Abstract
The duration of survival of both the S and C strains of Mycobacterium avium subsp. paratuberculosis in feces was quantified in contrasting climatic zones of New South Wales, Australia, and detailed environmental temperature data were collected. Known concentrations of S and C strains in feces placed on soil in polystyrene boxes were exposed to the environment with or without the provision of shade (70%) at Bathurst, Armidale, Condobolin, and Broken Hill, and subsamples taken every 2 weeks were cultured for the presence of M. avium subsp. paratuberculosis. The duration of survival ranged from a minimum of 1 week to a maximum of 16 weeks, and the provision of 70% shade was the most important factor in extending the survival time. The hazard of death for exposed compared to shaded samples was 20 and 9 times higher for the S and C strains, respectively. Site did not affect the survival of the C strain, but for the S strain, the hazard of death was 2.3 times higher at the two arid zone sites (Broken Hill and Condobolin) than at the two temperate zone sites (Bathurst and Armidale). Temperature measurements revealed maximum temperatures exceeding 60°C and large daily temperature ranges at the soil surface, particularly in exposed boxes.
INTRODUCTION
Mycobacterium avium subsp. paratuberculosis, the causative organism of Johne's disease (JD), is an obligate parasite of animals, meaning that its survival outside the host animal is finite (1). Transmission between animals is usually by the fecal-oral route; therefore, the length of survival of M. avium subsp. paratuberculosis in the environment is an important factor in both the risk and the rate of indirect disease transmission, particularly on pastures that have been destocked of infected animals and where there can be no new contamination. There are two distinct genotypes of M. avium subsp. paratuberculosis in Australia (2), an S strain that predominantly infects sheep and a C strain that primarily infects cattle. While cross infection of cattle with the S strain has been reported, this appears to be rare (3).
Most research on M. avium subsp. paratuberculosis survival in the environment has been conducted in the Northern Hemisphere with the C strain, but a previous study (4) has documented the environmental persistence of the S strain in the high-prevalence tablelands regions of New South Wales (NSW) in eastern Australia. In short, the length of survival of M. avium subsp. paratuberculosis around the Camden, Borenore, and Carcoar areas (34.0°S, 150.6°E; 33.2°S, 148.9°E; and 33.6°S, 149.1°E, respectively) ranged from a maximum of 55 weeks in fully shaded locations to a low of 2 weeks in unshaded plots where vegetation had been removed. The authors proposed that daily temperature flux or variation may be the factor correlated with the lack of shade that reduces environmental survival.
The prevalence of JD in sheep varies considerably across NSW, being highest in the higher-altitude, wetter (temperate) southeast tablelands regions and lowest in the drier, hotter (arid) western districts (5). Prevalence is also low in the northeast, encompassing the New England region, which is a temperate summer rainfall area. Two possible explanations for this uneven distribution are that historical trading patterns reduced the opportunity for disease spread or that M. avium subsp. paratuberculosis does not survive for extended periods in the environments typical of the low-prevalence regions, hence reducing the chances of disease being established in the destination flock. We hypothesized that the higher temperatures generally found in the lower-prevalence arid regions of NSW, especially in summer, may result in shorter environmental survival and that this in turn may help explain the lower levels of JD prevalence found there.
Given that the original trial (4) was limited to the high-prevalence tablelands region, the aim of this project was to quantify the survival of M. avium subsp. paratuberculosis across different climatic and JD prevalence regions of NSW and to document environmental temperature parameters for each environment. Knowledge of survival in the different climatic regions may allow simpler and more targeted risk management options for extensively grazed sheep and cattle.
MATERIALS AND METHODS
Trial design.
The trial utilized a two-by-two factorial design conducted at four sites incorporating two shade treatments (exposed and 70% shade), two strains of M. avium subsp. paratuberculosis (S and C), and four geographic sites (Bathurst, Armidale, Condobolin, and Broken Hill [33.4°S, 149.6°E; 30.5°S, 151.6°E; 33.1°S, 147.1°E; and 32°S, 141°E, respectively).
Beginning in September 2010, sheep and cattle feces containing known concentrations of viable M. avium subsp. paratuberculosis were placed on soil in open expanded-polystyrene boxes at the four field sites. The sites varied in both climate and the known prevalence of JD in sheep (Table 1). All sites were considered to have a low prevalence of bovine JD.
TABLE 1.
Climate and between-flock prevalence of ovine JD at the four sites studied
| Site | Climatic zonea | Mean annual rainfall (mm) | Mean annual minimum-maximum temp (°C) | Mean annual solar exposure (MJ m−2) | Altitude (m) | Flock-level prevalence of JD |
|---|---|---|---|---|---|---|
| Bathurst | Temperate | 638 | 6.8–19.8 | 18.1 | 713 | High |
| Armidale | Temperate | 801 | 3.6–19.7 | 18.5 | 1,053 | Low |
| Condobolin | Hot arid | 457 | 10.2–24.4 | 19.2 | 203 | Low |
| Broken Hill | Hot arid | 249 | 11.9–24.3 | 20.1 | 299 | Low |
As classified by the Australian Bureau of Meteorology.
At Bathurst, Condobolin, and Broken Hill, all boxes (25 by 45 by 20 cm) were filled to a depth of 15 cm with a common soil type sourced from Bathurst, while at Armidale, half of the boxes within each shade-strain treatment contained the Bathurst soil and the remainder were filled with soil sourced locally. A total of 16 boxes, arranged in 4 rows of 4 boxes each, were used at each site (to provide 4 replicates per strain-shade treatment). The boxes were laid with their long axes in an east-west orientation, and the 8 boxes on the south side were enclosed by a frame covered with woven polypropylene cloth designed to provide 70% shade, while the 8 boxes on the north side were left exposed. To prevent the disturbance of feces by rainfall events, all boxes were covered with a roof made from 10-cm-thick expanded polystyrene sandwiched between 1-mm-thick aluminum sheeting suspended on steel posts. The roof was sloped such that its height above the boxes varied from a low of 25 cm over the southern shaded boxes to 150 cm over the exposed northern boxes.
Collection and processing of infected feces.
Feces were collected daily for 10 days from six Merino wethers and for 5 days from one Friesian cow that were showing clinical signs of Johne's disease (weight loss, diarrhea) and that had previously been confirmed to be shedding M. avium subsp. paratuberculosis in their feces by an IS900 quantitative PCR (qPCR) assay (6). The sheep feces were collected directly into rectal bags, while the cattle feces were collected from a concrete yard, and all fecal samples were stored at 5°C during the collection period. All fecal collection procedures were approved by the University of Sydney Animal Ethics Committee. The sheep feces were thoroughly mixed with chaff to extend the volume (10.5 kg sheep feces to 0.5 kg chaff) and to ensure a consistent concentration of M. avium subsp. paratuberculosis throughout each sample. Cattle feces were thoroughly mixed but not extended with chaff. The concentration of M. avium subsp. paratuberculosis in the bulk samples for each species was enumerated by qPCR. Sixteen aliquots of feces (each of 200 g) were then transferred into plastic bags for transport within 24 h to each of the four trial sites, where they were seeded into their designated boxes. The sheep feces were distributed evenly over the soil surface (1.8 kg m−2), while the cattle feces were extruded to form two artificial fecal pats per box (100 g/pat).
Sampling and culture.
Feces were sampled from each box immediately following seeding, every 2 weeks until week 26, and then every 4 weeks until week 52. At each sampling, approximately 5 g of the fecal sample was transferred to a sterile plastic vial. Sheep samples were collected as individual fecal pellets randomly selected from across the soil surface of each box, while in the boxes with cattle samples, three small sections were broken from each pat. To eliminate the risk of cross contamination, disposable sterile wooden sticks and plastic gloves were used and replaced between the collection of each sample. Soil was not included in the sample. Samples were then transported to arrive at the laboratory within 2 days of collection. On arrival at the laboratory, the samples were cultured radiometrically as described previously (7, 8).
Samples of soil from two sources (Bathurst and Armidale) were thoroughly mixed, and a 1-kg sample was taken from each and sent for commercial chemical analysis (Incitec Pivot, Victoria, Australia).
Temperature data.
An automatic temperature data logger (LogTag temperature loggers; On Solution, Baulkham Hills, NSW, Australia) was placed on the soil surface in the center of each shaded and exposed section at each trial site, and these were programmed to record the temperature every 2 h for the duration of the experiment. These recordings were used to calculate the temperature variation between sites and treatments.
Statistical analyses. (i) Effect of exposure to sunlight on survival.
For each box used in the trial, the length of survival was calculated as the number of days between box seeding and 1 week after the last positive culture was recorded for that box (as no box was culture positive after moving from a sampling regime of once every 2 weeks to one of once a month). Survival analyses were conducted to evaluate the difference in survival times between groups. Kaplan-Meier survival curves were initially produced to compare survival between treatment groups (shade or exposed) and sites (Bathurst, Armidale, Condobolin, and Broken Hill) separately for the C and S strains and the effect of soil source only at the Armidale site. Univariable and multivariable Cox proportional hazard models were then built to evaluate the effect of treatment (shade or exposed) after adjusting for sites. The assumption of proportional hazards was visually evaluated from the Kaplan-Meier survival curves and then by creating time-dependent covariates (by adding an interaction between the explanatory variables and log time to infection or death) in the Cox proportional hazard model. Nonoverlapping survival curves and/or a nonsignificant interaction indicated that the assumption was valid. Hazard ratios and their 95% confidence intervals are reported.
(ii) Effect of exposure to sunlight on M. avium subsp. paratuberculosis concentration.
The concentration of viable M. avium subsp. paratuberculosis in culture-positive samples was estimated from the time taken to reach the peak growth index (number of days to a growth index of 999) by approximating the approach suggested by Reddacliff et al. (9): log M. avium subsp. paratuberculosis count = 9.25 − (0.185 · number of days to a growth index of 999). If the sample was positive but did not reach a growth index of 999, a value of 98 days (14 weeks) was assumed, while culture-negative samples were given a count of 0. The log of the M. avium subsp. paratuberculosis numbers was analyzed by fitting a linear mixed model in SAS with log M. avium subsp. paratuberculosis numbers as the outcome; group, species, time (days) since inoculation, and their interactions were analyzed as fixed effects; and site and box nested within site were analyzed as random effects. Nonsignificant interactions were removed from the final model. The autoregressive correlation structure was imposed on time since inoculation to account for repeated measures, but a simpler model was retained if this did not improve the model fit, as evaluated using the Akaike information criterion (AIC) and differences in the log likelihoods of the two models. Predicted log M. avium subsp. paratuberculosis concentrations for treatment groups and species for time since inoculation were calculated and plotted.
(iii) Effect of shade treatment and site on ground temperature.
Temperature data were summarized for the period beginning at box seeding and continuing until 7 days after the last culture-positive box was detected (from 21 September 2010 to 11 January 2011). These values include the daily mean, minimum, maximum, range, standard deviation, and coefficient of variation. Each variable was analyzed by analysis of variance using Genstat regression fitting shade treatment, site, and their interaction as fixed effects.
RESULTS
Both the sheep and cattle feces dried out rapidly over time and by 3 weeks after the start of the trial were hard and dry. A proportion of the pelleted sheep samples became buried up to 1 cm below the soil surface over time, while the cattle pats remained on the soil surface. However, only entire sheep pellets without attached soil were collected at each sampling, and the small sections of cattle pats sampled contained no obvious soil.
The soil sourced from Bathurst was a dark brown sandy loam, while that from Armidale was a light gray clay loam. Both were low in organic matter, and both were of neutral pH. Compared to the soil sourced from Armidale, the Bathurst-derived soil had a higher Colwell phosphorus level (43 versus 11 mg/kg), a lower phosphorus buffering index (40 versus 92), more calcium (7.0 versus 3.8 meq/100 g), and a higher calcium/magnesium ratio (2.3 versus 1.1).
Effect of exposure to sunlight on survival.
An initial analysis of the effect of soil source at the Armidale site was conducted, and this showed no significant difference (P = 0.51) in survival time; the median survival time was 54.5 days (95% confidence interval [CI], 7, 96 days) versus 62 days (95% CI, 7, 89 days) for Bathurst- and Armidale-derived soils, respectively. Consequently, soil type was not considered in further analyses. Because the concentration of M. avium subsp. paratuberculosis in the pooled sheep feces was much higher than that in the cattle feces (106 versus 102 M. avium subsp. paratuberculosis bacteria per gram of feces, as estimated by qPCR) and as the duration of microbial survival is likely proportional to the starting concentration, separate analyses of the survival of M. avium subsp. paratuberculosis were conducted for the S and C strains.
All samples collected at seeding were culture positive for M. avium subsp. paratuberculosis. The observed survival period ranged from a minimum of 7 days (1 week) to a maximum of 111 days (16 weeks). The median survival times for shade and site treatments and the univariable Cox proportional hazard ratios for each species are presented in Table 2. The Kaplan-Meier survival curves are presented in Fig. 1.
TABLE 2.
Univariable results of effect of shade treatment and site on environmental survival times of M. avium subsp. paratuberculosisa
| Parameter | Cattle |
Sheep |
||||
|---|---|---|---|---|---|---|
| Median survival time (days) | Hazard ratiob | P value | Median survival time (days) | Hazard ratio | P value | |
| Shade treatment | 0.001 | <0.001 | ||||
| Exposed | 7 (IE) | 8.65 (2.65, 39.58) | 20 (IE) | 14.25 (4.58, 54.56) | ||
| Shaded | 54 (33, 62) | 1.00 | 82 (62, 96) | 1.00 | ||
| Site | 0.53 | 0.70 | ||||
| Armidale | 41.0 (7, 76) | 1.00 | 75.5 (20, 96) | 1.00 | ||
| Bathurst | 20.0 (7, 47) | 1.47 (0.53, 4.09) | 61.5 (7, 96) | 1.05 (0.38, 2.86) | ||
| Condobolin | 20.0 (7, 62) | 1.14 (0.40, 3.19) | 41.0 (20, 76) | 1.70 (0.60, 4.81) | ||
| Broken Hill | 7.0 (7, 20) | 2.08 (0.73, 5.93) | 33.5 (7, 62) | 1.48 (0.51, 4.17) | ||
Data in parentheses are 95% confidence intervals. IE, inestimable.
Cox proportional hazard of death ratio.
FIG 1.
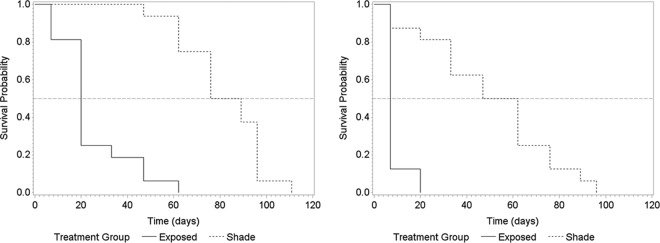
Kaplan-Meier survival curves for shade treatment for sheep (left) and cattle (right) strains of M. avium subsp. paratuberculosis.
With both strains, shade treatment had significant effects on the median survival time (P < 0.001). The hazard ratios presented in Table 2 suggest that the hazard of death in the exposed boxes was about 9 and 14 times greater than that in the shaded boxes for the C and S strains, respectively. The results of Kaplan-Meier analyses were similar (Fig. 2), as M. avium subsp. paratuberculosis in the exposed samples tended to die very quickly compared to the time to death in shaded samples, regardless of the strain.
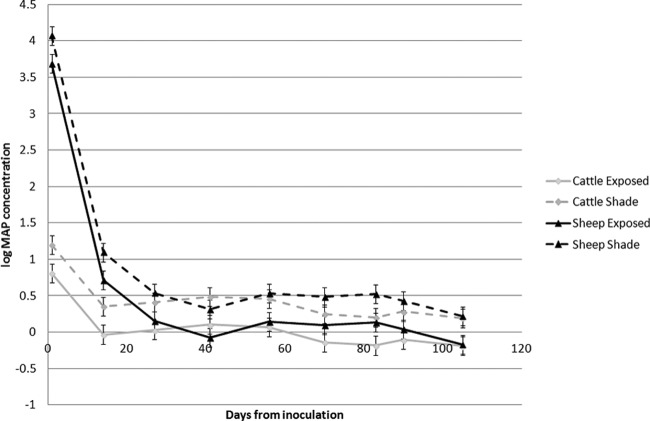
FIG 2.
Plot of log viable M. avium subsp. paratuberculosis (MAP) concentration (calculated from culture results) and the number of days from the time of inoculation for each shade treatment-strain group.
Site had no effect on survival time for either strain when modeled as four individual sites (P = 0.53 and 0.70 for the C and S strains, respectively) or when the two western arid sites of Broken Hill and Condobolin and the two eastern temperate sites of Bathurst and Armidale were combined into two respective arid and temperate sites (P = 0.55 and 0.25 for the C and S strains, respectively, based on the Cox proportional model).
Multivariable results.
In the multivariate model, for the C strain the shade treatment was significant (P < 0.01) but site was nonsignificant (P = 0.42; hazard ratio for arid versus temperate, 1.4; 95% CI, 0.65, 2.82). Exposed boxes had a 9 times greater hazard of death (95% CI, 2.7, 42.3) than unexposed boxes.
For the S strain, both shade treatment (P < 0.0001) and site (P = 0.049) were significant, suggesting that after adjusting for variation due to shade treatment, there is some evidence of a difference due to site for the S strain. Broken Hill and Condobolin together (arid) had 2.3 times (95% CI, 1.0, 5.2 times) the hazard of death compared to that for Bathurst and Armidale together (temperate), while exposed boxes had a 19.8 times (95% CI, 5.8, 83.8 times) greater hazard of death than shaded boxes, after adjusting for site.
Effect of exposure on viable M. avium subsp. paratuberculosis concentration.
In 30 of the 64 (47%) boxes, a pattern of several negative cultures (for 1 to 6 samplings) prior to a later positive culture was observed. When the log of the viable M. avium subsp. paratuberculosis count was analyzed as an outcome variable, all three variables—shade treatment, strain, and time (days) from inoculation—and the interaction between strain and time from inoculation were significant (all P values were <0.001). The predicted means plotted in Fig. 2 show the effect of the higher initial concentration in sheep feces but identify a dramatic reduction in the amount of viable M. avium subsp. paratuberculosis bacteria during the first fortnight after inoculation.
Temperature measurements.
The summary temperature statistics for the period from seeding to 1 week after the last positive culture are presented in Table 3. Shade had no effect on the minimum temperatures experienced but greatly reduced the maximum, resulting in a 50% reduction in the average daily temperature range and a 37% reduction in the daily temperature coefficient of variation. The temperature profiles were similar for the two temperate eastern sites and for the two arid western sites. However, there were differences between the regions, with the arid sites having higher minimum, maximum, and mean temperatures but not coefficients of variation of temperature.
TABLE 3.
Effect of shade treatment and site on mean daily temperature parameters
| Parameter | Tempa (°C) |
|||||
|---|---|---|---|---|---|---|
| Mean | Minimum | Maximum | Rangeb | SD | CV | |
| Shade treatment | ||||||
| Exposed | 20.0 ± 0.2 | 12.0 ± 0.2 | 34.7 ± 0.4 | 22.8 ± 0.4 | 7.5 ± 0.1 | 0.38 ± 0.01 |
| Shaded | 17.4 ± 0.2 | 12.1 ± 0.2 | 23.5 ± 0.4 | 11.3 ± 0.4 | 4.0 ± 0.1 | 0.24 ± 0.01 |
| Significance | <0.001 | NS | <0.001 | <0.001 | <0.001 | <0.001 |
| Site | ||||||
| Bathurst | 17.0 ± 0.3 | 10.3 ± 0.3 | 27.7 ± 0.6 | 17.4 ± 0.6 | 5.8 ± 0.2 | 0.35 ± 0.01 |
| Armidale | 15.4 ± 0.3 | 9.7 ± 0.3 | 24.2 ± 0.6 | 14.5 ± 0.6 | 5.0 ± 0.2 | 0.32 ± 0.01 |
| Condobolin | 21.7 ± 0.3 | 14.7 ± 0.3 | 33.6 ± 0.6 | 18.9 ± 0.6 | 6.3 ± 0.2 | 0.30 ± 0.01 |
| Broken Hill | 20.7 ± 0.3 | 13.4 ± 0.3 | 30.8 ± 0.6 | 17.4 ± 0.6 | 5.8 ± 0.2 | 0.29 ± 0.01 |
| Significance | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Data represent means for each parameter ± standard errors. CV, coefficient of variation; NS, not significant.
The range values represent the maximum minus the minimum.
Some very high temperatures were recorded on the soil surface, especially in the exposed boxes (Table 4), with maxima exceeding 60°C at 3 of the 4 sites. The temperature exceeded 55°C on 10 to 12 days at the arid sites and on 0 to 5 days at the temperate sites.
TABLE 4.
Number of days on which temperatures exceeded specific high temperatures and maximum temperatures recorded for each site
| Site | No. of days above: |
Maximum temp reached (°C) | ||
|---|---|---|---|---|
| 45°C | 50°C | 55°C | ||
| Bathurst | 31 | 15 | 5 | 63.8 |
| Armidale | 11 | 3 | 0 | 52.7 |
| Condobolin | 59 | 33 | 12 | 62.5 |
| Broken Hill | 31a | 23 | 10 | 61.0 |
Includes one shaded box.
DISCUSSION
This study supports the previous observations that the survival of M. avium subsp. paratuberculosis in the environment is extended but finite. The 2010-2011 summer weather across NSW was likely to have been favorable for the environmental survival of M. avium subsp. paratuberculosis due to the cloud cover and below-average temperatures. Rainfall was also above average, but soil moisture has not previously been associated with survival (4). Despite this, the length of survival observed in this study was considerably less than that observed in previous studies using both the S and C strains. Survival studies of the C strain have previously been limited to relatively protected in vitro sites in the Northern Hemisphere, where survival for periods of 8 to 17 months has been reported (10–12). With the S strain, we observed a 16-week maximum survival time; in comparison, Whittington et al. (4) observed a maximum survival time of 55 weeks in a similar environment using a concentration of the S strain of M. avium subsp. paratuberculosis of approximately 106 bacteria per gram of feces, similar to that used in the present study. However, Whittington et al. (4) observed the 55-week maximum under 100% shade, whereas we used only 70% shade. In the 11 experiments where Whittington et al. (4) used 70% shade, maximum survival was lower and ranged from 10 to 32 weeks, which is similar to the survival that we observed.
At all sites and for both strains, survival was significantly greater in feces located in shaded boxes than boxes directly exposed to sunlight, with the M. avium subsp. paratuberculosis bacteria in these sites having a 9- to 14-fold greater hazard of death. We measured temperature more frequently than other studies, and this revealed the dampening effects of shade on both the maximum ground temperature and the variability of ground temperatures under Australian conditions. In the arid trial sites, summer temperatures exceeded 60°C, with the temperatures in the unshaded boxes being above 50°C on up to 10 days. The provision of shade did not affect minimum ground temperatures, but it significantly reduced the maximum temperatures reached, consequently reducing measures of daily temperature variation, including the range, standard deviation, and coefficient of variation. These temperatures were measured on the soil surface, but given the dark color of the fecal pellets, it is possible that similar temperature profiles would have occurred inside the pellets, where most of the M. avium subsp. paratuberculosis would be found.
The high ground temperatures measured in this study approach those known to inactivate most M. avium subsp. paratuberculosis organisms during food processing. At 70°C, the D value, or the time required for a 90% reduction in the number of viable M. avium subsp. paratuberculosis bacteria, is about 15 s in milk (13), 1 min in phosphate-buffered saline (14), and less than 2 s in a muscle substrate, increasing to only 11 min at 60°C and 90 min at 55°C (15). In addition, it has been demonstrated (14) that in vitro survival at high temperatures is further reduced by cycling through periods of heating and cooling compared to that obtained when the sample is held at the higher temperature. Given the very high temperatures and the large range in daily temperatures observed in our study, it is perhaps not surprising that survival was curtailed, especially in the exposed boxes.
The decline in the number of viable M. avium subsp. paratuberculosis bacteria in positive cultures over time, as estimated by the rate of cell growth in culture, indicates a rapid decay in the viability of sampled M. avium subsp. paratuberculosis during the first 2 to 4 weeks following initial deposition in the environment, followed by a much lower rate of decline, and is similar to the biphasic decay previously reported (4). In both this study and that of Whittington et al. (4), the patterns of bacterial survival observed, where growth occurred following one or more negative cultures, suggest the possibility that some M. avium subsp. paratuberculosis may survive in a dormant state, requiring extended periods under favorable laboratory culture conditions for resuscitation. More recently, a spore-like morphotype of M. avium subsp. paratuberculosis has been described (17), suggesting a further mechanism for persistence in the environment.
Despite the observed impacts of exposure and elevated temperatures on survival, differences between sites were less evident. When the data were combined, the two arid sites experienced an elevated temperature profile (minimum, maximum, and mean) compared to the two temperate sites; however, the measures of temperature variation were similar. The higher temperatures were associated with shorter S-strain survival at the two arid western sites and a 2-fold increase in the hazard of death compared to the hazard of death at the two temperate eastern sites. Given that many sheep are grazed in intermediate climatic zones in eastern Australia, it is unlikely that the environmental persistence of M. avium subsp. paratuberculosis can fully explain the observed variation in the prevalence of JD across NSW.
These results suggest that, in the absence of shedding livestock, the high soil temperatures and temperature variation that occur in eastern Australia are likely to decontaminate the terrestrial landscape in a single summer. However, consideration should be given to the presence of completely shaded areas, especially those where significant fecal contamination has accumulated but that are sufficiently open to allow grazing by sheep and cattle. In particular, the concentration of M. avium subsp. paratuberculosis in dams and sediment may potentially pose a risk, as survival appears to be extended in these environments compared to that in soil (16). Because this study was conducted in a cooler than normal year, it may be that survival would be less in a more typical year, but further study would be required to confirm this.
ACKNOWLEDGMENTS
This work was supported by the NSW sheep industry through the NSW Ovine Johne's Disease Industry Fund and the NSW Rural Assistance Authority.
Technical assistance was provided by Greg Curran, Geoff Green, Kath Marsh, Anna Waldron, Ann-Michele Whittington, and Karren Plain. The infected cattle feces were kindly supplied by Jakob Malmo.
Footnotes
Published ahead of print 24 January 2014
REFERENCES
- 1.Thorel MF, Krichevsky M, Levy-Frebault VV. 1990. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int. J. Syst. Bacteriol. 40:254–260. 10.1099/00207713-40-3-254 [DOI] [PubMed] [Google Scholar]
- 2.Marsh IB, Bannantine JP, Paustian ML, Tizard ML, Kapur V, Whittington RJ. 2006. Genomic comparison of Mycobacterium avium subsp. paratuberculosis sheep and cattle strains by microarray hybridization. J. Bacteriol. 188:2290–2293. 10.1128/JB.188.6.2290-2293.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maloney BJ, Whittington RJ. 2008. Cross species transmission of ovine Johne's disease from sheep to cattle: an estimate of prevalence in exposed susceptible cattle. Aust. Vet. J. 86:117–123. 10.1111/j.1751-0813.2008.00272.x [DOI] [PubMed] [Google Scholar]
- 4.Whittington R, Marshall D, Nicholls P, Marsh I, Reddacliff L. 2004. Survival and dormancy of Mycobacterium avium subsp. paratuberculosis in the environment. Appl. Environ. Microbiol. 70:2989–3004. 10.1128/AEM.70.5.2989-3004.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NSW Department of Primary Industry. 2011. OJD prevalence areas 2011. NSW Department of Primary Industry; Sydney, NSW, Australia: http://www.dpi.nsw.gov.au/agriculture/livestock/health/specific/sheep/ojd/about/historical/ojd-prevalence-areas-jan2011 Accessed 21 August 13 [Google Scholar]
- 6.Marsh IB, Plain KM, Galea F, Waldron A, Whittington AM, Whittington RJ. 2012. New and improved direct PCR test for Johne's disease, abstr. ORO7. Abstr. Proc. 11th Int. Coll Paratuberc. Univ. Sydney [Google Scholar]
- 7.Whittington RJ, Marsh I, Turner MJ, McAllister S, Choy E, Eamens GJ, Marshall DJ, Ottaway S. 1998. Rapid detection of Mycobacterium paratuberculosis in clinical samples from ruminants and in spiked environmental samples by modified BACTEC 12B radiometric culture and direct confirmation by IS900 PCR. J. Clin. Microbiol. 36:701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whittington RJ, Marsh I, McAllister S, Turner MJ, Marshall DJ, Fraser CA. 1999. Evaluation of modified BACTEC 12B radiometric medium and solid media for the culture of Mycobacterium avium subsp. paratuberculosis from sheep. J. Clin. Microbiol. 37:1077–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddacliff LA, Nicholls PJ, Vadali A, Whittington RJ. 2003. Use of growth indices from radiometric culture for quantification of sheep strains of Mycobacterium avium subsp. paratuberculosis. Appl. Environ. Microbiol. 69:3510–3516. 10.1128/AEM.69.6.3510-3516.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorgenson JB. 1977. Survival of Mycobacterium paratuberculosis in slurry. Norsk. Vet. Med. 29:267–270 [PubMed] [Google Scholar]
- 11.Lovell R, Levi M, Francis J. 1944. Studies on the survival of Johne's bacilli. J. Comp. Pathol. 54:120–129. 10.1016/S0368-1742(44)80013-3 [DOI] [Google Scholar]
- 12.Larsen AR, Merkal RS, Vardaman TH. 1956. Survival time of Mycobacterium paratuberculosis. Am. J. Vet. Res. 17:549–551 [PubMed] [Google Scholar]
- 13.Sung N, Collins MT. 1998. Thermal tolerance of Mycobacterium paratuberculosis. Appl. Environ. Microbiol. 64:999–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gumber S, Whittington RJ. 2009. Analysis of the growth pattern, survival and proteome of Mycobacterium avium subsp. paratuberculosis following exposure to heat. Vet. Microbiol. 136:82–90. 10.1016/j.vetmic.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Whittington RJ, Waldron A, Warne D. 2010. Thermal inactivation profiles of Mycobacterium avium subsp. paratuberculosis in lamb skeletal muscle homogenate fluid. Int. J. Food Microbiol. 137:32–39. 10.1016/j.ijfoodmicro.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 16.Whittington RJ, Marsh I, Reddacliff L. 2005. Survival of Mycobacterium avium subsp. paratuberculosis in dam water and sediment. Appl. Environ. Microbiol. 71:5304–5308. 10.1128/AEM.71.9.5304-5308.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamont EA, Bannantine JP, Armién A, Ariyakumar DS, Sreevatsan S. 2012. Identification and characterization of a spore-like morphotype in chronically starved Mycobacterium avium subsp. paratuberculosis cultures. PLoS One 7:e30648. 10.1371/journal.pone.0030648 [DOI] [PMC free article] [PubMed] [Google Scholar]