Abstract
For HIV infection, anti-HIV drug combinations are typically used as highly active antiretroviral therapy (HAART), intended to maximize viral suppression. Three drugs used frequently in combination are the protease inhibitors lopinavir and ritonavir and the nucleotide reverse transcriptase inhibitor tenofovir. We have successfully developed a simple, efficient, and sensitive method to simultaneously extract and determine the concentrations of lopinavir, ritonavir, and tenofovir in plasma samples. The plasma extractions were performed using a liquid-liquid extraction followed by protein precipitation of the remaining aqueous layer. The collected fractions were combined, dried, and reconstituted in the mobile phase. The drugs were quantified using liquid chromatography coupled with tandem mass spectrometry. The assay was applied to a study of plasma drug levels in two primates (Macaca nemestrina). The bioanalytical assay was optimized and validated to exhibit a high extraction efficiency and good sensitivity and reproducibility. When the assay was applied in a primate study, all three drugs could be detected in plasma within minutes of subcutaneous dosing. This validated assay will be useful for evaluation of drug concentrations in an efficient, selective, and sensitive manner.
INTRODUCTION
Since the introduction of highly active antiretroviral combination therapy (HAART) in the late 1990s, the life expectancy and quality of life of human immunodeficiency virus (HIV)-infected patients have improved due to plasma virus load reductions to below detectable levels (1, 2). Most HAART treatments include two or three anti-HIV drugs targeted to different viral proteins. Typically, a nucleoside/nucleotide reverse transcriptase inhibitor (NRTI/NtRTI) is combined with a nonnucleoside reverse transcriptase inhibitor (NNRTI), intended to reduce the risk of harboring drug-resistant virus, or with one or more protein inhibitors (PIs) to attack the virus at multiple HIV enzyme targets. In addition to PIs, other, newer classes of drugs, such as integrase inhibitors, are also used in various drug combinations (3, 4).
Among the PIs used in HAART, lopinavir is formulated as a combination with a subtherapeutic dose of ritonavir in the product Kaletra. In Kaletra, ritonavir is intended to inhibit cytochrome P450 (CYP) 3A isoenzyme metabolism of lopinavir, thereby boosting lopinavir exposure (5). Ritonavir has also been shown to inhibit drug efflux transport by P-glycoprotein (PgP) and therefore could enhance cellular retention of lopinavir (6). Tenofovir, an NtRTI, is often prescribed with lopinavir and ritonavir. Tenofovir, given as tenofovir disoproxil fumarate as an oral prodrug to increase absorption but readily hydrolyzing to the tenofovir active form, is recommended in the WHO HIV/AIDS treatment guidelines as a combinational drug of HAART (3).
A number of reports have described bioanalytical assays for simultaneous extraction and detection of lopinavir and ritonavir, alone or in combination with additional PIs and NNRTIs, from plasma and/or cell samples (5, 7–10). Some assays detect tenofovir alone or in combination with other drugs, such as lamivudine (11–13). Two different analytical assays were employed to determine time course plasma drug concentrations in lopinavir-ritonavir and tenofovir drug interaction studies (14–16). The two-assay approach is labor-intensive and time-consuming and also requires an expanded plasma volume and other resources. While a one-step liquid chromatography-mass spectrometry (LC-MS) assay was previously developed that is capable of detecting 17 anti-HIV drugs in plasma (17), the ability to detect plasma drug levels consistently in patients taking lopinavir, ritonavir, and tenofovir is not clear.
One of the challenges in developing a single assay for these three drugs is the significant differences in hydrophobicity of the PIs (lopinavir and ritonavir) and the NtRTI (tenofovir). While lopinavir and ritonavir are hydrophobic, and hence insoluble in water, tenofovir is hydrophilic. This difference makes it challenging to extract the three drugs effectively and simultaneously from plasma and also to identify a suitable chromatographic column matrix for the separation. Thus, a creative solution is needed. We systematically addressed these issues and developed a single chromatographic assay to detect all three compounds, lopinavir, ritonavir, and tenofovir, simultaneously by using LC coupled with tandem MS (LC-MS/MS). The optimized method is capable of extracting and quantifying the plasma concentrations of the three drugs simultaneously with high efficiency, selectivity, and sensitivity.
MATERIALS AND METHODS
Chemicals.
Original standards of lopinavir, ritonavir, and tenofovir were provided by the NIH AIDS Research and Reference Reagent Program. Some later samples were purchased from Waterstone Technology (Carmel, IN). Cyheptamide was purchased from Sigma-Aldrich (St. Louis, MO). Acetic acid (HAc) was of glacial grade and was obtained from J. T. Baker (Center Valley, PA). Water, acetonitrile, methanol (all optima grade), and methylene chloride were obtained from Fisher Scientific (Pittsburgh, PA). Trifluoroacetic acid (TFA) was purchased from Aldrich (Milwaukee, WI). All other reagents were of analytical grade or higher.
Instrumentation.
The high-pressure liquid chromatograph (HPLC) consisted of two Shimadzu LC-20A pumps, a DGU-20A5 degasser, and a Shimadzu SIL-20AC HT autosampler. This was coupled to a 3200 QTRAP mass spectrometer from Applied Biosystems (Grand Island, NY), equipped with an electrospray ionization (ESI) TurboIonSpray source. The software to control the systems and to process the data was Analyst software, version 1.5.2 (ABSciex, Framingham, MA).
HPLC-MS method.
Separations were carried out on a Synergi column (100 × 2.0 mm; 4-μm particle size) (Phenomenex, Torrance, CA). A C8 guard column (4.0 × 2.0 mm) was used (Phenomenex). The separations were done under ambient temperature, and the flow rate was set to 0.35 ml/min. The injection volume was 5 μl. The mobile phase for the separations consisted of buffers A (water with 0.1% HAc) and B (acetonitrile with 0.1% HAc). The gradient program used was as follows: 3% buffer B for 2.0 min, 50% buffer B at 2.1 min, 100% buffer B at 4.0 min to 5.5 min, and 3% buffer B at 6.0 min, held until 8.5 min. The needle wash was a solution of methanol-water (50-50 [vol/vol]).
Analytes were monitored using multiple-reaction monitoring (MRM) mode for positive ions. In MRM, a selected precursor ion is fragmented to a specific ion, which is analyzed at the detector. By cycling through different precursor–fragment-ion transitions, additional selectivity is allowed beyond separation on the column. The following ion transitions were monitored: for lopinavir, m/z 629.4 → 447.2; for ritonavir, m/z 721.3 → 296.1; for tenofovir, m/z 288.1 → 176.1; and for the internal standard (IS), m/z 238.1 → 193.2. The detector parameters were as follows: curtain gas (N2), 30 psi; ion-spray voltage, 5 kV; temperature, 475°C; nebulizer gas (N2), 40 psi; dry gas (N2), 40 psi; and collision gas, set to medium.
Standard samples.
Standard samples were weighed in two different batches for all three drugs independently and dissolved to a concentration of 50 μg/ml. Lopinavir and ritonavir were kept in acetonitrile, while tenofovir was kept in a solution of water-acetonitrile (50-50 [vol/vol]). The stock solutions were stored at −20°C. Working solutions were diluted from the stock to 1 μg/ml in water-acetonitrile (50-50 [vol/vol]) and were kept at 4°C. One of the weighings was used to generate the calibration samples, while the other was used for quality control (QC) samples.
The internal standard (cyheptamide) was prepared in a stock solution of 250 μg/ml in acetonitrile and kept at −20°C. Working solutions of 10 μg/ml and 1 μg/ml were diluted from the stock and kept at 4°C.
Calibration samples were prepared in water-acetonitrile (90-10) with 0.1% HAc at 11 different concentrations, ranging from 1 ng/ml to 1,000 ng/ml. Quality control samples were prepared at low, medium, and high concentrations (5, 50, and 750 ng/ml).
Sample preparation.
A liquid-liquid extraction (LLE) method was developed for simultaneous extraction of all three drugs from plasma. Plasmas from primates were used and were stored at −80°C until use.
Two-hundred-microliter plasma samples were spiked with 20-μl aliquots of the working solution (1 μg/ml) to generate a standard curve with 3 QC concentrations (low, medium, and high [5, 50, and 750 ng/ml]). To the unknown samples, 20 μl water-acetonitrile (50-50) was added to bring the volume up to that of the spiked samples. Ten microliters of IS (1 μg/ml cyheptamide) was added. To this, 5 μl of 4 M KOH was added for pH adjustment. LLE was performed by adding 500 μl of methylene chloride, and the samples were vortexed for 5 min. The samples were then centrifuged in an Eppendorf centrifuge (Danfoss, Denmark) for 10 min at 14,000 rpm (20,800 × g) at 4°C, and the organic phase was collected. The LLE step was repeated once. To the remaining aqueous phase, 20 μl TFA was added in order to perform protein precipitation (PP). The sample was vortexed and centrifuged as described above. Two hundred microliters of the aqueous phase was collected and added to the earlier collected organic phase. This was dried under a stream of N2 gas at 40°C and reconstituted in water-acetonitrile (90-10) with 0.1% HAc.
Validation.
Validation of the assay was performed based on the Guidance for Industry, Bioanalytical Method Validation, issued by the U.S. Food and Drug Administration (FDA) (18). Parameters evaluated were linearity, sensitivity, accuracy, precision, stability, selectivity, and recovery.
The linearity was tested in the range of 1 to 1,000 ng/ml for all three drugs. For this determination, 11 different concentration points were injected in triplicate, in order from lowest to highest. The back-calculated accuracy had to be ±15%, or ±20% for the lowest value, for the standard curve to be accepted.
Sensitivity was determined by finding the limit of detection (LOD) and limit of quantification (LOQ) for the substances from samples not used in the calibration curve, determined according to the guidelines of the Clinical and Laboratory Standards Institute (19).
For determinations of intra- and interday accuracy and precision, QC samples were used at low, medium, and high concentrations. The accepted limit for both accuracy and precision was ±15% for all concentrations except for the lowest point, where it was ±20%.
For selectivity, blank plasma samples were tested to evaluate interferences at the retention times of the drugs of interest.
The recovery was calculated as the extraction yield, comparing extracted standards with standards spiked in extracted blank plasma, representing a 100% yield.
Primate study.
To confirm the method, nonhuman primates (pigtailed macaques [Macaca nemestrina]) were injected subcutaneously with a suspension consisting of lopinavir-ritonavir-tenofovir (20:14.3:13.6 mg/kg of body weight [10:5:15 molar ratio]). The aqueous suspension was composed of 8% dimethyl sulfoxide (DMSO), 0.1% Tween 20, 0.7% NaCl, and 20 mM NaHCO3. The primate studies were done under an approved Institutional Animal Care and Use Committee (IACUC) protocol.
Blood was collected at the following time points: 0 (predose), 0.5, 1, 3, 5, 8, and 24 h. The samples were obtained in K2-EDTA collection tubes and immediately put on ice. Whole blood was centrifuged at 1,200 rpm for 10 min (4°C), and the plasma was collected and subsequently transferred to cryovials and stored at −80°C until analysis.
RESULTS
Method development. (i) Optimization of support matrix for separation of lopinavir, ritonavir, and tenofovir.
To develop a single chromatographic assay in which all three analytes could be measured simultaneously, five different columns were evaluated. They included an Agilent Zorbax SB-C18 column (150 × 2.1 mm; 5-μm particle size), an Agilent Zorbax SB-C8 column (150 × 2.1 mm; 5-μm particle size), a Waters Acquity phenyl column (100 × 2.1 mm; 1.7-μm particle size), a Thermo Scientific Hypersil Gold aQ column (100 × 2.1 mm; 3-μm particle size), and a Phenomenex Synergi Polar-RP column (100 × 2.0 mm; 4-μm particle size). The Zorbax SB-C18 column was unable to bind tenofovir properly, and hence the drug eluted with the solvent front. In contrast, the Zorbax SB-C8 column was able to separate the protease inhibitors, but tenofovir did not show any peak. The Acquity phenyl column failed to bind tenofovir, and the compound eluted with the solvent front; also, the lopinavir and ritonavir peaks showed significant peak tailing. While the Hypersil Gold aQ column exhibited a peak for tenofovir, the intensity was low, and the lopinavir and ritonavir peaks showed high levels of variability. Only the Synergi column retained tenofovir effectively, along with lopinavir and ritonavir, and produced sharp peaks consistently and at high intensities for all three compounds. Therefore, the Synergi column was used for solvent and method optimization.
(ii) Optimization of mobile phase.
We first used combinations of acetonitrile and methanol with water. Acetonitrile was chosen over methanol because of its lower viscosity, allowing higher flow rates and shorter run times. Also, this choice provided a higher intensity for tenofovir, the analyte with the lowest sensitivity. For detection of tenofovir, an acidic solvent was necessary. Thus, we evaluated formic, acetic, and trifluoroacetic acids. Among them, acetic acid produced the highest sensitivity and peak heights.
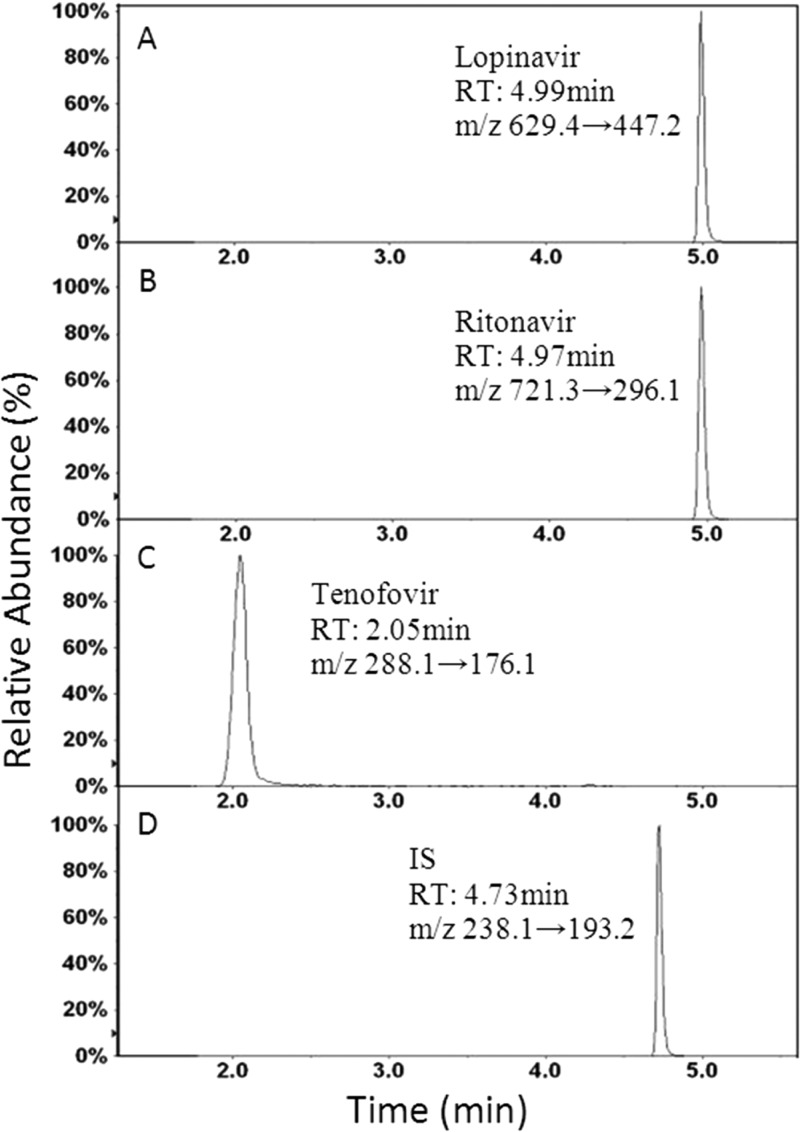
Typical HPLC-MS chromatograms under optimized conditions for the three compounds are shown in Fig. 1. Tenofovir bound to the column more weakly than the other compounds and eluted first, at 2.05 min (±0.1 min). Tenofovir was followed by cyheptamide (IS), at 4.73 min (±0.02 min); ritonavir, at 4.97 min (±0.05 min); and lopinavir, at 4.99 min (±0.05 min). With two compounds eluting at the same time, though separated by use of two different mass transition channels, there is a possibility of quenching of the signal at the detector. This was investigated at low, medium, and high concentrations and was found to be negligible. Based on the optimized LC-MS/MS conditions, we could detect all three compounds plus the IS by using a single-column chromatographic method. The optimized conditions were used for subsequent studies.
FIG 1.
Chromatograms of the drugs. (A) Lopinavir; (B) ritonavir; (C) tenofovir; (D) IS (cyheptamide). RT, retention time.
(iii) Optimization of extraction method.
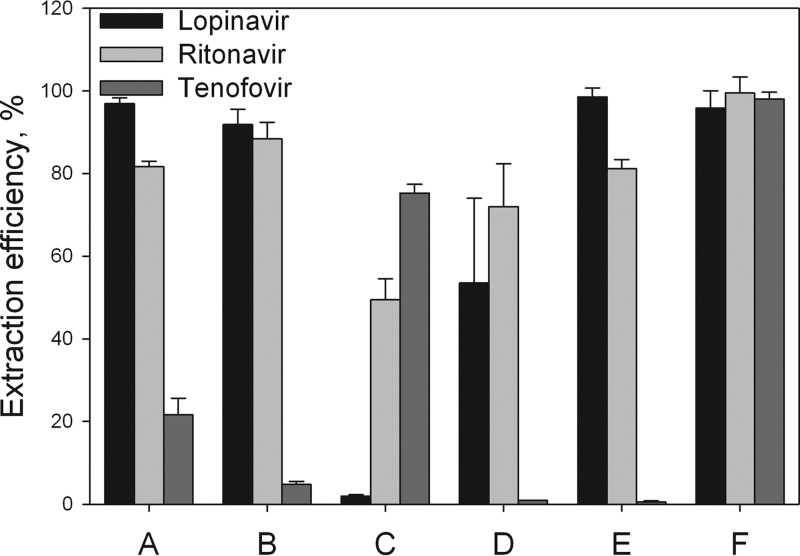
To simultaneously extract lopinavir, ritonavir, and tenofovir from plasma, we evaluated 6 different methods. The significant differences in hydrophobicity and solubility between the compounds presented a challenge. We compared 6 methods for recovery efficiency, and the results are presented in Fig. 2. The first attempt was a simple protein precipitation (PP) with acetonitrile (method A) or methanol (method B). While this PP approach could extract lopinavir and ritonavir, recovery of tenofovir was low. Next, we used TFA for PP (method C), and this gave a low level of recovery for lopinavir, about 50% recovery for ritonavir, and a high level of recovery for tenofovir. Therefore, we evaluated an LLE method using methylene chloride. The LLE was evaluated with addition of an acid (method D) or a base (method E) to transform drugs in charged or uncharged form. The acidic LLE extraction (method D) gave recoveries of about 55% for lopinavir and 75% for ritonavir, but the yield for tenofovir was very poor. The basified extraction (method E) gave satisfying results for lopinavir and ritonavir, but recovery of tenofovir was, again, below the accepted percentage. Therefore, to overcome this deficiency, the LLE method was combined with protein precipitation using TFA (method F). This final method was shown to give very high levels of recovery for all three compounds, in a reproducible manner, within a time frame of 1 h, and this method was chosen for subsequent assay validation.
FIG 2.
Different extraction methods investigated during assay development. (A) PP done with acetonitrile; (B) PP done with methanol; (C) PP done with trifluoroacetic acid; (D) LLE done under acidic conditions; (E) LLE done under basic conditions; (F) final method, as described in Materials and Methods. PP, protein precipitation; LLE, liquid-liquid extraction.
Assay validation.
The optimized assay is reproducible and exhibits linearity over a wide dynamic range. The linearity, measured over the range of 1 to 1,000 ng/ml, provided correlation coefficients (r2) of 0.99 or more for all three compounds.
Sensitivity is expressed via the LOD and LOQ. LOD was defined as the mean response in blank samples (n = 60) + 1.645 × the standard deviation (SD). This calculated value was confirmed by injection of standards (n = 6). The LOQ was set to where the coefficient of variation (CV) for 6 injected samples was ≤20%. The assay LODs were 5 (±1.2), 25 (±4.9), and 250 (±44.6) pg/ml, for lopinavir, ritonavir, and tenofovir, respectively, and the LOQs were 10 (±1.4), 50 (±1.9), and 500 (±12.2) pg/ml, respectively.
Table 1 presents both intra- and interday assay precisions and accuracies. For all three compounds, evaluated at 5, 50, and 750 ng/ml, the assay accuracies ranged from 98.8 to 105.3%, while the precision (CV) was less than 5% for both inter- and intraday comparisons. Thus, the assay is precise and accurate.
TABLE 1.
Intraday and interday precisions and accuracies of the assay to detect lopinavir, ritonavir, and tenofovir in undiluted standard samples at the indicated concentrations
| Parameter | Value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lopinavir |
Ritonavir |
Tenofovir |
|||||||
| 5 ng/ml | 50 ng/ml | 750 ng/ml | 5 ng/ml | 50 ng/ml | 750 ng/ml | 5 ng/ml | 50 ng/ml | 750 ng/ml | |
| Intra-assay comparisons (n = 6) | |||||||||
| Avg concn (ng/ml) | 5.0 | 49.5 | 748.2 | 5.0 | 51.7 | 754.9 | 5.0 | 49.8 | 752.0 |
| SD | 0.1 | 1.6 | 10.0 | 0.1 | 1.7 | 12.0 | 0.1 | 2.1 | 13.6 |
| CV (%) (precision) | 2.6 | 3.2 | 1.3 | 1.7 | 3.3 | 1.6 | 1.6 | 4.3 | 1.8 |
| Accuracy (%) | 99.7 | 99.1 | 99.8 | 99.8 | 103.4 | 100.7 | 100.1 | 99.5 | 100.3 |
| Interassay comparisons (n = 6) | |||||||||
| Avg concn (ng/ml) | 5.0 | 52.7 | 756.6 | 5.0 | 52.1 | 760.5 | 5.1 | 51.5 | 740.9 |
| SD | 0.1 | 1.1 | 10.2 | 0.1 | 2.1 | 10.4 | 0.0 | 2.0 | 7.6 |
| CV (%) (precision) | 1.4 | 2.1 | 1.4 | 2.3 | 4.0 | 1.4 | 0.8 | 3.9 | 1.0 |
| Accuracy (%) | 99.1 | 105.3 | 100.9 | 100.7 | 104.2 | 101.4 | 101.1 | 103.0 | 98.8 |
To ensure that the signals detected by MS were truly from the compounds of interest, the selectivity of the assay was investigated in order to find any possible interferences from other residues in the extracted plasma. No interferences were found at the retention times of the compounds when blank plasma samples were run, showing good selectivity for the assay.
We next determined run-to-run variation of this assay for drugs extracted from plasma. Again, three drug concentrations, 5, 50, and 750 ng/ml, were used. The results, expressed as recovery percentages for lopinavir, ritonavir, and tenofovir, are presented in Table 2. The recovery percentages were calculated based on comparisons between mock- and plasma-extracted drugs. With this approach, we found that all three drugs showed over 91% (range, 91.3 to 102.6%) recovery, with a CV of <5%.
TABLE 2.
Plasma sample extraction efficiencies of the assay for lopinavir, ritonavir, and tenofovir at the indicated concentrations
| Parameter | Value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lopinavir |
Ritonavir |
Tenofovir |
|||||||
| 5 ng/ml | 50 ng/ml | 750 ng/ml | 5 ng/ml | 50 ng/ml | 750 ng/ml | 5 ng/ml | 50 ng/ml | 750 ng/ml | |
| Calculated concn (ng/ml)a for run: | |||||||||
| 1 | 4.90 | 49.82 | 735.99 | 5.05 | 47.86 | 695.88 | 5.11 | 49.47 | 706.82 |
| 2 | 4.87 | 43.43 | 706.25 | 5.02 | 53.76 | 728.47 | 4.93 | 49.66 | 702.97 |
| 3 | 4.89 | 43.73 | 772.30 | 5.33 | 50.15 | 705.65 | 4.94 | 49.38 | 735.72 |
| Avg concn (ng/ml) | 4.89 | 45.66 | 738.18 | 5.13 | 50.59 | 710.00 | 4.99 | 49.50 | 715.17 |
| SD | 0.01 | 3.60 | 33.08 | 0.17 | 2.97 | 16.73 | 0.10 | 0.14 | 17.90 |
| CV (%) | 0.25 | 7.89 | 4.48 | 3.31 | 5.88 | 2.36 | 1.97 | 0.29 | 2.5 |
| Recovery (%) | 97.76 | 91.32 | 98.42 | 102.63 | 101.18 | 94.67 | 99.89 | 99.01 | 95.36 |
Values for spiked blank plasma extractions represented 100%.
With this sensitive and high recovery value, we used this validated assay to measure time course plasma concentrations of lopinavir, ritonavir, and tenofovir in primates dosed subcutaneously with these three drugs.
Time course plasma drug concentrations in primates.
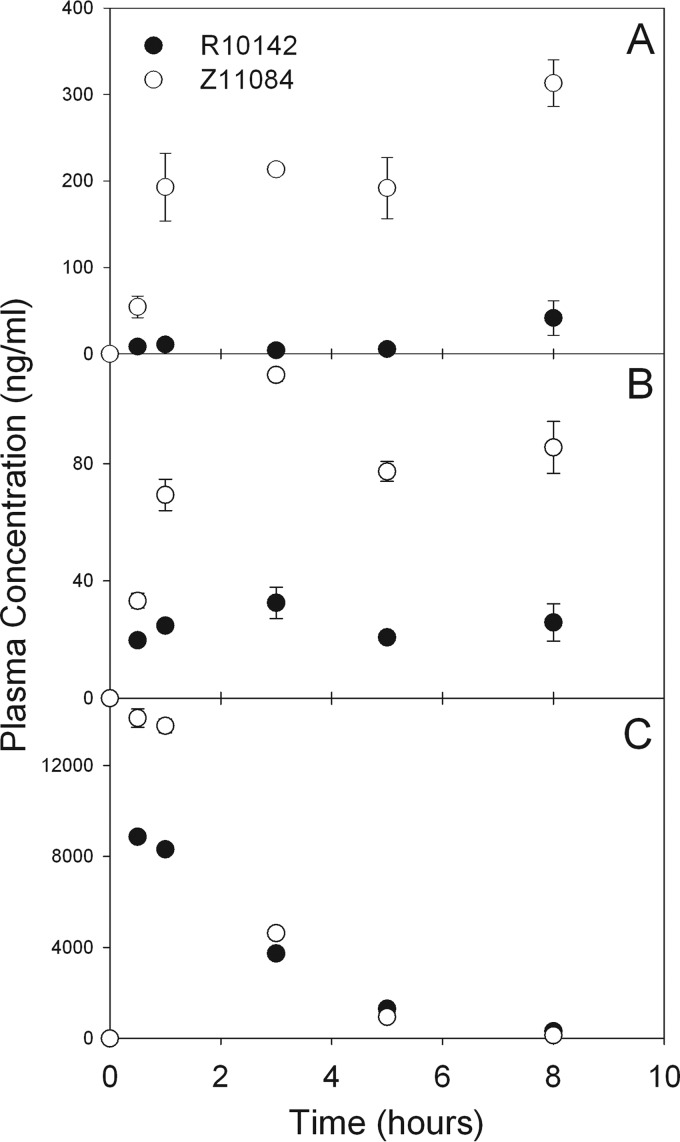
To measure the performance of the validated assay, we evaluated the plasma drug concentrations for two primates (pigtailed macaques [M. nemestrina]) after a single subcutaneous dose. Time course drug concentrations are presented in Fig. 3. All three drugs were detected in plasma within minutes of subcutaneous dosing. For the water-soluble drug tenofovir, the drug levels dropped precipitately after 8 h, while the more hydrophobic drugs lopinavir and ritonavir remained detectable for 24 h in the two primates. In addition, the drug levels extracted from plasma were reproducible, as the variations (expressed as SD for each time point) between two extractions and three LC-MS/MS runs were small. Collectively, these data indicate that this one-step, three-drug assay to determine plasma drug concentrations of lopinavir, ritonavir, and tenofovir is reproducible and sensitive for plasma drug analysis.
FIG 3.
Time course plasma concentrations of lopinavir (A), ritonavir (B), and tenofovir (C) in two primates (M. nemestrina). The two primates were administered lopinavir (20 mg/kg), ritonavir (11.4 mg/kg), and tenofovir (13.6 mg/kg) subcutaneously. Plasma samples were extracted with the validated assay as described in the text and were analyzed using LC-MS/MS. The data were analyzed for each drug at specific time points and presented as means ± SD (n = 6). The symbols (●, ○) represent the samples collected from two different primates.
DISCUSSION
While a number of anti-HIV drug assays have been developed to detect multiple HIV PIs or reverse transcriptase inhibitors in one assay, it is more challenging to detect both classes of drugs at the same time. PIs, such as lopinavir and ritonavir, are generally more hydrophobic, and NtRTIs, such as tenofovir, tend to be water soluble at physiologic pH. With the ability of a column matrix and MS/MS technique to separate the two PIs (lopinavir and ritonavir) as well as the NtRTI (tenofovir), we developed and optimized a single-step assay with a simplified extraction procedure to detect plasma drug concentrations of all three compounds in a single plasma sample source. The final assay was validated to be effective and sensitive. Also, the one-step assay method is reliable and reproducible, with a high accuracy, precision, and extraction recovery.
While a number of methods for extracting and detecting plasma lopinavir, ritonavir, and tenofovir have been described, most of these require two different assays. The use of two different assays not only is labor-intensive and time-consuming but also requires two sets of samples, which involves larger sample volumes and introduces additional variations. Our one-step method not only discerns all three drug concentrations from one sample, which could reduce variations in drug-drug interaction analyses, but also saves time, labor, and resources, making it a more sustainable approach. Jung et al. (17) developed an assay for extraction and detection of 17 antiretroviral drugs simultaneously, using the same principle of an LLE followed by PP, but with different solvents from those described for our assay. While their method appeared to be rapid and reliable, developing a single assay for a wide range of drugs may have reduced the extraction efficiency and sensitivity. With the reported extraction efficiency of 70% and variability in PI analysis, the report described some challenges in detecting steady-state plasma drug concentrations in patients for the three drugs reported here. In comparison, we have increased the extraction efficiency of tenofovir from 70% up to 95%. We have also increased the sensitivities for all three drugs included in our study, 1,000-fold, 200-fold, and 20-fold for lopinavir, ritonavir, and tenofovir, respectively, over those reported by Jung et al. (17). This enables us to detect consistently lower concentrations in plasma samples. This improvement could be a critically important factor in clinical settings, particularly in situations where plasma drug concentrations could vary due to induced drug metabolism or multifaceted disease conditions. We also showed in a primate study that our method is reliable and consistent, even for very low drug concentrations at extended time points.
With the reproducible and high extraction efficiency, the current assay employed MS/MS to provide additional resolution of lopinavir and ritonavir, two closely eluted drugs (Fig. 1). While the selectivity and sensitivity based on the detection limit achieved (Table 1) are respectable for detecting plasma drug concentrations, an even higher sensitivity may be required to detect intracellular drug concentrations in lymphocytes in blood and lymph nodes. Detection of intracellular drug levels could be particularly challenging because only a limited number of lymphocytes can be obtained from human lymph node biopsy samples, which are expected to be small. In this situation, this method could be adapted with LC-MS-QTRAP, which could selectively trap target analytes as they accelerate into the mass detector and boost the sensitivity results about another log. In addition, this method could also be adapted for extraction and detection of intracellular phosphorylated metabolites of tenofovir (both mono- and diphosphates). However, additional measures would be needed to stabilize these metabolites, which could readily be hydrolyzed in the extraction and MS ionization processes. These and other possible improvements are beyond the scope of this paper and are under investigation.
In our assay, we focused on detecting two protease inhibitors, lopinavir and ritonavir, plus the NtRTI tenofovir, because these three drugs are recommended as a key HAART combination in the most recent HIV/AIDS treatment guidelines. With some modifications, a one-step clinical assay such as that described here, but for other PI and NRTI drug combinations, such as darunavir or atazanavir with emtricitabine plus tenofovir, could be developed. However, such studies are also beyond the scope of this report.
In summary, using a single column and a combination of liquid extraction and protein precipitation, we successfully developed a one-step LC-MS assay to detect three analytes, lopinavir, ritonavir, and tenofovir, from a single plasma sample. This assay is reproducible, with excellent to outstanding extraction efficiencies for the hydrophobic drugs lopinavir and ritonavir as well as the hydrophilic drug tenofovir. This validated assay can be used to evaluate plasma drug concentrations in a sensitive, specific, and reproducible manner, with good consistency and precision.
ACKNOWLEDGMENTS
This study was supported by NIH grants AI-077390, AI-077390-S1, AI-077390-S2, and 1UL1-RR025014.
We thank Jennifer Freeling for planning and performing primate triple-drug-combination studies, Cuiling Shu for processing blood and plasma samples, and primate center staff members Michael Gough and Jason Ogle for their assistance in dosing and collecting blood samples.
Footnotes
Published ahead of print 24 February 2014
REFERENCES
- 1.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Ashman DJ, Holmberg SD, The HIV Outpatient Study Investigators 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853–860. 10.1056/NEJM199803263381301 [DOI] [PubMed] [Google Scholar]
- 2.Yeni P. 2006. Update on HAART in HIV. J. Hepatol. 44(Suppl 1):100–103. 10.1016/j.jhep.2005.11.021 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 9 September 2013, accession date Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. WHO, Geneva, Switzerland: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html [PubMed] [Google Scholar]
- 4.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Günthard HF, Hammer SM, Reiss P, Richman DD, Rizzardini G, Thomas DL, Jacobsen DM, Volberding PA. 2012. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society—USA Panel. JAMA 308:387–402. 10.1001/jama.2012.7961 [DOI] [PubMed] [Google Scholar]
- 5.Wang PG, Wei JS, Kim G, Chang M, El-Shourbagy T. 2006. Validation and application of a high-performance liquid chromatography-tandem mass spectrometric method for simultaneous quantification of lopinavir and ritonavir in human plasma using semi-automated 96-well liquid-liquid extraction. J. Chromatogr. A 1130:302–307. 10.1016/j.chroma.2006.07.071 [DOI] [PubMed] [Google Scholar]
- 6.Holmstock N, Annaert P, Augustijns P. 2012. Boosting of HIV protease inhibitors by ritonavir in the intestine: the relative role of cytochrome P450 and P-glycoprotein inhibition based on Caco-2 monolayers versus in situ intestinal perfusion in mice. Drug Metab. Dispos. 40:1473–1477. 10.1124/dmd.112.044677 [DOI] [PubMed] [Google Scholar]
- 7.Rouzes A, Berthoin K, Xuereb F, Djabarouti S, Pellegrin I, Pellergrin JL, Coupet AC, Augagneur S, Budzinski H, Saux MC, Breilh D. 2004. Simultaneous determination of the antiretroviral agents: amprenavir, lopinavir, ritonavir, saquinavir and efavirenz in human peripheral blood mononuclear cells by high-performance liquid chromatography-mass spectrometry. J. Chromatogr. B 813:209–216. 10.1016/j.jchromb.2004.09.041 [DOI] [PubMed] [Google Scholar]
- 8.Temghare GA, Shetye SS, Joshi SS. 2009. Rapid and sensitive method for quantitative determination of lopinavir and ritonavir in human plasma by liquid chromatography-tandem mass spectrometry. EJ. Chem. 6:223–230. 10.1155/2009/709478 [DOI] [Google Scholar]
- 9.Ehrhardt M, Möck M, Haefeli WE, Mikus G, Burhenne J. 2007. Monitoring of lopinavir and ritonavir in peripheral blood mononuclear cells, plasma and ultrafiltrate using a selective and highly sensitive LC/MS/MS assay. J. Chromatogr. B 850:249–258. 10.1016/j.jchromb.2006.11.037 [DOI] [PubMed] [Google Scholar]
- 10.Colombo S, Beguin A, Telenti A, Biollaz J, Buclin T, Rochat B, Decosterd LA. 2005. Intracellular measurements of anti-HIV drugs indinavir, amprenavir, saquinavir, ritonavir, nelfinavir, lopinavir, atazanavir, efavirenz and nevirapine in peripheral blood mononuclear cells by liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B 819:259–276. 10.1016/j.jchromb.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 11.Delahunty T, Bushman L, Fletcher CV. 2006. Sensitive assay for determining plasma tenofovir concentrations by LC/MS/MS. J. Chromatogr. B 830:6–12. 10.1016/j.jchromb.2005.10.015 [DOI] [PubMed] [Google Scholar]
- 12.Matta MK, Burugula L, Pilli NR, Inamadugu JK, Jvln SR. 2012. A novel LC-MS/MS method for simultaneous quantification of tenofovir and lamivudine in human plasma and its application to a pharmacokinetic study. Biomed. Chromatogr. 26:1202–1209. 10.1002/bmc.2679 [DOI] [PubMed] [Google Scholar]
- 13.Durand-Gasselin L, Van Rompay KK, Vela JE, Henne IN, Lee WA, Rhodes GR, Ray AS. 2009. Nucleotide analogue prodrug tenofovir disoproxil enhances lymphoid cell loading following oral administration in monkeys. Mol. Pharm. 6:1145–1151. 10.1021/mp900036s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. 2006. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. J. Acquir. Immune. Defic. Syndr. 43:278–283. 10.1097/01.qai.0000243103.03265.2b [DOI] [PubMed] [Google Scholar]
- 15.Kiser JJ, Carten ML, Aquilante CL, Anderson PL, Wolfe P, King TM, Delahunty T, Bushman LR, Fletcher CV. 2008. The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin. Pharmacol. Ther. 83:265–272. 10.1038/sj.clpt.6100269 [DOI] [PubMed] [Google Scholar]
- 16.Pruvost A, Negredo E, Théodoro F, Puig J, Levi M, Ayen R, Grassi J, Clotet B. 2009. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob. Agents Chemother. 53:1937–1943. 10.1128/AAC.01064-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung BH, Rezek NL, Bridges AS, Corbett AH, Kashuba ADM. 2007. Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 21:1095–1104. 10.1002/bmc.865 [DOI] [PubMed] [Google Scholar]
- 18.Department of Health and Human Services, U.S. Food and Drug Administration. May 2001. Guidance for industry, bioanalytical method validation. Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), Rockville, MD [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. October 2004. Protocols for determination of limits of detection and limits of quantification, approved guideline. CLSI document EP17-A CLSI, Wayne, PA [Google Scholar]