Abstract
Quorum sensing (QS) is a widespread mechanism of bacterial communication in which individual cells produce and respond to small chemical signals. In Agrobacterium tumefaciens, an acylhomoserine lactone-dependent QS mechanism is known to regulate the replication and conjugation of the tumor-inducing (Ti) plasmid. Most of the QS regulatory proteins are encoded within the Ti plasmid. Among them, TraI is the LuxI-type enzyme synthesizing the QS signal N-3-oxooctanoyl-l-homoserine lactone (3OC8HSL), TraR is the LuxR-type transcriptional factor that recognizes 3OC8HSL, and TraM is an antiactivator that antagonizes TraR. Recently, we identified a TraM homolog encoded by the traM2 gene in the chromosomal background of A. tumefaciens A6. In this study, we further identified additional homologs (TraI2 and TraR2) of TraI and TraR in this strain. We showed that similar to TraI, TraI2 could predominantly synthesize the QS signal 3OC8HSL. We also showed that TraR2 could recognize 3OC8HSL and activate the tra box-containing promoters as efficiently as TraR. Further analysis showed that traM2, traI2, and traR2 are physically linked on a mobile genetic element that is not related to the Ti plasmid. These findings indicate that A. tumefaciens A6 carries a second QS system that may play a redundant role in the regulation of the replication and conjugation of the Ti plasmid.
INTRODUCTION
Quorum sensing (QS) is a bacterial community genetic regulatory mechanism that controls diverse biological functions in different bacterial species. Among the various bacterial QS systems reported, the best-characterized one is the acylhomoserine lactone (AHL)-based QS system. In this system, the LuxI (I)- and LuxR (R)-type proteins appear to be the central components. The I-type protein is the AHL synthase, and the R-type protein is the AHL-responsive transcription factor; they are conserved in different bacterial species containing AHL-based QS systems (1). In most cases, at a low bacterial population density, the I-type enzyme produces a basal level of AHL signals that accumulate as bacterial cells proliferate and interact with a cognate R-type transcription factor. Subsequently, the R-AHL complexes induce higher-level expression of I-type enzymes, which boosts AHL production and activates the transcriptional expression of other QS-dependent genes (2). It is through QS that individual bacterial cells could behave as a coordinated community in performing various biological activities, such as the production of secondary metabolites, the synthesis of virulence factors, and the development of biofilms.
Agrobacterium tumefaciens has been extensively studied as a model pathogen for the investigation of microbe-host interactions. Upon recognizing the chemical signals produced by host plants, A. tumefaciens infects a variety of plants and causes the crown gall diseases, which result in substantial losses of agricultural production worldwide. During infection, a DNA fragment (T-DNA) is transferred from the bacterial cells into the host plant cells and integrated into the chromosomal DNA (3–5). T-DNA, together with the genes associated with its interkingdom gene transfer, is located on the Ti plasmid (2). Interestingly, many environmental Agrobacterium isolates do not harbor the Ti plasmid and hence are avirulent. Therefore, conjugative transfer of the Ti plasmid from pathogenic strains to plasmid-free strains could play a key role in maintaining and expanding the population of infectious A. tumefaciens (6–8). In A. tumefaciens, the key QS regulators are encoded by the Ti plasmid, which includes TraI and TraR. TraI synthesizes the AHL signal N-3-oxooctanoyl-l-homoserine lactone (3OC8HSL), which binds to and activates TraR (9–11). TraR then binds to the palindromic tra box and thereby activates a number of operons that encode proteins necessary for Ti plasmid replication and conjugation (7, 12–14). In this context, the QS system of A. tumefaciens is similar to the prototype LuxI-LuxR QS system of Vibrio species.
However, the regulatory mechanisms of the A. tumefaciens QS system appear to be more complicated than the prototype mechanisms. First, this QS system is normally not active until bacterial cells detect the conjugative opines produced by the crown gall tumors incited by the pathogen (15). Furthermore, when the TraR level is low, TraM binds to TraR and forms an inactive complex that sets off the QS system until the cell density is high (16–18). TraM, functioning as a TraR antiactivator, has been identified in part of the agrobacterial species. Therefore, species-specific opines and TraM constitute additional regulatory components of the QS system of A. tumefaciens and ensure that Ti plasmid conjugation occurs only under certain conditions. In addition to this species specificity, the QS system of A. tumefaciens also displays strain-specific features. For example, in octopine-type strains, the conjugative opine for QS-dependent Ti plasmid conjugative transfer is octopine (19), while in nopaline-type strains, it is agrocinopines A and B (20). In addition, in nopaline strain C58, traR is a member of a five-gene operon of pTiC58, which is expressed from a promoter regulated by the transcriptional repressor AccR. Repression by AccR is relieved in the presence of agrocinopine A or B, and the operon, including traR, is expressed (19). In contrast, in octopine strains, traR is located in a 14-member operon that is regulated by the transcription factor OccR (21). OccR acts as either a repressor or an activator, depending on the absence or presence of octopine, respectively, by binding to different positions of the promoter of the traR operon (22). Furthermore, in octopine strains, the QS system is also negatively regulated by TrlR, a truncated version of TraR. TrlR antagonizes the activity of TraR by the formation of inactive heterodimers (23, 24). In contrast, no TrlR homolog is found in nopaline strains. Moreover, while TraM encoded by the Ti plasmid is a conserved QS modulator in both octopine- and nopaline-type strains of A. tumefaciens, a TraM homolog (TraM2) has recently been identified as an extra TraR antiactivator for QS regulation. Like TrlR, TraM2 exists in only some of the octopine strains (25). Put together, these findings have illustrated the complexity and genetic variations of the A. tumefaciens QS system.
In this investigation, we further studied the regulation of QS in A. tumefaciens. Using genetic approaches, we showed that traR2 and traI2 encode a second QS system (QS2) in A. tumefaciens strain A6. Bioinformatic and biochemical analyses showed that TraI2 is a functional homolog of TraI that predominantly synthesizes 3OC8HSL and that TraR2 is a functional homolog of TraR that recognizes 3OC8HSL and activates the QS-responsive genes. A conjugal transfer assay showed that traR2 and traI2, together with the previously identified gene traM2, are located on a mobile genetic element. Phylogenetic analysis suggested that QS2 may represent an ancient paradigm of the QS systems of rhizobial species.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. A. tumefaciens strains were grown at 28°C in LB medium (containing [per liter] 10 g Bacto tryptone, 5 g yeast extract, and 10 g NaCl, pH 7.0) or in BM minimal medium (basic minimal-nutrient medium with 0.2% mannitol added as the sole carbon source and 0.2% ammonia sulfate as the sole nitrogen source unless otherwise indicated) (26). For bacterial growth assays, mannitol was replaced with octopine (0.2 g/liter). Escherichia coli strains were grown at 37°C in LB medium. Antibiotics were added at the following concentrations when required: kanamycin, 50 μg/ml (A. tumefaciens) or 100 μg/ml (E. coli); tetracycline, 5 μg/ml (A. tumefaciens) or 10 μg/ml (E. coli); rifampin, 100 μg/ml; ampicillin, 100 μg/ml; chloramphenicol, 50 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| A. tumefaciens | ||
| A6 | Wild-type octopine strain | A. Kerr |
| A6(traMk588) | A6 with traM replaced with traM of strain K588, which is dysfunctional | 25 |
| A6(traMk588 ΔtraM2) | A6(traMk588) carrying in-frame deletion of traM2 | 25 |
| A6(traMk588 ΔtraM2; traR2::Tn5) | A6(traMk588 ΔtraM2) carrying Tn5 insertion of traR2, Kanr | This study |
| A6(traMk588 pDSK-traR2) | A6(traMk588) with overexpression of traR2, Kanr | This study |
| NT1(pDSK-traR pLA-traG::lacZ) | Also named NT1(traR tra::lacZ749), AHL signal reporter strain, Kanr Tcr | 9 |
| A6(traMk588 pLA-traG::lacZ) | A6(traMk588) carrying AHL-responsive reporter plasmid pLA-traG::lacZ, Tcr | This study |
| A6(traMk588 ΔtraM2 pLA-traG::lacZ) | A6(traMk588 ΔtraM2) carrying pLA-traG::lacZ vector, Tcr | This study |
| A6(traMk588 ΔtraM2 traR2::Tn5 pLA-traG::lacZ) | A6(traMk588 ΔtraM2; traR2::Tn5) carrying pLA-traG::lacZ vector, Kanr Tcr | This study |
| A6(traMk588 pDSK-traR2 pLA-traG::lacZ) | A6(traMk588, pDSK-traR2) carrying pLA-traG::lacZ vector, Kanr Tcr | This study |
| C58C1 | Derivative of nopaline strain C58 cured of Ti plasmid used for conjugation analysis, Rifr | S. Q. Pan |
| Ach5C3 | Derivative of wild-type octopine strain Ach5, Ti plasmid cured, Rifr | S. K. Farrand |
| Ach5C3(pLA-traG::lacZ) | Ach5C3 carrying pLA-traG::lacZ vector, Tcr | This study |
| Ach5C3(pLA-traG::lacZ pDSK-traR2) | Ach5C3(pLA-traG::lacZ) carrying pDSK-traR2 vector, Kanr Tcr | This study |
| A6(traMk588, ΔtraI) | A6(traMk588) with in-frame deletion of traI gene | This study |
| A6(traMk588 ΔtraI traI2::Tn5) | A6(traMk588, ΔtraI) with Tn5 insertion of traI2, Knar | This study |
| A6(traMk588 ΔtraI traI2::Tn5 pLA-traI2) | A6(traMk588 ΔtraI traI2::Tn5) carrying plasmid pLA-traI2, Knar Tcr | This study |
| A6(traMk588 ΔtraI traI2::Tn5 pLA-traI) | A6(traMk588 ΔtraI traI2::Tn5) carrying plasmid pLA-traI, Knar Tcr | This study |
| C1A6MR1 | Transconjugant of A6(traMk588 ΔtraM2; traR2::Tn5) and C58C1, Rifr Kanr | This study |
| C1A6MR2 | Transconjugant of A6(traMk588 ΔtraM2; traR2::Tn5) and C58C1, Rifr Kanr | This study |
| C1A6MR3 | Transconjugant of A6(traMk588 ΔtraM2; traR2::Tn5) and C58C1, Rifr Kanr | This study |
| C58C1(pTiA6) | C58C1 carrying Ti plasmid of A6 | 25 |
| E. coli | ||
| DH5α (λpir) | supE44 ΔlacU169(ϕ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 λpir | Laboratory collection |
| BW020767(pRL27) | Harbors Tn5 for A. tumefaciens mutagenesis | 49 |
| Plasmids | ||
| pK18mobsacB | Broad-host-range gene replacement vector, Sucr Kanr | 50 |
| pK18-traI | pK18mobsacB harboring deleted traI-flanking region, Kanr | This study |
| pLAFR3 | IncP, broad-host-range cosmid vector, Tcr | 51 |
| pLA-traG::lacZ | pLAR3 carrying traG gene transcriptionally fused with promoterless lacZ, Tcr | 9 |
| pLA-traI | pLAFR3 harboring traI gene from A6, Tcr | This study |
| pLA-traI2 | pLAFR3 harboring traI2 gene from A6, Tcr | This study |
| pDSK | IncQ, broad-host-range plasmid, Kanr | 52 |
| pDSK-traR | pDSK harboring traR gene from A6, Kanr | 9 |
| pDSK-traR2 | pDSK harboring traR2 gene from A6, Kanr | This study |
Abbreviations: Ampr, ampicillin resistant; Kanr, kanamycin resistant; Tcr, tetracycline resistant; Rifr, rifampincin resistant; Sucr, sucrose resistant.
DNA manipulation and plasmid construction.
Plasmids were purified with the Plasmid Miniprep kit as recommended by the manufacturer (Qiagen). PCR product purification and DNA recovery from agarose gel were carried out with the QIAquick PCR Purification kit and the QIAquick Gel Extraction kit (Qiagen), respectively. For gene deletion construct pK18-traI, the DNA fragments flanking traI were separately amplified from A. tumefaciens strain A6 with two PCR primer pairs (5′-GCTCTAGAAGTGGGCGGGAACAGCG/5′-CGGGATCCTGGTGTTGGTATTGGTCGG and 5′-CGGGATCCGTTCGATTATCGCTGACG/5′-GCTCTAGATCTCGGCAATGATGGCATTG [enzyme cut sites are underlined]), digested with BamHI, and ligated with each other for PCR amplification with primers 5′-GCTCTAGAAGTGGGCGGGAACAGCG and 5′-GCTCTAGATCTCGGCAATGATGGCATTG. The PCR products were rescued from agarose gel and cut with XbaI for subsequent ligation with XbaI-digested vector pK18mobsacB. The resultant plasmid was screened by PCR and confirmed by DNA sequencing. For the preparation of expression constructs pLA-traI and pLA-traI2, traI and traI2, respectively, were amplified with PCR primer pairs 5′-CGGGATCCATGCTGATTCTGACCGTCTC/5′-CGGGATCCTCACGCCGCACTCCTCAAC and 5′-GGAATTCCATGCGGATCCTGACCGTTC/5′-GGAATTCACGCCGCGCTCCTCGCCG (enzyme cut sites are underlined), cut with BamHI and EcoRI, and then inserted into the pLAR3 vector by placing traI and traI2 separately under the control of the lac promoter carried by the vector. For the construction of expression plasmid pDSK-traR2, the traR2 gene was cloned from A. tumefaciens A6 with PCR primers 5′-ACCCACCTTACACATCAAGC/5′-CACAAGGTGCGGCATCGTAT, enzymatically digested, and linked into the vector pDSK by placing the gene under the control of the vector-borne lac promoter. pLA-traG::lacZ and pDSK-traR were isolated from A. tumefaciens NT1 (traR tra::lacZ749) (9).
Genetic manipulation of A. tumefaciens.
Plasmids were transformed into A. tumefaciens by electroporation unless otherwise specified. The in-frame deletion of traI from strain A6(traMk588) was carried out as described previously (25). Conjugation analysis was performed on solid plates with octopine (0.2 g/liter) induction as reported previously (25). Tn5 transposon mutagenesis of A. tumefaciens was performed as described previously (27). Ti plasmid conjugation transfer efficiency was examined by octopine induction on solid plates as described previously (25). Tumorigenicity assay of A. tumefaciens on plants was conducted as previously described (28).
Quantification of β-galactosidase activity.
Quantitative analysis of β-galactosidase activity in bacterial cultures of A. tumefaciens strains was conducted as previously described (29). β-Galactosidase activity was measured and expressed in units per 109 CFU.
Detection of AHLs.
The amount of AHL signals produced by bacterial cells was determined as described previously (30). The AHL indicator strain was A. tumefaciens NT1(traR tra::lacZ749), unless stated otherwise. Bacteria were grown overnight at 28°C for measurement of AHL production, and the amount of AHL production was calculated and expressed as the equivalent of 3OC8HSL as described previously (31, 32). Thin-layer chromatography (TLC) analysis of AHLs was carried out as reported previously (33). Briefly, bacterial strains were grown in 90 ml BM medium overnight and the supernatants were extracted with 200 ml ethyl acetate. The organic extracts were then condensed down to 180 μl in methanol. Two-microliter samples were then separated with a 50:50 methanol-water mixture on a C18 TLC plate with 1 μl (1 μM) standard 3OC8HSL. The signals were visualized by overlaying NT1(traR lacZ749) on the TLC plate.
RNA preparation and RT-PCR analysis.
Bacterial strains were cultivated in minimal medium at 28°C with shaking at 200 rpm. When the optical density at 600 nm (OD600) reached approximately 0.5, cells were collected and total RNA was isolated with the RNeasy Minikit (Qiagen). Residual DNA present in the RNA samples was removed by digestion with RNase-free DNase I. The quantity and quality of the RNA samples were examined with NanoDrop ND-1000 (NanoDrop Technologies) and by agarose gel electrophoresis. An aliquot of 0.2 μg of total RNA was serially 10-fold diluted and used as the template for one-step reverse transcription (RT)-PCR analysis (Qiagen). The PCR primer pairs used for RT-PCR analysis were as follows: traM2, 5′-GCGCGGACTTTCAAGCG/5′-AAACCCAGAAGTCCAACCAGC; traR2, 5′-GACTGCACAATACCCGGCC/5′-TCCGCTCCACGTAAACACTC; traI2, 5′-CCCACCTATGTCCTTGCG/5′-CGGTATTGTCAATCGCTACG; traI, 5′-CGACCAATACCAACACCAG/5′-GTTCGAAGCGAAGATCGG.
RESULTS
TraR2 is an additional regulator governing AHL production in A. tumefaciens strain A6.
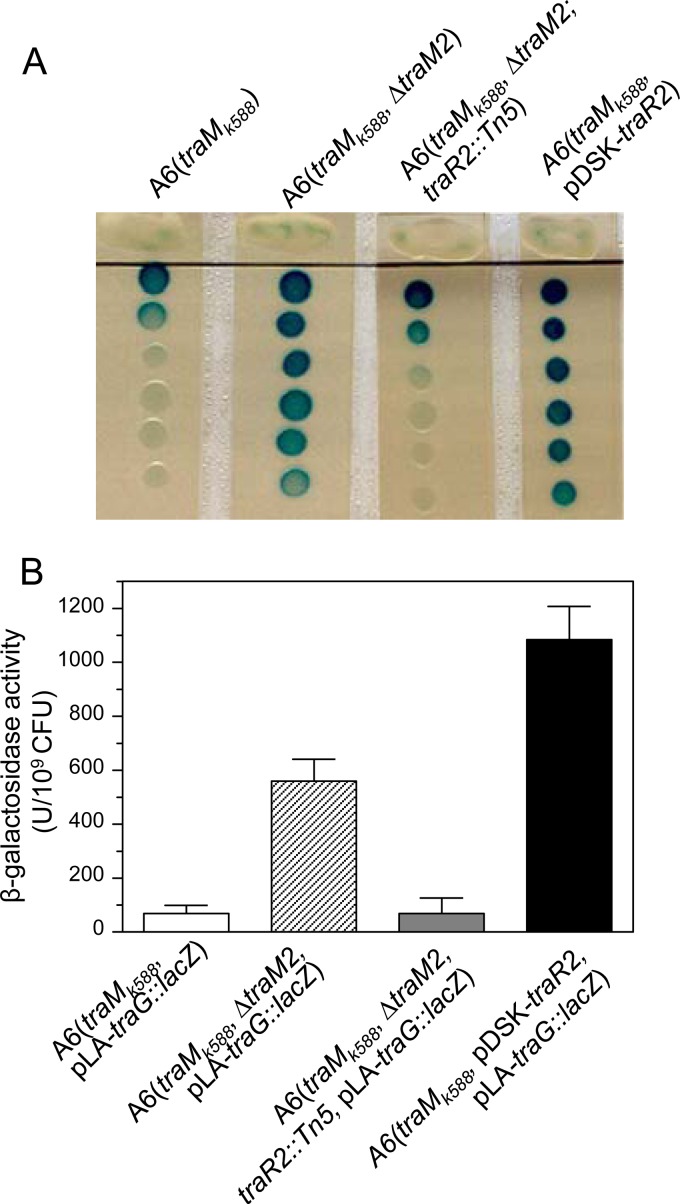
We showed previously that a single amino acid mutation (L54P) in the QS antiactivator TraM is responsible for the constitutive phenotype of A. tumefaciens K588 in QS; i.e., the strain could produce AHL signals and transfer the Ti plasmid in the absence of the cognate conjugal inducer octopine (25). K588 is a strain with a nopaline-type chromosomal background harboring an octopine-type Ti plasmid. To investigate the molecular basis of the QS-constitutive phenotype of A. tumefaciens K588, we introduced the L54P point mutation into TraM of A. tumefaciens wild-type strain A6 by allelic replacement to generate strain A6(traMk588). Because of the presence of traM2 in the chromosomal background of A6, however, A6(traMk588) exhibited QS-related phenotypes indistinguishable from those of strain A6, including the production of AHL signals and the efficiency of Ti plasmid conjugal transfer (25). When cultured on an agar plate, A6(traMk588) could produce a detectable amount of AHLs, and deletion of the second copy of traM (traM2) significantly increased its AHL production (Fig. 1A), which is consistent with the previous findings (25). In this study, we used strain A6(traMk588 ΔtraM2) to explore additional elements involved in QS regulation in A. tumefaciens by using A6(traMk588) as a control. Given that the AHL signals produced by A6(traMk588) in liquid medium without the addition of the inducer octopine were not detectable (25), solid agar plates were used to examine AHL production. Using A6(traMk588 ΔtraM2) as the parent strain, we carried out Tn5 transposon mutagenesis to screen for mutants with altered AHL production. The screening of >10,000 mutants identified 1 showing substantially decreased AHL production, which was comparable to that of A6(traMk588) (Fig. 1A). Analysis of the transposon-flanking sequences showed that the transposon was inserted into an open reading frame (ORF) that encodes a traR homolog, which was designated traR2.
FIG 1.
Characterization of the traR2 gene associated with AHL production in A. tumefaciens A6. (A) AHL production by A6(traMk588) and its derivatives on BM agar plates. (B) β-Galactosidase activity of A6(traMk588 pLA-traG::lacZ) and its derivatives grown in liquid BM medium.
To verify that the decrease in AHL production is due to the defect in traR2, we carried out a phenotypic analysis by the constitutive expression of traR2 in the mutant A6(traMk588). As shown in Fig. 1A, the traR2-overexpressing strain had dramatically enhanced AHL production, comparable to that of A6(traMk588 ΔtraM2), suggesting that TraR2 positively regulates the QS system in A. tumefaciens A6.
To further verify the involvement of traR2 in AHL production in A. tumefaciens A6, we transferred the low-copy-number reporter vector pLA-traG::lacZ, where the promoterless lacZ fragment was transcriptionally fused with the AHL-responsive promoter of the traG gene, into the traR2-deficient strains and then analyzed their AHL production as indicated by their β-galactosidase activity. The results showed that the β-galactosidase activity of A6(traMk588 ΔtraM2) was approximately 100 times that of A6(traMk588). However, when traR2 was mutated in the traM double mutant, its β-galactosidase activity was decreased considerably, to a level comparable to that of the traM single mutant (Fig. 1B). Moreover, overexpression of traR2 in A6(traMk588) dramatically enhanced its β-galactosidase activity to a level even higher than that of parent strain A6(traMk588 ΔtraM2) (Fig. 1B). These findings agree well with the AHL production results (Fig. 1A), supporting the notion that traR2 functions as a positive regulator of QS in A. tumefaciens A6.
In silico analysis showed that traR2 is about 67 and 68% identical to the traR gene of strain A6 at the nucleotide and peptide levels, respectively. The predicted secondary structures of TraR2 appear similar to those of TraR, containing 10 α-helixes and five β-sheets. Inspection of the N-terminal domain sequence of TraR2 showed that it is about 61% identical to the same domain of TraR, suggesting a high degree of conservation of AHL binding sites. Among the 13 amino acids important for ligand binding, 11 are identical between TraR and TraR2 (see Fig. S1B in the supplemental material).
Inspection of the C-terminal domain sequence also showed a high level of conservation between TraR2 and TraR of A. tumefaciens A6, whose amino acid sequences are about 71% identical. However, among the 10 key amino acids of TraR associated with DNA binding, TraR2 contains four varied residues (R206K, V207S, R210E, and M213R) (see Fig. S1B in the supplemental material). According to the X-ray crystal structure of TraR, the side chains of Arg206 and Arg210 make base-specific interactions, forming a hydrogen bond with the bases G and T in the Tra box, respectively, whereas the side chains of Val207 and Met213 form hydrophobic interactions with the backbone sugar of the bases G and T, respectively. Considering that all four of these residues are supposed to be determinants of the specific recognition by TraR of its cognate DNA target, alteration of these amino acids may indicate variations in the DNA recognition specificity and DNA binding affinity of these two QS regulators in A. tumefaciens A6.
The DNA sequences of the traR2-flanking segments were determined by the chromosome walking approach. The sequenced segment stretches a length of ∼6 kb and encodes five putative ORFs. Downstream of traR2, three ORFs were identified that are homologs of traM, traH, and part of traB of the Ti plasmid, respectively, with identities ranging from 66 to 69% (see Fig. S1A in the supplemental material). Moreover, the genetic location and transcriptional orientation of these homologs appeared similar to those of their counterparts in the Ti plasmid, suggesting a highly conserved syntenic organization. However, no obvious ORFs could be defined within the ∼3-kb region upstream of traR2, different from the case of the Ti plasmid, in which ophED is located immediately upstream of traR and constitutes a cotranscriptional operon with traR and upstream genes (see Fig. S1A in the supplemental material).
TraR2 is a functional 3OC8HSL receptor.
For further investigation of the biological roles of traR2 in A. tumefaciens, the traR2 gene was heterogeneously and constitutively activated by the lac promoter of pDSK in Ach5C3, an octopine-type A. tumefaciens strain cured of the Ti plasmid and thus lacking the TraR-, TraM-, and TraI-encoding components of the QS system carried by the Ti plasmid. The AHL reporter plasmid of pLA-traG::lacZ was then introduced to monitor the response of TraR2 to external AHL signals. As shown in Fig. 2, when chemically synthesized 3OC8HSL was added at various concentrations, Ach5C3 and Ach5C3(pLA-traG::lacZ) both exhibited only background levels of β-galactosidase activity. When 3OC8HSL was added to Ach5C3(pDSK-traR2 pLA-traG::lacZ), however, its β-galactosidase activity was substantially increased to a level comparable to that of control strain NT1(pDSK-traR pLA-traG::lacZ), in which the traR gene of the Ti plasmid was constitutively expressed by the lac promoter of vector pDSK. These findings demonstrate that TraR2 alone could also serve as an AHL receptor and activate the typical QS-dependent gene expression in A. tumefaciens. Additionally, it was also noticed that the β-galactosidase activity of Ach5C3(pDSK-traR2 pLA-traG::lacZ) was much lower at low concentrations of 3OC8HSL but became relatively higher at high concentrations of 3OC8HSL than that of NT1(pDSK-traR pLA-traG::lacZ), respectively (Fig. 2), suggesting that the divergence in the DNA-binding domain may affect their promoter binding affinity. These results may suggest that TraR and TraR2 could play different roles under various physiological conditions of AHL signals.
FIG 2.
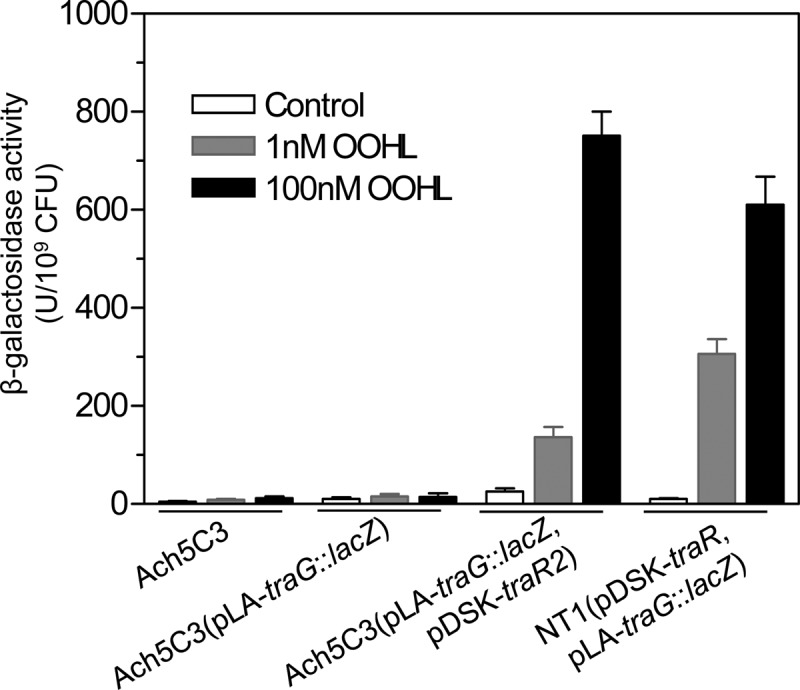
Functional analysis of TraR2. The β-galactosidase activities of Ach5C3 and its relevant derivatives were determined after incubation with various concentrations of 3OC8HSL signal molecules.
A. tumefaciens A6 contains a traI homolog traI2.
In species such as Rhizobium leguminosarum, several sets of AHL-type QS systems have been identified (36). The identification of second copies of traM and traR tempted us to assume that there may exist another copy of traI in A. tumefaciens A6. To test this assumption, we deleted the traI gene of the Ti plasmid from A6(traMk588) and then examined its AHL production. Deletion of traI did not significantly affect AHL production on solid BM plates, indicating the existence of another copy of traI in A. tumefaciens A6 (see Fig. 4A). For identification of the traI homolog, A6(traMk588 ΔtraI) was used as the parent strain for Tn5 transposon mutagenesis to screen for AHL-defective mutants. Among 5,000 transposon mutants screened, 1 was isolated that could not produce detectable AHLs on solid BM plates (Fig. 3). As could be expected, analysis of transposon-flanking sequences showed Tn5 inserted within a traI homolog, which was thus named traI2. The traI2 gene encodes a peptide of 212 amino acids with a deduced molecular mass of 23.6 kDa. Sequence alignment showed that the TraI2 peptide is about 69% identical to the peptide encoded by the traI gene of A. tumefaciens A6. Compared with TraI, both ends of TraI2 appeared highly variable while the central part was relatively conserved (see Fig. S2B in the supplemental material). Similar to the sequence downstream of traI of the Ti plasmid, the sequence downstream of traI2 contains the homologs of trbB, trbC, trbD, and trbE, respectively, which likely form a cotranscriptional operon together with traI. However, no typical ORFs could be found upstream of the traI2 gene, which was different from the corresponding region of the Ti plasmid, in which the rep operon containing repA, repB, and repC was divergently oriented in the region upstream of the traI gene (see Fig. S2A).
FIG 4.
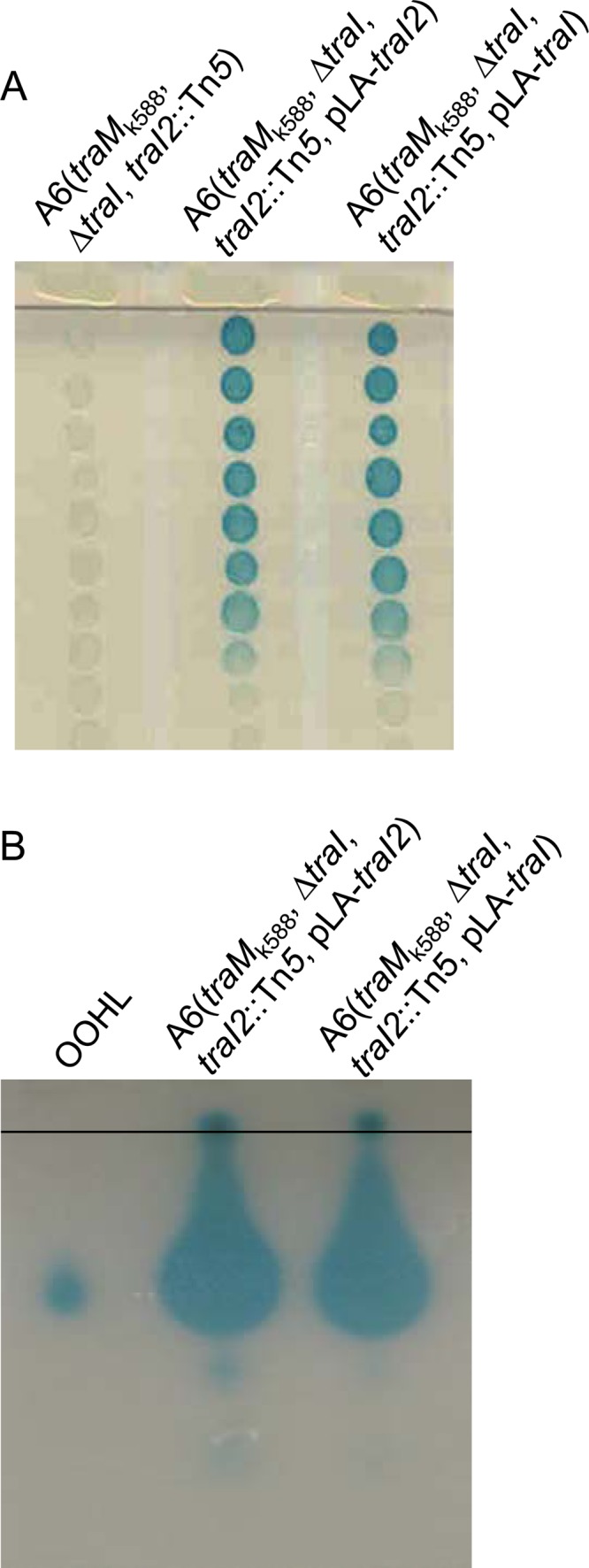
TraI2 is an AHL synthase. (A) AHL production by A6(traMk588 ΔtraI traI2::Tn5) and its derivatives on BM agar plates. (B) TLC analysis of the AHL molecules produced by A6(traMk588 ΔtraI traI2::Tn5) and its derivatives with chemically synthesized 3OC8HSL as the standard control.
FIG 3.
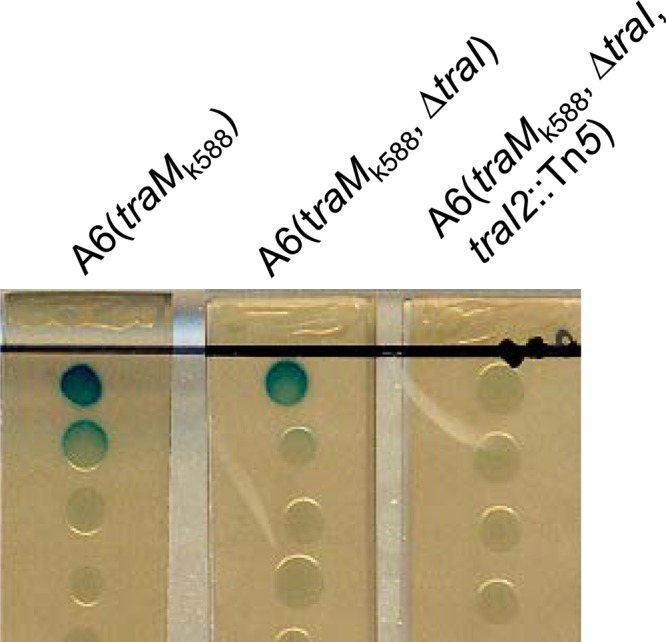
AHL production by A6(traMk588) and its derivatives on BM agar plates.
TraI2 is a 3OC8HSL synthase.
To examine the biological function of TraI2 in A. tumefaciens A6, we overexpressed the traI2 and traI genes, respectively, in the mutant A6(traMk588 ΔtraI traI2::Tn5) and determined its AHL production on solid BM plates. As shown in Fig. 4A, the traI2-overexpressing strain produced a large amount of AHLs, indistinguishable from that of the traI-overexpressing strain. This result demonstrates that TraI2 not only synthesized active AHLs but also synthesized the signals as efficiently as TraI in the background of octopine-type strain A6. Given that various active AHLs have been found in A. tumefaciens and the solid-plate-based bioassay system is not able to distinguish these signals, we used a TLC-based assay to analyze the AHLs produced by TraI2 and TraI in A. tumefaciens A6. The results showed that both TraI2 and TraI synthesized predominantly 3OC8HSL signals (Fig. 4B). These findings indicate that TraI2 is a functional 3OC8HSL synthase and thereby could participate in the regulation of Ti plasmid conjugal transfer.
QS2 is independent of octopine induction.
It has been well established that the transcription of traR on the Ti plasmid is induced by octopine and controlled by QS itself in octopine-type A. tumefaciens strains. In the presence of octopine, the transcription of traR is activated and its product, TraR, forms a complex with the AHL signal 3OC8HSL. The TraR-AHL complex further increases the transcriptional expression of traI, traM, and traR. In this way, the QS regulatory mechanism forms a positive feedback loop triggered by octopine. The parallel roles of TraM2 and TraM, TraR2 and TraR, and TraI2 and TraI tempted us to examine the potential relationship of octopine induction and QS2 regulation. For this purpose, we first examined the AHL production of A6(traMk588) and A6(traMk588 ΔtraI) with or without octopine induction. As shown in Fig. 5A, octopine treatment dramatically increased the AHL production of A6(traMk588) but only marginally increased that of A6(traMk588 ΔtraI). These sharply contrasting results did not seem to be in favor of the notion that QS2, which includes TraM2, TraR2, and TraI2, could be induced by octopine. For further validation, RT-PCR was carried to study the transcription of the QS2-related genes in strain A6(traMk588) with or without octopine. As a control, the transcription of traI was dramatically enhanced by octopine treatment; in contrast, however, the levels of traM2, traR2, and traI2 transcripts did not show obvious changes regardless of the presence or absence of octopine (Fig. 5B). Taken together, these data demonstrate that the regulation of QS2 is independent of octopine induction, which is different from the regulatory mechanism of the Ti plasmid-borne QS system.
FIG 5.
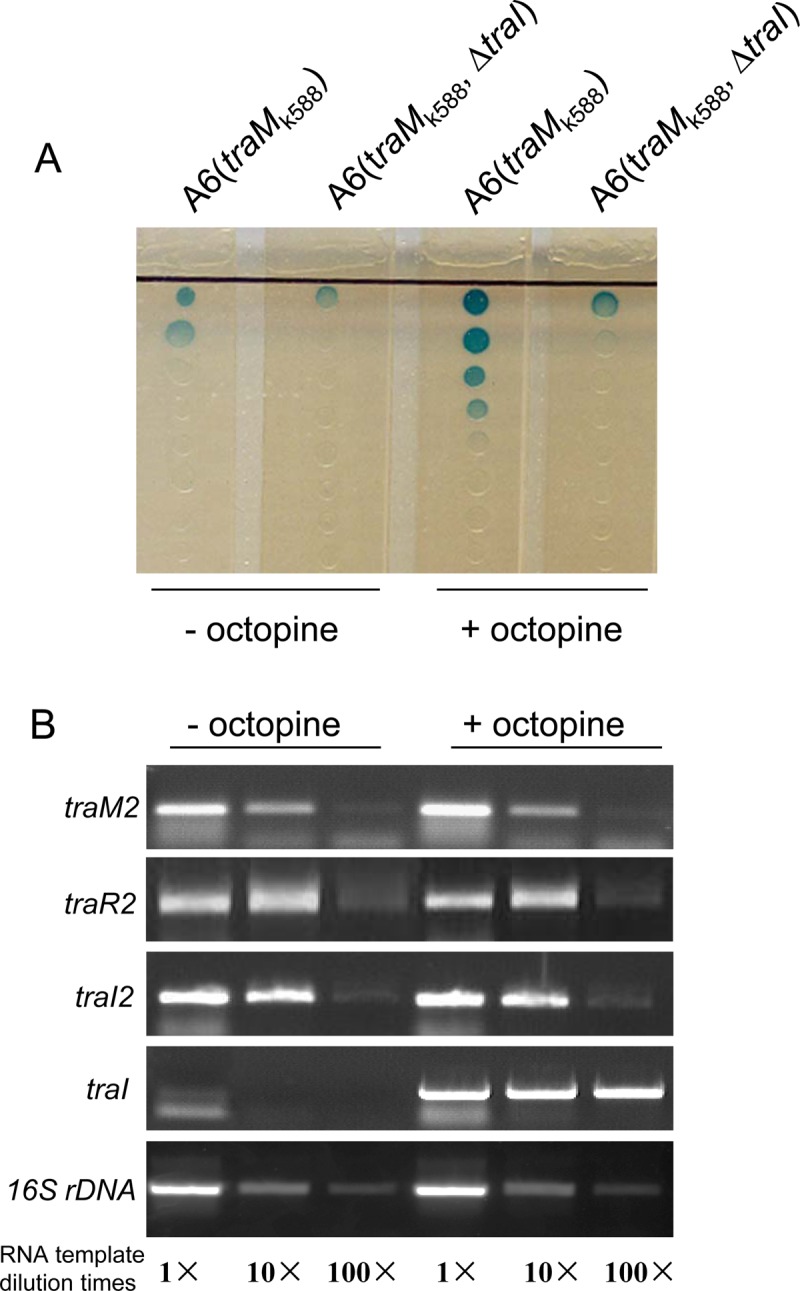
The QS2 system is independent of octopine induction in A. tumefaciens A6. (A) AHL production by A6(traMk588) and A6(traMk588 ΔtraI) with (+) or without (−) octopine induction on BM agar plates. (B) RT-PCR analysis of traM2, traR2, and traI2 expression with (+) or without (−) octopine induction with traI as a control. A6(pTiA6traMK588) cells were cultivated in BM medium at 28°C with or without octopine induction. Samples were collected at OD600 of 1.2 for total RNA isolation. RT-PCR analysis was carried out with the primers described in Materials and Methods. Three concentrations of RNA templates, including an original concentration of 20 ng/μl and its 10× and 100× dilutions, were used for the comparative RT-PCR analysis.
QS2 components are located on a transmissible genetic element.
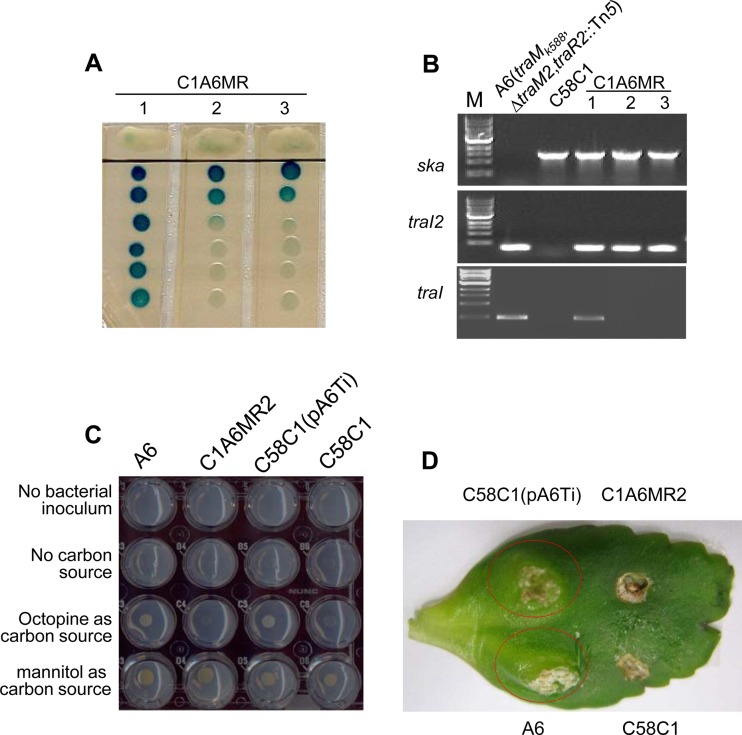
In A. tumefaciens strains, all of the QS regulatory genes are mapped on the Ti plasmid and thus these components are capable of transfer among the bacterial population via Ti plasmid conjugation. The tight linkage of traM2, traR2, and traI2 with other homologs of the tra and trb genes suggested that the QS2 components may be located on a plasmid that is transmissible. To examine this hypothesis, we carried out the Ti plasmid conjugation assay with A6(traMk588 ΔtraM2 traR2::Tn5) as the donor strain and C58C1 as the recipient strain. The donor strain was resistant to kanamycin because the Tn5 transposon was inserted into the traR2 gene while the recipient strain was resistant to rifampin because of spontaneous mutation. After conjugation overnight with octopine induction, transconjugants were screened on LB agar plates supplied with kanamycin and rifampin. After 3 days of incubation at 28°C, single colonies appeared on the plates at a frequency of approximately 3.5 × 10−8. Three transconjugants were randomly picked up for further phenotypic characterization. To test if traI2 was transferred into these transconjugants, we detected their AHL production on the plate and found that all three of the transconjugants could produce detectable AHLs (Fig. 6A). PCR analysis showed that all of these colonies contained the traI2 gene. As a control, the ska gene, which is present in C58C1 but absent from A6, could be successfully amplified from the transconjugants (Fig. 6B), supporting the idea that these colonies are nopaline-type C58C1 but not octopine-type A6 colonies. Additionally, the traI2 and traB2 segments, which are specific to the donor strain A6(traMk588 ΔtraM2 traR2::Tn5) but absent from the recipient strain C58C1, could also be amplified from these colonies, indicating that the transconjugants carried the traI2-containing fragment (Fig. 6B). Given that the traB2 gene is physically linked with traM2 and traR2 (see Fig. S1A in the supplemental material), the above-described results also support the idea that the QS2 components (namely, traM2, traR2, and traI2) may reside on a single transmissible genetic unit. It was noted that the traI gene, which is unique to the donor strain, could also be amplified from one of the transconjugants, suggesting that this transconjugant also contains the Ti plasmid, which may account for the AHL overproduction phenotype of this transconjugant (Fig. 6A). Put together, these data demonstrate that the QS2 components are located on a transmissible genetic element, but its identity remains elusive. Using the transconjugant carrying this transmissible element alone, we tested the potential roles of this mobile genetic element. However, the results showed that this transmissible unit neither enabled the bacterial species to utilize octopine as its sole carbon source (Fig. 6C) nor incited crown gall tumor formation on a host plant (Fig. 6D).
FIG 6.
The QS2 genes are located on a transmissible genetic element in A. tumefaciens A6. (A) AHL production by three transconjugants (C1A6MR1 to C1A6MR3) obtained after conjugal mating with A6(traMk588 ΔtraM2 traR2::Tn5) as the donor strain and C58C1 as the recipient strain. (B) PCR examination of the donor, the recipient, and their transconjugants. The ska gene, which is unique to nopaline strain C58C1 and absent from octopine strain A6, and the traI2 and traI fragments, which are present in A6 but absent from C58C1, were, respectively, amplified by PCR. Lanes M contained molecular size markers. (C) Bacterial growth of various A. tumefaciens strains with the carbon sources indicated. Results were recorded after 5 days of growth at 28°C. (D) Tumorigenicity assay of A. tumefaciens on kalanchoe leaves. Strains A6, C58C1, C1A6MR2, and C58C1(pTiA6) were grown in LB overnight and diluted to an OD600 of 1.0. Ten-microliter volumes of bacterial cultures were inoculated onto wounded kalanchoe leaves. The plant was grown at 24°C for 4 weeks before results were recorded, and the tumors incited by A6 and C58C1(pTiA6) are circled in red.
Using A6(traMk588 ΔtraM2 traR2::Tn5) as the donor strain and C58C1 as the recipient strain, we also conducted a conjugation assay in the absence of octopine. However, no transconjugants were obtained on LB agar plates containing kanamycin and rifampin. These results suggest that octopine was essential for inducing the transmission of a QS2-containing genetic element. In A. tumefaciens A6, octopine has been known to trigger the expression of TraR, which could directly activate the tra2 promoters, or it may activate only the tra promoters and subsequently the Tra proteins detect oriC for transmission of the QS2-containing genetic element. Further investigations are required to distinguish these two possibilities.
DISCUSSION
QS has been well established as a conserved regulatory mechanism for bacterial populations to coordinately regulate gene expression. The QS of A. tumefaciens could be one of the best-characterized paradigms of QS systems. Several proteins, including TraI, TraR, and TraM, constitute the crucial components of QS regulation in this bacterium. TraI is an enzyme that synthesizes the signal molecule 3OC8HSL, which activates TraR. Activated TraR induces the transcriptional expression of its target genes, including the tra, trb, and rep operons. Moreover, the transcription of traR is initiated by cognate conjugal opines (37). In the octopine strains, placing traI within the QS-responsive trb operon constitutes a classic QS positive feedback loop found in many Gram-negative bacteria (38). In addition, a negative feedback loop is also formed by the TraM antiactivator, whose transcription is directly activated by the QS system (16). Previous data showed that these QS regulatory genes, including traM, are all located on the Ti plasmid. Recently, we identified TraM2 as an additional QS regulator that is not encoded by the Ti plasmid (25). TraM2 and its paralog TraM dually control Ti plasmid conjugation in A. tumefaciens A6. In this study, we continued to use the A6 strain to study the QS systems in A. tumefaciens, and we identified TraR2 and TraI2 as additional QS regulators in this bacterial species. TraR2, like TraR, recognizes 3OC8HSL and activates the QS-responsive reporter (Fig. 2). TraI2, similar to TraI, efficiently synthesizes 3OC8HSL in A. tumefaciens A6 (Fig. 4). Together with previously identified TraM2, TraR2 and TraI2 appear to constitute QS2 in A. tumefaciens A6. However, the regulation of QS2 is independent of octopine (Fig. 5), which differs from that of the Ti plasmid-borne QS system and highlights the complexity of QS regulation in A. tumefaciens. The next challenges would be to unravel the molecular mechanisms involved in QS2 regulation and to determine the extent to which these two QS systems could interact.
Identification of QS2 in this study also highlights the variations of QS systems in A. tumefaciens. Several previous lines of evidence have demonstrated the strain-specific variations of QS regulation in A. tumefaciens. First, host plants produce different opines to specifically activate the QS systems of different A. tumefaciens strains. For example, octopine and agrocinopine are the QS inducers of strains A6 and C58, respectively (20). Second, opine receptors may act differently in different strains. In A6, the receptor is OccR, which could act as an activator. In C58, however, the receptor is AccR, which functions only as a repressor. Third, the TrlR antagonist is absent from the C58 strain, while the TraM antiactivator is present only in octopine strains. Furthermore, TraM2, a TraM paralog initially identified in A6, was identified in only some of the octopine strains. The identification of TraR2 and TraI2 in A6 but not in other strains, such as C58, B6, K588, and R10, adds another line of evidence for the strain specificity of the QS regulatory mechanisms in A. tumefaciens.
The identification of QS2 in A. tumefaciens is not the first example of a bacterial species with more than one AHL-type QS system. In R. leguminosarum, four QS systems have been identified and their roles are intertwined in a complex regulatory network regulating plasmid transfer, nodulation, bacterial growth, and other (unknown) functions (36, 37, 39, 40). In R. etli CNPAF512, two QS systems have been found that are involved in the regulation of symbiosome development and nitrogen fixation (41, 42). In these examples, each of the QS systems appeared to produce different AHL signal molecules and control different biological functions. In contrast, however, TraI2 of QS2 seemed to play a role identical to that of TraI of QS, with both synthesizing predominantly 3OC8HSL in A. tumefaciens A6. Moreover, TraR2 appeared to recognize 3OC8HSL and activate the tra box-containing promoters as efficiently as TraR. Put together, these results strongly suggest a signaling cross talk between QS and QS2 and we conclude that QS2 may play a role that is redundant with respect to that of QS in the regulation of Ti plasmid replication and conjugation. To date, however, we have not been able to rule out the possibility that QS2 is also involved in the regulation of other bacterial behaviors that are independent of QS control.
About 2 decades ago, we reported that octopine strains can be divided into two groups, transfer efficient (Trae) and transfer inefficient (Traie), on the basis of their Ti plasmid conjugal transfer efficiency (26). The Trae group includes strains A6, NCPPB1001, and K608, whereas the Traie group consists of B6S3, Ach5, and B6. It is interesting that Trae strain K608 is a conjugal transfer constitutive mutant (26) that is similar to strain K588, used in this study, which contains an L54P point mutation in TraM, and that QS2 genes were found in Trae strain A6 but not in Traie strains B6S3, Ach5, and B6 (25). Therefore, the identification of the QS2 system in strain A6 in this study and the previous findings on the impact of the L54P mutation in TraM on QS (25) seem to provide two molecular mechanisms that could affect the conjugal transfer efficiency of the Ti plasmid in A. tumefaciens.
A cluster of genes in symbiotic plasmid pNGR234a of Rhizobium sp. strain NGR234 has been found to be highly homologous to the tra and QS system of A. tumefaciens (43, 44). Given the fact that the key QS regulators, i.e., TraI, TraR, and TraM, identified in this Rhizobium strain are the orthologs of A. tumefaciens, the QS of Rhizobium sp. strain NGR234 is proposed to regulate the transfer of pNGR234a (37). It has been demonstrated that the TraI homolog synthesizes 3OC8HSL in NGR234 (45). However, a traI mutant still produces an AHL signal that is likely to be 3OC8HSL, suggesting the presence of one or more additional AHL synthetases in this bacterial strain. Further analysis showed that a synthetase gene should reside elsewhere in the genome, but its identity has not yet been characterized (37). Our findings that TraI2 resides outside the Ti plasmid and predominantly produces 3OC8HSL in A. tumefaciens A6 may imply that strain NGR234 may also encode a TraI homolog, i.e., TraI2, that is responsible for the observed 3OC8HSL production of its traI mutant.
The results of this study show that the QS2 component genes (traR2, traI2, and traM2) are physically linked on a transmissible genetic unit. Given that their counterparts traR, traI, and traM are carried by the Ti plasmid, which is conjugally mobile (38), we speculated that the QS2 system genes could also be contained by a plasmid similar to the Ti plasmid. Using an approach described previously for Ti plasmid isolation (26), however, we failed to isolate the putative plasmid. One possible explanation for this failure is that this plasmid is too large to be isolated by routine methods, and the other possibility is that the QS2 system is carried not on a plasmid but in an integrative conjugative element, similar to the transmissible QS system in Serratia marcescens (46). It has been suggested that the multiple QS systems in most bacteria appear to have been acquired from separate sources (47, 48). Further characterization of the mobile genetic element that carries the QS2 genes may shade light on the origin of the QS2 system and the biological roles of this intriguing mechanism.
Supplementary Material
Footnotes
Published ahead of print 24 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01351-13.
REFERENCES
- 1.Fuqua C, Greenberg EP. 2002. Listening in on bacteria: acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 3:685–695. 10.1038/nrm907 [DOI] [PubMed] [Google Scholar]
- 2.Zhang LH. 2003. Quorum quenching and proactive host defense. Trends Plant Sci. 8:238–244. 10.1016/S1360-1385(03)00063-3 [DOI] [PubMed] [Google Scholar]
- 3.Gheysen G, Montagu MV, Zambryski P. 1987. Integration of Agrobacterium tumefaciens transfer DNA (T-DNA) involves rearrangements of target plant DNA sequences. Proc. Natl. Acad. Sci. U. S. A. 84:6169–6173. 10.1073/pnas.84.17.6169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCullen CA, Binns AN. 2006. Agrobacterium tumefaciens and plant cell interactions and activities required for interkingdom macromolecular transfer. Annu. Rev. Cell Dev. Biol. 22:101–127. 10.1146/annurev.cellbio.22.011105.102022 [DOI] [PubMed] [Google Scholar]
- 5.Pitzschke A, Hirt H. 2010. New insights into an old story: Agrobacterium-induced tumour formation in plants by plant transformation. EMBO J. 29:1021–1032. 10.1038/emboj.2010.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai CS, Winans SC. 2010. LuxR-type quorum-sensing regulators that are detached from common scents. Mol. Microbiol. 77:1072–1082. 10.1111/j.1365-2958.2010.07279.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuqua WC, Winans SC. 1994. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 176:2796–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang I, Li PL, Zhang L, Piper KR, Cook DM, Tate ME, Farrand SK. 1994. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc. Natl. Acad. Sci. U. S. A. 91:4639–4643. 10.1073/pnas.91.11.4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piper KR, Beck von Bodman S, Farrand SK. 1993. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature 362:448–450. 10.1038/362448a0 [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Murphy PJ, Kerr A, Tate ME. 1993. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature 362:446–448. 10.1038/362446a0 [DOI] [PubMed] [Google Scholar]
- 11.Qin Y, Luo ZQ, Smyth AJ, Gao P, Beck von Bodman S, Farrand SK. 2000. Quorum-sensing signal binding results in dimerization of TraR and its release from membranes into the cytoplasm. EMBO J. 19:5212–5221. 10.1093/emboj/19.19.5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang I, Cook DM, Farrand SK. 1995. A new regulatory element modulates homoserine lactone-mediated autoinduction of Ti plasmid conjugal transfer. J. Bacteriol. 177:449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pappas KM, Winans SC. 2003. The RepA and RepB autorepressors and TraR play opposing roles in the regulation of a Ti plasmid repABC operon. Mol. Microbiol. 49:441–455. 10.1046/j.1365-2958.2003.03560.x [DOI] [PubMed] [Google Scholar]
- 14.Pappas KM, Winans SC. 2003. A LuxR-type regulator from Agrobacterium tumefaciens elevates Ti plasmid copy number by activating transcription of plasmid replication genes. Mol. Microbiol. 48:1059–1073. 10.1046/j.1365-2958.2003.03488.x [DOI] [PubMed] [Google Scholar]
- 15.Farrand SK. 1998. Conjugation in the Rhizobiaceae, p 199–233 In Spaink HP, Kondorosi A, Hooykaas PJJ. (ed), The Rhizobiaceae: molecular biology of model plant-associated bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands [Google Scholar]
- 16.Hwang I, Smyth AJ, Luo ZQ, Farrand SK. 1999. Modulating quorum sensing by antiactivation: TraM interacts with TraR to inhibit activation of Ti plasmid conjugal transfer genes. Mol. Microbiol. 34:282–294. 10.1046/j.1365-2958.1999.01595.x [DOI] [PubMed] [Google Scholar]
- 17.Luo ZQ, Qin Y, Farrand SK. 2000. The antiactivator TraM interferes with the autoinducer-dependent binding of TraR to DNA by interacting with the C-terminal region of the quorum-sensing activator. J. Biol. Chem. 275:7713–7722. 10.1074/jbc.275.11.7713 [DOI] [PubMed] [Google Scholar]
- 18.Piper KR, Farrand SK. 2000. Quorum sensing but not autoinduction of Ti plasmid conjugal transfer requires control by the opine regulon and the antiactivator TraM. J. Bacteriol. 182:1080–1088. 10.1128/JB.182.4.1080-1088.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petit A, Tempe J, Kerr A, Holsters M, Van Montagu M, Schell J. 1978. Substrate induction of conjugative activity of Agrobacterium tumefaciens Ti plasmids. Nature 271:570–572. 10.1038/271570a0 [DOI] [Google Scholar]
- 20.Ellis JG, Kerr A, Petit A, Tempe J. 1982. Conjugal transfer of nopaline and agropine Ti-plasmids—the role of agrocinopines. Mol. Gen. Genet. 186:269–274. 10.1007/BF00331861 [DOI] [Google Scholar]
- 21.Fuqua C, Winans SC. 1996. Localization of OccR-activated and TraR-activated promoters that express two ABC-type permeases and the traR gene of Ti plasmid pTiR10. Mol. Microbiol. 20:1199–1210. 10.1111/j.1365-2958.1996.tb02640.x [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Helmann JD, Winans SC. 1992. The A. tumefaciens transcriptional activator OccR causes a bend at a target promoter, which is partially relaxed by a plant tumor metabolite. Cell 69:659–667. 10.1016/0092-8674(92)90229-6 [DOI] [PubMed] [Google Scholar]
- 23.Chai Y, Zhu J, Winans SC. 2001. TrlR, a defective TraR-like protein of Agrobacterium tumefaciens, blocks TraR function in vitro by forming inactive TrlR:TraR dimers. Mol. Microbiol. 40:414–421. 10.1046/j.1365-2958.2001.02385.x [DOI] [PubMed] [Google Scholar]
- 24.Oger P, Kim KS, Sackett RL, Piper KR, Farrand SK. 1998. Octopine-type Ti plasmids code for a mannopine-inducible dominant-negative allele of traR, the quorum-sensing activator that regulates Ti plasmid conjugal transfer. Mol. Microbiol. 27:277–288. 10.1046/j.1365-2958.1998.00671.x [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Zhang HB, Chen G, Chen L, Zhang LH. 2006. Dual control of quorum sensing by two TraM-type antiactivators in Agrobacterium tumefaciens octopine strain A6. J. Bacteriol. 188:2435–2445. 10.1128/JB.188.7.2435-2445.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang LH, Kerr A. 1991. A diffusible compound can enhance conjugal transfer of the Ti plasmid in Agrobacterium tumefaciens. J. Bacteriol. 173:1867–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Zhang HB, Wang LH, Zhang LH. 2006. Succinic semialdehyde couples stress response to quorum-sensing signal decay in Agrobacterium tumefaciens. Mol. Microbiol. 62:45–56. 10.1111/j.1365-2958.2006.05351.x [DOI] [PubMed] [Google Scholar]
- 28.Xu XQ, Pan SQ. 2000. An Agrobacterium catalase is a virulence factor involved in tumorigenesis. Mol. Microbiol. 35:407–414. 10.1046/j.1365-2958.2000.01709.x [DOI] [PubMed] [Google Scholar]
- 29.Stachel SE, An G, Flores C, Nester EW. 1985. A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 4:891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong YH, Xu JL, Li XZ, Zhang LH. 2000. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. U. S. A. 97:3526–3531. 10.1073/pnas.97.7.3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang HB, Wang C, Zhang LH. 2004. The quormone degradation system of Agrobacterium tumefaciens is regulated by starvation signal and stress alarmone (p) ppGpp. Mol. Microbiol. 52:1389–1401. 10.1111/j.1365-2958.2004.04061.x [DOI] [PubMed] [Google Scholar]
- 32.Zhang HB, Wang LH, Zhang LH. 2002. Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. U. S. A. 99:4638–4643. 10.1073/pnas.022056699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang F, Wang LH, Wang J, Dong YH, Hu JY, Zhang LH. 2005. Quorum quenching enzyme activity is widely conserved in the sera of mammalian species. FEBS Lett. 579:3713–3717. 10.1016/j.febslet.2005.05.060 [DOI] [PubMed] [Google Scholar]
- 34. Reference deleted.
- 35. Reference deleted.
- 36.Rodelas B, Lithgow JK, Wisniewski-Dye F, Hardman A, Wilkinson A, Economou A, Williams P, Downie JA. 1999. Analysis of quorum-sensing-dependent control of rhizosphere-expressed (rhi) genes in Rhizobium leguminosarum bv. viciae. J. Bacteriol. 181:3816–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.González JE, Marketon MM. 2003. Quorum sensing in nitrogen-fixing rhizobia. Microbiol. Mol. Biol. Rev. 67:574–592. 10.1128/MMBR.67.4.574-592.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu J, Oger PM, Schrammeijer B, Hooykaas PJ, Farrand SK, Winans SC. 2000. The bases of crown gall tumorigenesis. J. Bacteriol. 182:3885–3895. 10.1128/JB.182.14.3885-3895.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lithgow JK, Wilkinson A, Hardman A, Rodelas B, Wisniewski-Dye F, Williams P, Downie JA. 2000. The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol. Microbiol. 37:81–97. 10.1046/j.1365-2958.2000.01960.x [DOI] [PubMed] [Google Scholar]
- 40.Wisniewski JP, Rathbun EA, Knox JP, Brewin NJ. 2000. Involvement of diamine oxidase and peroxidase in insolubilization of the extracellular matrix: implications for pea nodule initiation by Rhizobium leguminosarum. Mol. Plant Microbe Interact. 13:413–420. 10.1094/MPMI.2000.13.4.413 [DOI] [PubMed] [Google Scholar]
- 41.Rosemeyer V, Michiels J, Verreth C, Vanderleyden J. 1998. luxI- and luxR-homologous genes of Rhizobium etli CNPAF512 contribute to synthesis of autoinducer molecules and nodulation of Phaseolus vulgaris. J. Bacteriol. 180:815–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daniels R, De Vos DE, Desair J, Raedschelders G, Luyten E, Rosemeyer V, Verreth C, Schoeters E, Vanderleyden J, Michiels J. 2002. The cin quorum sensing locus of Rhizobium etli CNPAF512 affects growth and symbiotic nitrogen fixation. J. Biol. Chem. 277:462–468. 10.1074/jbc.M106655200 [DOI] [PubMed] [Google Scholar]
- 43.Farrand SK, Hwang I, Cook DM. 1996. The tra region of the nopaline-type Ti plasmid is a chimera with elements related to the transfer systems of RSF1010, RP4, and F. J. Bacteriol. 178:4233–4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freiberg C, Fellay R, Bairoch A, Broughton WJ, Rosenthal A, Perret X. 1997. Molecular basis of symbiosis between Rhizobium and legumes. Nature 387:394–401. 10.1038/387394a0 [DOI] [PubMed] [Google Scholar]
- 45.He X, Chang W, Pierce DL, Seib LO, Wagner J, Fuqua C. 2003. Quorum sensing in Rhizobium sp. strain NGR234 regulates conjugal transfer (tra) gene expression and influences growth rate. J. Bacteriol. 185:809–822. 10.1128/JB.185.3.809-822.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei JR, Tsai YH, Horng YT, Soo PC, Hsieh SC, Hsueh PR, Horng JT, Williams P, Lai HC. 2006. A mobile quorum-sensing system in Serratia marcescens. J. Bacteriol. 188:1518–1525. 10.1128/JB.188.4.1518-1525.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray KM, Garey JR. 2001. The evolution of bacterial LuxI and LuxR quorum sensing regulators. Microbiology 147:2379–2387 http://mic.sgmjournals.org/content/147/8/2379.long [DOI] [PubMed] [Google Scholar]
- 48.Lerat E, Moran NA. 2004. The evolutionary history of quorum-sensing systems in bacteria. Mol. Biol. Evol. 21:903–913. 10.1093/molbev/msh097 [DOI] [PubMed] [Google Scholar]
- 49.Larsen RA, Wilson MM, Guss AM, Metcalf WW. 2002. Genetic analysis of pigment biosynthesis in Xanthobacter autotrophicus Py2 using a new, highly efficient transposon mutagenesis system that is functional in a wide variety of bacteria. Arch. Microbiol. 178:193–201. 10.1007/s00203-002-0442-2 [DOI] [PubMed] [Google Scholar]
- 50.Simon R, Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Nat. Biotechnol. 1:784–791. 10.1038/nbt1183-784 [DOI] [Google Scholar]
- 51.Staskawicz B, Dahlbeck D, Keen N, Napoli C. 1987. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J. Bacteriol. 169:5789–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keen NT, Tamaki S, Kobayashi D, Trollinger D. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191–197. 10.1016/0378-1119(88)90117-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.