Abstract
Over the past decade, bacteria of the genus Acinetobacter have emerged as a leading cause of hospital-acquired infections. Outbreaks of Acinetobacter infections are considered to be caused exclusively by contamination and transmission in hospital environments. The natural habitats of clinically important multiresistant Acinetobacter spp. remain to be defined. In this paper, we report an incidental finding of a viable multidrug-resistant strain of Acinetobacter baumannii, related to clinical isolates, in acid paleosol from Croatia. The environmental isolate of A. baumannii showed 87% similarity to a clinical isolate originating from a hospital in this geographic area and was resistant to gentamicin, trimethoprim-sulfamethoxazole, ciprofloxacin, and levofloxacin. In paleosol, the isolate was able to survive a low pH (3.37), desiccation, and a high temperature (50°C). The probable source of A. baumannii in paleosol is illegally disposed waste of external origin situated in the abandoned quarry near the sampling site. The bacteria could have been leached from waste by storm water and thus infiltrated the paleosol.
INTRODUCTION
Bacteria of the genus Acinetobacter have been recognized as significant hospital pathogens since the late 1970s, but at that time they were easily treated, because they were susceptible to commonly used antimicrobials. Acinetobacter spp. have an increasing ability to develop resistance to commonly used antimicrobial agents, leading to limited options for antibiotic treatment (1). Three major overlapping populations of bacteria of the genus Acinetobacter are known: multiresistant isolates from hospitals and hospitalized patients (Acinetobacter baumannii, Acinetobacter genomic species 3 [recently renamed Acinetobacter pittii], and genomic species 13TU [recently renamed Acinetobacter nosocomialis]; all three together are known as the A. baumannii complex), sensitive isolates from human and animal skin and from foodstuffs (A. johnsonii, A. lwoffii, A. radioresistens), and sensitive isolates from soil and wastewater (A. calcoaceticus, A. johnsonii) (2). Over the past decade, bacteria of the genus Acinetobacter have emerged as a leading cause of hospital-acquired infections, and A. baumannii accounts for approximately 80% of all reported Acinetobacter infections (3). A. baumannii is among the pathogens targeted in the call by the Infectious Diseases Society of America to develop new antibiotics by 2020 (4).
The problems caused by multidrug-resistant Acinetobacter baumannii in hospital settings are exacerbated by their increasing ability to develop resistance to commonly used antimicrobial agents, their ability to form biofilms on abiotic surfaces, and their high degree of resistance to drying and disinfectants, leading to long-term persistence in the hospital environment (2). Possible sources for the occurrence of outbreaks include preinjury skin colonization, introduction from the environment at the time of injury, and acquisition after injury during treatment in hospitals (5). Contamination of the hospital environment, accompanied by the transmission of infection within the hospital, is generally recognized as the major source for the occurrence of outbreaks (6).
According to Bergey's Manual of Systematic Bacteriology, bacteria of the genus Acinetobacter are saprophytes occurring naturally in soil, water, sewage, and food (7). This statement has been cited in the introductions of papers dealing with clinical isolates of Acinetobacter spp. Such reports in the literature of the ubiquity of clinically important Acinetobacter spp. in natural environments, such as soil and water, are now recognized as misconceptions (1).
The prevalence of clinically important Acinetobacter spp. in nature and their potential to migrate into and/or out of the hospital environments are undefined to date. The natural habitats of clinically important multiresistant Acinetobacter spp. remain to be defined. Colonization of the digestive tracts of patients with multidrug-resistant Acinetobacter spp. in hospitals occurs at high rates (8). The digestive tracts of colonized patients could be an important epidemiologic reservoir of multiresistant Acinetobacter spp., from which bacteria could migrate through wastewater into the environment.
The Istrian peninsula represents the northwest part of the spacious Adriatic Carbonate Platform (9). This part of the platform is composed of a succession of carbonate deposits more than 2,000 m thick, of the Middle Jurassic (Bathonian) to the Eocene age, and is overlaid by Paleogene (Eocene) foraminiferal limestones, transitional beds (Globigerina marls), and flysch deposits. Carbonate deposits of the Istrian peninsula exhibit numerous exposure surfaces reflecting emergence. Greenish-gray clays associated with Late Aptian–Late Albian regional emergence show clear evidence of subaerial exposure and are considered paleosols (10, 11). In the Tri Jezerca quarry (Fig. 1), they are situated in paleokarst pits of Lower Aptian massive limestone, which is used as a building stone known as Istarski žuti (Istrian yellow). This stone is presently obtained from a few quarries in central Istria. However, the abandoned part of the Tri Jezerca quarry is currently used as an illegal landfill. Disposed waste of external origin found in this abandoned quarry may be an important threat to the environment. In this paper, we report an incidental finding of a viable multidrug-resistant strain of A. baumannii, related to clinical isolates, in paleosol from Istria, Croatia.
FIG 1.
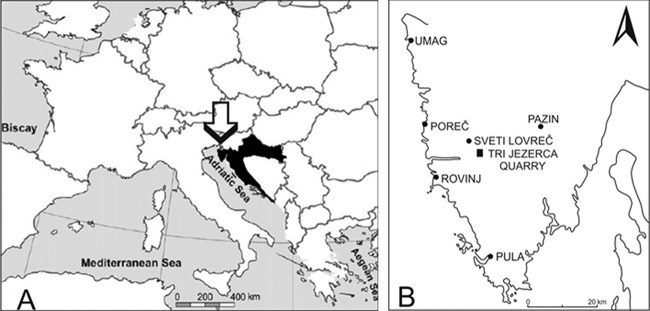
(A) Position of Croatia in Europe. (B) Istrian peninsula and location of the study site, the Tri Jezerca quarry. The nearest medical institutions are situated in Rovinj and Poreč.
MATERIALS AND METHODS
Characterization of paleosol.
The greenish-gray clays in the Tri Jezerca quarry are as much as 93 cm thick (Fig. 2). The paleosol profile was carefully cleaned and prepared for sampling. A disturbed paleosol sample was collected from the upper part of the profile (0 to 40 cm) immediately below the Lower Albian limestone and was air dried afterwards. An undisturbed paleosol sample (10 to 18 cm from the top) for thin-section preparation was taken using an 80- by 60- by 40-cm Kubiena box.
FIG 2.

Greenish-gray paleosol in the Tri Jezerca quarry. A clean profile was prepared for sampling.
Particle sizes were analyzed on the bulk sample after dispersion in water and ultrasonic treatment. Fractions of >45 μm were obtained by wet sieving. The <2-μm fraction was separated by sedimentation in a cylinder and was quantitatively obtained after the appropriate settling time. The remaining portion in the cylinder was calculated as the 2- to 45-μm fraction. The contents of major elements and several minor elements (Zr, Cr, Nb) were determined with an X-ray fluorescence (XRF) spectrometer on glass discs. The contents of trace elements were determined by inductively coupled plasma (ICP) mass spectrometry following multiacid digestion. The contents of sulfur and carbon were determined by a Leco analyzer. The mineral compositions of the bulk sample and the <2-μm fraction were determined by X-ray powder diffraction (XRD) using a Philips diffractometer (graphite monochromator, CuKα radiation, proportional counter). The XRD patterns of the bulk sample and the nonoriented <2-μm fraction were taken after air drying. The XRD patterns of the oriented <2-μm fraction were taken after the following treatments: (i) Mg saturation, (ii) K saturation, (iii) Mg saturation and ethylene glycol solvation, (iv) Mg saturation and glycerol solvation, (v) K saturation and dimethyl sulfoxide (DMSO) solvation, and (vi) heating for 2 h at 550°C. The identification of clay minerals was generally based on the methods outlined by Brown (12), Brindley and Brown (13), and Moore and Reynolds (14). The term “illitic material” is used as defined by Środoń (15) and Środoń and Eberl (16). The thin section was prepared in the Thomas Beckmann Laboratory (Schwülper-Lagesbüttel, Germany) and was analyzed using a Leica DM-LSP petrographic microscope with plane-polarized light (ppl) and cross-polarized light (xpl). An air-dried fragment of the paleosol sample was gold coated and was analyzed using a JEOL JSM-6510LV scanning electron microscope (SEM) fitted with an Oxford Instruments INCA system. Selected mineral grains from the sample fraction of >45 μm were also analyzed using SEM.
Cultivable bacteria native to paleosol.
The wet sample of paleosol was taken aseptically in May 2013, placed in a sterile glass bottle, and transferred to the laboratory within 4 h. The number of native cultivable bacteria was determined in a fresh sample of paleosol in duplicate. A 1.0-g aliquot of paleosol was placed in a tube containing 9 ml of sterile 0.05 M NaCl. The paleosol sample was suspended in the dark by shaking at 150 rpm for 24 h at 22°C. In this suspension, the number of aerobically grown heterotrophic neutrophilic bacteria was determined as CFU on nutrient agar (Biolife) after 5 days of incubation at 22°C. In order to determine the number of anaerobically grown heterotrophic neutrophilic bacteria, nutrient agar was cultivated under anaerobic conditions generated in an Anaerocult A system (Merck). The final pH value of the suspension of paleosol was measured using a WTW pH meter, model 330. In order to determine the number of acidophilic bacteria in paleosol, 1.0-, 0.5-, and 0.1-g aliquots of paleosol were placed in a tube with Leptospirillum HH medium (DSMZ medium 882) and were incubated in the dark for 7 days at 25°C. The presence of acidophilic bacteria was confirmed by scanning electron microscopy (JSM 5300 SEM; JEOL, Japan) after the dehydration of samples in an ethanol series.
Characterization of the A. baumannii environmental isolate.
A. baumannii was isolated from aerobically cultivated nutrient agar plates. The isolate was first characterized by routine bacteriological techniques: Gram staining, growth at 42°C, catalase and oxidase reactions, and biochemical characteristics in an API 20NE system (bioMérieux). Further identification was carried out by using the ATB 32GN and Vitek 2 systems (bioMérieux), according to standard procedures in microbiological laboratories, and was confirmed by the presence of OXA-69-like oxacillinase, a variant of OXA-51-type oxacillinase specific for A. baumannii (17, 18). Susceptibilities to β-lactams (imipenem, meropenem), β-lactam–β-lactamase inhibitor combinations (ampicillin-sulbactam), trimethoprim-sulfamethoxazole, aminoglycosides (amikacin, gentamicin, tobramycin), and fluoroquinolones (ciprofloxacin, levofloxacin) were determined by disc diffusion tests, the results of which were interpreted according to the European Committee on Antimicrobial Susceptibility Testing criteria (19), and were confirmed by MICs determined by using the AST-XN05 and AST-N233 testing cards for the Vitek 2 system or, for colistin and ampicillin-sulbactam, by E-tests (AB Biodisk).
The next step in the characterization of the A. baumannii environmental isolate was genotyping using pulsed-field gel electrophoresis (PFGE) in order to investigate its similarity to clinical strains. We compared the A. baumannii strain isolated from paleosol with three different isolates: a clinical isolate of A. baumannii from a general hospital in Pula, Istria, belonging to a multicenter collection and two clinical isolates belonging to European (international) clones I and II that are causing outbreaks in Croatia (20, 21). The general hospital in Pula is situated in the area where our environmental isolate was found, so we supposed that our environmental isolate could be similar to the clinical isolate from this region. This clinical isolate was collected in 2009 during multicenter collection of carbapenem-resistant isolates from southern Croatia and Istria. The PFGE analysis was performed according to the work of Seifert et al. (22) with ApaI (New England BioLabs) as the restriction enzyme, according to the manufacturer's instructions. The resulting macrorestriction digestion products were electrophoresed in a 1.4% agarose gel. PFGE fingerprinting patterns were analyzed using Molecular Analyst Fingerprinting software (Bio-Rad). Dice similarity coefficients were calculated using pairwise comparison of the PFGE profiles, with 1.5% optimization and a position tolerance of 1.0%. Dendrograms were created by using the unweighted-pair group method with arithmetic averages (UPGMA).
Biofilm formation by the environmental A. baumannii isolate was compared to that by the clinical isolate in the multicenter collection. Overnight cultures were diluted to an optical density at 600 nm (OD600) of 0.1 in nutrient broth, distributed in polypropylene tubes, and incubated at 37°C for 24 h without shaking. Biofilms were stained with 0.5% (wt/vol) crystal violet and were quantified spectrophotometrically at 550 nm after solubilization in 96% ethanol. Biofilm formation was quantified in duplicate. The OD550 values of experimental tubes were subtracted from those of the negative control, which contained nutrient broth without bacteria. The production of extracellular substances in isolates was confirmed by alcian blue staining of 24-h-old cultures cultivated in nutrient broth.
Influences of low pH, desiccation, and high temperatures on the A. baumannii environmental isolate.
In order to test the influence of low pH on the A. baumannii isolate, the isolate was pregrown on nutrient agar (BioLife) for 16 h at 37 ± 0.1°C. The biomass was then suspended in commercially available natural spring water (pH 8.29). The suspended biomass was inoculated in triplicate into tubes that contained 10 ml of either natural spring water, natural spring water adjusted to pH 3.40 with 1 M HCl, or natural spring water to which 1.0 g of air-dried paleosol had been added. The tubes were sealed and were incubated at 22 ± 0.1°C for 24 h with shaking at 150 rpm. The number of A. baumannii bacteria was determined in triplicate on nutrient agar after incubation at 37 ± 0.1°C for 24 h. The numbers of CFU were logarithmically transformed, and survival was calculated as (log CFU24 h/log CFUc0) × 100, where c0 is the initial concentration of bacteria. Statistical analyses were carried out using Statistica software, version 10.0 (StatSoft, Tulsa, OK, USA). The numbers of bacterial CFU were logarithmically transformed beforehand to normalize distribution and to equalize the variances of the parameters measured. Comparisons between samples were carried out using one-way analysis of variance (ANOVA), and subsequently the Duncan post hoc test was performed for pairwise comparisons. Statistical decisions were made at a significance level (P) of <0.05.
The influences of desiccation and high temperatures on the A. baumannii isolate were determined by drying the original sample of paleosol at 50°C for 72 h. A 1.0-g portion of dried paleosol was suspended in a tube containing 9 ml of sterile 0.05 M NaCl, and the numbers of A. baumannii bacteria were determined in triplicate on the nutrient agar after incubation at 42 ± 0.1°C for 24 h.
RESULTS
Characterization of paleosol.
Paleosol is composed predominantly of clay (particle diameter, <2 μm); silt particles (2 to 63 μm) and sand particles (>63 μm) make up less than 5% by weight (5 wt%). Clay minerals are the main constituents of paleosol (phyllosilicates and amorphous inorganic compounds, 95 wt%), while quartz (1 wt%), pyrite (2 wt%), gypsum (1 wt%), and jarosite (1 wt%) are present in silt and sand fractions and are minor mineral phases. The main clay mineral is illitic material (Fig. 3), which makes up 70 wt% of the clay fraction, followed by illite/smectite mixed-layer minerals (30 wt%). Pyrite fills former roots, burrows, and channels (Fig. 4) or is randomly distributed within the clay matrix (Fig. 5). The high content of illitic material corresponds well with the chemical data (Table 1): the K2O content is around 5 wt%, clearly indicating that the illitic material is the dominant mineral phase in the paleosol. Based on infrared studies, Ottner et al. (10) found that the smectites in illite/smectite mixed-layer minerals from clays in the Tri Jezerca quarry are aluminum-rich montmorillonites without iron substitution in the octahedral position. The values obtained for MgO (Table 1) match that finding well. Durn et al. (11) concluded that the clay mineral composition of paleosol in the Tri Jezerca quarry clearly indicates the influences of both pedogenic and diagenetic processes. They concluded that paleosols were probably seasonally marshy soils or permanently waterlogged soils. The high contents of V, Mo, U, Ni, and Zn (Table 2) are also in favor of an acidic reductive pedogenic paleoenvironment. Pyrite, which formed in paleosol both pedogenetically and diagenetically, is an unstable mineral phase at the surface. Pyrite oxidation (Fig. 6), which lowers the pH of the soil, results in the formation of the secondary minerals gypsum (the calcium originates from limestones below and/or above the paleosol) and jarosite (hydrous sulfate of potassium and iron).
FIG 3.

SEM photograph of illitic material in the freshly fractured surface of paleosol.
FIG 4.
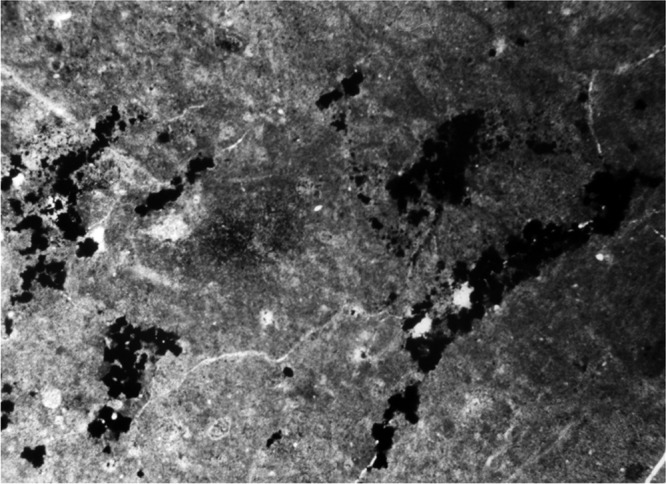
Root remains, burrows, and channels, mainly filled with pyrite (upper part of profile, 10 to 18 cm from the top). The section represents a length dimension of 3.3 mm.
FIG 5.

SEM photograph of pyrites in a clay matrix.
TABLE 1.
Chemical composition of the bulk sample of paleosola
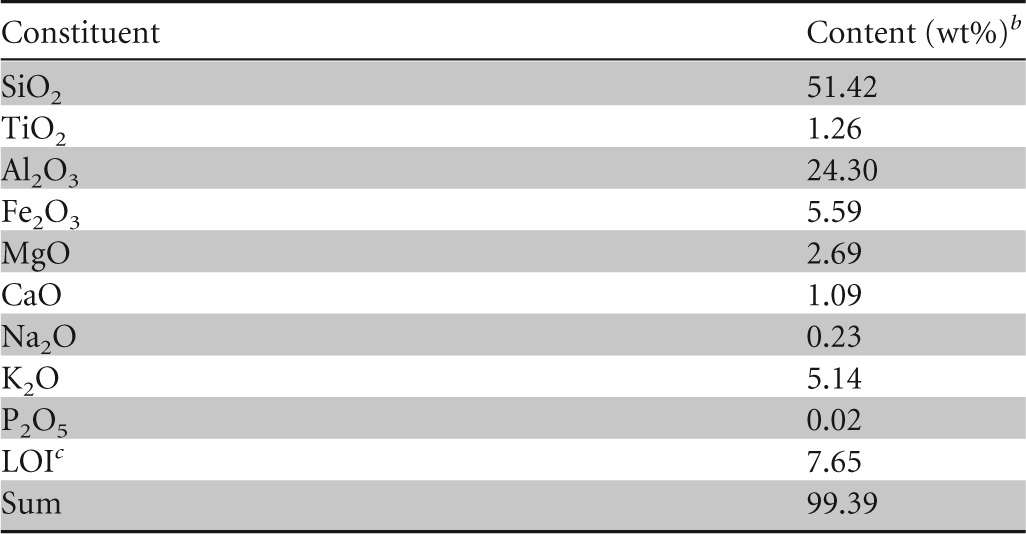
The color (dry) is 5BG4/1 according to the Munsell soil color charts (23).
wt%, percentage by weight.
Loss on ignition (1,000°C).
TABLE 2.
Contents of sulfur, carbon, and trace elements in the bulk sample of paleosol
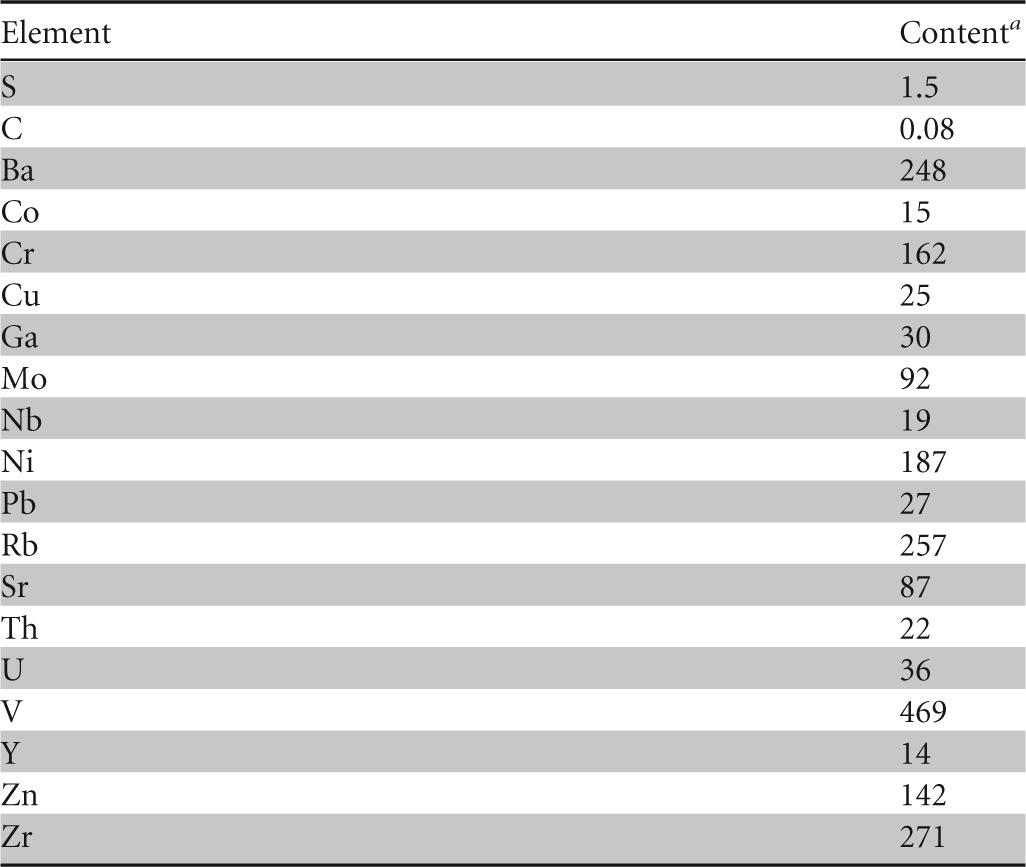
Amounts of S and C are expressed as percentages by weight; all other values are expressed as parts per million.
FIG 6.

SEM photograph of weathered pyrite.
Cultivable bacteria native to paleosol.
In the fresh sample of paleosol, no anaerobically grown heterotrophic neutrophilic bacteria were detected. Aerobic cultivation of heterotrophic neutrophilic bacteria resulted in the growth of 80 to 120 CFU per g of paleosol. The population was represented by only one morphological type of colony. The colonies grown were picked and were used for further characterization. The suspension of paleosol in 0.05 M NaCl was acidic, with a final pH of 2.55.
In the fresh sample of paleosol, acidophilic bacteria were detected by cultivation of 1.0- and 0.5-g aliquots of paleosol in Leptospirillum HH medium, which is used for the cultivation of Acidithiobacillus ferrooxidans and Leptospirillum spp. (24). The rod-shaped acidophilic bacteria in the extracellular polymeric matrix (Fig. 7) gave no results by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Microflex LT; Bruker Daltonics). The total organic carbon (TOC) concentration in the paleosol sample examined was 0.08 wt% (Table 2). Due to the low TOC concentration, the presence of acidophilic mesophilic heterotrophic bacteria (25) is unlikely. The acidophilic mesophilic chemolithotrophs A. ferrooxidans and Leptospirillum ferrooxidans are the most significant bacteria involved in the biological oxidation of pyrite and other sulfide minerals. Acidophilic bacteria that oxidize reduced sulfur compounds but not ferrous ion, such as Acidithiobacillus thiooxidans, support the leaching of minerals by the production of sulfuric acid. Those bacteria degrade sulfide minerals by attachment to minerals and enzymatic oxidation of ferrous ion or sulfide. The extracellular polymers, which are important in the process of bacterial attachment, may also play a role in the leaching of sulfide minerals (26).
FIG 7.
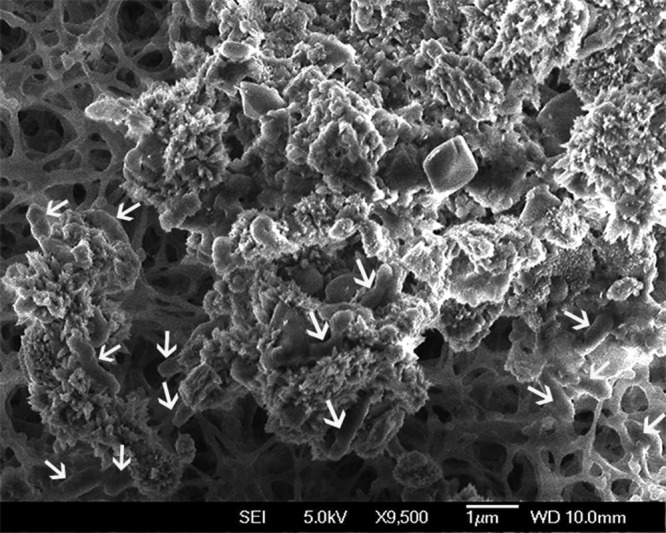
Acidophilic bacteria on the surface of paleosol surrounded by the network of the extracellular polymeric matrix. Arrows indicate bacterial cells.
Characterization of the A. baumannii isolate.
The colonies of aerobically grown heterotrophic neutrophilic bacteria were characterized by routine bacteriological techniques (Gram staining, growth at 42°C, catalase and oxidase reactions, biochemical characteristics in the API 20NE system) as A. baumannii. One colony was chosen for subsequent analyses by the ATB 32GN and Vitek 2 systems, and the presence of OXA-69-like oxacillinase was determined by PCR (18). Susceptibility testing by the disc diffusion method and MIC values revealed a resistance pattern close to those of clinical isolates of A. baumannii from most hospitals in Croatia according to the results of the Public Health Collegium Committee for Antibiotic Resistance Surveillance (27). The isolate was susceptible to ampicillin-sulbactam (MIC, 4 mg/liter), amikacin (MIC, <2 mg/liter), imipenem (MIC, 0.5 mg/liter), meropenem (MIC, 0.5 mg/liter), tobramycin (MIC, <1 mg/liter), and colistin (MIC, 0.5 mg/liter) but resistant to gentamicin (MIC, >16 mg/liter), trimethoprim-sulfamethoxazole (MIC, 160 mg/liter), ciprofloxacin (MIC, >4 mg/liter), and levofloxacin (MIC, 4 mg/liter) according to EUCAST criteria (19).
Molecular typing by PFGE confirmed that the environmental isolate of A. baumannii showed 87% similarity to the clinical isolate obtained from the general hospital in Pula (Fig. 8) and that these isolates represent a cluster within European clone I. European clone I has been recognized as a cause of nosocomial infection in Croatia for more than 10 years, and for a long time it has been the dominant clone causing hospital outbreaks (20). However, the carbapenem resistance pattern of the clinical isolate in Pula displays reduced susceptibility to imipenem and meropenem (MICs, 8 to 16 mg/liter), and it carries a blaOXA-69-like gene associated with ISAba1, a well-described mechanism of carbapenem resistance in Croatia in the past decade (20, 21).
FIG 8.
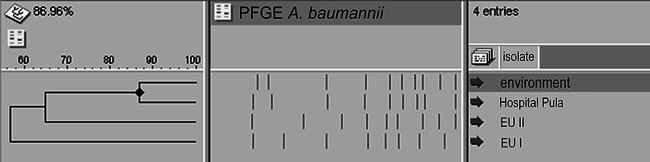
Dendrogram based on ApaI-digested DNA from different isolates of A. baumannii.
Quantification of biofilm formation resulted in OD550 values of 0.587 ± 0.100 for the environmental isolate and 0.022 ± 0.007 for the clinical isolate of A. baumannii. Since the difference in the OD550 between the environmental isolate and the negative control was 7.4 ± 0.9, while that between the clinical isolate and the negative control was 1.2 ± 0.1, the environmental isolate could be interpreted as biofilm forming, while the clinical isolate could be interpreted as a biofilm-negative strain. Alcian blue staining confirmed the presence of a thick layer of extracellular substances for the environmental isolate of A. baumannii, which were much more abundant than those for the clinical isolate (Fig. 9). The extracellular polysaccharides are important for the attachment of bacteria to particles and protect bacterial cells under unfavorable environmental conditions (28).
FIG 9.
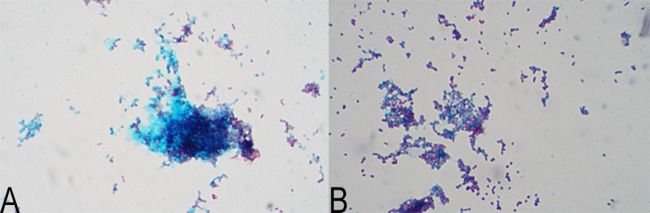
Thick layer of extracellular substances (blue) with embedded cells (red) of an environmental isolate (A) and less-abundant extracellular substances for a clinical isolate (B) of A. baumannii.
Influences of low pH, desiccation, and high temperatures on the A. baumannii isolate.
The influence of low pH (initially adjusted or generated after the addition of paleosol) on the environmental isolate of A. baumannii is shown in Table 3. The isolate showed 100% survival in natural spring water, which has a neutral pH. The survival of the isolate in spring water acidified by HCl was not significantly different from its survival in spring water acidified by paleosol. This finding suggests that low pH was the major reason for the reduction in the number of A. baumannii bacteria in contact with paleosol. The relatively high content of potentially toxic heavy metals in paleosol (Table 2) was not important for the survival of A. baumannii. The survival of A. baumannii in contact with paleosol (20 to 100 CFU/g) was high enough to support the findings of 80 to 120 CFU/g from fresh paleosol.
TABLE 3.
Survival of A. baumannii after 24 h of contact with natural spring water, natural spring water adjusted to pH 3.40, or natural spring water with added paleosol
| Sample | pH |
Survival (%)a | |
|---|---|---|---|
| Starting | Final | ||
| Natural spring water (positive control) | 8.29 ± 0.02 | 7.89 ± 0.12 | 100.9 ± 1.2 |
| Spring water adjusted to pH 3.40 | 3.40 ± 0.02 | 3.53 ± 0.09 | 8.9 ± 4.6* |
| Spring water with paleosol (1.0 g/10 ml) added | 8.29 ± 0.02 | 3.37 ± 0.03 | 10.5 ± 3.7* |
Survival was calculated as (log CFU24 h/log CFUc0) × 100, where c0 is the initial concentration of bacteria ([5.14 ± 1.08] × 107 CFU/ml). Asterisks indicate survival rates significantly lower than that with the positive control. No statistical difference was found between spring water at pH 3.40 and spring water with added paleosol.
In the paleosol sample that was dried at 50°C for 72 h, 60 to 100 CFU/g of aerobically grown heterotrophic neutrophilic bacteria was detected. Again, the population was represented by only one morphological type of colony. Characterization of the colony (performed as described for the fresh sample) showed that the bacterium isolated was A. baumannii. Obviously, desiccation and high temperatures had no significantly negative influence on the survival of the A. baumannii population in fresh paleosol.
DISCUSSION
Reports of the occurrence of viable clinically important multidrug-resistant Acinetobacter spp. in nature are scarce. While it is certainly true that A. baumannii can be isolated from patients and hospital environmental sources during outbreaks, this species has no known natural habitat outside the hospital (29). Multiresistant isolates of A. baumannii have been found in wastewater from hospitals in Brazil (30). A. baumannii has also been isolated from hospital solid waste (31). In a study on the occurrence of A. baumannii in the soil, screening of 49 soil samples resulted in the recovery of an Acinetobacter sp. from only 1 soil sample (5). This isolate was not genetically related to any clinical isolate. Such investigations may underestimate the significance of soil as a source of Acinetobacter spp. related to clinical strains, because the isolation procedure involved the placement of a swab in the soil sample, after which the swab was streaked across the agar plate. By such a procedure, a small quantity of soil is analyzed. The conditions and duration of the storage of archived soil samples may result in limited recovery of environmental isolates. The analysis of a large quantity of fresh soil holds promise for the successful isolation from the environment of Acinetobacter spp. related to clinical strains.
Our environmental isolate of A. baumannii showed similarity to the clinical isolate with which it was compared in the pattern of susceptibility to ampicillin-sulbactam, colistin, amikacin, and tobramycin. However, our environmental isolate was susceptible to carbapenems (both imipenem and meropenem), which is not unexpected given the main basis of carbapenem resistance in Croatia up to 2009 (20). According to the published data and surveillance follow-ups in the past 10 years, the main mechanism of carbapenem resistance was the presence of insertion sequence ISAba1 upstream of a blaOXA-51/69-like gene (20, 32). Since this mobile genetic element attains full expression under conditions of excessive consumption (overuse) of antibiotics (in this case, carbapenems), one would not expect to find this mechanism of resistance in an environmental isolate.
The nearest medical institutions to the Tri Jezerca quarry are situated at the seaside, more than 10 km away. Therefore, it is hard to believe that the A. baumannii strain isolated from paleosol originates from the hospital wastewaters (30). The probable source of A. baumannii in the upper part of the paleosol profile (0 to 40 cm) is illegally disposed of waste of external origin (31) in the abandoned quarry where the paleosol under investigation is situated. The bacteria could have been leached from waste by storm water and thus could have infiltrated the paleosol. In a wet environment, Acinetobacter spp. have the potential to survive for prolonged periods. At a relative humidity of 31%, strains of A. baumannii have survived as long as 36 days (33, 34). On a dry Formica surface, A. calcoaceticus has survived as long as 13 days (35). Thirty-nine of 118 Acinetobacter sp. isolates from human skin tolerated a temperature of 50°C (36). Acinetobacter spp. grow well in the pH range of 5 to 8 (7). Planktonic Acinetobacter junii did not survive a pH of 3 during 24 h of contact, while A. junii immobilized on zeolite particles survived successfully (28). There are no reports in the literature on the survival of Acinetobacter spp. on acid minerals. In the paleosol, A. baumannii is immobilized on soil particles. The immobilized cells are protected by clay particles in the soil, which can increase the survival of bacteria over that of planktonic cells. The extracellular polymeric substances of A. baumannii may have an additional protective role under unfavorable environmental conditions (low pH, desiccation, and high temperatures).
The input of A. baumannii in the reported paleosol was probably much greater than the amount detected (80 to 120 CFU/g of paleosol). Despite the wetness of the soil, only a part of the bacterial population survived, due to the low pH of paleosol. The survival of A. baumannii in a neutral or alkaline soil would be much greater. The finding in paleosol of an A. baumannii strain related to clinical isolates suggests that illegal landfill could be the source of multidrug-resistant bacteria in nature. Therefore, illegal landfills represent a threat to the environment. The detection of this isolate and its ability to survive in paleosol open up new options for finding the spread of these pathogens outside the hospital environment and reveal a potential new source of infection, even in immunocompetent hosts.
ACKNOWLEDGMENTS
This research was supported by the Ministry of Science, Education and Sports of the Republic of Croatia (projects 119-1191155-1203 and 195-1953068-2704).
We thank the staffs of all the Croatian collaborative centers for collecting clinical isolates of A. baumannii for multicenter investigations, especially Nada Barisic and Mirna Vranic-Ladavac (GHP).
Footnotes
Published ahead of print 28 February 2014
REFERENCES
- 1.Towner KJ. 2009. Acinetobacter: an old friend, but a new enemy. J. Hosp. Infect. 73:355–363. 10.1016/j.jhin.2009.03.032 [DOI] [PubMed] [Google Scholar]
- 2.Roca I, Espinal P, Vila-Farres X, Vila J. 2012. The Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menace. Front. Microbiol. 3:148. 10.3389/fmicb.2012.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camp C, Tatum OL. 2010. A review of Acinetobacter baumannii as a highly successful pathogen in times of war. Lab. Med. 41:649–657. 10.1309/LM90IJNDDDWRI3RE [DOI] [Google Scholar]
- 4.Gilbert DN, Guidos RJ, Boucher HW, Talbot GH, Spellberg B, Edwards JE, Scheld WM, Bradley JS, Bartlett JG. 2010. The 10 × '20 Initiative: pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin. Infect. Dis. 50:1081–1083. 10.1086/652237 [DOI] [PubMed] [Google Scholar]
- 5.Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, Fishbain J, Craft D, Riddell S, Lindler L, Mancuso J, Milstrey E, Bautista CT, Patel J, Ewell A, Hamilton T, Gaddy C, Tenney M, Christopher G, Petersen K, Endy T, Petruccelli B. 2007. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin. Infect. Dis. 44:1577–1584. 10.1086/518170 [DOI] [PubMed] [Google Scholar]
- 6.Fournier PE, Richet H. 2006. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 42:692–699. 10.1086/500202 [DOI] [PubMed] [Google Scholar]
- 7.Garrity GM, Brenner DJ, Krieg NR, Staley JT. 2005. Bergey's manual of systematic bacteriology, 2nd ed, vol 2, part B, p 425–437 Springer, New York, NY [Google Scholar]
- 8.Corbella X, Pujol M, Ayats J, Sendra M, Ardanuy C, Dominguez MA, Linares J, Ariza J, Gudiol F. 1996. Relevance of digestive tract colonization in the epidemiology of nosocomial infections due to multiresistant Acinetobacter baumannii. Clin. Infect. Dis. 23:329–334. 10.1093/clinids/23.2.329 [DOI] [PubMed] [Google Scholar]
- 9.Vlahovic I, Tisljar J, Velic I, Maticec D. 2005. Evolution of the Adriatic Carbonate Platform: palaeogeography, main events and depositional dynamics. Palaeogeogr. Palaeoclimatol. Palaeoecol. 220:333–360. 10.1016/j.palaeo.2005.01.011 [DOI] [Google Scholar]
- 10.Ottner F, Durn G, Schweighofer B, Tisljar J. 1999. Clay minerals in paleosols of Cretaceous age in Istria, Croatia. Chin. Sci. Bull. 44:145–151 [Google Scholar]
- 11.Durn G, Ottner F, Mindszenty A, Tisljar J, Mileusnic M. 2006. Clay mineralogy of bauxites and palaeosols in Istria formed during regional subaerial exposures of the Adriatic Carbonate Platform, p 3–30 In Vlahovic I, Tibljas D, Durn G. (ed), 3rd Mid-European Clay Conference: field trip guidebook. University of Zagreb, Faculty of Science and Faculty of Mining, Geology and Petroleum Engineering, Zagreb, Croatia [Google Scholar]
- 12.Brown G. 1961. The X-ray identification and crystal structures of clay minerals. Mineralogical Society, London, United Kingdom [Google Scholar]
- 13.Brindley GW, Brown G. 1980. Crystal structures of clay minerals and their X-ray identification. Mineralogical Society, London, United Kingdom [Google Scholar]
- 14.Moore DM, Reynolds RC. 1989. X-ray diffraction and the identification and analysis of clay minerals. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 15.Środoń J. 1984. X-ray powder diffraction identification of illitic materials. Clays Clay Miner. 32:337–349. 10.1346/CCMN.1984.0320501 [DOI] [Google Scholar]
- 16.Środoń J, Eberl DD. 1984. Illite. Rev. Miner. 13:495–544 [Google Scholar]
- 17.Schreckenberger PC, Daneshvar MI, Weyant RS, Hollis DG. 2003. Acinetobacter, Achromobacter, Chryseobacterium, Moraxella, and other nonfermentative gram-negative rods, p 749–779 In Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH. (ed), Manual of clinical microbiology, 8th ed. ASM Press, Washington DC [Google Scholar]
- 18.Evans BA, Hamouda A, Towner KJ, Amyes SGB. 2008. OXA-51-like β-lactamases and their association with particular epidemic lineages of Acinetobacter baumannii. Clin. Microbiol. Infect. 14:268–275. 10.1111/j.1469-0691.2007.01919.x [DOI] [PubMed] [Google Scholar]
- 19.European Committee on Antimicrobial Susceptibility Testing. April 2013. Reading guide: EUCAST disk diffusion method for antimicrobial susceptibility testing, version 3.0. [Google Scholar]
- 20.Goic-Barisic I, Bedenic B, Tonkic M, Novak A, Katic S, Kalenic S, Punda-Polic V, Towner KJ. 2009. Occurrence of OXA-107 and ISAba1 in carbapenem-resistant isolates of Acinetobacter baumannii from Croatia. J. Clin. Microbiol. 47:3348–3349. 10.1128/JCM.02394-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goic-Barisic I, Towner KJ, Kovacic A, Sisko-Kraljevic K, Tonkic M, Novak A, Punda-Polic V. 2011. Outbreak in Croatia caused by a new carbapenem-resistant clone of Acinetobacter baumannii producing OXA-72 carbapenemase. J. Hosp. Infect. 77:368–369. 10.1016/j.jhin.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 22.Seifert H, Dolzani L, Bressan R, van der Reijden T, van Strijen B, Stefanik D, Heersma H, Dijkshoorn L. 2005. Standardization and interlaboratory reproducibility assessment of pulsed-field gel electrophoresis-generated fingerprints of Acinetobacter baumannii. J. Clin. Microbiol. 43:4328–4335. 10.1128/JCM.43.9.4328-4335.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munsell Color. 1994. Munsell soil color charts, rev. ed. Macbeth Division of Kollmorgan Instruments, New Windsor, NY [Google Scholar]
- 24.Atlas RM. 2004. Handbook of microbiological media, 3rd ed. CRC Press, Boca Raton, FL [Google Scholar]
- 25.Bacelar-Nicolau P, Johnson DB. 1999. Leaching of pyrite by acidophilic heterotrophic iron-oxidizing bacteria in pure and mixed cultures. Appl. Environ. Microbiol. 65:585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sand W, Gerke T, Hallmann R, Schippers A. 1995. Sulfur chemistry, biofilm, and the (in)direct attack mechanism—a critical evaluation of bacterial leaching. Appl. Microbiol. Biotechnol. 43:961–966. 10.1007/BF00166909 [DOI] [Google Scholar]
- 27.Public Health Collegium Committee for Antibiotic Resistance Surveillance in Croatia. 2011. Antibiotic resistance in Croatia in 2009. Croatian Academy of Medical Sciences (AMZH), Zagreb, Croatia [Google Scholar]
- 28.Ivankovic T, Hrenovic J, Matonickin-Kepcija R. 2013. Resistance of bioparticles formed of phosphate-accumulating bacteria and zeolite to harsh environmental conditions. Biofouling 29:641–649. 10.1080/08927014.2013.786048 [DOI] [PubMed] [Google Scholar]
- 29.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582. 10.1128/CMR.00058-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira AE, Marchetti DP, De Oliveira LM, Gusatti CS, Fuentefria DB, Corcao G. 2011. Presence of OXA-23-producing isolates of Acinetobacter baumannii in wastewater from hospitals in southern Brazil. Microb. Drug Resist. 17:221–227. 10.1089/mdr.2010.0013 [DOI] [PubMed] [Google Scholar]
- 31.Hossain MS, Ab Rahman, NNN. Balakrishnan V, Puvanesuaran VR, Sarker MZI, Ab Kadir MO. 2013. Infectious risk assessment of unsafe handling practices and management of clinical solid waste. Int. J. Environ. Res. Public Health 10:556–567. 10.3390/ijerph10020556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, Livermore DM, Pitt TL. 2006. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol. Lett. 258:72–77. 10.1111/j.1574-6968.2006.00195.x [DOI] [PubMed] [Google Scholar]
- 33.Jawad A, Seifert H, Snelling AM, Heritage J, Hawkey PM. 1998. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J. Clin. Microbiol. 36:1938–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Espinal P, Marti S, Vila J. 2012. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J. Hosp. Infect. 80:56–60. 10.1016/j.jhin.2011.08.013 [DOI] [PubMed] [Google Scholar]
- 35.Getchell-White SI, Donowitz LG, Groschel DH. 1989. The inanimate environment of an intensive care unit as a potential source of nosocomial bacteria: evidence for long survival of Acinetobacter calcoaceticus. Infect. Control Hosp. Epidemiol. 10:402–407. 10.2307/30144208 [DOI] [PubMed] [Google Scholar]
- 36.Yavankar SP, Pardesi KR, Chopade BA. 2007. Species distribution and physiological characterization of Acinetobacter genospecies from healthy human skin of tribal population in India. Indian J. Med. Microbiol. 25:336–345. 10.4103/0255-0857.37335 [DOI] [PubMed] [Google Scholar]


