Abstract
Acm2, the major autolysin of Lactobacillus plantarum WCFS1, was recently found to be O-glycosylated with N-acetylhexosamine, likely N-acetylglucosamine (GlcNAc). In this study, we set out to identify the glycosylation machinery by employing a comparative genomics approach to identify Gtf1 homologues, which are involved in fimbria-associated protein 1 (Fap1) glycosylation in Streptococcus parasanguinis. This in silico approach resulted in the identification of 6 candidate L. plantarum WCFS1 genes with significant homology to Gtf1, namely, tagE1 to tagE6. These candidate genes were targeted by systematic gene deletion, followed by assessment of the consequences on glycosylation of Acm2. We observed a changed mobility of Acm2 on SDS-PAGE in the tagE5E6 deletion strain, while deletion of other tagE genes resulted in Acm2 mobility comparable to that of the wild type. Subsequent mass spectrometry analysis of excised and in-gel-digested Acm2 confirmed the loss of glycosylation on Acm2 in the tagE5E6 deletion mutant, whereas a lectin blot using GlcNAc-specific succinylated wheat germ agglutinin (sWGA) revealed that besides Acm2, tagE5E6 deletion also abolished all but one other sWGA-reactive, protease-sensitive signal. Only complementation of both tagE5 and tagE6 restored those sWGA lectin signals, establishing that TagE5 and TagE6 are both required for the glycosylation of Acm2 as well as the vast majority of other sWGA-reactive proteins. Finally, sWGA lectin blotting experiments using a panel of 8 other L. plantarum strains revealed that protein glycosylation is a common feature in L. plantarum strains. With the establishment of these enzymes as protein glycosyltransferases, we propose to rename TagE5 and TagE6 as GtfA and GtfB, respectively.
INTRODUCTION
Probiotics, of which the majority belong to the genera Lactobacillus and Bifidobacterium (1–3), have been defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (4). One mechanism by which these health benefits are mediated is through molecular interactions between probiotic bacteria and host cells, in which bacterial surface molecules appear to play a pivotal role (1–3). These surface effector molecules include canonical polymers such as wall teichoic acids (WTA) and lipoteichoic acids (LTA), peptidoglycan, and capsular polysaccharides, but also proteinaceous molecules (2, 5–7).
Many proteinaceous molecules have established functions associated with adhesion to intestinal mucus, such as the mucin-binding proteins (Mub) of Lactobacillus acidophilus NCFM (8) and Lactobacillus reuteri 1063 (9), a mucus adhesion-promoting protein (MapA) of L. reuteri 104R (10), and the mannose-specific adhesin (Msa) of Lactobacillus plantarum WCFS1 (11). Examples of proteins involved in adhesion to epithelial cells include the surface layer proteins of Lactobacillus brevis ATCC 8287 (12), Lactobacillus crispatus JCM 5810 (13), and Lactobacillus helveticus R0052 (14). In addition to their role in the capacity for adhesion to mucus and epithelial cells, some Lactobacillus surface proteins are able to bind with extracellular matrix (ECM), which is a complex structure surrounding epithelial cells and composed of various proteins, including laminin, collagen, and fibronectin. Reported examples include the collagen-binding protein of L. reuteri NCIB11951 (15) and fibronectin-binding protein A of L. acidophilus NCFM (8).
Other surface proteins have an impact on probiotic-host interactions via their immunomodulating capacity, for example, Msp1 and Msp2, two peptidoglycan hydrolases of Lactobacillus rhamnosus GG which promote epithelial homeostasis (16, 17). Recombinant Msp2 was also shown to prevent and ameliorate experimental colitis in mice by an epidermal growth factor receptor-dependent mechanism (18). Furthermore, surface layer protein A (SlpA) of L. acidophilus NCFM was documented to be recognized by the dendritic cell-specific ICAM-3-grabbing nonintegrin (DC-SIGN) receptor and as a consequence modulates human DCs and T cell functions, leading to regulatory T cell differentiation through increased interleukin 10 (IL-10) and reduced IL-12p70 production (19). A serine- and threonine-rich peptide (STp) harbored by protein D1 that is secreted by Lactobacillus plantarum BMCM12 represents another example of a proteinaceous effector molecule, as it was recently demonstrated to stimulate regulatory responses in human intestinal DCs (20).
The most common modification found in proteinaceous molecules is glycosylation, in which glycans can be attached to the amide nitrogen of asparagine, i.e., N-glycosylation, or to the hydroxyl oxygen of serine or threonine, i.e., O-glycosylation (21). Although protein glycosylation was initially studied exclusively for eukaryotes, bacterial protein glycosylation has recently received increasing attention, and it is now clear that bacteria can also modify proteins with diverse N-linked and O-linked glycan moieties (22–26). So far, most studies on bacterial protein glycosylation have focused on pathogenic organisms (23, 27–29), resulting in the identification of general glycosylation pathways (26), including an N-glycosylation pathway in Campylobacter jejuni (30, 31) and O-linked glycosylation systems in Neisseria gonorrhoeae (32, 33). Specific machineries responsible for O-glycosylation of abundant surface proteins such as flagellin and pilin have also been described for various pathogenic bacteria (34–36). Moreover, fimbria-associated protein 1 (Fap1), a serine-rich adhesin of Streptococcus parasanguinis, has been demonstrated to be heavily glycosylated with N-acetylglucosamine (GlcNAc) and glucose (37, 38). This glycosylation requires the concerted activities of two putative glycosyltransferases; Gtf1 and Gtf2 (37). More recent studies pinpointed that protein glycosylation also occurs in certain human intestine commensals, including several Bacteroides species (39, 40), and probiotic species such as L. plantarum WCFS1 (41, 42) and L. rhamnosus GG (43). More specifically, Msp1 of L. rhamnosus GG is O-glycosylated at serine residues 106 and 107, and its glycan moieties are recognized by the concanavalin A (ConA) lectin, which is specific for mannose and/or glucose moieties (43). Similarly, the major autolysin of L. plantarum WCFS1, Acm2, was shown to be O-glycosylated in its N-terminal alanine-, serine-, and threonine-rich region (AST domain), which could be selectively detected by using the GlcNAc-specific biotinylated succinylated wheat germ agglutinin (sWGA) lectin (41, 42). Intriguingly, AST domains are present in several other proteins encoded in the L. plantarum WCFS1 genome, including several other peptidoglycan hydrolases (41) and Lp_2145 (44), suggesting that these proteins could also be subjected to glycosylation (41). Indeed, a recent study found 10 novel glycoproteins in L. plantarum WCFS1, including 2 AST domain-containing peptidoglycan hydrolases (Lp_2162 and Lp_3421), 4 cytoplasmic proteins (DnaK, ELp_2152, FtsY, and FtsK1), and the secreted proteins Lp_2260 and Lp_1643 (45).
To date, no protein glycosylation machinery has been described for Lactobacillus species (41, 43). In this study, we employed a comparative genomics approach to identify Gtf1 homologues in the genome of L. plantarum WCFS1, resulting in the identification of 6 candidate genes [previously annotated as poly(glycerolphosphate) α-glucosyltransferases, i.e., tagE1 to tagE6] that might encode protein glycosyltransferases (46). These candidate genes were targeted by a gene deletion and complementation approach, after which we assessed the consequences of these genetic modifications for the presence of glycan moieties in proteins by employing the GlcNAc-specific lectin sWGA in blotting experiments. Moreover, we specifically assessed the impact of tagE5E6 deletion on the previously established glycosylation of Acm2 (41, 42) by mass spectrometry analysis (MS). These experiments revealed that TagE5 and TagE6 are both required for the glycosylation of proteins, including Acm2, in L. plantarum WCFS1. Moreover, expansion of our lectin blotting experiments to a panel of other L. plantarum strains revealed that protein glycosylation is widespread in this species. To the best of our knowledge, these results represent the first example of protein glycosylation machinery in a Lactobacillus species.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Lactobacillus plantarum strains were grown at 37°C in MRS broth (Difco, West Molesey, United Kingdom) without aeration. Escherichia coli strain TOP10 (Invitrogen, Bleiswijk, the Netherlands) was grown at 37°C in tryptone yeast broth (47) with aeration (48). Solid media were prepared by adding 1.5% (wt/vol) agar to the broths. Where appropriate, antibiotics were added for L. plantarum and E. coli at 10 μg/ml of chloramphenicol and 30 and 200 μg/ml of erythromycin, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristic(s)a | Reference or sourceb |
|---|---|---|
| Strains | ||
| L. plantarum | ||
| WCFS1 | Single-colony isolate of L. plantarum NCIMB8826 | 46 |
| ATCC 14917 | Isolate from pickled cabbage | ATCC |
| ATCC 8014 | Isolate from maize ensilage | ATCC |
| CIP104440 | Isolate from human stool | CIP |
| CIP104450 | Isolate from human stool | CIP |
| NC8 | Isolate from grass silage | 77 |
| NCIMB12120 | Isolate from Ogi, Nigeria | NCIMB |
| LP80 | Isolate from silage | 78 |
| LP85-2 | Origin from silage, France | 57 |
| Δacm2 mutant | NZ3557Cm; Cmr; derivative of WCFS1 containing a lox66-P32-cat-lox71-tagH9 replacement of acm2 (acm2::lox66-P32-cat-lox71-tagH9) | 41 |
| ΔtagE1 mutant | NZ3540Cm; Cmr; derivative of WCFS1 containing a lox66-P32-cat-lox71-tag8.5 replacement of tagE1 (tagE1:: lox66-P32-cat-lox71-tag8.5) | This study |
| ΔtagE2E3 mutant | NZ3541Cm; Cmr; derivative of WCFS1 containing a lox66-P32-cat-lox71-tagF10 replacement of tagE2E3 (tagE2E3::lox66-P32-cat-lox71-tagF10) | This study |
| ΔtagE4 mutant | NZ3542Cm; Cmr; derivative of WCFS1 containing a lox66-P32-cat-lox71-tagG1 replacement of tagE4 (tagE4:: lox66-P32-cat-lox71-tagG1) | This study |
| ΔtagE5E6 mutant | NZ3543Cm; Cmr; derivative of WCFS1 containing a lox66-P32-cat-lox71-tagG7 replacement of tagE5E6 (tagE5E6::lox66-P32-cat-lox71-tagG7) | This study |
| tagE5E6 complementation mutant | NZ8204CmEm; Cmr Emr; derivative of NZ3543Cm containing chromosomally integrated pNZ8204 at tRNASer site | This study |
| tagE6 complementation mutant | NZ8205CmEm; Cmr Emr; derivative of NZ3543Cm containing chromosomally integrated pNZ8205 at tRNASer site | This study |
| tagE5 complementation mutant | NZ8206CmEm; Cmr Emr; derivative of NZ3543Cm containing chromosomally integrated pNZ8206 at tRNASer site | This study |
| E. coli | ||
| TOP 10 | Cloning host; F− mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| Plasmids | ||
| pNZ5319 | Cmr Emr; mutagenesis vector for gene replacements in L. plantarum | 50 |
| pNZ3540 | Cmr Emr; pNZ5319 derivative containing homologous regions up- and downstream of tagE1 | This study |
| pNZ3541 | Cmr Emr; pNZ5319 derivative containing homologous regions up- and downstream of tagE2-E3 | This study |
| pNZ3542 | Cmr Emr; pNZ5319 derivative containing homologous regions up- and downstream of tagE4 | This study |
| pNZ3543 | Cmr Emr; pNZ5319 derivative containing homologous regions up- and downstream of tagE5E6 | This study |
| pMEC10 | Emr; integration plasmid | 79 |
| pNZ8204 | Emr; pMEC10 derivative harboring tagE5E6 | This study |
| pNZ8205 | Emr; pMEC10 derivative harboring tagE6 | This study |
| pNZ8206 | Emr; pMEC10 derivative harboring tagE5 | This study |
Cmr, chloramphenicol resistant; Emr, erythromycin resistant; Strr, streptomycin resistant.
ATCC, American Type Culture Collection, Manassas, VA; CIP, Collection de l'Institut Pasteur, Paris, France; NCIMB, National Collections of Industrial, Food and Marine Bacteria, Aberdeen, Scotland.
DNA manipulations.
Primers used are listed in Table 2 and were synthesized by Sigma-Aldrich (Zwijndrecht, the Netherlands). Standard procedures were used for DNA manipulations in E. coli (48). Plasmid DNA was isolated from E. coli using a JETSTAR kit (Genomed GmbH, Bad Oberhausen, Germany). L. plantarum DNA was isolated and transformed as described previously (49). PCR amplifications were performed using hot-start KOD polymerase (Novagen, Madison, WI). Amplicons were purified using the WizardSV Gel and PCR Clean-Up System (Promega, Leiden, the Netherlands). Restriction endonucleases (Fermentas GmbH, St. Leon-Rot, Germany), MSB Spin PCRapace (Invitek GmbH, Berlin, Germany), PCR master mix (Promega), and T4 DNA ligase (Invitrogen) were used as specified by the manufacturers.
TABLE 2.
Primers used in this study
| Primer | Sequencea | Reference or source |
|---|---|---|
| is128 tag-lox66-F3 | 5′-AAATCTACCGTTCGTATAATGTATG-3′ | 80 |
| is129 tag-lox71-R3 | 5′-CTCATGCCCGGGCTGTAACCG-3′ | 80 |
| 87 | 5′-GCCGACTGTACTTTCGGATCC-3′ | 50 |
| CreF | 5′-CGATACCGTTTACGAAATTGG-3′ | 50 |
| CreR | 5′-CTTGCTCATAAGTAACGGTAC-3′ | 50 |
| EryintF | 5′-TCAAATACAGCTTTTAGAACTGG-3′ | 50 |
| EryintR | 5′-ATCACAAACAGAATGATGTACC-3′ | 50 |
| tagE1-Up-F | 5′-GCCGCAACAACCATACTGGG-3′ | This study |
| tagE1-Up-R | 5′-GCATACATTATACGAACGGTAGATTTAAAATAATACATCACCTAGCCG-3′ | This study |
| tagE1-Down-F | 5′-CGGTTACAGCCCGGGCATGAGATAGCAGCACTTTAAGAACTGG-3′ | This study |
| tagE1-Down-R | 5′-GCGATTACATCGCCTTGGCG-3′ | This study |
| tagE1-out-F | 5′-GCTAGTCATGTCACGGATGC-3′ | This study |
| tagE1-out-R | 5′-TCACTCACAATAAATTCCCCC-3′ | This study |
| tagE2E3-Up-F | 5′-GCAATTACAATGTTGTGCGGC-3′ | This study |
| tagE2E3-Up-R | 5′-GCATACATTATACGAACGGTAGATTTGAAGTAAAACATACAGTCACCC-3′ | This study |
| tagE2E3-Down-F | 5′-CGGTTACAGCCCGGGCATGAGACGGCTTAAGTAGATTTGACGG-3′ | This study |
| tagE2E3-Down-R | 5′-AAGTGCGCGTTTTAGTACGC-3′ | This study |
| tagE2E3-out-F | 5′-TACGGTTATTTTCCGGCTCG-3′ | This study |
| tagE2E3-out-R | 5′-ATCGGTGGCCTTTTACTTGG-3′ | This study |
| tagE4-Up-F | 5′-CGTATCGATTGTTGACAGCG-3′ | This study |
| tagE4-Up-R | 5′-GCATACATTATACGAACGGTAGATTTATCGGCTAAACAACCACATGC-3′ | This study |
| tagE4-Down-F | 5′-CGGTTACAGCCCGGGCATGAGGAAATACATTTGCTACGCCCC-3′ | This study |
| tagE4-Down-R | 5′-CGAAGTGACGACTGCAAACG-3′ | This study |
| tagE4-out-F | 5′-CTTTCGTAGCCAAAATCGACG-3′ | This study |
| tagE4-out-R | 5′-CAAGAACAAGTCACAGCCGC-3′ | This study |
| tagE5E6-Up-F | 5′-ATTGGAAACGTTCTGTGCGG-3′ | This study |
| tagE5E6-Up-R | 5′-GCATACATTATACGAACGGTAGATTTGTTGTTCAGTGAATATCAAAAATGG-3′ | This study |
| tagE5E6-Down-F | 5′-CGGTTACAGCCCGGGCATGAGATAATACATTATTACTCGCTCCC-3′ | This study |
| tagE5E6-Down-R | 5′-AGTTGTTGATGAACTGCTGC-3′ | This study |
| tagE5E6-out-F | 5′-AAATAATAGTTAGGGGTGAACAC-3′ | This study |
| tagE5E6-out-R | 5′-CTTCAGCACTACTTGATGTGC-3′ | This study |
| tRNA | 5′-GCGAACCGGCTAATACCGGC-3′ | 81 |
| IC013 | 5′-AGCTAACAGACCGGTAGCTGCCAATGAAG-3′ | This study |
| IC014 | 5′-AACCAGAGCTCCTGGCTGCTACGTGAACCTAATTCC-3′ | This study |
| IC015 | 5′-TTTCCGAGCTCGCGTTACTAGTTTAGCCGGTGCCTG-3′ | This study |
| IC016 | 5′-TATTGGTTCACAAAAAAATTCATTATTACTCGCTCCCTTACACGA-3′ | This study |
| IC017 | 5′-CGTGTAAGGGAGCGAGTAATAATGAATTTTTTTGTGAACCAATATT-3′ | This study |
| IC021 | 5′-ACGCCACATGCAGTCGATCC-3′ | This study |
| IS169 | 5′-TTATCATATCCCGAGGACCG-3′ | 82 |
| IS247 | 5′-AGATTGTACTGAGAGTGCACC-3′ | This study |
| IS260 | 5′-GTTGAAAGAACCTGTACTCTCC-3′ | This study |
Underlined nucleotides indicate parts of the primers that are complementary to the is128-lox66-F3 and is129-lox71-R3 primers.
Construction of tagE deletion mutants.
The tagE deletion mutants were constructed as described previously (50), using a double-crossover strategy to replace the target tagE genes with a chloramphenicol resistance cassette (lox66-P32cat-lox71) (50). In this study, a derivative of the mutagenesis vector pNZ5319 (50), designated pNZ5319TAG (P. A. Bron et al., unpublished data), was used to introduce a unique DNA tag into the chromosome during gene deletion, which can be used for mutant tracking purposes in mixed populations (not relevant for the study presented here). The upstream and downstream flanking regions of each tagE gene set (tagE1, tagE2E3, tagE4, and tagE5E6) were amplified by PCR using tagEs-Up-F/R and tagEs-Down-F/R primers, respectively (Table 2). Each amplicon generated was subsequently joined by a second PCR to tag-lox66-P32cat-lox71 by a splicing by overlap extension strategy (51), using tagE-Up-F/tagE-Down-R primer pairs (Table 2). The resulting PCR products were digested with SwaI and Ecl136II and cloned into similarly digested pNZ5319TAG. The obtained mutagenesis plasmids were transformed into L. plantarum WCFS1 as described previously (49). The resulting integrants were assessed for a double-crossover integration event by using tagE-out-F/R primers (Table 2). For each of the mutant constructions a single colony displaying the anticipated genotype was selected, yielding the mutants NZ3540Cm (ΔtagE1), NZ3541Cm (ΔtagE2E3), NZ3542Cm (ΔtagE4), and NZ3543Cm (ΔtagE5E6).
Complementation of ΔtagE5E6.
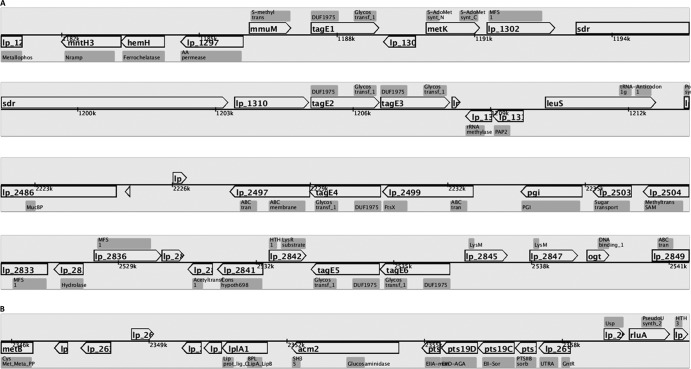
The genomic organization of tagE5 and tagE6 (lp_2843-2844) is shown in Fig. 1A. The tagE5E6 genes and the individual tagE6 gene of L. plantarum WCFS1 were amplified, including their native promoter (PtagE6, upstream of tagE6), using primers IC013/IC014 and IC013/IC015, respectively. Since tagE5 is also transcribed from the tagE6 promoter, the PtagE6 promoter and tagE5 were joined by splicing using an overlap extension strategy (51). The promoter was amplified by using primers IC013/IC016; primer IC016 contains the initial 23 nucleotides of tagE5. The tagE5 gene was amplified using primer IC017, which contains the terminal 21 nucleotides of the promoter region, and primer IC014. The two PCR products were mixed in a molar ratio of 1:1 and amplified using primers IC013/IC014 to join the promoter and tagE5. A SacI site was introduced by primer IC014 and IC015 downstream of tagE6 and tagE5, respectively. pMEC10 was digested by SacI and SfoI, whereas PCR products of tagE5E6, tagE6, and tagE5 were digested with SacI. Digested fragments were ligated using T4 DNA ligase. Subsequently, the ligation mixtures were transformed into E. coli TOP10; positive clones were selected by colony PCR (52) using primers IC013/IC015 for tagE6, IC014/IC017 for tagE5, and IS260/IS247 for tagE5E6. Resulting plasmids were designated pNZ8204, pNZ8205, and pNZ8206 for the complementation plasmid of tagE5E6, tagE6, and tagE5, respectively. Integrity of nucleotide sequences for each construct was confirmed by sequence analysis. Subsequently, the complementation plasmids were introduced into the ΔtagE5E6 strain by electroporation as described previously (49). Transformants were screened for chloramphenicol and erythromycin resistance, followed by PCR amplifications to confirm the chromosomal integration of introduced plasmid using primers tRNA/IC021 for NZ8204 and NZ8205 and primers tRNA/IC020 for NZ8206.
FIG 1.
Genetic organization views of tagE1-E6 and their neighboring genes (A) and acm2 (B) generated by Microbial Genomic context Viewer (MGcV) (76). The annotated Pfam domains of each gene are shown in gray.
Preparation of surface proteins and whole-cell extracts and proteinase K treatment.
Overnight cultures of L. plantarum strains were diluted in fresh MRS broth to an optical density at 600 nm (OD600) of 0.1. After 5 h of incubation at 37°C (OD600 of approximately 1.0), the exact OD600s of the cultures were determined and cells were harvested by centrifugation at 3,000 × g for 10 min at 4°C. For surface protein isolation, a procedure adapted from that of Fredriksen et al. (41) was used. Briefly, harvested cells were washed once with phosphate-buffered saline (PBS) to remove residual medium and resuspended in 1 ml of cold PBS. Surface proteins were extracted by gentle agitation at 600 rpm for 30 min using an Eppendorf thermomixer (Eppendorf, Hamburg, Germany). The supernatants were collected after centrifugation at 5,000 × g for 10 min. The surface proteins were precipitated from supernatants by addition of trichloroacetic acid (TCA) to a final concentration of 16% and an overnight incubation at 4°C, followed by centrifugation at 16,000 × g for 15 min. The precipitated proteins were washed with 200 μl of acetone and then air dried with open lids at 50°C. Dried protein pellets were solubilized in NuPAGE loading buffer and reducing agent (both from Invitrogen). The NuPAGE buffer volumes were normalized by OD600 measurement of original cultures to ensure that the samples represent the surface proteins from similar amounts of cells, and these samples were subsequently used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis and Coomassie brilliant blue staining (48).
For whole-cell extract samples, harvested cells were washed once with 50 mM sodium phosphate buffer, pH 7, to remove residual medium and subsequently resuspended in 50 mM sodium phosphate buffer, pH 7, to a final OD600 equivalent of 2.5. Cell suspensions of 1 ml were added to a screw-cap 2-ml tube containing 1-g zirconium beads. Cells were disrupted by three rounds of bead beating (30 s at speed 4.0) using a Fastprep cell disrupter (QBiogene Inc., Cedex, France), interspaced with cooling intervals on ice. The tubes were left for 5 min to allow zirconium bead sedimentation. The resulting supernatants were collected as whole-cell extracts and used in sWGA lectin blot experiments.
For proteinase K treatment, the whole-cell extract samples were treated with proteinase K (Qiagen GmbH, Hilden, Germany; final concentration of 50 μg/ml) for 10, 30, or 60 min at 37°C.
SDS-PAGE and lectin blot analyses.
SDS-PAGE and wet blotting were performed using the NuPAGE electrophoresis system (Invitrogen) and XCell II blot module (Invitrogen), respectively, as described in the user manuals. Whole-cell extracts were mixed with NuPAGE sample buffer and were separated under denaturing conditions on NuPAGENovex 4 to 12% bis-Tris gels with morpholinepropanesulfonic acid (MOPS) SDS running buffer (Invitrogen).
For visualization of surface proteins by Coomassie brilliant blue, the standard procedure was used (48).
For lectin blotting, the gels were transferred to nitrocellulose membranes (Thermo Scientific, Bremen, Germany) using a wet blotting method described in the NuPAGE manual (Invitrogen). The membranes were blocked with 3% bovine serum albumin (BSA) in PBS containing 0.05% Tween 20 (PBST) for 1 h at room temperature. The membranes were then incubated with biotinylated sWGA (Vector Laboratories, Burlingame, CA; final concentration of 14.3 μg/ml), Dolichos biflorus lectin (Sigma-Aldrich, Zwijndrecht, the Netherlands; final concentration of 14.3 μg/ml), or Lens culinaris lectin (EY Labs Inc., San Mateo, CA; final concentration of 5 μg/ml) in the blocking solution, followed by incubation with 0.1 nl/ml (1:10,000 dilution) of streptavidin poly-horseradish peroxidase (poly-HRP; ImmunoTools GmbH, Friesoythe, Germany). In between the incubations, the membranes were washed three times with PBST for 15 min. Precision Plus protein dual color standards (Bio-Rad, Richmond, CA) were used as a reference of molecular size. RNase B (New England BioLabs, Ipswich, MA) was used as a positive control for sWGA blotting. After the membranes were washed, they were developed by using Super Signal West Pico chemiluminescent substrate (Thermo Scientific) and Kodak BioMax Light film (Kodak, Rochester, NY).
Mass spectrometry.
The protein bands apparent around 100 kDa were excised from a Coomassie blue-stained gel (see above), followed by characterization of the glycosylation pattern using the same method as described by Rolain et al. (42). Briefly, the protein was in-gel digested with trypsin (Promega) for 16 h at 37°C. Digested peptides were recovered and vacuum dried (Speedvac SC200; Savant). Peptides were then dissolved in 0.025% (vol/vol) trifluoroacetic acid (TFA) and 5% (vol/vol) acetonitrile (ACN), desalted using a C18 Pep Map 100 precolumn (10 mm, 5-μm inside diameter [i.d.], 100 Å), and subsequently subjected to reverse-phase chromatography using Ultimate 3000 chromatography chain (LC Packings) with a C18 Pep Map 100 analytical column (150 mm, 3-μm i.d., 100 Å). Peptides were back-flushed onto the analytical column with a flow rate of 300 nl/min using a 180-min linear gradient from 8 to 76% (vol/vol) ACN in water containing 0.1% (vol/vol) TFA in 4% ACN–0.1% TFA and 0.085% (vol/vol) TFA in 80% ACN–0.1% TFA. The eluted peptides were mixed with α-cyano-4-hydrocinnamic acid (4 mg/ml in 70% ACN-0.1% TFA) and spotted directly onto a matrix-assisted laser desorption ionization (MALDI) target using a Probot system (LC Packings). The spotted plates were analyzed in reflector mode on an Applied Biosystems 4800 MALDI–time of flight (TOF)/TOF analyzer using a 200-Hz solid-state laser operating at 355 nm. MS spectra were obtained using a laser intensity of 3,600 and 2,000 laser shots per spot in the m/z range of 800 to 4,000, while MS/MS spectra were obtained by automatic selection of the 20 most intense precursor ions per spot using a laser intensity of 4,000 and 2,000 laser shots per precursor. Collision-induced dissociation was performed with an energy of 1 kV with air gas at a pressure of 1 × 106 torr. Data were collected using Applied Biosystems 4000 series Explorer software. LC-MS/MS data were processed using Applied Biosystems GPS Explorer 3.6 software.
For peptide identification, a local database containing Acm2 sequence was used, with the tolerance set to 200 ppm on the precursors and 0.3 Da on the fragments. One trypsin miscleavage was authorized. For modifications, methionine oxidation and N-acetylhexosamine (HexNAc) glycosylation (203.08 Da) on Ser, Thr, and Asn were selected. HexNAc-modified peptides were checked by manual de novo sequencing on the MS/MS fragmentation spectra. The data presented for the wild type (WT) were combined from 2 independent analyses, while those for the tagE5E6 deletion mutant were combined from 3 independent analyses.
RESULTS
Comparative genomics and mutagenesis of candidate protein glycosyltransferases.
Acm2 of Lactobacillus plantarum WCFS1 was previously established to be O-glycosylated with N-acetylhexosamines, most likely GlcNAc, at multiple positions in its AST domain (41, 42). Glycosylation with GlcNAc was also found in flagellin of Listeria monocytogenes (35) and Fap1 of S. parasanguinis (37, 38). The glycosylation with GlcNAc moieties in the latter species requires two genetically coupled functions, Gtf1 and Gtf2 (37). Bu et al. and Wu and Wu suggested that Gtf1 catalyzes GlcNAc glycosylation via its C-terminal glycosyltransferase domain, while Gtf2 might act as a chaperone to maintain correct folding of Gtf1 and to promote efficient glycosylation (37, 53). Based on these previous findings, we performed a BLASTP analysis (54, 55) using the Gtf1 sequence to identify candidate protein glycosyltransferases in the L. plantarum WCFS1 genome. Six genes (tagE1 to tagE6) which are annotated as poly(glycerolphosphate) α-glucosyltransferases, and consequently are thought to be involved in teichoic acid glycosylation (46), appeared the closest homologues of the Gtf1 protein, and all share more than 20% sequence identity with Gtf1. Two pairs of tagE genes are genetically coupled in the L. plantarum chromosome (tagE2-tagE3 and tagE5-tagE6) (Fig. 1). Therefore, all 6 tagE genes identified were targeted by gene deletion, with the notion that the genetically coupled tagE pairs were deleted jointly. This genetic engineering approach yielded four L. plantarum WCFS1 derivatives, NZ3540Cm (ΔtagE1), NZ3541Cm (ΔtagE2E3), NZ3542Cm (ΔtagE4), and NZ3543Cm (ΔtagE5E6).
Deletion of tagE5 and tagE6 abolishes protein glycosylation in L. plantarum WCFS1.
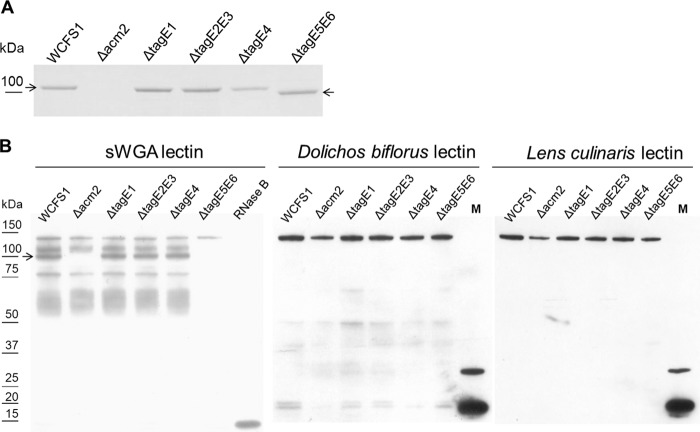
Surface proteins derived from the tagE deletion mutants, as well as the wild type and acm2 deletion mutant (41), were analyzed by SDS-PAGE. One protein band detected in the wild-type extract appeared to be absent in the sample derived from the acm2 deletion derivative, suggesting that this protein band represents Acm2 (Fig. 2A). To substantiate this suggestion, the band representing Acm2 was excised and in-gel digested with trypsin, and the resulting peptides were extracted and subjected to liquid chromatography coupled to mass spectrometry analysis (LC-MS/MS), which generated MS spectra that represented 75% and 69% coverages of the mature Acm2 protein sequence and its N-terminal glycosylated AST domain, respectively (see Fig. S1A in the supplemental material). Importantly, the MS spectra detected 5 different glycopeptides (designated glycos I, II, IV, V, and VI, according to nomenclature introduced by Rolain et al. [42] [Table 3]) that appeared all located in the AST domain and to be 1-, 2-, or 3-fold substituted with a molecule of an approximate mass of 203 Da, which corresponds to the previously suggested glycosylation with GlcNAc (41, 42, 45) (Table 3; see also Table S1 in the supplemental material). This observation is in agreement with the apparent molecular mass of wild-type Acm2, which was estimated to be approximately 100 kDa (Fig. 2), higher than the molecular mass of 78.9 kDa predicted on basis of the mature protein sequence. Moreover, we were able to identified some of the glycosylated residues (Table 3; see also Table S1), but not all. These glycosylated residues were all also found in the study of Rolain et al. (42). Interestingly, different glycosylated forms were found in glyco II (one or two HexNAc) and glyco VI (one, two, or three HexNAc), which might imply a dynamic level of glycosylation in Acm2. In the ΔtagE1, ΔtagE2E3, and ΔtagE4 mutants, the mobility of the Acm2 protein appeared to be unaffected compared to that of the wild-type strain. In contrast, the Acm2 protein present in the wild type was absent in the ΔtagE5E6 deletion strain, but a protein band of higher mobility (lower apparent molecular mass) appeared in the gel (Fig. 2A). These observations provide a first clue that TagE5 and/or TagE6 is involved in the glycosylation of Acm2. Indeed, the loss of glycosylation of Acm2 in the ΔtagE5E6 deletion strain could also be confirmed by LC-MS/MS, as the Acm2 protein band extracted from gel was used to generate MS spectra that enabled 52% and 59% coverages of the mature Acm2 protein sequence and its AST domain, respectively (Fig. S1B). Notably, 6 distinct peptides of the AST domain that contained proposed HexNAc glycosylations (42) (designated glycos II to VII [Table 3]) that were detected in the wild-type Acm2 protein spectra (glycos II, IV, V, and VI in this study and glyco VII in the work of Rolain et al. [42]) were also detected in the Acm2 protein spectra derived from the tagE5E6 deletion strain, although in the latter strain, these peptides consistently lacked the substitutions seen in the wild type (Table 3; see also Table S1). In addition, three peptides (glycos III, IV, and V) were detected exclusively in their nonglycosylated form in Acm2 isolated from the ΔtagE5E6 mutant (Table 3). These data reveal that the HexNAc-glycosylated peptides derived from Acm2 are detected only in their nonglycosylated form in the ΔtagE5E6 mutant (Table 3), supporting the role of TagE5E6 in the Acm2 glycosylation that is observed in the wild-type strain.
FIG 2.
(A) Coomassie brilliant blue-stained SDS-PAGE gel of surface proteins extracted from the tagE deletion mutants as well as from the wild type and acm2 deletion mutant to detect Acm2 (indicated by the arrow). (B) sWGA, Dolichos biflorus lectin, and Lens culinaris lectin blots of whole-cell extracts derived from the tagE deletion mutants, Lactobacillus plantarum WCFS1 (wild type), and the acm2 deletion mutant to assess glycan moieties. On the left side of the blot the protein sizes are indicated based on the Precision Plus protein dual color standard (Bio-Rad) molecular marker (data not shown). M, CandyCane glycoprotein molecular size marker. The arrow indicates Acm2.
TABLE 3.
Numbers of HexNAc on trypsinized Acm2 peptides isolated from the wild type or the TagE5E6 deletion mutant (ΔtagE5E6)
| Namea | Peptide sequenceb | Calculated [M + H]+c | WT |
ΔtagE5E6 mutant |
||||
|---|---|---|---|---|---|---|---|---|
| No. of HexNAcd | Observed m/z, WT | Δm (Da), WTe | No. of HexNAc | Observed m/z, WT | Δm (Da), ΔtagE5E6 mutante | |||
| Glyco I | GNSAASAASQQVTLSAGSQTETTAAGATDQSVASDGAK | 3,495.62 | 2 | 3,901.25 | 405.63 | ND | ||
| Glyco II | TDDQAESTSTTTATTSATSR | 2,030.89 | 0 | 2,031.62 | 0.73 | 0 | 2,031.67 | 0.78 |
| Glyco II | TDDQAESTSTTTATTSATSR | 2,030.89 | 1 | 2,234.68 | 203.79 | |||
| Glyco II | TDDQAESTSTTTATTSATSR | 2,030.89 | 2 | 2,437.74 | 406.85 | |||
| Glyco III | ADSTGPQSQSSASEAAK | 1,620.72 | ND | 0 | 1,621.55 | 0.83 | ||
| Glyco IV | DNAATSATADSTTSAVDQLDK | 2,080.94 | 2 | 2,487.75 | 406.81 | ND | ||
| Glyco V | ASAATSQASHSTTNETAK | 1,761.81 | 2 | 2,168.75 | 406.94 | 0 | 1,762.63 | 0.82 |
| Glyco VI | ASAAASQDSHVTTDQSSVTVTSEVAK | 2,576.22 | 0 | 2,576.86 | 0.64 | 0 | 2,576.9 | 0.68 |
| Glyco VI | ASAAASQDSHVTTDQSSVTVTSEVAK | 2,576.22 | 1 | 2,779.89 | 203.67 | |||
| Glyco VI | ASAAASQDSHVTTDQSSVTVTSEVAK | 2,576.22 | 2 | 2,982.94 | 406.72 | |||
| Glyco VI | ASAAASQDSHVTTDQSSVTVTSEVAK | 2,576.22 | 3 | 3,186 | 609.78 | |||
Glycopeptide number as reported previously (42).
The glycosylated amino acids that could be identified are underlined.
Calculated [M + H]+ values correspond to nonglycosylated peptides.
Number of HexNAc detected or not (ND, not detected) of peptides by LC-MS/MS from secreted Acm2 digested by trypsin.
Δm, difference between calculated and observed m/z values.
To further investigate this, we employed a lectin-based detection of glycan moieties using biotinylated sWGA, specific for GlcNAc, in a Western blot-like setup. This approach showed that an sWGA-recognized protein of approximately 100 kDa derived from the wild type was absent in the acm2 deletion strain, reconfirming the glycosylation of Acm2. Moreover, the glycans linked to these proteins react only with the GlcNAc-specific lectin sWGA and not with Dolichos biflorus lectin (specific for α-GalNAc) or with Lens culinaris lectin (specific for α-mannose) (Fig. 2B), implying that the glycan is most likely GlcNAc. Interestingly, the sWGA blot revealed signals other than Acm2 that appeared to be glycosylated and were detected in both wild-type- and acm2 mutant-derived whole-cell extracts. All these signals were lost when sWGA blotting experiments were performed using samples that were proteinase K treated, indicating that all glycan signals in the lectin blotting experiment were derived from proteinaceous molecules (Fig. 3). In addition, the sWGA blot revealed that deletion of tagE1, tagE2E3, or tagE4 did not affect protein glycosylation, since these mutants displayed the same banding pattern as was observed for the wild-type strain. In contrast, deletion of tagE5 and tagE6 abolished almost all detectable sWGA-specific signals, including that of Acm2, indicating that TagE5 and TagE6 play a critical role in the glycosylation of Acm2 and the additional proteins detected. Intriguingly, a single band with an apparent molecular mass of approximately 125 kDa not only appeared to be unaffected by the ΔtagE5E6 mutation but also was recognized by Dolichos biflorus and Lens culinaris lectins (Fig. 2B), implying that another, TagE5/E6-independent mechanism of glycosylation may be active for the glycosylation of this particular protein. Taken together, these results evidence the essential role of TagE5 and/or TagE6 for protein glycosylation in L. plantarum WCFS1.
FIG 3.
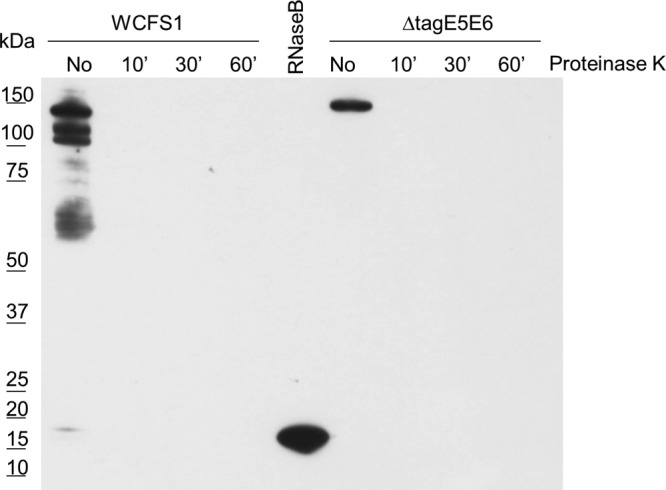
sWGA blot of whole-cell extracts derived from the wild type and tagE5E6 deletion mutant with or without proteinase K treatment for 10, 30, or 60 min. On the left side of the blot the protein sizes are indicated based on the Precision Plus protein dual color standard (Bio-Rad) molecular marker.
Both TagE5 and TagE6 are required for protein glycosylation in L. plantarum WCFS1.
To investigate whether TagE5, TagE6, or their concerted action is required for protein glycosylation in L. plantarum WCFS1, the ΔtagE5E6 mutant was complemented with tagE5, tagE6, or both genes. Complementations were achieved by integrating a single copy of the original gene(s) at a specific chromosomal site located downstream of the tRNASer locus, under the control of the native tagE6 promoter. Whole-cell extracts from the different complemented ΔtagE5E6 derivatives were analyzed by SDS-PAGE and sWGA blotting and compared to extracts derived from the wild type, as well as the Δacm2 and ΔtagE5E6 mutants. Complementation with either tagE5 or tagE6 did not restore protein glycosylation and generated the same banding patterns as observed for the ΔtagE5E6 strain (Fig. 4). However, complementation with the complete locus, encompassing both tagE5 and tagE6, restored the glycosylation not only of Acm2 but also of all other proteins that were detected in the wild-type banding pattern. These results indicate that glycosylation of proteins in L. plantarum WCFS1 requires both TagE5 and TagE6 activities.
FIG 4.
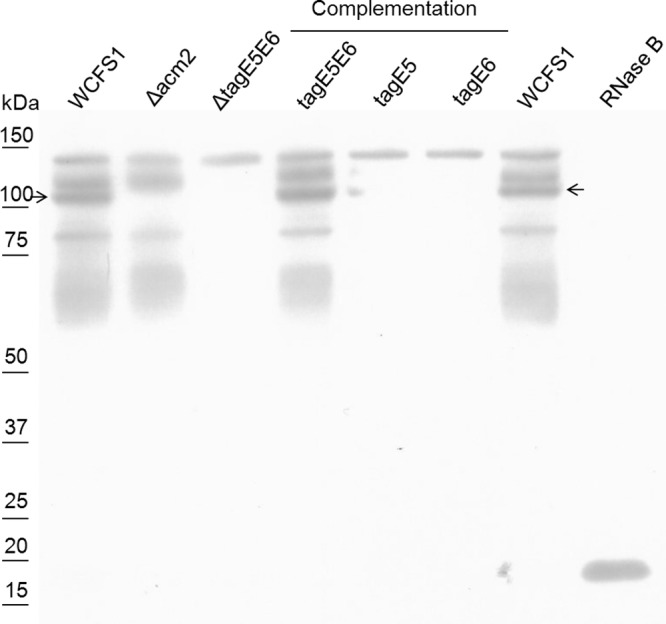
sWGA blot of the wild type, acm2 and tagE5E6 deletion mutants, and a panel of complemented mutants. On the left side of the blot the protein sizes are indicated based on the Precision Plus protein dual color standard (Bio-Rad) molecular marker (data not shown). The arrow indicates Acm2.
Protein glycosylation is a common feature in L. plantarum strains.
Using previously generated comparative genome hybridization (CGH) data for 42 L. plantarum strains (56, 57), we concluded that the 6 tagE genes recognized in the genome of L. plantarum WCFS1 appear to be conserved among all these 42 strains, with the notable exception of strain NCIMB12120, which appeared to lack genes that hybridize to the L. plantarum WCFS1 tagE4, tagE5, and tagE6 probes. To evaluate glycosylation of proteins in other L. plantarum strains, 9 of the 42 mentioned strains were selected, including NCIMB12120 and LP85-2 from Lactobacillus plantarum subsp. argentoratensis, for analysis of whole-cell extracts by SDS-PAGE and GlcNAc-specific sWGA blotting. Notably, these 9 strains were selected to maximize the coverage of the phylogenetic tree based on the whole-genome comparative genome hybridization data sets (56), as well as to include strains isolated from diverse niches (Table 1). All selected strains, including NCIMB12120, displayed sWGA-recognized glycosylated proteins that showed similar banding patterns on SDS-PAGE gels (Fig. 5). This result strongly suggests that glycosylation of proteins is a common feature in the species L. plantarum.
FIG 5.
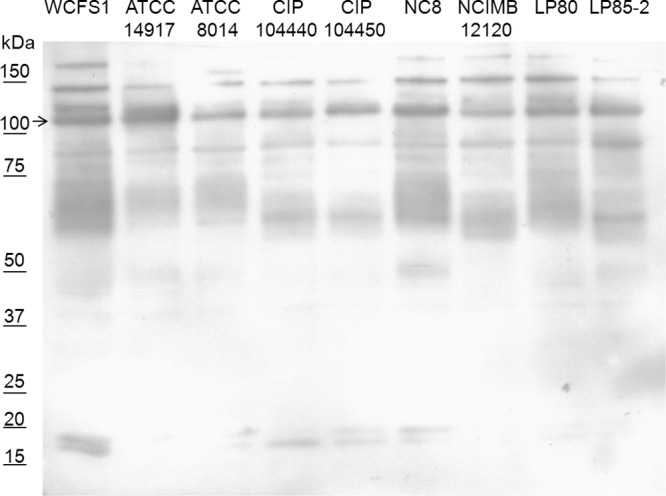
Assessment of 9 Lactobacillus plantarum strains for protein glycosylation, using whole-cell extract SDS-PAGE and sWGA lectin-based detection. On the left side of the blot the protein sizes are indicated based on the Precision Plus protein dual color standard (Bio-Rad) molecular marker (data not shown). The arrow indicates Acm2 of strain WCFS1.
DISCUSSION
Existing information on the protein glycosyltransferase Gtf1 in S. parasanguinis (37, 38, 41) enabled us to employ a comparative genomics approach, resulting in the identification of the 6 TagE orthologues as candidate protein glycosyltransferases in L. plantarum WCFS1. All 6 TagE proteins contain a GT1_gtfA-like domain designated cd04949 (58) at their C-terminal ends (46). This domain is named after gtfA in Streptococcus gordonii, in which it plays a role in the O-linked glycosylation, and this family containing this domain is most closely related to the GT1 family of glycosyltransferases (58). Limiting the amount of glycosyltransferases (50 annotated in the L. plantarum WCFS1 genome [46]) to the six TagE glycosyltransferases as most likely candidates for protein glycosylation enabled us to perform a systematic gene deletion and complementation strategy, followed by assessment of the consequences for protein glycosylation. This approach revealed that the concerted activities of TagE5 and TagE6 are required for the previously established glycosylation of Acm2 (41, 42), as well as other proteins. To the best of our knowledge, these results represent the first example of protein glycosylation machinery in a Lactobacillus species. The TagE proteins of L. plantarum WCFS1 are annotated according to their originally predicted function in teichoic acid glycosylation [poly(glycerolphosphate)-α-glucosyltransferases]. The glucose substitution levels in lipoteichoic acids (LTA) of L. plantarum WCFS1 are very low (59, 60), while glucose is a backbone constituent into the repeating unit of wall teichoic acids (WTA) that does not have additional glucose substitutions in this strain (60, 61). Neither LTA nor WTA of L. plantarum WCFS1 contain N-acetylhexosamine (59, 61), the glycan transferred by TagE5 and TagE6. Since the glucose substitution level is very low in LTA from L. plantarum WCFS1, we have isolated deacylated and dealanylated LTA (dd-LTA) to obtain better nuclear magnetic resonance (NMR) spectral resolution for signals from anomeric protons of sugar residues. The one-dimensional (1D) 1H NMR spectra revealed that dd-LTA isolated from ΔtagE5E6 mutants has the same level of glycosylation as LTA isolated from the WT (see Fig. S2A in the supplemental material). Moreover, 2D NMR spectra also showed that the glycosylation position of LTA is unaltered in the tagE5E6 deletion mutant (Fig. S2B). Therefore, with the establishment of TagE5 and TagE6 as dedicated protein glycosyltransferases, we propose to rename these enzymes (and genes) to GtfA (gtfA) and GtfB (gtfB), respectively.
Currently established bacterial O-linked glycosylation pathways employ either block or sequential transfer pathways for the addition of sugars to proteins (26). The block transfer pathway is exemplified by the glycosylation of Neisseria sp. pilin. This pathway assembles an oligosaccharide using nucleotide-activated sugars on a lipid anchor at the cytoplasmic side of the inner membrane. The assembled oligosaccharide is subsequently translocated across the inner membrane by a flippase to the periplasm, where the lipid-linked oligosaccharide is transferred to Ser/Thr residues of proteins (26, 33). On the other hand, the sequential transfer pathway, for example, employed in flagellar glycosylation of Campylobacter jejuni, transfers nucleotide-activated sugars individually onto Ser/Thr residues of proteins at the cytoplasm-inner membrane interface (26). Acm2 of L. plantarum WCFS1 undergoes cytoplasmic O-glycosylation with single N-acetylhexosamine moieties, likely GlcNAc, at multiple sites of its AST domain (41, 42). The fact that this glycosylation occurs in the cytoplasm might imply that the machinery responsible for Acm2 glycosylation is more similar to the sequential transfer pathway. Moreover, the glycosylation nature of Acm2 is similar to that of the glycosylation found in flagellin from L. monocytogenes, which is glycosylated with single GlcNAc at 3 to 6 sites (35), and in Fap1 fimbrial adhesin from S. parasanguinis, of which all the oligosaccharides are primed with GlcNAc (37). Interestingly, since the glycosyltransferases responsible for their glycosylations (GmaR for listerial flagellin [62] and Gtf1/Gtf2 for Fap1 [37, 53]) are predicted to be cytoplasmic proteins, the glycosylations of flagellin from L. monocytogenes and Fap1 from S. parasanguinis are also thought to occur in the cytoplasm. Notably, another example of a Lactobacillus glycoprotein, Msp1 of L. rhamnosus GG, was found to be glycosylated in the supernatant but not in the cytosolic fraction (43), hinting at species-specific O-glycosylation pathways in Lactobacillus species.
We have successfully identified the glycosyltransferases GtfA/B for the glycosylation of Acm2 based on the similar glycan moieties found in Fap1. However, the protein property and function are completely different between Acm2 and Fap1. Fap1 belongs to serine-rich repeat proteins (SRRPs), which are a family of surface-exposed adhesion-mediated proteins predominately found within the oral Streptococcus species (63). Currently, seven SRRPs have been researched, including Fap1 of S. parasanguinis, Has and GspB of S. gordonii, PsrP of Streptococcus pneumoniae, Srr-1 and Srr-2 of Streptococcus agalactiae, and SraP of Staphylococcus aureus (53). Each SRRP locus locates at a close proximity with a highly conserved core region, consisting of accessory secretory components and two essential glycosyltransferases (38). In this study, we found that GtfA and GtfB are required for the glycosylation of Acm2 as well as unidentified proteins other than SRRPs. Moreover, acm2 (lp_2645) locates in a distinct region of the chromosome, not linked to the gtfA/B (lp_2843/lp_2844) genes (Fig. 1), which is also distinct from Streptococcus SRRPs. Recently, the two glycosyltransferases, Gtf1 and Gtf2, of S. parasanguinis have been investigated, and it was found that the glycosylation of Fap1 requires the glycosyltransferase activity from Gtf1 together with the chaperone function of Gtf2 to maintain the correct folding of Gtf1 (53). However, GtfA and GtfB in L. plantarum are both homologues of Gtf1 in S. parasanguinis and display much lower similarity with the chaperone Gtf2. Although we have not experimentally excluded the possibility that the coexpression of GtfA or GtfB of L. plantarum WCFS1 is required for the correct folding of GtfB or GtfA, respectively, it does not seem likely that either GtfA or GtfB acts as a chaperone.
Comparative genome hybridization (CGH) data suggest that 6 orthologues of tagE genes are typically present in L. plantarum strains (56), with the notable exception of strain NCIMB12120, which appears to lack tagE4 and gtfA-gtfB. However, this strain still contains sWGA-recognized, glycosylated proteins, similar to the other 7 strains tested (Fig. 5). NCIMB12120 belongs to a subspecies (L. plantarum subsp. argentoratensis) different from reference strain WCFS1. Strains in this subspecies commonly have a smaller genome size (64) and appear to lack homologues of approximately 20% of the genes present in WCFS1 (57). Despite the apparent absence of tagE4 and gtfA-gtfB in NCIMB12120, the glycosylation of proteins apparently still occurs, suggesting that this strain (subspecies) contains genes with the same function that are of low homology and therefore were missed in the CGH analysis. Taken together, our data suggest that glycosylation and the presence of tagE genes are common features in L. plantarum strains. Moreover, the sequence and length of Acm2 are highly similar in all sequenced L. plantarum strains, e.g., WCFS1 (46), ST-III (65), JDM1 (66), and NC8 (67) (785 residues) and ATCC 14917 (781 residues). This suggests that the Acm2 proteins of different L. plantarum strains may all have similar sizes as well as similar degrees of glycosylation and are represented by the universal abundant protein band around 100 kDa (Fig. 5).
Other Lactobacillus species also harbor genetically coupled gtfA-gtfB homologues, for example, tagE2-tagE3 of Lactobacillus casei BL23, lsei_0891-lsei_0892 of L. casei ATCC 334 (68), and yohH-yohJ of L. rhamnosus GG (69). However, the genomes of other species, including Lactobacillus acidophilus NCFM (70), do not appear to contain gtfA-gtfB homologues, while Lactobacillus johnsonii NCC533 (71) and Lactobacillus delbrueckii subsp. bulgaricus ND02 (72) harbor a single gene displaying similarity with gtfA-gtfB. Although we successfully identified the role of GtfA-GtfB in glycosylation of proteins based on their sequence homology with Gtf1 of S. parasanguinis, sequence similarity alone did not provide a direct identification of this specific glycosyltransferase function, since all 6 TagE proteins display similar degrees of sequence homology with Gtf1. The role of the other 4 TagE glycosyltransferases in L. plantarum WCFS1 is currently unestablished but might involve the transfer of other glycan moieties to proteins or N-glycosylation. Indeed, among recently found glycoproteins in L. plantarum WCFS1, glycosylation of hexoses was also found in Lp_2162, Lp_3421, and DnaK, besides the HexNAc substitutions already established for Acm2 (45). Moreover, some lectin-based studies suggested the presence of glycoproteins modified with glycans other than GlcNAc, such as glycoproteins of L. acidophilus JCM1132T (recognized by β-galctoside-specific lectin) (73), SlpA of L. acidophilus NCFM (recognized by fucose- and mannose-specific lectins) (19, 74), and Msp1 of L. rhamnosus GG (recognized by glucose- and mannose-specific ConA lectin) (43). Furthermore, many Lactobacillus genomes contain the genes to produce multiple nucleotide-activated sugars, including UDP-glucose, UDP-galactose, sialic acid, and dTDP-rhamnose (6), suggesting the potential capacity to glycosylate proteins with diverse sugar moieties. Alternatively, WTA of L. plantarum WCFS1 contains glucose in its backbone (61), and biosynthesis of this structure could require the activity of specific TagE proteins, as predicted by the current annotation.
We have conclusively shown that protein glycosylation is a common feature in L. plantarum strains and does not target a single protein but modifies a much broader range of proteinaceous compounds. One important question remains unanswered: what is the biological role of protein glycosylation in lactobacilli? Earlier studies with pathogens showed that glycoproteins are often involved in adherence, pathogenicity, flagellum assembly, and protein stability (23). A more recent example illustrated that the glycans attached on surface layer proteins of Tannerella forsythia, which is implicated in periodontitis, modulate the function of DCs and suppress T-helper 17 responses (75). In light of this, it is intriguing that glycosylation of Msp1 of L. rhamnosus GG is not essential for its peptidoglycan hydrolyase activity (17, 43), nor for activating Akt signaling in Caco-2 cells (43), but does influence Msp1 protein stability and protein localization (43). Moreover, Lebeer et al. suggested the possibility of an indirect modulating role of the Msp1 glycan moieties in Akt activation via shielding bacteria and host interaction (43). Furthermore, the ConA and Aleuria aurantia (AAL)-reactive glycans on SlpA of L. acidophilus NCFM are essential for the modulation of T cell function and lead to more IL-4 production (19). Importantly, it was recently established that O-glycosylation of Acm2 in L. plantarum functions as a major negative modulator of Acm2 peptidoglycan hydrolase activity (42), which is the first evidence that glycosylation regulates the bacterial enzyme activity. In fact, we observed different glycoforms of glyco II and glyco VI (Table 3), which might imply a kinetic modulation of Acm2 hydrolase activity via O-glycosylation (42). Our future work will focus on recognizing the biological roles of glycosylation of other proteins in L. plantarum, especially in relation to its possible consequences for host-microbe interactions in the gastrointestinal tract.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lasse Fredriksen (Norwegian University of Life Sciences, Aas, Norway) for helpful information on performing lectin blotting experiments. We warmly thank Hervé Degand and the proteomic facility of the Institute of Life Sciences for technical assistance in the analysis of the glycosylation status of Acm2.
Work by the team of P.H. was supported by the Research Department of the Communauté française de Belgique (Concerted Research Action). T.R. held a doctoral fellowship from FRIA. P.H. is a Senior Research Associate of the FNRS. P.A.B. is partially employed within the research program of the Kluyver Centre for Genomics of Industrial Fermentation, which is part of the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research.
Footnotes
Published ahead of print 14 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01401-13.
REFERENCES
- 1.Marco ML, Pavan S, Kleerebezem M. 2006. Towards understanding molecular modes of probiotic action. Curr. Opin. Biotechnol. 17:204–210. 10.1016/j.copbio.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 2.Lebeer S, Vanderleyden J, De Keersmaecker SC. 2010. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat. Rev. Microbiol. 8:171–184. 10.1038/nrmicro2297 [DOI] [PubMed] [Google Scholar]
- 3.Bron PA, van Baarlen P, Kleerebezem M. 2012. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 10:66–78. 10.1038/nrmicro2690 [DOI] [PubMed] [Google Scholar]
- 4.FAO/WHO. 2002. Guidelines for the evaluation of probiotics in food. FAO/WHO, Geneva, Switzerland [Google Scholar]
- 5.Wells JM, Rossi O, Meijerink M, van Baarlen P. 2011. Epithelial crosstalk at the microbiota-mucosal interface. Proc. Natl. Acad. Sci. U. S. A. 108(Suppl 1):4607–4614. 10.1073/pnas.1000092107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleerebezem M, Hols P, Bernard E, Rolain T, Zhou M, Siezen RJ, Bron PA. 2010. The extracellular biology of the lactobacilli. FEMS Microbiol. Rev. 34:199–230. 10.1111/j.1574-6976.2009.00208.x [DOI] [PubMed] [Google Scholar]
- 7.Lee IC, Tomita S, Kleerebezem M, Bron PA. 2013. The quest for probiotic effector molecules—unraveling strain specificity at the molecular level. Pharmacol. Res. 69:61–74. 10.1016/j.phrs.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 8.Buck BL, Altermann E, Svingerud T, Klaenhammer TR. 2005. Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 71:8344–8351. 10.1128/AEM.71.12.8344-8351.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roos S, Jonsson H. 2002. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 148:433–442 [DOI] [PubMed] [Google Scholar]
- 10.Miyoshi Y, Okada S, Uchimura T, Satoh E. 2006. A mucus adhesion promoting protein, MapA, mediates the adhesion of Lactobacillus reuteri to Caco-2 human intestinal epithelial cells. Biosci. Biotechnol. Biochem. 70:1622–1628. 10.1271/bbb.50688 [DOI] [PubMed] [Google Scholar]
- 11.Pretzer G, Snel J, Molenaar D, Wiersma A, Bron PA, Lambert J, de Vos WM, van der Meer R, Smits MA, Kleerebezem M. 2005. Biodiversity-based identification and functional characterization of the mannose-specific adhesin of Lactobacillus plantarum. J. Bacteriol. 187:6128–6136. 10.1128/JB.187.17.6128-6136.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hynönen U, Westerlund-Wikstrom B, Palva A, Korhonen TK. 2002. Identification by flagellum display of an epithelial cell- and fibronectin-binding function in the SlpA surface protein of Lactobacillus brevis. J. Bacteriol. 184:3360–3367. 10.1128/JB.184.12.3360-3367.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antikainen J, Anton L, Sillanpaa J, Korhonen TK. 2002. Domains in the S-layer protein CbsA of Lactobacillus crispatus involved in adherence to collagens, laminin and lipoteichoic acids and in self-assembly. Mol. Microbiol. 46:381–394. 10.1046/j.1365-2958.2002.03180.x [DOI] [PubMed] [Google Scholar]
- 14.Johnson-Henry KC, Hagen KE, Gordonpour M, Tompkins TA, Sherman PM. 2007. Surface-layer protein extracts from Lactobacillus helveticus inhibit enterohaemorrhagic Escherichia coli O157:H7 adhesion to epithelial cells. Cell. Microbiol. 9:356–367. 10.1111/j.1462-5822.2006.00791.x [DOI] [PubMed] [Google Scholar]
- 15.Aleljung P, Shen W, Rozalska B, Hellman U, Ljungh A, Wadstrom T. 1994. Purification of collagen-binding proteins of Lactobacillus reuteri NCIB 11951. Curr. Microbiol. 28:231–236. 10.1007/BF01575966 [DOI] [PubMed] [Google Scholar]
- 16.Claes IJJ, Schoofs G, Regulski K, Courtin P, Chapot-Chartier M-P, Rolain T, Hols P, von Ossowski I, Reunanen J, de Vos WM, Palva A, Vanderleyden J, De Keersmaecker SCJ, Lebeer S. 2012. Genetic and biochemical characterization of the cell wall hydrolase activity of the major secreted protein of Lactobacillus rhamnosus GG. PLoS One 7:e31588. 10.1371/journal.pone.0031588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. 2007. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132:562–575. 10.1053/j.gastro.2006.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, Chaturvedi R, Peek RM, Wilson KT, Polk DB. 2011. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Invest. 121:2242–2253. 10.1172/JCI44031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konstantinov SR, Smidt H, de Vos WM, Bruijns SCM, Singh SK, Valence F, Molle D, Lortal S, Altermann E, Klaenhammer TR, van Kooyk Y. 2008. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. U. S. A. 105:19474–19479. 10.1073/pnas.0810305105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernardo D, Sanchez B, Al-Hassi HO, Mann ER, Urdaci MC, Knight SC, Margolles A. 2012. Microbiota/host crosstalk biomarkers: regulatory response of human intestinal dendritic cells exposed to lactobacillus extracellular encrypted peptide. PLoS One 7:e36262. 10.1371/journal.pone.0036262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drickamer K, Taylor ME. 1998. Evolving views of protein glycosylation. Trends Biochem. Sci. 23:321–324. 10.1016/S0968-0004(98)01246-8 [DOI] [PubMed] [Google Scholar]
- 22.Messner P. 2004. Prokaryotic glycoproteins: unexplored but important. J. Bacteriol. 186:2517–2519. 10.1128/JB.186.9.2517-2519.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szymanski CM, Wren BW. 2005. Protein glycosylation in bacterial mucosal pathogens. Nat. Rev. Microbiol. 3:225–237. 10.1038/nrmicro1100 [DOI] [PubMed] [Google Scholar]
- 24.Weerapana E, Imperiali B. 2006. Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology 16:91R–101R. 10.1093/glycob/cwj099 [DOI] [PubMed] [Google Scholar]
- 25.Abu-Qarn M, Eichler J, Sharon N. 2008. Not just for Eukarya anymore: protein glycosylation in Bacteria and Archaea. Curr. Opin. Struct. Biol. 18:544–550. 10.1016/j.sbi.2008.06.010 [DOI] [PubMed] [Google Scholar]
- 26.Nothaft H, Szymanski CM. 2010. Protein glycosylation in bacteria: sweeter than ever. Nat. Rev. Microbiol. 8:765–778. 10.1038/nrmicro2383 [DOI] [PubMed] [Google Scholar]
- 27.Moens S, Vanderleyden J. 1997. Glycoproteins in prokaryotes. Arch. Microbiol. 168:169–175. 10.1007/s002030050484 [DOI] [PubMed] [Google Scholar]
- 28.Schäffer C, Graninger M, Messner P. 2001. Prokaryotic glycosylation. Proteomics 1:248–261. [DOI] [PubMed] [Google Scholar]
- 29.Benz I, Schmidt MA. 2002. Never say never again: protein glycosylation in pathogenic bacteria. Mol. Microbiol. 45:267–276. 10.1046/j.1365-2958.2002.03030.x [DOI] [PubMed] [Google Scholar]
- 30.Szymanski CM, Yao R, Ewing CP, Trust TJ, Guerry P. 1999. Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol. Microbiol. 32:1022–1030. 10.1046/j.1365-2958.1999.01415.x [DOI] [PubMed] [Google Scholar]
- 31.Wacker M, Linton D, Hitchen PG, Nita-Lazar M, Haslam SM, North SJ, Panico M, Morris HR, Dell A, Wren BW, Aebi M. 2002. N-Linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science 298:1790–1793. 10.1126/science.298.5599.1790 [DOI] [PubMed] [Google Scholar]
- 32.Ku SC, Schulz BL, Power PM, Jennings MP. 2009. The pilin O-glycosylation pathway of pathogenic Neisseria is a general system that glycosylates AniA, an outer membrane nitrite reductase. Biochem. Biophys. Res. Commun. 378:84–89. 10.1016/j.bbrc.2008.11.025 [DOI] [PubMed] [Google Scholar]
- 33.Vik A, Aas FE, Anonsen JH, Bilsborough S, Schneider A, Egge-Jacobsen W, Koomey M. 2009. Broad spectrum O-linked protein glycosylation in the human pathogen Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. U. S. A. 106:4447–4452. 10.1073/pnas.0809504106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thibault P, Logan SM, Kelly JF, Brisson JR, Ewing CP, Trust TJ, Guerry P. 2001. Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J. Biol. Chem. 276:34862–34870. 10.1074/jbc.M104529200 [DOI] [PubMed] [Google Scholar]
- 35.Schirm M, Kalmokoff M, Aubry A, Thibault P, Sandoz M, Logan SM. 2004. Flagellin from Listeria monocytogenes is glycosylated with beta-O-linked N-acetylglucosamine. J. Bacteriol. 186:6721–6727. 10.1128/JB.186.20.6721-6727.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castric P, Cassels FJ, Carlson RW. 2001. Structural characterization of the Pseudomonas aeruginosa 1244 pilin glycan. J. Biol. Chem. 276:26479–26485. 10.1074/jbc.M102685200 [DOI] [PubMed] [Google Scholar]
- 37.Bu S, Li Y, Zhou M, Azadin P, Zeng M, Fives-Taylor P, Wu H. 2008. Interaction between two putative glycosyltransferases is required for glycosylation of a serine-rich streptococcal adhesin. J. Bacteriol. 190:1256–1266. 10.1128/JB.01078-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou M, Wu H. 2009. Glycosylation and biogenesis of a family of serine-rich bacterial adhesins. Microbiology 155:317–327. 10.1099/mic.0.025221-0 [DOI] [PubMed] [Google Scholar]
- 39.Fletcher CM, Coyne MJ, Bentley DL, Villa OF, Comstock LE. 2007. Phase-variable expression of a family of glycoproteins imparts a dynamic surface to a symbiont in its human intestinal ecosystem. Proc. Natl. Acad. Sci. U. S. A. 104:2413–2418. 10.1073/pnas.0608797104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fletcher CM, Coyne MJ, Villa OF, Chatzidaki-Livanis M, Comstock LE. 2009. A general O-glycosylation system important to the physiology of a major human intestinal symbiont. Cell 137:321–331. 10.1016/j.cell.2009.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fredriksen L, Mathiesen G, Moen A, Bron PA, Kleerebezem M, Eijsink VGH, Egge-Jacobsen W. 2012. The major autolysin Acm2 from Lactobacillus plantarum undergoes cytoplasmic O-glycosylation. J. Bacteriol. 194:325–333. 10.1128/JB.06314-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolain T, Bernard E, Beaussart A, Degand H, Courtin P, Egge-Jacobsen W, Bron PA, Morsomme P, Kleerebezem M, Chapot-Chartier MP, Dufrene YF, Hols P. 2013. O-glycosylation as a novel control mechanism of peptidoglycan hydrolase activity. J. Biol. Chem. 288:22233–22247. 10.1074/jbc.M113.470716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebeer S, Claes IJ, Balog CI, Schoofs G, Verhoeven TL, Nys K, von Ossowski I, de Vos WM, Tytgat HL, Agostinis P, Palva A, Van Damme EJ, Deelder AM, De Keersmaecker SC, Wuhrer M, Vanderleyden J. 2012. The major secreted protein MspI/p75 is O-glycosylated in Lactobacillus rhamnosus GG. Microb. Cell Fact. 11:15. 10.1186/1475-2859-11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Remus DM, Kleerebezem M, Bron PA. 2011. An intimate tête-à-tête—how probiotic lactobacilli communicate with the host. Eur. J. Pharmacol. 668(Suppl 1):S33–S42. 10.1016/j.ejphar.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 45.Fredriksen L, Moen A, Adzhubei A, Mathiesen G, Eijsink VGH, Egge-Jacobsen W. 2013. Lactobacillus plantarum WCFS1 O-linked protein glycosylation: an extended spectrum of target proteins and modification sites detected by mass spectrometry. Glycobiology 23:1439–1451. 10.1093/glycob/cwt071 [DOI] [PubMed] [Google Scholar]
- 46.Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MW, Stiekema W, Lankhorst RM, Bron PA, Hoffer SM, Groot MN, Kerkhoven R, de Vries M, Ursing B, de Vos WM, Siezen RJ. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. U. S. A. 100:1990–1995. 10.1073/pnas.0337704100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Killmann H, Herrmann C, Torun A, Jung G, Braun V. 2002. TonB of Escherichia coli activates FhuA through interaction with the beta-barrel. Microbiology 148:3497–3509 [DOI] [PubMed] [Google Scholar]
- 48.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 49.Josson K, Scheirlinck T, Michiels F, Platteeuw C, Stanssens P, Joos H, Dhaese P, Zabeau M, Mahillon J. 1989. Characterization of a Gram-positive broad-host-range plasmid isolated from Lactobacillus hilgardii. Plasmid 21:9–20. 10.1016/0147-619X(89)90082-6 [DOI] [PubMed] [Google Scholar]
- 50.Lambert JM, Bongers RS, Kleerebezem M. 2007. Cre-lox-based system for multiple gene deletions and selectable-marker removal in Lactobacillus plantarum. Appl. Environ. Microbiol. 73:1126–1135. 10.1128/AEM.01473-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horton RM. 1993. In vitro recombination and mutagenesis of DNA: SOEing together tailor-made genes. Methods Mol. Biol. 15:251–261 [DOI] [PubMed] [Google Scholar]
- 52.Sandhu GS, Precup JW, Kline BC. 1989. Rapid one-step characterization of recombinant vectors by direct analysis of transformed Escherichia coli colonies. Biotechniques 7:689–690 [PubMed] [Google Scholar]
- 53.Wu R, Wu H. 2011. A molecular chaperone mediates a two-protein enzyme complex and glycosylation of serine-rich streptococcal adhesins. J. Biol. Chem. 286:34923–34931. 10.1074/jbc.M111.239350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 55.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siezen RJ, Tzeneva VA, Castioni A, Wels M, Phan HT, Rademaker JL, Starrenburg MJ, Kleerebezem M, Molenaar D, van Hylckama Vlieg JE. 2010. Phenotypic and genomic diversity of Lactobacillus plantarum strains isolated from various environmental niches. Environ. Microbiol. 12:758–773. 10.1111/j.1462-2920.2009.02119.x [DOI] [PubMed] [Google Scholar]
- 57.Molenaar D, Bringel F, Schuren FH, de Vos WM, Siezen RJ, Kleerebezem M. 2005. Exploring Lactobacillus plantarum genome diversity by using microarrays. J. Bacteriol. 187:6119–6127. 10.1128/JB.187.17.6119-6127.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zhang N, Zheng C, Bryant SH. 2011. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 39:D225–D229. 10.1093/nar/gkq1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palumbo E, Deghorain M, Cocconcelli PS, Kleerebezem M, Geyer A, Hartung T, Morath S, Hols P. 2006. D-alanyl ester depletion of teichoic acids in Lactobacillus plantarum results in a major modification of lipoteichoic acid composition and cell wall perforations at the septum mediated by the Acm2 autolysin. J. Bacteriol. 188:3709–3715. 10.1128/JB.188.10.3709-3715.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andre G, Deghorain M, Bron PA, van Swam II, Kleerebezem M, Hols P, Dufrene YF. 2011. Fluorescence and atomic force microscopy imaging of wall teichoic acids in Lactobacillus plantarum. ACS Chem. Biol. 6:366–376. 10.1021/cb1003509 [DOI] [PubMed] [Google Scholar]
- 61.Tomita S, de Waard P, Bakx EJ, Schols HA, Kleerebezem M, Bron PA. 2013. The structure of an alternative wall teichoic acid produced by a Lactobacillus plantarum WCFS1 mutant contains a 1,5-linked poly(ribitol phosphate) backbone with 2-alpha-D-glucosyl substitutions. Carbohydr. Res. 370:67–71. 10.1016/j.carres.2013.01.018 [DOI] [PubMed] [Google Scholar]
- 62.Shen A, Kamp HD, Grundling A, Higgins DE. 2006. A bifunctional O-GlcNAc transferase governs flagellar motility through anti-repression. Genes Dev. 20:3283–3295. 10.1101/gad.1492606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lizcano A, Sanchez CJ, Orihuela CJ. 2012. A role for glycosylated serine-rich repeat proteins in gram-positive bacterial pathogenesis. Mol. Oral Microbiol. 27:257–269. 10.1111/j.2041-1014.2012.00653.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chevallier B, Hubert JC, Kammerer B. 1994. Determination of chromosome size and number of rrn loci in Lactobacillus plantarum by pulsed-field gel electrophoresis. FEMS Microbiol. Lett. 120:51–56. 10.1111/j.1574-6968.1994.tb07006.x [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Chen C, Ai L, Zhou F, Zhou Z, Wang L, Zhang H, Chen W, Guo B. 2011. Complete genome sequence of the probiotic Lactobacillus plantarum ST-III. J. Bacteriol. 193:313–314. 10.1128/JB.01159-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang ZY, Liu C, Zhu YZ, Zhong Y, Zhu YQ, Zheng HJ, Zhao GP, Wang SY, Guo XK. 2009. Complete genome sequence of Lactobacillus plantarum JDM1. J. Bacteriol. 191:5020–5021. 10.1128/JB.00587-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Axelsson L, Rud I, Naterstad K, Blom H, Renckens B, Boekhorst J, Kleerebezem M, van Hijum S, Siezen RJ. 2012. Genome sequence of the naturally plasmid-free Lactobacillus plantarum strain NC8 (CCUG 61730). J. Bacteriol. 194:2391–2392. 10.1128/JB.00141-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, Koonin E, Pavlov A, Pavlova N, Karamychev V, Polouchine N, Shakhova V, Grigoriev I, Lou Y, Rohksar D, Lucas S, Huang K, Goodstein DM, Hawkins T, Plengvidhya V, Welker D, Hughes J, Goh Y, Benson A, Baldwin K, Lee J-H, Díaz-Muñiz I, Dosti B, Smeianov V, Wechter W, Barabote R, Lorca G, Altermann E, Barrangou R, Ganesan B, Xie Y, Rawsthorne H, Tamir D, Parker C, Breidt F, Broadbent J, Hutkins R, O'Sullivan D, Steele J, Unlu G, Saier M, Klaenhammer T, Richardson P, Kozyavkin S, Weimer B, Mills D. 2006. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. U. S. A. 103:15611–15616. 10.1073/pnas.0607117103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, Satokari R, Vesterlund S, Hendrickx AP, Lebeer S, De Keersmaecker SC, Vanderleyden J, Hamalainen T, Laukkanen S, Salovuori N, Ritari J, Alatalo E, Korpela R, Mattila-Sandholm T, Lassig A, Hatakka K, Kinnunen KT, Karjalainen H, Saxelin M, Laakso K, Surakka A, Palva A, Salusjarvi T, Auvinen P, de Vos WM. 2009. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human- mucus binding protein. Proc. Natl. Acad. Sci. U. S. A. 106:17193–17198. 10.1073/pnas.0908876106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Altermann E, Russell WM, Azcarate-Peril MA, Barrangou R, Buck BL, McAuliffe O, Souther N, Dobson A, Duong T, Callanan M, Lick S, Hamrick A, Cano R, Klaenhammer TR. 2005. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc. Natl. Acad. Sci. U. S. A. 102:3906–3912. 10.1073/pnas.0409188102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pridmore RD, Berger B, Desiere F, Vilanova D, Barretto C, Pittet AC, Zwahlen MC, Rouvet M, Altermann E, Barrangou R, Mollet B, Mercenier A, Klaenhammer T, Arigoni F, Schell MA. 2004. The genome sequence of the probiotic intestinal bacterium Lactobacillus johnsonii NCC 533. Proc. Natl. Acad. Sci. U. S. A. 101:2512–2517. 10.1073/pnas.0307327101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun Z, Chen X, Wang J, Zhao W, Shao Y, Guo Z, Zhang X, Zhou Z, Sun T, Wang L, Meng H, Zhang H, Chen W. 2011. Complete genome sequence of Lactobacillus delbrueckii subsp. bulgaricus strain ND02. J. Bacteriol. 193:3426–3427. 10.1128/JB.05004-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukai T, Arihara K. 1994. Presence of intestinal lectin-binding glycoproteins on the cell surface of Lactobacillus acidophilus. Biosci. Biotechnol. Biochem. 58:1851–1854 [Google Scholar]
- 74.van Liempt E, Bank CM, Mehta P, Garcia-Vallejo JJ, Kawar ZS, Geyer R, Alvarez RA, Cummings RD, Kooyk Y, van Die I. 2006. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 580:6123–6131. 10.1016/j.febslet.2006.10.009 [DOI] [PubMed] [Google Scholar]
- 75.Settem RP, Honma K, Nakajima T, Phansopa C, Roy S, Stafford GP, Sharma A. 2013. A bacterial glycan core linked to surface (S)-layer proteins modulates host immunity through Th17 suppression. Mucosal Immunol. 6:415–426. 10.1038/mi.2012.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Overmars L, Kerkhoven R, Siezen RJ, Francke C. 2013. MGcV: the microbial genomic context viewer for comparative genome analysis. BMC Genomics 14:209. 10.1186/1471-2164-14-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aukrust T, Blom H. 1992. Transformation of Lactobacillus strains used in meat and vegetable fermentations. Food Res. Int. 25:253–261. 10.1016/0963-9969(92)90121-K [DOI] [Google Scholar]
- 78.Bringel F, Quenee P, Tailliez P. 2001. Polyphasic investigation of the diversity within Lactobacillus plantarum related strains revealed two L. plantarum subgroups. Syst. Appl. Microbiol. 24:561–571. 10.1078/0723-2020-00061 [DOI] [PubMed] [Google Scholar]
- 79.Pavan S, Hols P, Delcour J, Geoffroy MC, Grangette C, Kleerebezem M, Mercenier A. 2000. Adaptation of the nisin-controlled expression system in Lactobacillus plantarum: a tool to study in vivo biological effects. Appl. Environ. Microbiol. 66:4427–4432. 10.1128/AEM.66.10.4427-4432.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bron PA, Tomita S, van Swam II, Remus DM, Meijerink M, Wels M, Okada S, Wells JM, Kleerebezem M. 2012. Lactobacillus plantarum possesses the capability for wall teichoic acid backbone alditol switching. Microb. Cell Fact. 11:123. 10.1186/1475-2859-11-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bron PA, Benchimol MG, Lambert J, Palumbo E, Deghorain M, Delcour J, De Vos WM, Kleerebezem M, Hols P. 2002. Use of the alr gene as a food-grade selection marker in lactic acid bacteria. Appl. Environ. Microbiol. 68:5663–5670. 10.1128/AEM.68.11.5663-5670.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Bokhorst-van de Veen H, Lee IC, Marco ML, Wels M, Bron PA, Kleerebezem M. 2012. Modulation of Lactobacillus plantarum gastrointestinal robustness by fermentation conditions enables identification of bacterial robustness markers. PLoS One 7:e39053. 10.1371/journal.pone.0039053 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.