Abstract
Antimicrobial peptides have recently emerged as a promising new group to be evaluated in the therapeutic intervention of infectious diseases. This study evaluated the anti-infectious effect of the short, synthetic, broad-spectrum antimicrobial peptide PXL150 in a mouse model of staphylococcal surgical site infections. We found that administration of PXL150, formulated in an aqueous solution or in a hydroxypropyl cellulose gel, significantly reduced the bacterial counts in the wound compared with placebo treatment, warranting further investigations of the potential of this peptide as a novel local treatment of microbial infections.
TEXT
The dramatic increase in bacterial resistance to conventional antibiotics in recent years emphasizes the importance of identifying novel, more potent antimicrobial therapies. One disease area where bacteria cause significant mortality and morbidity is surgical site infections (SSIs) affecting either the incision or deep tissues at the operation site (1). Despite advances in environment and surgical practice, SSIs are the third most frequently reported nosocomial infections (2). According to a surveillance study performed in the United States, SSIs account for 14 to 16% of all nosocomial infections among hospital inpatients and for 38% of nosocomial infections among surgical patients (1, 2). Similarly, European data suggest that the incidence of SSIs may be as high as 20%, depending on the surgical procedure (3).
The pathogens responsible for SSIs depend on the procedure. Most commonly isolated bacteria in SSIs are Gram-positive cocci: Staphylococcus aureus is the most frequently isolated organism, followed by coagulase-negative staphylococci and Enterococcus spp. Approximately one-third of the isolates are Gram-negative bacilli, including Escherichia coli, Pseudomonas aeruginosa, and Enterobacter species (4). The most significant change in the microbiology of SSIs over recent years has been the increased involvement of antibiotic-resistant bacteria, in particular, methicillin-resistant S. aureus (MRSA), as well as a progressive variation in causative pathogens. A dramatic increase in MRSA infections has been reported in different geographic locations in connection with a wide range of surgical procedures (5–8). According to different surveillance studies, as many as 50% of S. aureus isolates are resistant to methicillin (9–11). The Gram-negative bacilli isolated from patients with SSIs also demonstrate an increased resistance profile (12, 13). Polymicrobial infections in SSIs are increasingly frequently reported, involving both Gram-positive and Gram-negative organisms, especially S. aureus together with P. aeruginosa (9). On the basis of this evidence, new antimicrobial agents with a wide spectrum of activity, a low potential for resistance development, and activity against resistant and multiresistant strains are required for the treatment of SSIs.
Antimicrobial peptides (AMPs), a group of natural compounds constituting an integral part of the innate immune response, have attracted attention as a possible novel strategy for treatment of infections (reviewed in reference 14). AMPs present several advantages over conventional antibiotics, including a rapid, broad-spectrum microbicidal activity against a wide range of microorganisms (15), low levels of induced resistance (16), and concomitant immunomodulatory activities (17).
We recently described a novel short synthetic AMP, PXL150, that exhibits broad-spectrum microbicidal action against both Gram-positive and Gram-negative bacteria, including resistant strains such as MRSA (18). S. aureus and MRSA failed to develop resistance to PXL150 under continued selection pressure. In human cell lines, PXL150 downregulated the secretion of the proinflammatory markers tumor necrosis factor alpha (TNF-α) and plasminogen activator inhibitor type 1 (PAI-1), suggesting that the microbicidal effect of the peptide is accompanied by anti-inflammatory properties. PXL150 in an aqueous solution demonstrated a pronounced anti-infectious effect in an in vivo model of full-thickness excision wounds infected with MRSA in rats and in an ex vivo model of pig skin infected with S. aureus (18).
To evaluate the pharmaceutical potential of PXL150 as a novel local treatment for SSIs, the antimicrobial effect of this peptide was investigated in a murine SSI model. In this model, a silk suture contaminated with the most common pathogen in SSIs, S. aureus, was implanted into an incision wound on the back of mice and assessment of the infection was performed by counting viable bacteria in the tissue homogenate. The same model has been used previously to assess the effect of systemic and topical antimicrobial agents, and the results observed have been shown to correlate closely with efficacy in clinical trials with human subjects (19–21). For further details of the experimental model used, see the supplemental material.
Sutures carrying an inoculum of S. aureus were able to cause marked infection in the wounds, which persisted during the whole 96-h period of observation. Consistent with previously published results (20), the viable count of bacteria in the wounds increased exponentially during the first 4 h and reached a plateau of approximately 107.5 CFU/wound at 24 h postinfection. Little variation between bacterial counts in individual wounds assessed over time was seen, indicating that a contaminated suture could cause a reproducible experimental infection (Fig. 1).
FIG 1.
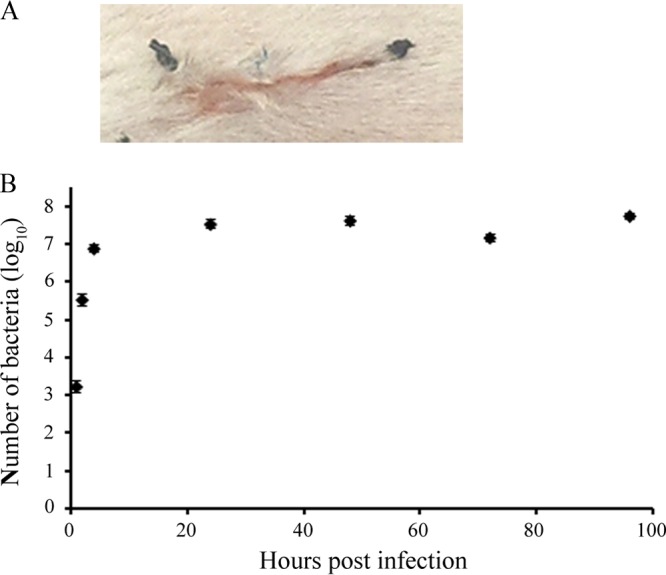
Infection establishment in a mouse SSI model. (A) A 1-cm-long, full-thickness incision wound was created on the dorsal side of the mouse. Approximately 1 cm of silk suture infected with S. aureus (approximately 5 × 103 cells/cm of suture) was placed into the wound and secured in the skin by knotting. One single nylon suture was attached over the middle of the incision. (B) Viable counts of bacteria per wound were analyzed in the tissue homogenate at 1, 2, 4, 24, 48, 72, and 96 h postinfection (n = 5 mice per time point).
First, the anti-infectious effect of aqueous solutions of PXL150 was evaluated in this model. On the basis of our previous studies suggesting that PXL150 causes rapid membrane depolarization and killing of target bacteria (18), viable counts in the wounds were assessed at 2 h posttreatment. A single treatment with PXL150 at a concentration of 2, 4, or 8.3 mg/g produced bacterial counts in wounds significantly lower than those obtained with placebo (treatment with only water corresponded to 5.8 ± 0.8 log10 CFU/wound). There were statistically significant differences between all of the concentrations of the peptide tested, demonstrating a clear dose-response effect of PXL150. Concentrations of 4 and 8.3 mg/g PXL150 killed >99% of the bacteria, compared with placebo (Fig. 2A). The mean number of CFU of S. aureus recovered from the wounds treated with the highest concentration of the peptide (8.3 mg/g) was more than 3 log10 lower than that recovered from the placebo-treated wounds. Moreover, PXL150 at the highest concentration eradicated S. aureus from four out of the five treated wounds (<30 CFU estimated from the original suspension). In this experiment, no antibiotic control was included, which is considered a limitation of this work. However, it has previously been shown that retapamulin and mupirocin ointments reduced the numbers of S. aureus bacteria in this model by 3 to 4 log10 CFU/wound following application two or three times per day for 5 to 7 days when the initial infection was comparable to that in this study (approximately 6 log10 CFU/wound) (21).
FIG 2.
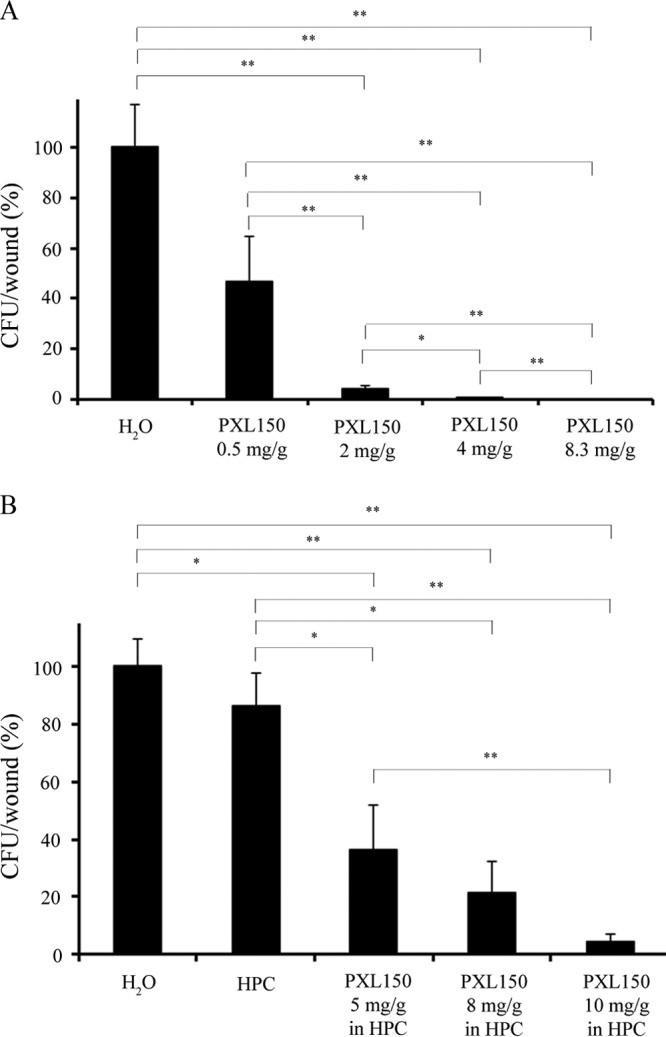
Antimicrobial dose-response effect of PXL150 administered in an aqueous solution (A) or in 1.5% HPC gel (B) in a mouse SSI model. Treatment was applied at 2 h postinfection, and analysis of bacterial survival in wounds was performed at 2 h posttreatment (n = 5 to 7 mice per group). *, P < 0.05; **, P < 0.01. HPC, hydroxypropyl cellulose.
As the next step, the anti-infectious effect of PXL150 at a concentration of 5, 8, or 10 mg/g formulated in 1.5% hydroxypropyl cellulose (HPC) gel was evaluated in this model. HPC is a nonionic, water-soluble polymer that is widely used as a thickening agent in pharmaceutical and cosmetic formulations in topical products (22). Previously it has been reported that placebo creams and ointments frequently affect the infection in this experimental model, leading to an increase in bacterial counts. This effect has been explained by the placebo formulation increasing the moisture content of the wound by preventing water loss or by the vehicle supporting the growth of pathogens per se (20). The vehicle used in this study (HPC gel) showed no effect by itself on bacterial counts, compared with the administration of water only (6.6 ± 0.1 log10 compared with 6.7 ± 0.1 log10, respectively). PXL150 in HPC gel exhibited a dose-dependent anti-infectious effect with statistically significant microbicidal activity observed at all of the concentrations of PXL150 tested, compared with treatment with water or HPC only (Fig. 2B). The highest concentration of PXL150 in HPC (10 mg/g) killed, on average, >95% of the bacteria, compared with water, while in three out of seven animals, bacterial counts were reduced by more than 99%. These results indicate that the microbicidal effect of PXL150 is preserved in the gel formulation. However, the lowest concentration of PXL150 that killed more than 95% of the bacteria was markedly higher when the peptide was formulated in HPC (10 mg/g) than when it was formulated in an aqueous solution (2 mg/g). The reason for this difference remains elusive; however, it is likely that the interaction of the cationic peptide with the HPC gel results in a slow release of PXL150, which results in an actual burst concentration of the peptide in the wound lower than that achieved with the administration of an aqueous solution of PXL150.
In all experiments, the treatment was applied at 2 h postinfection. This time point was selected on the basis of previous studies using a mouse surgical wound infection model (19–21), combined with our experiments on infection kinetics in the wound, suggesting that at 2 h postinfection, the exponential phase of bacterial growth with a high viable count of approximately 105.5 CFU/wound was reached (Fig. 1B). However, this time interval is too short to allow assessment of the effect against bacterial biofilm, which is considered a limitation of this work.
In summary, our data demonstrate the potential benefit of using the peptide PXL150 for local antibacterial treatment of SSIs. In a mouse model of the management of experimental surgical wound infections, which is viewed as a valuable tool for predicting the effect of topical antibiotics in humans (19–21), PXL150 was shown to be efficient in reducing bacterial counts of the most common pathogen in SSIs, S. aureus. These results, in combination with our previous studies showing that PXL150 has a broad antimicrobial spectrum, an advantageous safety profile, and a low potential for resistance development (18), support the progression of PXL150 as a novel topical treatment of microbial infections.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Pergamum AB, Solna, Sweden. At the time of the investigation, Joakim Håkansson, Camilla Björn, Kerstin Lindgren, Emma Sjöström, Veronika Sjöstrand, and Margit Mahlapuu were employees of the company. Pergamum contributed financially, including salaries and study costs, and by providing laboratory space.
Footnotes
Published ahead of print 3 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00143-14.
REFERENCES
- 1.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR, Hospital Infection Control Practices Advisory Committee 1999. Guideline for prevention of surgical site infection. Infect. Control Hosp. Epidemiol. 20:250–278, quiz 279–280. 10.1086/501620 [DOI] [PubMed] [Google Scholar]
- 2.Emori TG, Gaynes RP. 1993. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 6:428–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leaper DJ, van Goor H, Reilly J, Petrosillo N, Geiss HK, Torres AJ, Berger A. 2004. Surgical site infection—a European perspective of incidence and economic burden. Int. Wound J. 1:247–273. 10.1111/j.1742-4801.2004.00067.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirby JP, Mazuski JE. 2009. Prevention of surgical site infection. Surg. Clin. North Am. 89:365–389, viii. 10.1016/j.suc.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 5.Zoumalan RA, Rosenberg DB. 2008. Methicillin-resistant Staphylococcus aureus—positive surgical site infections in face-lift surgery. Arch. Facial Plast. Surg. 10:116–123. 10.1001/archfaci.10.2.116 [DOI] [PubMed] [Google Scholar]
- 6.Merrer J, Girou E, Lortat-Jacob A, Montravers P, Lucet JC, Groupe de Recherche sur l'Antibioprophylaxie en Chirurgie 2007. Surgical site infection after surgery to repair femoral neck fracture: a French multicenter retrospective study. Infect. Control Hosp. Epidemiol. 28:1169–1174. 10.1086/520745 [DOI] [PubMed] [Google Scholar]
- 7.Kourbatova EV, Halvosa JS, King MD, Ray SM, White N, Blumberg HM. 2005. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA 300 clone as a cause of health care-associated infections among patients with prosthetic joint infections. Am. J. Infect. Control 33:385–391. 10.1016/j.ajic.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 8.Sharma M, Berriel-Cass D, Baran J., Jr 2004. Sternal surgical-site infection following coronary artery bypass graft: prevalence, microbiology, and complications during a 42-month period. Infect. Control Hosp. Epidemiol. 25:468–471. 10.1086/502423 [DOI] [PubMed] [Google Scholar]
- 9.Giacometti A, Cirioni O, Schimizzi AM, Del Prete MS, Barchiesi F, D'Errico MM, Petrelli E, Scalise G. 2000. Epidemiology and microbiology of surgical wound infections. J. Clin. Microbiol. 38:918–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel M, Kumar RA, Stamm AM, Hoesley CJ, Moser SA, Waites KB. 2007. USA300 genotype community-associated methicillin-resistant Staphylococcus aureus as a cause of surgical site infections. J. Clin. Microbiol. 45:3431–3433. 10.1128/JCM.00902-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson DJ, Sexton DJ, Kanafani ZA, Auten G, Kaye KS. 2007. Severe surgical site infection in community hospitals: epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 28:1047–1053. 10.1086/520731 [DOI] [PubMed] [Google Scholar]
- 12.Kusachi S, Sumiyama Y, Arima Y, Yoshida Y, Tanaka H, Nakamura Y, Nagao J, Saida Y, Watanabe M, Watanabe R, Sato J. 2007. Isolated bacteria and drug susceptibility associated with the course of surgical site infections. J. Infect. Chemother. 13:166–171. 10.1007/s10156-007-0513-z [DOI] [PubMed] [Google Scholar]
- 13.Gaynes R, Edwards JR, National Nosocomial Infections Surveillance System 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41:848–854. 10.1086/432803 [DOI] [PubMed] [Google Scholar]
- 14.Fox JL. 2013. Antimicrobial peptides stage a comeback. Nat. Biotechnol. 31:379–382. 10.1038/nbt.2572 [DOI] [PubMed] [Google Scholar]
- 15.Reddy KV, Yedery RD, Aranha C. 2004. Antimicrobial peptides: premises and promises. Int. J. Antimicrob. Agents 24:536–547. 10.1016/j.ijantimicag.2004.09.005 [DOI] [PubMed] [Google Scholar]
- 16.Fjell CD, Hiss JA, Hancock RE, Schneider G. 2012. Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov. 11:37–51. 10.1038/nrd3591 [DOI] [PubMed] [Google Scholar]
- 17.Yeung AT, Gellatly SL, Hancock RE. 2011. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. 68:2161–2176. 10.1007/s00018-011-0710-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myhrman E, Hakansson J, Lindgren K, Bjorn C, Sjostrand V, Mahlapuu M. 2013. The novel antimicrobial peptide PXL150 in the local treatment of skin and soft tissue infections. Appl. Microbiol. Biotechnol. 97:3085–3096. 10.1007/s00253-012-4439-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gisby J, Bryant J. 2000. Efficacy of a new cream formulation of mupirocin: comparison with oral and topical agents in experimental skin infections. Antimicrob. Agents Chemother. 44:255–260. 10.1128/AAC.44.2.255-260.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McRipley RJ, Whitney RR. 1976. Characterization and quantitation of experimental surgical-wound infections used to evaluate topical antibacterial agents. Antimicrob. Agents Chemother. 10:38–44. 10.1128/AAC.10.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rittenhouse S, Singley C, Hoover J, Page R, Payne D. 2006. Use of the surgical wound infection model to determine the efficacious dosing regimen of retapamulin, a novel topical antibiotic. Antimicrob. Agents Chemother. 50:3886–3888. 10.1128/AAC.00183-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramachandran S, Chen S, Etzler F. 1999. Rheological characterization of hydroxypropylcellulose gels. Drug Dev. Ind. Pharm. 25:153–161. 10.1081/DDC-100102155 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


