Abstract
The purpose of this study was to define the pulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and formed colistin following intravenous (i.v.) and inhaled administration in cystic fibrosis (CF) patients. Six CF subjects were administered nebulized CMS doses of 2 and 4 million IU and an i.v. CMS infusion of 150 mg of colistin base activity. Blood plasma, sputum, and urine samples were collected for 12 to 24 h postdose. To assess the tolerability of the drug, lung function tests, blood serum creatinine concentrations, and adverse effect reports were recorded. All doses were well tolerated in the subjects. The pharmacokinetic parameters for CMS following i.v. delivery were consistent with previously reported values. Sputum concentrations of formed colistin were maintained at <1.0 mg/liter for 12 h postdose. Nebulization of CMS resulted in relatively high sputum concentrations of CMS and formed colistin compared to those resulting from i.v. administration. The systemic availability of CMS was low following nebulization of 2 and 4 million IU (7.93% ± 4.26% and 5.37% ± 1.36%, respectively), and the plasma colistin concentrations were below the limit of quantification. Less than 2 to 3% of the nebulized CMS dose was recovered in the urine samples in 24 h. The therapeutic availability and drug targeting index for CMS and colistin following inhalation compared to i.v. delivery were significantly greater than 1. Inhalation of CMS is an effective means of targeting CMS and formed colistin for delivery to the lungs, as high lung exposure and minimal systemic exposure were achieved in CF subjects.
INTRODUCTION
Cystic fibrosis (CF) is characterized by persistent airway colonization and subsequent chronic airway infections, most commonly caused by Pseudomonas aeruginosa. (1–3) The establishment of chronic airway infections results in a vicious cycle of infection and inflammation that leads to permanent lung damage, pulmonary insufficiency, and eventual mortality (3–5). Therefore, the effective management of CF lung infections using antibiotic therapy plays a crucial role in reducing the progression of lung function deterioration (1, 2, 4–6).
As a consequence of the rising rates of resistance to multiple antibiotics among P. aeruginosa isolates and the lack of new antibiotics available, colistin (also known as polymyxin E), which entered into clinical use in late 1950s, is now used increasingly in CF patients (7, 8). Colistin methanesulfonate (CMS), an inactive prodrug that converts to the antibacterial form of colistin (9), is administered intravenously (i.v.) or via inhalation to manage various stages of pulmonary colonization and infection with P. aeruginosa (1, 4, 5, 10–12). Intravenous administration of CMS relies on the achievement of sufficiently high exposure of formed colistin in the airways in order to elicit the required antibacterial effect, ideally without precipitating nephrotoxicity, which is the major dose-limiting adverse effect of systemically administered CMS (13). Delivery of the antibiotic via inhalation compared to i.v. administration has the potential to achieve higher concentrations within the respiratory tract while minimizing systemic exposure. Although CMS has been administered i.v. or by inhalation in CF patients for >2 decades, there is a paucity of comparative information on the pharmacokinetics of CMS and formed colistin following these modes of administration. While there are data in CF patients on the concentrations of colistin in blood plasma and/or sputum following dry powder inhalation and nebulized CMS (14–18) and of CMS/colistin in plasma and/or sputum following i.v. CMS, (19, 20), no studies have reported the relative concentrations of CMS, and in particular formed colistin, in plasma and sputum following i.v. versus pulmonary administration of CMS in the same patient. Thus, the targeting advantage that may be achieved by delivering CMS directly to the airway to maximize bacterial killing (and potentially reduce bacterial resistance) while minimizing systemic exposure (and the possibility of nephrotoxicity) has not been explored systematically in CF patients.
The principal aim of the current study was to examine the pulmonary and systemic pharmacokinetics of CMS and formed colistin following the administration of CMS by the i.v. and pulmonary routes in CF patients. On three separate occasions, each patient received two single doses of nebulized CMS (two escalating dose levels) and a single dose of i.v. CMS. The tolerability of the respective doses of nebulized and i.v. CMS was also monitored. Our study enabled an assessment of the targeting advantage that may be achieved by administering CMS directly to the airways for the treatment of pulmonary infections, which may be used to facilitate the optimization of dosing regimens in CF patients.
MATERIALS AND METHODS
Clinical setting and subjects.
The study population consisted of six inpatients at the CF service at Alfred Hospital (Melbourne, Australia). The study was approved by the human research ethics committees of Alfred Hospital and Monash University (Melbourne, Australia), and subjects provided informed written consent before participating in the study. Subjects were eligible to participate in the study if they (i) were ≥18 years of age, (ii) had a documented diagnosis of CF, (iii) had a positive respiratory culture of P. aeruginosa in the absence of other Gram-negative and/or Gram-positive organisms, (iv) were willing and able to use nebulized antibiotics, (v) had adequate birth control measures if sexually active while participating in the study, and (vi) had a baseline forced expiratory volume in 1 s (FEV1) that was 25 to 75% of the predicted value. Subjects were excluded if they had a history of allergy to colistin or polymyxin B or had experienced a recovery of Burkholderia cepacia in respiratory secretions over the previous 2 years. Inhaled and systemic CMS was not used by subjects for 1 to 2 weeks prior to commencement of the study. Prior to the commencement of this pharmacokinetic study, the subjects were stabilized for their lung infection, which was demonstrated by an improvement in clinical symptoms and lung function tests.
Study protocol. (i) Administration of nebulized and intravenous colistin methanesulfonate.
Subjects received each of the following single-dose treatments in the order indicated: (i) a nebulized CMS dose of 2 million IU (equivalent to 60 mg of colistin base activity [CBA] or 160 mg sodium CMS), (ii) a nebulized CMS dose of 4 million IU (120 mg of CBA or 320 mg sodium CMS), and (iii) an i.v. CMS dose of 150 mg of CBA (5 million IU or 400 mg sodium CMS). The dose equivalences of CMS in regard to IU (in millions) and mg of CBA and sodium CMS were reported previously (21). The dosing solutions for nebulization were prepared by reconstituting CMS (Tadim, Phebra Pty Ltd., NSW, Australia) in 4 ml of sterile 0.9% sodium chloride. The i.v. dose of 150 mg of CBA (Colistin Link parenteral; Link Pharmaceuticals Ltd., Auckland, New Zealand) was reconstituted with 2 ml of sterile water for injection and added to 100 ml of sterile 0.9% sodium chloride solution. There was a minimum 3-day washout period between each single-dose treatment, and the subsequent treatment was administered only if the preceding treatment was well tolerated.
All subjects received chest physiotherapy and salbutamol (2.5 to 5 mg via nebulizer) 1 h prior to the administration of inhaled CMS. For nebulization of CMS, a Salter Ultramist nebulizer (no. 8960) attached to an exhalation filter (no. 8980) connected to an outlet supplying air at a rate of 8 liters/min was utilized, and the mean nebulization time was 14 ± 4.0 min. Nebulization was carried out immediately following preparation of the dosing solution to minimize any potential in vitro CMS conversion to colistin (22). The subjects sat upright for the duration of the nebulization period to promote lung inflation. Immediately after preparation, the i.v. dose of CMS was infused over 45 min via a single-lumen peripherally inserted central catheter line. Following dose administration, the line was flushed with 30 ml of 0.9% sodium chloride. During the study period, the subjects were administered all routine medications for the management of their CF, including other antibiotics.
(ii) Sampling of blood, sputum, and urine.
Blood samples (5 ml) were collected predose and following nebulization of CMS at 0.5, 1, 2, 4, 8, and 12 h for subjects 1, 2, and 3. A request was made to amend the blood sampling times to 0.5, 1, 2, 4, 6, and 8 h postdose, and this was approved by the ethics committee for subjects 4, 5, and 6, as the CMS/colistin plasma concentrations were below the limit of quantification (LOQ) at 12 h postdose following nebulized CMS in subjects 1 to 3. Blood samples (5 ml) were collected predose and following the completion of i.v. infusion at 0.25, 0.5, 0.75, 1, 2, 4, 8, and 12 h. In all subjects, an additional 15 ml of blood was collected predose and at 12 h postdose for blood serum creatinine quantification and full blood examination. Sputum samples were obtained in subjects capable of spontaneously expectorating adequate amounts of sputum, both predose and at 1, 4, and 12 h following nebulized and i.v. administration. Urine samples voided over the 12-h predose period and 24-h postdose period were collected. To minimize any potential in vitro conversion of CMS to colistin (23), samples for CMS and colistin concentration analysis were immediately stored on ice and processed at 4°C prior to storage at −80°C, pending high-performance liquid chromatography (HPLC) analysis. The samples were analyzed for CMS and colistin concentrations within 4 months of the completion of the study (23).
(iii)Assessment of tolerability.
Following CMS administration, subjects were monitored for signs and symptoms of adverse effects. Lung function tests were carried out predose and 1 to 2 h following the completion of inhaled and i.v. CMS administration. The lung function parameters monitored were FEV1, forced vital capacity (FVC), minimum inspiratory pressure (MIP), and maximal expiratory pressure (MEP). Measurements of serum creatinine concentrations predose and 12 h following the completion of CMS administration were carried out to enable an estimation of the glomerular filtration rate (eGFR) for the assessment of nephrotoxicity (24, 25). Monitoring of blood pressure, heart rate, and oxygen saturation was conducted at baseline and throughout the 24-h postdose period. The subjects completed an adverse effect questionnaire 0.5 h following the completion of CMS administration to report any adverse effects and to rate the severity on a scale of 0 (no adverse effects) to 10. The subjects were asked specifically if they experienced any of the following adverse effects: cough, chest tightness, irritation of the throat, and wheezing following inhaled CMS delivery, as well as burning/tingling/numbness or problems with coordination/balance/clumsiness following i.v. delivery.
Bioanalytical methods. (i) Determination of colistin methanesulfonate and colistin in plasma and urine.
Concentrations of CMS and formed colistin in plasma and urine samples were measured using validated HPLC assays (26, 27), with minor modifications. Briefly, the analytical methods involved the quantification of colistin before and after forced in vitro conversion from CMS; the concentration of CMS was calculated as the difference between the two assay results and was adjusted for molecular weight (26, 27). The calibration standards and quality-control (QC) samples were from independently prepared stock solutions of the analytes (colistin sulfate and sodium CMS); the QC samples were used to assess the accuracy (% deviation from nominal QC concentration) and precision (coefficient of variation) of the assays. For the plasma assay, two colistin calibration curves (0.125 to 2 mg/liter and 0.125 to 8 mg/liter) were prepared in drug-free plasma to cover the range of colistin concentrations encountered after pulmonary and i.v. delivery, respectively. The CMS calibration standards ranged from 0.125 to 2 mg/liter and 0.78 to 50 mg/liter for the pulmonary and i.v. delivery routes, respectively. For the analysis of urine samples, calibration standards containing colistin and CMS concentrations ranging from 0.125 to 16 mg/liter were prepared. The subject urine samples with estimated CMS or colistin concentrations above the upper end of the respective calibration curve were diluted prior to reanalysis; the QC samples of 40 mg/liter colistin and 150 mg/liter CMS were similarly diluted to ensure satisfactory assay performance. The lowest concentration on the calibration curve was the LOQ. In all cases, the accuracy and precision were within ±15% for non-LOQ QCs and within ±20% for LOQ samples.
(ii) Determination of colistin methanesulfonate and colistin in sputum.
For the determination of CMS and formed colistin concentrations in sputum samples, the assay performed was similar to that described above, with minor modifications. For the colistin assay, colistin calibration standard or independently prepared QC working solutions were spiked into predetermined volumes of homogenized CMS/colistin-free sputum. Following vortexing, an alkaline sodium dodecyl sulfate (SDS) solution (1% SDS, 200 mM sodium hydroxide) (Sigma-Aldrich, MO, USA [28]) was added in a 1:1 ratio. The tube contents were vortexed and the samples left to stand in ice water for 5 min. Subsequently, protein was precipitated with a 1:1 ratio of acetonitrile, and the supernatant was transferred onto solid-phase extraction (SPE) C18 cartridges (Sep-Pak; Waters, MA, USA). Calibration standards were prepared containing colistin concentrations in sputum ranging from 0.125 to 16 mg/liter. As described above, a QC sample of 40 mg/liter colistin was diluted to ensure satisfactory assay performance for subject samples that required a similar dilution. For the CMS assay, two calibration curves (0.125 to 2 mg/liter and 5 to 320 mg/liter) were required to encompass the CMS sputum concentrations following i.v. and pulmonary delivery, respectively. The two CMS calibration curves were prepared in a similar manner to that of the colistin sputum assay except for a 5-fold dilution of sputum calibration standards, ranging from 5 to 320 mg/liter with blank cation-adjusted Mueller-Hinton broth. A QC sample of 500 mg/liter was diluted to assess the assay performance for subject samples above the calibration range that required the same dilution step. The accuracy and precision were within ±15% for non-LOQ QCs and within ±20% for the LOQ.
Data analysis.
Colistin concentrations in the subject samples were determined by multiplying the colistin sulfate concentrations obtained from the assay by the ratio of the molecular weights of colistin base and colistin sulfate (19). CMS concentrations in the subject samples were calculated as the difference between the measured colistin concentrations with and without the forced within-assay conversion of CMS to colistin and by adjusting for the molecular weights of colistin, sodium CMS, and CMS base, as described previously (19).
A noncompartmental analysis of the pharmacokinetic properties of CMS and formed colistin was performed using WinNonlin (version 5.3; Pharsight Corporation, NC, USA). The maximum concentration of colistin (Cmax) and time to reach maximum concentration (Tmax) were determined from the concentration-versus-time profiles following CMS administration. The terminal rate constant (λz) was calculated by linear least-squares regression analysis using the last three log-transformed concentration-versus-time points, while the corresponding half-life (t1/2) was calculated using 0.693/λz. The area under the concentration-versus-time profile from the time of initiation of i.v. infusion or nebulization to the last sampling time (AUC0-tlast) was calculated by the linear trapezoidal rule. The area under the concentration-versus-time to infinity profile (AUC0-∞) was calculated as the sum of AUC0-tlast and Clast/λz, where Clast is the concentration in the last sample. Clearance (CL) and the volume of distribution at steady state (Vss) of CMS following i.v. infusion were calculated using the formulas dosei.v./AUC0-∞ and dosei.v. × [AUMC0-∞/(AUC0-∞)2], respectively, where AUMC0-∞ is the area under the first-moment curve to infinite time. The percentage of the CMS dose recovered in urine in 24 h was calculated from the cumulative molar amount of CMS and colistin recovered in urine in 24 h divided by the CMS dose expressed in molar terms; the percentage recovered as colistin was determined in a corresponding manner. Systemic CMS availability (F) following inhaled CMS delivery was calculated using plasma data as [(AUC0-∞)pulm × dosei.v.]/[(AUC0-∞)i.v. × dosepulm], where dosepulm represents the nominal CMS dose that was nebulized. The systemic availability of CMS following inhaled CMS delivery was also calculated using urinary recovery data: [(Ae,0-24)pulm × dosei.v.]/[(Ae,0-24)i.v. × dosepulm] was the formula used, where Ae,0-24 is the cumulative amount of CMS and formed colistin, expressed in molar terms, recovered in urine in 24 h. The targeting advantage in terms of airway (sputum) exposure to CMS or formed colistin by the direct administration of CMS to the respiratory tract via nebulization, versus that achieved with i.v. administration of CMS, was estimated by calculations of therapeutic availability (TA) (29) and the drug targeting index (DTI) (29, 30). The TA was calculated by the ratio of the dose-normalized AUC in sputum to time t following nebulized and i.v. CMS administration (equation 1). The DTI was calculated by the ratio of the dose-normalized AUC in sputum and plasma to time t following nebulized CMS administration divided by the same ratio following i.v. CMS administration (equation 2). Time t refers to either 12 h or up to the scheduled 4-h postsampling time, as discussed in Results.
| (1) |
| (2) |
Statistical analysis was performed using SPSS Statistics (version 20; IBM, USA). Unless otherwise indicated, the group data are presented as the average ± standard deviation. Differences between the values of Cmax, Tmax, and the AUC following the two nebulized CMS doses and difference between the pre- and postdose values of lung function parameters and the eGFR were evaluated using paired-sample t tests. A P value of <0.05 was regarded as a statistically significant difference.
RESULTS
Six subjects were included in the study, and the subject demographic characteristics are summarized in Table 1. All subjects received all three study doses.
TABLE 1.
Demographic characteristics of enrolled subjects (n = 6)
| Subject | Age (yr) | Sex | Body wt (kg) | Race | eGFR (ml/min/1.73 m2) |
|---|---|---|---|---|---|
| 1 | 29 | Male | 70 | Caucasian | 127 |
| 2 | 20 | Male | 56 | Caucasian | 130 |
| 3 | 35 | Male | 71 | Caucasian | 103 |
| 4 | 31 | Male | 80 | Caucasian | 148 |
| 5 | 30 | Male | 85 | Caucasian | 116 |
| 6 | 27 | Male | 65 | Caucasian | 143 |
Pharmacokinetics following intravenous administration.
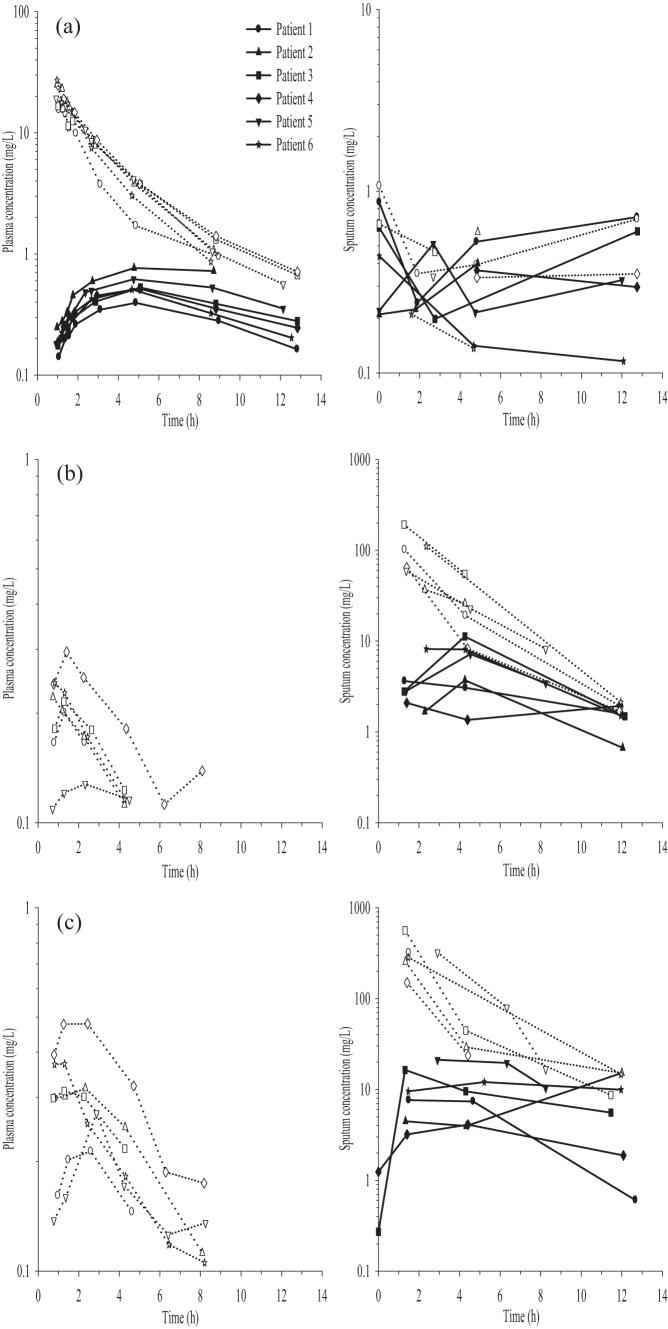
Notwithstanding that i.v. dosing was the third treatment for each subject, the pharmacokinetic data for this treatment are presented first because of the pivotal nature of i.v.-administered drugs in defining key parameters. The plasma concentration-versus-time profiles for CMS and formed colistin following i.v. CMS infusion are shown in Fig. 1a. The plasma pharmacokinetic parameters of CMS and formed colistin are presented in Table 2. The maximum plasma concentrations of formed colistin were reached within approximately 5 h following the initiation of i.v. infusion (Table 2 and Fig. 1a). The terminal half-life of CMS was shorter than that of colistin in each subject for whom the calculation was possible (Table 2). Of the i.v. dose of CMS, 40.0% ± 18.7% was recovered as CMS and colistin in urine collected over 24 h, with approximately half of the recovered CMS dose (19.5% ± 8.79%) in the form of colistin (Table 3).
FIG 1.
Plasma (left panels) and sputum (right panels) concentration-versus-time profiles of CMS (open symbols) and formed colistin (closed symbols) for each patient following i.v. CMS dose of 150 mg CBA (a) and nebulized CMS doses of 2 million IU (b) and 4 million IU (c). Note the different range of concentrations on the y axis across the panels. The concentrations of CMS and colistin present in predose sputum samples for the nebulized 4 million IU dose (second treatment arm) and the i.v. dose (third treatment arm) are shown at time zero in the respective panels.
TABLE 2.
Pharmacokinetic parameters of CMS and formed colistin in plasma following a single i.v. infusion of CMS (150 mg of CBA)
| Subject | Colistin methanesulfonate |
Colistin |
||||||
|---|---|---|---|---|---|---|---|---|
| AUC0-∞ (mg · h/liter) | CL (liters/h)a | Vss (liters)b | t1/2 (h)c | AUC0-∞ (mg · h/liter) | t1/2 (h)d | Cmax (mg/liter) | fTmax (h) | |
| 1 | 46.9 | 8.00 | 23.5 | 3.17 | 4.91 | 6.04 | 0.40 | 4.9 |
| 2 | 71.7 | 5.23 | 12.2 | 1.99 | NCe | NC | 0.77 | 4.8 |
| 3 | 62.3 | 6.02 | 21.4 | 3.12 | 8.14 | 8.42 | 0.53 | 5.1 |
| 4 | 75.1 | 4.99 | 16.1 | 3.23 | 7.28 | 7.38 | 0.52 | 5.1 |
| 5 | 65.2 | 5.75 | 16.6 | 2.55 | 10.2 | 9.03 | 0.62 | 4.8 |
| 6 | 65.1 | 5.76 | 12.0 | 1.92 | 5.87 | 5.84 | 0.51 | 4.7 |
The mean (SD) of the CL for all 6 subjects was 5.96 liters/h (1.07 liters/h).
The mean (SD) of the Vss for all 6 subjects was 16.9 liters (4.68 liters).
The mean (SD) of the t1/2 for colistin methanesulfonate for all 6 subjects was 2.66 h (0.60 h).
The mean (SD) of the t1/2 for colistin for all 6 subjects was 7.34 h (1.41 h).
NC, not calculated, the tlast for subject 2 was 8.71 h, as the 12-h sample could not be collected; thus, we were not able to estimate the t½ or AUC0-∞.
TABLE 3.
Percentage of CMS and colistin recovered in urine following administration of CMS by nebulization or i.v. infusion
| Administration route and dose | % CMS recovered in urine in 24 h as: |
|
|---|---|---|
| CMS + colistin | Colistin | |
| i.v. infusion of 150 mg of CBAa | 40.0 ± 18.7 | 19.5 ± 8.79 |
| Nebulized 2 million IU of CMSb | 1.30 ± 0.65 | 0.62 ± 0.26 |
| Nebulized 4 million IU of CMSc | 0.68 ± 0.26 | 0.29 ± 0.13 |
Subject 1 urine data excluded from analysis, as urine sample was collected until 12 h post-i.v. CMS delivery.
Subject 4 urine data excluded from analysis, as colistin concentrations were below LOQ of the assay.
Subject 5 urine data excluded from analysis, as colistin concentrations were below LOQ of the assay.
Quantifiable concentrations of CMS (two subjects; 0.66 and 1.07 mg/liter) and formed colistin (five subjects; 0.47 ± 0.28 mg/liter) were present in predose sputum samples prior to i.v. CMS administration, due to the persistence of CMS and formed colistin in sputum following nebulized delivery of CMS in the first two treatment arms. When only limited quantities of sputum were collected, colistin concentrations were preferentially determined, since colistin is the antibacterial moiety (9). The sputum CMS concentrations following i.v. administration of CMS ranged between 0.14 and 0.70 mg/liter, which were similar to the CMS concentrations in the predose sputum samples (Fig. 1a). Colistin sputum concentration-versus-time profiles following i.v. CMS infusion showed relatively little variability over time within individual subjects, and for all six subjects, they were within a narrow range of 0.12 to 0.72 mg/liter over the postdose sampling period; again, this is similar to the colistin concentrations present in the predose sputum (Fig. 1a).
Pharmacokinetics following nebulization.
Sputum concentration-versus-time profiles for CMS and formed colistin following the nebulization of 2 and 4 million IU of CMS in the subjects are shown in Fig. 1b and c, respectively. Sputum CMS concentrations of 94 ± 55 mg/liter and 315 ± 137 mg/liter were observed at the first sampling time following nebulized CMS doses of 2 and 4 million IU, respectively, with concentrations declining thereafter (Fig. 1b and c). In two subjects, quantifiable colistin concentrations (0.27 and 1.24 mg/liter) were present in predose sputum samples prior to nebulization of 4 million IU of CMS (the second treatment arm), again likely a result of a prolonged presence in the lung after the earlier nebulized administration of 2 million IU of CMS. The pharmacokinetic parameters of colistin in sputum following pulmonary CMS delivery are presented in Table 4. Peak sputum colistin concentrations were reached within 1.3 to 5.2 h following the initiation of both nebulized CMS doses, with the exception of subject 2, in whom the Tmax was observed at 12 h after nebulization of 4 million IU of CMS (Table 4 and Fig. 1b and c). Limited sputum samples and the assay sensitivity precluded a characterization of the CMS and colistin terminal phases in sputum. An estimation of CMS dose linearity in sputum was not possible due to the unavailability of data for CMS at later time points in some subjects (Fig. 1b and c) because of limited amounts of sputum, as mentioned above. For colistin, no significant difference was evident for the dose-normalized areas under the sputum concentration-versus-time profiles to tlast with doubling of the CMS dose (Table 4).
TABLE 4.
Pharmacokinetic parameters of formed colistin in sputum following nebulization of CMS
| Subject | Parameters with nebulized administration of CMS at a dose of: |
|||||
|---|---|---|---|---|---|---|
| 2 million IUa,b |
4 million IU |
|||||
| AUC0-tlast (mg · h/liter) | Cmax (mg/liter)c | Tmax (h) | AUC0-tlast (mg · h/liter)d | Cmax (mg/liter)e | Tmax (h) | |
| 1 | 30.3 | 3.63 | 1.3 | 61.5 | 7.63 | 1.5 |
| 2 | 24.3 | 3.72 | 4.3 | 88.9 | 15.2 | 12 |
| 3 | 73.1 | 11.3 | 4.3 | 104 | 16.4 | 1.3 |
| 4 | 18.9 | 2.09 | 1.4 | 37.0 | 4.10 | 4.4 |
| 5 | 37.5f | 7.18 | 4.5 | 129f | 21.2 | 2.9 |
| 6 | 62.2 | 8.15 | 2.4 | 122 | 12.1 | 5.2 |
No statistically significant difference for dose-normalized AUC0-tlast and dose-normalized Cmax and Tmax between the two nebulized CMS doses.
The mean (SD) of the AUC0-tlast for all 6 subjects was 41.8 mg · h/liter (24.3 mg · h/liter).
The mean (SD) of the Cmax for all 6 subjects was 6.00 mg/liter (3.45 mg/liter).
The mean (SD) of the AUC0-tlast for all 6 subjects was 82.6 mg · h/liter (33.7 mg · h/liter).
The mean (SD) of the Cmax for all 6 subjects was 12.8 mg/liter (6.19 mg/liter).
Subject 5 tlast following nebulized CMS doses was 8.3 h.
The plasma CMS concentrations following pulmonary delivery of both doses were relatively low (0.11 to 0.48 mg/liter), and there were no quantifiable concentrations of formed colistin in plasma, as shown in Fig. 1b and c. The pharmacokinetic parameters of CMS in plasma following pulmonary CMS delivery are presented in Table 5. The percentages of the CMS dose recovered in urine as CMS plus colistin following pulmonary delivery of 2 and 4 million IU of CMS were 1.30% ± 0.65% and 0.68% ± 0.26%, respectively, with approximately half of the recovered amount in the form of colistin (Table 3). The systemic availability values of CMS following the nebulization of 2 and 4 million IU of CMS, calculated from the areas under plasma concentration-versus-time curves to infinity, were 7.93% ± 4.26% and 5.37% ± 1.36%, respectively. Using the urine recovery data, the estimated systemic availability values for CMS were 3.01% ± 0.50% and 2.69% ± 2.20%, following nebulized CMS doses of 2 and 4 million IU, respectively.
TABLE 5.
Pharmacokinetic parameters of CMS in plasma following nebulization of 2 and 4 million IU of CMS
| Subject | Parameters with nebulized administration of CMS at dose of: |
|||||
|---|---|---|---|---|---|---|
| 2 million IU |
4 million IU |
|||||
| AUC0-∞ (mg · h/liter)a | Cmax (mg/liter)b | Tmax (h)a | AUC0-∞ (mg · h/liter)c | Cmax (mg/liter)d | Tmax (h) | |
| 1 | 1.39 | 0.20 | 1.3 | 1.67 | 0.21 | 2.6 |
| 2 | 1.16 | 0.22 | 0.74 | 2.41 | 0.32 | 2.3 |
| 3 | 1.27 | 0.22 | 1.3 | 2.53 | 0.31 | 1.3 |
| 4 | 3.53 | 0.30 | 1.4 | 3.53 | 0.48 | 1.3 |
| 5 | 3.77 | 0.13 | 2.3 | 4.10 | 0.27 | 2.9 |
| 6 | 1.26 | 0.24 | 0.82 | 2.46 | 0.37 | 0.8 |
No statistically significant difference for dose-normalized AUCo-∞ and Tmax between the two nebulized CMS doses. The mean (SD) of the AUCo-∞ for the 2 million IU dose for all 6 subjects was 2.06 mg · h/liter (1.24 mg · h/liter).
Statistically significant difference for dose-normalized Cmax between the two nebulized CMS doses (P < 0.05). The mean (SD) of the Cmax for the 2 million IU dose for all 6 subjects was 0.22 mg/liter (0.055 mg/liter).
The mean (SD) of the AUCo-∞ for the 4 million IU dose for all 6 subjects was 2.78 mg · h/liter (0.88 mg · h/liter).
The mean (SD) of the Cmax for the 4 million IU dose for all 6 subjects was 0.33 mg/liter (0.092 mg/liter).
Given the long residence times of CMS and colistin in sputum and the fact that following the nebulized CMS dose of 4 million IU, the predose sputum samples had quantifiable colistin concentrations, calculations of the TA and DTI were carried out using the lower of the two nebulized CMS doses, which was the first treatment arm. Following i.v. administration, the sputum exposure was calculated, including the CMS and colistin predose concentrations. The targeting advantage of delivering CMS via the pulmonary route compared to the i.v. route was demonstrated by the TA estimations of 387 and 24 for CMS and formed colistin, respectively. The corresponding respective DTI values for CMS and formed colistin were 15,952 and 35, respectively. To estimate the TA values for CMS and colistin, the sputum exposures following pulmonary and i.v. delivery were calculated from time zero to 12 h postdose. To estimate the DTI for colistin, as plasma colistin concentrations were below the LOQ following pulmonary delivery, the systemic exposure was calculated using a colistin LOQ concentration (0.125 mg/liter) from time zero to 12 h postdose. The DTI for CMS was estimated up to the scheduled 4-h postdose sampling time, as the plasma CMS concentrations after that time were below the LOQ.
Tolerability following nebulized and intravenous administration.
Inhaled CMS was generally well tolerated by the subjects, as demonstrated by a lack of change in the lung function parameters and eGFR, as shown in Table 6. Following inhalation of 2 million IU of CMS, subject 5 reported chest tightness, while subject 6 complained of cough and chest tightness after the higher CMS nebulized dose. Subject 2 reported the sensation of a lump in the throat following inhalation of 2 million IU of CMS, and on the morning of the third treatment arm (i.v. CMS administration), subject 2 presented with hemoptysis with an uncertain relationship to the earlier inhalational CMS treatments. The abovementioned adverse effects were minor in severity and transient. There were no remarkable changes in the lung function parameters in subjects 2, 5, and 6 following each respective treatment. Following i.v. CMS, subjects did not report any pulmonary adverse effects, and no significant changes in lung function parameters were evident (Table 6). Subject 2 reported transient dizziness 4 h after the administration of i.v. CMS. A small but statistically significant difference (P < 0.05) in the pre- and 12-h postdose eGFR was evident following i.v. CMS administration (Table 6).
TABLE 6.
Lung function parameters and glomerular filtration rates following administration of CMS by nebulization or i.v. infusiona
| Respiratory parametersb | Effects with nebulized administration of CMS at dose of: |
Effects with i.v. infusion of 150 mg of CBAc |
||||
|---|---|---|---|---|---|---|
| 2 million IU |
4 million IU |
|||||
| Predose | Postdosed | Predose | Postdosed | Predose | Postdosed | |
| FEV1e | 2.0 ± 0.65 | 1.9 ± 0.65 | 2.0 ± 0.61 | 2.1 ± 0.64f | 2.1 ± 0.62 | 2.1 ± 0.62 |
| FVCe | 3.6 ± 0.85 | 3.5 ± 0.94 | 3.9 ± 0.87 | 3.8 ± 0.93f | 3.9 ± 0.78 | 3.9 ± 0.88 |
| MIPe | 155 ± 64 | 151 ± 44 | 160 ± 40 | 161 ± 33 | 148 ± 39 | 153 ± 36 |
| MEPe | 157 ± 56 | 157 ± 55 | 158 ± 30 | 166 ± 38 | 152 ± 29 | 164 ± 30 |
| eGFRg | 127 ± 18 | 121 ± 20h | 133 ± 22 | 121 ± 37 | 145 ± 22 | 127 ± 31h |
Data represented as mean ± SD (n = 6).
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; MIP, minimum inspiratory pressure; MEP, maximum expiratory pressure.
For lung function parameters, n = 5, as subject 2 did not undergo lung function tests since he presented with hemoptysis.
One to two hours postadministration for lung function test; 12 h postadministration for eGFR.
No statistically significant difference between pre- and postdose measurements for FEV1, FVC, MIP, and MEP.
Due to time constraints in the lung function laboratory, n = 5, as FEV1 and FVC could not be carried out for subject 2.
No statistically significant difference was found between pre- and postdose measurements for eGFR following nebulized CMS delivery. A statistically significant difference was found between pre- and postdose measurements for eGFR following i.v. CMS delivery.
n = 4 for nebulized 2 million IU CMS and n = 5 for i.v. 150 mg of CBA.
DISCUSSION
This study was designed to evaluate the systemic and pulmonary pharmacokinetics of CMS and formed colistin following the administration of inhaled and i.v. CMS in CF subjects. The results provide the first quantitative description of the targeting advantage that may be achieved by administering CMS by inhalation for the treatment of a pulmonary infection.
Following i.v. CMS infusion (150 mg of colistin base activity [CBA]), the CL, Vss, and t1/2 values for CMS were consistent with previously reported values in CF patients following i.v. infusion of 30 to 60 mg CBA every 8 h (19) and in healthy volunteers following i.v. CMS infusion of 30 mg of CBA (31). The estimated terminal t1/2 of formed colistin, 7.34 ± 1.41 h, in this study was slightly longer than in previous reports (19, 31), and peak colistin plasma concentrations were observed at approximately 5 h following the initiation of i.v. infusion, compared to ∼1.3 to 2 h and 1 to 4 h in the reports by Li et al. (19) and Couet et al. (31), respectively. One possibility for the variation in the time to achievement of peak plasma concentrations of formed colistin is brand-to-brand differences in the ratio of fully and partially sulfomethylated CMS entities, which may impact the time course for the formation of colistin (32). Consistent with the findings by Li et al. (19) and Couet et al. (31), the colistin terminal half-life was ∼2.5-fold longer than that of CMS, which indicates that the disposition of formed colistin is not rate limited by formation from CMS. Following i.v. CMS infusion, 40.0% ± 18.7% of the CMS dose was recovered in urine in 24 h, with colistin representing approximately half of the recovered CMS dose (Table 3). As described previously, most of the colistin recovered in urine is expected to result from the conversion of CMS to colistin in the kidneys and bladder (31, 33).
There is a paucity of information available on the disposition of CMS and formed colistin in sputum following i.v. administration of CMS. In this study, the sputum concentrations of CMS and colistin after i.v. administration of CMS were relatively low and similar to those observed in the predose sputum samples; there was no convincing evidence for a time course that one would expect if there was substantial ingress from the systemic circulation followed by egress (Fig. 1a). Thus, it is apparent that the CMS and colistin concentrations observed were largely the result of carryover from the previously administered inhalational doses of CMS. Even so, the colistin sputum concentrations were below the MIC90 of 1.0 mg/liter for P. aeruginosa (34) across the 12-h postdose sampling period. In CF patients, intravenous CMS is used to treat acute exacerbations of lung infections, for which it is typically administered two to three times per day for several days (5, 10–12). It remains to be determined whether higher concentrations of formed colistin in sputum would be observed with such multiple-dose regimens. In critically ill patients, Imberti et al. (35) reported that under steady-state conditions, formed colistin concentrations were undetectable in bronchoalveolar lavage (BAL) fluid samples following i.v. CMS infusion of 60 mg CBA every 8 h. The authors reported a limit of detection (LOD) concentration of 0.05 mg/liter in BAL fluid, with no corresponding LOD concentration for lung epithelial lining fluid (ELF) (35). Referring to the original study that Imberti and colleagues referenced for their BAL procedure by Conte et al. (36), and utilizing the percentage of recovered BAL fluid for a standard BAL procedure (37), we approximated that the ELF LOD concentration for the assay was ∼5 mg/liter (i.e., BAL fluid concentration is ∼100-fold dilution of ELF concentration). Therefore, the undetectable BAL fluid concentrations of formed colistin in the study by Imberti et al. (35) must be interpreted with caution. The generation of higher concentrations of formed colistin in lung fluids may be achieved by increasing the i.v. dose of CMS, but this would be associated with a potential for increased nephrotoxicity, which is the major dose-limiting adverse effect for the i.v. route (13).
One of the major findings of this study was that pulmonary administration of 2 and 4 million IU of CMS resulted in much higher sputum concentrations of CMS and more importantly of the active antibacterial entity, colistin, than were observed after i.v. administration of a higher dose of CMS (Fig. 1b and c). With all subjects, the higher nebulized dose led to a proportional increase in the sputum concentration exposure profile for formed colistin. In the majority of subjects, the nebulization of 2 million IU of CMS resulted in colistin sputum concentrations above the MIC90 of 1.0 mg/liter for P. aeruginosa (34) for up to 12 h. Doubling the nebulized CMS dose resulted in sputum colistin concentrations of >3.0 mg/liter up to 12 h postadministration. A multiple-dosing regimen of inhaled CMS would lead to higher sputum colistin concentrations, due to accumulation of the drug. Ratjen et al. (16) reported significantly higher colistin concentrations (∼5 to 45 mg/liter) in sputum following nebulization of a single dose of 2 million IU of CMS in CF patients. It should be noted that their analytical method for quantitating colistin involved treating sputum with a relatively high concentration of trifluoroacetic acid (∼7%) (16). This may promote the conversion of CMS to colistin during the sample preparation procedures (16). More recently, Athanassa et al. (38) reported that following the nebulization of 1 million IU of CMS every 8 h in critically ill patients, ELF concentrations of colistin were within a similar range (∼1.0 to 15 mg/liter) to the concentrations observed in sputum in the current single-dose study. The variability in the reported colistin concentrations in sputum and ELF across the studies is likely a function of the efficiency of nebulized delivery, whether a study involved single or multiple dosing, the different biological matrices (sputum versus ELF), analytical issues as discussed above, and variation in the pathophysiological statuses of the respective patient populations.
Administration of CMS via the pulmonary route resulted in the achievement of very high CMS concentrations in sputum (∼50 to 500 mg/liter) approximately 1 h after nebulization (Fig. 1b and c). Over the ensuing hours, CMS in the lungs is expected to act as a reservoir for the ongoing conversion of CMS to colistin and in doing so to support the observed persistence of formed colistin in the lungs (Fig. 1b and c). For the treatment of pseudomonal respiratory infections, the prolonged presence of CMS/colistin in sputum has significant implications for therapeutic efficacy and therefore warrants further detailed investigation.
Despite the high and persistent concentrations of CMS and colistin in sputum following pulmonary delivery of CMS, the systemic exposures to CMS and colistin were minimal (Fig. 1b and c). Following the inhalation of 2 and 4 million IU of CMS, blood plasma concentrations of CMS were <0.5 mg/liter and quantifiable only up to 8 h postadministration (Fig. 1b and c); the systemic availability of inhaled CMS was <10%, as assessed by both the plasma concentration-versus-time and urinary recovery data. Importantly, colistin concentrations in plasma were below the LOQ of the assay (0.125 mg/liter) for the entire 12-h sampling period following the nebulization of 2 and 4 million IU CMS. Following nebulization, the relatively low systemic exposures of CMS and formed colistin compared to those from i.v. delivery are likely to result in a reduction of systemic adverse effects, such as nephrotoxicity.
Overall, these findings highlight the major advantage of pulmonary over i.v. delivery of CMS, as sputum concentrations of the active antibacterial entity, colistin, were maintained above the MIC90 of 1.0 mg/liter for P. aeruginosa (34) while systemic exposure to CMS and colistin was minimized. Following nebulization of CMS (doses of 2 or 4 million IU), the colistin sputum exposure was >10-fold higher than that achieved with i.v. administration, while colistin concentrations in plasma following pulmonary administration were below the LOQ of the assay. Calculations of the therapeutic availability (TA) and drug targeting index (DTI) provide an opportunity for a quantitative assessment of the benefits of local (inhalational) delivery over systemic (i.v.) administration. The TA (equation 1) provides an indication of the availability of CMS and colistin in sputum. The TA values for CMS (387) and colistin (24) were far greater than unity, which indicated that the sputum exposures for CMS and colistin following pulmonary administration were substantially higher than after i.v. administration of the same CMS dose. The effectiveness or degree of targeting achieved following administration via the pulmonary route compared to the i.v. route can be estimated by the DTI ratio (equation 2). For CMS and colistin, the respective DTI were 15,952 and 35, again values substantially higher than unity, indicating that a high degree of targeting to the lungs (greater sputum exposure and minimal plasma exposure) was achieved after inhalational delivery of CMS compared to i.v. administration. The targeting values calculated for TA and DTI underestimate the true targeting benefit for CMS and colistin following pulmonary delivery. In order to calculate the DTI, it is necessary to have a value for the mean plasma AUC for colistin following inhalational administration; however, all samples were below the LOQ (0.125 mg/liter). Therefore, a minimum value for AUC was calculated assuming that the samples contained 0.125 mg/liter of colistin at all times. Additionally, for the i.v. treatment, the predose sputum samples contained residual CMS and colistin from the prior nebulization treatment arms, and these values were included in the calculation of mean sputum AUC, which further contributes to the underestimation of both TA and DTI. For an antibiotic, such as colistin, which is a last line of defense against P. aeruginosa infections, the ability to target the lungs to achieve high local concentrations while simultaneously minimizing systemic exposure has a major advantage in terms of maximizing efficacy and minimizing the potential to develop resistance and nephrotoxicity. We recognize that both pulmonary exposure and systemic availability will be influenced by the efficiency of the nebulization equipment and process; nevertheless, the colistin-targeting advantage of inhalational versus i.v. administration of CMS is readily apparent.
In contrast to the low systemic availability of CMS and formed colistin (<2 to 3% of the nebulized CMS dose recovered in urine) observed in CF subjects in the current study, Marchand et al. (39) reported that the total dose of CMS administered by intratracheal nebulization to rats was absorbed into the systemic circulation either as CMS (systemic availability of ∼70%) or following CMS conversion to colistin in the lungs (39% of the nebulized CMS dose). Following the nebulization of sodium CMS (15 mg/kg of body weight) in rats, a ∼4-fold-higher plasma concentration-versus-time exposure was observed for formed colistin compared to i.v. administration of the same CMS dose (39). We recently reported similar observations in rats where the intratracheal method resulted in formed colistin exposure in ELF that was 8,000-fold higher than that in plasma after i.v. dosing (40). This is in contrast to CF subjects, in whom colistin concentrations in plasma were unquantifiable after CMS inhalation. The fraction of the CMS dose that was converted to colistin following i.v. delivery in rats was ∼13%, which suggests that following nebulization, the absorption of presystemically formed colistin contributes significantly to the systemic exposure of colistin (39). Such species differences (i.e., between rats and humans) can be attributed to the efficiency of the different nebulizer devices in delivering the total dose into the lungs, the physiological size of the rat versus the human lung, and the healthy versus infected status of the lungs, which can influence the barriers for absorption and impact clearance mechanisms (i.e., mucocilliary clearance, uptake by macrophages, and coughing).
Cystic fibrosis centers worldwide have adopted different inhaled CMS dosing regimens (dose and dosing interval), with current therapies ranging from 1 million IU of CMS twice daily to 2 million IU of CMS three times daily (1, 4, 5, 10). One reason for the variability in the inhaled dosage regimens is the lack of robust pharmacokinetic data to inform dosage selection. The inclusion of two inhaled CMS doses (2 and 4 million IU) in the present study has enabled a better understanding of the kinetics of CMS and colistin in sputum and plasma, and it has additionally shown that CMS inhalation at both dose levels was well tolerated by CF subjects.
In conclusion, we have for the first time demonstrated, in quantitative terms, the targeting advantage that may be achieved by nebulized delivery of CMS to the airways. Following pulmonary CMS administration, greater lung exposure and minimal systemic exposure were observed for CMS and importantly for colistin compared to that from i.v. administration, where systemic exposure was very substantially higher than the lung exposure. Thus, inhalation of CMS is an effective means of targeting colistin to the lungs to maximize antibacterial effect while minimizing systemic exposure and the potential for nephrotoxicity. This study and future studies will inform the design of inhalational CMS dosing regimens for CF patients.
ACKNOWLEDGMENTS
We thank the patients and their families who participated in this study. We also thank the Monash University research staff located at the Alfred Hospital for allowing us to utilize their laboratory facilities.
S.W.S.Y. was supported by a Monash Postgraduate Research Scholarship. This work was supported by internal funding.
Footnotes
Published ahead of print 18 February 2014
REFERENCES
- 1.Hansen CR, Pressler T, Høiby N. 2008. Early aggressive eradication therapy for intermittent Pseudomonas aeruginosa airway colonization in cystic fibrosis patients: 15 years experience. J. Cyst. Fibros. 7:523–530. 10.1016/j.jcf.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 2.Döring G. 2010. Prevention of Pseudomonas aeruginosa infection in cystic fibrosis patients. Int. J. Med. Microbiol. 300:573–577. 10.1016/j.ijmm.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 3.Høiby N, Ciofu O, Bjarnsholt T. 2010. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 5:1663–1674. 10.2217/fmb.10.125 [DOI] [PubMed] [Google Scholar]
- 4.Döring G, Conway SP, Heijerman HG, Hodson ME, Høiby N, Smyth A, Touw DJ. 2000. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur. Respir. J. 16:749–767. 10.1034/j.1399-3003.2000.16d30.x [DOI] [PubMed] [Google Scholar]
- 5.Döring G, Høiby N, Consensus Study Group 2004. Early intervention and prevention of lung disease in cystic fibrosis: a European consensus. J. Cyst. Fibros. 3:67–91. 10.1016/j.jcf.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 6.Høiby N. 2011. Recent advances in the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. BMC Med. 9:32. 10.1186/1741-7015-9-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nation RL, Li J. 2009. Colistin in the 21st century. Curr. Opin. Infect. Dis. 22:535–543. 10.1097/QCO.0b013e328332e672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. 2005. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 25:11–25. 10.1016/j.ijantimicag.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 9.Bergen PJ, Li J, Rayner CR, Nation RL. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1953–1958. 10.1128/AAC.00035-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee D, Stableforth D. 2000. The treatment of respiratory Pseudomonas infection in cystic fibrosis: what drug and which way? Drugs 60:1053–1064. 10.2165/00003495-200060050-00006 [DOI] [PubMed] [Google Scholar]
- 11.Ledson MJ, Gallagher MJ, Cowperthwaite C, Convery RP, Walshaw MJ. 1998. Four years' experience of intravenous colomycin in an adult cystic fibrosis unit. Eur. Respir. J. 12:592–594. 10.1183/09031936.98.12030592 [DOI] [PubMed] [Google Scholar]
- 12.Conway SP, Pond MN, Watson A, Etherington C, Robey HL, Goldman MH. 1997. Intravenous colistin sulphomethate in acute respiratory exacerbations in adult patients with cystic fibrosis. Thorax 52:987–993. 10.1136/thx.52.11.987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, Vishnepolsky M, Weintrob A, Wortmann G. 2009. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin. Infect. Dis. 48:1724–1728. 10.1086/599225 [DOI] [PubMed] [Google Scholar]
- 14.Westerman EM, De Boer AH, Le Brun PP, Touw DJ, Roldaan AC, Frijlink HW, Heijerman HG. 2007. Dry powder inhalation of colistin in cystic fibrosis patients: a single dose pilot study. J. Cyst. Fibros. 6:284–292. 10.1016/j.jcf.2006.10.010 [DOI] [PubMed] [Google Scholar]
- 15.Le Brun PP, de Boer AH, Mannes GP, de Fraîture DM, Brimicombe RW, Touw DJ, Vinks AA, Frijlink HW, Heijerman HG. 2002. Dry powder inhalation of antibiotics in cystic fibrosis therapy: part 2. Inhalation of a novel colistin dry powder formulation: a feasibility study in healthy volunteers and patients. Eur. J. Pharm. Biopharm. 54:25–32. 10.1016/S0939-6411(02)00044-9 [DOI] [PubMed] [Google Scholar]
- 16.Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, van Koningsbruggen S, Grasemann H. 2006. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J. Antimicrob. Chemother. 57:306–311. 10.1093/jac/dki461 [DOI] [PubMed] [Google Scholar]
- 17.Byrne N, Keavey P, Perry J, Gould F, Spencer D. 2003. Comparison of lung deposition of colomycin using the HaloLite and the Pari LC Plus nebulisers in patients with cystic fibrosis. Arch. Dis. Child. 88:715–718. 10.1136/adc.88.8.715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Brun PP, de Graaf AI, Vinks AA. 2000. High-performance liquid chromatographic method for the determination of colistin in serum. Ther. Drug Monit. 22:589–593. 10.1097/00007691-200010000-00014 [DOI] [PubMed] [Google Scholar]
- 19.Li J, Coulthard K, Milne R, Nation RL, Conway S, Peckham D, Etherington C, Turnidge J. 2003. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J. Antimicrob. Chemother. 52:987–992. 10.1093/jac/dkg468 [DOI] [PubMed] [Google Scholar]
- 20.Reed MD, Stern RC, O'Riordan MA, Blumer JL. 2001. The pharmacokinetics of colistin in patients with cystic fibrosis. J. Clin. Pharmacol. 41:645–654. 10.1177/00912700122010537 [DOI] [PubMed] [Google Scholar]
- 21.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 6:589–601. 10.1016/S1473-3099(06)70580-1 [DOI] [PubMed] [Google Scholar]
- 22.Wallace SJ, Li J, Rayner CR, Coulthard K, Nation RL. 2008. Stability of colistin methanesulfonate in pharmaceutical products and solutions for administration to patients. Antimicrob. Agents Chemother. 52:3047–3051. 10.1128/AAC.00103-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudhani RV, Nation RL, Li J. 2010. Evaluating the stability of colistin and colistin methanesulphonate in human plasma under different conditions of storage. J. Antimicrob. Chemother. 65:1412–1415. 10.1093/jac/dkq134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 130:461–470 [DOI] [PubMed] [Google Scholar]
- 25.National Kidney Foundation Kidney Disease Outcomes Quality Initiative. 2002. Part 5. Evaluation of laboratory measurements for clinical assessment of kidney disease. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. National Kidney Foundation, New York, NY [Google Scholar]
- 26.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Johnson DW. 2001. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J. Chromatogr. B. Biomed. Sci. Appl. 761:167–175. 10.1016/S0378-4347(01)00326-7 [DOI] [PubMed] [Google Scholar]
- 27.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Valentine J. 2002. Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob. Agents Chemother. 46:3304–3307. 10.1128/AAC.46.10.3304-3307.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birnboim HC, Doly J. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513–1523. 10.1093/nar/7.6.1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt CA, MacGregor RD, Siegel RA. 1986. Engineering targeted in vivo drug delivery. I. The physiological and physicochemical principles governing opportunities and limitations. Pharm. Res. 3:333–344 [DOI] [PubMed] [Google Scholar]
- 30.Stevens AJ, Martin SW, Brennan BS, McLachlan A, Gifford LA, Rowland M, Houston JB. 1995. Regional drug delivery II: relationship between drug targeting index and pharmacokinetic parameters for three non-steroidal anti-inflammatory drugs using the rat air pouch model of inflammation. Pharm. Res. 12:1987–1996. 10.1023/A:1016212510900 [DOI] [PubMed] [Google Scholar]
- 31.Couet W, Gregoire N, Gobin P, Saulnier PJ, Frasca D, Marchand S, Mimoz O. 2011. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin. Pharmacol. Ther. 89:875–879. 10.1038/clpt.2011.48 [DOI] [PubMed] [Google Scholar]
- 32.He H, Li J, Nation RL, Jacob J, Chen G, Lee HJ, Tsuji BT, Thompson PE, Roberts K, Velkov T, Li J. 2013. Pharmacokinetics of four different brands of colistimethate and formed colistin in rats. J. Antimicrob. Chemother. 68:2311–2317. 10.1093/jac/dkt207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. 2004. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J. Antimicrob. Chemother. 53:837–840. 10.1093/jac/dkh167 [DOI] [PubMed] [Google Scholar]
- 34.Gales AC, Jones RN, Sader HS. 2011. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program (2006–09). J. Antimicrob. Chemother. 66:2070–2074. 10.1093/jac/dkr239 [DOI] [PubMed] [Google Scholar]
- 35.Imberti R, Cusato M, Villani P, Carnevale L, Iotti GA, Langer M, Regazzi M. 2010. Steady-state pharmacokinetics and BAL concentration of colistin in critically ill patients after IV colistin methanesulfonate administration. Chest 138:1333–1339. 10.1378/chest.10-0463 [DOI] [PubMed] [Google Scholar]
- 36.Conte JE, Jr, Golden JA, Kipps J, Zurlinden E. 2002. Intrapulmonary pharmacokinetics of linezolid. Antimicrob. Agents Chemother. 46:1475–1480. 10.1128/AAC.46.5.1475-1480.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. (1985). 60:532–538 [DOI] [PubMed] [Google Scholar]
- 38.Athanassa ZE, Markantonis SL, Fousteri MZ, Myrianthefs PM, Boutzouka EG, Tsakris A, Baltopoulos GJ. 2012. Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med. 38:1779–1786. 10.1007/s00134-012-2628-7 [DOI] [PubMed] [Google Scholar]
- 39.Marchand S, Gobin P, Brillault J, Baptista S, Adier C, Olivier JC, Mimoz O, Couet W. 2010. Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats. Antimicrob. Agents Chemother. 54:3702–3707. 10.1128/AAC.00411-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.W S Yapa S, Li J, Porter CJH, Nation RL, Patel K, McIntosh MP. 2013. Population pharmacokinetics of colistin methanesulfonate in rats: achieving sustained lung concentrations of colistin for targeting respiratory infections. Antimicrob. Agents Chemother. 57:5087–5095. 10.1128/AAC.01127-13 [DOI] [PMC free article] [PubMed] [Google Scholar]