Abstract
We have analyzed 26 Shiga toxin-producing Escherichia coli (STEC) strains for Shiga toxin 2 (Stx2) production using matrix-assisted laser desorption ionization (MALDI)–tandem time of flight (TOF-TOF) tandem mass spectrometry (MS/MS) and top-down proteomic analysis. STEC strains were induced to overexpress Stx2 by overnight culturing on solid agar supplemented with either ciprofloxacin or mitomycin C. Harvested cells were lysed by bead beating, and unfractionated bacterial cell lysates were ionized by MALDI. The A2 fragment of the A subunit and the mature B subunit of Stx2 were analyzed by MS/MS. Sequence-specific fragment ions were used to identify amino acid subtypes of Stx2 using top-down proteomic analysis using software developed in-house at the U.S. Department of Agriculture (USDA). Stx2 subtypes (a, c, d, f, and g) were identified on the basis of the mass of the A2 fragment and the B subunit as well as from their sequence-specific fragment ions by MS/MS (postsource decay). Top-down proteomic identification was in agreement with DNA sequencing of the full Stx2 operon (stx2) for all strains. Top-down results were also compared to a bioassay using a Vero-d2EGFP cell line. Our results suggest that top-down proteomic identification is a rapid, highly specific technique for distinguishing Stx2 subtypes.
INTRODUCTION
Shiga toxin (Stx)-producing Escherichia coli (STEC) is linked increasingly to major outbreaks of food-borne illness (1–7). There are two primary Shiga toxin types: Shiga toxin 1 (Stx1) and Shiga toxin 2 (Stx2). Escherichia coli O157:H7 (strain EDL933) has both the Shiga toxin 1 gene (stx1) and the Shiga toxin 2 gene (stx2) as well as other virulence factors and has been the focus of much research and regulatory action (8). Other STECs have been linked also to food-borne outbreaks, have been added to the list of regulated serotypes (O26, O45, O103, O111, O121, and O145), and are referred to as the “non-O157” STECs (9, 10).
Stx is an AB5 toxin that is comprised of an A subunit and five identical B subunits. The B subunits form a noncovalent “donut” pentamer structure. The A subunit sits on one side of this structure, and a part of the A subunit (A2) is inserted into the “donut hole,” providing structural stability to the holotoxin complex. The holotoxin attaches to a cell surface receptor (globotriaosylceramide [Gb3]) and is internalized into the eukaryotic cell by endocytosis. The holotoxin then follows a retrograde pathway through the cell until it reaches the nucleus, where the A subunit is cleaved by a membrane-bound protease (furin) at a highly exposed 20-residue loop of its polypeptide backbone. A disulfide bond at the base of the loop is then reduced, and the catalytically active A1 fragment (∼28 kDa) of the A subunit is then released to the cytosol where it disrupts protein synthesis in the ribosome. The A2 fragment (∼5 kDa) remains with the pentamer of B subunits (11–14).
There are a number of amino acid subtypes for both Stx1 and Stx2. The Stx1 subtypes are Stx1a, Stx1c, and Stx1d. The Stx2 subtypes are Stx2a (8, 15), Stx2b and Stx2c (15), Stx2d (16), Stx2e (17, 18), Stx2f (19, 20), and Stx2g (21). A systematic nomenclature for classifying Stx1 and Stx2 has been proposed recently (22). Differences in amino acid sequences of the Stx2 subtypes have been linked to differences in toxicity in mammalian cells (15, 23–26). Stx2a, Stx2c, and Stx2d subtypes have been linked most often to human illness, whereas Stx2b, Stx2e, Stx2f, and Stx2g have been associated with nonhuman hosts (24).
A number of techniques have been developed to detect and identify stx genes or the presence of Stx toxins. PCR has been used as a sequenced-based technique for subtyping stx1 and stx2 genes (22, 27, 28). Monoclonal antibodies (MAbs) have been developed into an enzyme-linked immunosorbent assay (ELISA) for detection of the protein toxin (29, 30). Another novel approach combines antibodies and gene amplification for a sensitive immuno-PCR assay (31, 32). For example, a sandwich immunoassay utilizes antibody binding to the A and/or B subunit of the holotoxin and a DNA marker linked to a second anti-Stx2-A-subunit antibody which can be removed chemically and amplified by PCR (32). Alternatively, intact and functional Shiga toxin has been detected by its inhibitory effect on protein synthesis. For example, a Vero cell-based assay that utilizes an enhanced green fluorescent protein (d2EGFP) has been developed to measure the toxicity of Shiga toxin by inhibition of protein synthesis (24, 33).
Mass spectrometry (MS) has been used for analysis of Shiga toxin subtypes. Melton-Celsa et al. used matrix-assisted laser desorption ionization (MALDI) and high-resolution mass spectrometry to analyze the B subunit and the A2 fragment of the A subunit to demonstrate the effect of elastase treatment on Stx2a and Stx2dactivatable subtypes (16). Kitova et al. examined the stability of the Shiga toxin holotoxin in the gas phase using electrospray ionization and high-resolution mass spectrometry (34, 35).
We have developed a top-down proteomic technique for identification of protein biomarkers and toxins from unfractionated bacterial cell lysates using MALDI tandem time of flight (TOF-TOF) tandem mass spectrometry (MS/MS) and top-down analysis software developed in-house at the U.S. Department of Agriculture (USDA) (36, 37). We have applied this technique to identification of the B subunit and the A2 fragment of the A subunit of Stx2 in Escherichia coli O157:H7 (strain EDL933) (38, 39). By this approach, the STEC strain was grown overnight on solid medium supplemented with a DNA-damaging antibiotic that triggers the bacterial SOS response leading to overexpression of bacteriophage-encoded elements, including Stx2 (40). Cells were harvested, subjected to bead beating, and centrifuged, and the supernatant was spotted onto the MALDI target and analyzed by MS and MS/MS. Stx was identified from its sequence-specific fragment ions by comparison to a database of in silico fragment ions of proteins having the same molecular weight as that of the analyte (36).
In the current study, we apply this technique to the analysis of 26 STEC strains harboring Stx2a, Stx2c, Stx2d, Stx2f, and Stx2g subtypes to determine if it is possible to definitively identify sequence-specific subtypes by this approach.
(Portions of this work were presented at the 113th General Meeting of the American Society of Microbiology, 18 to 21 May 2013, Denver, CO [41].)
MATERIALS AND METHODS
Induction and extraction of Stx2 from Shiga toxin-producing Escherichia coli.
Table 1 summarizes the reference and environmental E. coli strains analyzed in this work (1, 42–47). Stx2 induction and sample preparation have been described in detail previously (38, 39). Briefly, bacterial cells from frozen stock were cultured in 25 ml of Luria-Bertani broth (LBB; Becton, Dickinson, Franklin Lakes, NJ) at 37°C for 4 to 6 h on an orbital shaker prior to culture overnight on Luria-Bertani agar (LBA; Becton, Dickinson, Franklin Lakes, NJ) supplemented with ciprofloxacin or mitomycin C. Antibiotic concentrations were optimized at subtherapeutic levels for each strain to maximize Stx2 induction that corresponded to the appearance of large isolated colonies in the bacterial lawn, whereas in the absence of antibiotic, only the bacterial lawn was observed. Optimal antibiotic concentrations for each strain varied from 10 to 60 ng/ml of ciprofloxacin or 800 to 2,500 ng/ml of mitomycin C. Bacterial cells were harvested with a 1-μl loop until it was filled flush with cells. Cells were then transferred to a 2.0-ml conical screw-cap microcentrifuge tube with an O-ring (Fisher Scientific, Houston, TX) that contained 300 μl of extraction solution (see below) and approximately 100 mg of 0.1-mm-diameter zirconia-silica beads (BioSpec Products, Bartlesville, OK).
TABLE 1.
Strains used in this study
| Strain | Serotype | Stx2 subtype | Additional information | Reference(s) |
|---|---|---|---|---|
| RM14735 | O104:H4 | a | Hamburg, Germany, outbreak strain, 2009 | 1 |
| RM12509 | O111:H8 | a | Strain EE5 from Statens Serum Institut | 42 |
| RM10638 | O157 | a | Cattle; feces | 43 |
| RM13368 | O104:H21 | a | Strain CDC 94-3024; isolated from a human with bloody diarrhea | 44, 45 |
| RM7787 | Unknown | c | Isolated from wild pig (feral) | 43 |
| RM8091 | Unknown | c | Isolated from cow feces | 43 |
| RM9482 | Unknown | c | Isolated from cattle | 43 |
| RM9483 | Unknown | c | Isolated from cattle | 43 |
| RM10058 | Unknown | c | Isolated from a cloacal swab from a brown-headed cowbird | 43 |
| RM10936 | Unknown | c | Isolated from cow feces | 43 |
| RM10648 | Unknown | c | Isolated from cow feces | 43 |
| RM7975 | Unknown | c | Isolated from a cow | 43 |
| RM8017 | Unknown | c | Isolated from cow feces | 43 |
| RM6976 | O157:H7 | c | Isolated from a Tule elk | 43 |
| RM6977 | O157:H7 | c | Isolated from a Tule elk | 43 |
| RM6978 | O157:H7 | c | Isolated from a Tule elk | 43 |
| RM12508 | O177:H25 | c or d | Strain DD4 from Statens Serum Institut | 42 |
| RM7006 | O91:H21 | d | Strain B2F1; isolated from human hemolytic-uremic syndrome source | 46, 47 |
| RM8013 | ONT:H7 | d | Isolated from cow feces | 43 |
| RM13503 | ONT:H21 | d | Human clinical isolate | |
| RM9508 | Unknown | f | Isolated from a deer mouse | 43 |
| RM9511 | Unknown | f | Isolated from a crow | 43 |
| RM9973 | Unknown | f | Isolated from crow feces | 43 |
| RM9974 | Unknown | f | Isolated from crow feces | 43 |
| RM9981 | Unknown | f | Isolated from a creek | 43 |
| RM10468 | Unknown | g | Isolated from cow feces | 43 |
For mass spectrometry analysis of the disulfide-bond-intact B subunit of Stx2, the extraction solution was high-pressure-liquid-chromatography (HPLC)-grade water (Honeywell, Burdick & Jackson, Muskegon, WI). Bacterial cells were lysed by bead beating (Mini-Beadbeater; BioSpec Products, Bartlesville, OK) for 2 min. Tubes were then centrifuged at 14,000 rpm (16,000 × g) for 2 min. The sample supernatant is adequate for mass spectrometry analysis without further treatment.
For mass spectrometry analysis of disulfide-bond-reduced B subunit, the extraction solution was 3 mM molecular-biology-grade CaCl2 (Sigma-Aldrich, St. Louis, MO) in HPLC-grade water. Bacterial cells were lysed by bead beating for 2 min and then centrifuged at 14,000 rpm (16,000 × g) for 2 min. Thirty microliters of sample supernatant was treated with 1 μl of 1.0 M dithiothreitol (DTT) and incubated in a water bath at 70°C for 10 min. The sample was then ready for mass spectrometry analysis. For analysis of the A2 fragment and disulfide-bond-reduced B subunit, 1.0 μl of furin (2,000 U ml−1; New England BioLabs, Ipswich, MA) was added to 30 μl of sample supernatant in 3 mM CaCl2, and the tube was vortexed briefly, centrifuged briefly, and incubated at 37°C for 1 h. The sample was then treated with 1.0 μl of 1 M DTT and incubated at 70°C for 10 min.
DNA sequencing of stx2 in STEC strains.
DNA sequencing of the full stx2 operon was performed on all STEC strains shown in Table 1. All subtypes were sequenced using primers shown in Table 2 on an ABI3130xl (Applied Biosystems) sequencer using POP7 polymer (Applied Biosystems; catalogue no. 4363785) and the BigDye Terminator v1.1 cycle sequencing kit (Applied Biosystems; catalogue no. 4337450) per the manufacturer's recommendations. Cultures were streaked for isolation on LBA (1.5% agar) and incubated overnight at 37°C. Well-defined single colonies were picked from the plates, suspended in 1 ml of sterile molecular-biology-grade water, boiled in a heating block at 100°C for 15 min, and then centrifuged at 18,000 × g for 5 min. Supernatant was transferred to a sterile 1.5-ml microcentrifuge tube, and the supernatant was used directly for PCR and stored at −20°C for further analysis. PCR was carried out in 50-μl reaction mixtures with 35.55 μl of water, 5 μl of 10× buffer (New England BioLabs; catalogue no. M0267), 5 μl of 25 mM MgCl2, 1.25 μl of 10 mM deoxynucleoside triphosphates (dNTPs), 1 μl of each primer (5 μM), 1 μl of boiled lysate, and 0.20 μl of Taq DNA polymerase (New England BioLabs; catalogue no. M0267). Thermocycler conditions were as follows: 95°C for 10 min followed by 30 cycles of 94°C for 30 s, 53°C for 30 s, and 72°C for 60 s, ending with 72°C for 3 min. PCR amplicons were stored at 4°C.
TABLE 2.
Primers used in this study
| Name | DNA sequence, 5′–3′ | Genomic coordinates | Additional information |
|---|---|---|---|
| Stx2F-22017 | GTCACAGCAGAACCTTACG | NAa | Reference 51 |
| Stx2R-22711 | ACCCACATACCACGAATCAG | NA | Reference 51 |
| Seq9F | GCAATCGGTCACTGGTTCG | NA | Reference 52 |
| Seq10R | CAGATTACACTTGTTACCCAC | NA | Reference 52 |
| WZStx2fF1 | CTGCCGGTTCAGACT | 10–24 of accession no. AB232172 | For PCR and sequencing of 2f variants |
| WZStx2fR1 | ACGAAAACACTGACCAA | 1352–1368 of accession no. AB232172 | For PCR and sequencing of 2f variants |
| WZStx2fF2 | TTCCTGGCGCTGCCG | 1–15 of accession no. AB232172 | For PCR and sequencing of 2f variants |
| WZstx2fR2 | AACAAAAGACGCGCA | 1295–1309 of accession no. AB232172 | For PCR and sequencing of 2f variants |
| WZstx2fF3 | TTGAATGTTAGAGGCCTTG | 260–278 of accession no. AB232172 | For PCR and sequencing of 2f variants |
| WZstx2fR3 | ACGGAGGTGGTTAAAT | 295–310 of accession no. AB232172 | For PCR and sequencing of 2f variants |
| WZstx2fF4 | TTGCAGTTTTATTCGGTCTC | 1029–1048 of accession no. AB232172 | For PCR and sequencing of 2f variants |
| WZstx2fR4 | GTCAACATCCTGAGCCGTCAT | 677–697 of accession no. AB232172 | For PCR and sequencing of 2f variants |
| WZstx2f6 | TCCAGAAACAAAAGACGCGCATA | 1293–1315 of accession no. AB232172 | For PCR and sequencing of 2f variants |
| WZstx2gF1 | AACGGATGATATTGCAGG | 80–97 of accession no. AY095209 | For PCR and sequencing of 2g variants |
| WZstx2gR1 | TAACAAATGCTCACTCTGAC | 1785–1804 of accession no. AY095209 | For PCR and sequencing of 2g variants |
| WZstx2gF2 | CGGGTGAATAAAGGAGTTAAG | 1420–1440 of accession no. AY095209 | For PCR and sequencing of 2g variants |
| WZstx2gR2 | ATTCAGTATAACGGCCACA | 1238–1256 of accession no. AY095209 | For PCR and sequencing of 2g variants |
| WZ stx2gF3 | AACGGATGATATTGCAGGAT | 80–99 of accession no. AY095209 | For PCR and sequencing of 2g variants |
| WZstx2gR3 | ACTGGACTTGATTGTGAC | 1643–1660 of accession no. AY095209 | For PCR and sequencing of 2g variants |
| WZstx2gF4 | ATGCAAATCAGTCGTCAC | 918–935 of accession no. AY095209 | For PCR and sequencing of 2g variants |
| WZstx2gF5 | GATAGACTTTTCGACTCAAC | 548–567 of accession no. AY095209 | For PCR and sequencing of 2g variants |
| WZstx2gF6 | CAACGCGCCATACTTATTC | 93–111 of accession no. AY286000 | For PCR and sequencing of 2g variants |
| WZstx2gR4 | ACACTGTTACCCACATACC | 1450–1468 of accession no. AY286000 | For PCR and sequencing of 2g variants |
| WZstx2gF7 | GCTTTTGCGGGCCTTTTTT | 119–137 of accession no. AY286000 | For PCR and sequencing of 2g variants |
| WZstx2gR5 | ACCCACATACCACGAATCA | 1442–1460 of accession no. AY286000 | For PCR and sequencing of 2g variants |
| WZ14F | CCGKCAACCTTCACTGTAAATGTG | 1353368–1353391 of accession no. AY286000 | For PCR and sequencing of 2a variants |
| WZstx2F1 | CACTCGGGCTTTTTTACA | 1353808–1353825 of accession no. AY286000 | For PCR and sequencing of 2a variants |
| WZstx2R1 | CATACCGCCATTAGCTCA | 1641076–1641093 of accession no. AY286000 | For PCR and sequencing of 2a variants |
| WZX07865F1 | TCCATTATCTGCATTATGC | 55–73 of accession no. AY286000 | For PCR and sequencing of 2a variants |
| WZX07865F2 | TCGACACGGGTTCGGTGGTACC | 1643–1664 of accession no. AY286000 | For PCR and sequencing of 2a variants |
| M13F | GTAAAACGACGGCCAG | NA | M13 cloning primers for insert confirmation |
| M13R | CAGGAAACAGCTATGAC | NA | M13 cloning primers for insert confirmation |
NA, not available.
DNA sequence analysis.
Nucleotide sequences of full stx2 operons, including intergenic regions, were analyzed using CLC Main Workbench 6.8.4 (CLC Bio). All sequencing reads were repeated at least three times. Stx2a and Stx2c subtypes were assembled using the stx2 operon of BP933W (NCBI accession no. X07865). Stx2d subtypes were assembled against the stx2 operon of strain B2F1 (accession no. AF479829), Stx2g subtypes were assembled against accession no. AY286000, and Stx2f subtypes were assembled against accession no. AB232172. Amino acid sequences for the combined A and B subunits were translated from predicted open reading frames. All nucleotide and amino acid sequences were uploaded into the CLC workbench for further analysis.
The full stx2 operon nucleotide sequences, including the intergenic regions, were analyzed using neighbor joining algorithm and the UPGMA (unweighted-pair group method using average linkages) algorithm for starting trees. Unrooted evolutionary trees were created from a cluster analysis using 100 bootstrap simulations. The substitution model chosen was HKY with a transition/transversion ratio of 2.0. Rate variation was included with 4 categories of substitution and a gamma distribution parameter of 1.0. These trees were compared with the phylogenetic trees generated with both the neighbor joining and UPGMA algorithms for discrepancies.
The amino acid sequences of the full Stx2 proteins without the intergenic region were analyzed with the neighbor joining algorithm using 100 bootstrap simulations to generate an unrooted tree. This analysis was repeated with the amino acid sequences, including the 20-residue loop that links A1 and A2 regions of the A subunit, the A2 fragment, and the mature B subunit (without signal peptide).
The amino acid sequences of the A2 fragment and the mature B subunit for each of the strains in Table 1 were processed and uploaded to the in silico database of the top-down analysis software described below.
Mass spectrometry.
A matrix-assisted laser desorption ionization–tandem time of flight (MALDI-TOF-TOF) mass spectrometer (4800 Plus; AB Sciex, Foster City, CA) was used in this work. Analysis of the A2 fragment of the A subunit and the mature B subunit of Stx2 using MALDI-TOF-TOF has been discussed in detail previously (38, 39). An 0.5-μl aliquot of bacterial cell lysate supernatant was spotted onto a 384-spot stainless steel MALDI plate. The spot diameter was 1.5 mm. The supernatant was allowed to dry at room temperature. The dried sample spot was then overlaid with an 0.5-μl aliquot of a 10-mg ml−1 solution of 3,5-dimethoxy-4-hydroxycinnamic acid, also called sinapinic acid (Protea Biosciences, Morgantown, WV). The matrix spot was then allowed to dry at room temperature. The MALDI plate was then ready for analysis.
MS data were acquired in linear mode. The instrument was externally calibrated as previously described (36). The A2 fragment of the A subunit and the B subunit of each Stx2 subtype were measured from their observed mass-to-charge ratio (m/z) in linear mode and compared to their theoretical molecular weight (MW) as calculated from DNA sequencing data. The singly charged (protonated) A2 fragment and B subunit were analyzed by tandem mass spectrometry (MS/MS) and postsource decay (PSD) in reflectron mode. The reflectron-mode mass analyzer was calibrated using PSD fragment ions of alkylated thioredoxin as described previously (48). MS and MS/MS data were processed as previously described (36). Processed MS/MS data were uploaded as an ASCII file to the top-down analysis software.
Top-down proteomic analysis.
The top-down proteomic analysis software has been described in detail in a previous publication (36). Briefly, Escherichia coli proteins (including bacteriophage-encoded proteins in E. coli genomes) were downloaded as a single multisequence FASTA file using the web-based TagIdent software program at the ExPASy website (www.expasy.org; release versions UniProtKB/Swiss-Prot Release 2013_08 of 24 July 2013 and UniProtKB/TrEMBL Release 2013_08 of 24 July 2013). The protein sequences downloaded were within a specified molecular mass (molecular mass ± 10 Da) of the m/z of the protein biomarker or toxin being identified. Previously, multisequence FASTA files were processed using a commercially available software program to generate individual files of in silico fragment ions of each protein sequence (36). These files were subsequently uploaded to our top-down analysis software. However, we recently developed software to streamline in silico database construction (USDA ISDB processor v1.2). A detailed description of in silico database construction is provided in the supplemental material, and a flowchart describing this process is shown in Fig. S1 in the supplemental material.
In the top-down analysis software, MS/MS data were compared against in silico MS/MS data derived from protein amino acid sequences of Escherichia coli downloaded from ExPASy/TagIdent as well as those sequences derived from our DNA sequencing. The parameters used to compare MS/MS data to in silico MS/MS data varied depending on the quality of the MS/MS data (reflected in the P value [49] and the percentage of matched MS/MS fragment ions). For most MS/MS data, a fragment ion intensity threshold of 1% was utilized, and the fragment ion error varied from 0.5 to 1.5 (Thompson). Weaker MS/MS data required the use of a higher signal intensity threshold to eliminate noise. For most analyses, a non-residue-specific comparison was used, i.e., all in silico fragment ions were compared to MS/MS data. However, with weaker-intensity MS/MS data a residue-specific ion (i.e., D-, E-, P-, or D-specific in silico fragment ions) was used for comparison. A residue-specific comparison was also useful to distinguish nearly identical protein sequences (36).
The ISDB software (as well as the top-down proteomic software) is available upon request (without charge) after completion of a material transfer agreement.
Vero-d2EGFP cell-based assay to detect Shiga toxin activity.
The assessment of Shiga toxin activity in the STEC strains was performed using a Vero cell line, Vero-d2EGFP, that harbored a destabilized variant (half-life [t1/2] = 2 h) of the enhanced green fluorescent protein (d2EGFP) (24, 33). To monitor the Stx-induced inhibition of protein synthesis, STEC strains were inoculated in 1 ml of sterile LBB (Difco, Detroit, MI), grown aerobically for 24 h at 37°C with shaking at 200 rpm, and then centrifuged at 3,000 × g for 15 min. The culture supernatants were filter sterilized using 0.45-μm polyvinylidene fluoride syringe filters (Durapore membranes; Millipore Corporation, Billerica, MA) and were frozen at −20°C until further use. One day prior to intoxication, the Vero-d2EGFP cells were seeded at 10,000 cells per well in Greiner black 96-well microplates with clear bottoms (VWR International, Aurora, CO) and were grown at 5% CO2 and 37°C under humidified conditions in Ham's F-12 medium, supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (Gibco BRL, Grand Island, NY) (24, 33). The Vero-d2EGFP cells were then exposed to Ham's F-12 complete medium containing 10-fold dilutions of Stx-containing, cell-free supernatants from each STEC strain and were incubated for 16 h at 37°C in a 5% CO2 humidified incubator. The EGFP fluorescence from the toxin-treated Vero-d2EGFP cells was measured on a Synergy HT multidetection microplate reader (BioTek, Winooski, VT) with the 485/20-nm excitation filter and the 528/20-nm emission filter (24, 33). Results from toxin-treated Vero-d2EGFP cells were expressed as percentages of the values obtained from control Vero-d2EGFP cells incubated without toxin. All measurements were performed in duplicate.
Nucleotide sequence accession numbers.
The sequences of the stx2 genes were deposited in GenBank under accession numbers KF932358 (RM10638), KF932362 (RM7787), KF932365 (RM8091), KF932366 (RM9482), KF932367 (RM9483), KF932368 (RM10058), KF932370 (RM10936), KF932369 (RM10648), KF932363 (RM7975), KF932364 (RM8017), KF932359 (RM6976), KF932360 (RM6977), KF932361 (RM6978), KF932371 (RM8013), KF932372 (RM13503), KF932373 (RM9508), KF932374 (RM9511), KF932375 (RM9973), KF932376 (RM9974), KF932377 (RM9981), and KF932378 (RM10468).
RESULTS AND DISCUSSION
Phylogenetic trees of DNA sequencing of Stx2 subtypes.
Figure 1A shows the phylogenetic tree for the full Stx2 operon nucleotide sequence. Figure 1B shows the phylogenetic tree for the full Stx2 operon translated sequence. Our top-down proteomic analysis identifies only the A2 fragment of the A subunit and the mature B subunit (i.e., signal peptide removed). We mimic in vitro the chemical steps that occur in vivo in order to detect the A2 fragment, i.e., furin digestion followed by disulfide-bond reduction, which confirms implicitly the presence of the highly accessible 20-residue loop that links the A1 and A2 regions, the recognition site (RXXR) at which furin cleaves the loop, and the disulfide bond at the base of the loop. In consequence, maximum likelihood phylogenetic trees were also generated for the nucleotide sequence coding for the 20-residue loop, the A2 fragment, and the B subunit but not including nucleotides of the intergenic region and the signal peptide of the B subunit. Figure 1C shows the phylogenetic tree comprised of the translated sequences of the 20-residue loop, the A2 fragment, and the B subunit (but not including its signal peptide). The three phylogenetic trees are quite similar in terms of their overall structure, with a few variations in the locations of some branches/nodes. Not surprisingly, there is greater complexity in the nucleotide sequence than in the translated amino acid sequence due to silent mutations.
FIG 1.
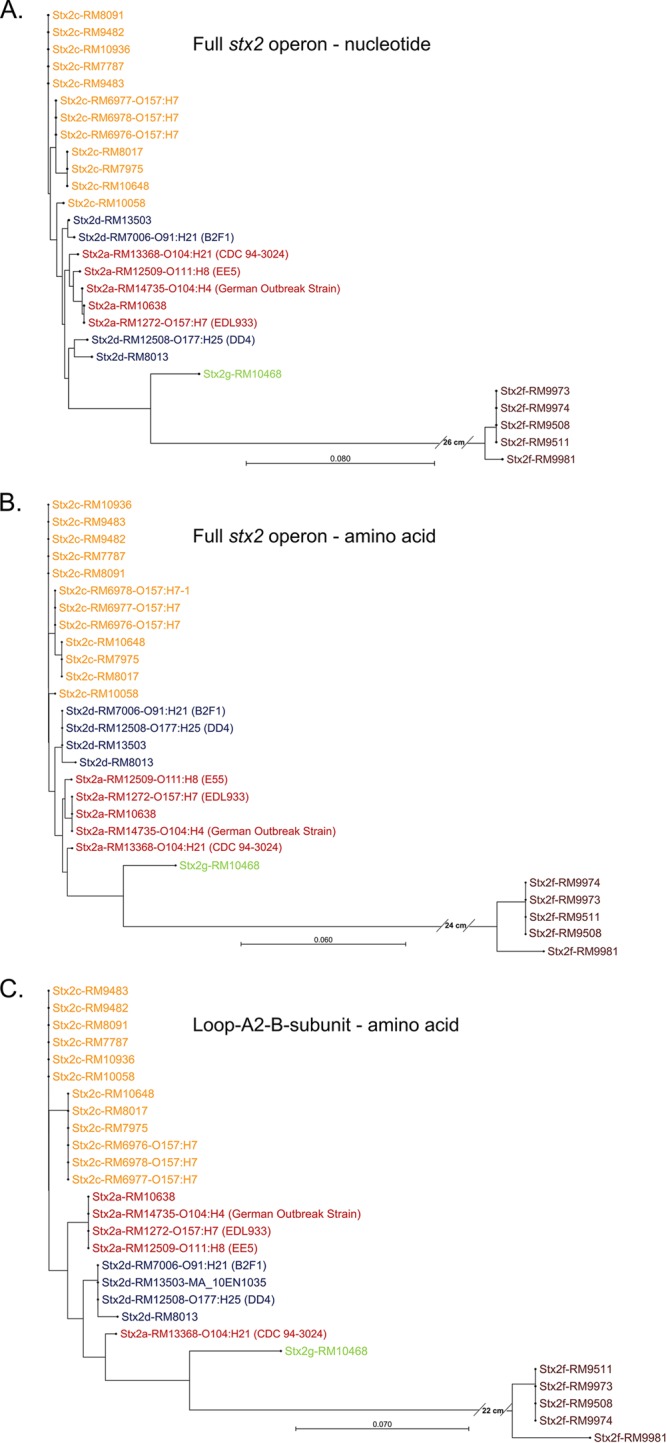
(A) Phylogenetic tree of nucleotide sequences of full stx2 operon for strains analyzed in this study. (B) Phylogenetic tree of translated amino acid sequence of full stx2 operon. (C) Phylogenetic tree of translated amino acid sequence of 20-residue loop and A2 fragment of A subunit and B subunit of Stx2.
Figure 2 shows the amino acid sequence alignment of the translated loop-A2-B shown in Fig. 1C. A cursory examination of Fig. 2 indicates that the amino acid differences across the A2 fragment and B subunit for these strains are sufficient to assign each sequence to a specific Stx2 subtype on the basis of the mass and sequence of the A2 fragment and the mature B subunit or the mature B subunit alone. Thus, detection of the A1 fragment (28 kDa) is not necessary to identify the Stx2 subtype (although it may be helpful to distinguish STEC strains within a subtype).
FIG 2.

Amino acid sequence alignment of the 20-residue loop, the A2 fragment of the A subunit, and the B subunit of Stx2 for strains analyzed in this study. The furin recognition site is boxed in red. The minimum recognition site is RXXR, which is not present in the Stx2f subtypes.
Figure S2 in the supplemental material shows the Stx2a, Stx2c, and Stx2d subtypes of Stx2 displayed as an unrooted radial tree.
Mass spectrometry (MS) and tandem mass spectrometry (MS/MS).
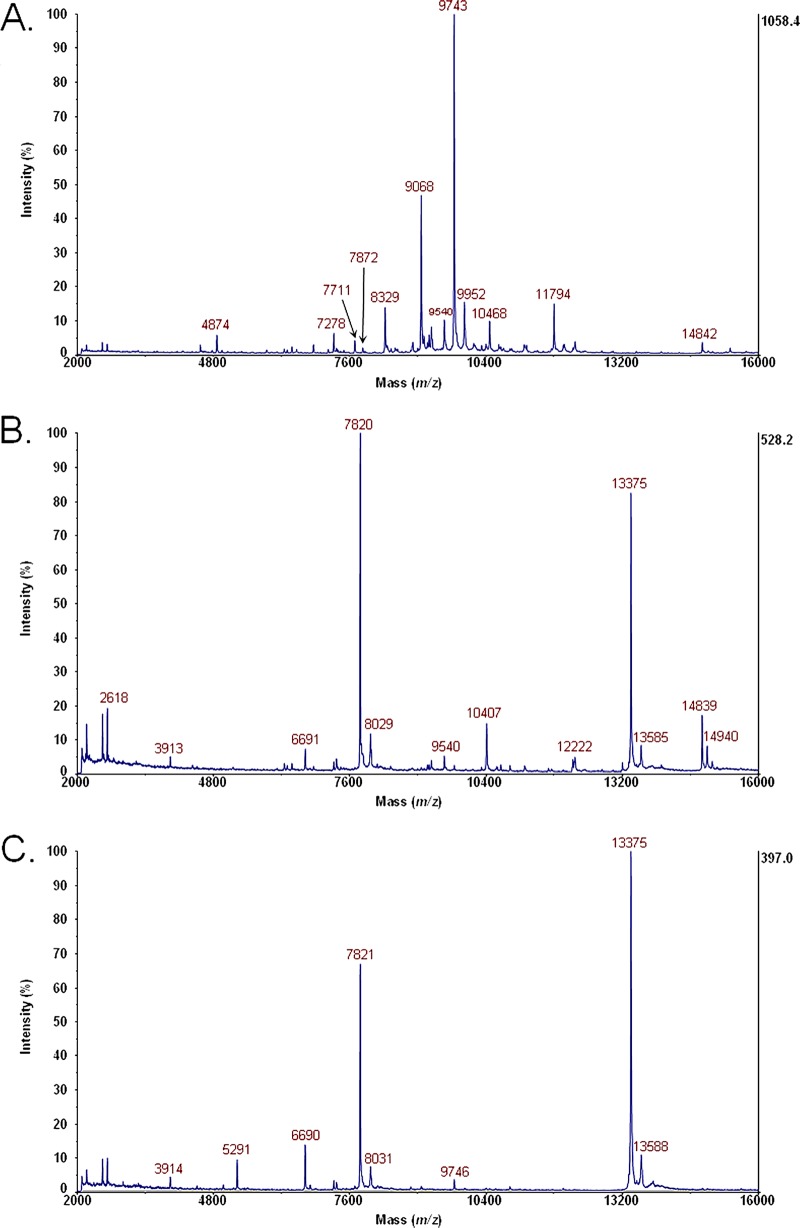
Figure 3A shows MS data for the bacterial cell lysate of E. coli O104:H4 (German outbreak strain) grown on LBA with no antibiotic. Figure 3B shows MS data for the bacterial cell lysate of E. coli O104:H4 grown on LBA supplemented with ciprofloxacin. The peak at m/z 7820 is the B subunit of Stx2. Figure 3C shows MS data for the bacterial cell lysate of E. coli O104:H4 grown on LBA supplemented with ciprofloxacin, digested with furin, and disulfide reduced. The peaks at m/z 7821 and m/z 5291 are the B subunit and A2 fragment of Stx2, respectively.
FIG 3.
(A) MS data for bacterial cell lysate of E. coli O104:H4 (German outbreak strain) grown on LBA with no antibiotic. (B) MS data for bacterial cell lysate of E. coli O104:H4 grown on LBA supplemented with ciprofloxacin. The peak at m/z 7820 is the B subunit of Stx2. (C) MS data for bacterial cell lysate of E. coli O104:H4 grown on LBA supplemented with ciprofloxacin, digested with furin, and disulfide reduced. The peaks at m/z 7821 and m/z 5291 are the B subunit and A2 fragment of Stx2, respectively.
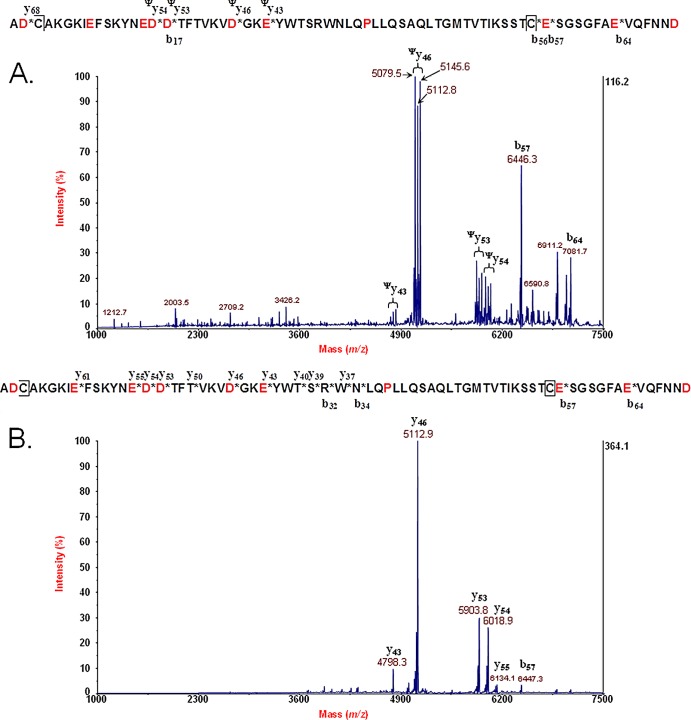
Figure 4A shows MS/MS data for the B subunit (disulfide bond intact) of Stx2 of E. coli O104:H4 (German outbreak strain). The mature amino acid sequence is provided above the spectrum. An asterisk in the figure indicates a site of polypeptide backbone fragmentation, and the ion type/number is provided above or below the sequence. A fragment ion with a ψ superscript indicates the presence of a fragment ion triplet that is due to fragmentation of the polypeptide backbone between the two cysteine residues involved in a disulfide bond and symmetric and asymmetric cleavage of the disulfide bond. Figure 4B shows MS/MS data for the B subunit (disulfide bond reduced) of Stx2 of E. coli O104:H4. The mature amino acid sequence is provided above. An asterisk in the figure indicates a site of polypeptide backbone fragmentation, and the ion type/number is provided above or below the sequence. Note that there are no fragment ion triplets as the disulfide bond is now reduced. The disulfide bond-reduced B-subunit MS/MS data were analyzed by top-down proteomic analysis.
FIG 4.
(A) MS/MS data for the B subunit (disulfide bond intact) of Stx2 of E. coli O104:H4 (German outbreak strain). The mature amino acid sequence is provided above the spectrum. An asterisk indicates a site of polypeptide backbone fragmentation, and the ion type/number is provided above or below the sequence. A fragment ion with a ψ superscript indicates the presence of a fragment ion triplet that is due to fragmentation of the polypeptide backbone between the two cysteine residues involved in a disulfide bond and symmetric and asymmetric cleavage of the disulfide bond. (B) MS/MS data for the B subunit (disulfide bond reduced) of Stx2 of E. coli O104:H4. The mature amino acid sequence is provided above. An asterisk indicates a site of polypeptide backbone fragmentation, and the ion type/number is provided above or below the sequence. Note that there are no fragment ion triplets as the disulfide bond is now reduced.
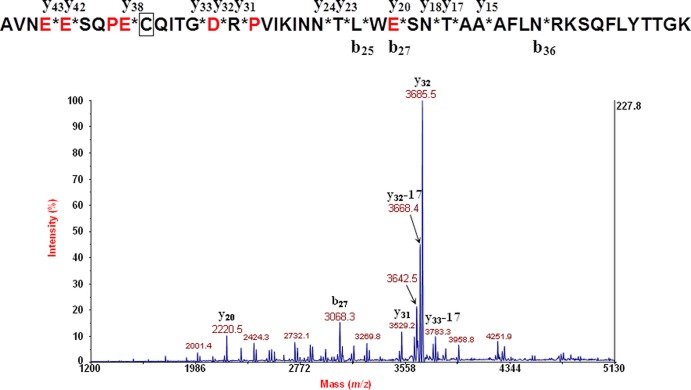
Figure 5 shows the MS/MS data for the A2 fragment of the A subunit of Stx2 of E. coli O104:H4 (German outbreak strain). The amino acid sequence is provided above the spectrum. An asterisk in the figure indicates a site of polypeptide backbone fragmentation, and the ion type/number is provided above or below the sequence. MS/MS data were analyzed by top-down proteomic analysis.
FIG 5.
MS/MS data for the A2 fragment of the A subunit of Stx2 of E. coli O104:H4 (German outbreak strain). The amino acid sequence is provided above the spectrum. An asterisk indicates a site of polypeptide backbone fragmentation, and the ion type/number is provided above or below the sequence.
The other strains in this study were analyzed by mass spectrometry in a similar fashion.
Top-down proteomic identification. (i) Stx2a subtype.
Top-down proteomic identifications from MS/MS data of the disulfide bond-reduced B subunit and the A2 fragment of the A subunit for all strains are summarized in Table 3. The high quality of the MS/MS data was due to the strong induction of Stx2 for E. coli O104:H4 (German outbreak strain, RM14735), which resulted in a very strong identification for both the A2 fragment (nominal mass of 5,285 Da) and the B subunit (nominal mass of 7,818 Da). Top-down analysis of the A2 fragment indicated this as either an Stx2a or an Stx2c subtype, whereas top-down identification of the B subunit indicated only an Stx2a subtype. Thus, top-down analysis indicates this as an Stx2a subtype, consistent with our DNA sequencing and previous reports by other researchers (1). This strain also had a strong response from the Vero-d2EGFP toxicity assay shown in Fig. 6.
TABLE 3.
Mass spectrometry and top-down proteomic identification
| Strain | Stx2 subtype(s) | A2 fragment |
B subunit |
||||
|---|---|---|---|---|---|---|---|
| DNA sequencing (Da) | MS (m/z) | MS/MS top-down identification, P (%), subtypes | DNA sequencing (Da) | MS (m/z) | MS/MS top-down identification, P (%), subtype(s) | ||
| RM14735 | a | 5,285 | 5,291 | 7.9E−17 (57.97), a, c | 7,818 | 7,819 | 1.4E−16 (61.36), a |
| RM12509 | a | 5,285 | 5,284 | 4.0E−21 (53.23), a, c | 7,818 | 7,817 | 2.3E−14 (52.94), a |
| RM10638 | a | 5,285 | 5,286 | 5.0E−10 (31.25), a, c | 7,818 | 7,816 | 4.6E−9 (43.48), a |
| RM13368 | a | 5,226 | 5,226 | 3.0E−16 (48.57), a, d, e | 7,818 | 7,817 | 1.4E−12 (38.78), a |
| RM7787 | c | 5,285 | 5,290 | 1.5E−10 (43.94), a, c | 7,773 | 7,774 | 1.7E−19 (42.70), c, d |
| RM8091 | c | 5,285 | 5,284 | 3.0E−11 (42.62), a, c | 7,773 | 7,773 | 5.2E−15 (45.33), c, d |
| RM9482 | c | 5,285 | 5,286 | 1.3E−14 (50.00), a, c | 7,773 | 7,775 | 1.7E−18 (51.39), c, d |
| RM9483 | c | 5,285 | 5,284 | 3.2E−5 (35.14), a, c | 7,773 | 7,773 | 1.5E−14 (46.38), c, d |
| RM10058 | a and c | 5,285 | 5,288 | 7.6E−9 (38.64), a, c | 7,773 | 7,775 | 3.7E−11 (33.64), c, d |
| RM10936 | c | 5,285 | 5,284 | 4.8E−11 (37.10), a, c | 7,773 | 7,773 | 1.9E−13 (46.77), c, d |
| RM10648 | c | 5,271 | 5,269 | 2.8E−7 (35.09), a, c | 7,773 | 7,772 | 6.6E−16 (47.89), c, d |
| RM7975 | c | 5,271 | 5,271 | 1.3E−8 (33.93), a, c | 7,773 | 7,774 | 1.0E−11 (38.10), c, d |
| RM8017 | c | 5,271 | 5,271 | 4.5E−7 (36.36), a, c | 7,773 | 7,774 | 2.4E−11 (57.58), c, d |
| RM6976 | c | 5,271 | 5,275 (weak), a, c | 7,773 | 7,778 | 1.7E−10 (34.02), c, d | |
| RM6977 | c | 5,271 | 5,275 (weak), a, c | 7,773 | 7,777 | 9.1E−9 (50.00), c, d | |
| RM6978 | c | 5,271 | 5,273 (weak) | 2.0E−4 (23.08), a, c | 7,773 | 7,779 | 1.6E−6 (20.45), c, d |
| RM12508 | c and/or d | 5,226 | 5,224 | 4.8E−11 (34.72), a, d, e | 7,773 | 7,772 | 1.3E−11 (23.88), c, d |
| RM7006 | d | 5,226 | NDa | 7,773 | 7,772 (weak) | ||
| RM13503 | d | 5,226 | 5,224 | 5.4E−13 (32.39), a, d, e | 7,773 | 7,773 | 8.0E−22 (53.85), c, d |
| RM8013 | d | 5,226 | 5,227 | 6.7E−6 (20.90), a, d, e | 7,715 | 7,714 | 3.9E−12 (37.78), d |
| RM9508 | f | 5,238 | ND | 7,555 | 7,552 | 2.2E−7 (13.21), f | |
| RM9511 | f | 5,238 | ND | 7,555 | 7,556 | 2.1E−4 (10.94), f | |
| RM9973 | f | 5,238 | ND | 7,555 | 7,556 | 1.6E−7 (14.63), f | |
| RM9974 | f | 5,238 | ND | 7,555 | 7,560 | 2.0E−6 (16.67), f | |
| RM9981 | f | 5,176 | ND | 7,582 | 7,581 (weak) | ||
| RM10468 | g | 5,348 | 5,348 (weak), g | 7,799 | 7,799 | 2.5E−6 (23.81), g | |
ND, not detected.
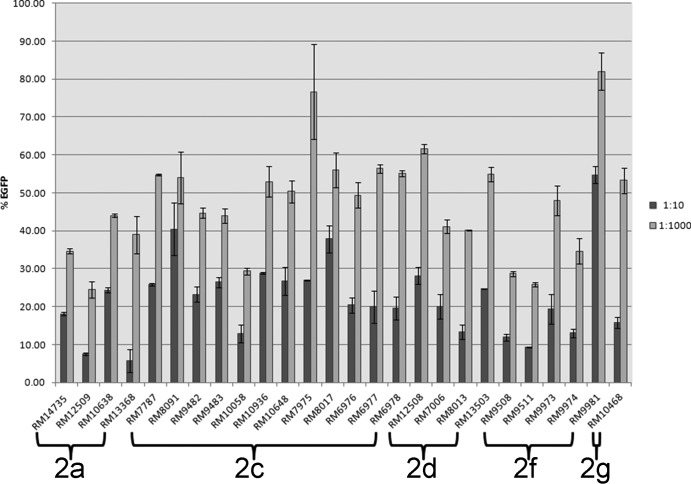
FIG 6.
Bar graph of EGFP fluorescence of toxin-treated Vero-d2EGFP cells at a 1:10 dilution (dark gray bars) and a 1:1,000 dilution (light gray bars) expressed as percentages of the EGFP fluorescence obtained from control Vero-d2EGFP cells incubated without toxin.
We analyzed three other Stx2a subtype strains, E. coli O111:H8 (strain EE5, Statens Serum Institut, RM12509) (42); E. coli strain RM10638, an environmental isolate (Salinas, CA) (43); and E. coli O104:H21 strain CDC 94-3024 (RM13368) (44, 45). E. coli O111:H8 gave a very strong top-down identification; however, two slightly different Stx2a sequences were possible. The first is A6MTT2_ECOLX Shiga toxin 2 B subunit with a sequence of MKKMFMAVLFALASVNAMAADCAKGKIEFSKYNEDDTFTVKVDGKEYWTSRWNLQPLLQSAQLTGMTVTLKSSTCESGSGFAEVQFNND, which is the correct sequence based on DNA sequencing for this strain with an average molecular mass of 7,817.64 Da (after removal of signal peptide shown by underlining). This sequence was found also to be the most common among the strains analyzed in this study. A nearly identical sequence was also equally scored/ranked: Q9RHP8_ECOLX Shiga toxin 2 B subunit MKKMFMAVLFALASVNAMAADCAQGKIEFSKYNEDDTFTVKVDGKEYWTSRWNLQPLLQSAQLTGMTVTIKSSTCESGSGFAEVQFNND with nearly the same average molecular mass of 7,817.60 Da.
The source organism of Q9RHP8_ECOLX is E. coli O157 strain NIID-2 (50). The differences in these two sequences are shown by boldface. The L↔I substitutions at residue 51, in the mature sequence, are geometric isomers that have exactly the same mass and are not distinguishable by our technique. The K↔Q substitutions at residue 5 have the same nominal mass but not the same exact mass, which makes them difficult to distinguish because these substitutions may not significantly affect protein ion fragmentation. A similar dual top identification was obtained for top-down analysis of the B subunit of E. coli strain RM10638 and E. coli O104:H21 strain CDC 94-3024 (RM13368). From the DNA sequencing of the A subunit for the NIID-2 strain, only 31 residues from the C terminus are shown, and thus, an incomplete A2 fragment is provided (50). Although our technique does not appear to distinguish these highly similar B-subunit sequences from the MS/MS data of these two Stx2a strains, they were distinguished correctly by the MS/MS data for the German outbreak strain, where they differed by only a single matched fragment ion, giving the correct sequence the most significant identification score.
On the basis of the B subunit alone, these three strains are identifiable as Stx2a subtypes. However, further taxonomic resolution was obtained by analysis of their A2 fragments. E. coli O111:H8 strain RM12509 and E. coli strain RM10638 shared the same A2 fragment sequence (5,285 Da) as the German outbreak strain and thus could be classified as either an Stx2a or an Stx2c subtype with the B subunit determining the identity of the subtype. Top-down analysis of the A2 fragment (5,226 Da) of E. coli O104:H21 strain RM13368 resulted in three possible subtypes: Stx2a, Stx2d, or Stx2e. Once again, the B subunit definitively identifies this as an Stx2a subtype.
Like the German outbreak strain, these three Stx2a subtype strains showed a strong response with the Vero-d2EGFP toxicity assay (Fig. 6). A “response” is defined here as a reduction in EGFP fluorescence, indicating that Stx2a is expressed by these STEC strains and that it is also having an effect at inhibiting protein synthesis in mammalian cells.
(ii) Stx2c subtype.
We analyzed 12 Stx2c subtype strains that were all environmental isolates from Salinas, CA. It is interesting that the sequences of the B subunits (nominal mass of 7,773 Da) for all of these strains were identical (Fig. 2). In contrast, there were two A2 sequences that differed by a single amino acid substitution, D↔E at residue 15, resulting in a nominal mass of 5,271 Da or 5,285 Da. As shown in Table 3, strains RM7787, RM8091, RM9482, RM9483, RM10058, and RM10936 were found to induce Stx2, strongly resulting in significant top-down identifications for both the A2 fragment (5,285 Da) and the B subunit (7,773 Da). The subtype common to both the A2 and B-subunit identifications was the Stx2c subtype; thus, identification of both the A2 and the B subunit was necessary to identify definitively the subtype, unlike the Stx2a subtype strains, which required only identification of the B subunit.
The next six Stx2c subtype strains analyzed have an amino acid substitution at residue 15 (D↔E) in the A2 fragment that not only changes the mass of A2 and some of its fragment ions but also changes protein ion fragmentation. Strains RM10648, RM7975, and RM8017 were also found to strongly induce Stx2, resulting in confident identifications of A2 (5,271 Da) and the B subunit (7,773 Da). Once again, identification of both the A2 fragment and the B subunit was necessary for definitive identification of the subtype. Strains RM6976, RM6977, and RM6978, which were found to be identical by pulsed-field gel electrophoresis (PFGE), all gave a weaker induction of Stx2 than did the other Stx2c subtypes. As shown in Table 3, the A2 fragment for RM6976 and RM6977 was so weak as to make MS/MS impossible. Only the mass of the A2 fragment could be measured, which facilitated distinguishing it as either an Stx2a or an Stx2c subtype. The A2 fragment of RM6978 was also weak, and MS/MS was possible but gave a poor signal-to-noise ratio (S/N). In consequence, a residue-specific analysis (D-specific) and a high noise threshold were used for top-down identification.
The stoichiometry of AB5 toxins means that the A2 fragment is 5 times less abundant than the B subunit. In consequence, we observed that when the Stx2 induction was weak for a particular strain, only the B subunit was detected. This is due primarily to the fact that samples were ionized from unfractionated (i.e., unpurified) bacterial cell lysates in order to simplify and accelerate sample preparation. As the B subunit was detected and identified by top-down analysis for these three strains coupled with MS of the A2 fragment, this was still sufficient to classify these strains as Stx2c subtypes.
All 12 Stx2c subtype strains gave a significant Stx response with the Vero-d2EGFP toxicity assay (Fig. 6).
(iii) Stx2d subtype.
We analyzed four Stx2d subtype strains. RM12508 is E. coli O177:[H25] strain DD4 (a reference strain from the Statens Serum Institut). Previous researchers have designated this strain as an Stx2c and/or Stx2d subtype (42). Stx was induced strongly in this strain, allowing top-down identification of both the A2 fragment and the B subunit. The subtype that was common to both the A2 fragment and the B-subunit identifications was the Stx2d subtype. An Stx2c subtype was not common to the two analyses, and we observed no evidence for multiple Stx2 subtypes in this strain; thus, we classify this strain as an Stx2d strain by our top-down technique.
RM7006 is E. coli O91:H21 strain B2F1, which has been characterized extensively and designated an Stx2d subtype (46, 47). Surprisingly, we obtained a poor Stx2 signal for this strain. MS analysis was successful on the B subunit only; the A2 fragment was not detected. Previous studies have reported Stx2 detection from this strain by antibiotic induction; therefore, we concluded that our sample preparation (growth on solid agar supplemented with antibiotic) may need to be modified for toxin detection from this strain. We discuss this subject at greater length under “Detection of Stx2 from unfractionated bacterial cell lysates by MALDI.”
RM13503 is an E. coli ONT:H21 clinical isolate. This strain induced Stx2 strongly and corresponded to a very significant top-down identification of both the A2 fragment and the B subunit. Stx2d was the only subtype common to both the A2 and B-subunit identifications; thus, top-down identification classifies this as an Stx2d subtype.
RM8013 is an E. coli ONT:H7 strain isolated from cow feces. It expressed Stx2 strongly after antibiotic exposure and yielded a significant top-down identification score for the A2 fragment and the B subunit. Interestingly, the B subunit had an A↔E substitution at residue 114 that was not common to the other Stx2d subtypes and corresponded to a nominal mass of 7,715 Da. The mass and sequence of the B subunit were sufficiently unique to identify this Stx2 as an Stx2d subtype without reference to the A2 fragment, which was identified by top-down analysis as an Stx2a, Stx2d, or Stx2e subtype.
All four Stx2d subtype strains resulted in significant responses in the Vero-d2EGFP toxicity assay (Fig. 6).
(iv) Stx2f subtype.
Strains RM9508, RM9511, RM9973, and RM9974 gave similar levels of Stx2 induction, which was not strong but adequate for top-down identification of the B subunit using a residue-specific analysis (D or D/E/P specific). The mass and sequence of the B subunit alone were sufficient to classify these strains as being Stx2f subtypes. The A2 fragment was not detected due to the lack of the minimum recognition site for cleavage by furin (i.e., RXXR) as shown in Fig. 2 (boxed in red). Instead, the Stx2f subtypes have YSVR, which is not sufficient for cleavage by furin.
The gene sequencing for strain RM9981 yielded a mass for the A2 fragment and the B subunit that was different from those of the other Stx2f subtype strains in this study. Unfortunately, Stx2 antibiotic induction in this strain was too weak for MS/MS on the B subunit even though a peak was detected at approximately the correct m/z in MS linear mode.
All of these Stx2f subtype strains provided significant responses in the Vero-d2EGFP toxicity assay (Fig. 6), with the exception of RM9981, which gave a weaker response and was consistent with mass spectrometry analysis.
(v) Stx2g subtype.
A single Stx2g subtype strain (RM10468, an environmental isolate) was analyzed in our study. Stx2 induction in this strain was weak but adequate for an identification of the B subunit using a residue-specific analysis (D specific). It was identified as an Stx2g subtype by top-down analysis. The A2 fragment was detected in MS mode at the correct m/z; however, the signal was too weak for MS/MS analysis. A significant response with the Vero-d2EGFP toxicity assay (Fig. 6) was obtained for this strain.
Analysis of a suspected STEC strain by top-down proteomic analysis.
We DNA sequenced the full Stx2 operon for all of the strains analyzed in this study. The translated amino acid sequences of Stx2 for these strains showed a high sequence similarity and were, in some cases, identical to sequences already present in public databases of other bacterial strains. For example, as shown in Fig. 1B, E. coli O104:H4 (German outbreak strain) has an amino acid sequence for the full Stx2 operon that is identical to the full Stx2 operon for E. coli O157:H7 strain EDL933, indicating that the Stx2 would have been identified by top-down identification without whole-genome sequencing of the strain or DNA sequencing of the stx2 gene. This approach suggests that it would be relatively straightforward to analyze any putative STEC strain by a similar approach. A positive top-down identification would confirm unambiguously the subtype as well as the fact that a specific antibiotic induces Stx2 in a particular bacterial strain.
Detection of Stx2 from unfractionated bacterial cell lysates by MALDI.
In the current study, we observed detectable levels of the B subunit of Stx2 in 26 STEC strains ionized by MALDI from unfractionated bacterial cell lysates. However, we did not detect Stx2 in 21 other STEC strains with a detectable response in the Vero-d2EGFP toxicity assay (data not shown). In 12 other STEC strains that did not give a detectable response by the Vero assay, Stx2 was also not detected by MALDI (data not shown). The lack of detection in these Vero assay-positive strains can be attributed to a lower-level induction in these strains as well as the fact that we are ionizing from an unfractionated bacterial cell lysate, i.e., a mixture of proteins. We suspect that Stx2 is present in these lysates but at a level below our detection by MALDI. Further sample method development may involve sample fractionation using liquid chromatography or molecular-weight-cutoff filters to increase the sensitivity of this technique for Stx2 detection and identification.
Cost, time, and labor of top-down analysis.
The primary cost, time, and labor of the top-down analysis involve culturing of a bacterial strain or a number of strains. Once cells are harvested, sample preparation takes 1 to 1.5 h. Once the mass spectrometer has been calibrated (45 min), data acquisition takes 5 s per spot in MS mode and 50 s per spot in MS/MS mode. The 384-spot stainless steel MALDI target can accommodate 128 samples or 128 strains (3 technical replicates per sample). Thus, the number of strains that can be analyzed is limited not by MS analysis but by the cost, time, and labor involved in culturing 128 strains. The only significant consumable mass spectrometry cost is the highly recrystallized MALDI matrix used, which is $15 per 10 mg, is sufficient for 1,000 to 2,000 spots depending on aliquot volume, and is thus pennies per strain. In practice, most DNA sequencing of bacterial strains also involves overnight culturing; thus, culturing costs are comparable. The cost of DNA sequencing is about $1.50 per reaction. MS data acquisition can be set up to run automatically; however, we prefer to acquire and analyze samples manually as each sample spot takes only seconds to collect and it allows the operator to evaluate the data in real time before the next acquisition.
Conclusions.
We have demonstrated the use of MALDI-TOF-TOF-MS/MS and top-down proteomic analysis for rapid, definitive identification of Stx2 subtypes in 26 STEC strains. Subtype identification involves detection, sequence-specific fragmentation, and identification of the A2 fragment of the A subunit and the mature B subunit. In some cases, identification of only the B subunit is sufficient for subtype identification. The larger catalytically active A1 fragment of the A subunit (28 kDa) did not ionize by MALDI from an unfractionated bacterial cell lysate. However, it was possible to identify the Stx2 subtype even in the absence of A1 ionization due to the fact that most of the amino acid substitutions across Stx2 subtypes occur in the A2 fragment and the B subunit.
After overnight culturing, sample preparation and data acquisition are very rapid. Our approach relies upon optimizing antibiotic induction of Stx2 to produce sufficient signal for MALDI-MS and MS/MS analysis. Our method may not be sufficiently sensitive to detect basal (noninduced) expression of Stx from an unfractionated bacterial cell lysate. In addition, we did not detect Stx2 in 21 STEC strains that gave a detectable response from our Vero-d2EGFP toxicity assay.
Although DNA sequencing of the full Stx2 operon was performed on all 26 STEC strains for the purposes of confirmation, we found that the translated amino acid sequences of the A2 fragment and the B subunit of Stx2 for these strains were fully homologous to sequences already present in public databases. Thus, DNA sequencing of these strains was not essential to identification of the Stx2 subtype using this approach.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by USDA-ARS CRIS project 5325-42000-047-00D.
We thank Pina Fratamico for providing E. coli O104:H21 strain CDC 94-3024 (RM13368) and Thomas Whittam for providing E. coli O91:H21 strain B2F1 (RM7006). We thank Lawrence Connolly (Massachusetts Department of Public Health) for providing E. coli O104:H4 (RM14735) and E. coli ONT:H27 (RM13503). We thank Michelle Swimley for assistance with the Vero-d2EGFP cell assay. We also thank Samarpita Walker for PFGE analysis.
Footnotes
Published ahead of print 28 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.04058-13.
REFERENCES
- 1.Bielaszewska M, Mellmann A, Zhang W, Köck R, Fruth A, Bauwens A, Peters G, Karch H. 2011. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect. Dis. 11:671–676. 10.1016/S1473-3099(11)70165-7 [DOI] [PubMed] [Google Scholar]
- 2.Balabanova Y, Klar S, Deleré Y, Wilking H, Faber MS, Lassen SG, Gilsdorf A, Dupke S, Nitschke M, Sayk F, Grunow R, Krause G. 2013. Serological evidence of asymptomatic infections during Escherichia coli O104:H4 outbreak in Germany in 2011. PLoS One 8:e73052. 10.1371/journal.pone.0073052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jay M, Garrett V, Mohle-Boetani JC, Barros M, Farrar JA, Rios R, Abbott S, Sowadsky R, Komatsu K, Mandrell R, Sobel J, Werner SB. 2004. A multistate outbreak of Escherichia coli O157: H7 infection linked to consumption of beef tacos at a fast-food restaurant chain. Clin. Infect. Dis. 39:1–7. 10.1086/421088 [DOI] [PubMed] [Google Scholar]
- 4.Cooley M, Carychao D, Crawford-Miksza L, Jay MT, Myers C, Rose C, Keys C, Farrar J, Mandrell RE. 2007. Incidence and tracking of Escherichia coli O157:H7 in a major produce production region in California. PLoS One 2:e1159. 10.1371/journal.pone.0001159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2006. Ongoing multistate outbreak of Escherichia coli serotype O157:H7 infections associated with consumption of fresh spinach—United States, September 2006. MMWR Morb. Mortal. Wkly. Rep. 55:1045–1046 [PubMed] [Google Scholar]
- 6.Beutin L, Hammerl JA, Strauch E, Reetz J, Dieckmann R, Kelner-Burgos Y, Martin A, Miko A, Strockbine NA, Lindstedt BA, Horn D, Monse H, Huettel B, Müller I, Stüber K, Reinhardt R. 2012. Spread of a distinct Stx2-encoding phage prototype among Escherichia coli O104:H4 strains from outbreaks in Germany, Norway, and Georgia. J. Virol. 86:10444–10455. 10.1128/JVI.00986-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laing CR, Zhang Y, Gilmour MW, Allen V, Johnson R, Thomas JE, Gannon VPJ. 2012. A comparison of Shiga-toxin 2 bacteriophage from classical enterohemorrhagic Escherichia coli serotypes and the German E. coli O104:H4 outbreak strain. PLoS One 7:e37362. 10.1371/journal.pone.0037362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL. 2005. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 11:603–609. 10.3201/eid1104.040739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mingle LA, Garcia DL, Root TP, Halse TA, Quinlan TM, Armstrong LR, Chiefari AK, Schoonmaker-Bopp DJ, Dumas NB, Limberger RJ, Musser KA. 2012. Enhanced identification and characterization of non-O157 Shiga toxin-producing Escherichia coli: a six-year study. Foodborne Pathog. Dis. 9:1028–1036. 10.1089/fpd.2012.1202 [DOI] [PubMed] [Google Scholar]
- 10.Kalchayanand N, Arthur TM, Bosilevac JM, Schmidt JW, Wang R, Shackelford SD, Wheeler TL. 2012. Evaluation of commonly used antimicrobial interventions for fresh beef inoculated with Shiga toxin-producing Escherichia coli serotypes O26, O45, O103, O111, O121, O145, and O157:H7. J. Food Prot. 75:1207–1212. 10.4315/0362-028X.JFP-11-531 [DOI] [PubMed] [Google Scholar]
- 11.O'Brien A, Holmes R. 1987. Shiga and Shiga-like toxins. Microbiol. Rev. 51:206–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johannes L, Römer W. 2010. Shiga toxins—from cell biology to biomedical applications. Nat. Rev. Microbiol. 8:105–116. 10.1038/nrmicro2279 [DOI] [PubMed] [Google Scholar]
- 13.Müthing J, Schweppe CH, Karch H, Friedrich AW. 2009. Shiga toxins, glycosphingolipid diversity, and endothelial cell injury. Thromb. Haemost. 101:252–264. 10.1160/TH08-05-0317 [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann P, Hülsewig M, Duvar S, Ziehr H, Mormann M, Peter-Katalinic J, Friedrich AW, Karach H, Müthing J. 2010. On the structural diversity of Shiga toxin glycosphingo-lipid receptors in lymphoid and myeloid cells determined by nanoelectrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 24:2295–2304. 10.1002/rcm.4636 [DOI] [PubMed] [Google Scholar]
- 15.De Sablet T, Bertin Y, Vareille M, Girardeau J-P, Garrivier A, Gobert AP, Martin C. 2008. Differential expression of stx2 variants in Shiga toxin-producing Escherichia coli belonging to seropathotypes A and C. Microbiology 154:176–186. 10.1099/mic.0.2007/009704-0 [DOI] [PubMed] [Google Scholar]
- 16.Melton-Celsa AR, Kokai-Kun JF, O'Brien AD. 2002. Activation of Shiga toxin type 2d (Stx2d) by elastase involves cleavage of the C-terminal two amino acids of the A2 peptide in the context of the appropriate B pentamer. Mol. Microbiol. 43:207–215. 10.1046/j.1365-2958.2002.02733.x [DOI] [PubMed] [Google Scholar]
- 17.Muniesa M, Recktenwald J, Bielaszewska M, Karch H, Schmidt H. 2000. Characterization of a Shiga toxin 2e-converting bacteriophage from an Escherichia coli strain of human origin. Infect. Immun. 68:4850–4855. 10.1128/IAI.68.9.4850-4855.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müthing J, Meisen I, Zhang W, Bielaszewska M, Mormann M, Bauerfeind R, Schmidt MA, Friedrich AW, Karch H. 2012. Promiscuous Shiga toxin 2e and its intimate relationship to Forssman. Glycobiology 22:849–862. 10.1093/glycob/cws009 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt H, Scheef J, Morabito S, Caprioli A, Wieler LH, Karch H. 2000. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl. Environ. Microbiol. 66:1205–1208. 10.1128/AEM.66.3.1205-1208.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skinner C, McMahon S, Rasooly R, Carter JM, He X. 2013. Purification and characterization of Shiga toxin 2f, an immunologically unrelated subtype of Shiga toxin 2. PLoS One 8:e59760. 10.1371/journal.pone.0059760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung P, Peiris J. 2003. A newly discovered verotoxin variant, VT2g, produced by bovine verocytotoxigenic Escherichia coli. Appl. Environ. Microbiol. 69:7549–7553. 10.1128/AEM.69.12.7549-7553.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheutz F, Teel LD, Beutin L, Piérard D, Buvens G, Karch H, Mellmann A, Caprioli A, Tozzoli R, Morabito S, Strockbine NA, Melton-Celsa AR, Sanchez M, Persson S, O'Brien AD. 2012. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J. Clin. Microbiol. 50:2951–2963. 10.1128/JCM.00860-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindgren SW, Samuel JE, Schmitt CK, O'Brien AD. 1994. The specific activities of Shiga-like toxin type II (SLT-II) and SLT-II-related toxins of enterohemorrhagic Escherichia coli differ when measured by Vero cell cytotoxicity but not by mouse lethality. Infect. Immun. 62:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quiñones B, Swimley MS. 2011. Use of a Vero cell-based fluorescent assay to assess relative toxicities of Shiga toxin 2 subtypes from Escherichia coli. Microb. Toxins 739:61–71. 10.1007/978-1-61779-102-4_6 [DOI] [PubMed] [Google Scholar]
- 25.Fuller CA, Pellino CA, Flagler MJ, Strasser JE, Weiss AA. 2011. Shiga toxin subtypes display dramatic differences in potency. Infect. Immun. 79:1329–1337. 10.1128/IAI.01182-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofer E, Cernela N, Stephan R. 2012. Shiga toxin subtypes associated with Shiga toxin-producing Escherichia coli strains isolated from red deer, roe deer, chamois, and ibex. Foodborne Pathog. Dis. 9:792–795. 10.1089/fpd.2012.1156 [DOI] [PubMed] [Google Scholar]
- 27.Fratamico PM, DebRoy C, Miyamoto T, Liu Y. 2009. PCR detection of enterohemorrhagic Escherichia coli O145 in food by targeting genes in the E. coli O145 O-antigen gene cluster and the Shiga toxin 1 and Shiga toxin 2 genes. Foodborne Pathog. Dis. 6:605–611. 10.1089/fpd.2008.0254 [DOI] [PubMed] [Google Scholar]
- 28.Feng P, Monday SR. 2000. Multiplex PCR for detection of trait and virulence factors in enterohemorrhagic Escherichia coli serotypes. Mol. Cell. Probes 14:333–337. 10.1006/mcpr.2000.0323 [DOI] [PubMed] [Google Scholar]
- 29.He X, McMahon S, Skinner C, Merrill P, Scotcher MC, Stanker LH. 2013. Development and characterization of monoclonal antibodies against Shiga toxin 2 and their application for toxin detection in milk. J. Immunol. Methods 389:18–28. 10.1016/j.jim.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 30.Li G, Hong J, Huo G, Ren X. 2010. Monoclonal antibodies against Stx1B subunit of Escherichia coli O157:H7 distinguish the bacterium from other bacteria. Lett. Appl. Microbiol. 51:499–503. 10.1111/j.1472-765X.2010.02919.x [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Bielaszewska M, Pulz M, Becker K, Friedrich AW, Karch H, Kuczius T. 2008. New immuno-PCR assay for detection of low concentrations of Shiga toxin 2 and its variants. J. Clin. Microbiol. 46:1292–1297. 10.1128/JCM.02271-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He X, Qi W, Quiñones B, McMahon S, Cooley M, Mandrell RE. 2011. Sensitive detection of Shiga toxin 2 and some of its variants in environmental samples by a novel immuno-PCR assay. Appl. Environ. Microbiol. 77:3558–3564. 10.1128/AEM.02205-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quiñones B, Massey S, Friedman M, Swimley MS, Teter K. 2009. Novel cell-based method to detect Shiga toxin 2 from Escherichia coli O157:H7 and inhibitors of toxin activity. Appl. Environ. Microbiol. 75:1410–1416. 10.1128/AEM.02230-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitova EN, Daneshfar R, Marcato P, Mulvey GL, Armstrong G, Klassen JS. 2005. Stability of the homopentameric B subunits of Shiga toxins 1 and 2 in solution and the gas phase as revealed by nanoelectrospray Fourier transform ion cyclotron resonance mass spectrometry. J. Am. Soc. Mass Spectrom. 16:1957–1968. 10.1016/j.jasms.2005.07.016 [DOI] [PubMed] [Google Scholar]
- 35.Kitova EN, Mulvey GL, Dingle T, Sinelnikov I, Wee S, Griener TP, Armstrong GD, Klassen JS. 2009. Assembly and stability of the Shiga toxins investigated by electrospray ionization mass spectrometry. Biochemistry 48:5365–5374. 10.1021/bi9003155 [DOI] [PubMed] [Google Scholar]
- 36.Fagerquist CK, Garbus BR, Williams KE, Bates AH, Boyle S, Harden LA. 2009. Web-based software for rapid top-down proteomic identification of protein biomarkers, with implications for bacterial identification. Appl. Environ. Microbiol. 75:4341–4353. 10.1128/AEM.00079-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fagerquist CK, Garbus BR, Miller WG, Williams KE, Yee E, Bates AH, Harden LA, Cooley MB, Mandrell RE. 2010. Rapid identification of protein biomarkers of Escherichia coli O157: H7 by matrix-assisted laser mass spectrometry and top-down proteomics. Anal. Chem. 82:2717–2725. 10.1021/ac902455d [DOI] [PubMed] [Google Scholar]
- 38.Fagerquist CK, Sultan O. 2011. Induction and identification of disulfide-intact and disulfide-reduced β-subunit of Shiga toxin 2 from Escherichia coli O157:H7 using MALDI-TOF-TOF-MS/MS and top-down proteomics. Analyst 136:1739–1746. 10.1039/c0an00909a [DOI] [PubMed] [Google Scholar]
- 39.Fagerquist CK, Sultan O. 2010. Top-down proteomic identification of furin-cleaved α-subunit of Shiga toxin 2 from Escherichia coli O157:H7 using MALDI-TOF-TOF-MS/MS. J. Biomed. Biotechnol. 2010:123460. 10.1155/2010/123460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michel B. 2005. After 30 years of study, the bacterial SOS response still surprises us. PLoS Biol. 3:e255. 10.1371/journal.pbio.0030255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fagerquist CK, Zaragoza WJ, Sultan O, Woo N, Quiñones B, Cooley MB, Mandrell RE. 2013. Distinguishing Shiga toxin 2 variants in Shiga toxin-producing E. coli (STEC) strains by MALDI-TOF-TOF-MS/MS and top-down proteomics, abstr ThP25-517 Abstr. 113th Gen. Meet. Am. Soc. Microbiol. [Google Scholar]
- 42.Feng PCH, Jinneman K, Scheutz F, Monday SR. 2011. Specificity of PCR and serological assays in the detection of Escherichia coli Shiga toxin subtypes. Appl. Environ. Microbiol. 77:6699–6702. 10.1128/AEM.00370-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooley MB, Jay-Russell M, Atwill ER, Carychao D, Nguyen K, Quiñones B, Patel R, Walker S, Swimley M, Pierre-Jerome E, Gordus AG, Mandrell RE. 2013. Development of a robust method for isolation of Shiga toxin-positive Escherichia coli (STEC) from fecal, plant, soil and water samples from a leafy greens production region in California. PLoS One 8:e65716. 10.1371/journal.pone.0065716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. 1995. Outbreak of acute gastroenteritis attributable to Escherichia coli serotype O104:H21—Helena, Montana, 1994. MMWR Morb. Mortal. Wkly. Rep. 44:501–503 [PubMed] [Google Scholar]
- 45.Feng P, Weagant SD, Monday SR. 2001. Genetic analysis for virulence factors in Escherichia coli O104:H21 that was implicated in an outbreak of hemorrhagic colitis. J. Clin. Microbiol. 39:24–28. 10.1128/JCM.39.1.24-28.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott ME, Melton-Celsa AR, O'Brien AD. 2003. Mutations in hns reduce the adherence of Shiga toxin-producing E. coli 091:H21 strain B2F1 to human colonic epithelial cells and increase the production of hemolysin. Microb. Pathog. 34:155–159. 10.1016/S0882-4010(03)00002-0 [DOI] [PubMed] [Google Scholar]
- 47.Teel LD, Melton-Celsa AR, Schmitt CK, O'Brien AD. 2002. One of two copies of the gene for the activatable Shiga toxin type 2d in Escherichia coli O91: H21 strain B2F1 is associated with an inducible bacteriophage. Infect. Immun. 70:4282–4291. 10.1128/IAI.70.8.4282-4291.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fagerquist CK, Sultan O. 2012. A new calibrant for matrix-assisted laser desorption/ionization time-of-flight-time-of-flight post-source decay tandem mass spectrometry of non-digested proteins for top-down proteomic analysis. Rapid Commun. Mass Spectrom. 26:1241–1248. 10.1002/rcm.6220 [DOI] [PubMed] [Google Scholar]
- 49.Demirev PA, Feldman AB, Kowalski P, Lin JS. 2005. Top-down proteomics for rapid identification of intact microorganisms. Anal. Chem. 77:7455–7461. 10.1021/ac051419g [DOI] [PubMed] [Google Scholar]
- 50.Miyahara M, Konuma H. 1999. Escherichia coli O157 strains which caused Japanese outbreaks have residues of bacteriophage sequences. Biol. Pharm. Bull. 22:1372–1375. 10.1248/bpb.22.1372 [DOI] [PubMed] [Google Scholar]
- 51.Zheng J, Cui S, Teel LD, Zhao S, Singh R, O'Brien AD, Meng J. 2008. Identification and characterization of Shiga toxin type 2 variants in Escherichia coli isolates from animals, food, and humans. Appl. Environ. Microbiol. 74:5645–5652. 10.1128/AEM.00503-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slanec T, Fruth A, Creuzburg K, Schmidt H. 2009. Molecular analysis of virulence profiles and Shiga toxin genes in food-borne Shiga toxin-producing Escherichia coli. Appl. Environ. Microbiol. 75:6187–6197. 10.1128/AEM.00874-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.