Abstract
Pepducins containing a fatty acid linked to an amino acid sequence derived from cytosolic parts of a G-protein-coupled receptor (GPCR) constitute a new group of lipopeptide tools in GPCR studies. Pepducins corresponding to the third intracellular loop of formyl peptide receptor 2 (FPR2) activate human neutrophils, and we show here that, in addition, these allosteric modulators of receptor activity also kill bacteria. The functional dualism of FPR2 pepducins could potentially be explored as a novel class of antibacterial drugs with immunomodulatory properties.
TEXT
Resistance to antibacterial drugs has led to an emergence of serious infections that increasingly become nontreatable by conventional antibiotics. As a consequence, there is a need for new classes of antimicrobial substances with novel modes of action (1). Molecules such as lipopeptides that in addition to having direct antimicrobial effects also possess immunomodulatory properties (2, 3) can possibly be used as the starting point for developing novel and potent antimicrobial drugs (4). The group of lipopeptides known as pepducins has been shown to possess immune regulatory functions (3, 5) but they have not been shown to directly kill bacteria. A typical pepducin, containing a fatty acid (normally palmitic acid) linked to a peptide chain with a sequence resembling one of the intracellular loops of a selected G-protein-coupled receptor (GPCR), specifically targets and allosterically modulates the function of the receptor from which the peptide sequence is derived (3, 5). The allosteric modulatory activity of a pepducin relies both on the lipid anchor and the peptide sequence and is proposed to be a result of an ability of the molecule to pass the cell membrane and interact with the signaling domain of the receptor exposed on the cytosolic side of the membrane (3, 5).
Neutrophils, the cells of the innate immune system first called into action during bacterial infections, eliminate/kill invading microorganisms, and the recruitment of neutrophils to sites of infection/inflammation is mediated by microbial- and host-derived chemoattractants (6). These attractants are recognized by different GPCRs, such as the formyl peptide receptors (FPRs) expressed on the neutrophil cell surface (7). Human neutrophils express two closely related FPRs, FPR1 and FPR2, that are important regulators of inflammation as well as innate defense reactions (6, 8).
The pepducin technology has allowed the identification of neutrophil-activating pepducins (F2Pals) with sequences identical to the third intracellular loop of FPR2, and F2Pals are clearly capable of interacting with this particular receptor (9, 10). In this study, we determined the direct antimicrobial activity of FPR2 pepducins and explored the structural/physicochemical property link of pepducins between receptor activation and bacterial killing. Our data show that FPR2 pepducins not only display FPR2-dependent immune stimulatory activity but also have direct bacterial killing capacity.
Bacterial growth and bacterial killing assays.
Escherichia coli and Staphylococcus aureus strains and clinical isolates were routinely grown in Luria broth (LB) to logarithmic growth phase. Two techniques, a modified inhibition zone assay (11) and standard dilution assay (12), were used for determination of MICs. The formula C = 0.468n/(ad2) was used to calculate the MIC (C) (μM) determined from the inhibition zone assay, where a (cm) is the thickness of the agar layer, d (cm) is the diameter of the inhibition zones, and n is the amount of compounds (nmol) added.
Neutrophils and assays to study their function.
Neutrophils were isolated from buffy coats obtained from apparently healthy adults on a Ficoll-Paque gradient. The effect of pepducins on neutrophil activation was examined by measuring the production of superoxide and mobilization of complement receptor 3 (CR3; CD11b/CD18) to the cell surface from intracellular granules (13).
Pepducins.
The pepducins and the nonpalmitoylated control peptides were synthesized by CASLO Laboratory (Lyngby, Denmark) by 9-fluorenylmethoxy carbonyl (Fmoc) solid-phase peptide synthesis, and the fatty acid was N-terminally linked on the resin as the last step. Peptides were purified by high-performance liquid chromatography (HPLC), and their sequences were verified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). The physicochemical properties of peptides (Table 1) were calculated (14).
TABLE 1.
The table displays amino acid sequences of the pepducins used in the studya
| Pepducin name | Fatty acid and peptide sequence | Peptide lengthb | Net charge | Isoelectric point | Hydrophobic moment | Mean hydrophobicity |
|---|---|---|---|---|---|---|
| F2Pal16 | Pal-KIHKKGMIKSSRPLRV | 16 | 5.1 | 12.44 | 0.67 | −0.78 |
| F2Pal12 | Pal-KIHKKGMIKSSR | 12 | 4.1 | 11.79 | 0.62 | −1.20 |
| F2Pal10 | Pal-KIHKKGMIKS | 10 | 3.1 | 10.98 | 0.30 | −0.91 |
| F2Pal8 | Pal-KIHKKGMI | 8 | 2.1 | 10.80 | 0.52 | −0.55 |
| F2Pal12K1→Q | Pal-QIHKKGMIKSSR | 12 | 3.1 | 11.72 | 0.58 | −1.16 |
| F2Pal12K4→Q | Pal-KIHQKGMIKSSR | 12 | 3.1 | 11.72 | 0.56 | −1.16 |
| F2Pal12K5→Q | Pal-KIHKQGMIKSSR | 12 | 3.1 | 11.72 | 0.59 | −1.16 |
| F2Pal12K9→Q | Pal-KIHKKGMIQSSR | 12 | 3.1 | 11.72 | 0.60 | −1.16 |
| F2Myr12 | Myr-KIHKKGMIKSSR | 12 | 4.1 | 11.79 | 0.62 | −1.20 |
| F2Lau12 | Lau-KIHKKGMIKSSR | 12 | 4.1 | 11.79 | 0.62 | −1.20 |
| F2-12 | KIHKKGMIKSSR | 12 | 5.1 | 11.80 | 0.62 | −1.20 |
| F1Pal16 | Pal-KIHKQGMIKSSRPLRV | 16 | 4.1 | 12.51 | 0.55 | −0.70 |
Physicochemical parameters are calculated from the method described in reference 14.
Length is the number of amino acids.
Neutrophils were activated to produce superoxide anions by the pepducin F2Pal16, with a peptide sequence identical to the entire third intracellular loop of FPR2 spanning from K227 to V242 (Table 2). When examined in an inhibition zone assay, the F2Pal16 pepducin killed both E. coli and S. aureus (Table 2). Pepducin variants (Table 1) including progressive C-terminal truncations of F2Pal16 were designed, synthesized, and examined for both neutrophil-activating capacity and bacterial killing activity. Removal of the last four amino acids from F2Pal16, generating F2Pal12, had minor effects on bacterial killing, but pepducins with even shorter peptide chains were less efficient in killing bacteria (Table 2). For comparison, the MIC value was 0.5 μg/ml for polymyxin B compared to 8 μg/ml for F2Pal12 for E. coli and 64 μg/ml for polymyxin B compared to 33 μg/ml for F2Pal12 for S. aureus, as determined in the standard dilution assay. When calculated from the inhibition zone assay, the MIC value of polymyxin B was 0.1 μg/ml compared to 28 μg/ml for F2Pal12 for E. coli and >50 mg/ml for polymyxin B compared to 56 μg/ml for F2Pal12 for S. aureus (Table 2).
TABLE 2.
Effect of the F2Pal variants on neutrophil activation and bacterial killing
| Pepducin name | Fatty acid and peptide sequence | Superoxide releasea | E. coli inhibitionb | S. aureus inhibitionb |
|---|---|---|---|---|
| F2Pal16 | Pal-KIHKKGMIKSSRPLRV | 10.7 ± 2.9 | 6.5 ± 0.5 | 5.8 ± 0.2 |
| F2Pal12 | Pal-KIHKKGMIKSSR | 52.7 ± 4.7 | 6.3 ± 0.3 | 3.3 ± 1.2 |
| F2Pal10 | Pal-KIHKKGMIKS | 85.6 ± 10.1 | 3.5 ± 0.3 | 2.5 ± 0.3 |
| F2Pal8 | Pal-KIHKKGMI | 0.7 ± 0.3 | 2.5 ± 0 | 2.7 ± 0.2 |
| F2Pal12K1→Q | Pal-QIHKKGMIKSSR | 0.6 ± 0.2 | 0 | 0 |
| F2Pal12K4→Q | Pal-KIHQKGMIKSSR | 1.4 ± 0.4 | 0 | 0 |
| F2Pal12K5→Q | Pal-KIHKQGMIKSSR | 0.9 ± 0.1 | 2.7 ± 0.3 | 0 |
| F2Pal12K9→Q | Pal-KIHKKGMIQSSR | 47.5 ± 7.7 | 1.8 ± 0.2 | 0 |
| F2Myr12 | Myr-KIHKKGMIKSSR | 32.6 ± 6.1 | 7.3 ± 0.6 | 3.3 ± 1.2 |
| F2Lau12 | Lau-KIHKKGMIKSSR | 2.5 ± 0.8 | 5.7 ± 0.6 | 0 |
| F2-12 | KIHKKGMIKSSR | 0 | 0 | 0 |
| F1Pal16 | Pal-KIHKQGLIKSSRPLRV | 0 | 5.7 ± 0.2 | 5 ± 0.3 |
| Polymyxin B | NAc | 7.5 ± 0.3 | 0 | |
Superoxide release upon neutrophil activation was measured by isoluminol-amplified chemiluminescence and presented as counts per minute × 106 (Mcpm).
Antibacterial activity of pepducin variants (3 μl, 6 nmol; 3.3 μg/ml for F2Pal12) or polymyxin B (16 μg/ml) was examined in the inhibition zone assay, and the zone diameters (mm) after subtracting the well diameter (3 mm) are shown. Polymyxin B at the highest concentration (50 mg/ml) examined did not exert any killing effect on S. aureus. Data are expressed as means ± standard errors of the means (SEM) (n > 3 experiments).
NA, not applicable.
For pepducins with 10 amino acids or more, there was an inverse relationship between the peptide length and the neutrophil-activating potency, the F2Pal10 pepducin being the most potent (Table 2). The 12-amino-acid F2Pal12 is thus the most potent pepducin with dual functions (Table 2). The neutrophil receptor identified to recognize the F2Pal pepducins was FPR2. Accordingly, the conventional FPR2 agonist WKYMVM as well as F2Pal12 induced secretions resulting in mobilization of CR3 to the cell surface (Fig. 1A) and activated the NADPH-oxidase (Fig. 1B) through FPR2-dependent processes. This conclusion is drawn from the facts that the FPR2-specific inhibitor PBP10 completely blocked the responses, whereas there were no effects of the FPR1-specific antagonist cyclosporin H (Fig. 1).
FIG 1.
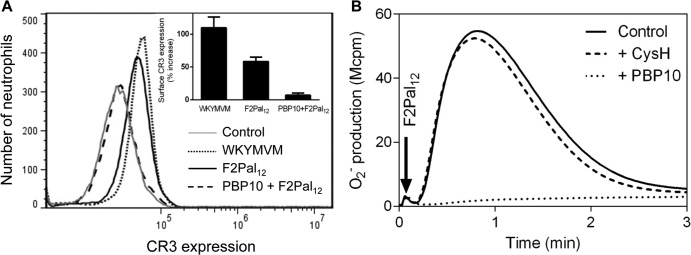
F2Pal12 induces CR3 upregulation and superoxide production in neutrophils through interaction with FPR2. (A) Neutrophils were treated with FPR2-specific agonist WKYMVM (0.1 μM final concentration) or F2Pal12 (1 μM final concentration) in the presence or absence of FPR2 inhibitor PBP10 (1 μM final concentration) and subjected to flow cytometry analysis of surface-exposed CR3. The histograms show CR3 staining in control (nontreated, gray line), WKYMVM-stimulated (dotted), and F2Pal12-treated neutrophils without (solid) or with (dashed) PBP10 from one representative experiment out of three. The results are summarized in the inset as the percentage of fluorescence increase from control (mean ± standard deviation [SD], n = 3 experiments). (B) The effects of FPR2 inhibitor PBP10 (1 μM final concentration, dotted line) and FPR1 antagonist cyclosporin H (1 μM final concentration, dashed line) on the release of superoxide anions from human neutrophils activated with F2Pal12 were determined. The control F2Pal12 response is also shown (solid line). The time for addition of the pepducin is indicated by the arrow, and the release of superoxide was followed continuously. Abscissa, time in minutes; ordinate, superoxide release (Mcpm, 106 counts per minute).
The impact of the overall charge and the hydrophobicity of the fatty acid on the killing and neutrophil-activating effects was next examined. The killing activity in relation to both E. coli and S. aureus was lost or largely reduced with pepducins in which one of the charged lysines was replaced by a neutral glutamine (Table 2). Three of the variants with reduced charge (F2Pal12K1→Q, F2Pal12K4→Q, and F2Pal12K5→Q) but not the fourth (F2Pal12K9→Q) also lacked the ability to activate neutrophils (Table 2), suggesting that the position of the charged amino acids rather than the net charge of the peptide determines the ability of F2Pal12 to generate proinflammatory signals from FPR2. This suggestion is also supported by the fact that a control pepducin with the same charge but another amino acid sequence compared to F2Pal12 (F1Pal16; Table 1) was unable to activate neutrophils but retained the microbial killing activity (Table 2). The naked peptide lacking the N-terminal palmitoyl group was completely deprived of activity (Table 2), suggesting that the fatty acid is essential both for bacterial killing and neutrophil activation. With regard to E. coli killing, a pepducin in which lauryl (F2Lau12) replaced the palmitoyl group was as potent as F2Pal12, and F2Myr12, in which the palmitoyl was replaced by a myristoyl, was actually more potent in killing E. coli than F2Pal12 (Table 2). There was no difference in S. aureus killing between F2Myr12 and F2Pal12, whereas F2Lau12 lacked all killing activity for this bacterium (Table 2). The antibacterial activity of pepducins was evident also against strains of clinical isolates of Pseudomonas aeruginosa from cystic fibrosis patients and of Enterococcus faecalis from patients suffering from sepsis (Table 3). It seems that the length of the fatty acid chain plays a more important role in neutrophil activation, as the activity of the F2Myr12 pepducin was reduced around 50% compared to that of F2Pal12, whereas F2Lau12 was almost inactive as measured by the release of superoxide (Table 2).
TABLE 3.
Antibacterial effect of pepducins and diagnostic routine antibiotics against clinical isolates
| Bacterial species (isolate no.) | Growth inhibition by pepducins (zone diam in mm)a |
Susceptibility pattern of antibiotics in the diagnostic routine panel (disc diffusion assay)b |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F2Pal12 | F2Myr12 | F2Pal16 | CAZ | MEM | TM | SXT | CIP | CO | AK | ATM | TZP | CTX | AM | VA | |
| Pseudomonas aeruginosa (1) | 5.2 ± 0.7 | 6.7 ± 0.7 | 8.0 ± 0.6 | S | I | R | R | R | S | R | I | S | R | ||
| P. aeruginosa (2) | 9.8 ± 0.5 | 13.3 ± 1.3 | 12.1 ± 0.1 | S | S | S | R | S | S | S | I | S | R | ||
| P. aeruginosa mucoid (3) | 8.3 ± 0.8 | 13.5 ± 0.3 | 9.4 ± 0.9 | S | S | S | R | S | S | S | I | S | R | ||
| P. aeruginosa mucoid (4) | 8.8 ± 0.6 | 12.3 ± 1.7 | 10.8 ± 0.8 | S | S | S | R | S | S | S | I | S | R | ||
| P. aeruginosa mucoid (5) | 7.8 ± 0.5 | 13.5 ± 0.9 | 10.8 ± 0.5 | S | S | S | R | S | S | S | I | S | R | ||
| Enterococcus faecalis (6) | 0 | 0 | 8.3 ± 1.3 | S | R | S | S | ||||||||
| E. faecalis (7) | 2.3 ± 1.2 | 2.0 ± 1.0 | 8.3 ± 0.9 | S | R | S | S | ||||||||
| E. faecalis (8) | 3.3 ± 1.8 | 2.3 ± 1.2 | 9.2 ± 0.8 | S | R | S | S | ||||||||
Zone sizes (mm) induced by pepducins (3 μl, 6 nmol) after subtracting the well diameter (3 mm) are given (mean ± SEM, n = 3 experiments).
S, susceptible; I, intermediate; R, resistant; CAZ, ceftazidime; MEM, meropenem; TM, tobramycin; SXT, trimethoprim-sulfamethoxazole; CIP, ciprofloxacin; CO, colistin; AK, amikacin; ATM, aztreonam; TZP, piperacillin-tazobactam; CTX, cefotaxime; AM, ampicillin; VA, vancomycin.
We show in this study that F2Pal pepducins can activate cells of the innate immune system through FPR2, the receptor from which the amino acid sequence in the lipopeptides was derived. The pepducins also possess direct microbial killing effects, a finding that may lead to new approaches and possibilities in the field of antimicrobial chemotherapy. Interestingly, many FPR2 ligands similar to FPR pepducins also have direct bacterial killing activity. These ligands include LL-37, the human member of the cathelicidin family, and the cecropin-like peptide Hp2-20 generated by Helicobacter pylori bacteria (15, 16). We demonstrate that the positive charge of the peptide chain in pepducins, but also the fatty acid to which it is linked, is of importance for bacterial killing. The charge may be required for binding to the anionic phospholipids present in bacterial membranes. The role of the acyl chain in bacterial killing may be due to its effect on hydrophobicity, but it may also actively participate in the formation of the defined conformation of the lipopeptides when bound to the membrane and that this somehow facilitates the perturbation and interaction with the membrane. Even though the killing mechanism of lipopeptides involves interaction between the fatty acid and the bacterial membrane (17, 18), the peptide part and fatty acid of the molecules obviously act together to determine the outcome. The inability of pepducins with a fatty acid shorter than 10 amino acids to mediate the dual effects is most likely due to the lack of interaction with the signaling domain of the receptor, and the interaction is also suboptimal when additional amino acids are added to the C terminus of the most potent neutrophil-activating pepducin, F2Pal10. The precise mechanism of modulation is still unclear regarding how pepducins pass through membranes and target the signaling interface at the level of the receptor. The fact that the respective cognate receptor is an absolute requirement for the pepducin effects strongly suggests that pepducins exert their action in close vicinity of the receptor and not by a direct effect on the signaling G proteins (3, 5, 9).
In summary, this study demonstrates a functional dualism, being directly antibacterial as well as activating innate immune cells, of pepducins containing a peptide sequence derived from one of the cytosolic parts of FPR2. Since pepducin technology provides the means to target specific GPCRs and thereby fine-tune innate immune responses, the antibacterial effect of pepducins described here means that receptor-targeted immunomodulatory and antibacterial chemotherapeutics could become a real alternative among anti-infective treatment strategies. It is imperative that future research be directed toward elucidating and enhancing beneficial inflammatory responses driven by antibacterial pepducins without potentiating chronic inflammation. It is tempting to speculate that a short-lived proinflammatory response of pepducins will increase efficiency in eliminating bacteria during the early stages of inflammation. Proinflammatory effects must, however, be controlled so that they do not contribute to the development or exacerbation of chronic inflammatory disorders.
ACKNOWLEDGMENTS
This work was supported by the Swedish Research Council, the King Gustaf V 80-Year Foundation, the Swedish government under the ALF agreement, the Clas Groschinsky Foundation, the Swedish Heart-Lung Foundation, and Ingabritt and Arne Lundberg's Research Foundation.
Footnotes
Published ahead of print 3 March 2014
REFERENCES
- 1.Rodriguez de Castro F, Naranjo OR, Marco JA, Violan JS. 2009. New antimicrobial molecules and new antibiotic strategies. Semin. Respir. Crit. Care Med. 30:161–171. 10.1055/s-0029-1202935 [DOI] [PubMed] [Google Scholar]
- 2.Mandal SM, Barbosa AE, Franco OL. 2013. Lipopeptides in microbial infection control: scope and reality for industry. Biotechnol. Adv. 31:338–345. 10.1016/j.biotechadv.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 3.O'Callaghan K, Kuliopulos A, Covic L. 2012. Turning receptors on and off with intracellular pepducins: new insights into G-protein-coupled receptor drug development. J. Biol. Chem. 287:12787–12796. 10.1074/jbc.R112.355461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jerala R. 2007. Synthetic lipopeptides: a novel class of anti-infectives. Expert Opin. Invest. Drugs 16:1159–1169. 10.1517/13543784.16.8.1159 [DOI] [PubMed] [Google Scholar]
- 5.Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. 2002. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc. Natl. Acad. Sci. U. S. A. 99:643–648. 10.1073/pnas.022460899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu H, Karlsson J, Bylund J, Movitz C, Karlsson A, Dahlgren C. 2006. Ligand recognition and activation of formyl peptide receptors in neutrophils. J. Leukoc. Biol. 79:247–256. 10.1189/jlb.0905498 [DOI] [PubMed] [Google Scholar]
- 7.Murphy PM. 1994. The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 12:593–633. 10.1146/annurev.iy.12.040194.003113 [DOI] [PubMed] [Google Scholar]
- 8.Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. 2009. International union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61:119–161. 10.1124/pr.109.001578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsman H, Bylund J, Oprea TI, Karlsson A, Boulay F, Rabiet MJ, Dahlgren C. 2013. The leukocyte chemotactic receptor FPR2, but not the closely related FPR1, is sensitive to cell-penetrating pepducins with amino acid sequences descending from the third intracellular receptor loop. Biochim. Biophys. Acta 1833:1914–1923. 10.1016/j.bbamcr.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 10.Forsman H, Andreasson E, Karlsson J, Boulay F, Rabiet MJ, Dahlgren C. 2012. Structural characterization and inhibitory profile of formyl peptide receptor 2 selective peptides descending from a PIP2-binding domain of gelsolin. J. Immunol. 189:629–637. 10.4049/jimmunol.1101616 [DOI] [PubMed] [Google Scholar]
- 11.Hultmark D, Engstrom A, Andersson K, Steiner H, Bennich H, Boman HG. 1983. Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. EMBO J. 2:571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gales AC, Reis AO, Jones RN. 2001. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines. J. Clin. Microbiol. 39:183–190. 10.1128/JCM.39.1.183-190.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bylund J, Bjornsdottir H, Sundqvist M, Karlsson A, Dahlgren C. 2014. Measurement of respiratory burst products, released or retained, during activation of professional phagocytes. Methods Mol. Biol. 1124:321–338. 10.1007/978-1-62703-845-4_21 [DOI] [PubMed] [Google Scholar]
- 14.Kyte J, Doolittle RF. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105–132. 10.1016/0022-2836(82)90515-0 [DOI] [PubMed] [Google Scholar]
- 15.Bylund J, Christophe T, Boulay F, Nystrom T, Karlsson A, Dahlgren C. 2001. Proinflammatory activity of a cecropin-like antibacterial peptide from Helicobacter pylori. Antimicrob. Agents Chemother. 45:1700–1704. 10.1128/AAC.45.6.1700-1704.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192:1069–1074. 10.1084/jem.192.7.1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezansoff AJ, Hunter HN, Jing W, Park IY, Kim SC, Vogel HJ. 2005. Interactions of the antimicrobial peptide Ac-FRWWHR-NH(2) with model membrane systems and bacterial cells. J. Pept. Res. 65:491–501. 10.1111/j.1399-3011.2005.00263.x [DOI] [PubMed] [Google Scholar]
- 18.Shai Y, Makovitzky A, Avrahami D. 2006. Host defense peptides and lipopeptides: modes of action and potential candidates for the treatment of bacterial and fungal infections. Curr. Protein Pept. Sci. 7:479–486. 10.2174/138920306779025620 [DOI] [PubMed] [Google Scholar]


