the AI-2 bioluminescence assay.
FIG 4.
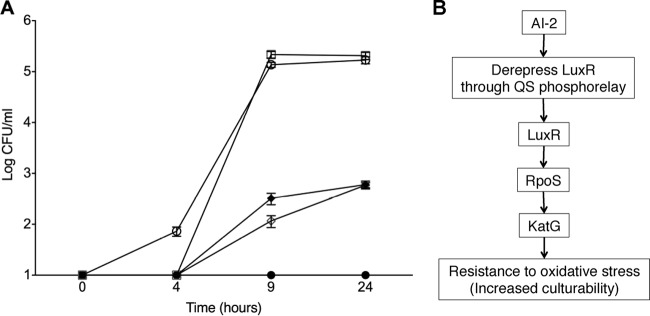
Resuscitation of V. vulnificus luxS and rpoS mutants during temperature upshift. (A) Culturability of luxS mutant (JDO1; closed circles), rpoS mutant (AH1; closed diamonds), and parent strain (C7184; open squares) after a resuscitation-inducing temperature upshift. JDO1 was unable to resuscitate within 24 h unless it was supplemented with exogenous AI-2 (open circles), indicating the requirement of AI-2 for the resuscitation of VBNC V. vulnificus. AH1 displayed a delayed ability to resuscitate, likely due to decreased survival at 4°C. Importantly, resuscitation of AH1 was not affected by the addition of AI-2 (open diamonds), indicating the importance of RpoS for AI-2-mediated resuscitation. (B) Proposed mechanism of resuscitation. An increase in AI-2 signaling causes the derepression of LuxR, a positive regulator of RpoS. Increased RpoS activity leads to an increase in production of catalase (KatG), which allows cells to be culturable on routine media containing H2O2.
Resuscitation mechanism.
It has previously been demonstrated that V. vulnificus cells in the VBNC state are nonculturable on laboratory media largely due to reduced catalase activity. This sensitizes the cells to the presence of reactive oxygen species and therefore prevents replication of these cells on media that routinely contain H2O2 (38). Furthermore, catalase production resumes upon resuscitation from the VBNC state, allowing renewed culturability (39). The stress-related alternate sigma factor RpoS is known to be necessary for the production of catalase (40) and to play an important role in resuscitation from the VBNC state (41). Furthermore, Joelsson et al. (42) reported that LuxR enhances the expression of rpoS. Another study found that expression of rpoS significantly increased during resuscitation from the VBNC state (43). Here we found that a V. vulnificus rpoS mutant had a restricted ability to resuscitate within 24 h. Importantly, the addition of AI-2 to a VBNC culture of the rpoS mutant did not induce early resuscitation (Fig. 4A) like that observed with VBNC cells of the wild-type parent. This finding indicates that RpoS is an important factor in AI-2-mediated resuscitation. Combining the existing literature with our current findings, we propose a model of resuscitation in which increased AI-2 signaling stimulates expression of rpoS through the action of LuxR. This subsequently induces the expression of catalase (katG), thereby allowing cells to overcome the toxic properties of H2O2 in bacterial media and to return to a culturable state (Fig. 4B). However, it cannot be excluded that quorum sensing has an effect on other processes related to dormancy, such as toxin-antitoxin regulation, which has been studied extensively in persister cell formation, another type of bacterial dormancy (44).
Conclusions.
A previous study demonstrated that artificially supplementing VBNC cells with quorum sensing autoinducers can resuscitate dormant Vibrio cholerae cells to improve their detectability (20). Here we provide evidence that interspecific quorum sensing autoinducers have the capability of resuscitating dormant bacterial populations both in vivo and in vitro. For the first time, we have elucidated the active interplay between autoinducer levels and VBNC dynamics in V. vulnificus.
Specifically, we found that VBNC cells resuscitate in response to a quorum sensing signal that is produced by cells within a dormant population when permissive conditions are reached. As proposed by Manina and McKinney (45), with the advent of real-time single-cell analysis technology, the role of quorum sensing in the resuscitation of dormant microorganisms may be studied with much greater resolution. This study may provide important insights for clinical intervention and for the development of innovative antimicrobial agents in the fight against drug-tolerant dormant cells. Overall, this study provides a greater understanding of the mechanisms employed by bacteria to exit from dormancy, which helps to explain the seasonality exhibited by bacterial populations in natural environments. These findings have important implications for microbial ecology and public health.
ACKNOWLEDGMENTS
We thank Bonnie L. Bassler for kindly providing the V. harveyi strains and Matthew W. Parrow for providing the synthetic AI-2 used in this study.
This material is based upon work supported by the U.S. Department of Agriculture, NIFA, under agreement 2010-65201-30959.
Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
We declare no conflicts of interest for this article.
Footnotes
Published ahead of print 7 February 2014
REFERENCES
- 1.Pinto D, Santos MA, Chambel L. 12 July 2013. Thirty years of viable but nonculturable state research: unsolved molecular mechanisms. Crit. Rev. Microbiol. 10.3109/1040841X.2013.794127 [DOI] [PubMed] [Google Scholar]
- 2.Oliver JD. 2010. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol. Rev. 34:415–425. 10.1111/j.1574-6976.2009.00200.x [DOI] [PubMed] [Google Scholar]
- 3.Oliver JD. 2005. The viable but nonculturable state in bacteria. J. Microbiol. 43:93–100 http://www.ncbi.nlm.nih.gov/pubmed/15765062 [PubMed] [Google Scholar]
- 4.Nowakowska J, Oliver JD. 2013. Resistance to environmental stresses by Vibrio vulnificus in the viable but nonculturable state. FEMS Microbiol. Ecol. 84:213–222. 10.1111/1574-6941.12052 [DOI] [PubMed] [Google Scholar]
- 5.Oliver JD, Bockian R. 1995. In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl. Environ. Microbiol. 61:2620–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaspar CW, Tamplin ML. 1993. Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl. Environ. Microbiol. 59:2425–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliver JD, Kaper JB. 2001. Vibrio species, p 263–300 In Doyle MP, Beuchat LR, Montville TJ. (ed), Food microbiology: fundamentals and frontiers. American Society for Microbiology, Washington, DC [Google Scholar]
- 8.CDC. 2007. Summary of human Vibrio cases reported to CDC. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 9.Blackwell KD, Oliver JD. 2008. The ecology of Vibrio vulnificus, Vibrio cholerae, and Vibrio parahaemolyticus in North Carolina estuaries. J. Microbiol. 46:146–153. 10.1007/s12275-007-0216-2 [DOI] [PubMed] [Google Scholar]
- 10.Oliver JD, Hite F, McDougald D, Andon NL, Simpson LM. 1995. Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Appl. Environ. Microbiol. 61:2624–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver JD. 2006. Vibrio vulnificus, p 349–366 In Thompson FL, Austin B, Swings J. (ed), The biology of vibrios. American Society for Microbiology, Washington, DC [Google Scholar]
- 12.Froelich B, Oliver JD. 2013. The interactions of Vibrio vulnificus and the oyster Crassostrea virginica. Microb. Ecol. 65:807–816. 10.1007/s00248-012-0162-3 [DOI] [PubMed] [Google Scholar]
- 13.Oliver JD. 2005. Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiol. Infect. 133:383–391. 10.1017/S0950268805003894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dechet AM, Yu PA, Koram N, Painter J. 2008. Nonfoodborne Vibrio infections: an important cause of morbidity and mortality in the United States, 1997–2006. Clin. Infect. Dis. 46:970–976. 10.1086/529148 [DOI] [PubMed] [Google Scholar]
- 15.Jones MK, Oliver JD. 2009. Vibrio vulnificus: disease and pathogenesis. Infect. Immun. 77:1723–1733. 10.1128/IAI.01046-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliver JD. 2013. Vibrio vulnificus: death on the half shell. A personal journey with the pathogen and its ecology. Microb. Ecol. 65:793–799. 10.1007/s00248-012-0140-9 [DOI] [PubMed] [Google Scholar]
- 17.Feldhusen F. 2000. The role of seafood in bacterial foodborne diseases. Microb. Infect. Inst. Pasteur 2:1651–1660. 10.1016/S1286-4579(00)01321-6 [DOI] [PubMed] [Google Scholar]
- 18.Whitesides MD, Oliver JD. 1997. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl. Environ. Microbiol. 63:1002–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Froelich B, Oliver JD. 2013. Increases of Vibrio spp. in oysters upon addition of exogenous bacteria. Appl. Environ. Microbiol. 79:5208–5213. 10.1128/AEM.01110-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bari SM, Roky MK, Mohiuddin M, Kamruzzaman M, Mekalanos JJ, Faruque SM. 2013. Quorum-sensing autoinducers resuscitate dormant Vibrio cholerae in environmental water samples. Proc. Natl. Acad. Sci. U. S. A. 110:9926–9931. 10.1073/pnas.1307697110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassler BL. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2:582–587. 10.1016/S1369-5274(99)00025-9 [DOI] [PubMed] [Google Scholar]
- 22.Withers H, Swift S, Williams P. 2001. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria. Curr. Opin. Microbiol. 4:186–193. 10.1016/S1369-5274(00)00187-9 [DOI] [PubMed] [Google Scholar]
- 23.Antunes LC, Ferreira RB, Buckner MM, Finlay BB. 2010. Quorum sensing in bacterial virulence. Microbiology 156:2271–2282. 10.1099/mic.0.038794-0 [DOI] [PubMed] [Google Scholar]
- 24.McDougald D, Srinivasan S, Rice SA, Kjelleberg S. 2003. Signal-mediated cross-talk regulates stress adaptation in Vibrio species. Microbiology 149:1923–1933. 10.1099/mic.0.26321-0 [DOI] [PubMed] [Google Scholar]
- 25.Rutherford ST, Bassler BL. 2012. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2:a012427. 10.1101/cshperspect.a012427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham E, Obyrne C, Oliver JD. 2009. Effect of weak acids on Listeria monocytogenes survival: evidence for a viable but nonculturable state in response to low pH. Food Control 20:1141–1144. 10.1016/j.foodcont.2009.03.005 [DOI] [Google Scholar]
- 27.Beam DM. 2004. The role of AI-2 in Vibrio vulnificus. M.S. thesis University of North Carolina at Charlotte, Charlotte, NC [Google Scholar]
- 28.Hulsmann A, Rosche TM, Kong IS, Hassan HM, Beam DM, Oliver JD. 2003. RpoS-dependent stress response and exoenzyme production in Vibrio vulnificus. Appl. Environ. Microbiol. 69:6114–6120. 10.1128/AEM.69.10.6114-6120.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg EP, Hastings JW, Ulitzur S. 1979. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch. Microbiol. 120:87–91. 10.1007/BF00409093 [DOI] [Google Scholar]
- 30.Williams TC, Froelich B, Oliver JD. 2013. A new culture-based method for the improved identification of Vibrio vulnificus from environmental samples, reducing the need for molecular confirmation. J. Microbiol. Methods 93:277–283. 10.1016/j.mimet.2013.03.023 [DOI] [PubMed] [Google Scholar]
- 31.Tsuchikama K, Lowery CA, Janda KD. 2011. Probing autoinducer-2 based quorum sensing: the biological consequences of molecules unable to traverse equilibrium states. J. Org. Chem. 76:6981–6989. 10.1021/jo200882k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brackman G, Defoirdt T, Miyamoto C, Bossier P, Van Calenbergh S, Nelis H, Coenye T. 2008. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 8:149. 10.1186/1471-2180-8-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassler BL, Greenberg EP, Stevens AM. 1997. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 179:4043–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaprelyants AS, Kell DB. 1993. Dormancy in stationary-phase cultures of Micrococcus luteus: flow cytometric analysis of starvation and resuscitation. Appl. Environ. Microbiol. 59:3187–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDougald D, Rice SA, Kjelleberg S. 2000. The marine pathogen Vibrio vulnificus encodes a putative homologue of the Vibrio harveyi regulatory gene, luxR: a genetic and phylogenetic comparison. Gene 248:213–221. 10.1016/S0378-1119(00)00117-7 [DOI] [PubMed] [Google Scholar]
- 36.Buerger S, Spoering A, Gavrish E, Leslin C, Ling L, Epstein SS. 2012. Microbial scout hypothesis, stochastic exit from dormancy, and the nature of slow growers. Appl. Environ. Microbiol. 78:3221–3228. 10.1128/AEM.07307-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epstein SS. 2009. Microbial awakenings. Nature 457:1083. 10.1038/4571083a [DOI] [PubMed] [Google Scholar]
- 38.Kong IS, Bates TC, Hulsmann A, Hassan H, Smith BE, Oliver JD. 2004. Role of catalase and oxyR in the viable but nonculturable state of Vibrio vulnificus. FEMS Microbiol. Ecol. 50:133–142. 10.1016/j.femsec.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 39.Smith B, Oliver JD. 2006. In situ and in vitro gene expression by Vibrio vulnificus during entry into, persistence within, and resuscitation from the viable but nonculturable state. Appl. Environ. Microbiol. 72:1445–1451. 10.1128/AEM.72.2.1445-1451.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park KJ, Kang MJ, Kim SH, Lee HJ, Lim JK, Choi SH, Park SJ, Lee KH. 2004. Isolation and characterization of rpoS from a pathogenic bacterium, Vibrio vulnificus: role of sigmaS in survival of exponential-phase cells under oxidative stress. J. Bacteriol. 186:3304–3312. 10.1128/JB.186.11.3304-3312.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boaretti M, Lleo MM, Bonato B, Signoretto C, Canepari P. 2003. Involvement of rpoS in the survival of Escherichia coli in the viable but non-culturable state. Environ. Microbiol. 5:986–996. 10.1046/j.1462-2920.2003.00497.x [DOI] [PubMed] [Google Scholar]
- 42.Joelsson A, Kan B, Zhu J. 2007. Quorum sensing enhances the stress response in Vibrio cholerae. Appl. Environ. Microbiol. 73:3742–3746. 10.1128/AEM.02804-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coutard F, Lozach S, Pommepuy M, Hervio-Heath D. 2007. Real-time reverse transcription-PCR for transcriptional expression analysis of virulence and housekeeping genes in viable but nonculturable Vibrio parahaemolyticus after recovery of culturability. Appl. Environ. Microbiol. 73:5183–5189. 10.1128/AEM.02776-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maisonneuve E, Castro-Camargo M, Gerdes K. 2013. (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell 154:1140–1150. 10.1016/j.cell.2013.07.048 [DOI] [PubMed] [Google Scholar]
- 45.Manina G, McKinney JD. 2013. A single-cell perspective on non-growing but metabolically active (NGMA) bacteria. Curr. Top. Microbiol. Immunol. 374:135–161. 10.1007/82_2013_333 [DOI] [PubMed] [Google Scholar]