Abstract
Functional expression in heterologous hosts is often less successful for integral membrane proteins than for soluble proteins. Here, two Ambrosiozyma monospora transporters were successfully expressed in Saccharomyces cerevisiae as tagged proteins. Growth of A. monospora on l-arabinose instead of glucose caused transport activities of l-arabinose, l-arabitol, and ribitol, measured using l-[1-3H]arabinose, l-[14C]arabitol, and [14C]ribitol of demonstrated purity. A. monospora LAT1 and LAT2 genes were cloned earlier by using their ability to improve the growth of genetically engineered Saccharomyces cerevisiae on l-arabinose. However, the l-arabinose and pentitol transport activities of S. cerevisiae carrying LAT1 or LAT2 are only slightly greater than those of control strains. S. cerevisiae carrying the LAT1 or LAT2 gene fused in frame to the genes for green fluorescent protein (GFP) or red fluorescent protein (mCherry) or adenylate kinase (AK) exhibited large (>3-fold for LAT1; >20-fold for LAT2) increases in transport activities. Lat1-mCherry transported l-arabinose with high affinity (Km ≈ 0.03 mM) and l-arabitol and ribitol with very low affinity (Km ≥ 75 mM). The Lat2-GFP, Lat2-mCherry, and Lat2-AK fusion proteins could not transport l-arabinose but were high-affinity pentitol transporters (Kms ≈ 0.2 mM). The l-arabinose and pentitol transport activities of A. monospora could not be completely explained by any combination of the observed properties of tagged Lat1 and Lat2, suggesting either that tagging and expression in a foreign membrane alters the transport kinetics of Lat1 and/or Lat2 or that A. monospora contains at least one more l-arabinose transporter.
INTRODUCTION
Although soluble proteins can usually be successfully expressed in heterologous hosts, functional expression of integral membrane proteins frequently cannot be achieved, or it fails to provide adequate levels of activity in heterologous hosts. Reasons for this failure include incompatibility between the heterologous protein and the host's trafficking machinery, inappropriate lipid composition of the host's membrane, and instability of foreign proteins in the host membrane. Various approaches to this problem have been reported (1–4). One consequence is that even when the intracellular steps of catabolic pathways for novel substrates have been engineered into a favored production organism, the catabolic rate may be limited by slow transport, which can be difficult to accelerate by engineering a heterologous transporter into the host.
Both redox-neutral and oxidoreductive catabolic pathways for l-arabinose have been introduced into Saccharomyces cerevisiae (5, 6) with the objective of improving the productivity of (fuel) ethanol production from cheap biomass containing pentose residues. The resulting genetically engineered strains could grow on l-arabinose and produce ethanol, but only slowly. Efforts have been made to improve the performance of l-arabinose-utilizing strains by rational engineering and by accelerated evolution (7, 8). The slow consumption of l-arabinose is probably caused, at least in part, by slow transport of l-arabinose into the cell. S. cerevisiae contains at least three transporters capable of carrying l-arabinose, the Gal2 galactose transporter (9) and the hexose transporters Hxt9 and Hxt10 (10). Glucose is a better substrate than l-arabinose for these endogenous transporters, so that in mixed-sugar fermentations, glucose competes with l-arabinose, which is not fermented until most glucose has been consumed. Screens for heterologous l-arabinose transporter genes have been conducted by looking for improved growth on l-arabinose of engineered strains of S. cerevisiae containing an l-arabinose catabolic pathway. Subtil and Boles (10) found two genes, ARAT from Scheffersomyces stipitis (Pichia stipitis) and STP2 from the plant Arabidopsis thaliana, which encoded l-arabinose transporters that did not carry glucose (Stp2) or carried it poorly (AraT). Verho et al. (11) found two genes, LAT1 and LAT2, from the yeast Ambrosiozyma monospora. Although LAT1 and LAT2 stimulated growth on l-arabinose, the growth rates were low, and the apparent l-arabinose transport activities of S. cerevisiae strains carrying either gene were much lower than that of A. monospora. We reinvestigated this system with the aim of improving the functional expression of LAT1 and LAT2 in the heterologous host, S. cerevisiae. We found that the addition of C-terminal tags to the recombinant transporters caused large increases in their transport activity, resulting in specific activities of the same order as observed in A. monospora.
During this work, we found that the commercial radioactive reagent supplied to Verho et al. (11) as l-[1-14C]arabinose was actually a mixture of two radioactive pentitols containing little or no l-[1-14C]arabinose. In the present work, repurified reagents (l-[1-3H]arabinose, [14C]ribitol, and l-[14C]arabitol) were used to characterize the substrate specificities and transport kinetics of the fusion proteins encoded by LAT1 and LAT2 linked 3′ in frame to the open reading frames (ORFs) of green fluorescent protein (GFP) or red fluorescent protein (mCherry) or adenylate kinase (AK).
MATERIALS AND METHODS
Water was deionized and filtered through active carbon using the Milli-Q water system (Millipore Corporation, Billerica, MA, USA).
Radioactive compounds for transport assays.
Two lots of l-[1-14C]arabinose were examined by GC-MS (gas chromatography-mass spectrometry) as described below. One lot was purchased from Moravek Biochemicals (Brea, CA, USA) (catalog no. MC 2019; lot 165-155-054-A-20020115-SB), and the other was a gift from American Radiolabeled Chemicals (catalog no. ARC 1041; lot 110523). Their radioactive purities were claimed by the manufacturers to be ≥99%. For both lots, GC-MS revealed the characteristic 4 peaks corresponding to the furan and pyran forms of α- and β-arabinose (12) and with the same retention times as those of authentic l-arabinose. The mass spectra of the four peaks were essentially identical and the same as those of authentic l-arabinose. With the small amounts of (carrier-free) radioactive materials available, the MS spectra could not be distinguished from those of other pentoses, but certain features distinguished them from the spectra of pentitols (e.g., signals at m/z = 307 and 319 were weak or absent for these 1-14C-labeled reagents and for pentoses, but strong for pentitols).
Unless otherwise stated, l-arabinose transport was measured using l-[1-3H]arabinose (catalog no. ART 0806; lots 110609 and 120209) purchased from American Radiolabeled Chemicals (claimed radiochemical purity of ≥99%) and repurified by high-performance liquid chromatography (HPLC system I; see below). Analysis of l-[1-3H]arabinose was hindered by the very small amount of material (250 μCi was 1.9 μg). The GC profile was disturbed by reagent peaks but contained 4 peaks with retention times similar to that of authentic l-arabinose. Each peak yielded the same characteristic pentose mass spectrum.
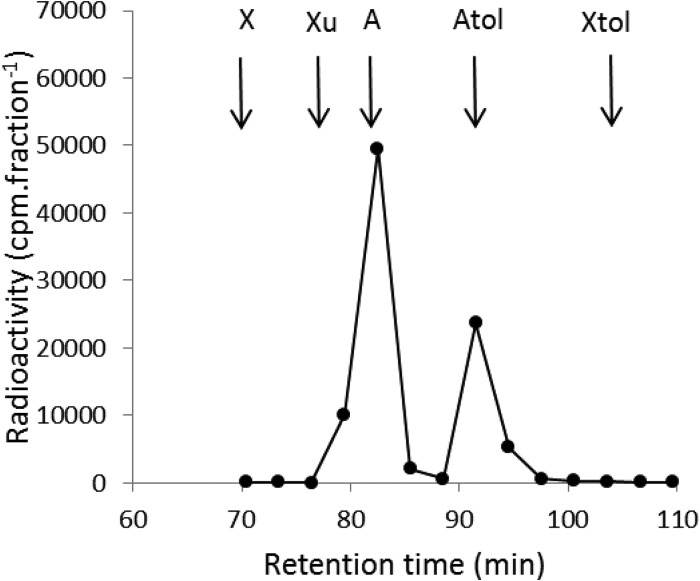
l-[14C]arabitol was purified by HPLC system II (see below) from two lots of radioactive material purchased from American Radiolabeled Chemicals as l-[1-14C]arabinose (catalog no. ARC 1041; lots 011110 and 101028). During this purification, the Atol compound had the same retention time as that of authentic l-arabitol and was resolved from d-xylose, l-xylulose, l-arabinose, and xylitol (Fig. 1). GC-MS analyses of compound Atol gave a single peak with the MS of a pentitol, although the dominant peaks in some regions of the spectrum (e.g., at m/z 307/309 and 319/321) were 2 units higher than for the library spectrum of arabitol, suggesting that these signals represent 14C-labeled fragments. A mixture of compound Atol with ribitol was resolved by GC-MS (ribitol eluting slightly after compound Atol), whereas a mixture of compound Atol with authentic l-arabitol was not resolved, confirming the identity of the Atol compound as arabitol.
FIG 1.
HPLC analysis of l-[1-14C]arabinose (catalog no. ARC 1041 [American Radiolabeled Chemicals]; lot 011110). A solution containing 1 mg · ml−1 each of d-xylose (X), l-xylulose (Xu), l-arabinose (A), l-arabitol (Atol), and xylitol (Xtol) and 1.9 × 106 cpm · ml of l-[1-14C]arabinose (catalog no. ARC 1041; lot 011110) was prepared in water, and 45 μl was injected into the HPLC system. After 70 min at a flow rate of 0.15 ml · min−1, 440-μl fractions were collected, and their radioactivities were determined. The retention times of the standards are indicated by the arrows.
[14C]ribitol was also purified by HPLC system II from l-[1-14C]arabinose (catalog no. ARC 1041; lots 011110 and 101028). During this purification, compound A was resolved from l-arabitol, xylitol, xylose, and xylulose, but not from l-arabinose (Fig. 1). GC-MS analyses of compound A gave a single peak with the same retention time as authentic ribitol and the MS of a pentitol. A mixture of compound A with l-arabitol was resolved by GC-MS, whereas a mixture of compound A with ribitol was not resolved, confirming the identity of compound A as [14C]ribitol.
HPLC system I: purification of l-[1-3H]arabinose.
A 125-μl portion (125 μCi; 0.95 μg) of l-[1-3H]arabinose (catalog no. ART0806; lot 120209) was diluted with 450 μl water and freeze-dried to remove ethanol. The residue was dissolved in 120 μl water. Three 40-μl portions were separately injected into a Waters Alliance 2690 separation module using a CarboSep CHO-882 column (Transgenomic, Omaha, NE, USA) with elution by water at 90°C and 0.5 ml · min−1. Fractions of 1.5 ml were collected, and their radioactivity was measured by scintillation counting. Eighty-seven percent of the radioactivity was recovered in fractions 5 and 6 (the expected position of l-arabinose), and 9% of the radioactivity was recovered in fraction 7. Minor peaks were found in fractions 3 (0.8%) and 9 plus 10 (1.2%). Other expected elution positions were fraction 7 for ribitol, fraction 9 for arabitol, fraction 11 for ribose, and fraction 12 for xylitol. Fractions 5 and 6 from all three runs were stored individually at −80°C and are referred to below as repurified l-[1-3H]arabinose.
HPLC system II: analysis of radioactive material in l-[1-14C]arabinose (catalog no. ARC1041; lots 011110 and 101028) and preparation of l-[14C]arabitol and [14C]ribitol.
For analysis, a 250-μl solution containing 1 mg · ml−1 each of d-xylose, l-xylulose, l-arabinose, l-arabitol, and xylitol in water was labeled by the addition of 2 μl (4.8 × 105 cpm) of lot 011110 or 101028 of catalog no. ARC 1041, supposedly l-[1-14C]arabinose. The mixtures were analyzed by injecting 45 μl onto Bio-Rad Aminex HPX-87N and Waters Sugar-Pak 1 columns arranged in series with elution by water at 0.15 ml · min−1 and 40°C and detection by photodiode array at 202 nm. Fractions of 440 μl were collected, and their radioactivities were measured by scintillation counting. From lot 011110, two peaks of radioactivity were recovered, 68% (compound A) with a retention time of 82 min, close to that of authentic l-arabinose, and 32% (compound Atol) with a retention time of 91.5 min, close to that of authentic l-arabitol (Fig. 1). For lot 101028, the compound A peak contained 35% of the total radioactivity, and the compound Atol peak contained 58% of the total radioactivity.
For purification, portions of lots 011110 and 101028 (catalog no. ARC 1041) were separately diluted 2-fold with water and freeze-dried to remove ethanol. The residues were dissolved in water (half the original volume), and 45-μl portions were fractionated by the HPLC system II described above (without carrier pentoses and pentitols). The radioactivities of the fractions (440 μl) were measured, and fractions from five replicate runs corresponding to compounds A and Atol in Fig. 1 were separately pooled, freeze-dried, dissolved in 200 μl water, stored at −80°C, and used as [14C]ribitol (compound A) and l-[14C]arabitol (compound Atol). The HPLC system used to purify these compounds does not distinguish between the l and d isomers of arabitol. The chemical synthesis of l-[1-14C]arabinose (catalog no. ARC 1041) was by treatment of l-erythrose with cyanide followed by hydrolysis to a mixture of ribonic and l-arabinoic acids, which are then reduced (S. Gupta, President of American Radiolabeled Chemicals, personal communication), so that the arabitol accidentally produced (presumably because the reduction proceeded too far) is expected to be the l isomer.
GC-MS analyses.
Samples were evaporated to dryness before silylation. Silylation was done by adding 100 μl of chlorotrimethylsilane and 100 μl of N,O-Bis(trimethylsilyl)trifluoroacetamide and heating at 70°C for 60 min. Derivatized samples (1 μl) were analyzed by gas chromatography-mass spectrometry (Agilent 6890 series gas chromatograph combined with Agilent 5973Network mass selective detector [MSD] [Agilent Technologies, USA] and Combipal injector [Varian Inc., USA]). Analytes were injected in split mode (30:1) at 200°C and separated on a ZB-5HT Inferno capillary column (30 m by 0.25 mm) with a phase thickness of 0.25 μm (Phenomenex, Denmark). Helium was used as the carrier gas at a constant flow of 0.9 ml · min−1. The temperature program was 70°C for 3 min and then a 10°C · min−1 gradient to 320°C. The MSD was operated in electron impact mode at 70 eV and in the full scan at m/z 40 to 550. The ion source temperature was 230°C, and the interface was 280°C. Compounds were identified by using authentic standards and comparison to the Palisade Complete Mass Spectral Library with more than 600,000 mass spectra (Palisade Mass Spectrometry, USA, and NIST 08 Scientific Instrument Services, Inc., NJ, USA).
Yeast strains.
Ambrosiozyma monospora (NRRL Y-1484) was obtained from the Agricultural Research Service (ARS) Culture Collection (http://nrrl.ncaur.usda.gov/). The S. cerevisiae strains were CEN.PK2-1D and the engineered strains listed in Table 1 derived from CEN.PK2-1D.
TABLE 1.
Genetically engineered S. cerevisiae strains used in this studya
| Strain | Relevant gene(s) | Plasmid(s) | Host | Reference |
|---|---|---|---|---|
| H2984 | 5 integrated genes for l-arabinose pathway | None | CEN.PK2-1D | Verho et al. (11) |
| H3169 | pLAT1 | pFL60 | H2984 | Verho et al. (11) |
| H3187 | pLAT2 | pFL60 | H2984 | Verho et al. (11) |
| H4387 | pLAT1 and pLAT2 | B2158 and B2159 | CEN.PK2-1D | This work |
| H4388 | pLAT1-mCherry | B2158 | CEN.PK2-1D | This work |
| H4389 | None (control) | B2158 | CEN.PK2-1D | This work |
| H4390 | pLAT2-GFP | B2159 | CEN.PK2-1D | This work |
| H4391 | None (control) | B2159 | CEN.PK2-1D | This work |
| H4392 | pLAT1-mCherry and pLAT2-GFP | B2158 and B2159 | CEN.PK2-1D | This work |
| H4393 | None (control) | B2158 and B2159 | CEN.PK2-1D | This work |
| H4394 | LAT2-GFP | M4297 | CEN.PK2-1D | This work |
| H4395 | LAT2-GFP | M4297 | H2984 | This work |
| H4399 | LAT2 partial deletant | M4297 | CEN.PK2-1D | This work |
| H4400 | LAT2-1/2GFP | M4297 | CEN.PK2-1D | This work |
| H4402 | pLAT2-mCherry | B2158 | CEN.PK2-1D | This work |
| H4403 | pLAT2-AK | B2158 | CEN.PK2-1D | This work |
Multicopy plasmids (pFL60, B2158, and B2159) also carried the indicated genes. Integration vector M4297 targeted the indicated genes at the S. cerevisiae HO locus.
Strain construction.
The construction of yeast expression vectors containing the A. monospora LAT1 and LAT2 genes has been described previously (11). LAT1 and LAT2 were amplified by PCR from these expression vectors. The GFP gene was amplified from the pSM1 vector (B2155) containing the enhanced green fluorescent protein (13). The mCherry gene was amplified from pRSETB_mCherry plasmid (a kind gift from R. Tsien) containing the red fluorescent protein variant called mCherry (14). The S. cerevisiae adenylate kinase gene, ADK1, was amplified from genomic DNA. Primers used to amplify these genes are listed in Table 2.
TABLE 2.
Primers for amplification of the genes
| Oligonucleotide primera | Sequence |
|---|---|
| Lat1_pyx.5F | CTTAAATCTATAACTACAAAAAACACATACAGATGCGGTTACCTAAAGTTTACAACC |
| Lat1.5R | GTTATCCTCCTCGCCCTTGCTCACCATCGCCGCACTAGTAGCAAAATTATGATTCTCATCAATTG |
| mCherry_Lat1.3F | CAATTGATGAGAATCATAATTTTGCTACTAGTGCGGCGATGGTGAGCAAGGGCGAGGAGGATAAC |
| mCherry_Lat1.3R | GTTAGCTAGCTGAGCTCGACTCTAGAGGATCCTACTTGTACAGCTCGTCCATGCCG |
| Lat2_pyx.5F | CTTAAATCTATAACTACAAAAAACACATACAGATGGGTCAGTTTATTGAAAAATTCAG |
| Lat2.5R | GAACAGCTCCTCGCCCTTGCTCACCATCGCCGCACTAGTAACACTACTTACAGAGTCTTTGAG |
| EGFP_Lat2.3F | CTCAAAGACTCTGTAAGTAGTGTTACTAGTGCGGCGATGGTGAGCAAGGGCGAGGAGCTGTTC |
| EGFP_Lat2.3R | GTTAGCTAGCTGAGCTCGACTCTAGAGGATCCTACTTGTACAGCTCGTCATGCCG |
| Lat2int.5F | GAAACTGACAGGTGGTTTGTTACGCATGCTAATGCAAAGGAGCCTATATACCTTTG |
| Lat2_adk.5R | CCATTCTAATGGATTCTGAGCTAGACATCGCCGCACTAGTAACACTACTTACAGAGTCTTTGAG |
| ADK1.F | CTCAAAGACTCTGTAAGTAGTGTTACTAGTGCGGCGATGTCTAGCTCAGAATCCATTAGAATGG |
| ADK1.R | CGTTCATTGTTCCTTATTCAGTTAGCTAGCTTAATCCTTACCTAGCTTGTTCAAGATGTCAGCC |
At the end of the oligonucleotide primer names, F stands for forward and R stands for reverse. EGFP stands for enhanced green fluorescent protein.
For expression from a multicopy plasmid, LAT1-mCherry, LAT2-GFP, LAT2-AK, and LAT2-mCherry fusion constructs were cloned into vectors B2158 and B2159, which are yeast expression vectors generated from pYX242 and pXY212 (R&D Systems, United Kingdom), respectively, by changing the multiple cloning site (15). They contain the constitutive TPI1 promoter and LEU2 (B2158) or URA3 (B2159) as the selection marker. A linker (5′-ACTAGTGCGGCG-3′) was added between the fusion partners. S. cerevisiae strain FY834 (16) was used for recombination cloning (17). The resulting plasmids were then amplified in Escherichia coli, and S. cerevisiae strain CEN.PK2-1D was transformed with these plasmids, making the strains shown in Table 1. The plasmid for the integrated LAT2-GFP expression construct was made by digesting the LAT2-GFP multicopy plasmid with AatII and PvuII and ligating the fragment containing the LAT2-GFP fusion gene as a blunt-end fragment into SmaI-digested M4287, which is a yeast vector for targeted integration of desired sequence at the S. cerevisiae HO locus (18). 3′-modified versions of LAT2 (LAT2 partial deletant; with 186 bp removed from the 3′ terminus of LAT2 and a new stop codon) and of LAT2-GFP (LAT2-1/2GFP [i.e., approximately half the GFP gene fused to LAT2]; with 297 bp removed from the 3′ terminus of GFP and a new stop codon) were constructed from PCR fragments and ligated into plasmid M4287. Strain CEN.PK2-1D was transformed with the integrating plasmids (Table 1).
Growth of yeast strains.
Strains were stored at −80°C as stock suspensions containing 200 mg (wet weight) of fresh yeast · ml of 30% (vol/vol) glycerol−1 and were grown at 25°C with orbital shaking at 170 rpm. For S. cerevisiae transformants containing multicopy plasmids, synthetic complete medium (19) lacking uracil or leucine or both and containing 20 g glucose · liter−1 was used. Also, the hosts and transformants with integrated genes were usually grown in synthetic complete medium, but where stated, they were grown in YPD (10 g yeast extract, 20 g peptone, and 20 g glucose [all amounts shown per liter]). Media (100 ml in 250-ml Erlenmeyer flasks) were inoculated with 10 to 400 μl of yeast stock suspensions. S. cerevisiae strains were harvested after 20 h at an optical density (optical density at 600 nm [OD600]) between 3 and 8, corresponding to between 0.8 and 2 mg (dry weight) yeast · ml−1. A. monospora was grown in yeast nitrogen base (YNB) containing either 40 g l-arabinose · liter−1 (harvested at an OD600 of 2.2 to 6.9) or where stated 20 g glucose · liter−1 (harvested at an OD600 of 5.0 to 5.6).
Transport assays.
Radioactive substrates were prepared at specific radioactivities between 20 and 300 cpm · nmol−1. The unlabeled components were l-arabinose (BioUltra; catalog no. 10893 [Sigma]) (≥99.5%), l-arabitol (catalog no. A-3506; Sigma) (≥98%), and ribitol (adonitol) (catalog no. A-5502; Sigma) (≥99%). Unless stated otherwise, the labeled components were repurified l-[1-3H]arabinose, purified l-[14C]arabitol, and purified [14C]ribitol prepared as described above.
Yeast samples for transport assays were harvested by centrifugation for 9 min at 9,000 × g. The pellets were resuspended in 25 ml of ice-cold water and centrifuged again. The pellets were again washed with 25 ml of ice-cold water, weighed, and suspended in 0.1 M tartaric acid–Tris buffer, pH 4.2, to a concentration of 270 mg (wet weight) fresh yeast · ml−1. Transport assays were performed at 20°C essentially by the method of Serrano (20) and modified by Guimarães et al. (21). Reactions were started by adding 30 μl of yeast suspension to 30 μl of reaction mixture containing radiolabeled substrate and any additions in 15-ml glass tubes with conical bottoms. Reactions were stopped by the addition of 10 ml ice-cold water. The yeast was immediately collected by filtration through wet glass microfiber filters (Whatman GF/C). The filters were washed with a further 10 ml ice-cold water, and transferred to scintillation cocktail (Optiphase Hisafe3 from PerkinElmer, Waltham, MA, USA), and the radioactivity was counted. Reaction times were between 10 s and 5 min (usually 20 or 60 s) and were chosen to give rates close to initial rates (observed rates were at least 90% of those observed with 50% shorter reaction times). Each independent sample was assayed in duplicate. Blanks (zero time values) were determined by first adding 10 ml of ice-cold water to the 30-μl reaction mixture and then adding the 30-μl yeast suspension and immediately filtering. Rates were normalized to the yeast mass (dry weight) determined by drying water-washed yeast pellets at 105°C. Median estimates of Km and Vmax were obtained by a computerized version of the direct linear plot (22).
RESULTS
Transport of l-arabinose, l-arabitol, and ribitol by Ambrosiozyma monospora.
Growth of A. monospora on l-arabinose instead of glucose caused the appearance of transport activities of l-arabinose, l-arabitol, and ribitol (Table 3). Similar l-arabinose transport activities were obtained using repurified l-[1-3H]arabinose or the l-[1-14C]arabinose reagent lot 110523 from American Radiolabeled Chemicals shown above to consist predominantly of [14C]arabinose. Much higher apparent l-arabinose transport activities (200 to 1,000 μmol · min−1 · g [dry weight]−1 yeast at 20 to 100 mM l-arabinose) were obtained using the defective ARC product (catalog no. 1041; lots 011110 and 101028) of so-called l-[14C]arabinose, shown above to consist of mixtures of l-[14C]arabitol and [14C]ribitol. These (erroneous) values agreed with the activity (640 μmol · min−1 · g [dry weight] yeast−1 at 100 mM l-arabinose) reported by Verho et al. (11), who also used the defective ARC product (catalog no. 1041; lot 011110).
TABLE 3.
Transport of l-arabinose, l-arabitol, and ribitol by Ambrosiozyma monosporaa
| Substrate | Concn (mM) | Transport rateb for yeast grown on: |
|
|---|---|---|---|
| Glucose | l-Arabinose | ||
| l-[1-14C]arabinose (ARC lot 110523) | 20 | 0.24 ± 0.01 | 22.7 ± 8.4 |
| Repurified l-[1-3H]arabinose | 20 | NA | 19.1 ± 4.0 |
| Repurified l-[1-3H]arabinose | 5 | NA | 17.9 ± 6.1 |
| l-[14C]arabitol | 10 | 0.0 ± 0.3 | 12.9 ± 3.2 |
| 0.2 | NA | 3.1 ± 1.1 | |
| [14C]ribitol | 10 | 0.42 ± 0.08 | 14.1 ± 4.9 |
| 0.2 | NA | 4.3 ± 0.4 | |
Yeast was grown on YNB containing glucose (20 g · liter−1) or l-arabinose (40 g · liter−1).
Transport rates (in micromoles · minute−1 · gram [dry weight] yeast−1) are means ± standard deviations (SDs) (n = 2 for glucose-grown yeast; n = 2 to 6 for l-arabinose-grown yeast). NA, not analyzed.
Transport of l-arabinose labeled with repurified l-[1-3H]arabinose exhibited Michaelis-Menten kinetics between 0.07 and 20 mM with a Km of 0.12 ± 0.02 mM (average ± half the range of two independent experiments).
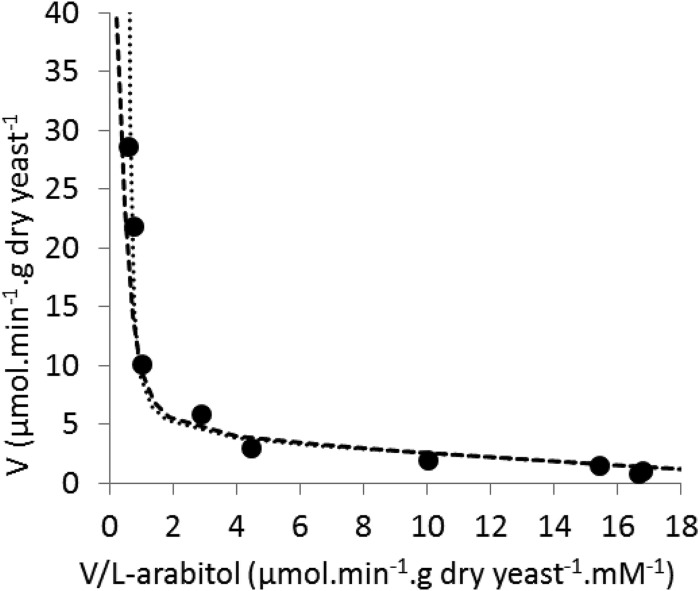
Transport of l-arabitol exhibited biphasic kinetics. Data below 1 mM could be fitted by a Km of 0.16 mM, but data above 10 mM indicated a much higher Km. Data between 50 and 0.05 mM were fitted by the sum of a high-affinity system (Km of 0.16 mM and Vmax of 3.9 μmol · min−1 · g [dry weight] yeast−1) and a low-affinity system. The data were fitted closely by assuming either that the low-affinity system had a Km of 75 mM and Vmax of 49 μmol · min−1 · g (dry weight) yeast−1 or that it was nonsaturable with a first-order rate constant of 0.55 μmol · min−1 · g (dry weight) yeast−1 · mM−1 (Fig. 2). The concentration dependence of ribitol transport was not studied.
FIG 2.
Biphasic kinetics of l-arabitol transport into A. monospora. The initial transport rates (V) were measured between 0.05 and 50 mM l-arabitol. The Eadie-Hofstee plot shows experimental points (●) fitted by curves calculated for the sum of either two Michaelis-Menten systems, a high-affinity system with a Km of 0.16 mM and a Vmax of 3.9 μmol · min−1 · g (dry weight) yeast−1 plus a low-affinity system with a Km of 75 mM and a Vmax of 49 μmol · min−1 · g (dry weight) yeast−1 (dashed line) or the same high-affinity system plus a first-order system with a k of 0.55 μmol · min−1 · g (dry weight) yeast−1 · mM l-arabitol−1 (dotted line).
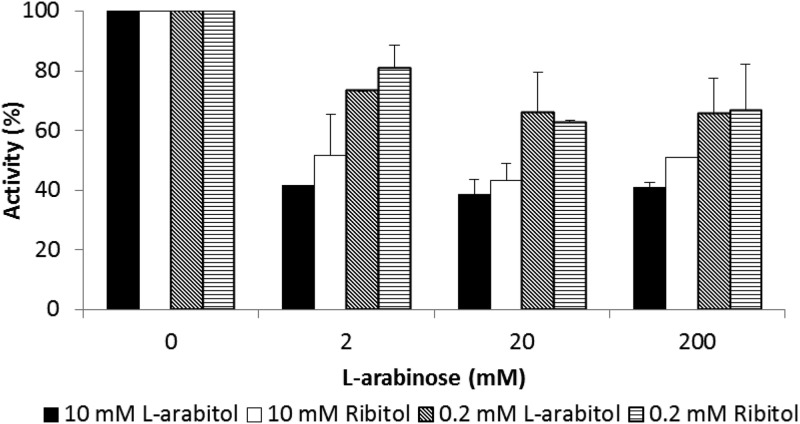
The transports of l-arabitol and ribitol were both strongly inhibited by l-arabinose (Fig. 3). These inhibitions were partial, reaching maximum values by 20 mM l-arabinose. The maximum inhibition was greater (about 60%) at 10 mM pentitol than at 0.2 mM pentitol (about 35%). The results of Fig. 2 and 3 suggested that pentitols are carried by at least two transporters in A. monospora, a low-affinity system (Km of ≥75 mM) that is strongly inhibited by l-arabinose and a high-affinity system (Km ≈ 0.16 mM) that is less sensitive to l-arabinose.
FIG 3.
Inhibition by l-arabinose of pentitol transport by A. monospora. Pentitol transport rates measured at 10 and 0.2 mM pentitol with or without l-arabinose are expressed as percentages of the rates without l-arabinose. Error bars show the standard deviations (SDs) of independent duplicate or triplicate assays.
Transport of l-arabitol and ribitol by transformants carrying LAT1 and LAT2.
Verho et al. (11) cloned the LAT1 and LAT2 genes from A. monospora into H2984, a CEN.PK2-1D derivative containing an integrated arabinose pathway. The apparent l-arabinose transport activity of the transformants (H3169 and H3187) was only slightly higher than that of the controls and much less than that of A. monospora (11). By using repurified l-[1-3H]arabinose as the substrate, we also found very low l-arabinose transport activities (<0.3 μmol · min−1 · g [dry weight] yeast−1). Because the commercial reagent used by Verho et al. (11) was found to be a mixture of [14C]ribitol and l-[14C]arabitol here (see above), we now measured pentitol transport in these transformants using purified [14C]ribitol and l-[14C]arabitol. Compared to strains CEN.PK2-1D and H2984, strains H3169 (containing LAT1) and H3187 (containing LAT2) had 2- to 7-fold-greater transport activities at 10 mM l-arabitol or ribitol (Table 4), but the activities were small compared to experimental errors and <3% of the corresponding activities of A. monospora (Table 3). The transformants had essentially no activity at 0.2 mM pentitol. CEN.PK2-1D was also simultaneously transformed with both LAT1 and LAT2 on separate plasmids. Compared to strains containing either gene alone, this double transformant (H4387) exhibited slightly higher transport activity of the pentitols (0.47 and 0.55 μmol · min−1 · g [dry weight] yeast−1 at 10 mM l-arabitol or ribitol, respectively; not shown in Table 4). However, there was no significant synergy between the two genes, making it unlikely that the gene products Lat1 and Lat2 are essential subunits of a heterodimeric pentitol transporter.
TABLE 4.
Transport of ribitol and l-arabitol by control strains and transformants carrying LAT1 or LAT2
| Substrate | Concn (mM) | Transport ratea |
|||
|---|---|---|---|---|---|
| Host strains | Empty plasmidb | H3169 (pLAT1) | H3187 (pLAT2) | ||
| l-Arabitol | 10 | 0.04 ± 0.02 | 0.05 ± 0.06 | 0.38 ± 0.13 | 0.11 ± 0.07 |
| 0.2 | NA | 0.01 | 0.01 ± 0.00 | 0.02 ± 0.01 | |
| Ribitol | 10 | 0.04 ± 0.06 | 0.06 ± 0.09 | 0.33 ± 0.08 | 0.18 ± 0.02 |
| 0.2 | NA | −0.02 | 0.00 ± 0.00 | 0.02 ± 0.00 | |
Initial rates of transport (μmol · min−1 · g [dry weight] yeast−1) were measured with l-[14C]arabitol and [14C]ribitol at the indicated concentrations. Host strains include both strain CEN.PK2-1D and strain H2984, which had similar activities. Data are means ± SDs (n = 2 to 7) or single experiments where no SDs are shown. NA, not analyzed.
Strain CEN.PK2-1D carrying the empty plasmids, B2158 and B2159.
Transport of pentitols and l-arabinose by transformants containing Lat1 and Lat2 with C-terminal fusions.
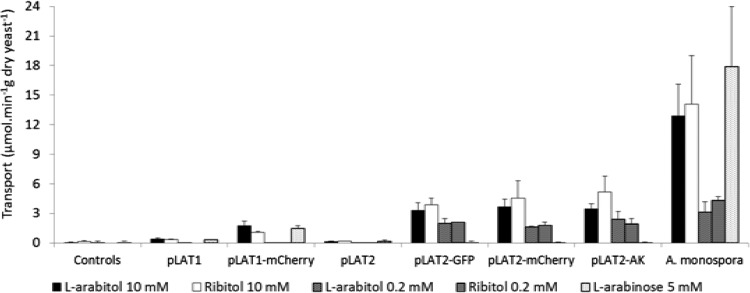
Because CEN.PK2-1D and H2984 transformants carrying LAT1 or LAT2 had such low pentitol transport activities, these genes were fused in frame to the genes for mCherry (red fluorescent protein) or GFP (green fluorescent protein). The resulting fusions were transformed into strain CEN.PK2-1D. Confocal fluorescence microscopy (see Fig. S1 in the supplemental material) showed that for strain H4388, the pLat1-mCherry fusion protein was mainly localized to the plasma membrane, although a few cells also contained some fluorescent intracellular structures. For strain H4390, carrying pLAT2-GFP, the plasma membrane was also strongly fluorescent, but in this case, most cells also exhibited a diffuse intracellular fluorescence. Unexpectedly, these fusions and a similar fusion with adenylate kinase (Lat2-AK; adenylate kinase was chosen to explore the generality of the effect, because like mCherry and GFP, it is a relatively small and compact protein) caused large increases in transport activities (Fig. 4).
FIG 4.
Effect of C-terminal tags on the transport activities of Lat1 and Lat2. Initial transport rates were assayed at 10 mM or 0.2 mM pentitol or 5 mM l-arabinose (labeled with repurified l-[1-3H]arabinose). Error bars show the SDs of 2 to 7 independent replicates. Control strains include both untransformed hosts and hosts carrying empty plasmids. Transformants carried plasmids containing the unmodified transporter genes (pLAT1 and pLAT2) or transporter genes fused in frame to sequences encoding red fluorescent protein, green fluorescent protein, or adenylate kinase (pLAT1-mCherry, pLAT2-mCherry, pLAT2-GFP, or pLAT2-AK). A. monospora was grown on 40 g l-arabinose · liter−1, and the other yeasts were grown on 20 g glucose · liter−1.
Compared to the strain (H3169) carrying unmodified LAT1, strain H4388 carrying the LAT1-mCherry fusion on a multicopy plasmid had 3- to 5-fold-greater transport activities of 10 mM ribitol, 10 mM l-arabitol, and 5 mM l-arabinose (labeled with repurified l-[1-3H]arabinose) but still had negligible activity at 0.2 mM pentitol. Compared to the strain (H3187) carrying unmodified LAT2, strains H4390, H4402, and H4403 carrying, respectively, LAT2-GFP, LAT2-mCherry, and LAT2-AK on multicopy plasmids showed 20- to 35-fold increases in transport activity at 10 mM ribitol or l-arabitol and even larger increases in transport at 0.2 mM pentitol. Within experimental error, the three C-terminal fusions, GFP, mCherry, and AK, were equally effective at increasing pentitol transport activity. Transformants carrying these LAT2 fusions showed no increase in transport activity of 5 mM l-arabinose. Strains H4394 and H4395, with LAT2-GFP integrated into the CEN.PK2-1D and H2984 background, respectively, also exhibited higher pentitol transport than strain H3187 (activities for 10 mM ribitol, 10 mM l-arabitol, 0.2 mM ribitol, and 0.2 mM l-arabitol were increased by 7-, 15-, 25-, and 30-fold, respectively; data not shown in Fig. 4). Thus, tagging LAT2 with GFP increased pentitol transport in both the unmodified CEN.PK2-1D background and in strain H2984, which contains an integrated l-arabinose pathway (11). Strain H4400, with integrated LAT2-1/2GFP (i.e., approximately half the GFP gene fused to LAT2) showed similar increases in pentitol transport (1.6 and 0.71 μmol · min−1 · g [dry weight] yeast−1, respectively, at 10 mM ribitol and l-arabitol; data not shown in Fig. 4), so that a complete, functional, Gfp protein was not required to obtain increased pentitol transport.
The increased activity caused by C-terminal tags suggested that in S. cerevisiae unmodified Lat1 and Lat2 might be subject to inactivation targeted at their C termini. Lat2 (11; GenBank accession number AY923869) contains potential phosphorylation sites (including amino acids 528, 548, and 551) and ubiquitinylation sites (including amino acids 521, 540, and 546) potentially involved in endocytosis of the transporter (23). We attempted to test whether these sites were responsible for the low activity of LAT2 transformants by deleting the terminal 186 nucleotides coding for amino acids 491 to 552. However, strain H4399 containing this truncated LAT2 still had very low pentitol transport activity (<0.1 μmol · min−1 · g [dry weight] yeast−1 at 10 mM ribitol or l-arabitol).
Kinetic properties of the tagged Lat1 and Lat2 transporters.
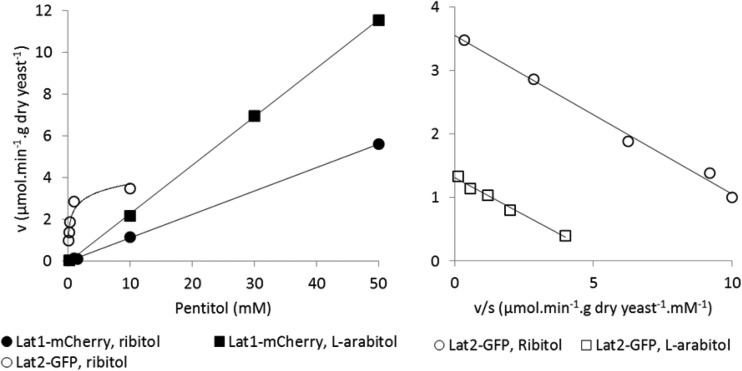
Strains bearing LAT2-GFP (either integrated or on a multicopy plasmid) transported l-arabitol and ribitol with Michaelis-Menten kinetics between 0.1 and 10 mM pentitol (Fig. 5). The Km for l-arabitol was 0.26 ± 0.13 mM (n = 2) for the strain with integrated LAT2-GFP and 0.21 ± 0.02 mM (n = 2) for the strain with LAT2-GFP on a multicopy plasmid. The Km for ribitol was 0.22 ± 0.03 mM (n = 3) for the strain with LAT2-GFP on a multicopy plasmid.
FIG 5.
Pentitol concentration dependency of Lat1 and Lat2 fusion proteins. (Left) Effect of substrate concentration on ribitol transport by strains H4388 and H4390, which bear LAT1-mCherry and LAT2-GFP, respectively, on multicopy plasmids, and on l-arabitol transport by strain H4388. The slopes of the trend lines for ribitol and l-arabitol transport by Lat1-mCherry are 0.11 and 0.23 μmol · min−1 · g (dry weight) yeast−1 · mM−1, respectively. (Right) Eadie-Hofstee plots (0.1 to 10 mM substrate) for ribitol transport by strain H4390 and l-arabitol transport by strain H4394 (integrated LAT2-GFP).
Strains bearing LAT2-GFP did not transport 20 mM l-arabinose faster than control strains when the l-arabinose was labeled with repurified l-[1-3H]arabinose (0.27 ± 0.05 μmol · min−1 · g [dry weight] yeast−1; n = 3) or with the commercial l-[1-14C]arabinose from Moravek (0.22 ± 0.17 μmol · min−1 · g [dry weight] yeast−1; n = 3). In contrast, higher apparent rates of transport were observed when the 20 mM l-arabinose was labeled with commercial (not repurified) l-[1-3H]arabinose from ARC (lot 110609; 5.3 ± 0.6 μmol · min−1 · g [dry weight] yeast−1; n = 7) or with commercial l-[1-14C]arabinose from ARC (lot 110523; 2.2 ± 0.4 μmol · min−1 · g [dry weight] yeast−1; n = 4). Similar results were obtained with Lat2-mCherry and Lat2-AK. These high apparent rates of transport were presumably caused by traces of radioactive impurities. This was confirmed for the commercial l-[1-3H]arabinose. During repurification of this reagent, 0.8% of the total radioactivity eluted in fraction 3 and 0.6% in fraction 10 (see above). “Labeling” 20 mM l-arabinose with fraction 3 or 10 caused very high (27 and 179 μmol · min−1 · g [dry weight] yeast−1) apparent rates of l-arabinose transport with the strain carrying LAT2-GFP.
Strain H4388, which bears LAT1-mCherry on a multicopy plasmid, did not show Michaelis-Menten kinetics toward ribitol and l-arabitol. Instead the transport rate was proportional to the pentitol concentration up to at least 50 mM, with first-order rate constants of 0.11 (ribitol) or 0.23 (l-arabitol) μmol · min−1 · g (dry weight) yeast−1 · mM−1 (Fig. 5). This strain transported l-arabinose labeled with repurified l-[1-3H]arabinose. Between 0.02 and 1 mM l-arabinose, the initial rates of uptake were much greater than those observed with strain CEN.PK2-1D bearing the empty plasmid and were fitted by a Km of about 0.03 mM and Vmax of 1.1 μmol · min−1 · g (dry weight) yeast−1 (data not shown). Above 5 mM l-arabinose, the strain (H4389) with the empty plasmid exhibited significant rates of (low-affinity) l-arabinose transport causing biphasic kinetics for l-arabinose transport by the strain bearing LAT1-mCherry. The biphasic nature of the kinetics of the LAT1-mCherry strain largely disappeared when the observed rates were corrected for the rate of the control strain (data not shown).
Lat1-mCherry and Lat2-GFP had different patterns of inhibition by various sugars and sugar alcohols, whereas Lat2-GFP and Lat2-AK behaved in the same way (Table 5). l-Arabinose (but not d-arabinose) strongly inhibited transport of ribitol and l-arabitol by Lat1-mCherry but did not inhibit transport of 10 mM ribitol by Lat2-GFP and Lat2-AK and only weakly inhibited transport of 0.2 mM ribitol by Lat2-GFP. This is consistent with the observations above that Lat1-mCherry is a high-affinity transporter of l-arabinose, whereas Lat2-GFP did not carry l-arabinose. d-Arabitol and xylitol did not inhibit transport of ribitol or l-arabitol by Lat1-mCherry. In contrast, d-arabitol and xylitol markedly inhibited the transport of ribitol by tagged Lat2. This would be expected if Lat2 has broad specificity and can carry all four pentitols. Transport of ribitol and l-arabitol by Lat1-mCherry was strongly inhibited by d-galactose and d-glucose, which had, however, little effect on ribitol transport by tagged Lat2. Several l-arabinose transporters can also carry d-galactose or d-glucose (9, 10), and Lat1 may belong to this group.
TABLE 5.
Effects of sugars and other sugar alcohols on the transport of pentitols by Lat1-mCherry, Lat2-GFP, and Lat2-AK
| Potential inhibitor | Concn (mM) | Transport activitya |
||||
|---|---|---|---|---|---|---|
| H4388 (Lat1-mCherry) |
H4390 (Lat2-GFP) |
H4403 (Lat2-AK) |
||||
| 10 mM ribitol | 10 mM l-arabitol | 10 mM ribitol | 0.2 mM ribitol | 10 mM ribitol | ||
| No inhibitor | 100 | 100 | 100 | 100 | 100 | |
| l-Arabinose | 2 | 13.8 ± 4.8 | 10.0 ± 0.2 | NA | NA | NA |
| 20 | 10.4 ± 2.0 | 1.7 ± 0.8 | 102 ± 4 | 97.1 ± 1.9 | NA | |
| 200 | 10.3 | 0 | 114 ± 12 | 84.6 ± 3.6 | 101 | |
| d-Arabinose | 20 | 95 | 82 | NA | NA | NA |
| d-Arabitol | 100 | NA | 94.3 ± 0.7 | NA | NA | NA |
| 20 | 100 ± 2 | 97.3 ± 5.0 | 90 | 15.2 ± 1.2 | 82.7 ± 3.7 | |
| Xylitol | 20 | 101 ± 3 | 96.8 ± 4.8 | 52 | 3.4 ± 0.4 | 56.3 ± 7.3 |
| d-Galactose | 20 | 22.2 ± 3.3 | 9.8 ± 1.4 | NA | 86.7 ± 6.7 | 98.0 ± 1.0 |
| d-Glucose | 20 | 19.4 ± 0.2 | 21 | NA | 95.5 ± 5.5 | NA |
Transport of ribitol (10 and 0.2 mM) and l-arabitol (10 mM) by strains H4388, H4390, and H4403, which bear the indicated tagged transporters, was measured in the presence or absence of potential inhibitors at the concentrations shown. In each column, the observed transport activities are shown as percentages of the activity in the absence of potential inhibitors. Data are averages of independent duplicates ± half the ranges or single experiments. NA, not analyzed.
DISCUSSION
S. cerevisiae carrying the unmodified genes LAT1 and LAT2 from Ambrosiozyma monospora exhibited transport activities of pentitols and l-arabinose that were barely greater than those of control strains. The increases in activity compared to controls were <3% of the transport activities observed in A. monospora (Table 4). The addition of mCherry, GFP, or AK tags increased the pentitol transport activities by 3- to 5-fold for S. cerevisiae carrying LAT1 and by more than 20-fold for S. cerevisiae carrying LAT2, resulting in specific activities 10 to 25% of those in A. monospora (Fig. 4) and permitting kinetic characterization of the transport activities. Lat1-mCherry was shown to be a high-affinity transporter of l-arabinose and low-affinity or nonsaturable transporter of l-arabitol and ribitol (Fig. 5). Lat2-GFP, Lat2-mCherry, and Lat2-AK were shown to be high-affinity transporters of these pentitols (Fig. 5) but to have little or no ability to transport l-arabinose.
These transport results were obtained by using repurified radioactive reagents to label the substrates. Two lots of commercial [14C]arabinose were shown to consist primarily of [14C]ribitol and l-[14C]arabitol. Knoshaug et al. (24) have reported that reagent from the same source was a mixture of l-[14C]arabinose, l-[14C]arabitol, and [14C]xylitol. In the present work, some other commercial lots of radioactive l-arabinose, which consisted primarily of [14C]arabinose or l-[3H]arabinose, nevertheless gave erroneously high transport results, which were shown in one case to be caused by traces (<1%) of radioactive impurities that were transported by tagged Lat2. Commercial radioactive maltotriose has also been shown to contain traces of labeled impurities that cause gross overestimation of maltotriose transport by S. cerevisiae (25). Clearly, radioactive transport assays can require very high (well over 99%) purity of the label in cases where the studied organism possesses transporters of the radioactive impurities.
The large increases in transport activity caused by tagging the heterologous Lat1 or Lat2 proteins contrast with findings that GFP tags have only small effects on the activity of homologous transporters in S. cerevisiae. Of two yeast glucose transporters, Hxt2-GFP had 30% greater Vmax (and 40% smaller Km) than unmodified Hxt2 (26), and Hxt7-GFP had the same Vmax (and 40% greater Km) as unmodified Hxt7 (27). The S. cerevisiae phosphate permease Pho84-GFP had a 50% smaller Vmax (and 25% smaller Km) than unmodified Pho84 (28). In this laboratory, the maltose transport activity of Agt1 transporters was found to be essentially unchanged by the addition of C-terminal GFP tags (29). However, the (heterologous) expression level of rat neurotensin in E. coli was increased up to 20-fold by the addition of a variety of C-terminal tags, the most effective including thioredoxin (1). These authors considered that improved stability was the probable mechanism and noted that thioredoxin itself is an unusually stable and compact protein. Mistic is a Bacillus subtilis protein that autonomously integrates into the cell membrane without using the translocon machinery. Mistic tags have been used to direct the integration of heterologous proteins into the B. subtilis membrane (3). We have not investigated the mechanism by which the present tags increased the transport activity of Lat1 and Lat2 proteins. Confocal fluorescence microscopy showed that Lat1-mCherry was predominantly located in the plasma membrane, whereas Lat2-GFP was both associated with the plasma membrane and diffusely spread through the intracellular space (see Fig. S1 in the supplemental material), but we do not know the distribution of untagged Lat1 and Lat2. The observation that tagging Lat2 with GFP, mCherry, or AK caused similar transport activity levels (Fig. 4) is consistent with improved trafficking of Lat2 to the plasma membrane and/or its stabilization after membrane insertion rather than an increase in the catalytic turnover number of individual Lat2 molecules (which would involve specific interactions between Lat2 and each tag). Like thioredoxin, Gfp (21 kDa), mCherry (27 kDa), and adenylate kinase (24 kDa) are relatively small, compact, and stable proteins. They may be useful as tags to facilitate the functional expression in yeast of other heterologous transporters.
Growth of A. monospora on l-arabinose instead of glucose caused the appearance of transport systems for l-arabinose and pentitols, and some of the properties of these transport systems correspond to the kinetic characteristics of tagged Lat1 and Lat2 as observed in S. cerevisiae. Thus, Lat1-mCherry was a high-affinity l-arabinose transporter that may account for the high-affinity transport of l-arabinose by A. monospora, although its Km (0.03 mM) was 3-fold smaller than that observed with A. monospora. Lat1-mCherry was also a low-affinity or nonsaturable transporter of ribitol and l-arabitol that may account for the low-affinity component of l-arabitol transport by A. monospora. The tagged Lat2 transporters, which cannot carry l-arabinose, appear to account for the high-affinity component of ribitol and l-arabitol transport by A. monospora. However, the characteristics of pentitol and l-arabinose transport by A. monospora cannot be completely explained by any combination of the observed characteristics of tagged Lat1 and Lat2 in S. cerevisiae. For example, although the specific activity of pentitol transport at 0.2 mM by S. cerevisiae carrying tagged Lat2 was over 50% of that seen in A. monospora (Fig. 4) and tagged Lat1 has negligible activity at 0.2 mM pentitol, Lat2 cannot account for all the A. monospora activity at 0.2 mM pentitol. This is because the A. monospora activity at 0.2 mM pentitol is partially (35%) inhibited by l-arabinose (Fig. 3), whereas tagged Lat2 activity toward pentitols is insensitive to l-arabinose (Table 5). Thus, A. monospora must also contain a high-affinity pentitol transporter that can be inhibited by l-arabinose. Second, for both A. monospora and tagged Lat1 in S. cerevisiae, the specific activity of l-arabinose transport at 5 mM was close to that of pentitol transport at 10 mM (Fig. 4), so that if Lat1 would account for all the l-arabinose transport of A. monospora, it would also account for all the pentitol transport at 10 mM. However, Lat2, which is required to explain pentitol transport by A. monospora at 0.2 mM, must also contribute a large fraction of its pentitol transport at 10 mM. We conclude that either the catalytic characteristics of Lat1 or Lat2 or both are radically altered by the addition of the C-terminal tags and expression in the S. cerevisiae cell membrane, or A. monospora contains at least one more l-arabinose transporter. Like Lat1 and Lat2, this transporter should be synthesized during growth of A. monospora on l-arabinose instead of glucose, and the arguments above suggest that it may also be a high-affinity pentitol transporter.
Supplementary Material
ACKNOWLEDGMENTS
We thank Christopher Landowski for his critical interest and for theoretical analysis of the C-terminal sequence of Lat2 and Virve Vidgren for the confocal fluorescence microscopy. We thank Tapani Suortti for analysis of commercial samples called l-[1-14C]arabinose and for purification of [14C]ribitol and l-[14C]arabitol and Juha-Pekka Pitkänen for analysis and purification of l-[1-3H]arabinose. The skilled technical assistance of Pirjo Tähtinen, Liisa Änäkäinen. and Marjo Öster is gratefully acknowledged.
We thank the Academy of Finland for financial support (no. 133854).
Footnotes
Published ahead of print 21 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.04067-13.
REFERENCES
- 1.Tucker J, Grisshammer R. 1996. Purification of a rat neurotensin receptor expressed in Escherichia coli. Biochem. J. 317:891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opekarová M, Tanner W. 2003. Specific lipid requirements of membrane proteins: a putative bottleneck in heterologous expression. Biochim. Biophys. Acta 1610:11–22. 10.1016/S0005-2736(02)00708-3 [DOI] [PubMed] [Google Scholar]
- 3.Roosild TP, Greenwald J, Vega M, Castronovo S, Riek R, Choe S. 2005. NMR structure of Mistic, a membrane-integrating protein for membrane protein expression. Science 307:1317–1321. 10.1126/science.1106392 [DOI] [PubMed] [Google Scholar]
- 4.Midgett CR, Madden DR. 2007. Breaking the bottleneck: eukaryotic membrane protein expression for high-resolution structural studies. J. Struct. Biol. 160:265–274. 10.1016/j.jsb.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 5.Becker J, Boles E. 2003. A modified Saccharomyces cerevisiae strain that consumes l-arabinose and produces ethanol. Appl. Environ. Microbiol. 69:4144–4150. 10.1128/AEM.69.7.4144-4150.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richard P, Verho R, Putkonen M, Londesborough J, Penttilä M. 2003. Production of ethanol from L-arabinose by Saccharomyces cerevisiae containing a fungal L-arabinose pathway. FEMS Yeast Res. 3:185–189. 10.1016/S1567-1356(02)00184-8 [DOI] [PubMed] [Google Scholar]
- 7.Wisselink HW, Toirkens MJ, del Rosario Franco Beriel M, Winkler AA, van Dijken JP, Pronk JT, van Maris AJ. 2007. Engineering of Saccharomyces cerevisiae for efficient anaerobic alcoholic fermentation of l-arabinose. Appl. Environ. Microbiol. 73:4881–4891. 10.1128/AEM.00177-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wisselink HW, Toirkens MJ, Wu O, Pronk JT, van Maris AJ. 2009. Novel evolutionary engineering approach for accelerated utilization of glucose, xylose, and arabinose mixtures by engineered Saccharomyces cerevisiae strains. Appl. Environ. Microbiol. 75:907–914. 10.1128/AEM.02268-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kou S-C, Christensen MS, Cirillo VP. 1970. Galactose transport in Saccharomyces cerevisiae. II. Characteristics of galactose uptake and exchange in galactokinaseless cells. J. Bacteriol. 103:671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subtil T, Boles E. 2011. Improving L-arabinose utilization of pentose fermenting Saccharomyces cerevisiae cells by heterologous expression of L-arabinose transporting sugar transporters. Biotechnol. Biofuels 4:38–47. 10.1186/1754-6834-4-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verho R, Penttilä M, Richard P. 2011. Cloning of two genes (LAT1,2) encoding specific l-arabinose transporters of the l-arabinose fermenting yeast Ambrosiozyma monospora. Appl. Biochem. Biotechnol. 164:604–611. 10.1007/s12010-011-9161-y [DOI] [PubMed] [Google Scholar]
- 12.Sweeley CC, Bentley R, Makita M, Wells WW. 1963. Gas-chromatography of trimethyl derivatives of sugars and related substances. J. Am. Chem. Soc. 85:2497–2507. 10.1021/ja00899a032 [DOI] [Google Scholar]
- 13.Pöggeler S, Masloff S, Hoff B, Mayrhofer S, Kück U. 2003. Versatile EGFP reporter plasmids for cellular localization of recombinant gene products in filamentous fungi. Curr. Genet. 43:54–61 [DOI] [PubMed] [Google Scholar]
- 14.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 12:1524–1525 [DOI] [PubMed] [Google Scholar]
- 15.Verho R, Putkonen M, Londesborough J, Penttilä M, Richard P. 2004. A novel NADH-linked l-xylulose reductase in the l-arabinose catabolic pathway of yeast. J. Biol. Chem. 279:14746–14751. 10.1074/jbc.M312533200 [DOI] [PubMed] [Google Scholar]
- 16.Winston F, Dollard C, Ricupero-Hovasse SL. 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11:53–55. 10.1002/yea.320110107 [DOI] [PubMed] [Google Scholar]
- 17.Oldenburg KR, Vo KT, Michaelis S, Paddon C. 1997. Recombination-mediated PCR-directed plasmid construction in vivo in yeast. Nucleic Acids Res. 25:451–452. 10.1093/nar/25.2.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voth WP, Richards JD, Shaw JM, Stillman DJ. 2001. Yeast vectors for integration at the HO locus. Nucleic Acids Res. 29:E59. 10.1093/nar/29.12.e59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherman F, Fink G, Hicks JB. 1983. Methods in yeast genetics. A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 20.Serrano R. 1977. Energy requirements for maltose transport in yeast. Eur. J. Biochem. 80:97–102. 10.1111/j.1432-1033.1977.tb11861.x [DOI] [PubMed] [Google Scholar]
- 21.Guimarães PMR, Multanen J-P, Domingues L, Teixeira JA, Londesborough J. 2008. Stimulation of zero-trans rates of lactose and maltose uptake into yeasts by preincubation with hexose to increase the adenylate energy charge. Appl. Environ. Microbiol. 74:3076–3084. 10.1128/AEM.00188-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenthal R, Cornish-Bowden A. 1974. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem. J. 139:715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicke L, Dunn R. 2003. Regulations of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19:141–192. 10.1146/annurev.cellbio.19.110701.154617 [DOI] [PubMed] [Google Scholar]
- 24.Knoshaug EP, Franden MA, Stambuk BU, Zhang M, Singh A. 2009. Utilization and transport of l-arabinose by non-Saccharomyces yeasts. Cellulose 16:729–741. 10.1007/s10570-009-9319-8 [DOI] [Google Scholar]
- 25.Dietvorst J, Londesborough J, Steensma HY. 2005. Maltose utilization in lager yeast strains: MTT1 encodes a maltotriose transporter. Yeast 22:775–788. 10.1002/yea.1279 [DOI] [PubMed] [Google Scholar]
- 26.Kruckeberg AL, Ye L, Berden JA, van Dam K. 1999. Functional expression, quantification and cellular localization of the Hxt2 hexose transporter of Saccharomyces cerevisiae tagged with a green fluorescent protein. Biochem. J. 339:299–307. 10.1042/0264-6021:3390299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye L, Berden JA, van Dam K, Kruckeberg AL. 2001. Expression and activity of the Hxt7 high-affinity hexose transporter of Saccharomyces cerevisiae. Yeast 18:1257–1267. 10.1002/yea.771 [DOI] [PubMed] [Google Scholar]
- 28.Petersson J, Pattison J, Kruckeberg AL, Berden JA, Persson BL. 1999. Intracellular localization of an active green fluorescent protein-tagged Pho84 phosphate permease in Saccharomyces cerevisiae. FEBS Lett. 462:37–42. 10.1016/S0014-5793(99)01471-4 [DOI] [PubMed] [Google Scholar]
- 29.Vidgren V, Viljanen K, Mattinen L, Rautio J, Londesborough J. Three Agt1 transporters from brewer's yeasts exhibit different temperature-dependencies for maltose transport over the range of brewery temperatures (0°C to 20°C). FEMS Yeast Res., in press [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.