Abstract
Metronidazole resistance in the sexually transmitted parasite Trichomonas vaginalis is a problematic public health issue. We have identified single nucleotide polymorphisms (SNPs) in two nitroreductase genes (ntr4Tv and ntr6Tv) associated with resistance. These SNPs were associated with one of two distinct T. vaginalis populations identified by multilocus sequence typing, yet one SNP (ntr6Tv A238T), which results in a premature stop codon, was associated with resistance independent of population structure and may be of diagnostic value.
TEXT
Trichomonas vaginalis is a flagellated, anaerobic protozoan and is the most common nonviral sexually transmitted pathogen, with ∼3.7 million Americans infected (1). Patients suffering from infection are treated with metronidazole, a prodrug that is reduced to its active nitro radical anion form by anaerobic metabolism. Metronidazole resistance has been observed in 4.3 to 9.6% of clinical T. vaginalis isolates in the United States (1–3). The mechanism of metronidazole resistance in T. vaginalis is unknown. Laboratory-generated resistance is associated with downregulation of enzymes thought to reduce metronidazole, such as pyruvate-ferredoxin oxidoreductase (PFOR) and ferredoxin, as well as shrinking of the hydrogenosome, a mitochondrion-related organelle where these enzymes are located in T. vaginalis (4, 5). However, resistant clinical isolates harbor normal-sized hydrogenosomes and do not exhibit reduced transcription of the PFOR or ferredoxin genes (4, 5). In addition, disruption of the gene encoding ferredoxin did not cause a resistant phenotype (6). Other studies have demonstrated that laboratory-generated resistance is associated with reduced thioredoxin reductase activity and free flavins, both of which are proposed to reduce metronidazole as well (7, 8), and decreased flavin reductase activity has been observed in resistant clinical isolates (9). Lastly, another study identified 11 nitroreductase genes in the T. vaginalis genome (6), at least one of which was capable of metronidazole reduction (10). We hypothesized that loss of function of one or more of these nitroreductase proteins could mediate metronidazole resistance in some T. vaginalis isolates. To test this hypothesis, we obtained 200 T. vaginalis isolates from the American Type Culture Collection (n = 5) and the Centers for Disease Control and Prevention (n = 195). The characteristics of these isolates are described in Table S1 in the supplemental material. Metronidazole minimal lethal concentration (MLC) values were determined by the method of Narcisi and Secor (11). Briefly, isolates were grown in 0.2 to 400 μg metronidazole under aerobic conditions at 37°C for 48 h, at which point viability was determined by checking for motility by microscopy. Metronidazole resistance in T. vaginalis is categorized as low (MLC, 50 to 100 μg/ml), moderate (MLC, 200 μg/ml), or high (MLC, ≥400 μg/ml) (3, 12).
In preliminary experiments, we PCR amplified and sequenced the ntrTv genes from between 24 and 50 isolates in order to identify polymorphisms that were associated with resistance. For DNA isolation, T. vaginalis was grown anaerobically at 37°C in modified basal Diamonds medium (13) with 100 U/ml penicillin, 100 μg/ml streptomycin, and 5% heat-inactivated horse serum. To extract DNA, ∼106 trichomonads were pelleted by centrifugation and resuspended in breaking buffer (2% Triton X-100, 1% SDS, 100 mM NaCl, 10 mM Tris [pH 8], 1 mM EDTA). Then 200 μl of acid-washed glass beads (425 to 600 μm) and 200 μl of phenol-chloroform-isoamyl alcohol (25:24:1) were added, the sample was vortexed for 2 min, and 200 μl of Tris-EDTA (TE) was added. The phenol-chloroform extraction was repeated twice and DNA was precipitated with ethanol and sodium acetate, washed with ethanol, and resuspended in nuclease-free water. PCRs were performed in 40-μl volumes with 2.5 units FideliTaq (Affymetrix), 4 μl 10× buffer, 625 nM of each primer, 200 μM deoxynucleoside triphosphates (dNTPs), 2.5 mM MgCl2, and 2 μl template. Reaction conditions were 95°C for 5 min; 35 cycles at 95°C for 1 min, 50°C for 1 min, and 68°C for 2 min; and then a final cycle at 68°C for 5 min. The primers used for PCR amplification and sequencing are listed in Table S2 in the supplemental material. Of the 40-μl reaction mixture, 20 μl was resolved on an agarose gel to confirm amplification, and the remaining 20 μl was purified using Wizard SV gel and the PCR Clean-Up system (Promega) and sequenced on an ABI3130 genetic analyzer (Applied Biosystems) according to the manufacturer's instructions. Each amplicon was sequenced with the same primers used for amplification, and both forward and reverse sequences were obtained for each amplicon.
We found that ntr1Tv, ntr2Tv, ntr5Tv, ntr7Tv, ntr8Tv, ntr11Tv, and ntr12Tv were largely nonpolymorphic compared with the published sequence of the type strain G3 (14). However, we did detect single nucleotide polymorphisms (SNPs) in ntr4Tv, ntr6Tv, ntr9Tv, and ntr10Tv (Table 1). These SNPs are denoted throughout this report with the first letter indicating the nucleotide or amino acid present in genome of the reference strain G3, the number indicating the position of the change relative to the open reading frame, and the second letter indicating the variant nucleotide or amino acid. Of these SNPs (amino acid changes), G76C (D26H), C213G (Y71STOP), and C318A (H106Q) in ntr4Tv (P ≤ 0.014) and A238T (K80STOP), G427C (E143Q), and T476C (V159A) in ntr6Tv (P ≤ 0.0001) were associated with metronidazole resistance. As only these two genes harbored SNPs significantly associated with resistance, we focused on them for the remainder of the study.
TABLE 1.
SNPs identified in ntrTv genes
| Gene | SNP | Amino acid change | No. (%) of resistant isolates with: |
No. (%) of sensitive isolates with: |
Pa | ||
|---|---|---|---|---|---|---|---|
| SNP present | SNP absent | SNP present | SNP absent | ||||
| ntr3Tv | A176T | Q59L | 1 (3.1) | 31 (97) | 0 (0.0) | 33 (100) | |
| G199A | G67R | 21 (66) | 11 (34) | 22 (69) | 10 (31) | ||
| G214A | G72R | 0 (0.0) | 32 (100) | 2 (6.1) | 31 (94) | ||
| G274A | D92N | 9 (28) | 23 (72) | 4 (12) | 30 (88) | ||
| A385G | S129G | 31 (97) | 1 (3.1) | 33 (97) | 1 (2.9) | ||
| T506C | L169P | 0 (0.0) | 32 (100) | 2 (5.9) | 32 (94) | ||
| A515C | E171A | 0 (0.0) | 32 (100) | 1 (2.9) | 33 (97) | ||
| ntr4Tv | T152A | V51D | 3 (6.0) | 47 (94) | 5 (10) | 45 (90) | |
| G208C | E70Q | 3 (6.0) | 47 (94) | 5 (10) | 45 (90) | ||
| G76C | D26H | 30 (60) | 20 (40) | 16 (32) | 34 (68) | 0.0056 | |
| C213G | Y71STOP | 33 (66) | 17 (34) | 17 (34) | 33 (66) | 0.0016 | |
| C318A | H106Q | 24 (48) | 26 (52) | 12 (24) | 38 (76) | 0.014 | |
| ntr6Tv | G259A | E87K | 6 (12) | 44 (88) | 1 (2.0) | 49 (98) | |
| T458C | V153A | 1 (2.0) | 49 (98) | 1 (2.0) | 49 (98) | ||
| A238T | K80STOP | 20 (40) | 30 (60) | 2 (4.0) | 48 (96) | <0.0001 | |
| G427C | E143Q | 38 (76) | 12 (24) | 18 (36) | 32 (64) | 0.0001 | |
| T476C | V159A | 20 (40) | 30 (60) | 2 (4.0) | 48 (96) | <0.0001 | |
| ntr9Tv | C288A | F96L | 1 (3.7) | 26 (96) | 0 (0.0) | 28 (100) | |
| T350C | V117A | 4 (15) | 23 (85) | 0 (0.0) | 27 (100) | ||
| ntr10Tv | T395C | V132A | 0 (0.0) | 17 (100) | 1 (14) | 6 (86) | |
P values determined by two-tailed Fisher's exact test.
Further analysis revealed that the metronidazole resistance-associated SNPs were frequently associated within both ntr4Tv and ntr6Tv, thus generating haplotypes (Table 2). For example, in ntr6Tv, A238T (K80STOP) and T476C (V159A) were found only together, along with G427C (E143Q) (22/100 isolates); however, G427C (E143Q) was found singly (34/100 isolates). Similarly, in ntr4Tv, G76C (D26H) and C213G (Y71STOP) were always found together (51/100 isolates, 36 of which also contained C318A [H106Q]). In addition, ntr4Tv and ntr6Tv haplotypes were associated with each other (P < 0.0001). For example, 14/22 isolates with all three resistance-associated ntr6Tv SNPs also had all three resistance-associated ntr4Tv SNPs, and 28/35 isolates with the reference ntr6Tv sequence also had the reference ntr4Tv sequence.
TABLE 2.
Association of ntr4Tv and ntr6Tv haplotypes
| ntr4Tv haplotypea |
ntr6Tv haplotype (no. of isolates positive) (resistant [n = 50], susceptible [n = 50]) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Referenceb |
G259A |
G427C |
A238T/G427C/T476C |
Total |
||||||
| R | S | R | S | R | S | R | S | R | S | |
| Reference | 4 | 24 | 6 | 2 | 3 | 4 | 2 | 0 | 15 | 30 |
| G76C/C213G | 0 | 0 | 0 | 1 | 5 | 3 | 5 | 1 | 10 | 5 |
| G76C/C213G/C318A | 0 | 3 | 0 | 0 | 10 | 9 | 13 | 1 | 23 | 13 |
| T152A/G208C | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Total | 6 | 29 | 6 | 3 | 18 | 16 | 20 | 2 | 50 | 50 |
The ntr4Tv haplotype is displayed vertically, and the ntr6Tv haplotype is displayed horizontally. Numbers refer to the specific counts of resistant and sensitive isolates where the combination of haplotypes is found.
Reference sequence refers to that found in the sequenced G3 isolate. R indicates metronidazole resistant (MLC ≥ 50 μg/ml), and S indicates metronidazole susceptible.
Based upon our findings with regard to ntr4Tv and ntr6Tv, we extended our analysis to the other 100 isolates in our collection. We focused on the ntr4Tv C213G (Y71STOP) and ntr6Tv A238T (K80STOP) SNPs, as they showed the strongest association with resistance for each gene (Table 1) and are predicted to cause nonsense mutations, thus providing a plausible biological mechanism for why they would be associated with metronidazole resistance. To this end, we developed quantitative real-time PCRs (qPCRs) to rapidly detect the presence of either the SNP of interest or the corresponding reference sequence. Reactions were performed in a 20-μl volume using iTaq master mix (Bio-Rad), 1 μM each primer, 200 nM probe, and 2 μl of template DNA. Reactions were performed on the MX3000P qPCR system (Agilent) using the following cycling conditions: 95°C for 2 min, followed by 40 cycles at 95°C for 15 s and 63°C for 45 s. The qPCR results were 100% concordant for the 100 isolates whose ntr4Tv and ntr6Tv genes had been sequenced. The combined results of sequencing and qPCR assays are listed in Table 3. The presence of metronidazole resistance was highly associated with the ntrTv genotype (P < 0.0001) (chi-square test). Neither SNP was present in 57% of susceptible isolates and 29% of resistant isolates, whereas both SNPs were present together in 30% of resistant isolates and only 4.1% of susceptible isolates (P < 0.0001 for both comparisons) (two-tailed Fisher's exact test). In contrast, the combination of ntr4Tv C213G (Y71STOP) and an intact ntr6Tv gene was present in 39% of susceptible isolates and 38% of resistant isolates. Lastly, two isolates (both resistant) harbored ntr6Tv A238T (K80STOP) alone and three isolates could be not be genotyped with regard to both genes.
TABLE 3.
Association of ntr4Tv C213G (Y71STOP) and ntr6Tv A238T (K80STOP) SNPs with metronidazole resistance
| Phenotype | No. (%) of isolates with indicated genotype |
|||
|---|---|---|---|---|
| ntr4Tv reference and ntr6Tv referencea | ntr4Tv C213G (Y71STOP) SNP and ntr6Tv reference | ntr4Tv reference and ntr6Tv A238T (K80STOP) SNP | ntr4Tv C213G (Y71STOP) SNP and ntr6Tv A238T (K80STOP) SNP | |
| Total | 85 (43) | 76 (39) | 2 (1.0) | 34 (17) |
| Susceptible | 56 (57) | 38 (39) | 0 (0) | 4 (4.1) |
| Resistantb | 29 (29) | 38 (38) | 2 (2.0) | 30 (30) |
| Low | 4 (18) | 13 (59) | 0 (0) | 5 (23) |
| Moderate | 10 (33) | 10 (33) | 0 (0) | 10 (33) |
| High | 15 (32) | 15 (32) | 2 (4.3) | 15 (32) |
Reference sequence refers to that found in the sequenced G3 isolate.
Resistance is defined as low (MLC, ≤100 μg/ml), moderate (MLC, 200 μg/ml), or high (MLC ≥400 μg/ml); isolates with MLC values between 100 and 400 μg/ml were categorized as moderate.
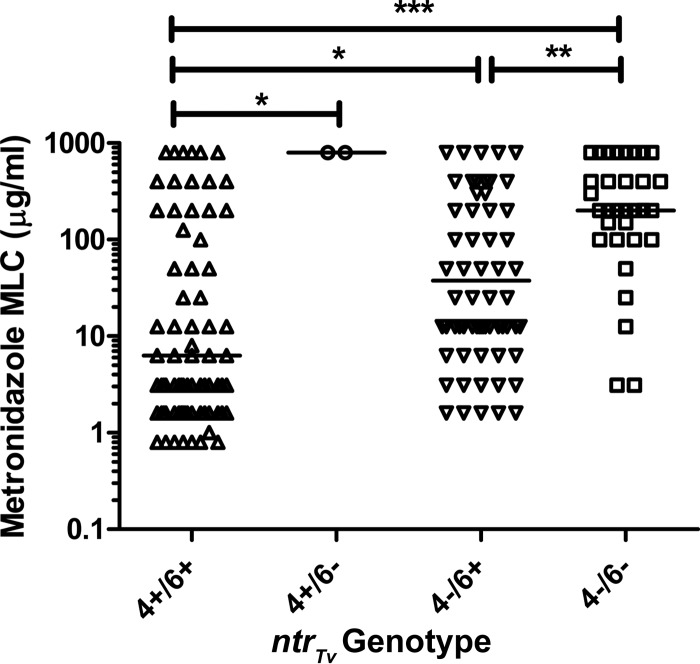
However, among resistant isolates, there was no association between the ntrTv genotype and the degree of resistance. In addition, we analyzed the distribution of MLC values in each genotypic group and found that MLC values were significantly associated with the ntrTv genotype (P < 0.0001, Kruskal-Wallis test) (Fig. 1). MLC values were lower in the isolates lacking both SNPs than in the isolates in each of the other groups (P ≤ 0.05), especially the isolates harboring both SNPs (P < 0.001) (Dunn's posttest used for all pairwise comparisons). In turn, isolates with both SNPs had higher MLC values than those harboring only ntr4Tv C213G (Y71STOP) (P < 0.01).
FIG 1.
Metronidazole susceptibility in 197 isolates stratified by ntrTv genotype. Intact open reading frames (ORFs) are represented by +, and the presence of stop codons is indicated by −. Horizontal bars represent median values. Isolates with a range of MLC values (e.g., 100 to 200 μg/ml) are plotted using their mean MLC values. Isolates with an MLC value of >400 μg/ml are assigned a value of 800 μg/ml for display purposes. *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
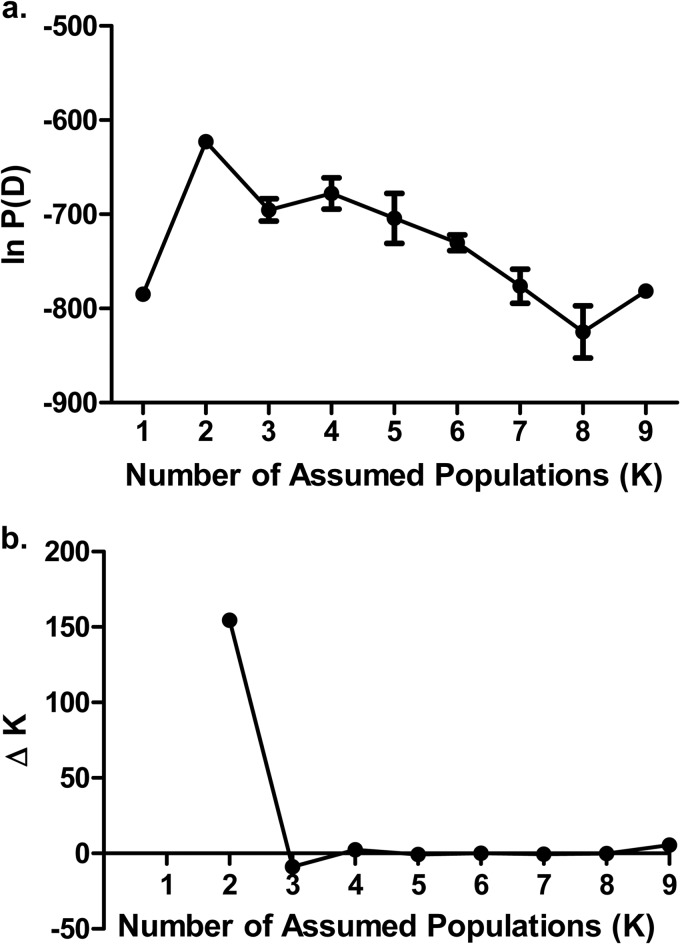
Although we had identified SNPs with strong associations with metronidazole resistance, it is important that they be interpreted in light of the T. vaginalis population structure. Prior studies using microsatellite genotyping and multilocus sequence typing (MLST) have shown that T. vaginalis has a two-type population structure, with one population highly similar to an inferred ancestral population (type 1) and a second population that is more divergent and exhibits a higher degree of metronidazole resistance (type 2) (10, 14). To determine the population structure of our isolate collection, we utilized the MLST methodology (10). Briefly, we PCR amplified and sequenced seven different loci using template DNA from 100 isolates (50 each metronidazole resistant and susceptible), using the primers listed in Table 1. Reaction mixtures were the same as those used for ntrTv gene amplification. Reaction conditions were as follows: 95°C for 5 min; followed by 35 cycles of 95°C for 1 min, 55°C for 1 min, and 68°C for 2 min; followed by 68°C for 5 min. PCR products were analyzed both by gel electrophoresis and by purification using Wizard SV gel and the PCR Clean-Up system (Promega) followed by sequencing on the ABI3130 genetic analyzer (Applied Biosystems) according to the manufacturer's instructions. Both forward and reverse sequences were obtained for each amplicon. Each allele for the seven different loci was assigned a numerical identifier, and these data were then analyzed using the program Structure 2.3 (15). Each run consisted of 100,000 burn-in simulations followed by data collection on 100,000 simulations; five independent runs were performed for each assumed population structure. To determine the population structure, the program was run using an assumed population structure of 2 to 9, and the lnP(D) (log probability) and ΔK (second-order rate of change in log probability) were determined. We found that an assumed population structure of 2 resulted in the highest log probability and that the transition from 2 to 3 populations resulted in the highest value for ΔK (Fig. 2). Based on these results, our isolate collection represented 2 populations, similar to those of the T. vaginalis collections analyzed in other studies (Fig. 3a) (10, 14).
FIG 2.
Determination of T. vaginalis population using Structure 2.3 to analyze SNP data from 100 isolates. (a) Mean values ± standard error of the means (SEM) (n = 5) for log probability of assumed population structure. (b) Second-order rate of change in likelihood (ΔK) between the number of assumed populations.
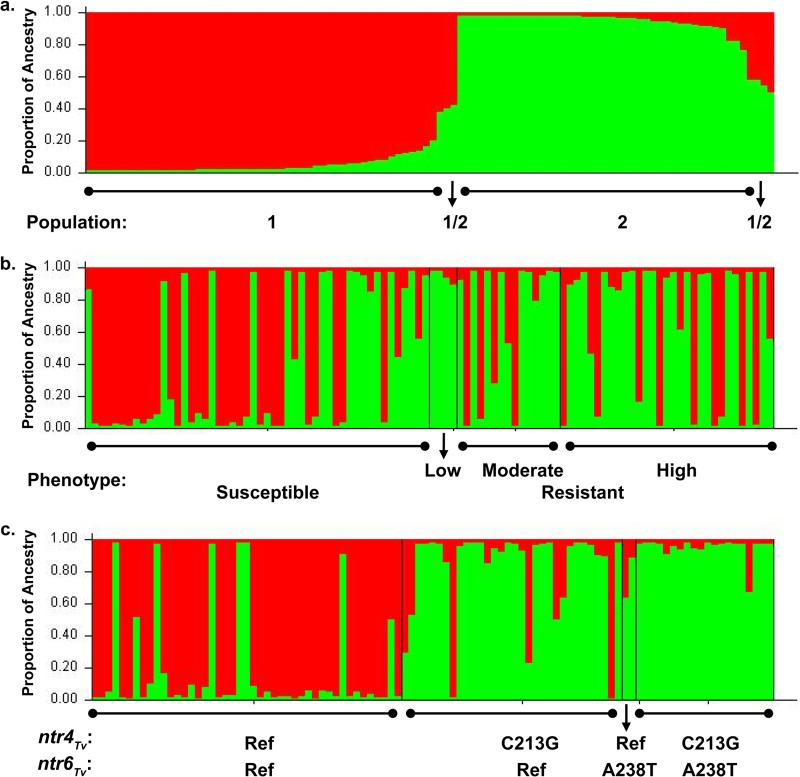
FIG 3.
T. vaginalis population structure. Red represents ancestry from population 1, and green represents ancestry from population 2. (a) Total population sorted by Q (ancestry vector) (100 isolates), (b) population sorted by ascending MLC values (100 isolates), and (c) population sorted by ntrTv genotype (99 isolates, 1 excluded due to undetermined ntr6Tv genotype).
The populations were analyzed with respect to the fixation index (FST), a 0-to-1 scale of population differentiation due to genetic structure, where 0 represents free interbreeding between populations and 1 indicates that all genetic differences between the populations are due to population structure, and the populations share no genetic diversity. Of the two inferred populations, population 1 was highly similar to an inferred ancestral population (where FST = 0.018 ± 0.0066), whereas the other population (population 2) was highly divergent from the inferred ancestral population (FST = 0.51 ± 0.020) (mean ± standard error). Based upon this analysis, we infer that populations 1 and 2 correspond to population types 1 and 2 identified by microsatellite analysis (14). Using an inferred ancestry cutoff of 0.75, we assigned 42 isolates to population 1 and 51 isolates to population 2, and 7 isolates consisted of an admixture of both populations (≥0.25 inferred ancestry from both populations).
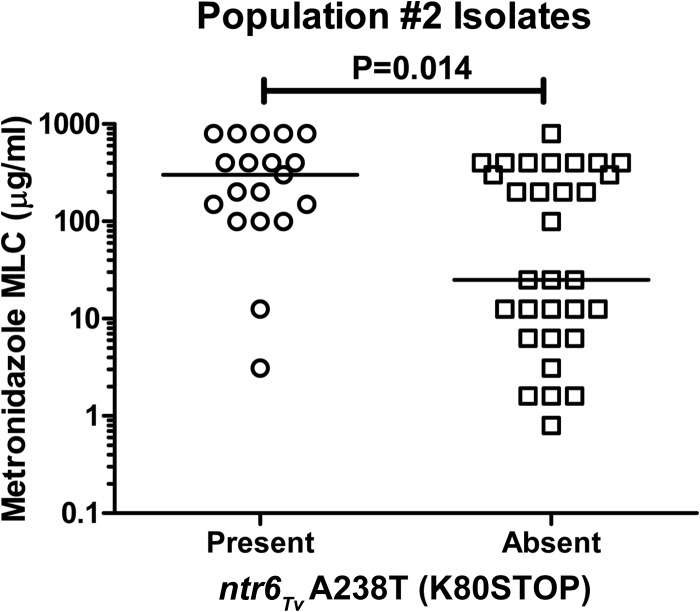
The prevalence of metronidazole resistance was highly dependent upon population structure (Fig. 3b); 33% of isolates in population 1 (14/43 isolates) were metronidazole resistant compared to 64% (32/50 isolates) of isolates in population 2 (P = 0.0032, two-tailed Fisher's exact test) (Table 4). These results are consistent with the prior microsatellite typing study that found the more recently diverged T. vaginalis population (type 1) had an elevated mean metronidazole MLC (14). The ntr4Tv C213G (Y71STOP) and ntr6Tv A238T (K80STOP) SNPs were also both strongly associated with population structure (Fig. 3c); both were far more prevalent in population 2 (88% and 38%, respectively) than in population 1 (4.7% and 0%, respectively) (P < 0.0001 for both comparisons, two-tailed Fisher's exact test). Accordingly, we found that the group of isolates lacking both SNPs had only 20% population 2 ancestry, whereas isolates with either ntr4Tv C213G (Y71STOP) or both SNPs had 85% and 95% population 2 ancestry, respectively. These results indicate that the observed association between these SNPs and metronidazole resistance (Table 3) may be a secondary effect due to their association with population 2, which is in turn associated with resistance. However, within population 2, the ntr6Tv A238T (K80STOP) SNP was present in 17/38 (45%) of resistant isolates and only 2/22 (9.1%) of susceptible isolates, indicating a significant association with resistance even after controlling for population structure (P = 0.0084, two-tailed Fisher's exact test). Analysis of the distribution of MLC values within population 2 also revealed a significant difference between isolates harboring the ntr6Tv A238T (K80STOP) SNP and those strains that did not possess this SNP (Fig. 4).
TABLE 4.
Association of metronidazole resistance and ntrTv genotypes with inferred populations
| Population | Characteristic (no. [%]) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total | ntr4Tv C213G (Y71STOP) | ntr6Tv A238T (K80STOP) | Metronidazole susceptible | Metronidazole resistanta |
||||
| Total | Low | Moderate | High | |||||
| 1 | 43 (100) | 2 (4.7) | 0 (0) | 29 (67) | 14 (33) | 0 (0) | 5 (12) | 9 (21) |
| 2 | 50 (100) | 44 (88) | 19 (38) | 18 (36) | 32 (64) | 4 (8) | 11 (22) | 17 (34) |
| 1/2 | 7 (100) | 4 (57) | 2 (29) | 3 (43) | 4 (57) | 0 (0) | 3 (43) | 1 (14) |
Resistance is defined as low (MLC ≤ 100 μg/ml), moderate (MLC > 200 μg/ml), or high (MLC ≥ 400 μg/ml); isolates with MLC values between 100 and 400 μg/ml were categorized as moderate.
FIG 4.
Distribution of metronidazole MLC values within 50 population 2 isolates by presence or absence of ntr6Tv A238T (K80STOP). Horizontal bars represent median values. Isolates with range of MLC values (e.g., 100 to 200 μg/ml) are plotted using their mean MLC values. Isolates with an MLC value of >400 μg/ml are assigned a value of 800 μg/ml for display purposes. P value determined by Mann-Whitney U test.
In summary, we have identified stop codons in genes encoding two putative nitroreductases that are associated with metronidazole resistance. However, the T. vaginalis genome encodes 11 putative nitroreductases, of which more than one may reduce metronidazole. In the initial study characterizing these genes, the authors found that of the 11 genes, the 3 that were transcribed at the highest level were ntr4Tv, ntr6Tv, and ntr10Tv (10). As the presence of stop codons in ntr4Tv and ntr6Tv was strongly associated with resistance, we speculate that in these strains ntr10Tv may be expressed at a low level or lack sufficient nitroreductase activity to convert metronidazole to its active form. It is important to note that although we have identified an association between stop codons in these genes and resistance, there is not sufficient evidence to conclude that these changes are the actual cause of resistance; additional studies modulating expression of these genes in T. vaginalis will be necessary to further test that hypothesis. Additionally, metronidazole resistance does occur in isolates with intact ntr4Tv and ntr6Tv genes, indicating that multiple resistance mechanisms may be responsible for resistance. For example, one study linked resistance to infection of T. vaginalis by Mycoplasma (16); however, the mechanism by which infection would impart resistance is unclear, and this association was not found in a subsequent study (17). Another study found that infection by a double-stranded RNA virus was associated with a lower mean metronidazole MLC value (2); however, the virus is also strongly associated with population 1, being present in 73% of isolates in this population compared to only 2.5% of isolates in population 2 (18). Further studies will be required to determine the relative contribution of population ancestry, ntrTv genotype, and other factors to metronidazole resistance.
Lastly, the ntr6Tv A238T (K80STOP) SNP may have clinical utility in identifying metronidazole-resistant T. vaginalis in a rapid culture-independent fashion. As this SNP was present in 32/99 (32%) resistant isolates and only 4/98 (4.1%) susceptible isolates, its detection has a sensitivity of 32%, specificity of 96%, positive predictive value of 89%, and negative predictive value of 58%. Therefore, this SNP may be useful as a confirmatory test for suspected metronidazole resistance in treatment-refractory cases, especially in light of a recent study finding that identification of metronidazole resistance provided useful information to clinicians and led to improved patient outcomes (18). Our future efforts will focus on investigating the cellular role of ntr4Tv and ntr6Tv in metronidazole susceptibility, as well as the identification of additional resistance markers to improve culture-independent detection of T. vaginalis metronidazole resistance in the clinic.
Supplementary Material
Footnotes
Published ahead of print 18 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02370-13.
REFERENCES
- 1.Snipes LJ, Gamard PM, Narcisi EM, Beard CB, Lehmann T, Secor WE. 2000. Molecular epidemiology of metronidazole resistance in a population of Trichomonas vaginalis clinical isolates. J. Clin. Microbiol. 38:3004–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkcaldy RD, Augostini P, Asbel LE, Bernstein KT, Kerani RP, Mettenbrink CJ, Pathela P, Schwebke JR, Secor WE, Workowski KA, Davis D, Braxton J, Weinstock HS. 2012. Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD Surveillance Network, 2009–2010. Emerg. Infect. Dis. 18:939–943. 10.3201/eid1806.111590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwebke JR, Barrientes FJ. 2006. Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob. Agents Chemother. 50:4209–4210. 10.1128/AAC.00814-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mead JR, Fernadez M, Romagnoli PA, Secor WE. 2006. Use of Trichomonas vaginalis clinical isolates to evaluate correlation of gene expression and metronidazole resistance. J. Parasitol. 92:196–199. 10.1645/GE-616R.1 [DOI] [PubMed] [Google Scholar]
- 5.Wright JM, Webb RI, O'Donoghue P, Upcroft P, Upcroft JA. 2010. Hydrogenosomes of laboratory-induced metronidazole-resistant Trichomonas vaginalis lines are downsized while those from clinically metronidazole-resistant isolates are not. J. Eukaryot. Microbiol. 57:171–176. 10.1111/j.1550-7408.2009.00455.x [DOI] [PubMed] [Google Scholar]
- 6.Carlton JM, Hirt RP, Silva JC, Delcher AL, Schatz M, Zhao Q, Wortman JR, Bidwell SL, Alsmark UC, Besteiro S, Sicheritz-Ponten T, Noel CJ, Dacks JB, Foster PG, Simillion C, Van de Peer Y, Miranda-Saavedra D, Barton GJ, Westrop GD, Muller S, Dessi D, Fiori PL, Ren Q, Paulsen I, Zhang H, Bastida-Corcuera FD, Simoes-Barbosa A, Brown MT, Hayes RD, Mukherjee M, Okumura CY, Schneider R, Smith AJ, Vanacova S, Villalvazo M, Haas BJ, Pertea M, Feldblyum TV, Utterback TR, Shu CL, Osoegawa K, de Jong PJ, Hrdy I, Horvathova L, Zubacova Z, Dolezal P, Malik SB, Logsdon JM, Jr, Henze K, Gupta A, Wang CC, Dunne RL, Upcroft JA, Upcroft P, White O, Salzberg SL, Tang P, Chiu CH, Lee YS, Embley TM, Coombs GH, Mottram JC, Tachezy J, Fraser-Liggett CM, Johnson PJ. 2007. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 315:207–212. 10.1126/science.1132894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leitsch D, Kolarich D, Binder M, Stadlmann J, Altmann F, Duchene M. 2009. Trichomonas vaginalis: metronidazole and other nitroimidazole drugs are reduced by the flavin enzyme thioredoxin reductase and disrupt the cellular redox system. Implications for nitroimidazole toxicity and resistance. Mol. Microbiol. 72:518–536 [DOI] [PubMed] [Google Scholar]
- 8.Leitsch D, Kolarich D, Duchene M. 2010. The flavin inhibitor diphenyleneiodonium renders Trichomonas vaginalis resistant to metronidazole, inhibits thioredoxin reductase and flavin reductase, and shuts off hydrogenosomal enzymatic pathways. Mol. Biochem. Parasitol. 171:17–24. 10.1016/j.molbiopara.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 9.Leitsch D, Drinic M, Kolarich D, Duchene M. 2012. Down-regulation of flavin reductase and alcohol dehydrogenase-1 (ADH1) in metronidazole-resistant isolates of Trichomonas vaginalis. Mol. Biochem. Parasitol. 183:177–183. 10.1016/j.molbiopara.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelius DC, Robinson DA, Muzny CA, Mena LA, Aanensen DM, Lushbaugh WB, Meade JC. 2012. Genetic characterization of Trichomonas vaginalis isolates by use of multilocus sequence typing. J. Clin. Microbiol. 50:3293–3300. 10.1128/JCM.00643-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narcisi EM, Secor WE. 1996. In vitro effect of tinidazole and furazolidone on metronidazole-resistant Trichomonas vaginalis. Antimicrob. Agents Chemother. 40:1121–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lossick JG, Muller M, Gorrell TE. 1986. In vitro drug susceptibility and doses of metronidazole required for cure in cases of refractory vaginal trichomoniasis. J. Infect. Dis. 153:948–955. 10.1093/infdis/153.5.948 [DOI] [PubMed] [Google Scholar]
- 13.Diamond LS. 1957. The establishment of various trichomonads of animals and man in axenic cultures. J. Parasitol. 43:488–490 [PubMed] [Google Scholar]
- 14.Conrad MD, Gorman AW, Schillinger JA, Fiori PL, Arroyo R, Malla N, Dubey ML, Gonzalez J, Blank S, Secor WE, Carlton JM. 2012. Extensive genetic diversity, unique population structure and evidence of genetic exchange in the sexually transmitted parasite Trichomonas vaginalis. PLoS Negl. Trop. Dis. 6:e1573. 10.1371/journal.pntd.0001573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubisz MJ, Falush D, Stephens M, Pritchard JK. 2009. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 9:1322–1332. 10.1111/j.1755-0998.2009.02591.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao JC, Xie LF, Fang SL, Gao MY, Zhu Y, Song LY, Zhong HM, Lun ZR. 2006. Symbiosis of Mycoplasma hominis in Trichomonas vaginalis may link metronidazole resistance in vitro. Parasitol. Res. 100:123–130. 10.1007/s00436-006-0215-y [DOI] [PubMed] [Google Scholar]
- 17.Butler SE, Augostini P, Secor WE. 2010. Mycoplasma hominis infection of Trichomonas vaginalis is not associated with metronidazole-resistant trichomoniasis in clinical isolates from the United States. Parasitol. Res. 107:1023–1027. 10.1007/s00436-010-1975-y [DOI] [PubMed] [Google Scholar]
- 18.Bosserman EA, Helms DJ, Mosure DJ, Secor WE, Workowski KA. 2011. Utility of antimicrobial susceptibility testing in Trichomonas vaginalis-infected women with clinical treatment failure. Sex. Transm. Dis. 38:983–987. 10.1097/OLQ.0b013e318224db39 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.