Abstract
The percentage of the dosing interval that the non-protein-bound plasma concentration is above the MIC (%fT>MIC) for cephalosporins has been shown to correlate with microbiological outcomes in preclinical studies. However, clinical data are scarce. Using data from a randomized double-blind phase 3 clinical trial, we explored the relationship of ceftobiprole exposure with microbiological and clinical outcomes in patients with nosocomial pneumonia. The individual ceftobiprole exposure was determined for different pharmacokinetic (PK)/pharmacodynamic (PD) indices using individual pharmacokinetic data and a previously published population model. The MICs used in the analysis were the highest MICs for any bacterium cultured at baseline or the end of treatment (EOT). Outcomes were microbiological cure at EOT and clinical cure at test of cure (TOC). Multiple logistic regression (MLR) and classification and regression tree (CART) analyses were applied to determine the relationships among exposure, patient characteristics, and outcomes. MLR indicated that the %fT>MIC of ceftobiprole was the best predictor for both microbiological eradication and clinical cure. CART analysis showed a breakpoint value of 51.1% (n = 159; P = 0.0024) for clinical cure, whereas it was 62.2% (n = 251; P < 0.0001) for microbiological eradication. Other factors also contributed, particularly to clinical outcome. These included the difference between VAP and non-VAP patients, systemic inflammatory response syndrome (SIRS), creatinine clearance, the use of anti-Pseudomonas combination therapy, and Acute Physiology and Chronic Health Evaluation II (APACHE-II) score. There is a strong correlation between microbiological eradication and clinical cure with exposure to ceftobiprole. The %fT>MIC required to result in a favorable clinical outcome is >51% of the dosing interval, which is in line with the values found for microbiological eradication, the comparator ceftazidime, and preclinical models.
INTRODUCTION
Ceftobiprole, the active moiety of its prodrug ceftobiprole medocaril, is a broad-spectrum cephalosporin active against most Gram-positive microorganisms, including methicillin-resistant Staphylococcus aureus (MRSA), as well as Gram-negative microorganisms. Similar to other β-lactams, studies in animals and in vitro pharmacokinetic (PK) models have shown that the percentage of the dosing interval that the maximum concentration of the free, unbound fraction of drug in serum is above the MIC (%fT>MIC) is the PK/pharmacodynamic (PD) index that best correlates with drug-related response (1). A %fT>MIC of 60 to 65% of the dosing interval has been identified in mouse infection models as the target of near-maximal bacterial killing, and a %fT>MIC of 40% best predicted bacteriostasis at 24 h for cephalosporins in general (2–4). These %fT>MIC values are often regarded as PD targets for drug exposure and as the values on which to base clinical breakpoints, and in general as targets to reach during therapy (5). Whereas data to support the use of these targets in clinical infections in humans for quinolones and glycopeptides have appeared over the last decade (6–8), the clinical evidence for beta-lactams was limited until recently, when a correlation between exposure to ceftazidime and microbiological eradication as well as clinical cure was demonstrated in patients with nosocomial pneumonia. Patients in whom a %fT>MIC of at least 44.9% was reached had a higher probability of bacterial eradication than patients with a lower exposure (9), and a significant relationship between %fT>MIC and clinical cure was also demonstrated. In addition, in a trial comparing continuous infusion versus intermittent infusion, Dulhunty et al. (10) demonstrated that the former was superior. In the present study, we investigated the relationships between microbiological eradication as well as clinical cure and exposure to ceftobiprole, using the data generated in a large phase 3 clinical trial.
MATERIALS AND METHODS
Trial, data set, and treatment.
This was a post hoc analysis of a clinical trial conducted between 2005 and 2007 to compare the efficacy of ceftobiprole to that of a combination of ceftazidime and linezolid for treatment of nosocomial pneumonia (NP), including ventilator-associated pneumonia (VAP). It was a randomized, double-blind, multicenter global phase 3 study involving 781 subjects (trial database, NCT00210964), of which all data recorded in the trial database were provided to the authors. The methodology of the trial is described elsewhere (9). The sample size of the original study was based on a noninferiority design with a margin of 15% and a power of 90%, with a total number of 770 subjects needed for randomization into both arms of the study. Included subjects were randomly assigned to treatment centrally in a 1:1 ratio to ceftobiprole (500 mg every 8 h, 2-h intravenous [i.v.] infusion) or linezolid (600 mg every 12 h, 1-h i.v. infusion) plus ceftazidime (2 g every 8 h, 2-h i.v. infusion) for 7 to 14 days. Subjects were stratified by infection type (non-ventilator-associated pneumonia [VAP] and VAP) and by Acute Physiology and Chronic Health Evaluation II (APACHE II) scores (scores of 8 to 19 and 20 to 25, respectively). Subjects who had VAP were further stratified by time to randomization after onset of mechanical ventilation (<5 or ≥5 days of ventilation). An independent data-monitoring committee was established to monitor data on an ongoing basis. Combination therapy (levofloxacin, amikacin, or gentamicin) was permitted in subjects at risk for pseudomonal infections. Subjects were evaluated before the start of therapy (baseline [BL]), during therapy, and at the end of therapy (EOT) within 24 h after the last administration of the study drug. A test-of-cure (TOC) evaluation was performed 7 to 14 days after the EOT visit.
None of the patients were excluded from the current analysis.
Microbiology.
Cultures were taken at BL, at EOT, and if possible at the TOC visit. Microbiological procedures were conducted according to local practice. Duplicate isolates from all assessments were stored and shipped to the central laboratory (Eurofins, Inc., Chantilly, VA) for pathogen identification and antimicrobial susceptibility testing using broth microdilution (11). Suitable specimens for ventilated subjects included those obtained by bronchoscopy with bronchoalveolar lavage/protected-brush sampling or transtracheal/endotracheal aspiration and for nonventilated patients included those obtained by deep expectoration or nasotracheal aspiration. Subsequently, the samples were processed according to local practices. Subjects with insufficient or unsuitable sputum specimens should have had the specimen repeated within 24 h of randomization. MICs determined in the central laboratory were used in the present analyses.
Calculation of individual PK parameter estimates and PK/PD indices.
A nonlinear mixed-effects modeling (NONMEM) population pharmacokinetic model as previously described was used (12). Briefly, data were best described by a 3-compartment model and included two covariates, creatinine clearance on clearance and age on the central volume of distribution. For patients with at least one serum sample, individual PK parameters were estimated by NONMEM as post hoc Bayesian estimates (n = 52). For patients without individual NONMEM estimates, parameter values were estimated using covariates (n = 312) as determined in the population PK model based on 171 individuals. Subsequently, the PK/PD indices %fT>MIC, area under the concentration-time curve for the free, unbound fraction of a drug (fAUC), fAUC/MIC, maximum concentration of the free, unbound fraction of drug in serum (fCmax), and fCmax/MIC were calculated for each individual patient using the KinFun106 program (Medimatics, Maastricht, the Netherlands). KinFun is based on an equation solver built in Excel using standard 2- and 3-compartment pharmacokinetic models and was used to calculate measures of exposure (%fT>MIC and fAUC/MIC) using estimates for the PK parameters, protein binding, and MICs. Steady state was assumed for calculations. A protein binding of 16% was used (13).
The indices were determined based on MICs in two different ways. The first was by taking the highest MIC of any pathogen cultured at BL. The second was by taking the microorganism with the highest MIC value at either BL or EOT (BL/EOT). Both Gram-negative and Gram-positive bacteria were included in the analysis.
Treatment outcome.
Various measures of outcome were used. At EOT the microbiological outcome was defined in two different ways. The first measure was eradication at EOT of the microorganism(s) cultured at baseline (outcome-BL-microorganism). For the second measure, if any microorganism was cultured at EOT, treatment was considered to have failed and was therefore independent of a positive or negative culture at baseline (outcome-EOT-culture). The analysis was performed for all patients in the intent-to-treat population (ITT) with a positive culture and for which the PK/PD indices could be derived. They represent the ITT PK/PD subset. At the TOC visit, both microbiological eradication and clinical outcome as well as clinically evaluable (CE) and microbiologically evaluable (ME) designations were used as defined per protocol and as described previously (9).
Statistical analysis.
PK/PD indices were correlated with microbiological outcome at EOT. For correlations of exposures with outcomes at TOC visit, the ME population was studied for microbiological outcome and the CE population for clinical outcome.
CART analysis to differentiate between lower and higher response rates and (multiple) logistic regression were performed using SAS (JMP) software version 9.02 and SAS 9.2 (SAS Institute, Cary, NC). Tests of significance for CART analysis were performed using Fisher's test (2 sided) as implemented in GraphPad Prism version 5.0 (GraphPad Software, Inc., San Diego, CA). For the multiple logistic regression, data for %fT>MIC, fAUC/MIC, fCmax/MIC, volume of distribution at steady state, APACHE II score, age, sex, body weight, body mass index, height, albumin, white-blood-cell count, creatinine clearance, creatinine, C-reactive protein (CRP), systemic inflammatory response syndrome (SIRS), and combination therapy with an antipseudomonal antibiotic and infection type (non-VAP/VAP) were entered in the model with entry levels of 0.25 and stay of 0.1, and subsequently both forward and backward selections were performed.
RESULTS
Patient population and demographics.
The total NP trial database consisted of 391 patients treated with ceftobiprole. There were 257 patients with a positive culture at BL and 267 at BL/EOT. For 27 patients, pharmacokinetic data were not available or could not be estimated because creatinine clearance was not available. Thus, PK/PD indices were calculated based on BL cultures for 243 individuals and based on BL/EOT cultures for 251 individuals. The demographic data of these patients are shown in Table 1.
TABLE 1.
Demographic data of patients with a positive culture in the NP trial database
| Parameter | Valuesa for patients used in analysis with: |
|
|---|---|---|
| PK/PD indices (n = 251) | Positive culture at BL/EOT (n = 267) | |
| Age (yr) | 60.3 (19–92) (251) | 59.7 (19–92) (267) |
| Sex (no. male/no. female) | 180/71 | 189/78 |
| Weight (kg) | 73.3 (29–129) (251) | 73.1 (29–129) (265) |
| BMIb (kg/m2) | 25.5 (11.3–42.9) (245) | 25.4 (11.3–42.9) (259) |
| Creatinine clearance (ml/min) | 106 (17.0–475) (250) | 106 (17.0–475) (250) |
| Infection type (VAPc/non-VAP) | 69/182 | 79/188 |
| SIRS (no. yes/no. no) | 189/62 | 197/70 |
| APACHE II score | 14.0 (8–26) (251) | 14.1 (8–26) (267) |
| Anti-Pseudomonas combination therapy (no. yes/no. no) | 47/204 | 51/216 |
Values for all parameters except sex, infection type, and anti-Pseudomonas combination therapy are given as the mean (range) (number of patients).
BMI, body mass index.
VAP, ventilator-associated pneumonia.
Microbiology.
Of the 257 patients with positive culture at baseline, 177 patients had a positive culture with at least one Gram-negative microorganism and 130 with at least one Gram-positive microorganism. Of the 267 patients with positive cultures at BL/EOT, 189 patients had at least one Gram-negative microorganism and 133 patients had at least one Gram-positive microorganism in the cultures, respectively. In most patients with a positive culture, 1 or 2 microorganisms were identified, but with up to 7 different microorganisms per patient (Table 2).
TABLE 2.
Species cultured at BL and BL/EOT
| Species with the highest MICs | BL cultures (n = 257 positive cultures) |
BL/EOT cultures (n = 267 positive cultures) |
||
|---|---|---|---|---|
| No. of the highest MICs | Range of highest ceftobiprole MICs (mg/liter)a | No. of the highest MICs | Range of highest ceftobiprole MICs (mg/liter)a | |
| Gram-negative bacteria | ||||
| Acinetobacter spp. | 20 | 0.12–32 | 29 | 0.12–32 |
| Citrobacter spp. | 4 | 0.06–8 | 4 | 0.06–8 |
| Enterobacter spp. | 18 | 0.03–32 | 15 | 0.03–32 |
| Escherichia coli | 32 | 0.03–32 | 33 | 0.03–32 |
| Haemophilus influenzae | 5 | 0.008–0.12 | 5 | 0.008–0.12 |
| Klebsiella spp. | 32 | 0.03–32 | 37 | 0.03–32 |
| Pseudomonas spp. | 45 | 0.25–32 | 46 | 0.5–32 |
| Proteus spp. | 6 | 0.015–32 | 6 | 0.03–32 |
| Serratia spp. | 8 | 0.12–32 | 7 | 0.12–32 |
| Stenotrophomonas maltophilia | 7 | 32 | 10 | 32 |
| Miscellaneous spp. | 12 | 0.008–32 | 13 | 0.008–32 |
| Gram-positive bacteria | ||||
| Corynebacterium spp. | 2 | 0.12b | 2 | 0.12b |
| Enterococcus spp. | 5 | 0.25–32 | 5 | 0.25–32 |
| Staphylococcus aureus | 97 | 0.12–8 | 100 | 0.12–8 |
| Staphylococcus spp. (excluding S. aureus) | 12 | 0.12–4 | 12 | 0.12–4 |
| Streptococcus pneumoniae | 9 | 0.008–1 | 9 | 0.008–1 |
| Streptococcus spp. (excluding S. pneumoniae) | 7 | 0.002–1 | 7 | 0.03–1 |
MICs of >16 mg/liter were recorded as 32 mg/liter.
In one strain, the MIC was not determined.
Exposure-response (PK/PD) analysis at end of treatment: microbiological outcome.
Multiple logistic regression (MLR) analysis using stepwise selection was performed to determine factors contributing to explaining exposure-response relationships. For eradication of BL microorganisms at EOT, the most significant factor entered in the model was %fT>MIC (P = 0.0003). However, creatinine clearance was also significant (P = 0.0039) (Table 3). Since the trial was stratified for non-VAP or VAP, a separate MLR analysis in each of the subgroups was performed. For non-VAP patients, %fT>MIC was the most significant variable entered in the model (P = 0.0033), but the MIC by itself was also significant (P = 0.0321). For VAP patients there were no significant predictors.
TABLE 3.
Multiple logistic regression analysis of factors determining microbiological eradication of BL and BL/EOT microorganisms at EOT
| Culture time (n) and covariate | Coefficient | SE | Chi square | P |
|---|---|---|---|---|
| BL (242) | ||||
| Intercept | 1.5395 | 0.4271 | 12.9934 | 0.0003 |
| %fT>MIC | 0.0145 | 0.00403 | 12.9630 | 0.0003 |
| Creatinine clearance | −0.00726 | 0.00251 | 8.3395 | 0.0039 |
| BL/EOT (251) | ||||
| Intercept | −0.8538 | 0.3738 | 5.2163 | 0.0224 |
| %fT>MIC | 0.0253 | 0.00363 | 48.5401 | <0.0001 |
| SIRS | 0.7497 | 0.3778 | 3.9370 | 0.0472 |
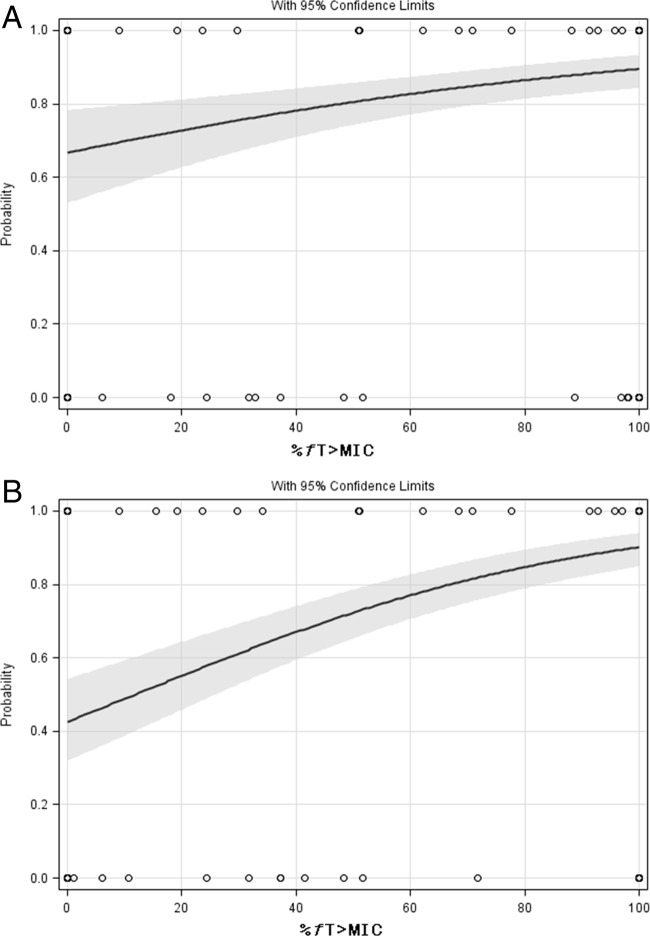
For microorganisms cultured at BL/EOT, %fT>MIC was the most significant predictor (P < 0.0001) using MLR, but SIRS was also marginally significant (P = 0.0472) (Table 3). In the subgroup analysis, %fT>MIC was the most significant predictor (P < 0.0001) in the non-VAP subgroup but SIRS was also marginally significant (P = 0.0476). In the VAP subgroup, %fT>MIC was the only significant predictor (P = 0.0013). Subsequent univariate analyses showed significant correlations between %fT>MIC and microbiological eradication at EOT (Fig. 1A and B). The Hosmer and Lemeshow goodness-of-fit test was nonsignificant, indicating a good fit.
FIG 1.
Univariate logistic regression analysis of %fT>MIC for microbiological eradication at EOT for the exposure based on BL MICs (A) (n = 243) and based on BL/EOT MICs (B) (n = 251) for all patients. (A) P = 0.0002. The gray area represents the 95% prediction interval. (B) P < 0.0001. The gray area represents the 95% prediction interval.
To determine the optimal split value for microbiological outcome for each potential predictor, a CART analysis was performed (Table 4). Both the highest MIC value of the microorganism cultured at BL and the highest MIC value of microorganisms cultured at BL/EOT were significantly correlated with microbiological eradication at EOT. All three PK/PD indices were also significantly associated with microbiological eradication at EOT. A %fT>MIC of at least 51.1% was correlated with microbiological eradication at EOT for patients in both the non-VAP subgroup and the VAP subgroup (P < 0.0001 and P = 0.0004, respectively).
TABLE 4.
Results of CART analysis for microbiological eradication (outcome) at EOT for a BL and BL/EOT microorganism
| Outcome and predictor | Split value | Successa in group with value more than the split value (no.) | Failureb in group with value more than the split value (no.) | Successa in group with value less than or equal to the split value (no.) | Failureb in group with value less than or equal to the split value (no.) | Pc |
|---|---|---|---|---|---|---|
| Microbiological eradication at EOT of BL microorganismd (243 patients) | ||||||
| MICe | 2 | 69 | 27 | 136 | 11 | <0.0001 |
| %fT>MIC | 100 | 157 | 14 | 48 | 24 | <0.0001 |
| %fT>MICf | 51.2 | 168 | 37 | 19 | 19 | <0.0001 |
| fAUC/MIC | 197.8 | 103 | 4 | 102 | 34 | <0.0001 |
| fCmax/MIC | 9.31 | 150 | 12 | 55 | 26 | <0.0001 |
| Microbiological eradication at EOTg (251 patients) | ||||||
| MICe | 8 | 47 | 46 | 143 | 15 | <0.0001 |
| %fT>MIC | 62.2 | 154 | 16 | 36 | 45 | <0.0001 |
| fAUC/MIC | 12.1 | 154 | 16 | 36 | 45 | <0.0001 |
| fCmax/MIC | 2.22 | 155 | 16 | 35 | 45 | <0.0001 |
Failure instead of success for MIC.
Success instead of failure for MIC.
The P value refers to the significance of the split value.
Outcome: eradication at end of treatment of the microorganisms cultured at baseline.
In 2-fold dilution series.
Alternative split.
Outcome: positive or negative culture at end of treatment.
Exposure-response (PK/PD) analysis at test of cure clinical outcome.
To determine the most important predictor(s) of clinical cure, MLR was performed. Using the BL MIC values to calculate the exposure, the most significant predictor was non-VAP versus VAP patient (P < 0.0001). Other significant independent predictors were SIRS (P = 0.0049), anti-Pseudomonas combination therapy (P = 0.0177), and %fT>MIC (P = 0.0445) (Table 5). Because the difference between non-VAP and VAP patients was the most significant predictor and a stratification factor during the trial, a separate MLR analysis was performed for each of the two subgroups. In the non-VAP subgroup, SIRS (P = 0.0025), anti-Pseudomonas combination therapy (P = 0.0324), and %fT>MIC (P = 0.0401) were independent significant predictors of clinical cure at TOC, whereas there was no significant predictor in the VAP subgroup.
TABLE 5.
Multiple logistic regression analysis of factors determining clinical cure at TOC for all patients
| Culture time (n) and covariate | Coefficient | SE | Chi square | P |
|---|---|---|---|---|
| BL (159) | ||||
| Intercept | −0.3157 | 0.5505 | 0.3289 | 0.5663 |
| NP vs VAP patient | −1.9829 | 0.4611 | 18.4919 | <0.0001 |
| SIRS | 1.2103 | 0.4300 | 7.9238 | 0.0049 |
| Anti-Pseudomonas combination therapy | −1.1350 | 0.4785 | 5.6256 | 0.0177 |
| %fT>MIC | 0.0103 | 0.00514 | 4.0376 | 0.0445 |
| BL/EOT (166) | ||||
| Intercept | 1.5638 | 0.6930 | 5.0926 | 0.0240 |
| NP vs VAP patient | −2.0851 | 0.4464 | 21.8203 | <0.0001 |
| %fT>MIC | 0.0118 | 0.00431 | 7.4787 | 0.0062 |
| APACHE score | −0.0892 | 0.0429 | 4.3208 | 0.0376 |
When the exposure to ceftobiprole was based on BL/EOT MICs, MLR showed significance for non-VAP patient versus VAP patient (P < 0.0001), %fT>MIC (P = 0.0062), and APACHE II score (P = 0.0376) (Table 5). A separate subgroup analysis for VAP and non-VAP revealed a single predictor for clinical cure at TOC, %fT>MIC (P = 0.0023, n = 130), in the non-VAP subgroup. The APACHE II score was not significant in this subgroup. Within the VAP subgroup there were no significant predictors (n = 36).
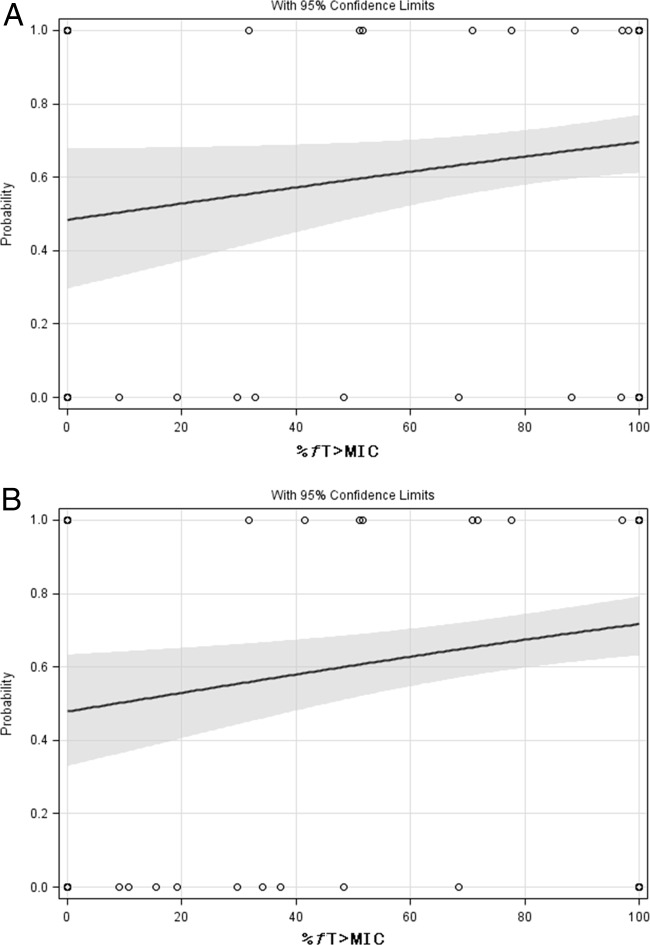
Univariate logistic regression of %fT>MIC for the total population showed that the relationship with clinical cure was not quite significant (P = 0.0537) (Fig. 2A) if based on BL, whereas it was highly significant for BL/EOT microorganism (P = 0.0088) (Fig. 2B). For both analyses, the Hosmer and Lemeshow test was not significant, indicating no lack of fit. Subgroup analysis within the non-VAP subgroup showed significant relationships for BL as well as BL/EOT (P = 0.0144 and P = 0.0023, respectively). Within the VAP subgroups, these relationships were nonsignificant. This analysis was further substantiated by the CART analysis (Table 6) showing that clinical cure was not significantly associated with the MIC of the BL microorganism, but was significantly correlated with the MIC of the BL/EOT cultures. Significant relationships were demonstrated between exposure based on BL and BL/EOT MICs. The %fT>MIC was the index best correlated with clinical cure, with a split value of 51.1 %fT>MIC, P is 0.0243 for BL and P is 0.0029 for BL/EOT, respectively.
FIG 2.
Univariate logistic regression analysis of %fT>MIC and clinical cure at TOC for exposures based on BL MICs (A) (n = 159) and based on BL/EOT MICs (B) (n = 166) for the total population. (A) P = 0.0537. The gray area represents the 95% prediction interval. (B) P = 0.0088. The gray area represents the 95% prediction interval.
TABLE 6.
Results of CART analysis for clinical cure (outcome) at the TOC visit with predictors based on microorganisms cultured at BL and eradicated at EOT or microorganisms cultured at BL/EOT and eradicated at EOT
| Outcome (n) and predictor | Split value | Successa in group with value more than the split value (no.) | Failureb in group with value more than the split value (no.) | Successa in group with value less than or equal to the split value (no.) | Failureb in group with value less than or equal to the split value (no.) | Pc |
|---|---|---|---|---|---|---|
| Clinical cure at TOC based on BL cultures (159) | ||||||
| MICd (mg/liter) | 8 | 96 | 53 | 9 | 1 | 0.1002 |
| %fT>MIC | 51.1 | 93 | 40 | 12 | 14 | 0.0243 |
| fAUC/MIC | 3,838 | 96 | 54 | 9 | 0 | 0.0288 |
| fCmax/MIC | 2.19 | 88 | 33 | 21 | 17 | 0.048 |
| Clinical cure at TOC based on BL/EOT cultures (166) | ||||||
| MIC (mg/liter) | 8 | 84 | 33 | 25 | 24 | 0.0124 |
| %fT>MIC | 51.1 | 89 | 34 | 20 | 23 | 0.0029 |
| fAUC/MIC | 10.7 | 89 | 34 | 20 | 23 | 0.0029 |
| fCmax/MIC | 2.19 | 89 | 34 | 20 | 23 | 0.0029 |
Failure instead of success for MIC.
Success instead of failure for MIC.
The P value refers to the significance of the split value.
In 2-fold dilution series.
DISCUSSION
Analysis of a phase 3 study in patients with nosocomial pneumonia showed that microbiological outcome at EOT and clinical outcome at TOC in patients treated with ceftobiprole were significantly correlated with exposure to this drug. %fT>MIC was the PK/PD index correlated with the highest probability of microbiological and clinical cure. A high probability of clinical cure at TOC was attained at values of >51.1% fT>MIC, whereas for microbiological cure slightly higher values were required to achieve the highest probability of microbiological eradication at EOT (62.2% fT>MIC for BL/EOT cultures). Whereas for microbiological eradication at EOT only %fT>MIC was a significant predictor for success outcome, other patient-related factors significantly contributed to clinical cure at TOC as well. These results compare well with the PK/PD analyses of ceftazidime and Gram-negative microorganisms in the comparator arm of the trial, where %fT>MIC was also identified as the parameter correlating with outcome in patients (9).
To calculate the PK/PD indices, we used the highest MIC encountered, reasoning that outcome would be related to the bacteria with the lowest exposure in case multiple microorganisms were cultured (9, 14). The cultures that were used were taken at BL and at EOT. We did not take TOC cultures into account. There is a significant time lapse between EOT and TOC, with significant recolonization or disappearance of the microorganism that cannot be attributed directly to therapy. These are very likely interfering with the actual effect of exposure during and immediately after treatment. However, changes in microbiology or MICs during therapy can be considered a direct effect of treatment. We therefore used two different data sets to determine the highest MIC of cultured microorganisms, one at BL and the other including MICs of microorganisms at the end of therapy. Likewise, we defined two different microbiological outcome measures. In the first outcome measure we took the conventional approach and determined whether microorganisms cultured at BL were eradicated at EOT. In addition, we defined the second outcome measure from combined BL and EOT cultures. Both approaches resulted in %fT>MIC as the only significant PK/PD index predictive for a favorable outcome, but the BL/EOT approach was more significant. This is predictable, as most likely patients become colonized before or during therapy with microorganisms that have relatively high MICs. The results indicate that these patients have a higher probability of microbiological as well as clinical failure.
The exposure curve of %fT>MIC as a function of the MIC is relatively steep. Because of the relatively long duration of infusion (2 h) and half-life (approximately 3 h) of ceftobiprole, the concentration time curve is relatively flat. The consequence hereof is that concentrations are either above the MIC or below the MIC for the full duration of the dosing interval for the majority of patients, corresponding to %fT>MIC values of 100 and 0, respectively. This is coming close to what is observed for patients receiving continuous infusion. A similar distribution of %fT>MIC versus MIC was observed for ceftazidime infused over 2 h in the comparator arm of the trial (9). This phenomenon is especially the case for the outcome measure based on BL cultures only, because of the relatively high number of microorganisms with low MICs. As a result, the split values as determined by CART analysis can be influenced by relatively small changes in the numbers. For example, microbiological eradication of BL microorganisms at EOT is significantly associated with exposure to ceftobiprole, with a split value of 100% fT>MIC, but there is also a second split value of 51% fT>MIC, which is also significant and concurs with values described elsewhere, i.e., 45% fT>MIC for ceftazidime and Gram-negative microorganisms in the comparator arm (9, 15).
Similar to the exposure-response relationship found for other antimicrobials (6, 7, 16, 17), the relationship found here indicates that optimal exposure increases the likelihood of microbiological eradication. In patients with insufficient exposure to ceftobiprole, the probability of microbiological eradication is approximately 44%, whereas the probability of microbiological eradication in patients with an exposure of 100% is 91%. Therefore, one might conclude that the additional effect of optimal treatment with ceftobiprole is approximately 47%. A similar observation was made with ceftazidime, for which at very low exposure (up to 25% fT>MIC) almost half the patient population is microbiologically cured (9). For both compounds, factors contributing to this effect include the use of other antibiotics, the immune systems of the patients, and the state of the disease. This has similarly been described for other antibiotics (3).
Similar to our results in humans, a thigh infection model in neutropenic mice showed that the PK/PD index best correlated with effect is %fT>MIC. The %fT>MIC required for a static effect varied between 14% and 48% of the dosing interval (15). In these experiments, the %fT>MIC required was twice as long for Enterobacteriaceae as for Staphylococcus aureus or Streptococcus pneumoniae (15). A higher percentage of time above the MIC required for ceftobiprole against Enterobacteriaceae than required against S. aureus and S. pneumoniae was also reported in other studies (18–20). These studies also showed no differences in efficacy between the thigh model and the lung model. The values found in this study, in which a %fT>MIC values of 51 to 62% and 51% were required for microbiological or clinical response, respectively, also concur with those found for ceftazidime, where a %fT>MIC of 45% of the dosing interval for the eradication of Gram-negative microorganisms has been described earlier in patients with nosocomial pneumonia (9).
The split values found for microbiological eradication and clinical cure were comparable. However, multiple logistic regression analysis indicated that VAP status by itself was the most important predictor for clinical cure, even with %fT>MIC as a significant predictor in the univariate analysis. Although within the non-VAP subgroup %fT>MIC was an independent predictor for clinical cure, it was not in the VAP subgroup. This could not be explained by differences in pharmacokinetics between the two subgroups, as we did not find differences in exposure. The split value for microbiological eradication of 51.1% was similar in both the VAP and non-VAP -subgroups, and the proportions of patients above and below the split value were not significantly different in the non-VAP and VAP subgroups (68% versus 70%).
Since we determined the correlation between the exposure of the comparator ceftazidime and clinical outcomes for patients infected with Gram-negative bacteria in an earlier analysis (9), we explored whether significant differences between ceftazidime and ceftobiprole exposures could explain the difference in outcome in the VAP subgroup. In the ceftobiprole arm, 34 patients had exposures higher than the split value and 19 had exposures below this value, whereas in the ceftazidime arm these numbers were 26 and 23, respectively, indicating similar exposures in the VAP subgroup between patients treated with ceftobiprole or with ceftazidime (Fisher's exact test, P = 0.315). There appears to be no explanation within the PK/PD analysis for the difference between the two subgroups.
We conclude that there is a strong correlation between microbiological eradication as well as clinical cure with exposure to ceftobiprole. The %fT>MIC that is required to result in likely favorable clinical outcome is >51%, which is in line with the value found for microbiological eradication and values found in preclinical models.
ACKNOWLEDGMENTS
We thank Anne-Hortense Schmitt-Hoffmann and Mark Jones for fruitful discussions.
This work was supported by an unrestricted grant from Basilea Pharmaceutica International, Ltd. Part of this work was financially supported by the European Union project Saturn (grant Health-F3-2009-241796).
J.W.M. has been a consultant and/or received research funding from Angelini, Merck & Co., Cubist, Jansen-Cilag, Astra-Zeneca, Basilea, Pfizer, and Polyphor. A.E.M. and N.P. declare that they have no conflicts of interest.
Footnotes
Published ahead of print 10 February 2014
REFERENCES
- 1.Andes D, Craig WA. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376–1383. 10.1128/AAC.50.4.1376-1383.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug'. Nat. Rev. Microbiol. 2:289–300. 10.1038/nrmicro862 [DOI] [PubMed] [Google Scholar]
- 3.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10; quiz 11–12. 10.1086/516284 [DOI] [PubMed] [Google Scholar]
- 4.Mouton JW, Punt N, Vinks AA. 2007. Concentration-effect relationship of ceftazidime explains why the time above the MIC is 40 percent for a static effect in vivo. Antimicrob. Agents Chemother. 51:3449–3451. 10.1128/AAC.01586-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouton JW, Brown DF, Apfalter P, Canton R, Giske CG, Ivanova M, MacGowan AP, Rodloff A, Soussy CJ, Steinbakk M, Kahlmeter G. 2012. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin. Microbiol. Infect. 18:E37–E45. 10.1111/j.1469-0691.2011.03752.x [DOI] [PubMed] [Google Scholar]
- 6.Bhavnani SM, Forrest A, Hammel JP, Drusano GL, Rubino CM, Ambrose PG. 2008. Pharmacokinetics-pharmacodynamics of quinolones against Streptococcus pneumoniae in patients with community-acquired pneumonia. Diagn. Microbiol. Infect. Dis. 62:99–101. 10.1016/j.diagmicrobio.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 7.Ambrose PG, Grasela DM, Grasela TH, Passarell J, Mayer HB, Pierce PF. 2001. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob. Agents Chemother. 45:2793–2797. 10.1128/AAC.45.10.2793-2797.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86. 10.1086/510079 [DOI] [PubMed] [Google Scholar]
- 9.Muller AE, Punt N, Mouton JW. 2013. Optimal exposures of ceftazidime predict the probability of microbiological and clinical outcome in the treatment of nosocomial pneumonia. J. Antimicrob. Chemother. 68:900–906. 10.1093/jac/dks468 [DOI] [PubMed] [Google Scholar]
- 10.Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, Shirwadkar C, Eastwood GM, Myburgh J, Paterson DL, Lipman J. 2013. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin. Infect. Dis. 56:236–244. 10.1093/cid/cis856 [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. CLSI M07-A8 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 12.Muller AE, Schmitt-Hoffmann AH, Punt N, Mouton JW. 2013. Monte Carlo simulations based on phase 1 studies predict target attainment of ceftobiprole in nosocomial pneumonia patients: a validation study. Antimicrob. Agents Chemother. 57:2047–2053. 10.1128/AAC.02292-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murthy B, Schmitt-Hoffmann A. 2008. Pharmacokinetics and pharmacodynamics of ceftobiprole, an anti-MRSA cephalosporin with broad-spectrum activity. Clin. Pharmacokinet. 47:21–33. 10.2165/00003088-200847010-00003 [DOI] [PubMed] [Google Scholar]
- 14.Mouton JW, Jacobs N, Tiddens H, Horrevorts AM. 2005. Pharmacodynamics of tobramycin in patients with cystic fibrosis. Diagn. Microbiol. Infect. Dis. 52:123–127. 10.1016/j.diagmicrobio.2005.02.011 [DOI] [PubMed] [Google Scholar]
- 15.Craig WA, Andes DR. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob. Agents Chemother. 52:3492–3496. 10.1128/AAC.01273-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preston SL, Drusano GL, Berman AL, Fowler CL, Chow AT, Dornseif B, Reichl V, Natarajan J, Corrado M. 1998. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125–129. 10.1001/jama.279.2.125 [DOI] [PubMed] [Google Scholar]
- 17.Kimko H, Xu X, Nandy P, Samtani MN, Strauss RS, Bagchi P, Noel GJ. 2009. Pharmacodynamic profiling of ceftobiprole for treatment of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 53:3371–3374. 10.1128/AAC.01653-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig WA. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89–96. 10.1016/0732-8893(95)00053-D [DOI] [PubMed] [Google Scholar]
- 19.Azoulay-Dupuis E, Bedos JP, Mohler J, Schmitt-Hoffmann A, Schleimer M, Shapiro S. 2004. Efficacy of BAL5788, a prodrug of cephalosporin BAL9141, in a mouse model of acute pneumococcal pneumonia. Antimicrob. Agents Chemother. 48:1105–1111. 10.1128/AAC.48.4.1105-1111.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. North Am. 17:479–501. 10.1016/S0891-5520(03)00065-5 [DOI] [PubMed] [Google Scholar]