Abstract
The growing number of microbial pathogens resistant to available antibiotics is a serious threat to human life. Among them is the bacterium Staphylococcus aureus, which colonizes keratinocytes, the most abundant cell type in the epidermis. Its intracellular accumulation complicates treatments against resulting infections, mainly due to the limited diffusion of conventional drugs into the cells. Temporins A (Ta) and B (Tb) are short frog skin antimicrobial peptides (AMPs). Despite extensive studies regarding their antimicrobial activity, very little is known about their activity on infected cells or involvement in various immunomodulatory functions. Here we show that Tb kills both ATCC-derived and multidrug-resistant clinical isolates of S. aureus within infected HaCaT keratinocytes (80% and 40% bacterial mortality, respectively) at a nontoxic concentration, i.e., 16 μM, whereas a weaker effect is displayed by Ta. Furthermore, the peptides prevent killing of keratinocytes by the invading bacteria. Further studies revealed that both temporins promote wound healing in a monolayer of HaCaT cells, with front speed migrations of 19 μm/h and 12 μm/h for Ta and Tb, respectively. Migration is inhibited by mitomycin C and involves the epidermal growth factor receptor (EGFR) signaling pathway. Finally, confocal fluorescence microscopy indicated that the peptides diffuse into the cells. By combining antibacterial and wound-healing activities, Ta and Tb may act as multifunctional mediators of innate immunity in humans. Particularly, their nonendogenous origin may reduce microbial resistance to them as well as the risk of autoimmune diseases in mammals.
INTRODUCTION
The emergence of a variety of microorganisms resistant to conventional drugs, particularly in hospital settings, has become a global health problem in recent years. This has led to the search for novel anti-infection compounds with new modes of action (1–3). Ribosomally made antimicrobial peptides (AMPs), which are produced by almost all living species from unicellular to pluricellular organisms (both plants and animals), are currently considered a potential source for the development of new antibiotics (4–11). Most of them are small cationic molecules (<10 kDa) with diverse sequences and structures and a strong activity against a vast number of microbial pathogens (8, 12). They represent key factors of the innate immune system (13), and many of them display, among other properties, a direct antimicrobial activity, mostly by membrane perturbation of the target cell or via cellular internalization, followed by killing of the pathogen (12, 14–16). In addition, they control host physiological functions such as inflammation, angiogenesis, wound healing, and neutralization of toxic microbial components, e.g., lipopolysaccharide and lipoteichoic acids (17–19). In particular, endogenous AMPs (e.g., the mammalian cathelicidin LL-37, which is expressed in different human tissues, including the skin) have been extensively studied for therapeutic purposes (20). For example, topical vitamin D3 application has a long history as a treatment for skin disorders (21), inducing the expression of LL-37 in human keratinocytes and thereby increasing the activities of these cells against intracellular bacteria, i.e., Staphylococcus aureus (22, 23). However, elevated levels of endogenous AMPs appear to be correlated with autoimmune diseases, e.g., psoriasis and lupus erythematosus (20, 24–26). The rational solution to avoid such an outcome could be the use of nonendogenous AMPs. In line with this, we investigated in this study the antimicrobial and immunomodulatory activities of nonmammalian AMPs, namely, two members of the amphibian skin temporin family, temporin A (Ta) and temporin B (Tb). Amphibian AMPs are synthesized by dermal glands and stored within granules that are released by a holocrine-type mechanism upon stress or physical injury (27–29). Ta and Tb are short (13 residues long), linear, and mildly cationic (net charge, +3 at neutral pH) AMPs particularly active against Gram-positive bacteria and Leishmania parasites (30). They both adopt an α-helical conformation in a membrane-mimicking environment (31–33); they are able to perturb the membrane of microbial cells (34), and in contrast with the highly cytotoxic isoform temporin L (35), Ta and Tb are practically nonhemolytic up to concentrations (i.e., 12 μM and 32 μM, respectively) 5-fold higher than their MICs against Gram-positive bacteria (34, 36).
However, whereas considerable literature is available on the antimicrobial properties of temporins, very little is known about their activity on infected cells or about additional features that may contribute to their defense mechanism against pathogens. We studied and compared the activities of Ta and Tb against S. aureus within infected HaCaT keratinocytes, as well as their ability to promote closure of a wound field produced in a keratinocyte monolayer. Importantly, these two functions make Ta and Tb potential template candidates for the development of a new therapeutic formulation against skin infections.
MATERIALS AND METHODS
Reagents.
TrypLE Express was purchased from Invitrogen (Life Technologies Europe, Monza, Italy); 3(4,5-dimethylthiazol-2yl)2,5-diphenyltetrazolium bromide (MTT), Triton X-100, trypan blue, AG1478, mitomycin C, and Hoechst 33258 were all from Sigma-Aldrich (St. Louis, MO).
Peptides.
Ta (FLPLIGRVLSGIL-NH2) and Tb (LLPIVGNLLKSLL-NH2) were synthesized by the 9-fluorenylmethoxy carbonyl (Fmoc) solid-phase method on Rink amide resin, using an ABI 433A automatic peptide synthesizer. Cleavage of the peptides from the MBHA [amino-(4-methylphenyl)methyl polystyrene, 4-methylbenzhydrylamine] resin resulted in the amidation of the C terminus. To label the peptides with rhodamine, the Fmoc protecting group was removed from the N termini of the resin-bound peptides by incubation with piperidine for 12 min, whereas all the other reactive amine groups of the attached peptides were kept protected. The resin-bound peptides were washed twice with N,N-dimethylformamide (DMF) and then treated with rhodamine-N-hydroxysuccinimide (2 eq), in anhydrous DMF containing 2% N,N-diisopropylethylamine, leading to the formation of a resin-bound N-rhodamine peptide. After 24 h, the resin was washed thoroughly with DMF and then with methylene chloride. The two rhodamine-labeled Ta (rho-Ta) and Tb (rho-Tb) peptides were then cleaved from the resin. All the peptides were purified by reversed-phase high-performance liquid chromatography on a C18 reversed-phase Bio-Rad semipreparative column (250 by 10 mm; 300-Å pore size; 5-μm particle size). The column was eluted with a 40-min linear gradient of 20 to 60% acetonitrile in water, containing 0.05% (vol/vol) trifluoroacetic acid, at a flow rate of 1.8 ml/min. The purified peptides were further subjected to amino acid analysis and electrospray mass spectrometry to confirm their compositions and molecular weights.
Microorganisms.
The following two strains were used in our experiments: the reference S. aureus ATCC 25923 and the clinical S. aureus isolate (no. 2069) from human soft tissue. The latter is resistant to methicillin (MRSA), gentamicin, levofloxacin, moxifloxacin, and rifampin and was kindly provided by Vincenzo Savini (Ospedale Santo Spirito, Pescara, Italy).
Cell culture.
A well-established line of human immortalized keratinocytes (HaCaT cell line; ATCC, USA) was used throughout the study. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), l-glutamine (4 mM), and 0.05 mg/ml of gentamicin, at 37°C and 5% CO2, in 25-cm2 flasks.
Cell toxicity assay.
The toxic effect of the peptides on HaCaT cells was evaluated using the MTT colorimetric method (37). MTT is a tetrazolium salt which is reduced to a colored formazan product by mitochondrial reductases, giving a purple color. The intensity of the color is directly proportional to the number of metabolically active cells. Keratinocytes were plated in triplicate wells of a microtiter plate, at 4 × 104 cells/well in DMEM supplemented with 4 mM glutamine (DMEMg) and 2% FBS without antibiotic. After overnight incubation at 37°C in a 5% CO2 atmosphere, the medium was replaced with 100 μl of fresh serum-free DMEMg containing the peptides at different concentrations. The plate was incubated for 2 h at 37°C in a 5% CO2 atmosphere. Then, DMEMg was removed and replaced with Hank's buffer (136 mM NaCl; 4.2 mM Na2HPO4; 4.4 mM KH2PO4; 5.4 mM KCl; 4.1 mM NaHCO3, pH 7.2; supplemented with 20 mM d-glucose) containing 0.5 mg/ml of MTT. After 4 h of incubation, the formazan crystals were dissolved by adding 100 μl of acidified isopropanol according to reference 38, and absorbance of each well was measured at 570 nm using a microplate reader (Infinite M200; Tecan, Salzburg, Austria).
Cell infection and peptide effect on intracellular bacteria.
About 40,000 HaCaT keratinocytes resuspended in DMEMg supplemented with 2% FBS were seeded in 96-well plates and grown overnight to confluence. At this point, each well contained approximately 100,000 cells. The bacterial strain S. aureus ATCC 25923 or the clinical isolate 2069 was grown in Luria-Bertani broth at 37°C with mild shaking and then harvested by centrifugation. The pellet was resuspended at a final cell density of 1 × 107 CFU/ml and gently sonicated. One hundred microliters of bacterial suspension was coincubated with keratinocytes for 2 h at 37°C and 5% CO2. The medium was aspirated and the cells were washed three times with phosphate-buffered saline (PBS) to remove nonadherent bacteria. In order to kill extracellular bacteria, cells were incubated for 1 h or 15 min, respectively, in serum-free DMEMg supplemented with 100 μg/ml of gentamicin (for the ATCC strain) or 10 μg/ml of lysostaphin (39) (for the gentamicin-resistant clinical isolate). Afterwards, the medium was aspirated and the infected cells were washed three times, as described above. One hundred microliters of peptide solution in serum-free DMEMg, or water (in control samples), was added to each well and the plate was incubated for 2 h (or 24 h) at 37°C and 5% CO2. Then, the peptide solution was removed and the cells were washed again with PBS three times and lysed with 100 μl of 0.1% Triton X-100 in PBS for 5 min at room temperature. The resulting bacterial suspension was sonicated in a water bath for 10 min, to break up possible clumps (40), and appropriate dilutions were plated on agar plates for colony counts; the CFU were counted after 24 h at 37°C. In parallel, the number of keratinocytes was evaluated before and after bacterial infection and peptide treatment. Briefly, cells were detached from each well by adding 20 μl of TrypLE Express. After 20 min of incubation at 37°C and 5% CO2, 180 μl of DMEMg supplemented with 10% FBS was added. Afterwards, an appropriate aliquot was counted under a microscope.
Cell migration assay.
Cell migration was studied as follows. HaCaT cells (40,000) suspended in DMEMg supplemented with 10% FBS were seeded on each side of an ibidi culture insert for live cell analysis (ibidi, Munich, Germany). Inserts were placed into 3-mm dishes and incubated at 37°C and 5% CO2 to allow cells grow to confluence. Afterwards, inserts were removed with sterile tweezers to create a cell-free area (“wound”) of approximately 500 μm; 1 ml of serum-free DMEMg supplemented or not with the peptide at different concentrations was added. Cells were allowed to migrate in an appropriate incubator. At 0, 3, 6, 9, and 12 h, fields of the injury area were visualized microscopically under an inverted microscope (Olympus CKX41) at a magnification of ×4 and photographed with a Color View II digital camera. The percentage of cell-covered area at each time was determined by the Wimasis Image Analysis program. The migration speed was evaluated according to Wimasis' instructions. Wound closure assays were also conducted in the presence of the cell proliferation inhibitor mitomycin C (20 μM) (41, 42), whereas the involvement of epidermal growth factor receptor (EGFR) in temporin-induced keratinocyte migration was analyzed by pretreating cells with 0.2 μM AG1478 inhibitor (43, 44).
Fluorescence studies.
HaCaT keratinocytes (40,000) were seeded on coverslips for 24 h in DMEMg supplemented with 10% FBS at 37°C and 5% CO2. After 24 h, cells were washed with PBS and treated with rho-Ta or rho-Tb (4 μM in serum-free DMEMg) at 37°C and 5% CO2. After different time periods (30 min and 3, 6, 9, and 12 h), cells were washed with PBS and fixed with 3.7% formaldehyde for 10 min at +4°C. Afterwards, they were washed with PBS, treated with 0.1% Triton X-100 for 10 min, and stained with 2 μg/ml of Hoechst 33258 for 10 min at room temperature (45). The coverslips were placed on a glass slide with buffered glycerol and visualized under a fluorescence confocal microscope (Olympus FV1000). Hoechst- and rhodamine-labeled peptides were visualized using laser wavelengths of 405 and 559 nm, respectively. All images were taken using an objective lens of 60× and zoom 3.
Statistical analyses.
Data were collected from at least three independent experiments. Quantitative data are expressed as means ± standard errors (SE). Statistical analysis was performed using two-way analysis of variance (ANOVA) with PRISM software (GraphPad, San Diego, CA). Differences were considered to be statistically significant at a P value of <0.05. The levels of statistical significance are indicated in the figure legends.
RESULTS
Peptide effect on cell viability.
The effect of Ta and Tb on the survival of metabolically active HaCaT cells was studied by the MTT-based assay. As shown in Table 1, Tb was devoid of toxic effects at a concentration range of 2 μM to 16 μM. However, when tested at higher concentrations, i.e., 32 μM or 64 μM, it was found to decrease the percentage of metabolically active cells by approximately 43% or 92%, respectively. On the other hand, the isoform Ta caused a 46% to 94% decrease at concentrations ranging from 16 μM to 64 μM. Note that when HaCaT cells were incubated with Ta for a longer time (48 h), as recently reported by Baranska-Rybak and colleagues (46), the peptide did not show any toxic effect up to a concentration of 25 μg/ml (∼18 μM). This is presumably due to its proteolytic degradation during such a long time.
TABLE 1.
Effects of temporins at different concentrations on the number of metabolically active HaCaT cells
| Peptide concn (μM) | Metabolically active cells (%)a |
|
|---|---|---|
| Ta | Tb | |
| 2 | 104 ± 1.2 | 104 ± 2 |
| 4 | 103 ± 2.2 | 101 ± 3.2 |
| 8 | 99 ± 3.3 | 102 ± 3.5 |
| 16 | 54 ± 1.76 | 99.97 ± 2.6 |
| 32 | 42 ± 2.2 | 57.56 ± 3.8 |
| 64 | 6.1 ± 0.7 | 8.0 ± 0.83 |
The number of metabolically active cells was determined by the MTT assay and is expressed as percentage with respect to the non-peptide-treated control cells (see Materials and Methods).
Activity on S. aureus-infected HaCaT keratinocytes.
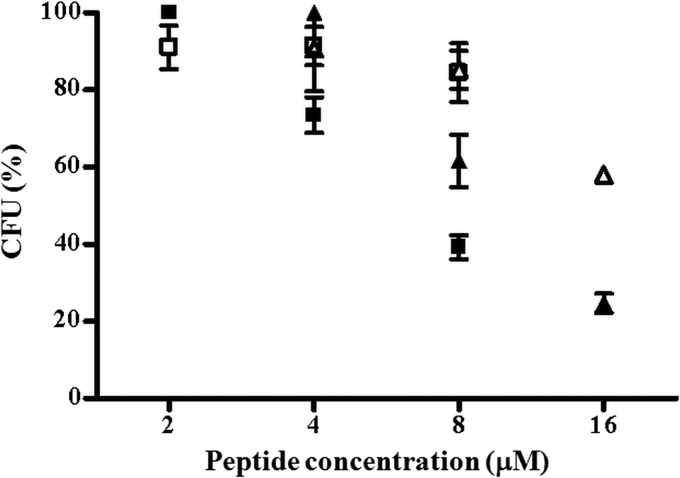
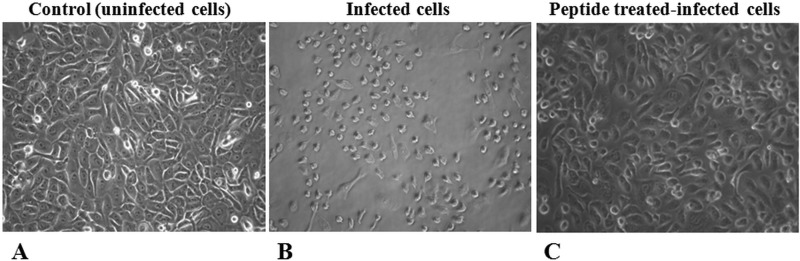
To get insight into the ability of both temporins to exhibit activity against ingested S. aureus cells, HaCaT keratinocytes were infected with S. aureus ATCC 25923 or a soft tissue isolate (see Materials and Methods) and subsequently treated with the peptides at different concentrations nontoxic for keratinocytes. Note that Ta and Tb had the same MIC, i.e., 4 μM (data not shown), against both S. aureus strains. The two temporins reduced the number of internalized bacteria in a dose-dependent manner, with Tb causing ∼80% or 40% killing of the ATCC strain or the MRSA clinical isolate, respectively, at the highest concentration used (16 μM) and after 2 h (Fig. 1). In comparison, a lower bactericidal effect on the intracellular S. aureus was displayed by Ta (∼60% or 20% killing of the ATCC 25923 or 2069 strain, respectively) at its highest noncytotoxic concentration (8 μM) (Table 1). It is worthwhile noting that bacterial invasion induced approximately 25% killing of keratinocytes, 3 h after their infection by S. aureus, whereas peptide treatment did not compromise the viability of infected cells (data not shown). As highlighted in Fig. 2, the killing of infected keratinocytes became more pronounced 1 day after their exposure to the bacterium, presumably due to S. aureus-induced apoptotic cell death (47), which was strongly prevented by treatment with both temporins.
FIG 1.
Bacterial survival within infected HaCaT keratinocytes upon peptide treatment. HaCaT cells were infected with 1 × 107 CFU/ml of S. aureus ATCC 25923 (filled symbols) or the MRSA clinical isolate (empty symbols) for 2 h and treated with Ta (squares) or Tb (triangles) at different concentrations. Afterwards, keratinocytes were lysed with 0.1% Triton X-100 and aliquots were plated on agar plates for counting of bacteria. The number of viable bacterial cells is expressed as a percentage with respect to the number of intracellular bacteria in the corresponding control samples (non-peptide-treated infected cells).
FIG 2.
Light microscopy images of HaCaT keratinocytes. About 100,000 cells were infected with S. aureus ATCC 25923 (1 × 107 CFU/ml) for 2 h, washed with PBS, treated with gentamicin to remove extracellular bacteria, and incubated with (C) or without (B) Tb (16 µM) in serum-free DMEMg for 24 h at 37°C and 5% CO2. (See Materials and Methods for additional details.) Similar results were obtained with Ta (8 µM) and therefore are not shown. Control samples were uninfected cells not treated with peptide (A).
Cell migration assay.
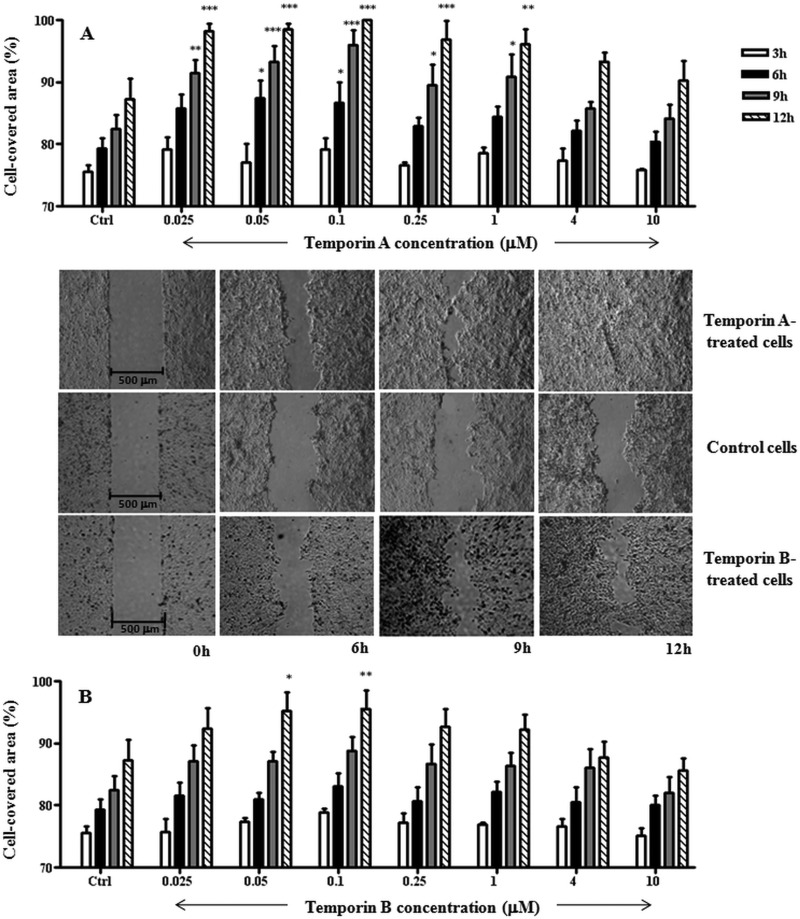
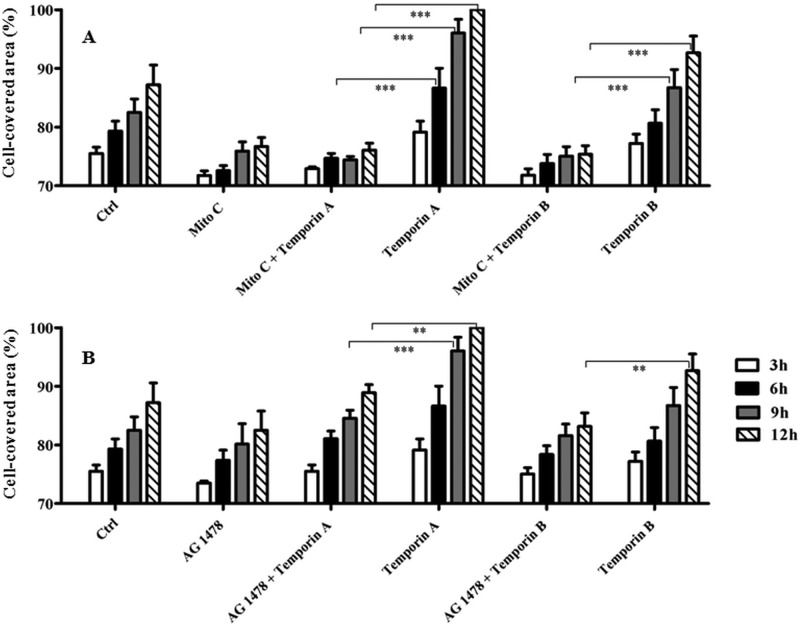
Next, we performed an in vitro wound healing assay based on special cell culture inserts (see Materials and Methods) to determine the temporins' capability to provoke migration of the human keratinocyte-derived HaCaT cells. As indicated in Fig. 3A, Ta significantly stimulated cell migration within 12 h, at a concentration range from 0.025 μM to 1 μM, with a bell-shaped dose-response curve. The maximal cell-covered area was observed 9 to 12 h after peptide addition. The optimal concentration allowing the complete coverage of the wound field was 0.1 μM (see micrographs in Fig. 3), with a front speed migration of 19 μm/h. Conversely, we obtained a weaker effect in the case of Tb (see micrographs in Fig. 3), where a statistical significance was reached only at 0.1 μM (Fig. 3B) with a migration speed estimated to be equal to 12 μm/h. Afterwards, in order to know whether the “gap” closure was influenced by an increased proliferation of keratinocytes upon their exposure to the temporins, the wound healing assay was carried out in the presence of 20 μM mitomycin C to block cell proliferation. As shown in Fig. 4A, mitomycin C strongly inhibited the migratory activity of keratinocytes induced by 0.1 μM Ta or Tb, as indicated by the lower percentages of cell-covered area. This suggests that proliferation of HaCaT cells highly contributes to the wound-healing effect produced by both peptides. Furthermore, we tested whether the temporin-induced migration of these cells involved the EGFR signaling pathway, as previously described for other AMPs, such as hBD2, -3, and -4 (48) or LL-37 not only on keratinocytes (43) but also on corneal (49) and airway epithelial (50) cells. For that purpose, we analyzed the effect of AG1478 on the closure of the wound field. AG1478 is an inhibitor of the EGFR tyrosine kinase (43, 44) and blocks the activation of EGFR, which is an essential regulator of keratinocyte biology, including proliferation, differentiation, and migration (51–53). Interestingly, pretreatment of HaCaT cells with 0.2 μM AG1478 clearly prevented cell migration induced by Ta or Tb (Fig. 4B).
FIG 3.
Effects of Ta (A) and Tb (B) on the closure of a wound field produced in a monolayer of HaCaT cells. (See Materials and Methods for additional details.) HaCaT cells were seeded in each side of an ibidi culture insert and grown to confluence. Afterwards, they were treated or not with the peptide at different concentrations, as indicated. Cells were photographed at the time of insert removal (0 h) and examined for cell migration after 3, 6, 9, and 12 h from peptide addition. The percentage of cell-covered area at each time point is reported on the y axis. The control (Ctrl) consisted of cells not treated with the peptide. All data are the means of at least three independent experiments ± SE. The levels of statistical significance between Ctrl and treated samples are indicated as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001. Micrographs show representative results of wound closure induced upon treatment of keratinocytes with 0.1 μM Ta or Tb with respect to the Ctrl sample.
FIG 4.
Effect of cell proliferation (A) or intracellular signaling (B) inhibitors on temporin-mediated closure of a wound field produced in a HaCaT keratinocyte monolayer. After removal of the ibidi culture insert, HaCaT cell monolayers were preincubated with 20 µM mitomycin C (Mito C) for 90 min (A) or 0.2 µM AG1478 for 10 min (B) and subsequently treated or not with 0.1 μM peptide, as indicated. In parallel, cells treated with the peptide alone were included for comparison. Cells incubated with medium served as a control (Ctrl). As indicated in the legend to Fig. 3, samples were photographed at different time intervals and the percentage of cell-covered area was calculated and reported on the y axis. All data are the means of at least three independent experiments ± SE. The levels of statistical significance between groups are indicated as follows: **, P < 0.01, and ***, P < 0.001.
Fluorescence studies.
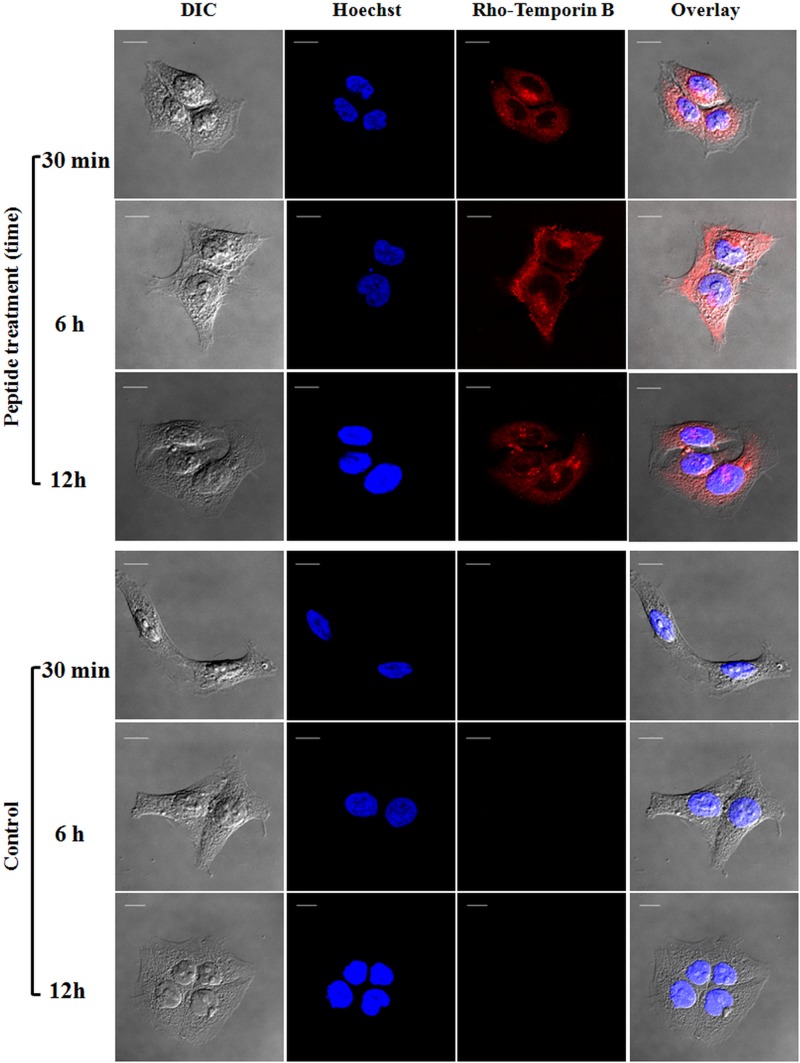
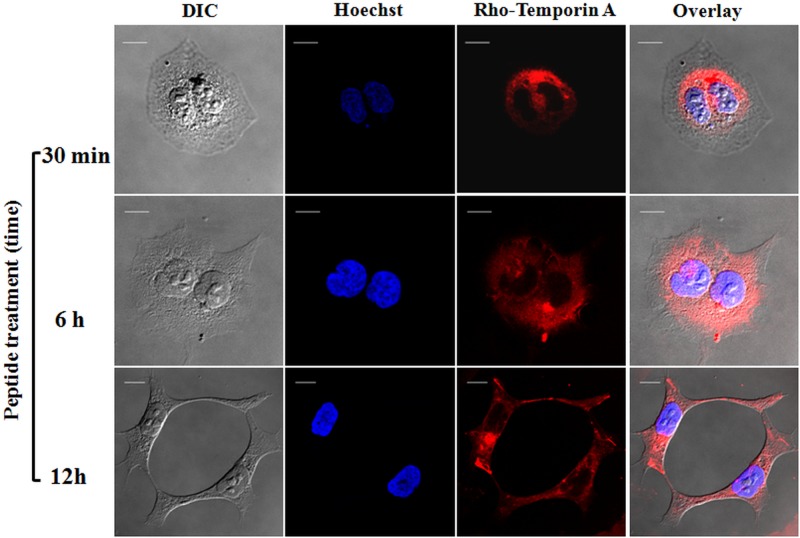
To discern whether temporins were located on the surface of HaCaT keratinocytes or internalized into the cytoplasm or nucleus, rhodamine-labeled peptides were employed and the results were visualized by confocal fluorescence microscopy. Cells were also stained with Hoechst dye for nuclear detection. As shown in Fig. 5, rho-Tb appeared to be evenly distributed in the cytosol of the cells within 30 min of its addition, without entering into the nucleus. Similar results were obtained after a longer incubation time with the peptide (6 h and 12 h) (Fig. 5), and similar behavior was also observed for rho-Ta at all the time intervals (Fig. 6).
FIG 5.
Confocal laser scanning microscopy images of HaCaT cells treated with rho-Tb at different times (30 min, 6 h, and 12 h). After peptide treatment, cells were stained with Hoechst for nuclear detection. The first column shows differential interference contrast (DIC) images, the second one shows the Hoechst fluorescence signal, the third column shows the rhodamine-labeled peptide signal, and the last column shows the overlay of the two fluorescence probes. All images are z section images taken from the mid-cell height. All bars represent 10 μm.
FIG 6.
Confocal laser scanning microscopy images of HaCaT cells treated with rho-Ta at different times (30 min, 6 h, and 12 h). After peptide treatment, cells were stained with Hoechst as described in the legend to Fig. 5. All images are z section images taken from the mid-cell height. All bars represent 10 μm.
DISCUSSION
Among microorganisms displaying increasing resistance to currently available antibiotics is the Gram-positive bacterium S. aureus, which was originally considered an extracellular pathogen. However, there is clear evidence that it can enter host cells to facilitate escape from immune attack, cellular barriers, and AMPs (54). Transient or persistent infections can occur in healthy individuals (e.g., impetigo and folliculitis), as well as in those with skin lesions due to wounds, inserted medical devices, or chronic autoimmune diseases (55). S. aureus can also become more invasive and cause more severe illnesses, including pneumonia, endocarditis, and sepsis (56). The fundamental role played by S. aureus in the colonization and infection processes of the skin is attributed to its interaction with keratinocytes. As indicated by Mempel and colleagues (57), it is able to adhere to pilus-like projections of the keratinocyte cell wall, followed by attachment and generation of endosomal vesicles in the cytoplasm, usually containing a few bacteria. Probably because of their nonphagocytotic nature, keratinocytes cannot effectively kill the internalized bacteria; and the intracellular persistence of Gram-positive cocci constitutes a potent virulence mechanism for various epithelial cell types (58). Intracellular accumulation of S. aureus can compromise the efficacy of antimicrobial agents, many of which (i.e., beta-lactams) hardly enter the cells or do not reach the same intracellular compartment as the bacteria (59). Furthermore, the antimicrobial activity of drugs may be impaired by the intracellular milieu or by changes of the bacterial metabolism (60–62). In addition, the increasing incidence of antibiotic-resistant S. aureus strains has complicated the therapy against their infections (63, 64). Here we reported on the ability of Ta and Tb to kill bacteria, once internalized by human keratinocyte-derived HaCaT cells, as well as on their capacity to promote cell migration. Keratinocytes are the major cell line in the epidermis, forming a barrier between the internal tissues of the host and the external environment; when the epidermis is disrupted by wounding, microbial pathogens can easily invade the body. The data reveal that both Ta and Tb, although to different extents, can reduce in a dose-dependent manner the number of S. aureus cells inside HaCaT keratinocytes. Tb, the more active peptide, kills ∼80% of the ATCC-derived bacterial cells at its highest noncytotoxic dosage (16 μM) within 2 h (Fig. 1) and without injuring the host cells. Yet a lower bactericidal activity is displayed toward the MRSA clinical isolate (Fig. 1). Furthermore, Ta and Tb promote the closure of a wound field produced in a keratinocyte monolayer, with a reversed order of efficacy (Fig. 3). This process is highly dependent on the peptide-induced cell proliferation (Fig. 4A). Note that in in vitro assays, it is difficult to simulate real wounds, in which debris from dead cells might play a role in their migration and in which the interaction among different cell types takes place. However, it is worth considering that the usage of cell culture inserts to produce a gap within a monolayer of cells provides an objective and highly reproducible experimental milieu to allow a quantitative evaluation of cell migration compared to what can be obtained by the classical method involving scratching the cell monolayer with a plastic pipette tip. According to what has been reported for LL-37 (43) and hBD peptides (48), temporins induce migration of HaCaT keratinocytes, which appears to be mediated by EGFR (Fig. 4B). After interaction with the plasma membrane, both temporins could directly or indirectly stimulate cell surface EGFR and activate downstream signaling cascades. In addition to this property, Ta has the ability to stimulate migration of human leukocytes, as reported previously (65): Ta was found to induce monocyte migration with a bell-shaped dose-response curve, with an optimal dose of 0.25 μM. This is similar to what we found for the immortalized human keratinocytes. However, the phagocyte-attracting activity of Ta is mediated by a Giα protein-coupled receptor (65).
When used at a sublethal dosage, the two temporins can enter HaCaT keratinocytes, as soon as within 30 min, and diffuse into the cytoplasm (Fig. 5 and 6). Importantly, this is the first evidence showing the intracellular localization of members of the temporin family in normal mammalian cells without an effect on their viability. Although it is not yet clear what the mechanism of action subtending the killing activity of intracellular microbial pathogens is, it is assumed to occur through a direct effect of the internalized peptide on the bacteria. Nevertheless, we cannot rule out the possibility that additional events encompassing the activation of the infected cell can assist in the killing of bacteria. Interestingly, despite the sequence similarity between the two temporins and their common α-helical structure (31), Ta is more potent in triggering migration of HaCaT cells, whereas Tb reduces the number of intracellular staphylococci (either from the ATCC-derived strain or the MRSA clinical isolate) more efficiently than Ta, when tested at a concentration equal to or 2-fold lower than the highest noncytotoxic dose used against keratinocytes (i.e., 16 μM and 8 μM for Tb and Ta, respectively). The difference in promoting migration might be due to differences between the two peptides in the activation of or binding affinity to receptors involved in the intracellular signaling pathway controlling the keratinocytes' migration or proliferation. Otherwise, the difference in the antibacterial activities might be due to differences in the ability of the two temporins to penetrate HaCaT cells and/or to differences in the antimicrobial effectiveness within the intracellular environment, despite their equal MICs for cell-free living S. aureus bacteria (see above). By combining antibacterial and wound-healing activities, Ta and Tb may act as multifunctional mediators of innate immunity in humans. In summary, temporins have several advantages that make them attractive candidates for the generation of new therapeutics to treat S. aureus-related epithelial (skin) infections as well as for the future development of cell-penetrating peptides to be employed for drug delivery. These include (i) a long-lasting existence in nature as active molecules which are able to exert a direct antimicrobial activity, which should guarantee the success of the antimicrobial efficacy of temporin-based anti-infective agents; (ii) a membrane-perturbing activity on bacteria, which should limit the induction of microbial resistance (27); (iii) the ability to kill both reference S. aureus and MRSA strains, once internalized by human epidermal cells (i.e., immortalized keratinocytes) and to treat them; (iv) the ability to stimulate migration of these cells; (v) a chemoattractic property for human monocytes (65); (vi) an exogenous (nonmammalian) nature, which should allow beneficial effects in clinical medicine, reducing the possible risk of inducing an autoimmune response; and, last but not least, (vii) a small size, which should allow a low production cost.
ACKNOWLEDGMENTS
We thank Vincenzo Savini (Department of Clinical Microbiology, Ospedale Santo Spirito, Pescara, Italy) for providing the clinical isolate of S. aureus and Giovanni Di Bonaventura (University “G. D'Annunzio,” Chieti, Italy) for his comments and suggestions on the infection experiments. We also thank Donatella Barra for critical reading of the manuscript.
This work was partially supported by grants from Sapienza Università di Roma (to M.L.M.) and the Israel Ministry of Health, grant no. 7291 (to Y.S.).
Footnotes
Published ahead of print 10 February 2014
REFERENCES
- 1.Chen LF, Chopra T, Kaye KS. 2009. Pathogens resistant to antibacterial agents. Infect. Dis. Clin. North Am. 23:817–845. 10.1016/j.idc.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 2.Kaye KS. 2012. Antimicrobial de-escalation strategies in hospitalized patients with pneumonia, intra-abdominal infections, and bacteremia. J. Hosp. Med. 7(Suppl 1):S13–S21. 10.1002/jhm.983 [DOI] [PubMed] [Google Scholar]
- 3.Yeh YC, Yeh KM, Lin TY, Chiu SK, Yang YS, Wang YC, Lin JC. 2012. Impact of vancomycin MIC creep on patients with methicillin-resistant Staphylococcus aureus bacteremia. J. Microbiol. Immunol. Infect. 45:214–220. 10.1016/j.jmii.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 4.Hancock RE, Sahl HG. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557. 10.1038/nbt1267 [DOI] [PubMed] [Google Scholar]
- 5.Nijnik A, Hancock RE. 2009. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr. Opin. Hematol. 16:41–47. 10.1097/MOH.0b013e32831ac517 [DOI] [PubMed] [Google Scholar]
- 6.Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Teran LM. 2010. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin. Immunol. 135:1–11. 10.1016/j.clim.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 7.Alba A, Lopez-Abarrategui C, Otero-Gonzalez AJ. 2012. Host defense peptides: an alternative as antiinfective and immunomodulatory therapeutics. Biopolymers 98:251–267. 10.1002/bip.22076 [DOI] [PubMed] [Google Scholar]
- 8.Yeung AT, Gellatly SL, Hancock RE. 2011. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. 68:2161–2176. 10.1007/s00018-011-0710-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fjell CD, Hiss JA, Hancock RE, Schneider G. 2012. Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov. 11:37–51. 10.1038/nrd3591 [DOI] [PubMed] [Google Scholar]
- 10.Haney EF, Hancock RB. 2013. Peptide design for antimicrobial and immunomodulatory applications. Biopolymers 100:572–583. 10.1002/bip.22250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guralp SA, Murgha YE, Rouillard JM, Gulari E. 2013. From design to screening: a new antimicrobial peptide discovery pipeline. PLoS One 8:e59305. 10.1371/journal.pone.0059305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395. 10.1038/415389a [DOI] [PubMed] [Google Scholar]
- 13.Lai Y, Gallo RL. 2009. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 30:131–141. 10.1016/j.it.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hale JD, Hancock RE. 2007. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev. Anti Infect. Ther. 5:951–959. 10.1586/14787210.5.6.951 [DOI] [PubMed] [Google Scholar]
- 15.Dempsey CE, Hawrani A, Howe RA, Walsh TR. 2010. Amphipathic antimicrobial peptides—from biophysics to therapeutics? Protein Pept. Lett. 17:1334–1344. 10.2174/0929866511009011334 [DOI] [PubMed] [Google Scholar]
- 16.Mangoni ML, Shai Y. 2011. Short native antimicrobial peptides and engineered ultrashort lipopeptides: similarities and differences in cell specificities and modes of action. Cell. Mol. Life Sci. 68:2267–2280. 10.1007/s00018-011-0718-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenfeld Y, Sahl HG, Shai Y. 2008. Parameters involved in antimicrobial and endotoxin detoxification activities of antimicrobial peptides. Biochemistry 47:6468–6478. 10.1021/bi800450f [DOI] [PubMed] [Google Scholar]
- 18.Mangoni ML, Epand RF, Rosenfeld Y, Peleg A, Barra D, Epand RM, Shai Y. 2008. Lipopolysaccharide, a key molecule involved in the synergism between temporins in inhibiting bacterial growth and in endotoxin neutralization. J. Biol. Chem. 283:22907–22917. 10.1074/jbc.M800495200 [DOI] [PubMed] [Google Scholar]
- 19.Easton DM, Nijnik A, Mayer ML, Hancock RE. 2009. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 27:582–590. 10.1016/j.tibtech.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Does AM, Bergman P, Agerberth B, Lindbom L. 2012. Induction of the human cathelicidin LL-37 as a novel treatment against bacterial infections. J. Leukoc. Biol. 92:735–742. 10.1189/jlb.0412178 [DOI] [PubMed] [Google Scholar]
- 21.Zasloff M. 2006. Fighting infections with vitamin D. Nat. Med. 12:388–390. 10.1038/nm0406-388 [DOI] [PubMed] [Google Scholar]
- 22.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. 2006. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology 118:509–519. 10.1111/j.1365-2567.2006.02399.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schauber J, Oda Y, Buchau AS, Yun QC, Steinmeyer A, Zugel U, Bikle DD, Gallo RL. 2008. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J. Invest. Dermatol. 128:816–824. 10.1038/sj.jid.5701102 [DOI] [PubMed] [Google Scholar]
- 24.Dombrowski Y, Peric M, Koglin S, Ruzicka T, Schauber J. 2010. Control of cutaneous antimicrobial peptides by vitamin D3. Arch. Dermatol. Res. 302:401–408. 10.1007/s00403-010-1045-4 [DOI] [PubMed] [Google Scholar]
- 25.Gilliet M, Lande R. 2008. Antimicrobial peptides and self-DNA in autoimmune skin inflammation. Curr. Opin. Immunol. 20:401–407. 10.1016/j.coi.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 26.Sun CL, Zhang FZ, Li P, Bi LQ. 2011. LL-37 expression in the skin in systemic lupus erythematosus. Lupus 20:904–911. 10.1177/0961203311398515 [DOI] [PubMed] [Google Scholar]
- 27.Mangoni ML. 2006. Temporins, anti-infective peptides with expanding properties. Cell. Mol. Life Sci. 63:1060–1069. 10.1007/s00018-005-5536-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conlon JM, Iwamuro S, King JD. 2009. Dermal cytolytic peptides and the system of innate immunity in anurans. Ann. N. Y. Acad. Sci. 1163:75–82. 10.1111/j.1749-6632.2008.03618.x [DOI] [PubMed] [Google Scholar]
- 29.Conlon JM. 2011. The contribution of skin antimicrobial peptides to the system of innate immunity in anurans. Cell Tissue Res. 343:201–212. 10.1007/s00441-010-1014-4 [DOI] [PubMed] [Google Scholar]
- 30.Mangoni ML, Saugar JM, Dellisanti M, Barra D, Simmaco M, Rivas L. 2005. Temporins, small antimicrobial peptides with leishmanicidal activity. J. Biol. Chem. 280:984–990. 10.1074/jbc.M410795200 [DOI] [PubMed] [Google Scholar]
- 31.Mangoni ML, Rinaldi AC, Di Giulio A, Mignogna G, Bozzi A, Barra D, Simmaco M. 2000. Structure-function relationships of temporins, small antimicrobial peptides from amphibian skin. Eur. J. Biochem. 267:1447–1454. 10.1046/j.1432-1327.2000.01143.x [DOI] [PubMed] [Google Scholar]
- 32.Bhunia A, Saravanan R, Mohanram H, Mangoni ML, Bhattacharjya S. 2011. NMR structures and interactions of temporin-1Tl and temporin-1Tb with lipopolysaccharide micelles: mechanistic insights into outer membrane permeabilization and synergistic activity. J. Biol. Chem. 286:24394–24406. 10.1074/jbc.M110.189662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saravanan R, Joshi M, Mohanram H, Bhunia A, Mangoni ML, Bhattacharjya S. 2013. NMR structure of temporin-1 ta in lipopolysaccharide micelles: mechanistic insight into inactivation by outer membrane. PLoS One 8:e72718. 10.1371/journal.pone.0072718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carotenuto A, Malfi S, Saviello MR, Campiglia P, Gomez-Monterrey I, Mangoni ML, Gaddi LM, Novellino E, Grieco P. 2008. A different molecular mechanism underlying antimicrobial and hemolytic actions of temporins A and L. J. Med. Chem. 51:2354–2362. 10.1021/jm701604t [DOI] [PubMed] [Google Scholar]
- 35.Rinaldi AC, Mangoni ML, Rufo A, Luzi C, Barra D, Zhao H, Kinnunen PK, Bozzi A, Di Giulio A, Simmaco M. 2002. Temporin L: antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem. J. 368:91–100. 10.1042/BJ20020806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uccelletti D, Zanni E, Marcellini L, Palleschi C, Barra D, Mangoni ML. 2010. Anti-Pseudomonas activity of frog skin antimicrobial peptides in a Caenorhabditis elegans infection model: a plausible mode of action in vitro and in vivo. Antimicrob. Agents Chemother. 54:3853–3860. 10.1128/AAC.00154-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grieco P, Carotenuto A, Auriemma L, Limatola A, Di Maro S, Merlino F, Mangoni ML, Luca V, Di Grazia A, Gatti S, Campiglia P, Gomez-Monterrey I, Novellino E, Catania A. 2013. Novel alpha-MSH peptide analogues with broad spectrum antimicrobial activity. PLoS One 8:e61614. 10.1371/journal.pone.0061614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Islas-Rodrìguez AE, Marcellini L, Orioni B, Barra D, Stella L, Mangoni ML. 2009. Esculentin 1–21: a linear antimicrobial peptide from frog skin with inhibitory effect on bovine mastitis-causing bacteria. J. Pept. Sci. 15:607–614. 10.1002/psc.1148 [DOI] [PubMed] [Google Scholar]
- 39.von Eiff C, Becker K, Metze D, Lubritz G, Hockmann J, Schwarz T, Peters G. 2001. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease. Clin. Infect. Dis. 32:1643–1647. 10.1086/320519 [DOI] [PubMed] [Google Scholar]
- 40.Boles BR, Horswill AR. 2008. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 4:e1000052. 10.1371/journal.ppat.1000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chieng-Yane P, Bocquet A, Letienne R, Bourbon T, Sablayrolles S, Perez M, Hatem SN, Lompre AM, Le Grand B, David-Dufilho M. 2011. Protease-activated receptor-1 antagonist F 16618 reduces arterial restenosis by down-regulation of tumor necrosis factor alpha and matrix metalloproteinase 7 expression, migration, and proliferation of vascular smooth muscle cells. J. Pharmacol. Exp. Ther. 336:643–651. 10.1124/jpet.110.175182 [DOI] [PubMed] [Google Scholar]
- 42.Wang YW, Ren JH, Xia K, Wang SH, Yin TF, Xie DH, Li LH. 2012. Effect of mitomycin on normal dermal fibroblast and HaCat cell: an in vitro study. J. Zhejiang Univ. Sci. B 13:997–1005. 10.1631/jzus.B1200055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, Yahata Y, Dai X, Tohyama M, Nagai H, Yang L, Higashiyama S, Yoshimura A, Sugai M, Hashimoto K. 2005. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J. Immunol. 175:4662–4668 [DOI] [PubMed] [Google Scholar]
- 44.Hoq MI, Niyonsaba F, Ushio H, Aung G, Okumura K, Ogawa H. 2011. Human catestatin enhances migration and proliferation of normal human epidermal keratinocytes. J. Dermatol. Sci. 64:108–118. 10.1016/j.jdermsci.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 45.Spohn D, Rossler OG, Philipp SE, Raubuch M, Kitajima S, Griesemer D, Hoth M, Thiel G. 2010. Thapsigargin induces expression of activating transcription factor 3 in human keratinocytes involving C2+ ions and c-Jun N-terminal protein kinase. Mol. Pharmacol. 78:865–876. 10.1124/mol.110.067637 [DOI] [PubMed] [Google Scholar]
- 46.Baranska-Rybak W, Pikula M, Dawgul M, Kamysz W, Trzonkowski P, Roszkiewicz J. 2013. Safety profile of antimicrobial peptides: camel, citropin, protegrin, temporin A and lipopeptide on HaCaT keratinocytes. Acta Pol. Pharm. 70:795–801 [PubMed] [Google Scholar]
- 47.Nuzzo I, Sanges MR, Folgore A, Carratelli CR. 2000. Apoptosis of human keratinocytes after bacterial invasion. FEMS Immunol. Med. Microbiol. 27:235–240. 10.1111/j.1574-695X.2000.tb01435.x [DOI] [PubMed] [Google Scholar]
- 48.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, Nagaoka I, Okumura K, Ogawa H. 2007. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Invest. Dermatol. 127:594–604. 10.1038/sj.jid.5700599 [DOI] [PubMed] [Google Scholar]
- 49.Huang LC, Petkova TD, Reins RY, Proske RJ, McDermott AM. 2006. Multifunctional roles of human cathelicidin (LL-37) at the ocular surface. Invest. Ophthalmol. Vis. Sci. 47:2369–2380. 10.1167/iovs.05-1649 [DOI] [PubMed] [Google Scholar]
- 50.Shaykhiev R, Beisswenger C, Kandler K, Senske J, Puchner A, Damm T, Behr J, Bals R. 2005. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L842–L848. 10.1152/ajplung.00286.2004 [DOI] [PubMed] [Google Scholar]
- 51.Jost M, Kari C, Rodeck U. 2000. The EGF receptor—an essential regulator of multiple epidermal functions. Eur. J. Dermatol. 10:505–510 [PubMed] [Google Scholar]
- 52.de Giorgi V, Sestini S, Massi D, Ghersetich I, Lotti T. 2007. Keratinocyte growth factor receptors. Dermatol. Clin. 25:477–485. 10.1016/j.det.2007.06.017 [DOI] [PubMed] [Google Scholar]
- 53.Pastore S, Mascia F, Mariani V, Girolomoni G. 2008. The epidermal growth factor receptor system in skin repair and inflammation. J. Invest. Dermatol. 128:1365–1374. 10.1038/sj.jid.5701184 [DOI] [PubMed] [Google Scholar]
- 54.Edwards AM, Potter U, Meenan NA, Potts JR, Massey RC. 2011. Staphylococcus aureus keratinocyte invasion is dependent upon multiple high-affinity fibronectin-binding repeats within FnBPA. PLoS One 6:e18899. 10.1371/journal.pone.0018899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brook I. 2002. Secondary bacterial infections complicating skin lesions. J. Med. Microbiol. 51:808–812 [DOI] [PubMed] [Google Scholar]
- 56.Krishna S, Miller LS. 2012. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin. Immunopathol. 34:261–280. 10.1007/s00281-011-0292-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mempel M, Schnopp C, Hojka M, Fesq H, Weidinger S, Schaller M, Korting HC, Ring J, Abeck D. 2002. Invasion of human keratinocytes by Staphylococcus aureus and intracellular bacterial persistence represent haemolysin-independent virulence mechanisms that are followed by features of necrotic and apoptotic keratinocyte cell death. Br. J. Dermatol. 146:943–951. 10.1046/j.1365-2133.2002.04752.x [DOI] [PubMed] [Google Scholar]
- 58.Wesson CA, Liou LE, Todd KM, Bohach GA, Trumble WR, Bayles KW. 1998. Staphylococcus aureus Agr and Sar global regulators influence internalization and induction of apoptosis. Infect. Immun. 66:5238–5243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brinch KS, Sandberg A, Baudoux P, Van Bambeke F, Tulkens PM, Frimodt-Moller N, Hoiby N, Kristensen HH. 2009. Plectasin shows intracellular activity against Staphylococcus aureus in human THP-1 monocytes and in a mouse peritonitis model. Antimicrob. Agents Chemother. 53:4801–4808. 10.1128/AAC.00685-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Bambeke F, Carryn S, Seral C, Chanteux H, Tyteca D, Mingeot-Leclercq MP, Tulkens PM. 2004. Cellular pharmacokinetics and pharmacodynamics of the glycopeptide antibiotic oritavancin (LY333328) in a model of J774 mouse macrophages. Antimicrob. Agents Chemother. 48:2853–2860. 10.1128/AAC.48.8.2853-2860.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob. Agents Chemother. 50:841–851. 10.1128/AAC.50.3.841-851.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Bambeke F, Barcia-Macay M, Lemaire S, Tulkens PM. 2006. Cellular pharmacodynamics and pharmacokinetics of antibiotics: current views and perspectives. Curr. Opin. Drug Discov. Devel. 9:218–230 [PubMed] [Google Scholar]
- 63.Appelbaum PC. 2006. MRSA—the tip of the iceberg. Clin. Microbiol. Infect. 12(Suppl 2):3–10. 10.1111/j.1469-0691.2006.01343.x [DOI] [PubMed] [Google Scholar]
- 64.Appelbaum PC. 2006. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 12(Suppl 1):16–23. 10.1111/j.1469-0691.2006.01344.x [DOI] [PubMed] [Google Scholar]
- 65.Chen Q, Wade D, Kurosaka K, Wang ZY, Oppenheim JJ, Yang D. 2004. Temporin A and related frog antimicrobial peptides use formyl peptide receptor-like 1 as a receptor to chemoattract phagocytes. J. Immunol. 173:2652–2659 [DOI] [PubMed] [Google Scholar]