Abstract
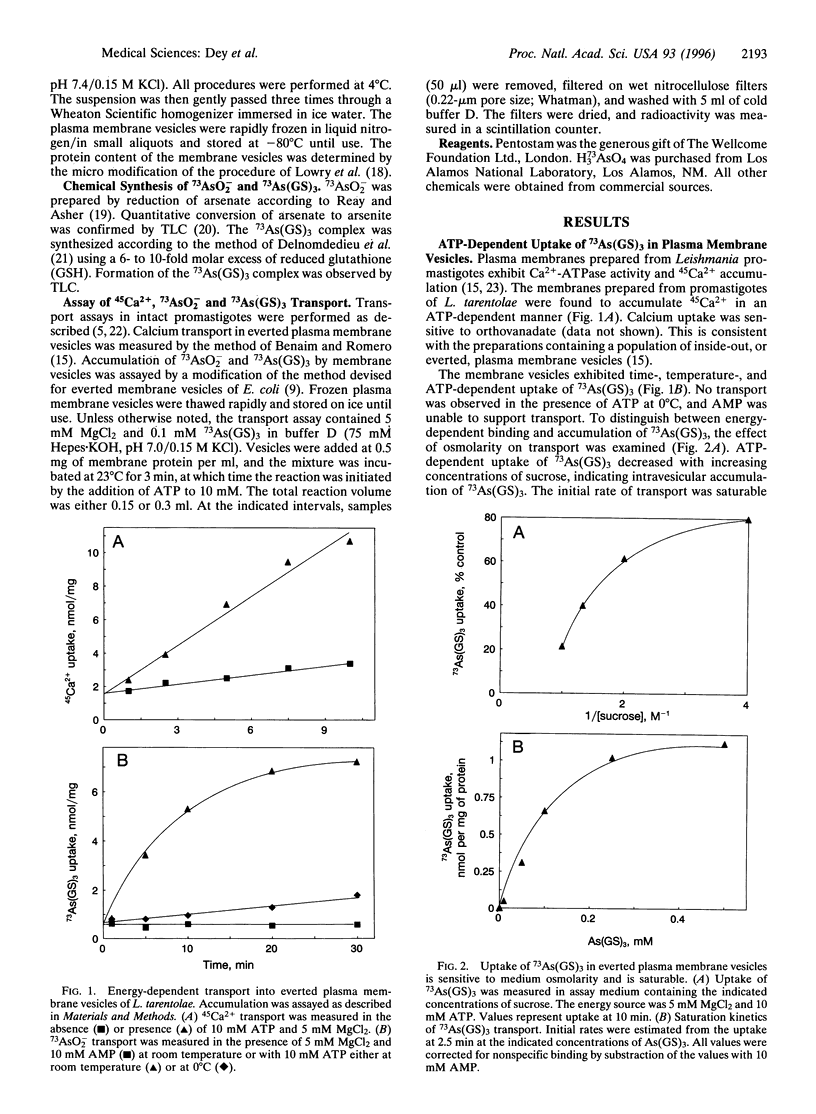
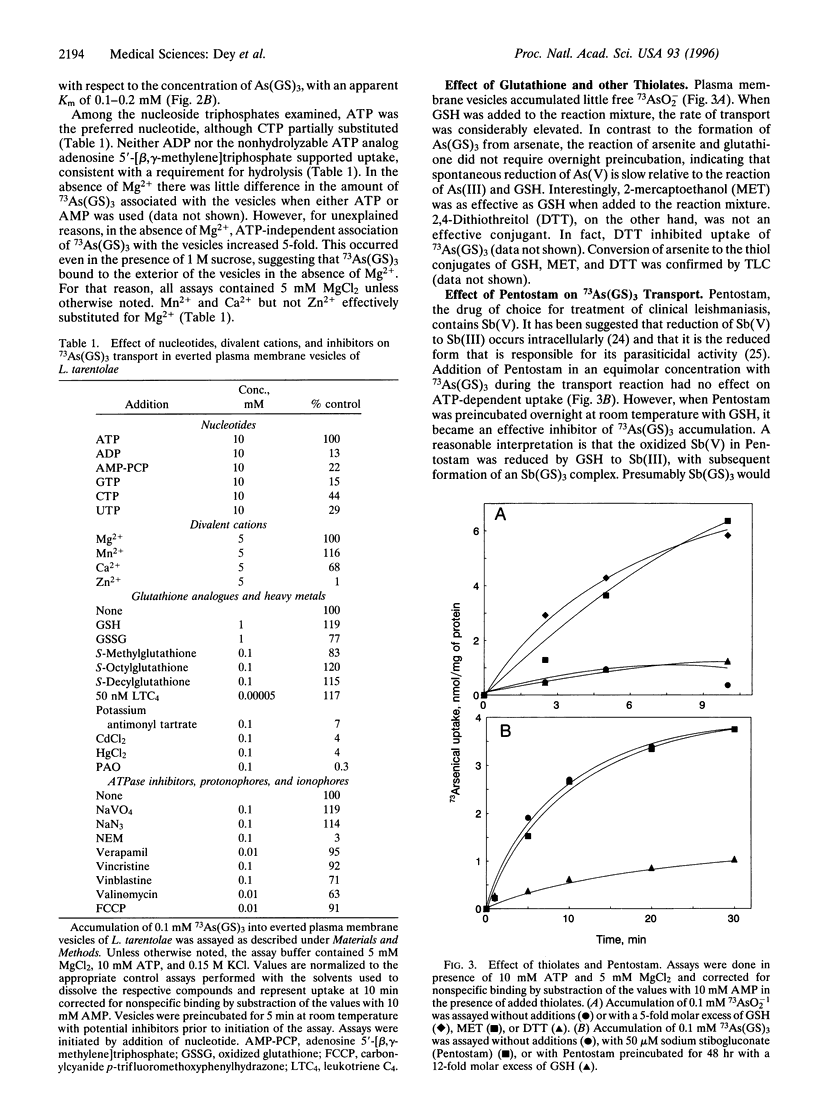
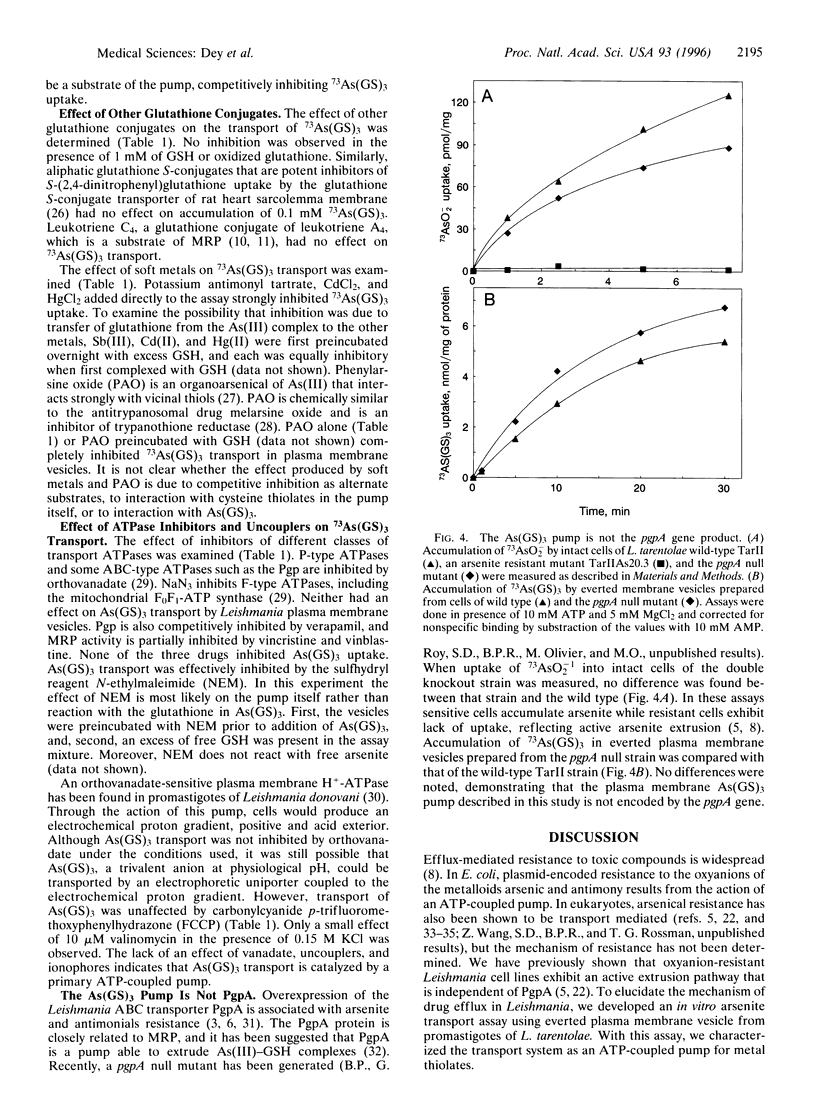
Membrane preparations enriched in plasma membrane vesicles prepared from promastigotes of Leishmania tarentolae were shown to accumulate thiolate derivatives of 73As(III). Free arsenite was transported at a low rate, but rapid accumulation was observed after reaction with reduced glutathione (GSH) conditions that favor the formation of As(GS)3. Accumulation required ATP but not electrochemical energy, indicating that As(GS)3 is transported by an ATP-coupled pump. Pentostam, a Sb(V)-containing drug that is one of the first-line therapeutic agents for treatment of leishmaniasis, inhibited uptake after reaction with GSH. Vesicles prepared from a strain in which both copies of the pgpA genes were disrupted accumulated As(GS)3 at wild-type levels, demonstrating that the PgpA protein is not the As(GS)3 pump. These results have important implications for the mechanism of drug resistance in the trypanosomatidae, suggesting that a plasma membrane As(GS)3 pump catalyzes active extrusion of metal thiolates, including the Pentostam-glutathione conjugate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford R. W., Desjeux P., Deraadt P. Estimation of population at risk of infection and number of cases of Leishmaniasis. Parasitol Today. 1992 Mar;8(3):104–105. doi: 10.1016/0169-4758(92)90249-2. [DOI] [PubMed] [Google Scholar]
- Ayté J., Gil-Gómez G., Haro D., Marrero P. F., Hegardt F. G. Rat mitochondrial and cytosolic 3-hydroxy-3-methylglutaryl-CoA synthases are encoded by two different genes. Proc Natl Acad Sci U S A. 1990 May;87(10):3874–3878. doi: 10.1073/pnas.87.10.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaim G., Romero P. J. A calcium pump in plasma membrane vesicles from Leishmania braziliensis. Biochim Biophys Acta. 1990 Aug 10;1027(1):79–84. doi: 10.1016/0005-2736(90)90051-o. [DOI] [PubMed] [Google Scholar]
- Bergot M. O., Diaz-Guerra M. J., Puzenat N., Raymondjean M., Kahn A. Cis-regulation of the L-type pyruvate kinase gene promoter by glucose, insulin and cyclic AMP. Nucleic Acids Res. 1992 Apr 25;20(8):1871–1877. doi: 10.1093/nar/20.8.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. D., Grogl M. Leishmania mexicana: chemistry and biochemistry of sodium stibogluconate (Pentostam). Exp Parasitol. 1988 Oct;67(1):96–103. doi: 10.1016/0014-4894(88)90012-4. [DOI] [PubMed] [Google Scholar]
- Borst P., Ouellette M. New mechanisms of drug resistance in parasitic protozoa. Annu Rev Microbiol. 1995;49:427–460. doi: 10.1146/annurev.mi.49.100195.002235. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Beverley S. M. Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J Biol Chem. 1991 Oct 5;266(28):18427–18430. [PubMed] [Google Scholar]
- Callahan H. L., Roberts W. L., Rainey P. M., Beverley S. M. The PGPA gene of Leishmania major mediates antimony (SbIII) resistance by decreasing influx and not by increasing efflux. Mol Biochem Parasitol. 1994 Nov;68(1):145–149. doi: 10.1016/0166-6851(94)00154-5. [DOI] [PubMed] [Google Scholar]
- Casals N., Roca N., Guerrero M., Gil-Gómez G., Ayté J., Ciudad C. J., Hegardt F. G. Regulation of the expression of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene. Its role in the control of ketogenesis. Biochem J. 1992 Apr 1;283(Pt 1):261–264. doi: 10.1042/bj2830261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen B. E., Ramos H., Gamargo M., Urbina J. The water and ionic permeability induced by polyene antibiotics across plasma membrane vesicles from Leishmania sp. Biochim Biophys Acta. 1986 Aug 7;860(1):57–65. doi: 10.1016/0005-2736(86)90498-0. [DOI] [PubMed] [Google Scholar]
- Croft S. L., Neame K. D., Homewood C. A. Accumulation of [125Sb]sodium stibogluconate by Leishmania mexicana amazonensis and Leishmania donovani in vitro. Comp Biochem Physiol C. 1981;68C(1):95–98. doi: 10.1016/0306-4492(81)90043-5. [DOI] [PubMed] [Google Scholar]
- Cuif M. H., Cognet M., Boquet D., Tremp G., Kahn A., Vaulont S. Elements responsible for hormonal control and tissue specificity of L-type pyruvate kinase gene expression in transgenic mice. Mol Cell Biol. 1992 Nov;12(11):4852–4861. doi: 10.1128/mcb.12.11.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuif M. H., Porteu A., Kahn A., Vaulont S. Exploration of a liver-specific, glucose/insulin-responsive promoter in transgenic mice. J Biol Chem. 1993 Jul 5;268(19):13769–13772. [PubMed] [Google Scholar]
- Cunningham M. L., Zvelebil M. J., Fairlamb A. H. Mechanism of inhibition of trypanothione reductase and glutathione reductase by trivalent organic arsenicals. Eur J Biochem. 1994 Apr 1;221(1):285–295. doi: 10.1111/j.1432-1033.1994.tb18740.x. [DOI] [PubMed] [Google Scholar]
- Delnomdedieu M., Basti M. M., Otvos J. D., Thomas D. J. Reduction and binding of arsenate and dimethylarsinate by glutathione: a magnetic resonance study. Chem Biol Interact. 1994 Feb;90(2):139–155. doi: 10.1016/0009-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Dey S., Dou D., Rosen B. P. ATP-dependent arsenite transport in everted membrane vesicles of Escherichia coli. J Biol Chem. 1994 Oct 14;269(41):25442–25446. [PubMed] [Google Scholar]
- Dey S., Papadopoulou B., Haimeur A., Roy G., Grondin K., Dou D., Rosen B. P., Ouellette M. High level arsenite resistance in Leishmania tarentolae is mediated by an active extrusion system. Mol Biochem Parasitol. 1994 Sep;67(1):49–57. doi: 10.1016/0166-6851(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Esser V., Britton C. H., Weis B. C., Foster D. W., McGarry J. D. Cloning, sequencing, and expression of a cDNA encoding rat liver carnitine palmitoyltransferase I. Direct evidence that a single polypeptide is involved in inhibitor interaction and catalytic function. J Biol Chem. 1993 Mar 15;268(8):5817–5822. [PubMed] [Google Scholar]
- Fairlamb A. H., Blackburn P., Ulrich P., Chait B. T., Cerami A. Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science. 1985 Mar 22;227(4693):1485–1487. doi: 10.1126/science.3883489. [DOI] [PubMed] [Google Scholar]
- Girard J., Perdereau D., Foufelle F., Prip-Buus C., Ferré P. Regulation of lipogenic enzyme gene expression by nutrients and hormones. FASEB J. 1994 Jan;8(1):36–42. doi: 10.1096/fasebj.8.1.7905448. [DOI] [PubMed] [Google Scholar]
- Gladysheva T. B., Oden K. L., Rosen B. P. Properties of the arsenate reductase of plasmid R773. Biochemistry. 1994 Jun 14;33(23):7288–7293. doi: 10.1021/bi00189a033. [DOI] [PubMed] [Google Scholar]
- Goodwin L. G., Page J. E. A study of the excretion of organic antimonials using a polarographic procedure. Biochem J. 1943 Jul;37(2):198–209. doi: 10.1042/bj0370198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogl M., Thomason T. N., Franke E. D. Drug resistance in leishmaniasis: its implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am J Trop Med Hyg. 1992 Jul;47(1):117–126. doi: 10.4269/ajtmh.1992.47.117. [DOI] [PubMed] [Google Scholar]
- Grondin K., Papadopoulou B., Ouellette M. Homologous recombination between direct repeat sequences yields P-glycoprotein containing amplicons in arsenite resistant Leishmania. Nucleic Acids Res. 1993 Apr 25;21(8):1895–1901. doi: 10.1093/nar/21.8.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Schmid W., Schütz G. Transcriptional activation of the rat liver tyrosine aminotransferase gene by cAMP. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6637–6641. doi: 10.1073/pnas.81.21.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. D., Lane M. D. Iodophenylarsine oxide and arsenical affinity chromatography: new probes for dithiol proteins. Application to tubulins and to components of the insulin receptor-glucose transporter signal transduction pathway. J Biol Chem. 1992 Jul 15;267(20):14005–14011. [PubMed] [Google Scholar]
- Iynedjian P. B. Mammalian glucokinase and its gene. Biochem J. 1993 Jul 1;293(Pt 1):1–13. doi: 10.1042/bj2930001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen H. M., Strähle U., Gloss B., Stewart F., Schmid W., Boshart M., Miksicek R., Schütz G. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell. 1987 Apr 10;49(1):29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- Ji G., Silver S. Reduction of arsenate to arsenite by the ArsC protein of the arsenic resistance operon of Staphylococcus aureus plasmid pI258. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9474–9478. doi: 10.1073/pnas.89.20.9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G. J., Dang C. V. Function of the c-Myc oncoprotein. FASEB J. 1992 Sep;6(12):3065–3072. doi: 10.1096/fasebj.6.12.1521738. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larner J. Insulin and the stimulation of glycogen synthesis. The road from glycogen structure to glycogen synthase to cyclic AMP-dependent protein kinase to insulin mediators. Adv Enzymol Relat Areas Mol Biol. 1990;63:173–231. doi: 10.1002/9780470123096.ch3. [DOI] [PubMed] [Google Scholar]
- Le Cam A., Guillouzo A., Freychet P. Ultrastructual and biochemical studies of isolated adult rat hepatocytes prepared under hypoxic conditions. Cryopreservation of hepatocytes. Exp Cell Res. 1976 Mar 15;98(2):382–395. doi: 10.1016/0014-4827(76)90448-1. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Wei M. L., Chang W. J., Ho I. C., Lo J. F., Jan K. Y., Huang H. Elevation of glutathione levels and glutathione S-transferase activity in arsenic-resistant Chinese hamster ovary cells. In Vitro Cell Dev Biol. 1989 May;25(5):442–448. doi: 10.1007/BF02624629. [DOI] [PubMed] [Google Scholar]
- Leier I., Jedlitschky G., Buchholz U., Cole S. P., Deeley R. G., Keppler D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem. 1994 Nov 11;269(45):27807–27810. [PubMed] [Google Scholar]
- Liu J. S., Park E. A., Gurney A. L., Roesler W. J., Hanson R. W. Cyclic AMP induction of phosphoenolpyruvate carboxykinase (GTP) gene transcription is mediated by multiple promoter elements. J Biol Chem. 1991 Oct 5;266(28):19095–19102. [PubMed] [Google Scholar]
- Liu M. L., Gibbs E. M., McCoid S. C., Milici A. J., Stukenbrok H. A., McPherson R. K., Treadway J. L., Pessin J. E. Transgenic mice expressing the human GLUT4/muscle-fat facilitative glucose transporter protein exhibit efficient glycemic control. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11346–11350. doi: 10.1073/pnas.90.23.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Thompson K. S., Towle H. C. Carbohydrate regulation of the rat L-type pyruvate kinase gene requires two nuclear factors: LF-A1 and a member of the c-myc family. J Biol Chem. 1993 Jun 15;268(17):12787–12795. [PubMed] [Google Scholar]
- Légaré D., Hettema E., Ouellette M. The P-glycoprotein-related gene family in Leishmania. Mol Biochem Parasitol. 1994 Nov;68(1):81–91. doi: 10.1016/0166-6851(94)00156-1. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part I. Myc. Genes Dev. 1990 Dec;4(12A):2025–2035. doi: 10.1101/gad.4.12a.2025. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Bossone S. A., Patel A. J. myc function and regulation. Annu Rev Biochem. 1992;61:809–860. doi: 10.1146/annurev.bi.61.070192.004113. [DOI] [PubMed] [Google Scholar]
- Marshall B. A., Ren J. M., Johnson D. W., Gibbs E. M., Lillquist J. S., Soeller W. C., Holloszy J. O., Mueckler M. Germline manipulation of glucose homeostasis via alteration of glucose transporter levels in skeletal muscle. J Biol Chem. 1993 Sep 5;268(25):18442–18445. [PubMed] [Google Scholar]
- Mazumder S., Mukherjee T., Ghosh J., Ray M., Bhaduri A. Allosteric modulation of Leishmania donovani plasma membrane Ca(2+)-ATPase by endogenous calmodulin. J Biol Chem. 1992 Sep 15;267(26):18440–18446. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992 Oct 30;258(5083):766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- Messina J. L. Inhibition and stimulation of c-myc gene transcription by insulin in rat hepatoma cells. Insulin alters the intragenic pausing of c-myc transcription. J Biol Chem. 1991 Sep 25;266(27):17995–18001. [PubMed] [Google Scholar]
- Müller M., Meijer C., Zaman G. J., Borst P., Scheper R. J., Mulder N. H., de Vries E. G., Jansen P. L. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):13033–13037. doi: 10.1073/pnas.91.26.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. M., Lucas P. C., Forest C. D., Magnuson M. A., Granner D. K. Identification of a sequence in the PEPCK gene that mediates a negative effect of insulin on transcription. Science. 1990 Aug 3;249(4968):533–537. doi: 10.1126/science.2166335. [DOI] [PubMed] [Google Scholar]
- Oden K. L., Gladysheva T. B., Rosen B. P. Arsenate reduction mediated by the plasmid-encoded ArsC protein is coupled to glutathione. Mol Microbiol. 1994 Apr;12(2):301–306. doi: 10.1111/j.1365-2958.1994.tb01018.x. [DOI] [PubMed] [Google Scholar]
- Oka Y., Asano T., Shibasaki Y., Lin J. L., Tsukuda K., Akanuma Y., Takaku F. Increased liver glucose-transporter protein and mRNA in streptozocin-induced diabetic rats. Diabetes. 1990 Apr;39(4):441–446. doi: 10.2337/diab.39.4.441. [DOI] [PubMed] [Google Scholar]
- Olliaro P. L., Bryceson A. D. Practical progress and new drugs for changing patterns of leishmaniasis. Parasitol Today. 1993 Sep;9(9):323–328. doi: 10.1016/0169-4758(93)90231-4. [DOI] [PubMed] [Google Scholar]
- Ortiz D. F., Ruscitti T., McCue K. F., Ow D. W. Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J Biol Chem. 1995 Mar 3;270(9):4721–4728. doi: 10.1074/jbc.270.9.4721. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Hettema E., Wüst D., Fase-Fowler F., Borst P. Direct and inverted DNA repeats associated with P-glycoprotein gene amplification in drug resistant Leishmania. EMBO J. 1991 Apr;10(4):1009–1016. doi: 10.1002/j.1460-2075.1991.tb08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Papadopoulou B. Mechanisms of drug resistance in Leishmania. Parasitol Today. 1993 May;9(5):150–153. doi: 10.1016/0169-4758(93)90135-3. [DOI] [PubMed] [Google Scholar]
- Papadopoulou B., Roy G., Dey S., Rosen B. P., Ouellette M. Contribution of the Leishmania P-glycoprotein-related gene ltpgpA to oxyanion resistance. J Biol Chem. 1994 Apr 22;269(16):11980–11986. [PubMed] [Google Scholar]
- Pikula S., Hayden J. B., Awasthi S., Awasthi Y. C., Zimniak P. Organic anion-transporting ATPase of rat liver. I. Purification, photoaffinity labeling, and regulation by phosphorylation. J Biol Chem. 1994 Nov 4;269(44):27566–27573. [PubMed] [Google Scholar]
- Pilkis S. J., Granner D. K. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- Printz R. L., Magnuson M. A., Granner D. K. Mammalian glucokinase. Annu Rev Nutr. 1993;13:463–496. doi: 10.1146/annurev.nu.13.070193.002335. [DOI] [PubMed] [Google Scholar]
- Roach P. J. Control of glycogen synthase by hierarchal protein phosphorylation. FASEB J. 1990 Sep;4(12):2961–2968. [PubMed] [Google Scholar]
- Rosen B. P., Bhattacharjee H., Shi W. Mechanisms of metalloregulation of an anion-translocating ATPase. J Bioenerg Biomembr. 1995 Feb;27(1):85–91. doi: 10.1007/BF02110335. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Cripe T. P., Koch S. R., Andreone T. L., Petersen D. D., Beale E. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984 Dec 25;259(24):15242–15251. [PubMed] [Google Scholar]
- Shih H. M., Towle H. C. Definition of the carbohydrate response element of the rat S14 gene. Evidence for a common factor required for carbohydrate regulation of hepatic genes. J Biol Chem. 1992 Jul 5;267(19):13222–13228. [PubMed] [Google Scholar]
- Shih H., Towle H. C. Definition of the carbohydrate response element of the rat S14 gene. Context of the CACGTG motif determines the specificity of carbohydrate regulation. J Biol Chem. 1994 Mar 25;269(12):9380–9387. [PubMed] [Google Scholar]
- Singh A. K., Liu H. Y., Lee S. T. Atomic absorption spectrophotometric measurement of intracellular arsenite in arsenite-resistant Leishmania. Mol Biochem Parasitol. 1994 Jul;66(1):161–164. doi: 10.1016/0166-6851(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Spies H. S., Steenkamp D. J. Thiols of intracellular pathogens. Identification of ovothiol A in Leishmania donovani and structural analysis of a novel thiol from Mycobacterium bovis. Eur J Biochem. 1994 Aug 15;224(1):203–213. doi: 10.1111/j.1432-1033.1994.tb20013.x. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Fahrlander P. D., Tesser P. M., Marcu K. B. Nucleotide sequence comparison of normal and translocated murine c-myc genes. Nature. 1984 Aug 2;310(5976):423–425. doi: 10.1038/310423a0. [DOI] [PubMed] [Google Scholar]
- Urbina J. A., Vivas J., Ramos H., Larralde G., Aguilar Z., Avilán L. Alteration of lipid order profile and permeability of plasma membranes from Trypanosoma cruzi epimastigotes grown in the presence of ketoconazole. Mol Biochem Parasitol. 1988 Aug;30(2):185–195. doi: 10.1016/0166-6851(88)90111-9. [DOI] [PubMed] [Google Scholar]
- Valera A., Bosch F. Glucokinase expression in rat hepatoma cells induces glucose uptake and is rate limiting in glucose utilization. Eur J Biochem. 1994 Jun 1;222(2):533–539. doi: 10.1111/j.1432-1033.1994.tb18895.x. [DOI] [PubMed] [Google Scholar]
- Valera A., Fillat C., Costa C., Sabater J., Visa J., Pujol A., Bosch F. Regulated expression of human insulin in the liver of transgenic mice corrects diabetic alterations. FASEB J. 1994 Apr 1;8(6):440–447. doi: 10.1096/fasebj.8.6.8168695. [DOI] [PubMed] [Google Scholar]
- Valera A., Pelegrin M., Asins G., Fillat C., Sabater J., Pujol A., Hegardt F. G., Bosch F. Overexpression of mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase in transgenic mice causes hepatic hyperketogenesis. J Biol Chem. 1994 Mar 4;269(9):6267–6270. [PubMed] [Google Scholar]
- Valera A., Pujol A., Gregori X., Riu E., Visa J., Bosch F. Evidence from transgenic mice that myc regulates hepatic glycolysis. FASEB J. 1995 Aug;9(11):1067–1078. doi: 10.1096/fasebj.9.11.7649406. [DOI] [PubMed] [Google Scholar]
- Valera A., Rodriguez-Gil J. E., Bosch F. Vanadate treatment restores the expression of genes for key enzymes in the glucose and ketone bodies metabolism in the liver of diabetic rats. J Clin Invest. 1993 Jul;92(1):4–11. doi: 10.1172/JCI116580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaulont S., Kahn A. Transcriptional control of metabolic regulation genes by carbohydrates. FASEB J. 1994 Jan;8(1):28–35. doi: 10.1096/fasebj.8.1.8299888. [DOI] [PubMed] [Google Scholar]
- White T. C., Fase-Fowler F., van Luenen H., Calafat J., Borst P. The H circles of Leishmania tarentolae are a unique amplifiable system of oligomeric DNAs associated with drug resistance. J Biol Chem. 1988 Nov 15;263(32):16977–16983. [PubMed] [Google Scholar]
- Woeltje K. F., Esser V., Weis B. C., Cox W. F., Schroeder J. G., Liao S. T., Foster D. W., McGarry J. D. Inter-tissue and inter-species characteristics of the mitochondrial carnitine palmitoyltransferase enzyme system. J Biol Chem. 1990 Jun 25;265(18):10714–10719. [PubMed] [Google Scholar]
- Zaman G. J., Lankelma J., van Tellingen O., Beijnen J., Dekker H., Paulusma C., Oude Elferink R. P., Baas F., Borst P. Role of glutathione in the export of compounds from cells by the multidrug-resistance-associated protein. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7690–7694. doi: 10.1073/pnas.92.17.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Dwyer D. M. Identification of a surface membrane proton-translocating ATPase in promastigotes of the parasitic protozoan Leishmania donovani. Biochem J. 1988 Nov 15;256(1):13–21. doi: 10.1042/bj2560013. [DOI] [PMC free article] [PubMed] [Google Scholar]



