Abstract
In this study, oritavancin had modal MIC, MIC50, and MIC90 values of 0.03, 0.03, and 0.06 μg/ml, respectively, against Staphylococcus aureus. Similar results (MIC50/90, 0.03/0.06 μg/ml) were observed against methicillin-resistant and -susceptible isolates and those demonstrating multidrug-resistant (MDR) and non-MDR phenotypes. When oritavancin (MIC50/90, 0.06/0.12 mg/ml) was tested against S. aureus with elevated MIC values for daptomycin (i.e., 1 to 4 mg/ml) and vancomycin (i.e., 2 mg/ml), it showed MIC results 2-fold higher than those for the more susceptible vancomycin or daptomycin counterparts (MIC50/90, 0.03/0.06 mg/ml), yet it inhibited these isolates at ≤0.25 mg/ml.
TEXT
Bacteremia is a significant cause of mortality among hospitalized patients worldwide. Gram-positive isolates are frequent causes of bacteremia, and Staphylococcus aureus and Staphylococcus epidermidis are the most common causative pathogens. Between 11 and 53% of S. aureus bacteremia cases lead to complications, which are associated with a high rate of recurrence, morbidity, and mortality, as well as a high risk of developing metastatic infections (1). In addition, S. aureus bacteremia remains a major cause of life-threatening complications in patients with cancer (2). Vancomycin has been the main option for treating serious infections caused by S. aureus (3). However, despite its consistent and potent in vitro activity, several studies have reported increased treatment failure and mortality for those infections caused by organisms exhibiting MIC values at the upper end of the wild-type distribution (≥1.5 μg/ml) (4).
These life-threatening situations require the timely prescription of efficient and potent agents, and not many options are currently available for treating serious Gram-positive infections. The Infectious Diseases Society of America (IDSA) guidelines for methicillin-resistant S. aureus (MRSA) infections recommend vancomycin or daptomycin for uncomplicated bacteremia, while higher doses of daptomycin are recommended for complicated bacteremia in combination with gentamicin, rifampin, linezolid, sulfamethoxazole-trimethoprim (TMP-SMX), or a β-lactam antibiotic for persistent MRSA bacteremia and cases that do not respond to vancomycin treatment (5). Oritavancin is a lipoglycopeptide in the final stages of clinical development for the treatment of patients with acute bacterial skin and skin structure infections (ABSSSI) (see http://ir.themedicinescompany.com/phoenix.zhtml?c=122204&p=irol-newsArticle&ID=1834647&highlight=). This study reports the in vitro activities of oritavancin and other agents tested against a large collection of S. aureus isolates recovered from blood specimens in hospitalized patients in U.S. and European centers.
A total of 9,115 S. aureus isolates (5,382 and 3,733 from the United States and Europe, respectively) were included in this study. These isolates were collected from hospitalized patients in 31 and 34 medical centers in the United States and Europe, respectively. The isolates were recovered from blood specimens over a 5-year period (2008 to 2012) and submitted to JMI Laboratories (North Liberty, IA, USA) as part of the SENTRY Antimicrobial Surveillance Program. The isolates were primarily identified by the participating laboratory, these identifications were confirmed by the reference monitoring laboratory (JMI Laboratories) using standard algorithms and Vitek 2 (bioMérieux, Hazelwood, MO, USA), and the identifications were also supported by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF-MS) (Bruker Daltonics, Bremen, Germany).
The isolates were tested for susceptibility by broth microdilution following the Clinical and Laboratory Standards Institute (CLSI) M07-A9 document (6). Testing was performed using panels manufactured by Thermo Fisher Scientific (Cleveland, OH, USA). These panels provide results equivalent to the CLSI-approved broth microdilution method supplemented with 0.002% polysorbate 80. The bacterial inoculum density was monitored by colony counts to ensure there was an adequate number of cells for each testing event. Validation of the MIC values was performed by concurrent testing of CLSI-recommended quality control (QC) reference strains (S. aureus strain ATCC 29213 and Enterococcus faecalis strain ATCC 29212) (7). All QC results were within published acceptable ranges. MIC interpretations were based on the CLSI M100-S23 (2013) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) (2013) breakpoint criteria (see reference 7 and http://www.eucast.org/clinical_breakpoints/). S. aureus isolates that were resistant (according to CLSI criteria) to at least four drug classes were defined as multidrug resistant (MDR).
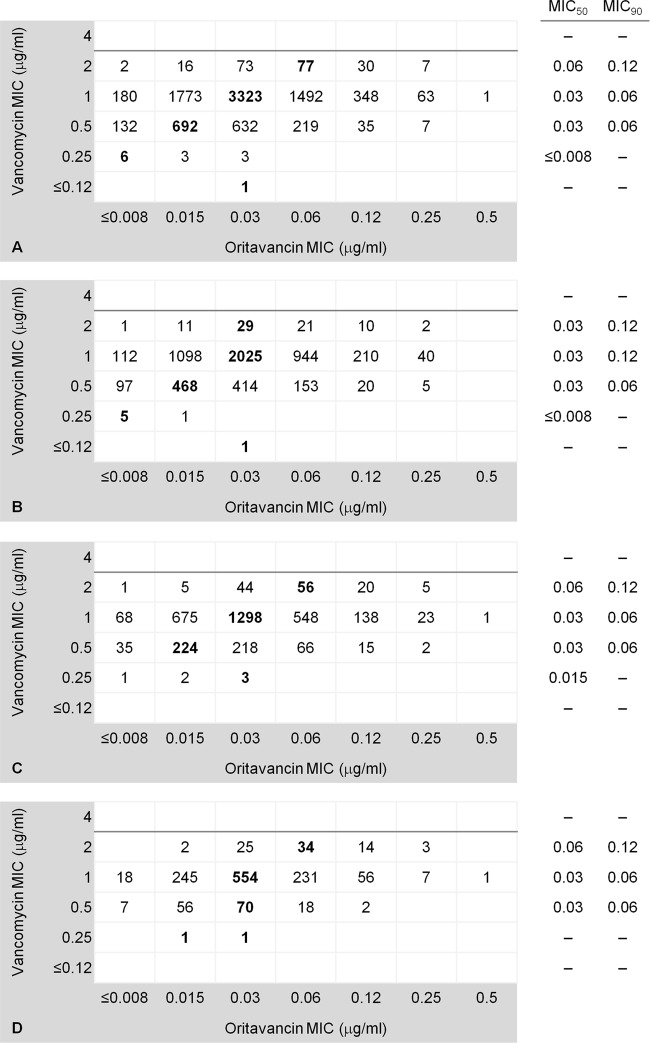
The analysis presented herein was performed according to the methicillin (oxacillin), vancomycin, and daptomycin susceptibilities and MDR phenotype. Most MRSA (72.3%) and MDR isolates (72.0%) and those with elevated MIC results for vancomycin (72.2%) and daptomycin (73.6%) originated from U.S. sites. Oritavancin was highly active when tested against all S. aureus and exhibited modal MIC, MIC50, and MIC90 values of 0.03, 0.03, and 0.06 μg/ml, respectively (Table 1). Similar MIC results were observed against methicillin-resistant and -susceptible isolates and those demonstrating MDR and non-MDR phenotypes (Table 1). Oritavancin (MIC50/90, 0.06/0.12 μg/ml) tested against those isolates with elevated MIC results for daptomycin (1 to 4 μg/ml) and vancomycin (2 μg/ml) showed MIC values 2-fold higher than those of the respective counterpart group (MIC50/90, 0.03/0.06 μg/ml) (Table 1). Oritavancin also demonstrated MIC50 and MIC90 values of 0.06 and 0.12 μg/ml, respectively, when tested against S. aureus, displaying vancomycin MIC values of 2 μg/ml within the MRSA and MDR populations (Fig. 1C and D). In contrast, those isolates exhibiting vancomycin MIC results of 0.25 and 0.5 μg/ml had oritavancin modal MIC values of ≤0.008 and 0.015 μg/ml, respectively (Fig. 1A).
TABLE 1.
Antimicrobial activity and MIC distribution of oritavancin when tested against 9,115 clinical isolates of S. aureus, including resistant subsets causing bacteremia as part of the 2008-2012 international oritavancin surveillance program
| Antimicrobial resistance of S. aureus isolates tested (no. of isolates tested)a | MIC (μg/ml) |
No. (cumulative %) inhibited at an MIC (μg/ml) ofb: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 50% | 90% | ≤0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | |
| All isolates (9,115) | 0.03 | 0.06 | 320 (3.5) | 2,484 (30.8) | 4,032 (75.0) | 1,788 (94.6) | 413 (99.1) | 77 (100) | 1 (100) |
| MSSA (5,667) | 0.03 | 0.06 | 215 (3.8) | 1,578 (31.6) | 2,469 (75.2) | 1,118 (94.9) | 240 (99.2) | 47 (100) | |
| MRSA (3,448) | 0.03 | 0.06 | 105 (3.0) | 906 (29.3) | 1,563 (74.7) | 670 (94.1) | 173 (99.1) | 30 (100) | 1 (100) |
| Vancomycin, ≤1 mg/ml (8,910) | 0.03 | 0.06 | 318 (3.6) | 2,468 (31.3) | 3,959 (75.7) | 1,711 (94.9) | 383 (99.2) | 70 (100) | 1 (100) |
| Vancomycin, 2 mg/ml (205) | 0.06 | 0.12 | 2 (1.0) | 16 (8.8) | 73 (44.4) | 77(82.0) | 30 (96.6) | 7 (100) | |
| Daptomycin, ≤0.5 mg/ml (9,014) | 0.03 | 0.06 | 318 (3.5) | 2,474 (31.0) | 3,996 (75.3) | 1,751 (94.7) | 403 (99.2) | 71 (100) | 1 (100) |
| Daptomycin, ≥1 g/ml (100) | 0.06 | 0.12 | 2 (2.0) | 10 (12.0) | 36 (48.0) | 37 (85.0) | 9 (94.0) | 6 (100) | |
| MDR (1,345) | 0.03 | 0.06 | 25 (1.9) | 304 (24.5) | 650 (72.8) | 283 (93.8) | 72 (99.2) | 10 (99.9) | 1 (100) |
| Non-MDR (7,770) | 0.03 | 0.06 | 295 (3.8) | 2,180 (31.9) | 3,382 (75.4) | 1,505 (94.7) | 341 (99.1) | 67 (100) | |
MSSA, methicillin-susceptible S. aureus; MRSA, methicillin-resistant S. aureus; MDR, multidrug-resistant isolates displaying resistance (CLSI criteria) to at least four anti-Gram-positive agents. Isolates exhibiting elevated MICs of daptomycin include results at 1 (91 isolates), 2 (eight isolates), and 4 μg/ml (one isolate).
Modal MICs are shown in bold type.
FIG 1.
Scatter diagram of oritavancin MIC results plotted against the vancomycin MIC values when tested against 9,115 S. aureus clinical isolates (A), methicillin-susceptible (B) and methicillin-resistant (C) isolates, and a subset of multidrug-resistant S. aureus isolates (D). The horizontal darker line represents the vancomycin breakpoint for susceptibility (≤2 μg/ml) of S. aureus, according to the CLSI. The oritavancin modal MIC results for each vancomycin MIC are in bold type, and the MIC50 and MIC90 values are shown if available.
Overall, 37.8% of all tested isolates were MRSA (46.3 and 25.6% in the United States and Europe, respectively). Among the comparator agents, erythromycin, clindamycin, and levofloxacin demonstrated limited antimicrobial coverage (≤82.8% susceptible) when tested against S. aureus, while vancomycin, teicoplanin, tetracycline, daptomycin, linezolid, and TMP-SMX showed in vitro activity (92.5 to 100.0% susceptible) (Table 2). Similar antimicrobial coverage profiles were obtained for MRSA isolates from the United States and Europe. Oritavancin (MIC50/90, 0.03/0.06 μg/ml) was equally active when tested against MRSA isolates from the United States (75.9% inhibited at ≤0.03 μg/ml) and Europe (73.7% inhibited at ≤0.03 μg/ml). Moreover, this drug was 8-fold-more active than daptomycin (MIC50/90, 0.25/0.5 μg/ml) and 16- to 32-fold-more potent than vancomycin (MIC50/90, 1/1 μg/ml) and linezolid (MIC50/90, 1/2 μg/ml) against MRSA, regardless of geographic region (Table 2). The vast majority of MDR strains (98.4%), as defined by the utilized criteria, were also MRSA (Table 2). Oritavancin (MIC50/90, 0.03/0.06 μg/ml) had MIC results 8- to 32-fold lower than those of active comparators, such as vancomycin (MIC50/90, 1/1 μg/ml), daptomycin (MIC50/90, 0.25/0.5 μg/ml), and linezolid (MIC50/90, 1/2 μg/ml) against MDR strains (Table 2). TMP-SMX (MIC50/90, ≤0.5/≤0.5 μg/ml; 94.2% susceptible) was also active in vitro against the MDR subset.
TABLE 2.
Antimicrobial activities of oritavancin and comparator agents tested against S. aureus clinical isolates, including resistant subsets causing bacteremia as part of the 2008-2012 international oritavancin surveillance program
| Isolates (source; no. tested) and antimicrobial agenta | MIC range (μg/ml) | MIC (μg/ml) |
% susceptible/intermediate/resistantb |
||
|---|---|---|---|---|---|
| 50% | 90% | CLSI | EUCAST | ||
| All S. aureus isolates (9,115) | |||||
| Oritavancin | ≤0.008–0.5 | 0.03 | 0.06 | −/−/−c | −/−/− |
| Oxacillin | ≤0.25 to >2 | 0.5 | >2 | 62.2/0.0/37.8 | 62.2/0.0/37.8 |
| Vancomycin | ≤0.12–2 | 1 | 1 | 100.0/0.0/0.0 | 100.0/0.0/0.0 |
| Teicoplanin | ≤2–8 | ≤2 | ≤2 | 100.0/0.0/0.0 | 99.6/0.0/0.4 |
| Erythromycin | ≤0.25 to >2 | 0.5 | >2 | 53.3/1.6/45.1 | 53.8/0.5/45.7 |
| Clindamycin | ≤0.25 to >2 | ≤0.25 | >2 | 83.3/0.3/16.4 | 82.8/0.5/16.7 |
| Tetracycline | ≤2 to >8 | ≤2 | ≤2 | 93.7/0.7/5.6 | 92.6/0.5/6.9 |
| Levofloxacin | ≤0.5 to >4 | ≤0.5 | >4 | 63.8/0.9/35.3 | 63.8/0.9/35.3 |
| Daptomycin | ≤0.06–4 | 0.25 | 0.5 | 99.9/−/− | 99.9/0.0/0.1 |
| Linezolid | ≤0.12 to >8 | 1 | 2 | >99.9/0.0/<0.1 | >99.9/0.0/<0.1 |
| TMP-SMX | ≤0.5 to >2 | ≤0.5 | ≤0.5 | 98.6/0.0/1.4 | 98.6/0.1/1.3 |
| MRSA (USA; 2,492) | |||||
| Oritavancin | ≤0.008–0.25 | 0.03 | 0.06 | −/−/− | −/−/− |
| Vancomycin | 0.25–2 | 1 | 1 | 100.0/0.0/0.0 | 100.0/0.0/0.0 |
| Teicoplanin | ≤2–8 | ≤2 | ≤2 | 100.0/0.0/0.0 | 99.5/0.0/0.5 |
| Erythromycin | ≤0.25 to >2 | >2 | >2 | 9.0/1.1/89.9 | 9.3/0.4/90.3 |
| Clindamycin | ≤0.25 to >2 | ≤0.25 | >2 | 61.9/0.3/37.8 | 61.2/0.7/38.1 |
| Tetracycline | ≤2 to >8 | ≤2 | ≤2 | 95.0/0.5/4.5 | 92.5/1.6/5.9 |
| Levofloxacin | ≤0.5 to >4 | >4 | >4 | 22.4/1.2/76.4 | 22.4/1.2/76.4 |
| Daptomycin | ≤0.06–4 | 0.25 | 0.5 | 99.6/−/− | 99.6/0.0/0.4 |
| Linezolid | 0.25 to >8 | 1 | 2 | >99.9/0.0/<0.1 | >99.9/0.0/<0.1 |
| TMP-SMX | ≤0.5 to >2 | ≤0.5 | ≤0.5 | 97.7/0.0/2.3 | 97.7/0.3/2.0 |
| MRSA (Europe; 956) | |||||
| Oritavancin | ≤0.008–0.5 | 0.03 | 0.06 | −/−/− | −/−/− |
| Vancomycin | 0.25–2 | 1 | 1 | 100.0/0.0/0.0 | 100.0/0.0/0.0 |
| Teicoplanin | ≤2–8 | ≤2 | ≤2 | 100.0/0.0/0.0 | 98.6/0.0/1.4 |
| Erythromycin | ≤0.25 to >2 | >2 | >2 | 29.8/2.2/68.0 | 30.6/0.6/68.8 |
| Clindamycin | ≤0.25 to >2 | ≤0.25 | >2 | 62.0/0.7/37.3 | 61.3/0.7/38.0 |
| Tetracycline | ≤2 to >8 | ≤2 | >8 | 81.5/1.1/17.4 | 80.8/0.2/19.0 |
| Levofloxacin | ≤0.5 to >4 | >4 | >4 | 10.6/1.1/88.3 | 10.6/1.1/88.3 |
| Daptomycin | 0.12–1 | 0.25 | 0.5 | 100.0/−/− | 100.0/0.0/0.0 |
| Linezolid | 0.25–2 | 1 | 2 | 100.0/0.0/0.0 | 100.0/0.0/0.0 |
| TMP-SMX | ≤0.5 to >2 | ≤0.5 | ≤0.5 | 97.7/0.0/2.3 | 97.7/0.0/2.3 |
| MDR (1,345) | |||||
| Oritavancin | ≤0.008–0.5 | 0.03 | 0.06 | −/−/− | −/−/− |
| Oxacillin | ≤0.25 to >2 | >2 | >2 | 1.6/0.0/98.4 | 1.6/0.0/98.4 |
| Vancomycin | 0.25–2 | 1 | 1 | 100.0/0.0/0.0 | 100.0/0.0/0.0 |
| Teicoplanin | ≤2–8 | ≤2 | ≤2 | 100.0/0.0/0.0 | 98.7/0.0/1.3 |
| Erythromycin | ≤0.25 to >2 | >2 | >2 | 0.1/0.2/99.7 | 0.1/0.1/99.8 |
| Clindamycin | ≤0.25 to >2 | >2 | >2 | 4.4/0.1/95.5 | 4.3/0.1/95.6 |
| Tetracycline | ≤2 to >8 | ≤2 | >8 | 85.9/0.6/13.5 | 82.1/2.6/15.3 |
| Levofloxacin | ≤0.5 to >4 | >4 | >4 | 0.6/0.1/99.3 | 0.6/0.1/99.3 |
| Daptomycin | 0.12–4 | 0.25 | 0.5 | 99.4/−/− | 99.4/0.0/0.6 |
| Linezolid | 0.25 to >8 | 1 | 2 | 99.9/0.0/0.1 | 99.9/0.0/0.1 |
| TMP-SMX | ≤0.5 to >2 | ≤0.5 | ≤0.5 | 94.2/0.0/5.8 | 94.2/0.4/5.4 |
TMP-SMX, trimethoprim-sulfamethoxazole; MRSA, methicillin-resistant S. aureus; MDR, multidrug-resistant isolates displaying resistance (CLSI criteria) to at least four anti-Gram-positive agents.
Breakpoint criteria according to the CLSI (document M100-S23, 2013) and EUCAST (2013).
−, breakpoint not available.
Limited options are currently available for MRSA bacteremia, especially that caused by isolates with decreased susceptibility to vancomycin. Clinical experience has suggested using high doses of daptomycin (8 to 10 mg/kg of body weight) for improved clinical and in vitro efficacy compared with standard dosing regimens (8). The efficacy and safety of a single dose of intravenous oritavancin therapy compared with twice-daily doses of vancomycin (7 to 10 days) for the treatment of patients with ABSSSI were assessed through phase 3 clinical trials (SOLO-I and SOLO-II). The preliminary data analysis showed oritavancin to be noninferior to vancomycin (http://ir.themedicinescompany.com/phoenix.zhtml?c=122204&p=irol-newsArticle&ID=1834647&highlight=). Overall, the oritavancin modal MIC, MIC50, and MIC90 results increased with increasing vancomycin MIC values when tested against S. aureus and resistant subsets. These observations are not unexpected, since the two molecules possess overlapping mechanisms of action (9), and in fact, these results were previously reported (10). However, the oritavancin in vitro potency described here was greater than that of the comparators (e.g., vancomycin) for treating MRSA infections and inhibited >99.9% of S. aureus isolates at ≤0.25 μg/ml, including those with decreased susceptibility to vancomycin and daptomycin. These results warrant further investigation to determine the role of oritavancin for difficult-to-treat MRSA infections.
ACKNOWLEDGMENTS
This surveillance study was sponsored by an educational/research grant from The Medicines Company (Parsippany, NJ) via the SENTRY Antimicrobial Surveillance Program platform. JMI Laboratories also received compensation fees for services with regard to manuscript preparation, which was funded by The Medicines Company. The Medicines Company had no involvement in the collection, analysis, or interpretation of the data. JMI Laboratories, Inc., received research and educational grants in 2010 to 2012 from Achaogen, Aires, the American Proficiency Institute (API), Anacor, Astellas, AstraZeneca, bioMérieux, Cempra, Cerexa, ContraFect, Cubist, Dipexium, Enanta, Furiex, GlaxoSmithKline, Johnson & Johnson, LegoChem Biosciences, Inc., Meiji Seika Kaisha, Nabriva, Novartis, Pfizer, PPD Therapeutics, Premier Research Group, Rempex, Rib-X Pharmaceuticals, Seachaid, Shionogi, The Medicines Company, Theravance, Thermo Fisher, and other corporations. Some JMI employees are advisors/consultants for Astellas, Cubist, Pfizer, Cempra, Cerexa-Forest, and Theravance. We have no disclosures in regard to speakers' bureaus or stock options.
Footnotes
Published ahead of print 18 February 2014
REFERENCES
- 1.Keynan Y, Rubinstein E. 2013. Staphylococcus aureus bacteremia, risk factors, complications, and management. Crit. Care Clin. 29:547–562. 10.1016/j.ccc.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 2.Montassier E, Batard E, Gastinne T, Potel G, de La Cochetière MF. 2013. Recent changes in bacteremia in patients with cancer: a systematic review of epidemiology and antibiotic resistance. Eur. J. Clin. Microbiol. Infect. Dis. 32:841–850. 10.1007/s10096-013-1819-7 [DOI] [PubMed] [Google Scholar]
- 3.Joo J, Yamaki J, Lou M, Hshieh S, Chu T, Shriner KA, Wong-Beringer A. 2013. Early response assessment to guide management of methicillin-resistant Staphylococcus aureus bloodstream infections with vancomycin therapy. Clin. Ther. 35:995–1004. 10.1016/j.clinthera.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 4.van Hal SJ, Lodise TP, Paterson DL. 2012. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin. Infect. Dis. 54:755–771. 10.1093/cid/cir935 [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:285–292. 10.1093/cid/cir034 [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—9th ed. Approved standard M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing; 23rd informational supplement. CLSI document M100-S23 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8.Gould IM, Miró JM, Rybak MJ. 2013. Daptomycin: the role of high-dose and combination therapy for Gram-positive infections. Int. J. Antimicrob. Agents 42:202–210. 10.1016/j.ijantimicag.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 9.Zhanel GG, Schweizer F, Karlowsky JA. 2012. Oritavancin: mechanism of action. Clin. Infect. Dis. 54(Suppl 3):S214–S219. 10.1093/cid/cir920 [DOI] [PubMed] [Google Scholar]
- 10.Arhin FF, Draghi DC, Pillar CM, Moeck G, Sahm DF. 2012. Correlation between oritavancin and vancomycin minimum inhibitory concentrations in staphylococci. Int. J. Antimicrob. Agents 40:562–563. 10.1016/j.ijantimicag.2012.06.019 [DOI] [PubMed] [Google Scholar]