Abstract
Leishmania donovani is the causative agent of the potentially fatal disease visceral leishmaniasis (VL). Chemotherapeutic options available to treat VL are limited and often face parasite resistance, inconsistent efficacy, and toxic side effects. Paromomycin (PMM) was recently introduced to treat VL as a monotherapy and in combination therapy. It is vital to understand the mechanisms of PMM resistance to safeguard the drug. In the present study, we utilized experimentally generated PMM-resistant L. donovani to elucidate the mechanisms of resistance and parasite biology. We found increased membrane fluidity accompanied by decreased intracellular drug accumulation in the PMM-resistant parasites. There were marked increases in gene expression of ATP-binding cassette (ABC) transporters (MDR1 and MRPA) and protein phosphatase 2A that evince increased drug efflux. Further, evaluation of parasite tolerance toward host leishmanicidal mechanisms revealed PMM-resistant parasites as being more tolerant to nitrosative stress at the promastigote and amastigote stages. The PMM-resistant parasites also predicted a better survival capacity, as indicated by resistance to complement-mediated lysis and increased stimulation of host interleukin-10 (IL-10) expression. The susceptibilities of PMM-resistant isolates to other antileishmanial agents (sodium antimony gluconate and miltefosine) remained unchanged. The data implicated the roles of altered membrane fluidity, decreased drug accumulation, increased expression of ABC transporters, and greater tolerance of parasites to host defense mechanisms in conferring PMM resistance in Leishmania.
INTRODUCTION
Visceral leishmaniasis (VL) is a protozoan disease caused by members of the Leishmania donovani complex that is lethal in the absence of treatment. The disease is endemic in 70 countries, with a total of 200 million people at risk and an estimated 500,000 new infections annually in all age groups (1, 2). More than 90% of the estimated VL cases occur in India, Bangladesh, Nepal, Sudan, Ethiopia, and Brazil, with India alone bearing almost 50% of the world's total disease burden (2). Chemotherapy remains the mainstay of VL control, but there is only a limited arsenal of available drugs. Increased resistance against antimonials has been reported from many parts of the world, especially from the State of Bihar, India, where up to 65% of patients did not respond to treatment (3). Amphotericin B is another highly effective antileishmanial agent; however, it is associated with severe side effects and requires hospitalization (4). Miltefosine (MIL) was recently introduced as the first oral agent against VL. High cost, teratogenic potential, and reports of relapses following MIL treatment in VL raise concerns regarding the utility of MIL in VL control (5–7).
Paromomycin (PMM) is an aminoglycoside antibiotic that has been used as an oral, topical, and parenteral drug for treatment of bacterial and parasitic infections. PMM was registered in 2006 in India for VL treatment. Phase IV trials confirmed the safety and efficacy of PMM to treat VL, with a cure rate of 94.2% (8). The drug has also been effective in combination therapy (9, 10). It was shown that antimony-resistant and -sensitive isolates are equally susceptible to PMM (11). Studies aimed toward understanding the mechanisms of PMM resistance have been mostly confined to fungal and bacterial diseases (12, 13). PMM resistance in prokaryotes has been associated with various mechanisms, such as decreased drug accumulation, mutations at the ribosomal binding sites, or enzymatic inactivation of the drug. There are limited studies on PMM resistance in Leishmania, which evince the involvement of mitochondrial membrane potential and decreases in drug uptake (14–16). In our previous report, we compared the outcomes and stability of experimental PMM resistance induction in promastigotes and intracellular amastigotes (17). Here, we exploited laboratory-generated PMM-resistant (PMM-R) parasites to examine changes in membrane fluidity, drug accumulation, mutations in rRNA genes, and mRNA expression of ATP-binding cassette (ABC) transporter genes (MDR1 and MRPA) and protein phosphatase 2A (PP2A). Additionally, we investigated the tolerance of PMM-resistant parasites to host defense mechanisms, including nitrosative, oxidative, and complement-mediated stresses.
MATERIALS AND METHODS
Culture of parasites.
A clinical isolate of L. donovani, BHU573, was obtained from splenic aspiration fluid from an antimony-resistant patient reporting to the Kala-azar Medical Research Center (Muzaffarpur, Bihar, India), under the guidelines of the ethics committee of the institution. Using a cloned line of the antimony-resistant (PMM-sensitive [PMM-S]) clinical isolate BHU573, PMM resistance was artificially induced at the promastigote stage by stepwise increases in drug concentrations up to 97 μM PMM, as described previously (17). Promastigotes were cultured at 25°C in M199 medium with 25 mM HEPES (pH 7.4) supplemented with 10% fetal bovine serum (FBS), 100 IU penicillin G, and 100 μg/ml streptomycin (18).
Drug susceptibility assay.
The drug sensitivity of parasites was assessed in intracellular amastigotes as described previously (19). Briefly, primary peritoneal macrophages were extracted from BALB/c mice and plated at 2 × 105 cells per well in RPMI 1640 medium supplemented with 10% FBS, in 16-well chamber slides, and were incubated at 37°C in 5% CO2. Twenty-four hours later, the medium was gently removed and the macrophages were infected with late-log-phase promastigotes at a ratio of 10 parasites per macrophage, in a volume of 200 μl complete RPMI 1640 medium. After 24 h of infection, noninternalized parasites were washed off and different concentrations of PMM (0, 10, 20, 40, 80, and 120 μM), MIL (0, 1, 2, 5, 10, 20, and 30 μM), or sodium antimony gluconate (SAG) (0, 5, 10, 20, 40, and 80 μg/ml) were applied in triplicate. After 48 h of incubation, the slides were fixed with methanol and stained with Diff-Quik solution. The numbers of amastigotes per cell were counted in 100 macrophages, and percent killing was calculated by sigmoidal regression analysis (Origin 6.0).
Measurement of membrane anisotropy.
The fluidity of the membranes was estimated by measuring anisotropy using diphenylhexatriene (DPH), a fluorescence probe (19). The PMM-S and PMM-R parasites were incubated with 2 mM DPH (2 × 106 cells/ml) for 2 h at 37°C, followed by washing with phosphate-buffered saline (PBS) and fixing with 2% paraformaldehyde for 15 min. The cells were washed again and resuspended in PBS, and the fluorescence anisotropy (FA) was measured with a spectrofluorometer (PerkinElmer). The fluorescence anisotropy (FA) value was calculated using the equation FA = [I∥ − (G × Iperp)]/[I∥ + (2 × G × Iperp)], where G is the intensity ratio of the vertical to horizontal components of the emission when the sample is excited with horizontally polarized light and I∥ and Iperp are the fluorescent intensities oriented parallel and perpendicular, respectively, to the direction of polarization of the excited light (19).
Drug accumulation assay.
PMM uptake was studied according to the previously described protocol (16), with slight modifications. Briefly, promastigotes (108 cells/ml) were treated with 100 μM PMM for various times (0 to 90 min). Cells harvested at different time points (0, 30, 60, and 90 min) were washed twice with PBS and digested by overnight incubation in 2 N HNO3. The parasites were washed twice with PBS and digested by overnight incubation in 2 N HNO3, and the supernatant was analyzed for PMM content using liquid chromatography-mass spectrometry (LC-MS).
RNA isolation and real-time PCR.
Total RNA was isolated from promastigotes using TRIzol reagent (Invitrogen) and was reverse transcribed. All real-time PCRs were performed in triplicate in a 25-μl volume, using SYBR green for detection in an ABI Prism 7500 Sequence Detection System (Applied Biosystems). The analysis of gene expression was performed using the 2−ΔΔCT method, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and cystathionine β-synthase (CBS) as endogenous controls (20, 21). The primers used for real-time PCR were GAAGTACACGGTGGAGGCTG (forward) and CGCTGATCACGACCTTCTTC (reverse) for GAPDH, CGCCGATGTCAACTGGATG (forward) and GCTCCTTCTTCAGCGTGTCG (reverse) for CBS, CGAAGTGGGAAGTGAATGGT (forward) and CAGCATGTAGCCCATCAAGA (reverse) for MDR1, GCGCAGCCGTTTGTGCTTGTGG (forward) and TTGCGTACGTCGCGATGGTGC (reverse) for MRPA, and GCGAAGGAGATTTTCACGAG (forward) and CGCCCATGAAGACGTAGTTT (reverse) for PP2A.
Full-gene sequencing of rRNA.
Mutations in the LinJ.27.rRNA6 gene were evaluated by DNA sequence analysis of the PCR amplicons for PMM-S and PMM-R parasites. Amplicons were passed through a Qiagen PCR purification kit before being sequenced on an ABI Prism 3730 automated DNA sequencer (version 3.0) at the sequencing facility of Delhi University (New Delhi, India). Sequences were analyzed using a sequence-editing tool (DNASTAR). To minimize sequencing errors, forward and reverse primers were utilized for sequencing, and the experiment was performed thrice using biological replicates.
Promastigote nitrosative and oxidative stress tests.
The promastigote nitrosative and oxidative stress tests were performed as described elsewhere (22). Assays were performed in sterile 96-well microtiter plates using promastigotes (105 cells/well) and different concentrations of stress-inducing agents, i.e., hydrogen peroxide (H2O2) (9.76 to 10,000 μM), 3-morpholinosydnonimine (SIN-1) (1.95 to 2,000 μM), and S-nitroso-N-acetyl-dl-penicillamine (SNAP) (0.98 to 1000 μM). After 72 h of incubation at 25°C, 20 μl resazurin (0.0125%) was added to each well and the plates were incubated further for 18 to 24 h except for those with SIN-1, for which the incubation period was 1 h. Cell viability was measured fluorometrically (excitation wavelength, 550 nm; emission wavelength, 590 nm) using a fluorometer (Tecan). The results were expressed as percent reduction in parasite viability, compared with untreated controls, and the 50% inhibitory concentration (IC50) was calculated with Origin 6.0. All experiments were repeated twice in quadruplicate.
Amastigote stress tests.
Evaluation of tolerance capacity against nitrosative stress and gamma interferon (IFN-γ)/lipopolysaccharide (LPS)-induced leishmanicidal activity at the amastigote stage was carried out using peritoneal macrophages infected with promastigotes, as described above. After 24 h of infection, noninternalized parasites were washed off and plates were incubated at 37°C for 48 h after the addition of different concentrations of SNAP (0, 25, 50, 100, 200, 400, or 800 μM) or IFN-γ (0.1 to 50 U/ml) plus LPS (0.1 to 50 ng/ml) (23). Slides were fixed with methanol and stained with Diff-Quik solutions. The number of amastigotes per cell was counted in 100 macrophages, to calculate the mean survival rate at each concentration.
Complement-mediated cell lysis.
Promastigotes (106 cells/ml in M199 medium with 20% FBS) were added to 96-well plates containing serial dilutions of freshly isolated human serum (0.78 to 50%), and the plates were incubated at 37°C for 60 min. Plates were incubated at 25°C for 24 h after the addition of 40 μl of cold EDTA and 24 μl of resazurin, and then fluorescence was measured using a fluorometer (Tecan) (24).
Cytokine analysis.
Peritoneal macrophages stimulated with IFN-γ/LPS were infected with PMM-S or PMM-R parasites. After 48 h of infection, the supernatants were collected and interleukin-10 (IL-10) levels were estimated with a sandwich enzyme-linked immunosorbent assay (ELISA) (e-Biosciences), in accordance with the manufacturer's instructions.
Statistical analysis.
Results are expressed as mean ± standard deviation (SD). Student's t test was performed to evaluate significance, and P values of <0.05 were considered significant.
Ethics approval.
The study was approved by the Ethics Committee of the Institute of Medical Sciences, Banaras Hindu University (Varanasi, India).
RESULTS
Susceptibility profile of PMM-R parasites with antileishmanial agents.
PMM-R parasites showed a remarkable decrease (>5-fold) in susceptibility toward PMM as amastigotes, compared with PMM-S parasites (17), whereas susceptibility to other antileishmanial agents (MIL and SAG) remained unaffected (Table 1). Five clones were established from PMM-R parasites, and all exhibited susceptibility profiles similar to that of the parental strain, revealing a homogeneous population.
TABLE 1.
Susceptibility profiles of PMM-R and PMM-S parasites with antileishmanial agents
| Isolatea | IC50 (mean ±SD) forb: |
||
|---|---|---|---|
| PMM (μM) | SAG (μg/ml) | MIL (μM) | |
| PMM-S | 11 ± 1 | 26.13 ± 0.74 | 5.73 ± 0.66 |
| PMM-R | 61 ± 7 | 29.01 ± 1.28 | 6.12 ± 0.22 |
PMM-S, paromomycin sensitive; PMM-R, paromomycin resistant.
Values represent mean ± SD values from three separate assays.
Increased membrane fluidity of PMM-R parasites.
The fluorescence anisotropy value of PMM-R isolates was 2.5 times lower than that of PMM-S isolates, indicating membrane modifications in the parasites upon induction of PMM resistance (Fig. 1). As anisotropy is inversely proportional to membrane fluidity, the data indicated increased membrane fluidity in the PMM-R parasites, compared with PMM-S parasites.
FIG 1.
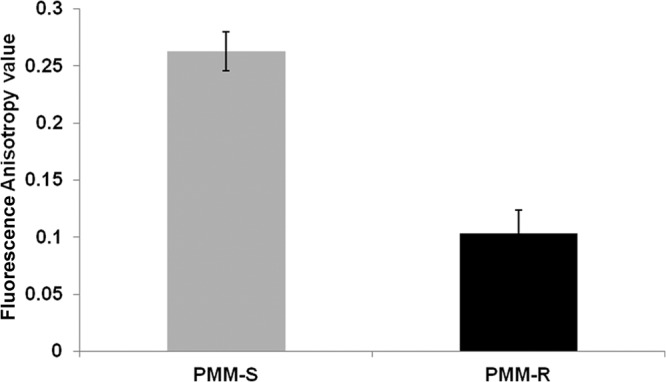
Fluorescence anisotropy measurements for PMM-R promastigotes. The fluorescence anisotropy (FA) values were measured using DPH as the probe. The fluorophore was excited at 365 nm and emission intensity was recorded at 435 nm, using a spectrofluorometer. Data represent mean ± SD FA values from three independent experiments.
Decreased PMM accumulation in PMM-R parasites.
The changes in the membranes of PMM-R isolates paved the way for analysis of the intracellular levels of PMM. A comparison of drug accumulation in PMM-R and PMM-S parasites at different time points (up to 90 min) showed an approximately 3-fold decrease in drug accumulation in the PMM-R strain (Fig. 2).
FIG 2.
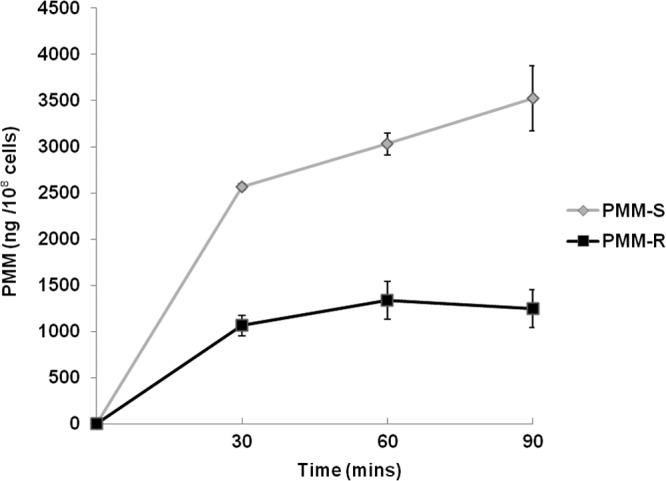
Drug accumulation in PMM-R promastigotes. PMM accumulation was estimated at different time points, using LC-MS, following incubation of the parasites with 100 μM PMM. Values represent the mean ± SD of two different experiments.
Increased expression of MDR1, MRPA, and protein phosphatase 2A in PMM resistance.
In view of the role of the ABC gene family in drug resistance in Leishmania, we analyzed the expression of the MDR1 and MRPA genes in PMM-resistant parasites. Marked increases in the levels of MDR1 (6.83 ± 3.01-fold) and MRPA (11.47 ± 0.22-fold) expression were observed in PMM-R parasites, in comparison with PMM-S parasites. Additionally, protein phosphatase 2A, which has a probable role in activating the expression of these transporters (25), was found to be highly upregulated (4.47 ± 0.71-fold) in PMM-R parasites (Fig. 3).
FIG 3.
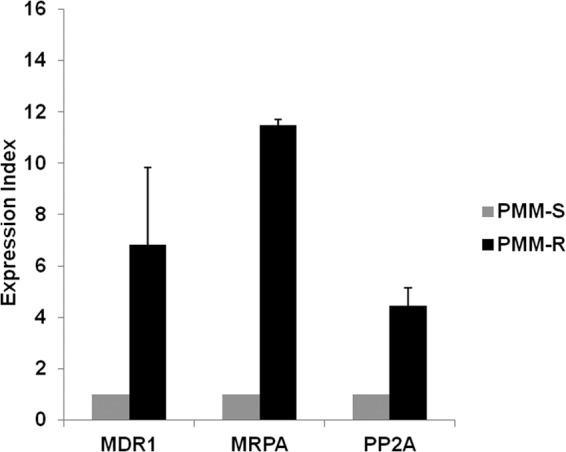
Expression of MDR1, MRPA, and PP2A in PMM-R isolates. Real-time PCR expression analysis of L. donovani ABC transporter (MDR1 and MRPA) and protein phosphatase 2A (PP2A) genes was performed by using GAPDH and CBS as internal controls. The graph shows the expression index, defined as gene expression relative to that of PMM-S parasites. Data represent the mean ± SD of the results of three independent experiments.
Sequence analysis of rRNA genes.
PCR sequencing of rRNA genes was carried out in order to analyze the presence of previously known mutations associated with PMM resistance. The results revealed identical sequences in PMM-R and PMM-S parasites.
Increased tolerance of PMM-R parasites to nitrosative stress at both promastigote and amastigote stages.
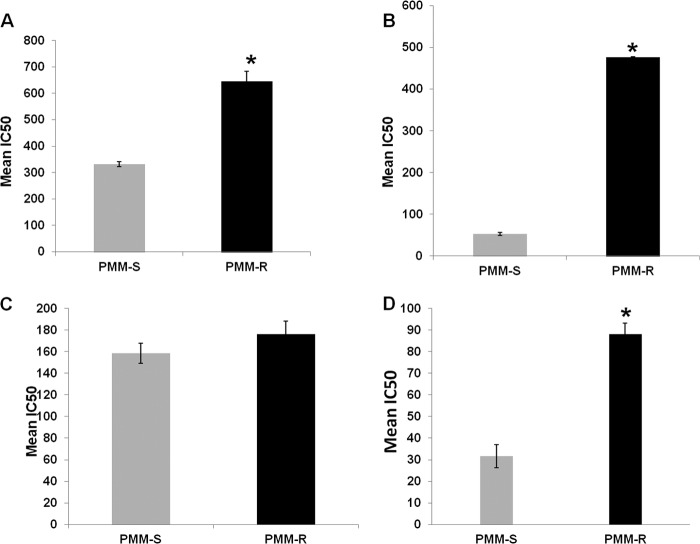
We compared the responses of the PMM-R and PMM-S strains to nitrosative and oxidative stress. PMM-R promastigotes were significantly more tolerant to SIN-1 (nitric oxide [NO] plus O2 donor) and SNAP (NO donor) than were PMM-S promastigotes. The resistant parasites were twice as tolerant (P = 0.045) to NO/O2 stress, with a mean IC50 of 646.14 ± 36.57 μM, compared with the PMM-S strain (IC50, 328 ± 4.24 μM) (Fig. 4A). The PMM-R parasites exhibited significant decreases in susceptibility (9-fold; P = 0.0048) to NO stress, in comparison with PMM-S parasites, with mean ± SD IC50 values of 476.58 ± 1.06 μM and 52.95 ± 3.48 μM, respectively (Fig. 4B). In contrast, the susceptibilities of PMM-R and PMM-S promastigotes to H2O2 were similar, with mean IC50 values of 176.03 ± 12.27 μM and 158.57 ± 9.10 μM, respectively (Fig. 4C). Furthermore, the responses of PMM-R parasites to nitrosative stress (NO) were evaluated at the amastigote stage; the PMM-R parasites showed significantly lower susceptibility (P = 0.0028) to nitrosative stress (NO) at the amastigote stage, compared with PMM-S parasites, with mean ± SD IC50 values of 88.12 ± 5.17 μM and 31.55 ± 5.35 μM, respectively (Fig. 4D).
FIG 4.
In vitro susceptibility of PMM-R isolates to nitrosative and oxidative stress. (A to C) The susceptibility of PMM-R and PMM-S parasites to 3-morpholinosydnonimine (SIN-1) (A), S-nitroso-N-acetyl-dl-penicillamine (SNAP) (B), and hydrogen peroxide (H2O2) (C) at the promastigote stage was determined using the resazurin assay. (D) The susceptibility at the intracellular amastigote stage was determined after incubation with different concentrations of SNAP, using peritoneal macrophages. Each bar represents the mean ± SD IC50 of two independent experiments. *, P < 0.05.
Increased tolerance of PMM-R parasites to IFN-γ/LPS-mediated leishmanicidal activity.
The mean infectivity rates for PMM-S and PMM-R isolates were determined in macrophages treated with different concentrations of IFN-γ and LPS (IFN-γ, 0.1, 1, 5, 10, and 50 U/ml; LPS, 0.1, 1, 5, 10, and 50 ng/ml). We observed significantly (P < 0.05) higher parasite burdens at concentrations above 5 U/ml IFN and 5 ng/ml LPS in macrophages infected with PMM-R isolates, compared with PMM-S parasites, indicating resistance of PMM-R isolates to macrophage killing mechanisms (Fig. 5).
FIG 5.
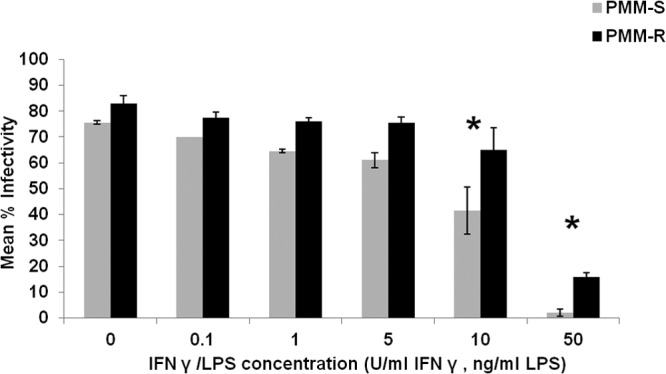
PMM-R parasite burden in macrophages treated with IFN-γ and LPS. Mean percent infectivity was calculated by infecting peritoneal macrophages with PMM-R and PMM-S promastigotes and treating them with different concentrations of IFN-γ and LPS or leaving them untreated (control). After 48 h, the percentage of infected cells was determined. The graph represents mean ± SD percent infectivity from two independent experiments in triplicate. *, P < 0.05.
Increased resistance of PMM-R parasites to complement-mediated lysis.
The PMM-R isolates showed significantly higher (P = 0.005) tolerance to complement-mediated lysis (mean IC50, 4.02% ± 0.26%) than did PMM-S parasites (mean IC50, 2.08% ± 0.16%), which indicates greater survival capability (since complement-mediated lysis is one of the first immune mechanisms encountered by promastigotes upon inoculation into the vertebrate host) and thus contributes to the fitness of PMM-R parasites (Fig. 6).
FIG 6.
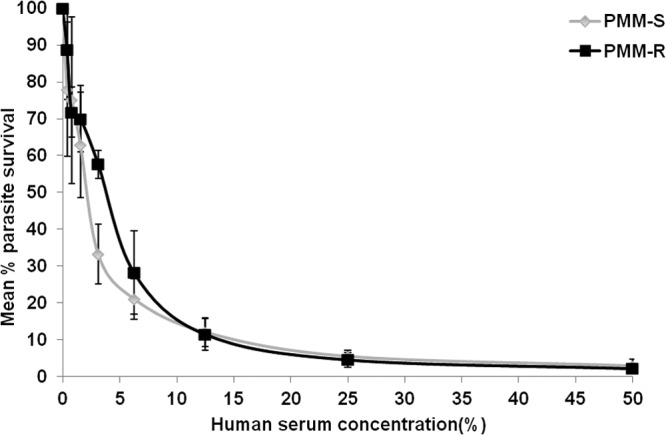
Resistance of PMM-R parasites to complement-mediated lysis. Parasites were incubated with fresh human serum, and parasite survival rates were measured at different serum concentrations. The assay was performed thrice in triplicate. Values given are mean ± SD percent survival.
Increased host IL-10 levels elicited by PMM-R parasites.
IL-10 plays an important role in promoting parasite survival in the host. We assessed IL-10 levels in the supernatants of macrophages infected with PMM-R and PMM-S isolates and found significantly greater IL-10 production (P = 0.03) in the host cells in response to PMM-R versus PMM-S parasites (Fig. 7).
FIG 7.
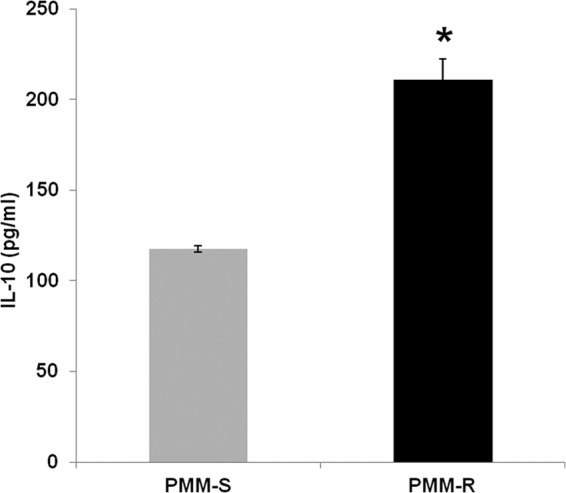
Estimation of IL-10 levels elicited in PMM-R parasite-infected macrophages. IL-10 levels in the supernatants of macrophages infected with PMM-R or PMM-S parasites were quantified by ELISA. The graph represents mean ± SD IL-10 levels produced in three independent experiments, each in triplicate. *, P < 0.05.
DISCUSSION
The present study aimed to explore the mechanisms of experimental PMM resistance in L. donovani and to evaluate the intrinsic susceptibility of the PMM-resistant parasites to host microbicidal activity. The laboratory-generated PMM-resistant strain showed a marked (>5-fold) decrease in susceptibility to PMM; however, the PMM-resistant parasites did not show any cross-tolerance toward SAG and MIL.
Several studies have established the increased expression of ATP-binding cassette (ABC) transporter genes such as MRPA and MDR1 in SAG-resistant and amphotericin B-resistant L. donovani (20, 26, 27). MDR1 and MRPA share a fundamental structure composed of transmembrane domains and nucleotide-binding domains, which are known to be involved in xenobiotic detoxification. The present study revealed remarkable increases in the expression of both MDR1 and MRPA in PMM-resistant parasites. The two transporters were reported to be modulated in Cryptosporidium parvum in the presence of PMM and cyclosporine (28). ABC transporter genes are regulated by protein phosphatase, which may activate expression of the transporter genes or may modulate the drug-pumping activity or substrate specificity of the transporters. It was demonstrated that increased expression of Sit4 protein phosphatase conferred PMM resistance in Kluyveromyces lactis, mediated by ABC transporters (25). Here we observed a marked increase in the expression of protein phosphatase 2A (a Sit4 protein phosphatase homologue in Leishmania) in paromomycin-resistant L. donovani, compared with its sensitive counterpart. Further studies based on overexpression of the ABC transporters are needed to verify transporter involvement in PMM resistance.
Resistance to drugs often results in modifications of the membrane structure and lipid composition, as observed in SAG-resistant and amphotericin B-resistant L. donovani (19, 26, 29) Here we observed a significant increase in membrane fluidity in paromomycin-resistant L. donovani, indicating alteration of the membrane composition of the parasites. Further, the resistant parasites showed marked decreases in intracellular drug accumulation, in line with the previous findings that indicated the association of PMM resistance with decreased drug accumulation (14, 16). The decreased drug accumulation detected in PMM-resistant parasites in the present study could be due to increased drug efflux, decreased drug uptake, or increased drug metabolism.
The importance of reactive oxygen species and nitrogen species in the host defense system in the context of antimony resistance in VL led to the hypothesis that resistance might be linked to parasite tolerance to stress imposed by macrophages (22). We investigated the susceptibility of the PMM-R parasites to macrophage killing mechanisms. Parasites are exposed to nitrosative and oxidative stresses upon phagocytosis by host macrophages; therefore, SNAP, SIN-1, and H2O2 were used to impersonate the stress imposed by macrophages. The PMM-R parasites showed significantly increased tolerance to NO as well as NO plus O2 at both the promastigote and amastigote stages, in comparison with PMM-S isolates, although the susceptibility to oxidative stress remained unaltered. IFN-γ secreted by macrophages synergizes to upregulate production of reactive nitrogen intermediates that mediate leishmanicidal activity (30). Analysis of parasite infectivity in macrophages incubated with IFN-γ and LPS revealed dose-dependent suppressive effects on both PMM-R and PMM-S parasites; however, the PMM-R strain was significantly more resistant to the macrophages' antileishmanial killing mechanisms at higher stimulations (≥5 U/ml IFN-γ and ≥5 ng/ml LPS). IL-10 has roles in parasite persistence and inhibition of host leishmanicidal activity (31). Here we observed greater stimulation of host IL-10 with PMM-R parasites than with PMM-S parasites. In addition, the PMM-R isolate resisted complement-mediated lysis, in comparison with PMM-S parasites. Taken together, these findings indicate that experimentally induced PMM-resistant promastigotes were significantly more tolerant to host leishmanicidal activity than were sensitive parasites, which would confer survival benefits to the parasites. In view of the earlier observation that the PMM-R strain behaved differently when induction was performed at the promastigote versus amastigote stage (17), it would be interesting to compare the types of adaptations with paromomycin resistance induced in amastigotes.
Our results seem to indicate that PMM-R parasites generated from an antimony-resistant isolate pushed adaptations (such as increased membrane fluidity and increased tolerance to leishmanicidal mechanisms) that were already present as a consequence of sodium stibogluconate resistance (19). Overall, the study showed that PMM resistance in Leishmania is a multifactorial phenomenon involving ABC transporters and components of nitrosative stress defense mechanisms, which may be exploited to judiciously develop specific inhibitors.
ACKNOWLEDGMENTS
The work has been funded by the European Commission Kaladrug-R project (grant EC-FP7-222895).
We report no conflicts of interest.
Footnotes
Published ahead of print 18 February 2014
REFERENCES
- 1.Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Desjeux P, Wasunna MK, Bryceson AD. 2002. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2:494–501. 10.1016/S1473-3099(02)00347-X [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundar S, More DK, Singh MK, Singh VP, Sharma S, Makharia A, Kumar PC, Murray HW. 2000. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 31:1104–1107. 10.1086/318121 [DOI] [PubMed] [Google Scholar]
- 4.Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. 2010. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N. Engl. J. Med. 362:504–512. 10.1056/NEJMoa0903627 [DOI] [PubMed] [Google Scholar]
- 5.Bhandari V, Kulshrestha A, Deep DK, Stark O, Prajapati VK, Ramesh V, Sundar S, Schonian G, Dujardin JC, Salotra P. 2012. Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis. PLoS Negl. Trop. Dis. 6:e1657. 10.1371/journal.pntd.0001657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundar S, Singh A, Rai M, Prajapati VK, Singh AK, Ostyn B, Boelaert M, Dujardin JC, Chakravarty J. 2012. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin. Infect. Dis. 55:543–550. 10.1093/cid/cis474 [DOI] [PubMed] [Google Scholar]
- 7.Rijal S, Ostyn B, Uranw S, Rai K, Bhattarai NR, Dorlo TP, Beijnen JH, Vanaerschot M, Decuypere S, Dhakal SS, Das ML, Karki P, Singh R, Boelaert M, Dujardin JC. 2013. Increasing failure of miltefosine in the treatment of kala-azar in Nepal and the potential role of parasite drug resistance, re-infection or non-compliance. Clin. Infect. Dis. 56:1530–1538. 10.1093/cid/cit102 [DOI] [PubMed] [Google Scholar]
- 8.Sinha PK, Jha TK, Thakur CP, Nath D, Mukherjee S, Aditya AK, Sundar S. 2011. Phase 4 pharmacovigilance trial of paromomycin injection for the treatment of visceral leishmaniasis in India. J. Trop. Med. 2011:645203. 10.1155/2011/645203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olliaro PL. 2010. Drug combinations for visceral leishmaniasis. Curr. Opin. Infect. Dis. 23:595–602. 10.1097/QCO.0b013e32833fca9d [DOI] [PubMed] [Google Scholar]
- 10.Sundar S, Sinha PK, Rai M, Verma DK, Nawin K, Alam S, Chakravarty J, Vaillant M, Verma N, Pandey K, Kumari P, Lal CS, Arora R, Sharma B, Ellis S, Strub-Wourgaft N, Balasegaram M, Olliaro P, Das P, Modabber F. 2011. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet 377:477–486. 10.1016/S0140-6736(10)62050-8 [DOI] [PubMed] [Google Scholar]
- 11.Kulshrestha A, Singh R, Kumar D, Negi NS, Salotra P. 2011. Antimony-resistant clinical isolates of Leishmania donovani are susceptible to paromomycin and sitamaquine. Antimicrob. Agents Chemother. 55:2916–2921. 10.1128/AAC.00812-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Stasio EA, Moazed D, Noller HF, Dahlberg AE. 1989. Mutations in 16S ribosomal RNA disrupt antibiotic-RNA interactions. EMBO J. 8:1213–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda K, Kondo S, Okanishi M, Utahara R, Umezawa H. 1968. Isolation of paromamine inactivated by Pseudomonas aeruginosa. J. Antibiot. (Tokyo) 21:458–459. 10.7164/antibiotics.21.458 [DOI] [PubMed] [Google Scholar]
- 14.Jhingran A, Chawla B, Saxena S, Barrett MP, Madhubala R. 2009. Paromomycin: uptake and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 164:111–117. 10.1016/j.molbiopara.2008.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maarouf M, Lawrence F, Brown S, Robert-Gero M. 1997. Biochemical alterations in paromomycin-treated Leishmania donovani promastigotes. Parasitol. Res. 83:198–202. 10.1007/s004360050232 [DOI] [PubMed] [Google Scholar]
- 16.Maarouf M, Adeline MT, Solignac M, Vautrin D, Robert-Gero M. 1998. Development and characterization of paromomycin-resistant Leishmania donovani promastigotes. Parasite 5:167–173 [DOI] [PubMed] [Google Scholar]
- 17.Hendrickx S, Inocêncio da Luz RA, Bhandari V, Kuypers K, Shaw CD, Lonchamp J, Salotra P, Carter K, Sundar S, Rijal S, Dujardin JC, Cos P, Maes L. 2012. Experimental induction of paromomycin resistance in antimony-resistant strains of L. donovani: outcome dependent on in vitro selection protocol. PLoS Negl. Trop. Dis. 6:e1664. 10.1371/journal.pntd.0001664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh R, Kumar D, Duncan RC, Nakhasi HL, Salotra P. 2010. Overexpression of histone H2A modulates drug susceptibility in Leishmania parasites. Int. J. Antimicrob. Agents 36:50–57. 10.1016/j.ijantimicag.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 19.Mukhopadhyay R, Mukherjee S, Mukherjee B, Naskar K, Mondal D, Decuypere S, Ostyn B, Prajapati VK, Sundar S, Dujardin JC, Roy S. 2011. Characterisation of antimony-resistant Leishmania donovani isolates: biochemical and biophysical studies and interaction with host cells. Int. J. Parasitol. 41:1311–1321. 10.1016/j.ijpara.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 20.Kumar D, Singh R, Bhandari V, Kulshrestha A, Negi NS, Salotra P. 2012. Biomarkers of antimony resistance: need for expression analysis of multiple genes to distinguish resistance phenotype in clinical isolates of Leishmania donovani. Parasitol. Res. 111:223–230. 10.1007/s00436-012-2823-z [DOI] [PubMed] [Google Scholar]
- 21.Decuypere S, Vanaerschot M, Rijal S, Yardley V, Maes L, de Doncker S, Chappuis F, Dujardin JC. 2008. Gene expression profiling of Leishmania (Leishmania) donovani: overcoming technical variation and exploiting biological variation. Parasitology 135:183–194. 10.1017/S0031182007003782 [DOI] [PubMed] [Google Scholar]
- 22.Vanaerschot M, Maes I, Ouakad M, Adaui V, Maes L, De Doncker S, Rijal S, Chappuis F, Dujardin JC, Decuypere S. 2010. Linking in vitro and in vivo survival of clinical Leishmania donovani strains. PLoS One 5:e12211. 10.1371/journal.pone.0012211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter KC, Hutchison S, Boitelle A, Murray HW, Sundar S, Mullen AB. 2005. Sodium stibogluconate resistance in Leishmania donovani correlates with greater tolerance to macrophage antileishmanial responses and trivalent antimony therapy. Parasitology 131:747–757. 10.1017/S0031182005008486 [DOI] [PubMed] [Google Scholar]
- 24.Ouakad M, Vanaerschot M, Rijal S, Sundar S, Speybroeck N, Kestens L, Boel L, De Doncker S, Maes I, Decuypere S, Dujardin JC. 2011. Increased metacyclogenesis of antimony-resistant Leishmania donovani clinical lines. Parasitology 138:1392–1399. 10.1017/S0031182011001120 [DOI] [PubMed] [Google Scholar]
- 25.Chen XJ, Bauer BE, Kuchler K, Clark-Walker GD. 2000. Positive and negative control of multidrug resistance by the Sit4 protein phosphatase in Kluyveromyces lactis. J. Biol. Chem. 275:14865–14872. 10.1074/jbc.275.20.14865 [DOI] [PubMed] [Google Scholar]
- 26.Purkait B, Kumar A, Nandi N, Sardar AH, Das S, Kumar S, Pandey K, Ravidas V, Kumar M, De T, Singh D, Das P. 2012. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob. Agents Chemother. 56:1031–1041. 10.1128/AAC.00030-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leprohon P, Legare D, Girard I, Papadopoulou B, Ouellette M. 2006. Modulation of Leishmania ABC protein gene expression through life stages and among drug-resistant parasites. Eukaryot. Cell 5:1713–1725. 10.1128/EC.00152-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benitez AJ, McNair N, Mead J. 2007. Modulation of gene expression of three Cryptosporidium parvum ATP-binding cassette transporters in response to drug treatment. Parasitol. Res. 101:1611–1616. 10.1007/s00436-007-0701-x [DOI] [PubMed] [Google Scholar]
- 29.t'Kindt R, Scheltema RA, Jankevics A, Brunker K, Rijal S, Dujardin JC, Breitling R, Watson DG, Coombs GH, Decuypere S. 2010. Metabolomics to unveil and understand phenotypic diversity between pathogen populations. PLoS Negl. Trop. Dis. 4:e904. 10.1371/journal.pntd.0000904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray HW, Delph-Etienne S. 2000. Roles of endogenous gamma interferon and macrophage microbicidal mechanisms in host response to chemotherapy in experimental visceral leishmaniasis. Infect. Immun. 68:288–293. 10.1128/IAI.68.1.288-293.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee B, Mukhopadhyay R, Bannerjee B, Chowdhury S, Mukherjee S, Naskar K, Allam US, Chakravortty D, Sundar S, Dujardin JC, Roy S. 2013. Antimony-resistant but not antimony-sensitive Leishmania donovani up-regulates host IL-10 to overexpress multidrug-resistant protein 1. Proc. Natl. Acad. Sci. U. S. A. 110:E575–E582. 10.1073/pnas.1213839110 [DOI] [PMC free article] [PubMed] [Google Scholar]