Abstract
Leishmaniasis chemotherapy remains very challenging. The high cost of active drugs, along with the severity of their side effects and the increasing failure rate of the current therapeutic schemes, calls for the discovery of new active drugs and schemes of treatment. The use of combination therapy has gained much attention in recent years as a possible strategy for overcoming the various shortcomings in the present arsenal. We recently described the effectiveness of tamoxifen in murine models of leishmaniasis, and here, we investigated the interactions between tamoxifen and amphotericin B, one of the most potent drugs used in leishmaniasis treatment. The in vitro interactions were indifferent for the association of tamoxifen and amphotericin B. The association was also assayed in vivo in Leishmania amazonensis-infected BALB/c mice and was found to yield at least additive effects at low doses of both drugs.
INTRODUCTION
Leishmaniasis affects 12 million people worldwide in 98 countries. Clinical presentations are highly variable, ranging from localized cutaneous lesions to severe diffuse tegumentary forms or to the life-threatening visceral form. The treatment of both cutaneous and visceral leishmaniasis depends heavily on expensive and toxic drugs, most of which have to be administered by the parenteral route. The first-line treatment is still based on pentavalent antimonials in many areas, but parasite resistance is now widespread in some regions of the Indian subcontinent. Evidence of decreased efficacy of antimonials in other regions of the world (1–3) and of geographical variations in parasite susceptibility to drugs is also emerging (4). In regions with a high incidence of antimonial resistance, amphotericin B and miltefosine became first-choice drugs, but there are also difficulties with these drugs, related to toxicity or to the possibility of selecting resistant strains (5).
Difficulties in treatment of the various clinical forms of leishmaniasis and the spread of resistance to drugs, mainly observed in areas where anthroponotic transmission is predominant, led to the present trend of utilizing combination therapy, as has been the case for many other infectious diseases. In leishmaniasis, this trend is justified because the available drugs belong to different chemical classes and are believed to have different targets on the parasite and combined therapy has the potential to reduce collateral effects and therefore increase treatment compliance. Furthermore, combination therapy may improve the efficacy of therapeutic schemes in patients with concomitant infections, as is the case in HIV-infected patients, who generally do not respond well to classical therapy.
Several combinations have been tried or are under trial in India and East Africa. For example, a shorter therapeutic scheme of combined sodium stibogluconate (SSG) and paromomycin has been tried in Africa and shown to be as effective as a longer scheme of SSG alone (6). A trial is under way in East Africa to determine the efficacy of miltefosine in combination with SSG and liposomal amphotericin B (7). However, there is concern about the emergence of resistance even for combination therapy (8), since it has been shown that stepwise in vitro selection is an efficient method to obtain resistant lines of Leishmania donovani against all drugs in use today, including amphotericin B (9). Amphotericin B resistance has also been detected in clinical isolates (10).
Therefore, the search for new alternative drugs useful for treating leishmaniasis is still necessary. We have previously shown that the selective estrogen receptor modulator (SERM) tamoxifen is effective in the treatment of cutaneous and visceral leishmaniasis in animal models (11, 12). Here we investigated the interactions of tamoxifen and amphotericin B, one of the drugs most widely used for the treatment of leishmaniasis.
MATERIALS AND METHODS
Parasites and macrophages.
Leishmania amazonensis (MHOM/BR/1973/M2269) promastigotes were grown in M-199 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 25 mM HEPES (pH 6.9), 12 mM NaHCO3, 7.6 mM hemin, 50 U/ml penicillin, and 50 μg/ml streptomycin at 25°C. Promastigotes of a L. amazonensis transgenic line expressing luciferase (La-LUC) (13) were grown in the same medium supplemented with 32 μg/ml G418 and incubated at 25°C. Bone marrow-derived macrophages (BMDM) were obtained from BALB/c mice as described previously (14).
Drugs.
Amphotericin B deoxycholate was a gift from Cristália (Sao Paulo, Brazil). Tamoxifen and tamoxifen citrate were purchased from Sigma-Aldrich. Stock solutions of amphotericin B (10 mM) were prepared in sterile water and kept at −20°C. Tamoxifen stock solutions (10 mM) were prepared in ethanol. Dilutions from the stock solutions were done in culture media. For in vivo experiments, stock solutions of tamoxifen citrate and amphotericin B were prepared in saline every day.
Evaluation of in vitro antileishmanial activity.
Promastigotes were counted in a Neubauer hemocytometer and seeded at 2 × 106/well in a final volume of 200 μl. The plates were incubated for 24 h at 25°C, and the viability of promastigotes was verified by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (15). Data analysis and 50% effective concentration (EC50) determinations were performed with Graph-Pad Prism 5.0 software.
For assays with intracellular amastigotes, macrophages were seeded in 96-well plates (8 × 104 macrophages per well) containing RPMI 1640 medium with 10% FCS and allowed to adhere overnight at 37°C and 5% CO2. Infections were initiated by adding La-LUC stationary-phase promastigotes at a ratio of 20 promastigotes per macrophage. Infected macrophage cultures were kept at 33°C and 5% CO2 for 3 h in RPMI 1640 medium with 10% FCS and then were washed twice with sterile phosphate-buffered saline (PBS) to remove free promastigotes. Infected BMDM were treated for 48 h. Enzyme detection was performed with the One-Glo luciferase assay system (Promega Corporation) according to the manufacturer's instructions. Briefly, medium was removed without disturbing the adherent cells and replaced by 100 μl of PBS. To each well, 20 μl of One-Glo reagent was added at room temperature followed by homogenization by pipetting the mixture up and down six times. Luminescence units were determined immediately after adding the substrate in a POLARstar Omega reader (BMG Labtech, Ortenberg, Germany). The luminescence reading from treated wells was used to calculate sigmoidal regression curves using untreated infected macrophages as a control.
Determination of drug interactions.
The interactions between drugs were evaluated in vitro by a modified isobologram method (16, 17). EC50s were used to determine the maximum concentrations of individual drugs, ensuring that the EC50 was at the midpoint of the serial dilution. The highest concentrations of solutions were prepared in proportions of 5:0, 4:1, 3:2, 2:3, 1:4, and 0:5 of tamoxifen and amphotericin B, respectively, which were serially diluted (base 2) to the seventh well of the microplate in triplicate. Sigmoidal regression curves were used to determine the EC50 as described above. For each ratio, an EC50 was calculated for each of the drugs. Two or three independent experiments were performed for each drug combination and susceptibility assay.
Determination of FIC index and isobologram construction.
Fractional inhibitory concentrations (FICs) at the EC50 were calculated as [EC50 when in combination]/[EC50 of drug alone]. The sum of the FICs (ΣFIC) was calculated with the equation ΣFIC = FIC drug A + FIC drug B (17). The mean sum of the FICs (xΣFIC) was calculated as the average of the ΣFICs. Isobolograms were built by plotting the FIC for each drug ratio. The ΣFIC was used to classify the interaction as recommended by Odds (18). Interactions were considered synergic for an xΣFIC of ≤0.5, indifferent with an xΣFIC between 0.5 and ≤4, and antagonistic for an xΣFIC of >4.
Evaluation of drug interactions in vivo.
Animal experiments were approved by the Ethical Committee on Animal Experimentation (protocol 178/2012). Female BALB/c mice (4 to 5 weeks old) were inoculated with 1 × 106 stationary-phase La-LUC promastigotes at the base of the tail. Five weeks after infection, mice were randomly assigned to experimental groups (n = 6). Doses used in vivo were 6.5 or 26 mg/kg of body weight/day tamoxifen and 1.2 or 4 mg/kg/day amphotericin B. Combinations were tested using a low-dose scheme (1.2 mg/kg/day amphotericin B plus 6.5 mg/kg/day tamoxifen) and a high-dose scheme (4 mg/kg/day amphotericin B plus 26 mg/kg/day tamoxifen). Treated groups received intraperitoneal injections containing the drugs in a 200-μl final volume. All animals received 20 doses of the assigned scheme, given on weekdays.
Disease progression was evaluated once a week by recording the average diameter of the tail measured as the mean of tail base diameters in horizontal and vertical directions. Measurements were taken with a caliper (Mitutoyo Corp., Japan). The treatment outcome was evaluated at the end of the treatment (9 weeks postinfection) through luciferase detection by bioimaging (IVIS Spectrum; Caliper Life Sciences, Inc., MA) as described previously (13). Briefly, prior to imaging, mice received 75 mg/kg luciferin (VivoGlo Luciferin; Promega) intraperitoneally. Imaging was performed 20 min later, through the high-resolution mode from a fixed-size region of interest. Results were quantified with Living Image software, version 4.3.1 (Caliper Life Sciences, Inc.), and results are expressed as the number of photons/s/cm2.
Statistical analysis.
Data were analyzed for statistical significance by one-way analysis of variance (ANOVA), followed by the Fisher posttest. Statistical analyses were performed using GraphPad Prism 5 software.
RESULTS
In vitro associations of amphotericin B and tamoxifen.
Wild-type or luciferase-expressing parasites were used to determine the EC50s against L. amazonensis promastigotes and intracellular amastigotes, respectively. The transgenic parasites express luciferase constitutively and show the same infectivity in vitro and in vivo as well as susceptibility to drugs as the parental line (13). Tamoxifen EC50s against promastigotes and amastigotes were 13.3 and 4.5 μM, respectively. Amphotericin B EC50s were determined to be 118.5 and 63.5 nM for promastigotes and amastigotes, respectively (Fig. 1 and data not shown).
FIG 1.
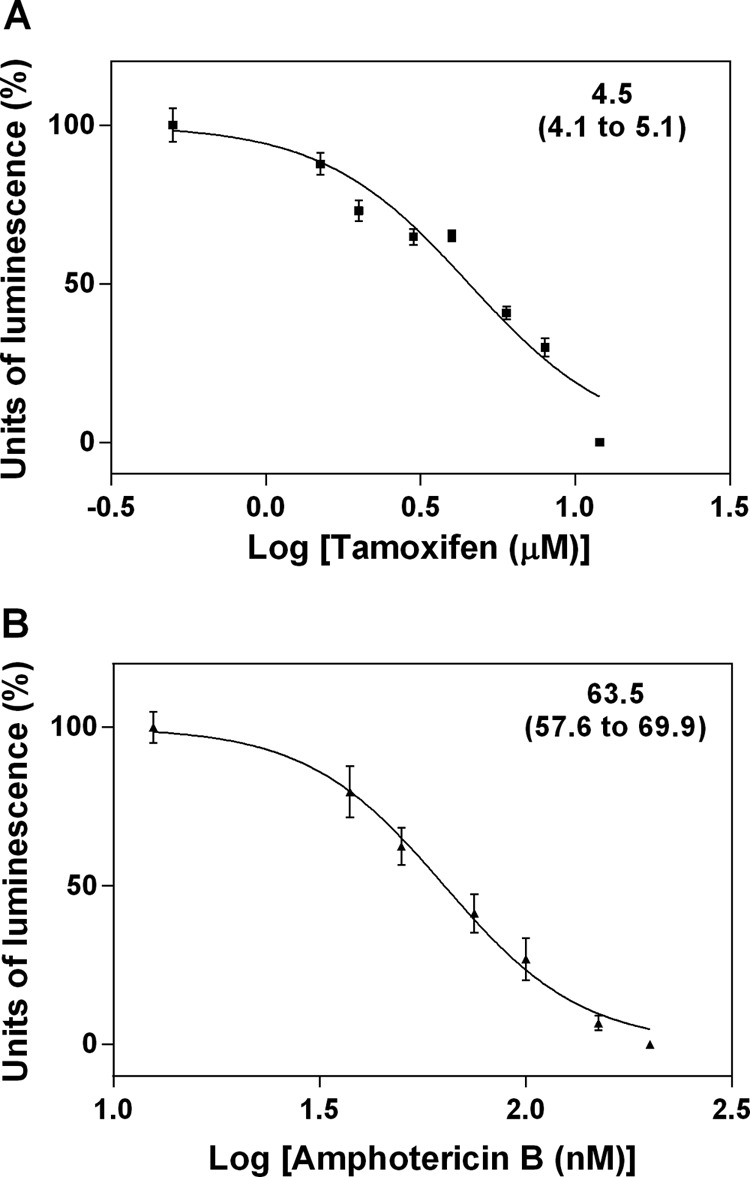
EC50 for L. amazonensis intracellular amastigotes. Infected BMDM were treated with tamoxifen (A) or amphotericin B (B) for 48 h at 33°C. The numbers of amastigotes were determined by luciferase quantification. Relative light units for control untreated cells were considered 100%. Values shown are the means and standard deviations of triplicates from one experiment representative of at least three independent experiments. The EC50s and 95% confidence intervals are indicated in the upper right corners.
The associations between tamoxifen and amphotericin B against L. amazonensis promastigotes and intracellular amastigotes were evaluated using the fixed-ratio isobologram method (16, 17). This experimental setup allowed the determination of FIC values for each combination (Table 1). The xΣFICs for all combinations were calculated as 1.23 when tested against promastigotes and 0.74 against intracellular amastigotes. According to Odds (18), this indicates an indifferent interaction. This observation was confirmed by plotting the individual FICs in isobolograms (Fig. 2), where all points are close to the additivity line.
TABLE 1.
EC50s and FICs of tamoxifen-amphotericin B combinations against L. amazonensis
| Form | Combination ratio (%)a |
EC50 (95% CI) ofb: |
FIC ofc: |
ΣFICd | |||
|---|---|---|---|---|---|---|---|
| Tam | AmB | Tam (μM) | AmB (nM) | Tam | AmB | ||
| Promastigotese | 100 | 0 | 13.3 (12.6–14.1) | ||||
| 80 | 20 | 13.2 (12.5–13.9) | 23.6 (22.5–24.7) | 0.99 | 0.20 | 1.19 | |
| 60 | 40 | 11.2 (10.4–12.1) | 53.0 (50.0–56.2) | 0.84 | 0.45 | 1.29 | |
| 40 | 60 | 7.7 (6.8–8.7) | 82.7 (75.1–91.0) | 0.58 | 0.70 | 1.28 | |
| 20 | 80 | 3.7 (3.1–4.3) | 105.9 (91.6–122.5) | 0.27 | 0.90 | 1.17 | |
| 0 | 100 | 118.5 (111.3–126.2) | |||||
| Intracellular amastigotesf | 100 | 0 | 4.5 (4.0–5.1) | ||||
| 80 | 20 | 2.6 (2.1–3.3) | 2.2 (1.7–2.8) | 0.57 | 0.04 | 0.61 | |
| 60 | 40 | 1.7 (1.4–1.9) | 11.7 (10.0–13.8) | 0.37 | 0.20 | 0.57 | |
| 40 | 60 | 0.8 (0.7–1.0) | 36.7 (29.6–45.6) | 0.18 | 0.63 | 0.81 | |
| 20 | 80 | 0.1 (0.1–0.2) | 55.3 (49.6–61.8) | 0.03 | 0.95 | 0.98 | |
| 0 | 100 | 63.5 (57.6–70.0) | |||||
Tam, tamoxifen; AmB, amphotericin B.
CI, confidence interval.
FIC, fractional inhibitory concentration at the indicated EC50.
ΣFIC, sum of FICs.
In vitro activities of tamoxifen and amphotericin B against promastigotes was determined by the MTT assay.
Activity against intracellular amastigotes was determined in infected macrophages by luminescence.
FIG 2.
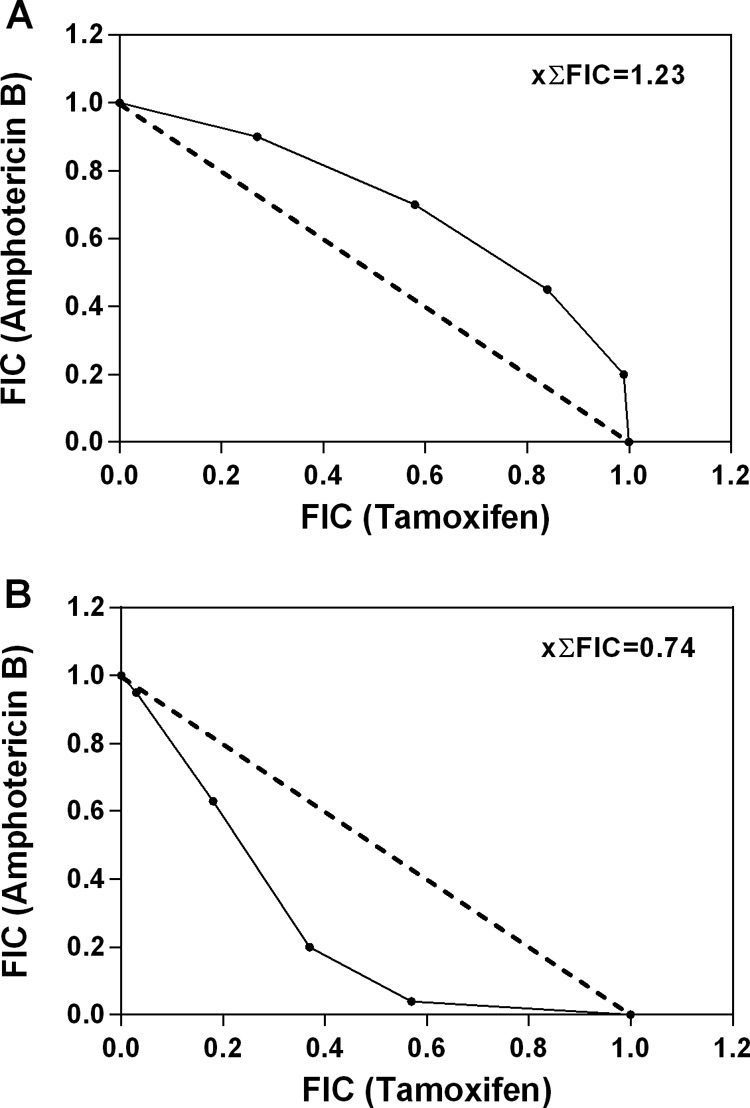
Isobolograms representing in vitro interactions between tamoxifen and amphotericin B against L. amazonensis promastigotes (A) and intracellular amastigotes (B). Assays were performed by a fixed-ratio method based on the EC50s, with the combinations being tested at constant ratios of 5:0, 4:1, 3:2, 2:3, 1:4, and 0:5. Results shown are from one experiment performed in triplicate, representative of at least two independent experiments. Plots were compared to a theoretical line that produced a sum of the FICs of 1 at all ratios tested (dashed line), which represents an additive effect of both compounds. The mean sum of the FICs (xΣFIC) for all interactions tested is shown at the upper right corner.
Tamoxifen in combination with amphotericin B in vivo.
The L. amazonensis-infected BALB/c mouse was the experimental model employed for in vivo efficacy studies. The half-maximal effective dose (ED50) was initially determined for each of the drugs given alone. Tamoxifen and amphotericin B ED50s were determined to be 13.2 mg/kg/day (95% confidence interval, 12.2 to 15.2 mg/kg/day) and 2.2 mg/kg/day (95% confidence interval, 1.1 to 4.4 mg/kg/day), respectively (reference 13 and data not shown).
The therapeutic efficacy of the association was evaluated by comparing animals treated with doses close to the maximal tolerated doses of tamoxifen and amphotericin B in this model (26 mg/kg/day and 4 mg/kg/day, respectively) with those treated with the association scheme employing half of the calculated ED50 for each drug (6.5 mg/kg/day tamoxifen and 1.2 mg/kg/day amphotericin B).
Representative animals from each group are shown in Fig. 3, together with the parasite burden determination through luciferase quantification. The mean sizes of the lesions in groups treated with the combination schemes were reduced compared with the sizes of lesions in animals treated with each of the drugs alone (Fig. 4A). As for the parasite burden evaluated through luciferase quantification, a significant reduction was observed in the group treated with the combined low-dose scheme compared with that in the amphotericin B alone or untreated group (Fig. 4B). The data clearly show that the association of 1.2 mg/kg/day amphotericin B and 6.5 mg/kg/day tamoxifen produced an improved outcome compared with that for tamoxifen or amphotericin B given alone at the same doses.
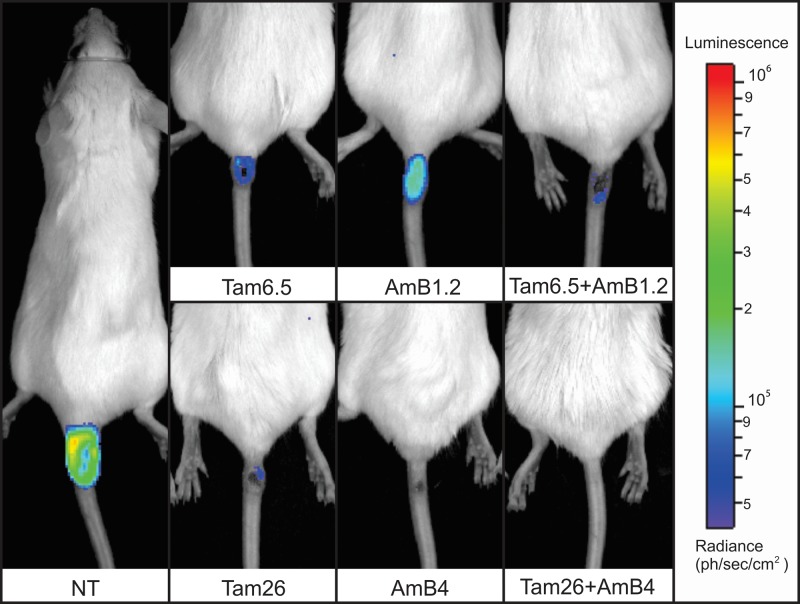
FIG 3.
In vivo evaluation of parasite burden after combined therapy with tamoxifen and amphotericin B. Treatment was initiated 5 weeks postinfection. Images were collected after the end of treatment at 9 weeks postinfection. Images are of representative animals treated with tamoxifen and amphotericin B (alone or in association). The bar on the right shows a pseudo-color scale representing light intensities. NT, untreated; Tam6.5, Tam26, treated with 6.5 or 26 mg/kg/day tamoxifen alone; AmB1.2, AmB4, treated with 1.2 or 4 mg/kg/day amphotericin B alone; Tam+AmB, treated with combinations of the two drugs in the doses indicated.
FIG 4.
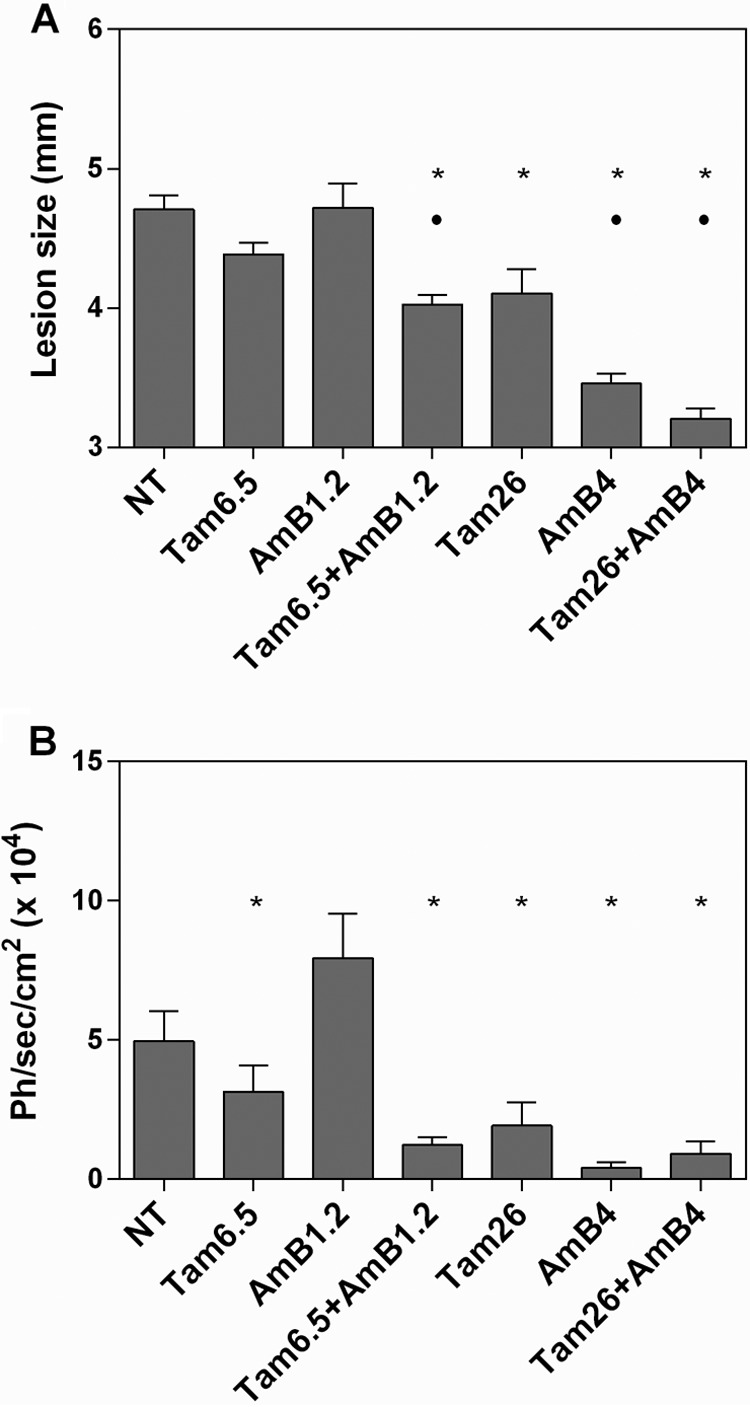
Combined therapy with tamoxifen and amphotericin B. Evaluation of lesion size (A) and parasite burden (B) in mice (n = 6) treated with tamoxifen (6.5 or 26 mg/kg/day) and/or amphotericin B (1.2 or 4 mg/kg/day) at the end of treatment. NT, untreated; Tam6.5, Tam26, treated with 6.5 or 26 mg/kg/day tamoxifen alone; AmB1.2, AmB4, treated with 1.2 or 4 mg/kg/day amphotericin B alone; Tam+AmB, treated with combinations of the two drugs in the doses indicated; Ph/sec/cm2, photons per second per square centimeter. *, P < 0.05 versus the untreated group and versus amphotericin B 1.2 mg/kg/day; •, P < 0.05 versus tamoxifen 6.5 mg/kg/day.
For groups treated with amphotericin B and tamoxifen at the highest dose, combined therapy produced better clinical outcomes than those for the untreated or treated with each drug alone groups. The parasite burden for the combination treatment at high doses was not significantly different from that for the groups treated with single drugs at the same doses (Fig. 4).
DISCUSSION
Combination therapy has been advocated for the treatment of several infectious diseases such as malaria, tuberculosis, AIDS, and, more recently, leishmaniasis. The combination approach may increase effectiveness, especially in immunocompromised patients (19), and may reduce the length of treatment and administered doses. Consequently, the undesirable effects and costs are decreased. Combination therapy is also important in delaying the selection of resistant parasites (20).
Tamoxifen is a SERM that has been in use as a therapeutic and prophylactic agent in breast cancer therapy for decades. Tamoxifen has also been studied as an alternative treatment in other conditions such as retroperitoneal fibrosis (21) and bipolar disease (22, 23). It is a low-cost orally administered drug with a good safety profile, especially in short-term therapeutic schemes, such as the ones proposed in the treatment of bipolar disorder. We previously described the effectiveness of tamoxifen given for 15 to 20 days in the treatment of established cutaneous and visceral leishmaniasis in animal models (11, 12). The work described herein was performed to investigate whether tamoxifen would be a good partner in combination therapy. We chose to test tamoxifen in combination with amphotericin B, one of the most widely used drugs in leishmaniasis treatment nowadays. Amphotericin B is effective, especially for visceral leishmaniasis, but toxicity is considerable (24). The selection of amphotericin B-resistant strains was considered unlikely until recently when some reports suggested that it was possible (9, 10).
In vitro interactions between tamoxifen and amphotericin B, studied through fixed-ratio dilutions and isobologram analysis, were shown to be indifferent or additive. Parameters for the analysis and classification of drug interaction profiles in vivo are less clear-cut than those for in vitro studies. In vivo synergism has been suggested to occur when the effect of the drug combination is significantly more pronounced than the sum of the effects of each agent alone (25). In an attempt to quantify in vivo the effects of combining tamoxifen and amphotericin B, we calculated the percentages of reduction in lesion size and parasite burden for each treated group compared with those for the untreated controls. While the lesion size was reduced by 25% in the 6.5 mg/kg/day tamoxifen group and by 0% in the 1.2 mg/kg/day amphotericin B group, there was a 55% reduction in the group assigned to low-dose combined therapy. When parasite burdens were considered, there was a 36% reduction in the 6.5 mg/kg/day tamoxifen group and an increase in the 1.2 mg/kg/day amphotericin B group, while a 75% reduction was noted in the group treated with the low-dose combined scheme. Therefore, in both cases, the effect of combined therapy was superior to the sum of effects of the individual drugs, suggesting that tamoxifen and amphotericin B have additive and possibly synergistic behavior in vivo.
A potential lack of correlation between in vitro (additive) and in vivo (additive/synergistic) interaction profiles is not unexpected since several factors come into play in vivo that cannot have an effect in vitro, such as pharmacokinetics, metabolism, distribution, and a possible role of the immune system in controlling the infection.
In mice, the elimination half-life of micellar amphotericin B is 89 min (26), while the rate of elimination from serum following a single dose of tamoxifen in mice is 11.9 h (27). Tamoxifen is rapidly metabolized into N-desmethyltamoxifen and 4-hydroxytamoxifen, both of which are active and have plasma half-lives of 9.6 and 6.0 h, respectively. The different rates of elimination may provide an extended effect for the combination scheme.
Another factor that needs to be taken into account is the mechanism of action. It is generally accepted that amphotericin B interacts with membrane sterols and with higher affinity with ergosterol, which is abundant in parasite membranes (28). That results in membrane disorganization, possibly through pore formation (28, 29). Amphotericin B also leads to lipid peroxidation and generation of free radicals (30–32).
Tamoxifen's mechanism of action against Leishmania is still unclear, but in mammalian cells this highly lipophilic molecule interacts with the membrane lipids (33). Therefore, altered membrane physiology might extend the lesions caused by amphotericin B. On the other hand, tamoxifen has been shown to prevent lipid peroxidation (34) and to behave as an antioxidant (35, 36), which might reduce the adverse effects of amphotericin B.
In conclusion, the data presented herein indicate clearly that tamoxifen does not hinder amphotericin B activity and that lower doses of the two drugs combined result in good clinical and parasitological responses.
ACKNOWLEDGMENTS
We thank the CEFAP-ICB for making the bioimaging equipment available.
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grant 2011/20484-7), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant 473343/2012-6), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). C.T.T. is supported by the FAPESP (fellowship 2011/18858-6).
Footnotes
Published ahead of print 18 February 2014
REFERENCES
- 1.Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, Miranda-Verastegui C, Lazo M, Loayza-Muro R, De Doncker S, Maurer A, Chappuis F, Dujardin JC, Llanos-Cuentas A. 2007. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J. Infect. Dis. 195:1846–1851. 10.1086/518041 [DOI] [PubMed] [Google Scholar]
- 2.Chrusciak-Talhari A, Dietze R, Chrusciak Talhari C, Da Silva RM, Gadelha Yamashita EP, de Oliveira Penna G, Lima Machado PR, Talhari S. 2011. Randomized controlled clinical trial to access efficacy and safety of miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania (Viannia) guyanensis in Manaus, Brazil. Am. J. Trop. Med. Hyg. 84:255–260. 10.4269/ajtmh.2011.10-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neves LO, Talhari AC, Gadelha EP, Silva Junior RM, Guerra JA, Ferreira LC, Talhari S. 2011. A randomized clinical trial comparing meglumine antimoniate, pentamidine and amphotericin B for the treatment of cutaneous leishmaniasis by Leishmania guyanensis. An. Bras. Dermatol. 86:1092–1101. 10.1590/S0365-05962011000600005 [DOI] [PubMed] [Google Scholar]
- 4.Hailu A, Musa A, Wasunna M, Balasegaram M, Yifru S, Mengistu G, Hurissa Z, Hailu W, Weldegebreal T, Tesfaye S, Makonnen E, Khalil E, Ahmed O, Fadlalla A, El-Hassan A, Raheem M, Mueller M, Koummuki Y, Rashid J, Mbui J, Mucee G, Njoroge S, Manduku V, Musibi A, Mutuma G, Kirui F, Lodenyo H, Mutea D, Kirigi G, Edwards T, Smith P, Muthami L, Royce C, Ellis S, Alobo M, Omollo R, Kesusu J, Owiti R, Kinuthia J. 2010. Geographical variation in the response of visceral leishmaniasis to paromomycin in East Africa: a multicentre, open-label, randomized trial. PLoS Negl. Trop. Dis. 4:e709. 10.1371/journal.pntd.0000709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhandari V, Kulshrestha A, Deep DK, Stark O, Prajapati VK, Ramesh V, Sundar S, Schonian G, Dujardin JC, Salotra P. 2012. Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis. PLoS Negl. Trop. Dis. 6:e1657. 10.1371/journal.pntd.0001657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musa A, Khalil E, Hailu A, Olobo J, Balasegaram M, Omollo R, Edwards T, Rashid J, Mbui J, Musa B, Abuzaid AA, Ahmed O, Fadlalla A, El-Hassan A, Mueller M, Mucee G, Njoroge S, Manduku V, Mutuma G, Apadet L, Lodenyo H, Mutea D, Kirigi G, Yifru S, Mengistu G, Hurissa Z, Hailu W, Weldegebreal T, Tafes H, Mekonnen Y, Makonnen E, Ndegwa S, Sagaki P, Kimutai R, Kesusu J, Owiti R, Ellis S, Wasunna M. 2012. Sodium stibogluconate (SSG) and paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: a randomised controlled trial. PLoS Negl. Trop. Dis. 6:e1674. 10.1371/journal.pntd.0001674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omollo R, Alexander N, Edwards T, Khalil EA, Younis BM, Abuzaid AA, Wasunna M, Njoroge N, Kinoti D, Kirigi G, Dorlo TP, Ellis S, Balasegaram M, Musa AM. 2011. Safety and efficacy of miltefosine alone and in combination with sodium stibogluconate and liposomal amphotericin B for the treatment of primary visceral leishmaniasis in East Africa: study protocol for a randomized controlled trial. Trials 12:166. 10.1186/1745-6215-12-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Hernández R, Manzano JI, Castanys S, Gamarro F. 2012. Leishmania donovani develops resistance to drug combinations. PLoS Negl. Trop. Dis. 6:e1974. 10.1371/journal.pntd.0001974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mbongo N, Loiseau PM, Billion MA, Robert-Gero M. 1998. Mechanism of amphotericin B resistance in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 42:352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purkait B, Kumar A, Nandi N, Sardar AH, Das S, Kumar S, Pandey K, Ravidas V, Kumar M, De T, Singh D, Das P. 2012. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob. Agents Chemother. 56:1031–1041. 10.1128/AAC.00030-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miguel DC, Yokoyama-Yasunaka JK, Uliana SR. 2008. Tamoxifen is effective in the treatment of Leishmania amazonensis infections in mice. PLoS Negl. Trop. Dis. 2:e249. 10.1371/journal.pntd.0000249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miguel DC, Zauli-Nascimento RC, Yokoyama-Yasunaka JK, Katz S, Barbieri CL, Uliana SR. 2009. Tamoxifen as a potential antileishmanial agent: efficacy in the treatment of Leishmania braziliensis and Leishmania chagasi infections. J. Antimicrob. Chemother. 63:365–368. 10.1093/jac/dkn509 [DOI] [PubMed] [Google Scholar]
- 13.Reimão JQ, Trinconi CT, Yokoyama-Yasunaka JK, Miguel DC, Kalil SP, Uliana SR. 2013. Parasite burden in Leishmania (Leishmania) amazonensis-infected mice: validation of luciferase as a quantitative tool. J. Microbiol. Methods 93:95–101. 10.1016/j.mimet.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 14.Zamboni DS, Rabinovitch M. 2003. Nitric oxide partially controls Coxiella burnetii phase II infection in mouse primary macrophages. Infect. Immun. 71:1225–1233. 10.1128/IAI.71.3.1225-1233.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zauli-Nascimento RC, Miguel DC, Yokoyama-Yasunaka JK, Pereira LI, Pelli de Oliveira MA, Ribeiro-Dias F, Dorta ML, Uliana SR. 2010. In vitro sensitivity of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis Brazilian isolates to meglumine antimoniate and amphotericin B. Trop. Med. Int. Health 15:68–76. 10.1111/j.1365-3156.2009.02414.x [DOI] [PubMed] [Google Scholar]
- 16.Fivelman QL, Adagu IS, Warhurst DC. 2004. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 48:4097–4102. 10.1128/AAC.48.11.4097-4102.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seifert K, Croft SL. 2006. In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob. Agents Chemother. 50:73–79. 10.1128/AAC.50.1.73-79.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. 10.1093/jac/dkg301 [DOI] [PubMed] [Google Scholar]
- 19.Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet JP, Gradoni L, Ter Horst R, Lopez-Velez R, Moreno J. 2008. The relationship between leishmaniasis and AIDS: the second 10 years. Clin. Microbiol. Rev. 21:334–359. 10.1128/CMR.00061-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olliaro PL. 2010. Drug combinations for visceral leishmaniasis. Curr. Opin. Infect. Dis. 23:595–602. 10.1097/QCO.0b013e32833fca9d [DOI] [PubMed] [Google Scholar]
- 21.van Bommel EF, Pelkmans LG, van Damme H, Hendriksz TR. 2013. Long-term safety and efficacy of a tamoxifen-based treatment strategy for idiopathic retroperitoneal fibrosis. Eur. J. Intern. Med. 24:444–450. 10.1016/j.ejim.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 22.Yildiz A, Vieta E, Leucht S, Baldessarini RJ. 2011. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology 36:375–389. 10.1038/npp.2010.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yildiz A, Guleryuz S, Ankerst DP, Ongur D, Renshaw PF. 2008. Protein kinase C inhibition in the treatment of mania: a double-blind, placebo-controlled trial of tamoxifen. Arch. Gen. Psychiatry 65:255–263. 10.1001/archgenpsychiatry.2007.43 [DOI] [PubMed] [Google Scholar]
- 24.Alvar J, Croft S, Olliaro P. 2006. Chemotherapy in the treatment and control of leishmaniasis. Adv. Parasitol. 61:223–274. 10.1016/S0065-308X(05)61006-8 [DOI] [PubMed] [Google Scholar]
- 25.Fantin B, Carbon C. 1992. In vivo antibiotic synergism: contribution of animal models. Antimicrob. Agents Chemother. 36:907–912. 10.1128/AAC.36.5.907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falk R, Grunwald J, Hoffman A, Domb AJ, Polacheck I. 2004. Distribution of amphotericin B-arabinogalactan conjugate in mouse tissue and its therapeutic efficacy against murine aspergillosis. Antimicrob. Agents Chemother. 48:3606–3609. 10.1128/AAC.48.9.3606-3609.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson SP, Langan-Fahey SM, Johnson DA, Jordan VC. 1991. Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab. Dispos. 19:36–43 [PubMed] [Google Scholar]
- 28.Saha AK, Mukherjee T, Bhaduri A. 1986. Mechanism of action of amphotericin B on Leishmania donovani promastigotes. Mol. Biochem. Parasitol. 19:195–200. 10.1016/0166-6851(86)90001-0 [DOI] [PubMed] [Google Scholar]
- 29.Ramos H, Valdivieso E, Gamargo M, Dagger F, Cohen BE. 1996. Amphotericin B kills unicellular leishmanias by forming aqueous pores permeable to small cations and anions. J. Membr. Biol. 152:65–75. 10.1007/s002329900086 [DOI] [PubMed] [Google Scholar]
- 30.Brajtburg J, Powderly WG, Kobayashi GS, Medoff G. 1990. Amphotericin B: current understanding of mechanisms of action. Antimicrob. Agents Chemother. 34:183–188. 10.1128/AAC.34.2.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokol-Anderson ML, Brajtburg J, Medoff G. 1986. Amphotericin B-induced oxidative damage and killing of Candida albicans. J. Infect. Dis. 154:76–83. 10.1093/infdis/154.1.76 [DOI] [PubMed] [Google Scholar]
- 32.Lamy-Freund MT, Ferreira VF, Schreier S. 1985. Mechanism of inactivation of the polyene antibiotic amphotericin B. Evidence for radical formation in the process of autooxidation. J. Antibiot. (Tokyo) 38:753–757 [DOI] [PubMed] [Google Scholar]
- 33.Custódio JB, Almeida LM, Madeira VM. 1993. The active metabolite hydroxytamoxifen of the anticancer drug tamoxifen induces structural changes in membranes. Biochim. Biophys. Acta 1153:308–314. 10.1016/0005-2736(93)90420-5 [DOI] [PubMed] [Google Scholar]
- 34.Wiseman H, Laughton MJ, Arnstein HR, Cannon M, Halliwell B. 1990. The antioxidant action of tamoxifen and its metabolites. Inhibition of lipid peroxidation. FEBS Lett. 263:192–194 [DOI] [PubMed] [Google Scholar]
- 35.Custodio JB, Dinis TC, Almeida LM, Madeira VM. 1994. Tamoxifen and hydroxytamoxifen as intramembraneous inhibitors of lipid peroxidation. Evidence for peroxyl radical scavenging activity. Biochem. Pharmacol. 47:1989–1998 [DOI] [PubMed] [Google Scholar]
- 36.Thangaraju M, Vijayalakshmi T, Sachdanandam P. 1994. Effect of tamoxifen on lipid peroxide and antioxidative system in postmenopausal women with breast cancer. Cancer 74:78–82. [DOI] [PubMed] [Google Scholar]