Abstract
Increasing antimicrobial resistance reduces treatment options for implant-associated infections caused by methicillin-resistant Staphylococcus aureus (MRSA). We evaluated the activity of fosfomycin alone and in combination with vancomycin, daptomycin, rifampin, and tigecycline against MRSA (ATCC 43300) in a foreign-body (implantable cage) infection model. The MICs of the individual agents were as follows: fosfomycin, 1 μg/ml; daptomycin, 0.125 μg/ml; vancomycin, 1 μg/ml; rifampin, 0.04 μg/ml; and tigecycline, 0.125 μg/ml. Microcalorimetry showed synergistic activity of fosfomycin and rifampin at subinhibitory concentrations against planktonic and biofilm MRSA. In time-kill curves, fosfomycin exhibited time-dependent activity against MRSA with a reduction of 2.5 log10 CFU/ml at 128 × the MIC. In the animal model, planktonic bacteria in cage fluid were reduced by <1 log10 CFU/ml with fosfomycin and tigecycline, 1.7 log10 with daptomycin, 2.2 log10 with fosfomycin-tigecycline and fosfomycin-vancomycin, 3.8 log10 with fosfomycin-daptomycin, and >6.0 log10 with daptomycin-rifampin and fosfomycin-rifampin. Daptomycin-rifampin cured 67% of cage-associated infections and fosfomycin-rifampin cured 83%, whereas all single drugs (fosfomycin, daptomycin, and tigecycline) and rifampin-free fosfomycin combinations showed no cure of MRSA cage-associated infections. No emergence of fosfomycin resistance was observed in animals; however, a 4-fold increase in fosfomycin MIC (from 2 to 16 μg/ml) occurred in the fosfomycin-vancomycin group. In summary, the highest eradication of MRSA cage-associated infections was achieved with fosfomycin in combination with rifampin (83%). Fosfomycin may be used in combination with rifampin against MRSA implant-associated infections, but it cannot replace rifampin as an antibiofilm agent.
INTRODUCTION
Device-associated infections are difficult to treat because of biofilm formation on the implant surface, consisting of adherent bacteria embedded in a polymeric matrix (1, 2). Bacteria in biofilms enter into a stationary growth phase and become up to 1,000 times more resistant to antibiotics. Staphylococci are causing more than 50% of periprosthetic joint infections (PJI) (3, 4). Rifampin was shown to exhibit good activity against staphylococcal biofilms (3, 5) and became (in combination) the standard treatment for PJI (3). For methicillin-resistant Staphylococcus aureus (MRSA), treatment options are limited and include glycopeptides, lipopepdides, oxazolidinones and “fifth-generation” cephalosporins, such as ceftobiprole and ceftaroline (4, 6). However, some studies suggested antagonistic activity between rifampin and either vancomycin or linezolid against MRSA biofilms (7, 8). Therefore, further investigations of the activity of rifampin combinations against MRSA biofilms are needed.
Fosfomycin is an old antibiotic with broad-spectrum and bactericidal activity against Gram-positive and Gram-negative bacteria, including MRSA, glycopeptide-intermediate S. aureus (GISA), and vancomycin-resistant enterococci (VRE) (9, 10). It penetrates well into bone and soft tissue and has been demonstrated to be effective in acute and chronic osteomyelitis (11). However, its role in the treatment of implant-associated infections is controversial, and it is recommended for treatment only by individual experts.
Fosfomycin acts by inhibiting the early phase synthesis of peptidoglycan by blocking the formation of N-acetylmuramic acid (12). Because of its unique mode of action, there is no cross-resistance between fosfomycin and other antibacterial agents. Indeed, surveillance studies have revealed that the frequency of fosfomycin resistance in clinical isolates remains low. Because of emergence of resistance, fosfomycin is mainly used in combination with other classes of antibiotics (13).
We evaluated the efficacy of fosfomycin, alone and in combination with vancomycin, daptomycin, tigecycline, and rifampin, against planktonic and biofilm MRSA, both in vitro and in a foreign-body infection model. This animal model has been previously used for studying the activity of antimicrobial combinations against implant-associated infections and is predictive for clinical outcome (14–19).
(Part of these results were presented at the 22nd European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], London, United Kingdom, 30 March to 3 April 2012 [20], the 13th Congress of the European Federation of National Associations of Orthopaedics and Traumatology [EFORT], Berlin, Germany, 23 to 25 May 2012 [43], and the 31st Annual Meeting of the European Bone and Joint Infection Society [EBJIS], Montreux, Switzerland, 20 to 22 September 2012 [44].)
MATERIALS AND METHODS
Test strain.
A methicillin-resistant S. aureus (MRSA) strain, ATCC 43300, was used, as in previous studies (15). Bacteria were preserved on cryovial beads at −70°C (Microbank; Pro-Lab Diagnostics, Richmond Hill, Ontario, Canada). For the preparation of the inoculum, a single bead was incubated for 7 h at 37°C in 1 ml sterile Trypticase soy broth (TSB) (Becton, Dickinson and Company, Le Pont de Claix, France), afterwards diluted 1:100 in TSB and incubated overnight at 37°C. Bacteria were washed three times by centrifugation at 3,800 rpm at 20°C for 10 min and resuspended in sterile normal saline. The suspension was adjusted to a McFarland standard turbidity of 0.5 and an optical density of 0.100. The exact bacterial count was determined by CFU counting from plating of serial 1:10 dilutions.
Antimicrobial agents.
Fosfomycin (InfectoPharm, Heppenheim, Germany), tigecycline (Pfizer Pharma, Zürich, Switzerland), and daptomycin (Novartis Pharma Schweiz, Basel, Switzerland) were supplied by the manufacturers. Rifampin (Sandoz, Steinhausen, Switzerland) and vancomycin (TEVA Pharma, Aesch, Switzerland) were purchased. All antibiotics were dissolved in sterile pyrogen-free water, except daptomycin, which was prepared in sterile normal saline.
Antimicrobial susceptibility.
The MIC was measured in triplicate by Etest (bioMérieux SA, Lyon, France). After incubation at 37°C for 18 h, the MIC was determined where the inhibition ellipse intersected the scale of the strip. For fosfomycin testing, glucose-6-phosphate (G6P) is commercially incorporated in the Etest strips.
Time-kill studies.
The activity of fosfomycin was evaluated by time-kill studies using an inoculum of ∼5 × 105 CFU/ml of MRSA. Antibiotic dilutions were prepared in 10 ml of Mueller-Hinton broth (MHB) adjusted to final concentrations of 1×, 8×, 32×, 64×, and 128× the MIC of the test strain. MHB without antibiotics served as a growth control; MHB without MRSA served as a negative control. The CFU were enumerated after 0, 2, 4, 6, 8, and 24 h of incubation at 37°C by plating aliquots of appropriate dilutions on Mueller-Hinton agar (MHA). The quantification limit was set at 200 CFU/ml. Killing was expressed by reduction in log10 CFU/ml. Experiments were performed in triplicate.
Evaluation of antimicrobial activity by microcalorimetry.
The activity of fosfomycin (alone and in combination) on planktonic and biofilm MRSA was measured by microcalorimetry (21, 22). A 48-channel batch isothermal microcalorimeter (thermal activity monitor, model 3102 TAM III; TA instruments, New Castle, DE) was used, measuring the heat generated by bacterial growth in real time. Heat flow below 10 μW was attributed to nonbacterial heat production (e.g., degradation of medium), while values above 10 μW followed the bacterial growth with an exponential increase of heat flow. Airtight sealed glass ampoules containing planktonic or biofilm MRSA (adherent to glass beads), antibiotics, and the appropriate medium were introduced in individual channels and lowered to an equilibrium position for 15 min to reach a temperature of 37°C. Heat flow was recorded separately for each channel every 10 s for 24 h. All experiments were performed in duplicate.
(i) Planktonic MRSA.
For planktonic MRSA, microcalorimetric ampoules containing 3 ml of MHB, supplemented with 25 mg/liter G6P for fosfomycin and 50 mg/liter Ca2+ for daptomycin and 2-fold antimicrobial dilutions were inoculated with 1 × 105 to 5 × 105 CFU/ml. The minimal heat inhibitory concentration for planktonic MRSA (MHICplank) was determined as the lowest antimicrobial concentration inhibiting heat production for 24 h. Synergism was evaluated by adding fosfomycin at subinhibitory concentrations to 1×, 0.5×, and 0.25× the MHICplank of vancomycin, daptomycin, rifampin, and tigecycline. The activity of fosfomycin was considered synergistic when growth of MRSA was inhibited at a concentration of 0.25× the MHICplank of the second drug. This definition was extrapolated from the definition of in vitro synergism used by standard techniques (45).
(ii) Biofilm MRSA.
For biofilm MRSA, porous glass beads (VitraPor; ROBU, Hattert, Germany) were placed in 10 ml MHB, inoculated with 2 or 3 colonies of MRSA, and incubated for 24 h at 37°C. Beads had the following characteristics: diameter, 4 mm; pore size, 40 to 100 μm; surface area, approximately 60 cm2. After 24 h, beads were washed with sterile saline three times, followed by exposure to serial dilutions of antimicrobials for another 24 h. Beads were then washed three times and placed in calorimeter ampoules with MHB, and heat flow was measured for 24 h. The minimal heat inhibitory concentration for biofilm MRSA (MHICbiofilm) was defined as the lowest antimicrobial concentration killing biofilm bacteria, without regrowth during 24 h of incubation, indicated by the absence of heat production in the microcalorimeter. Synergism was evaluated by adding fosfomycin at a subinhibitory concentration to 1×, 0.5×, or 0.25× the MHICbiofilm of vancomycin, daptomycin, rifampin, and tigecycline, as described above for planktonic MRSA.
Animal model.
A foreign-body infection model using male albino guinea pigs (Charles River, Sulzfeld, Germany) was used (23). Animal experiments were performed according to Swiss veterinary law, and the study protocol had been approved by the Institutional Animal Care and Use Committee. Briefly, four sterile polyfluorotetrathylene (Teflon) cages (cylinders of 32-mm length by 10-mm diameter), perforated with 130 regularly spaced holes 1 mm in diameter (Angst-Pfister, Zurich, Switzerland) were subcutaneously implanted in the flanks of guinea pigs, weighing between 450 and 500 g. Surgery was performed under aseptic conditions. After complete wound healing (2 weeks after implantation), the sterility of the cage was determined by aspirating fluid from each cage and plating on the blood agar plate. Cages were infected by percutaneous injection of 200 μl bacterial suspension containing 3.1 × 106 CFU of MRSA (i.e., day 0). Cage fluid was aspirated 3 days later, before the initiation of antimicrobial therapy, in order to confirm the infection and quantify the initial bacterial count.
Treatment groups.
Antimicrobial treatment was started 72 h after infection (i.e., day 3). Three animals (each implanted with four cages) were randomized into each of the following treatment groups: fosfomycin (150 mg/kg), daptomycin (50 mg/kg), tigecycline (10 mg/kg), daptomycin (50 mg/kg) plus fosfomycin (150 mg/kg), vancomycin (15 mg/kg) plus fosfomycin (150 mg/kg), daptomycin (50 mg/kg) plus rifampin (12.5 mg/kg), rifampin (12.5 mg/kg) plus fosfomycin (150 mg/kg), tigecycline (10 mg/kg) plus fosfomycin (150 mg/kg), and normal saline (control). Antimicrobials were administered intraperitoneally every 12 h for 4 days (i.e., a total of 8 doses), except daptomycin, which was administered every 24 h (i.e., a total of 4 doses). The dosing regimens were chosen based on pharmacokinetic studies performed with mice, rats, guinea pigs, and rabbits, in order to mimic concentration profiles achievable in humans (14–17, 24–32).
Evaluation of activity against planktonic bacteria.
Bacterial counts were determined (i) immediately before the start of therapy (i.e., day 3), (ii) during therapy, before the last dose of antibiotic (i.e., day 6), and (iii) 5 days after the end of therapy (i.e., day 11). The activity of each treatment regimen was expressed quantitatively as the difference in planktonic bacterial counts in cage fluid on day 6 or day 11 compared to the count on day 3: i.e., Δlog10 CFU/ml = log10 CFU/ml (day 6 or 11) − log10 CFU/ml (day 3). In addition, the activity against planktonic MRSA was expressed qualitatively as the clearance rate, defined as the percentage of aspirated cage fluid samples without MRSA growth divided by all cage fluids in each treatment group.
Evaluation of activity against biofilm bacteria.
Cages were aseptically removed on day 11 (i.e., 5 days after end of treatment) and were incubated in 5 ml TSB at 37°C for 48 h. After vigorous vortexing, 100-μl aliquots of the cage cultures were plated on blood agar plates and incubated aerobically for 48 h. Cure rate (%) was defined as the number of explanted cages without MRSA growth divided by the total number of cages in each treatment group.
Emergence of antimicrobial resistance in vivo.
All MRSA isolates from treatment failures were screened for emergence of resistance to fosfomycin, vancomycin, daptomycin, rifampin, and tigecycline. For this purpose, multiple colonies were collected from the agar plate, suspended in normal saline to an inoculum corresponding to the turbidity of a McFarland standard of 0.5, and plated on an agar plate. An Etest (bioMérieux SA, Lyon, France) was performed according to the manufacturer's instructions.
Statistical analysis.
Nonparametric continuous variables were expressed as the median and interquartile range (IQR). Comparisons between groups were performed by Mann-Whitney U test for continuous variables or chi-square tests for categorical variables. Statistical significance was defined as P < 0.05. Data were processed in GraphPad Prism (version 6.01; GraphPad Software, La Jolla, CA).
RESULTS
Antimicrobial susceptibility testing.
The susceptibilities of MRSA (ATCC 43300) to tested antimicrobials are presented in Table 1. MRSA was susceptible to all tested antimicrobials, according to EUCAST guidelines (33). Rifampin exhibited the lowest MIC (0.04 μg/ml), followed by tigecycline and daptomycin (both 0.125 μg/ml).
TABLE 1.
Antimicrobial susceptibility of MRSA strain ATCC 43300 determined by Etest and microcalorimetry
| Antimicrobial | Etest MIC (μg/ml) | MHIC (μg/ml) for MRSAa |
||
|---|---|---|---|---|
| Planktonic | Biofilm | Biofilm/planktonic | ||
| Fosfomycin | 1 | 32 | 4,096 | 128 |
| Daptomycin | 0.125 | 0.125 | 40 | 320 |
| Vancomycin | 1 | 1 | >1,024 | >1,024 |
| Rifampin | 0.04 | 0.08 | 164 | 2,050 |
| Tigecycline | 0.125 | 0.125 | 128 | 1,024 |
MHIC, minimal heat inhibitory concentration determined by microcalorimetry.
Antimicrobial activity by microcalorimetry.
The MIC values determined by Etests correlated well with the MHICplank measured by microcalorimetry, except for fosfomycin, where 32× higher values were determined by microcalorimetry (32 μg/ml) than by Etest (1 μg/ml) (Table 1). MHICbiofilm for all tested antimicrobials are above reachable concentrations in clinical practice and are at least 128× higher than the MHICplank. Figure 1 shows the synergistic activity of fosfomycin and rifampin by microcalorimetry. Synergistic activity against planktonic MRSA was observed when fosfomycin at a subinhibitory concentration (0.06× the MHICplank, 2 μg/ml) was combined with rifampin at 0.02 μg/ml, corresponding to 0.25× the MHICplank (Fig. 1A). Similarly, synergistic activity was observed against biofilm MRSA strains when fosfomycin at a subinhibitory concentration (0.25× the MHICbiofilm, 1,024 μg/ml) was combined with rifampin at 40 μg/ml, corresponding to 0.25× the MHICbiofilm (Fig. 1B).
FIG 1.
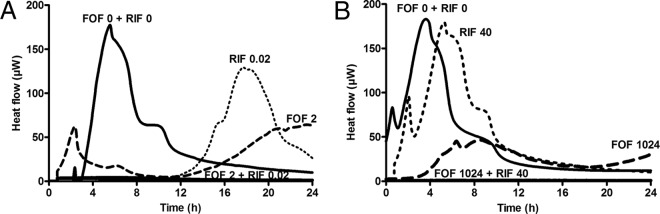
Evaluation of synergistic activity of fosfomycin (FOF) and rifampin (RIF) by microcalorimetry on planktonic (A) and biofilm (B) MRSA. Numbers above curves represent drug concentrations (μg/ml). Bacterial growth-related heat was suppressed by subinhibitory concentrations of fosfomycin (2 μg/ml for planktonic MRSA and 1,024 μg/ml for biofilm MRSA), combined with a subinhibitory concentration of rifampin (0.25× the MHIC).
Time-kill studies.
Figure 2 shows the time-kill curves against planktonic MRSA. Bacterial counts increased to 5 × 108 CFU/ml after 24 h without antimicrobials (growth control). Fosfomycin showed time-dependent activity against MRSA with a maximum decrease of 2.5 log10 CFU/ml at 128× the MIC at 24 h. At 8× and 32× the MIC, an initial bacterial reduction was observed between 4 and 8 h, but regrowth occurred at 24 h.
FIG 2.
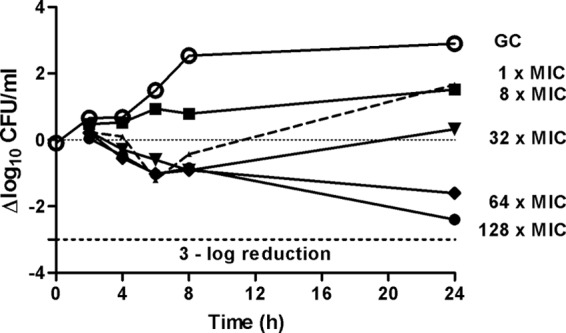
Time-kill studies for fosfomycin against MRSA at 1×, 8×, 32×, 64×, and 128× the MIC. The fosfomycin MIC was 1 μg/ml. The dotted line indicates a 3-log-CFU reduction. GC, growth control.
Infection profile in animal studies.
The median bacterial count before the start of antimicrobial treatment was 6.5 log10 CFU/ml (IQR, 5.8 to 6.8 log10 CFU/ml). Neither bacterial clearance (Fig. 3A) nor spontaneous cure occurred in the untreated animals. Compared to day 3, the median bacterial count in untreated animals increased by 1.1 (IQR, 0.6 to 1.6) log10 CFU/ml on day 6 and by 1.2 (0.9 to 1.7) log10 CFU/ml on day 11.
FIG 3.
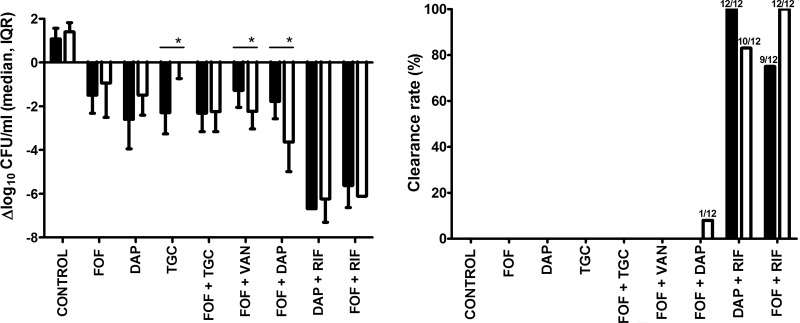
Antimicrobial activity against planktonic MRSA. (A) Bacterial counts during therapy (closed bars) and 5 days after the end of therapy (open bars), compared to the initial bacterial counts on day 3 (before treatment). Positive values denote net bacterial growth, and negative values denote net bacterial killing. Values represent medians and interquartile ranges (IQR). Asterisks indicate therapy regimens exhibiting significant differences in bacterial load during therapy versus 5 days after the end of therapy (P < 0.05). (B) Clearance rate of planktonic MRSA in aspirated cage fluids, during therapy (closed bars), and 5 days after the end of therapy (open bars). For each treatment group, 12 cages were used (i.e., 3 animals each implanted with 4 cages). The numbers of MRSA-free cage fluid samples divided by the total number of cages in each therapy group are indicated on the bars. Control, untreated animals; DAP, daptomycin; FOF, fosfomycin; RIF, rifampin; TGC, tigecycline; VAN, vancomycin.
Antimicrobial activity against planktonic bacteria.
Figure 3A shows the difference in planktonic bacteria on day 6 (during treatment) and day 11 (after treatment) compared to day 3 (before treatment). None of the single drugs or combinations of fosfomycin-vancomycin and fosfomycin-tigecycline reduced bacterial counts in the cage fluid by ≥3 log10 CFU/ml. On day 11, fosfomycin changed the bacterial count by a median of −0.3 (IQR, −2.4 to 0.2) log10 CFU/ml, daptomycin by −1.7 (IQR, −2.1 to −1.1) log10 CFU/ml, and tigecycline by −0.2 (IQR, −0.6 to 0.8) log10 CFU/ml. The combinations daptomycin-rifampin and fosfomycin-rifampin were the most active treatments against planktonic bacteria, followed by fosfomycin-daptomycin. Interestingly, 5 days after end of therapy, planktonic bacteria increased in the daptomycin (P = 0.06) and tigecycline (P = 0.002) single-drug groups but remained low in rifampin-containing combinations or were further reduced in the fosfomycin-vancomycin (P = 0.01) and fosfomycin-daptomycin (P = 0.006) groups, decreasing the median bacterial loads by 2.2 (IQR, −2.9 to −1.8) log10 CFU/ml and by 3.5 (IQR, −4 to −2.8) log10 CFU/ml, respectively.
The combination fosfomycin-daptomycin cleared MRSA from 1 of 12 cage fluids (8%) on day 11 (Fig. 3B). When rifampin was added to fosfomycin, the planktonic MRSA was cleared in 9 of 12 cage fluids (75%) during treatment (day 6) and from all 12 cage fluids (100%) after treatment (day 11). When rifampin was added to daptomycin, the clearance rates were 100% during treatment and 83% after treatment.
Antimicrobial activity against biofilm bacteria.
Figure 4 shows the cure rate of MRSA biofilms. Cure of cage-associated infections was achieved only with rifampin-containing treatment regimens, namely, rifampin-daptomycin (67%) and fosfomycin-rifampin (83%). All the other treatment groups, including single drugs (fosfomycin, daptomycin, and tigecycline) and rifampin-free fosfomycin combinations, were unable to eradicate biofilm MRSA from cages.
FIG 4.
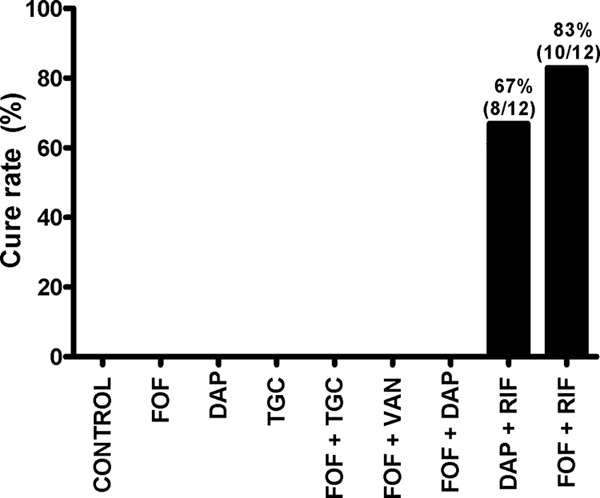
Antimicrobial activity against biofilm MRSA. For each treatment group, 3 animals each implanted with 4 cages were used. Numbers denote cure rates of cage-associated MRSA infections. Numbers of sterile explanted cages divided by the total number of cages in each therapy group are indicated on the bars. Control, untreated animals; DAP, daptomycin; FOF, fosfomycin; RIF, rifampin; TGC, tigecycline; VAN, vancomycin.
Emergence of antimicrobial resistance in vivo.
No emergence of fosfomycin resistance (MIC, ≤16 μg/ml) was observed in animals receiving single- or combined-drug treatment regimens. However, a 4-fold increase in fosfomycin MIC (i.e., from 2 μg/ml to 16 μg/ml) was observed in the fosfomycin-vancomycin group (in 3 of 12 strains). Single-drug treatment with daptomycin also led to a 4-fold increase in MIC (i.e., from 0.25 μg/ml to 1 μg/ml). In untreated animals, no MIC increase was observed to any of the antimicrobials tested.
DISCUSSION
In this experimental model, the combination of fosfomycin and rifampin showed the best activity against MRSA cage-associated infections. The clearance rate of planktonic MRSA was 100%, and the cure rate of biofilm MRSA was 83%. In combination with antibiotics other than rifampin (vancomycin, daptomycin, and tigecycline), fosfomycin showed no clearance and no cure of MRSA. This observation suggests that fosfomycin is an important combination agent with rifampin but cannot replace it in the treatment of MRSA biofilm infections.
Interestingly, the fosfomycin-rifampin combination was superior to all previously tested rifampin combinations in this animal model, against both planktonic and biofilm MRSA. For example, the cure rates of the combinations are as follows: levofloxacin plus rifampin, 58%; vancomycin plus rifampin, 8%; linezolid plus rifampin, 0%; and rifampin and high-dose daptomycin, 67%. The better activity with the fosfomycin-rifampin combination may be explained by the antibiofilm activity of rifampin (5), combined with favorable characteristics of fosfomycin in biofilms, such as good penetration in cage fluid (12), a presumed immunomodulatory effect (11), and the ability to break up biofilms and enhance the permeability of other antibiotics (13).
In a recent study from Garrigós et al. (32), a rat model was used for the evaluation of fosfomycin activity for treatment of an experimental MRSA foreign-body infection. The combination of a standard and a high dose of daptomycin (equivalent to 6 and 10 mg/kg in humans) with fosfomycin exerted a synergistic effect both in vitro and in vivo. Fosfomycin-rifampin was as effective as daptomycin-fosfomycin. A limitation of this model is that approximately 10% of spontaneous cure of cage infections occurs in rats, which does not happen in humans. Indeed, in this study 40 of 260 infected cages were spontaneously cured before the start of treatment and needed to be excluded from the study. The cure rate of the fosfomycin-daptomycin combination in the study by Garrigós et al. was 80%, as opposed to 0% in our study. Differences in the setups of the two experimental models may explain this difference, such as strain selection, animal choice, implant characteristic, duration of antimicrobial treatment, pharmacokinetic differences, and methods for evaluation of cure rates (sonication of the glass coverslips of cages, as opposed to culturing of the whole explanted cages in TSB for 48 h and vortexing before plating). Moreover, it was shown that the combination of fosfomycin and daptomycin in humans typically cannot eradicate MRSA when the implant is retained; in contrast, rifampin-combination regimens reached high cure rates (34–37).
In the treatment of S. aureus endocarditis, fosfomycin showed synergistic activity with high-dose daptomycin (38). Its small molecule enables excellent diffusion in tissue, including bone (12), which is likely due to structural similarity of fosfomycin to hydroxyapatite (39). However, the role of fosfomycin in the treatment of PJI and its potential capacity to replace rifampin are unclear. In an animal model of osteomyelitis (27), there was no synergistic or antagonistic effect reported between fosfomycin and daptomycin.
In vitro, fosfomycin showed good bactericidal activity on staphylococci and exhibited a synergistic effect with other antistaphylococcal drugs (40). However, fosfomycin seems to have better activity against Escherichia coli than MRSA as a single drug (41). The fosfomycin MIC of our test MRSA strain, as determined by Etest (1 μg/ml), was lower than the MIC values of most strains found in the EUCAST report (33). However, the MHICplank was at the limit of susceptibility (32 μg/ml) when evaluated in ampoules containing MHB supplemented with glucose-6-phosphate. It is known that susceptibility tests of fosfomycin performed in MHB can give inconsistent results and are not yet standardized (11). The MHICbiofilms for fosfomycin are much higher than those achievable in clinical practice. However, synergism was found between fosfomycin and rifampin, against both planktonic and biofilm MRSA. In other studies, standard assays for evaluation of drug-drug interactions (checkerboard and time-kill curve assays) showed synergistic activity for fosfomycin-daptomycin (11, 32) but inconsistent synergistic activity for fosfomycin-rifampin (40), vancomycin-rifampin (8, 42), and vancomycin-fosfomycin (13, 28, 40) combinations. In these studies, heterogeneous definitions of synergism, different strains, and only planktonic MRSA were used. Importantly, vancomycin and rifampin showed an antagonistic effect against MRSA biofilm (ATCC 43300), evaluated by parametric response surface analysis (8). A potential explanation for the antagonism between vancomycin and rifampin is that inhibition of RNA synthesis by rifampin can delay the activity of the cell-wall-active antibiotics suggested (42).
In addition to pharmacokinetic issues, an increase in fosfomycin MICs was observed in animals treated with fosfomycin-vancomycin, but no resistant strains were found. In the study from Garrigós et al. (32), therapy with fosfomycin alone led quickly to emergence of fosfomycin resistance, and resistance to rifampin occurred during rifampin therapy, including in the vancomycin-rifampin combination group. In another study from Baldoni et al. (16), using an MRSA guinea pig model, no emergence of rifampin resistance was observed in vivo when rifampin was administered alone or in combination.
In conclusion, the highest eradication rate of MRSA cage-associated infections was achieved with fosfomycin in combination with rifampin (83%), which was superior to other rifampin combinations, including those with daptomycin, vancomycin, levofloxacin, and linezolid. These results suggest that fosfomycin may be a useful combination partner to rifampin but cannot replace rifampin in the treatment of MRSA implant-associated infections.
ACKNOWLEDGMENTS
We thank Elena Maiolo and Inês Santos Ferreira from the Infectious Diseases Research Laboratory for useful comments and laboratory assistance.
This study was supported by a training fellowship from the European Society for Clinical Microbiology and Infectious Diseases (ESCMID; www.escmid.org) to Raluca Mihailescu and an educational grant from InfectoPharm, Heppenheim, Germany.
Footnotes
Published ahead of print 18 February 2014
REFERENCES
- 1.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193. 10.1128/CMR.15.2.167-193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95–108. 10.1038/nrmicro821 [DOI] [PubMed] [Google Scholar]
- 3.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645–1654. 10.1056/NEJMra040181 [DOI] [PubMed] [Google Scholar]
- 4.Salgado CD, Dash S, Cantey JR, Marculescu CE. 2007. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin. Orthop. Relat. Res. 461:48–53. 10.1097/BLO.0b013e3181123d4e [DOI] [PubMed] [Google Scholar]
- 5.Trampuz A, Widmer AF. 2006. Infections associated with orthopedic implants. Curr. Opin. Infect. Dis. 19:349–356. 10.1097/01.qco.0000235161.85925.e8 [DOI] [PubMed] [Google Scholar]
- 6.Soriano A, Garcia S, Bori G, Almela M, Gallart X, Macule F, Sierra J, Martinez JA, Suso S, Mensa J. 2006. Treatment of acute post-surgical infection of joint arthroplasty. Clin. Microbiol. Infect. 12:930–933. 10.1111/j.1469-0691.2006.01463.x [DOI] [PubMed] [Google Scholar]
- 7.Grohs P, Kitzis MD, Gutmann L. 2003. In vitro bactericidal activities of linezolid in combination with vancomycin, gentamicin, ciprofloxacin, fusidic acid, and rifampin against Staphylococcus aureus. Antimicrob. Agents Chemother. 47:418–420. 10.1128/AAC.47.1.418-420.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salem AH, Elkhatib WF, Noreddin AM. 2011. Pharmacodynamic assessment of vancomycin-rifampicin combination against methicillin resistant Staphylococcus aureus biofilm: a parametric response surface analysis. J. Pharm. Pharmacol. 63:73–79. 10.1111/j.2042-7158.2010.01183.x [DOI] [PubMed] [Google Scholar]
- 9.Carlet J, Mainardi JL. 2012. Antibacterial agents: back to the future? Can we live with only colistin, co-trimoxazole and fosfomycin? Clin. Microbiol. Infect. 18:1–3. 10.1111/j.1469-0691.2011.03702.x [DOI] [PubMed] [Google Scholar]
- 10.Cai Y, Fan Y, Wang R, An MM, Liang BB. 2009. Synergistic effects of aminoglycosides and fosfomycin on Pseudomonas aeruginosa in vitro and biofilm infections in a rat model. J. Antimicrob. Chemother. 64:563–566. 10.1093/jac/dkp224 [DOI] [PubMed] [Google Scholar]
- 11.Popovic M, Steinort D, Pillai S, Joukhadar C. 2010. Fosfomycin: an old, new friend? Eur. J. Clin. Microbiol. Infect. Dis. 29:127–142. 10.1007/s10096-009-0833-2 [DOI] [PubMed] [Google Scholar]
- 12.Raz R. 2012. Fosfomycin: an old—new antibiotic. Clin. Microbiol. Infect. 18:4–7. 10.1111/j.1469-0691.2011.03636.x [DOI] [PubMed] [Google Scholar]
- 13.Kastoris AC, Rafailidis PI, Vouloumanou EK, Gkegkes ID, Falagas ME. 2010. Synergy of fosfomycin with other antibiotics for Gram-positive and Gram-negative bacteria. Eur. J. Clin. Pharmacol. 66:359–368. 10.1007/s00228-010-0794-5 [DOI] [PubMed] [Google Scholar]
- 14.Trampuz A, Murphy CK, Rothstein DM, Widmer AF, Landmann R, Zimmerli W. 2007. Efficacy of a novel rifamycin derivative, ABI-0043, against Staphylococcus aureus in an experimental model of foreign-body infection. Antimicrob. Agents Chemother. 51:2540–2545. 10.1128/AAC.00120-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John AK, Baldoni D, Haschke M, Rentsch K, Schaerli P, Zimmerli W, Trampuz A. 2009. Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: importance of combination with rifampin. Antimicrob. Agents Chemother. 53:2719–2724. 10.1128/AAC.00047-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldoni D, Haschke M, Rajacic Z, Zimmerli W, Trampuz A. 2009. Linezolid alone or combined with rifampin against methicillin-resistant Staphylococcus aureus in experimental foreign-body infection. Antimicrob. Agents Chemother. 53:1142–1148. 10.1128/AAC.00775-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furustrand Tafin U, Majic I, Zalila Belkhodja C, Betrisey B, Corvec S, Zimmerli W, Trampuz A. 2011. Gentamicin improves the activities of daptomycin and vancomycin against Enterococcus faecalis in vitro and in an experimental foreign-body infection model. Antimicrob. Agents Chemother. 55:4821–4827. 10.1128/AAC.00141-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. 2012. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob. Agents Chemother. 56:1885–1891. 10.1128/AAC.05552-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliva A, Tafin UF, Maiolo EM, Jeddari S, Betrisey B, Trampuz A. 2014. Activity of fosfomycin and rifampin on planktonic and adherent Enterococcus faecalis in an experimental foreign-body infection model. Antimicrob. Agents Chemother. 58:1284–1293. 10.1128/AAC.02583-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mihailescu R, Furustrand Tafin U, Bétrisey B, Rio L, Maiolo E, Borens O, Trampuz A. 2011. High efficacy of fosfomycin-rifampin combination against methicillin-resistant Staphylococcus aureus in an experimental model of foreign-body infection, abstr P2062 22nd Eur Congr. Clin. Microbiol. Infect. Dis., London, United Kingdom, 30 March to 3 April 2012 [Google Scholar]
- 21.Boling EA, Blanchard GC, Russell WJ. 1973. Bacterial identification by microcalorimetry. Nature 241:472–473. 10.1038/241472a0 [DOI] [PubMed] [Google Scholar]
- 22.von Ah U, Wirz D, Daniels AU. 2009. Isothermal micro calorimetry—a new method for MIC determinations: results for 12 antibiotics and reference strains of E. coli and S. aureus. BMC Microbiol. 9:106. 10.1186/1471-2180-9-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmerli W, Waldvogel FA, Vaudaux P, Nydegger UE. 1982. Pathogenesis of foreign body infection: description and characteristics of an animal model. J. Infect. Dis. 146:487–497. 10.1093/infdis/146.4.487 [DOI] [PubMed] [Google Scholar]
- 24.Blaser J, Vergeres P, Widmer AF, Zimmerli W. 1995. In vivo verification of in vitro model of antibiotic treatment of device-related infection. Antimicrob. Agents Chemother. 39:1134–1139. 10.1128/AAC.39.5.1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widmer AF, Gaechter A, Ochsner PE, Zimmerli W. 1992. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin. Infect. Dis. 14:1251–1253. 10.1093/clinids/14.6.1251 [DOI] [PubMed] [Google Scholar]
- 26.Poeppl W, Tobudic S, Lingscheid T, Plasenzotti R, Kozakowski N, Lagler H, Georgopoulos A, Burgmann H. 2011. Daptomycin, fosfomycin, or both for treatment of methicillin-resistant Staphylococcus aureus osteomyelitis in an experimental rat model. Antimicrob. Agents Chemother. 55:4999–5003. 10.1128/AAC.00584-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poeppl W, Tobudic S, Lingscheid T, Plasenzotti R, Kozakowski N, Georgopoulos A, Burgmann H. 2011. Efficacy of fosfomycin in experimental osteomyelitis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 55:931–933. 10.1128/AAC.00881-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pachon-Ibanez ME, Ribes S, Dominguez MA, Fernandez R, Tubau F, Ariza J, Gudiol F, Cabellos C. 2011. Efficacy of fosfomycin and its combination with linezolid, vancomycin and imipenem in an experimental peritonitis model caused by a Staphylococcus aureus strain with reduced susceptibility to vancomycin. Eur. J. Clin. Microbiol. Infect. Dis. 30:89–95. 10.1007/s10096-010-1058-0 [DOI] [PubMed] [Google Scholar]
- 29.Ribes S, Taberner F, Domenech A, Cabellos C, Tubau F, Linares J, Viladrich PF, Gudiol F. 2006. Evaluation of fosfomycin alone and in combination with ceftriaxone or vancomycin in an experimental model of meningitis caused by two strains of cephalosporin-resistant Streptococcus pneumoniae. J. Antimicrob. Chemother. 57:931–936. 10.1093/jac/dkl047 [DOI] [PubMed] [Google Scholar]
- 30.Niska JA, Shahbazian JH, Ramos RI, Pribaz JR, Billi F, Francis KP, Miller LS. 2012. Daptomycin and tigecycline have broader effective dose ranges than vancomycin as prophylaxis against a Staphylococcus aureus surgical implant infection in mice. Antimicrob. Agents Chemother. 56:2590–2597. 10.1128/AAC.06291-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin LY, Lazzarini L, Li F, Stevens CM, Calhoun JH. 2005. Comparative evaluation of tigecycline and vancomycin, with and without rifampicin, in the treatment of methicillin-resistant Staphylococcus aureus experimental osteomyelitis in a rabbit model. J. Antimicrob. Chemother. 55:995–1002. 10.1093/jac/dki109 [DOI] [PubMed] [Google Scholar]
- 32.Garrigos C, Murillo O, Lora-Tamayo J, Verdaguer R, Tubau F, Cabellos C, Cabo J, Ariza J. 2013. Fosfomycin-daptomycin and other fosfomycin combinations as alternative therapies in experimental foreign body infection by methicillin resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 57:606–610. 10.1128/AAC.01570-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.EUCAST. 2012. Clinical breakpoints. EUCAST, European Society of Clinical Microbiology and Infectious Diseases; http://www.eucast.org/clinical_breakpoints/ [Google Scholar]
- 34.Giulieri SG, Graber P, Ochsner PE, Zimmerli W. 2004. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection 32:222–228. 10.1007/s15010-004-4020-1 [DOI] [PubMed] [Google Scholar]
- 35.Betsch BY, Eggli S, Siebenrock KA, Tauber MG, Muhlemann K. 2008. Treatment of joint prosthesis infection in accordance with current recommendations improves outcome. Clin. Infect. Dis. 46:1221–1226. 10.1086/529436 [DOI] [PubMed] [Google Scholar]
- 36.El Helou OC, Berbari EF, Lahr BD, Eckel-Passow JE, Razonable RR, Sia IG, Virk A, Walker RC, Steckelberg JM, Wilson WR, Hanssen AD, Osmon DR. 2010. Efficacy and safety of rifampin containing regimen for staphylococcal prosthetic joint infections treated with debridement and retention. Eur. J. Clin. Microbiol. Infect. Dis. 29:961–967. 10.1007/s10096-010-0952-9 [DOI] [PubMed] [Google Scholar]
- 37.Achermann Y, Vogt M, Spormann C, Kolling C, Remschmidt C, Wust J, Simmen B, Trampuz A. 2011. Characteristics and outcome of 27 elbow periprosthetic joint infections: results from a 14-year cohort study of 358 elbow prostheses. Clin. Microbiol. Infect. 17:432–438. 10.1111/j.1469-0691.2010.03243.x [DOI] [PubMed] [Google Scholar]
- 38.Miro JM, Entenza JM, Del Rio A, Velasco M, Castaneda X, Garcia de la Maria C, Giddey M, Armero Y, Pericas JM, Cervera C, Mestres CA, Almela M, Falces C, Marco F, Moreillon P, Moreno A, Hospital Clinic Experimental Endocarditis Study Group 2012. High-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 56:4511–4515. 10.1128/AAC.06449-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schintler MV, Traunmuller F, Metzler J, Kreuzwirt G, Spendel S, Mauric O, Popovic M, Scharnagl E, Joukhadar C. 2009. High fosfomycin concentrations in bone and peripheral soft tissue in diabetic patients presenting with bacterial foot infection. J. Antimicrob. Chemother. 64:574–578. 10.1093/jac/dkp230 [DOI] [PubMed] [Google Scholar]
- 40.Grif K, Dierich MP, Pfaller K, Miglioli PA, Allerberger F. 2001. In vitro activity of fosfomycin in combination with various antistaphylococcal substances. J. Antimicrob. Chemother. 48:209–217. 10.1093/jac/48.2.209 [DOI] [PubMed] [Google Scholar]
- 41.Corvec S, Furustrand Tafin U, Betrisey B, Borens O, Trampuz A. 2013. Activities of fosfomycin, tigecycline, colistin, and gentamicin against extended-spectrum-beta-lactamase-producing Escherichia coli in a foreign-body infection model. Antimicrob. Agents Chemother. 57:1421–1427. 10.1128/AAC.01718-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaPlante KL, Woodmansee S. 2009. Activities of daptomycin and vancomycin alone and in combination with rifampin and gentamicin against biofilm-forming methicillin-resistant Staphylococcus aureus isolates in an experimental model of endocarditis. Antimicrob. Agents Chemother. 53:3880–3886. 10.1128/AAC.00134-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mihailescu R, Furustrand Tafin U, Betrisey B, Rio L, Maiolo E, Bizzini A, Borens O, Trampuz A. 2012. High efficacy of fosfomycin-rifampin combination against methicillin-resistant Staphylococcus aureus (MRSA) in an experimental model of foreign-body infection, abstr 134 Abstr 31st Eur Bone Joint Infect Soc Congr. European Bone and Joint Infection Society, Utrecht, The Netherlands [Google Scholar]
- 44.Mihailescu R, Furustrand Tafin U, Betrisey B, Trampuz A, Borens O. 2012. High efficacy of fosfomycin in combination with rifampin against methicillin-resistant Staphylococcus aureus (MRSA) in the animal model of infection. Abstr 13th Eur Fed Natl Assoc Orthoped Traumatol Congr. European Federation of National Associations of Orthopaedics and Traumatology, Rolle, Switzerland [Google Scholar]
- 45.Lorian V. 2011. Antibiotics in laboratory medicine, 5th ed. Lippincott Williams & Wilkins Co., Philadelphia, PA [Google Scholar]


