Abstract
The nontyphoidal Salmonella enterica serovar Dublin is adapted to cattle but infrequently infects humans, very often resulting in invasive infections with high levels of morbidity and mortality. A Salmonella-induced intestinal acute inflammatory response is postulated as a mechanism to prevent bacterial dissemination to systemic sites. In S. enterica serovar Typhimurium, flagella contribute to this response by providing motility and FliC-mediated activation of pattern recognition receptors. In this study, we found 4 Salmonella enterica isolates, with the antigenic formula 9,12:−:−, that, based on fliC sequence and multilocus sequence type (MLST) analyses, are aflagellate S. Dublin isolates. Interestingly, all were obtained from human bloodstream infections. Thus, we investigated the potential role of flagella in the unusual invasiveness exhibited by S. Dublin in humans by analyzing flagellation and proinflammatory properties of a collection of 10 S. Dublin human clinical isolates. We found that 4 of 7 blood isolates were aflagellate due to significantly reduced levels of fliC expression, whereas all 3 isolates from other sources were flagellated. Lack of flagella correlated with a reduced ability of triggering interleukin-8 (IL-8) and CCL20 chemokine expression in human intestinal Caco-2 cells and with reduced early inflammation in the ceca of streptomycin-pretreated C57/BL6 mice. These results indicate that flagella contribute to the host intestinal inflammatory response to Salmonella serovar Dublin and suggest that their absence may contribute to its systemic dissemination through dampening of the gut immune response. Analysis of FliC production in a collection of cattle isolates indicated that the aflagellate phenotype is widely distributed in field isolates of S. Dublin.
INTRODUCTION
Salmonellosis by nontyphoidal salmonellae (NTS) is among the most common food-borne infections worldwide, with an estimated global incidence of 93.8 million cases of gastroenteritis and 155,000 deaths per year (1). Among the >2,500 serotypes assigned to the species Salmonella enterica, those able to cause disease in humans are divided into typhoidal serotypes (mainly Typhi and Paratyphi A) and hundreds of NTS serotypes, among which the most prevalent worldwide are Enteritidis and Typhimurium (2, 3). The typhoidal serotypes are restricted to the human host and cause invasive disease in immunocompetent individuals, while NTS typically have a broad vertebrate host range and cause predominantly a self-limiting gastroenteritis, characterized by acute intestinal inflammation and diarrhea, in humans. However, depending on the immune status of the host and the serovar and particular strain involved, NTS can enter the bloodstream and cause systemic disease. In addition, NTS are consistently the most common bacteria isolated from the bloodstream in both adults and children presenting with fever in sub-Saharan Africa, and this is associated with HIV, malaria, and malnutrition (4). In Uruguay, from a total of 495 NTS clinical isolates received at the National Salmonella Centre (NSC) from 2008 to 2012, 96 (19.4%) were obtained from bloodstream infections (NSC, unpublished data).
When the invasive index is considered, i.e., the percentage of blood isolates relative to the total number of isolates for each Salmonella serovar in a defined geographical region, it becomes evident that certain NTS serovars are more invasive than others (2, 5, 6). This is the case for Salmonella serovars Dublin and Choleraesuis, which are consistently reported worldwide, with invasive indexes of >40%, while the invasive indexes of the more ubiquitous Salmonella serovars Enteritidis and Typhimurium rarely exceed 5% (2, 5, 7, 8).
S. enterica serovar Dublin (formula 1,9,12 [Vi]:g,p:−) is strongly adapted to cattle, where it causes systemic infections and abortion (9). However, human infections with S. Dublin occur at low frequency, and these are often severe and may be fatal, especially in AIDS patients and other immunocompromised persons (10–12). The bacterial factors responsible for this invasive phenotype of S. Dublin in humans are unknown.
The difference in clinical outcome between typhoidal salmonellae and NTS is thought to be due partly to the way both groups of serovars interact with the gut epithelium, with the NTS causing an acute local inflammatory reaction with a strong neutrophil influx that prevents systemic spread of the bacteria (2, 13, 14).
For Salmonella enterica serovar Typhimurium, several bacterial factors have been described as important for triggering the inflammatory response at the gut in calves and streptomycin-pretreated mice, including SPI-1 (15–17) and SPI-2 (18–20) effectors and flagella (21, 22).
The flagellin protein (FliC) is the main structural component of the flagellar filament and is also the agonist of the pattern recognition receptors Toll-like receptor 5 (TLR5) and Naip5/NLRC4 (23–27). In model human intestinal epithelia, it has been demonstrated that S. Typhimurium FliC binding to TLR5 triggers a proinflammatory response characterized by upregulation of expression of interleukin 8 (IL-8), CCL20 (macrophage inflammatory protein 3α [MIP-3α]), and several other proinflammatory chemokines (25, 28) that recruit neutrophils and dendritic cells into the subepithelial compartment (29, 30). In vivo, it has been reported that in streptomycin-pretreated mice, S. Typhimurium flagellin knockout mutants trigger diminished intestinal inflammation compared to that with wild-type bacteria at early time points postinfection (p.i.) (21, 31). Similar findings were obtained with calf ligated ileal loops (22, 32) and chickens (33). Interestingly, a mechanism has been described for the invasive serovar S. Typhi through which fliC expression is repressed when the bacterium encounters conditions of tissue osmolarity (but not the higher osmolarity present in the intestinal lumen), thus enabling it to overcome the mucosal barrier at the intestine through innate immunity evasion (34).
The aim of this work was to elucidate bacterial factors responsible for the unusual invasiveness exhibited by S. Dublin in humans, focusing on the role of flagella. Thus, we characterized the motility, flagellar expression, and proinflammatory properties of a set of S. Dublin clinical isolates obtained at different times and from invasive or localized infections and evaluated the correlation between flagellar expression and the source of the isolate (blood versus nonblood). Our results revealed the existence of a large proportion of aflagellate strains among the clinical isolates of this serovar, a phenotype that correlated with impaired proinflammatory properties in vitro and in vivo. Note that all aflagellate strains were isolated from invasive cases of salmonellosis.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Uruguayan clinical isolates of S. Dublin were obtained from the NSC and the Bacteriology Unit of the Ministry of Public Health (MPH) collections (Table 1). These were all the human isolates of this serovar available in both collections and included seven isolated from blood and three from other sources (feces or urine). “Invasive” cases were defined as those in which Salmonella had been isolated from the bloodstream. An isolate obtained from urine was not considered invasive because we lacked the clinical data to distinguish between invasive infection and colonization or contamination of urine. Where available, data about the patients from whom the isolates were obtained are depicted in Table 1.
TABLE 1.
S. Dublin field isolates used in this study
| Isolate | Yr of isolation | Sourceb | Patient informationb |
|---|---|---|---|
| Human isolatesa | |||
| SDu1 | 1995 | Blood | ND |
| SDu2 | 2004 | Blood | ND |
| SDu3 | 2006 | Blood | Female, 41 years old, asthmatic, diabetic |
| SDu4 | 2008 | Blood | Young man, drug addict |
| SDu5 | 2000 | Feces | ND |
| SDu6 | 2005 | Feces | Male, 40 years old |
| SDu7 | 2008 | Blood | Male, 1 year old |
| SDu8 | 2011 | Blood | Male, 76 years old |
| SDu9 | 2011 | Blood | Female, 50 years old |
| SDu10 | 2011 | Urine | Male |
| Bovine isolates | |||
| 75/95 | 1995 | ND | |
| 56/96 | 1996 | Calf lymph | |
| 57/96 | 1996 | Calf lung | |
| 69/96 | 1996 | Calf | |
| 72/96 | 1996 | Calf | |
| 73/96 | 1996 | Bile | |
| 74/96 | 1996 | Liver | |
| 75/96 | 1996 | Calf | |
| 76/96 | 1996 | Calf | |
| 79/98 | 1998 | ND | |
| 210/00 | 2000 | Calf | |
| 73/04 | 2004 | Calf | |
| 74/04 | 2004 | Calf | |
| 75/04 | 2004 | Calf | |
| 99/04 | 2004 | Fetus |
All human isolates were of ST10 according to MLST.
ND, no data are available.
S. Dublin cattle isolates were obtained from the NSC and are shown in Table 1. All isolates were confirmed biochemically and serologically at the NSC and the Bacteriology Unit of the MPH. In those cases where no agglutination with anti-H serum was obtained, the sequence of the fliC hypervariable region was determined using primers Gfor and Grev (see Table 3) and compared with those available at the GenBank database to confirm the Dublin serotype. In addition, multilocus sequence typing (MLST) was performed as described at http://mlst.ucc.ie/mlst/dbs/Senterica/documents/primersEnterica_html (35).
TABLE 3.
Primers used in this study
| Primer | Sequence (5′ → 3′) | Amplicon size (bp) | Reference |
|---|---|---|---|
| Gfor | GTGATCTGAAATCCAGCTTCAAG | 509 | 58 |
| Grev | AAGTTTCGCACTCTCGTTTTTGG | ||
| icdA F | TGGTATCGGTGTTGATGTCACTC | 140 | Modified from reference 59 |
| icdA R | CATCCTGGCCGTAAACCTGTGTG | ||
| fliA F | GCATCGAACTGGTGACTGAAGAAC | 149 | 59 |
| fliA R | GAGCTCTTCCTGGTAATACAGCGT | ||
| fliC F | AGATCACCTTAGCTGGCAAAACC | 164 | This work |
| fliC R | CCCCAGAGAAGAACGAACTGC | ||
| mB-actin_F | GCTTCTTTGCAGCTCCTTCGT | 68 | 60 |
| mB-actin_R | CGTCATCCATGGCGAACTG | ||
| mCXCL1_F | CTTGGTTCAGAAAATTGTCCAAAA | 84 | 60 |
| mCXCL1_R | ACGGTGCCATCAGAGCAGTCT | ||
| mIL-17A_F | CTCCAGAAGGCCCTCAGACTAC | 69 | 60 |
| mIL-17A_R | GGGTCTTCATTGCGGTGG | ||
| mIFNg_F | TCAGCAACAGCAAGGCGAAA | 143 | 61 |
| mIFNg_R | CCGCTTCCTGAGGCTGGAT | ||
| mCCL20_F | TTTTGGGATGGAATTGGACAC | 69 | 60 |
| mCCL20_R | TGCAGGTGAAGCCTTCAACC | ||
| mTNFalfa_F | CATCTTCTCAAAATTCGAGTGACAA | 63 | 60 |
| mTNFalfa_R | CCTCCACTTGGTGGTTTGCT | ||
| mLcn2_F | CCATCTATGAGCTACAAGAGAACAAT | 89 | 62 |
| mLcn2_R | TCTGATCCAGTAGCGACAGC | ||
| mS100.A9_F | CACCCTGAGCAAGAAGGAAT | 95 | 62 |
| mS100.A9_R | TGTCATTTATGAGGGCTTCATTT |
The hypervariable fliC sequences of the aflagellate S. Dublin isolates described in this study were identical to those of the two sequenced S. Dublin strains available in the GenBank database (strains CT_02021853 and SD3246) and differed in three single nucleotide polymorphisms (SNPs) from the same region of S. Enteritidis strain P125109, whose complete genome is also available in GenBank. Moreover, MLST analysis revealed that all 10 S. Dublin human isolates used in this study belong to ST10 (Table 1), which is the predominant ST reported for S. Dublin (35).
Luria-Bertani (LB) broth and LB agar (Sigma) were used for routine cultures of S. Dublin at 37°C in an orbital shaking incubator (200 rpm). Isolates were stored in replicates at −80°C in LB broth containing 25% glycerol.
Bacterial constructs are depicted in Table 2. An SDu5 derivative fliC knockout mutant was constructed by P22 transduction of a fliC::kan cassette from S. Enteritidis strain PT4 P125109 fliC::kan (36). The resulting strain, SDu5 fliC::kan, was resistant to kanamycin and lacked motility, as verified by plating on soft agar and microscopic visualization.
TABLE 2.
Strain constructs used in this study
| Strain | Description |
|---|---|
| SDu5 fliC::kan | SDu5 derivative containing fliC::kan (Kanr) |
| SDu3 Strr | SDu3 derivative containing aadA (Strr) |
| SDu5 Strr | SDu5 derivative containing aadA (Strr) |
| SDu5 Strr fliC::kan | SDu5 derivative containing aadA and fliC::kan (Kanr Strr) |
For animal studies, field isolates were made streptomycin resistant by P22 phage transduction of the aadA gene from the streptomycin-resistant S. Typhimurium strain SL1344 (http://www.sanger.ac.uk/Projects/Microbes/). The resulting strains were able to grow in 500 μg/ml of streptomycin and were verified by anti-O-antigen serum agglutination and motility testing. In addition, growth curves were performed to verify that genetic manipulation did not affect the growth properties of the original isolates. An SDu5 fliC::kan derivative of the previously constructed streptomycin-resistant SDu5 strain was obtained by P22 transduction. The resulting strain was resistant to kanamycin and lacked motility; the rest of the analyzed parameters (growth rate and O-antigen agglutination) were unchanged compared to those of the parental strain.
For mouse infection experiments, bacteria were grown overnight (o/n) at 200 rpm and 37°C in LB broth containing 50 μg/ml of streptomycin; the o/n cultures were diluted 1:20 in the same medium plus 0.3 M NaCl and subcultured for 4 h with mild aeration (100 rpm).
Motility tests.
Motility tests were performed as described by Yim et al. (37). Briefly, 2 μl of overnight culture grown in LB broth was spotted onto the surface of an LB plate containing 0.3% agar (semisolid agar) and incubated for 6 h at 37°C. Those isolates showing no halo of growth (indistinguishable from a fliC knockout strain) were considered nonmotile. Values are expressed as diameters of growth (in mm) obtained after 6 h of incubation at 37°C. The assays were repeated three times, and the results were confirmed by phase-contrast microscopic visualization of mid-log-phase bacterial cultures grown in LB broth.
Protein analysis.
For preparation of total protein extracts, log-phase bacterial cultures (optical density at 600 nm [OD600] = 0.4 to 0.6) were centrifuged, resuspended in phosphate-buffered saline (PBS) containing 0.15 mM phenylmethylsulfonyl fluoride (PMSF) and 0.5 mM EDTA, sonicated, and centrifuged again to remove unbroken cells. The supernatants (cleared lysates) were quantified by the Bradford assay (Sigma).
For Western blot analysis, 25 μg of total protein extract was loaded onto a 12% SDS-PAGE gel and analyzed by Western blotting using mouse anti-flagellar antigen Hg antibody (Bio-Stat, United Kingdom) and ECL Prime Western blotting detection reagent (GE Healthcare). As an internal control, an anti-DnaK monoclonal antibody was used (AbCam, United Kingdom).
Flagellar staining.
For detection of flagella in live cells, we performed a previously described method using Alexa Fluor 594-carboxylic acid succinimidyl ester (Molecular Probes), an amino-specific fluorescent dye (37). Briefly, overnight cultures of bacteria grown in LB broth at 37°C and 200 rpm were diluted 1/100 in fresh medium and grown under the same conditions to mid-log phase (OD600 = 0.4 to 0.6). The protocol was then followed exactly as described previously (37). The samples were visualized on an Olympus FV300 confocal laser scanning microscope with Fluoview software 4.3, a 100× oil immersion objective (numerical aperture [NA] = 1.35), and 543/610-nm excitation/emission wavelengths. A minimum of 10 fields were recorded for each isolate, using an image size of 1,024 by 1,024 pixels in the x-y plane, with a pixel size of 70 nm. Images were deconvolved with Huygens Professional software.
Cell lines, media, and growth conditions.
The human colon carcinoma Caco-2 cell line was obtained from the American Type Culture Collection (ATCC). The cells were maintained in minimal essential medium with Earle's salts (high glucose; 4.5 g/liter) (PAA Laboratories) supplemented with 2 mM l-glutamine and 20% fetal calf serum (FCS) at 37°C in 5% CO2, at up to 80% confluence.
The Caco-Rumbo reporter cell line consists of the Caco-2 cell line stably transfected with a plasmid harboring the luciferase gene under the control of the human CCL20 promoter (38) and was kindly provided by J. C. Sirard (Université de Lille Nord, France). It was cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM; PAA Laboratories) supplemented with 10% FCS, 1 mM sodium pyruvate, 1× nonessential amino acids, and 0.7 mg/ml G418 (Invitrogen) at 37°C in 5% CO2.
Analysis of the nucleotide sequence of fliC and its promoter region.
To analyze the nucleotide sequence of fliC and its promoter region, we purified genomic DNA from overnight bacterial cultures grown in LB by using a DNeasy Blood and Tissue kit (Qiagen). One hundred nanograms of this DNA was used as the template to PCR amplify a 1,713-bp region comprising the entire fliC coding sequence, as well as 152 bp upstream from the start codon and 42 bp downstream from the stop codon, using primers fliC1 and fliC4 (Table 3), and the resulting amplicon was sequenced using primers fliC1, fliC2, fliC3, and fliC4.
Quantitative real-time PCR (qRT-PCR).
For bacterial mRNA quantifications, strains were grown to mid-log phase and total RNA was extracted using an RNeasy minikit (Qiagen), with a pretreatment with RNAprotect bacterial reagent (Qiagen). One microgram of this RNA was treated with DNase (Invitrogen) and reverse transcribed using Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen) and random primers in a 20-μl reaction mixture, and 2 μl of a 1/32 dilution of this reaction mixture was used for real-time PCR using Sybr green (QuantiTect; Qiagen) in a Corbett thermocycler. Primer sequences used are presented in Table 3. The cycling program was as follows: 15 min at 95°C and 45 cycles of 15 s at 95°C, 30 s at 57°C, and 30 s at 72°C. For the analysis, we used the comparative threshold cycle (CT) method for relative mRNA quantitation (39). Briefly, the CT obtained for the fliC or fliA gene was normalized to the CT obtained for icdA in each sample, giving the ΔCT (ΔCT = CT for fliC or fliA − CT for icdA). The corresponding 2−ΔCT value was calculated for each strain, and the 2−ΔCT values were compared to the 2−ΔCT value obtained for an arbitrary selected motile reference strain (SDu5) to obtain the fold variation in mRNA abundance of the sample relative to the reference (fold variation = 2−ΔCT for sample/2−ΔCT for reference). Each isolate was assayed in triplicate. Non-reverse-transcribed controls rendered no detectable CT values or were amplified at least 10 cycles later than the corresponding reverse-transcribed samples.
For Caco-2 mRNA quantifications, cells were infected with log-phase bacteria at a multiplicity of infection (MOI) of ∼30:1, the plates were centrifuged for 5 min at 200 × g, and invasion was allowed to proceed for 1 h at 37°C in 5% CO2. After three PBS washes, gentamicin (100 μg/ml)-containing medium was added for 1.5 h. The antibiotic concentration was then changed to 10 μg/ml, and the cells were incubated for an additional 1.5 h. At 3 h postinfection, the cells were gently washed 3 times with prewarmed PBS and resuspended in TRIzol (Invitrogen) for extraction of total RNA. After reverse transcription with random hexamers and real-time PCR using specific primers, CT values were normalized to the values for the 18S rRNA gene and referred to values for uninfected cells. Total RNA extraction, reverse transcription, and qRT-PCR were carried out as previously reported (36).
For mRNA quantification in the ceca of infected mice, fractions of the middle cecum were immediately removed after sacrifice, embedded in TRIzol (Invitrogen), and stored at −80°C. For total RNA extraction, the samples were homogenized using an Ultraturrax instrument, and then the protocol indicated by the manufacturer was followed. One microgram of the resulting RNA was treated with DNase and reverse transcribed using MMLV reverse transcriptase as described above. Two microliters of a 1/5 dilution of this reaction mixture was used for real-time PCR using Sybr green (QuantiTect; Qiagen) in an ABI 7400 thermocycler (Applied Biosystems). Primer sequences used are shown in Table 3; the final concentration of primers in the reaction mixture was 0.9 μM. The cycling program was as follows: 15 min at 95°C followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. We applied the CT method for relative mRNA quantitation using the β-actin housekeeping gene, and the mRNA levels for each group of infected mice were referred to the levels obtained for the uninfected (streptomycin-pretreated) group. The experiment was done two times, using five animals per group each time.
Luciferase assays.
The Caco-Rumbo cell line was stimulated with log-phase bacteria at an MOI of ∼100 to 200:1, and the infection was allowed to proceed similarly to that described for Caco-2 mRNA measurements. At 6 h p.i., the luciferase activity in cell extracts was measured using the One Glo luciferase assay (Promega) and a Luminoskan Ascent microplate luminometer (Thermo Scientific). Purified FliC (1.0 μg/ml) from S. Typhimurium, generously provided by J. C. Sirard, was used as a positive control. The luminescence obtained for the stimulated cells was normalized to the luminescence obtained for the unstimulated cells, with the latter value set to 1. Each isolate was assayed in quadruplicate.
Animal experiments.
Animal experiments were performed as described by Barthel et al. (40). Briefly, groups of five 6- to 8-week-old female C57BL/6 mice (provided by the National Division of Veterinary Laboratories, Uruguay) were pretreated with 25 mg of streptomycin 24 h prior to infection with ∼5 × 107 CFU of the indicated bacterial strain. At 24 h p.i., mice were sacrificed, and fractions of the distal cecum were embedded in OCT compound (Sakura), immediately frozen in liquid nitrogen, and stored at −80°C. Cecal pathology was evaluated in hematoxylin and eosin (H&E)-stained cryosections (5 to 8 μm thick), in a blinded manner, by following exactly the histopathological score scheme described by Stecher et al. (21). The parameters evaluated were destruction of the epithelial cell layer, submucosal edema, reduction in number of goblet cells, and infiltration of polymorphonuclear (PMN) cells. In addition, fractions of the middle cecum were immediately removed after sacrifice, embedded in TRIzol (Invitrogen), and stored at −80°C for subsequent total RNA extraction and qRT-PCR analysis as described above. Bacterial loads in ceca and spleens were analyzed by homogenizing the organ contents in PBS containing 0.5% Tergitol and plating appropriate dilutions on MacConkey agar plates containing 50 μg/ml streptomycin.
Experiments with animals were performed according to national guidelines for animal experimentation that meet the international guiding principles for biomedical research involving animals, and all protocols were approved by the university ethics committee.
Statistical analysis.
For analysis of differences in motility, transcriptional responses to infection of Caco-2 cells, and fliC mRNA levels, one-way analysis of variance (ANOVA) with Dunnet's multiple-comparison posttest was used (GraphPad Prism 4.0 software), with P values of <0.05 considered to be statistically significant. For analysis of differences in transcriptional responses to infection of mice, we used the Mann-Whitney U test (GraphPad Prism 4.0 software), with P values (two-tailed) of <0.05 considered to be statistically significant.
RESULTS
Motility characterization of S. Dublin clinical isolates and determination of flagellar expression.
Ten S. Dublin isolates recovered from human sources in Uruguay from 1995 to 2011 were evaluated (Table 1). These were all S. Dublin human isolates available in the two national Salmonella collections.
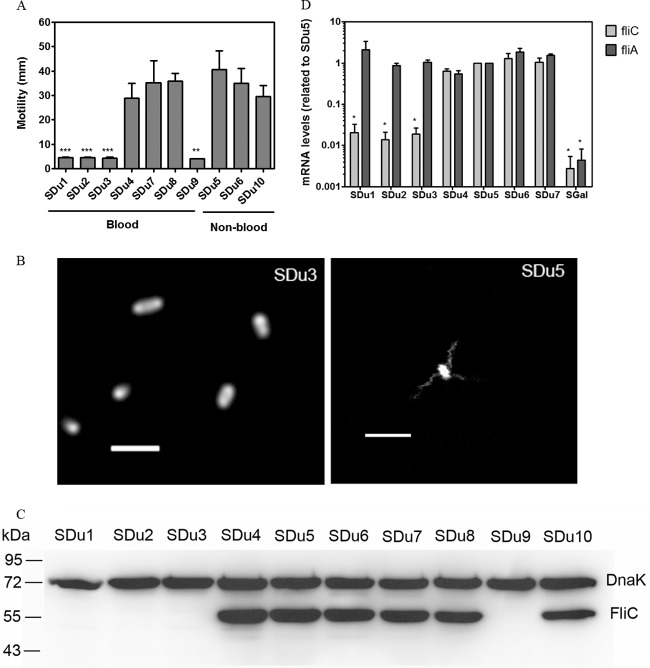
Four of the 10 analyzed isolates were nonmotile (Fig. 1A), and all of these were isolated from blood. In order to evaluate flagellation, we performed fluorescence microscopic analysis of two selected isolates (SDu3 and SDu5, which are nonmotile and motile, respectively) stained with an amino-specific fluorescent dye. The peritrichous flagellar filaments of isolate SDu5 were readily visualized, while no filaments at all were observed in SDu3 (Fig. 1B). Furthermore, Western blotting revealed undetectable levels of flagellin in total bacterial extracts of the nonmotile isolates (Fig. 1C), indicating that the absence of flagella was due to reduced flagellin synthesis or increased degradation, not to defects in export of subunits or in filament assembly. To gain further information about this phenotype, we quantified fliC mRNA levels by qRT-PCR analysis of a selected set of isolates (SDu1 to SDu7). The results indicated that the levels of fliC mRNA were significantly reduced in the nonmotile isolates compared to the motile ones (about 50- to 100-fold reductions) (Fig. 1D), which is consistent with the hypothesis of reduced flagellin synthesis. Interestingly, the complete nucleotide sequences of fliC and its promoter region in isolates SDu1 to SDu7 were identical (data not shown). We also quantified the mRNA levels for fliA, coding for the sigma factor responsible for transcription of flagellar class III genes (which include fliC [41]), in isolates SDu1 to SDu7 (Fig. 1D). There were no significant differences among isolates, suggesting that the repression occurred at the last level of the flagellar regulatory hierarchy.
FIG 1.
(A) Motility measurement of S. Dublin isolates. The diameter of the halo of growth (in mm) after 6 h at 37°C is plotted for each isolate. Results (means and standard deviations [SD]) from three independent experiments are shown. Asterisks indicate significant differences relative to SDu5. **, P < 0.01; ***, P < 0.001. (B) Fluorescence labeling of flagellar filaments in live cells of isolates SDu3 and SDu5. Bars, 2 μm. (C) Western blot analysis of total protein extracts of S. Dublin isolates grown in LB to mid-log phase, using anti-flagellin antibody. Detection of DnaK was used to verify equal loading of samples. Sizes of molecular mass markers are indicated in kDa. (D) fliC and fliA mRNA level quantification in S. Dublin isolates grown in LB to mid-log phase. Results (means and standard errors [SE]) from three independent experiments are shown. *, significant difference relative to SDu5 (P < 0.05). SGal is a strain of the aflagellate serovar S. Gallinarum.
To investigate the reversibility of the nonmotile phenotype, we performed successive daily passages of the SDu3 isolate in semisolid agar, but this strain did not recover motility, at least after 12 passages, suggesting an irreversible mechanism.
In summary, all these results indicate that the absence of motility observed in S. Dublin blood isolates is due to a significant reduction in fliC expression which results in impaired synthesis of flagellin and, consequently, a lack of flagellar filaments at the bacterial surface.
In vitro evaluation of the proinflammatory properties of S. Dublin clinical isolates.
Since Salmonella flagellin is regarded as the major proinflammatory determinant in epithelial cells (28, 42), and because all four aflagellate strains were isolated from invasive infections, we hypothesized that the lack of flagella would impair the proinflammatory properties of S. Dublin on the gut epithelium, therefore promoting its systemic dissemination. First, we sought to determine if the different S. Dublin isolates would trigger different proinflammatory responses in model human epithelia. Thus, we quantified the mRNA levels for IL-8 and CCL20 in cultured intestinal epithelial Caco-2 cells infected with four selected S. Dublin isolates (two aflagellate and two flagellated isolates) and compared them to the levels in uninfected cells. While the two flagellated isolates elicited transcriptional induction of IL-8 and CCL20 in Caco-2 cells, the two aflagellate isolates failed to do so (Fig. 2A). We further analyzed the induction of CCL20 expression in Caco-2 cells in response to infection by all S. Dublin isolates by using a CCL20-luciferase reporter cell line. As expected, a fliC knockout mutant triggered no luciferase activity (Fig. 2B). A perfect correlation between the presence of flagellin in S. Dublin isolates and upregulation of expression from the CCL20 promoter was found (compare Fig. 2B with Fig. 1C), suggesting that repression of fliC expression in aflagellate S. Dublin isolates is maintained during interaction with epithelial cells. Quantification of fliC mRNA levels in S. Dublin isolates incubated in the presence of Caco-2 cells by qRT-PCR confirmed this suggestion (data not shown).
FIG 2.
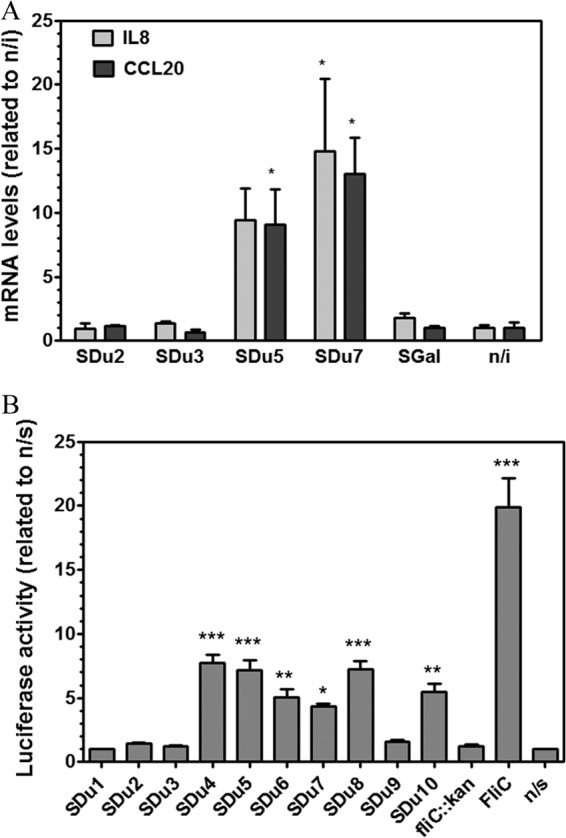
(A) Analysis of the Caco-2 transcriptional response to infection by S. Dublin flagellated or aflagellate isolates. Caco-2 cells were infected with isolate SDu2, SDu3, SDu5, or SDu7, and at 3 h p.i., the levels of mRNA transcripts for CCL20 and IL-8 were measured by qRT-PCR. SGal is a strain of the aflagellate serovar S. Gallinarum. n/i, noninfected cells. *, statistical difference relative to noninfected cells (P < 0.05). Means and SE for two independent experiments are shown. (B) Analysis of Caco-2 transcriptional response (CCL20 expression) to infection by all S. Dublin clinical isolates. Purified S. Typhimurium flagellin (FliC) and an SDu5 derivative fliC knockout mutant (fliC::kan) were used as positive and negative controls, respectively; induction was performed for 6 h. Luciferase activity was determined and normalized to the activity obtained for the nonstimulated control (n/s), with this value considered to be 1. Means and SE for one representative experiment performed in quadruplicate are shown. Asterisks indicate significant differences relative to the nonstimulated control. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Evaluation of the proinflammatory properties of S. Dublin clinical isolates in vivo.
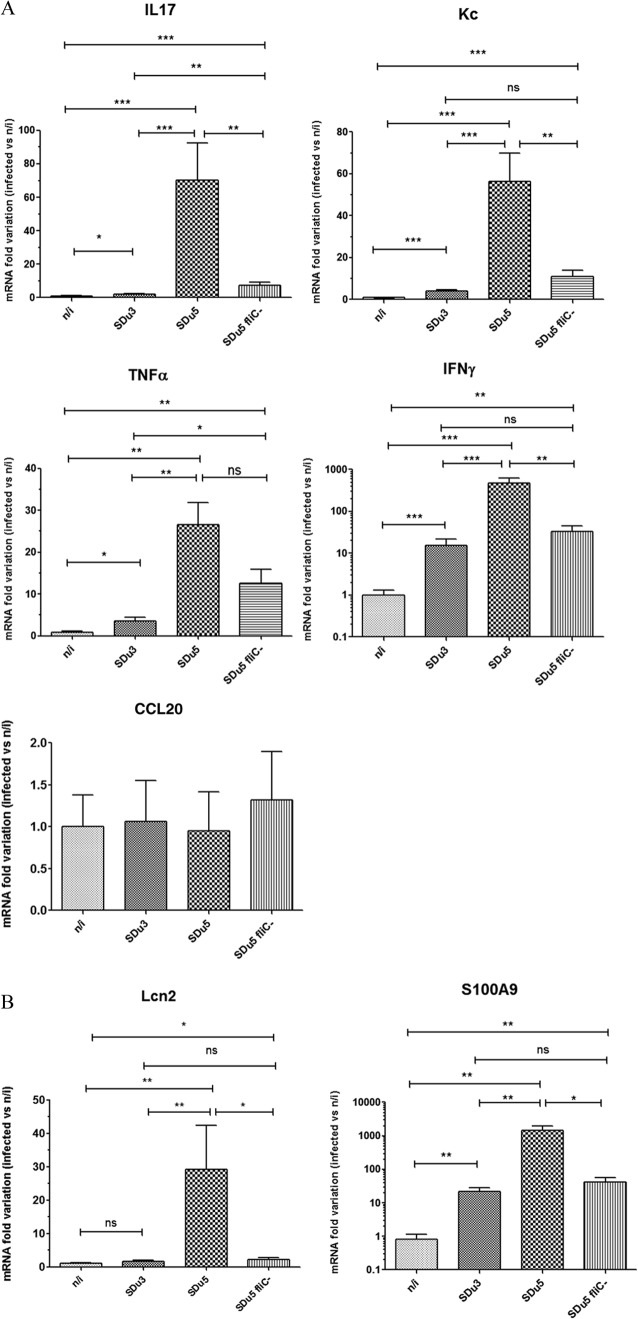
Results of in vitro studies showed a correlation between FliC production and induction of the intestinal epithelial proinflammatory gene program and indicated that the lack of fliC expression observed when S. Dublin isolates were grown in LB broth remained in the presence of epithelial cells. To evaluate if these phenotypes were reproduced in vivo, we analyzed proinflammatory gene expression and histological pathology in the ceca of streptomycin-pretreated mice 24 h after oral infection with two selected S. Dublin isolates (one nonflagellated and one flagellated isolate) and compared the results to those for mock-infected animals. We decided to analyze the ceca of streptomycin-pretreated mice because severe pathological inflammatory changes are restricted to this organ (40). We selected the time point of 24 h postinfection because it has been shown that the differences in pathology caused by a nonflagellated mutant of S. Typhimurium compared to the wild type are more pronounced at early times p.i. (21). Both S. Dublin isolates efficiently colonized the cecum within 24 h p.i. (for SDu3, 1.97 × 109 ± 5.14 × 108 CFU/g of cecum content; and for SDu5, 1.32 × 109 ± 3.24 × 108 CFU/g of cecum content), and no statistically significant differences in bacterial numbers were observed between the isolates. Mice infected with the flagellated isolate SDu5 showed a dramatic and statistically significant increase in cecal mRNA levels for IL-17 (70-fold), keratinocyte-derived chemokine (encoded by Kc, the murine homologue of human IL-8) (56-fold), tumor necrosis factor alpha (TNF-α) (27-fold), and gamma interferon (IFN-γ) (470-fold) compared to mock-infected animals (Fig. 3A). Infection with the aflagellate isolate SDu3 also elicited significant induction of expression of IL-17 (2-fold), Kc (4-fold), TNF-α (3.6-fold), and IFN-γ (15-fold) compared to the case in mock-infected mice, indicating that despite the absence of flagella and motility, the SDu3 isolate was still able to elicit an innate immune response. However, the increases in the cecal mRNA levels for all four cytokines were significantly lower than those elicited by infection with SDu5. SDu3 bacteria recovered from the cecum contents of infected mice were still devoid of motility (data not shown), supporting the hypothesis that the aflagellate phenotype is maintained during interaction with host tissues. In contrast to the observation in cell culture, Mip-3a (Ccl20) expression was not significantly induced in the ceca of streptomycin-pretreated mice infected with either S. Dublin isolate (Fig. 3A). Although this may be considered surprising, because it is postulated that induction of CCL20 production may contribute to orchestrating host responses during Salmonella infection through dendritic cell recruitment to the follicle-associated epithelium, similar results were indeed previously reported by Winter et al. (32).
FIG 3.
mRNA variation at 24 h p.i. in the cecal mucosa of mice infected with S. Dublin, determined by qRT-PCR. Values are expressed as fold changes of mRNA levels for the indicated genes in infected mice relative to their levels in mock-infected animals. Note that the y axes of the IFN-γ and S100.A9 graphs show log10 values. Means and SE for two independent experiments, with five animals per group in each experiment, are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not statistically significant. (A) mRNA quantification of genes coding for proinflammatory cytokines and chemokines. (B) mRNA quantification of genes coding for antimicrobial proteins.
Other genes involved in defense responses, such as those encoding antimicrobial peptides and proteins, have also been reported to be induced in the murine intestine upon S. Typhimurium infection (43). Thus, we also quantified the mRNA levels for two antimicrobial proteins involved in iron and zinc deprivation, i.e., lipocalin-2 (encoded by Lcn2) and calprotectin (one of whose subunits is encoded by S100.a9), respectively. Transcript levels for lipocalin-2 were significantly upregulated in the ceca of mice infected with the flagellated isolate (29-fold) compared to those of uninfected mice, whereas those animals infected with the aflagellate isolate did not show significant upregulation of this antimicrobial protein (Fig. 3B). Concerning S100.A9, its expression was significantly induced in mice infected with both isolates relative to that in mock-infected animals, but the induction was significantly lower with SDu3 (22-fold) than with SDu5 (1,441-fold).
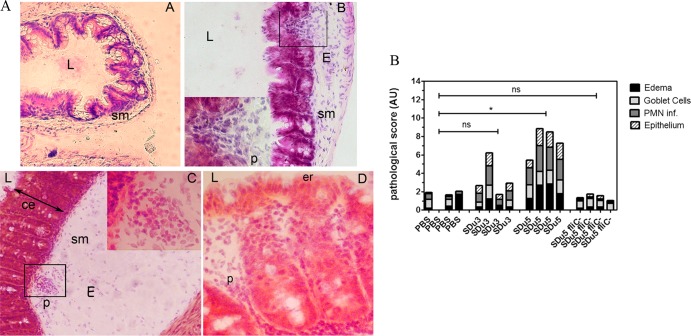
Cecal pathology in S. Dublin-infected animals was also analyzed by scoring the signs of inflammation in H&E-stained cryosections. Despite the similar colonization levels, mice infected with SDu5 developed a severe colitis at 24 h p.i., with high levels of PMN cell recruitment, epithelial damage, and submucosal edema, whereas the ceca of SDu3-infected mice showed milder signs of inflammation (Fig. 4A and B). Taken together, these results suggest that lack of flagella results in S. Dublin isolates still being able to trigger intestinal proinflammatory and antimicrobial responses in vivo, but this ability is severely impaired compared to the responses induced by flagellated isolates.
FIG 4.
Cecal pathology in H&E-stained tissue cryosections of streptomycin-pretreated S. Dublin-infected mice at 24 h p.i. (A) Representative histology of the cecum of a mock-infected mouse (A), a mouse infected with SDu3 (B), and a mouse infected with SDu5 (C and D). L, intestinal lumen; sm, submucosa; E, edema; p, PMN cell; ce, crypt elongation; er, erosion of the epithelial layer. Note the larger extent of edema in the submucosa, as well as PMN cell infiltration and epithelial erosion, in the cecum of the SDu5-infected mouse than in that of the mock- or SDu3-infected mouse. Magnification, ×200 (A to C) or ×400 (D and insets of parts B and C). (B) Histological scores for changes in the cecum. Tissue cryosections were scored for edema in the submucosa (black bars), reduction in the number of goblet cells (light gray bars), PMN cell infiltration (dark gray bars), and desquamation and erosion of the epithelial monolayer (dashed bars). Values are expressed as stacked vertical bars; each bar corresponds to one animal. PBS, mock-infected mice. The results of statistical analysis of the total score value (the sum of the separate scores) for each isolate- infected group relative to that of the mock-infected group are shown (Mann-Whitney test). *, P < 0.05; ns, not statistically significant.
In order to verify if flagellation was the underlying cause of the observed differences in mucosal innate immune responses elicited by isolates SDu5 and SDu3, we constructed an isogenic mutant of SDu5 carrying fliC inactivated by a kanamycin cassette and tested it in the mouse model of colitis. Since S. Dublin has monophasic flagella, inactivation of the fliC gene renders it a completely aflagellate strain. Similar to the previously tested isolates, the mutant strain efficiently colonized the ceca of infected mice within 24 h p.i. (1.09 × 109 ± 2.86 × 108 CFU/g of cecum content). As shown in Fig. 3A and B, the aflagellate SDu5 mutant induced significantly less cecal proinflammatory and antimicrobial gene expression than the parental strain. Moreover, there were no significant differences between cecal mRNA levels for Kc, IFN-γ, Lcn2, and S100.A9 in mice infected with SDu5 fliC::kan and those infected with SDu3, indicating that the aflagellate phenotype results in impaired proinflammatory properties regardless of the genetic background. In addition, the ceca of mice infected with the SDu5 fliC::kan mutant revealed no significant pathological changes compared to SDu3- and mock-infected mice (Fig. 4B). These results demonstrate that flagellation is indeed the major property underlying the differences in the ability of S. Dublin isolates to induce mucosal immune responses. However, the SDu5 fliC::kan mutant and SDu3 elicited significantly different levels of expression of IL-17 and TNF-α (Fig. 3A), suggesting that other factors could also have minor contributions to S. Dublin's proinflammatory properties.
Concerning the ability to spread to systemic sites, while slightly larger numbers of SDu5 than SDu3 were recovered from spleens of streptomycin-pretreated mice at day 3 p.i., the differences were not statistically significant (for SDu5, 2.00 × 105 ± 6.94 × 104 CFU/organ; and for SDu3, 7.93 × 104 ± 3.06 × 104 CFU/organ).
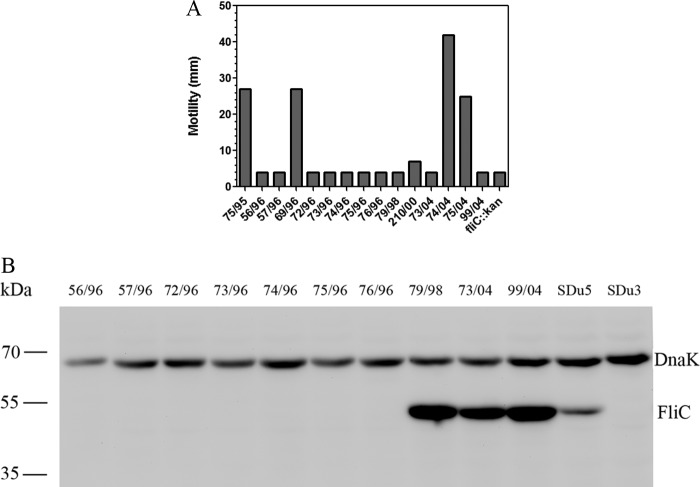
Motility properties of S. Dublin isolates from cattle.
Since S. Dublin is a cattle-adapted serovar, we decided to analyze the presence/absence of flagella in a collection of S. Dublin isolates obtained from cattle in Uruguay in order to evaluate whether the aflagellate phenotype is a specific trait of human isolates or is widely distributed in nature. Ten of 15 analyzed isolates, obtained from 1995 through 2004, showed a total absence of motility (Fig. 5A). Western blotting using an anti-FliC antibody indicated that 7 of these 10 nonmotile isolates did not produce flagellin, similar to the case observed in human isolates (Fig. 5B). However, the remaining 3 nonmotile cattle isolates did produce flagellin yet were unable to move.
FIG 5.
(A) Motility analysis of 15 isolates of S. Dublin obtained from cattle. An SDu5 derivative fliC knockout mutant (fliC::kan) was included as a negative control. (B) Western blotting of total protein extracts from the 10 nonmotile S. Dublin isolates obtained from cattle, using a specific anti-FliC antibody. Detection of DnaK was used to verify equal loading of samples. Sizes of molecular mass markers are indicated in kDa.
DISCUSSION
Flagella have classically been regarded as important virulence factors, mainly because they allow bacteria to move toward favorable environments and to colonize host tissues (44). More recently, evidence has indicated that flagella also participate actively in other processes, such as adhesion, biofilm formation, translocation of virulence factors, and modulation of both the innate and adaptive immune systems (44).
The role of flagella in the pathogenesis of Salmonella has been investigated extensively, and the results vary depending on the experimental model and serovar involved. It has been reported that S. Typhimurium mutants harboring paralyzed flagella, in contrast to aflagellate mutants, are slightly attenuated in the mouse typhoid model upon oral infection but not when the mice are challenged via the intraperitoneal route (45). In another study, an S. Typhimurium flhD mutant (the master flagellar regulator gene), but not a fliC fljB mutant (which lacks flagella but not the flagellar secretion apparatus), was significantly more virulent than the parental strain in the mouse model of typhoid fever (22). Moreover, an S. Typhimurium mutant deficient in the anti-sigma factor flgM gene, which overproduces flagellin, was attenuated in vivo in mice (46). More recently, Lai et al. reported that the attenuation of flgM-deficient Salmonella in vivo is due to recognition of flagellin by the innate immune system, predominantly by caspase-1-dependent pathways (47).
Using the model of murine colitis, it has been demonstrated that S. Typhimurium flagella contribute to early cecal inflammation, predominantly by providing motility, and promote enhanced bacterial growth by allowing S. Typhimurium to benefit from the nutrients released in the inflamed intestine (21, 48). However, the systemic infection which occurred parallel to the enterocolitis did not depend significantly on flagellum function (21). More recently, Winter and colleagues reported that S. Typhimurium flagellin pattern recognition contributes to initiating inflammatory responses in the bovine ileal mucosa but not the mouse mucosa (32).
Most studies have been conducted using artificially constructed mutants of S. Typhimurium and animal models, mainly murine models, and little is known about the role of flagella in naturally occurring isolates of less prevalent Salmonella serovars. In this work, we report that 4 of 10 human isolates and 7 of 15 cattle isolates of Salmonella enterica serovar Dublin lack flagella, are therefore nonmotile, and have the antigenic formula 9,12:−:−. These isolates had a fliC hypervariable region sequence identical to that of S. Dublin strains available in the GenBank database and belonged to the same ST as most S. Dublin isolates worldwide (ST10), and they were therefore confirmed as belonging to this serovar. The lack of flagella in human isolates was due to an impaired fliC expression that resulted in undetectable levels of FliC protein in total bacterial extracts. However, comparative analysis of the nucleotide sequence of fliC and its promoter region between flagellated and nonflagellated isolates revealed no differences, nor did the mRNA levels coding for FliA, the alternate sigma factor responsible for fliC transcription. These results suggest that the repression of flagellar gene expression occurs at the last level of the regulatory cascade and may be associated with impairment of the synthesis/stability of FliA or its interaction with FlgM, the corresponding anti-sigma factor (41).
It has been reported that the TviA protein of S. Typhi, encoded in SPI-7, is able to repress fliC expression under tissue osmolarity conditions (34). We previously analyzed the SDu1 to SDu3 and SDu5 isolates by comparative genomic hybridization using a Salmonella pan-array, but no signal for tviA was detected in the genomes of these isolates (49). This indicates that a different mechanism mediates fliC repression in S. Dublin.
In a previous work, we found that among a collection of 266 S. Enteritidis field isolates, 27% of the strains were nonmotile (37). This percentage included strains that had structurally normal but paralyzed flagella, meaning that the percentage of aflagellate isolates was even lower. The results presented in this work showing 11 aflagellate strains among 25 field isolates (considering human and cattle isolates together) of S. Dublin indicate an unusually high frequency of naturally occurring aflagellate isolates in this Salmonella serovar.
Lack of flagella does not seem to be a specific trait of S. Dublin field isolates from Uruguay. Selander et al. reported that all but 1 of 114 nonmotile isolates of serotype 1,9,12:−:− recovered from cattle and swine in the United States in 1988 and 1989 were Du1 (the major type of electrophoretic type assigned for S. Dublin according to multilocus enzyme electrophoresis [MLEE] analysis) and had been identified provisionally as S. Dublin at the National Veterinary Services Laboratories (50). Similarly, Franklin et al. reported that five 1,9,12:−:− Salmonella isolates recovered from cattle in Sweden were identified as S. Dublin based on restriction plasmid profiles (51). Taken together, these results support the hypothesis of a nonessential role for flagella in the infectious cycle of S. Dublin. In line with this, a previous study using a fliC transposon mutant showed that flagella play a minor role in extra-animal survival of S. Dublin, a feature considered important for zoonotic pathogens (52). More recently, the same group investigated the role of flagella during S. Dublin infection in the murine model, using the fliC knockout mutant together with the wild-type parental strain in coinfection assays (53). They found that 4 to 5 days after oral infection, the fliC mutant was recovered from the spleens of infected animals in significantly smaller numbers than those of the wild type, but this difference was modest, and no effect was observed after intraperitoneal challenge. The authors concluded that these small differences were sufficient enough to provide the selective force for stable maintenance of the flagellar apparatus in this serovar. To our knowledge, no studies have addressed the role of S. Dublin flagella during infection in cattle, the natural host, but results of the present study indicate that 7 of 15 isolates recovered from cattle were indeed nonflagellated. Based on the results of others and the present study, it is tempting to speculate that the flagellar apparatus may be suffering a process of loss in this serovar, maybe because its function is not essential for the infectious cycle or its absence is advantageous under specific circumstances. Notably, the aflagellate phenotype we observed in S. Dublin clinical isolates was irreversible in semisolid agar, an environmental condition where the selective pressure is in favor of a motile phenotype. Moreover, it remained during bacterial interaction with cultured epithelial cells and host tissues, suggesting that fliC expression is permanently repressed in these isolates and is not due to a regulatory mechanism specific for a particular environmental condition.
Host-restricted and host-adapted serovars seem to have lost gene functions that were previously active in their broad-host-range ancestors, and they are generally more prone to cause invasive disease than ubiquitous serovars (9, 54, 55). Indeed, we have shown by comparative genomics that S. Dublin has a larger number of pseudogenes than its close relative but ubiquitous serovar S. Enteritidis (49). Moreover, Salmonella enterica serovar Gallinarum, a serovar closely related to S. Dublin and S. Enteritidis that is avian restricted and causes an invasive typhoid-like disease in chickens, is nonflagellated due to pseudogene accumulation in flagellar genes (54). It has been postulated that the lack of flagella may promote S. Gallinarum's ability to disseminate systemically by avoiding the flagellin-TLR5-induced proinflammatory responses in the gut (56). Consistent with this hypothesis, Iqbal et al. reported that an aflagellate S. Typhimurium fliM mutant established early enhanced systemic infection in chickens, as well as inducing less IL-1β mRNA and PMN cell infiltration at the gut during the early stages of infection than the wild type (33). Conversely, a flagellated mutant of S. Gallinarum colonized efficiently and caused pathological changes in the ceca of infected birds, as well as producing less mortality than the wild-type strain (57). Similarly, for S. Typhi, a molecular mechanism mediated by TviA has been described that represses fliC expression when the bacterium encounters human tissue osmolarity, i.e., during interaction with the epithelium, where it could be sensed by pattern recognition receptors (34). In the same study, the authors reported that an S. Typhimurium mutant expressing tviA (which is normally absent in this serovar) induced lower levels of CXCL-1 chemokine expression than the wild type in human model epithelia and showed more invasiveness in an avian model of infection (34). In the present work, we showed that S. Dublin isolates lacking flagella were not able to induce the expression of the CCL20 and IL-8 chemokines in cultured human intestinal epithelial cells, confirming the results obtained by Eaves-Pyles et al. (42) (i.e., similar to what has been described for S. Typhimurium, FliC is also the main determinant in S. Dublin for induction of these chemokines in intestinal epithelial cells). In addition, a naturally aflagellate isolate as well as a flagellin knockout mutant caused milder pathological changes and significantly less proinflammatory cytokine and antimicrobial gene expression in the ceca of infected mice than a flagellated isolate at early phases of infection. These data indicate, as reported for S. Typhimurium (21), that S. Dublin flagella are necessary for efficient induction of early cecal inflammation in streptomycin-pretreated mice.
In spite of the remarkably different mucosal responses elicited by SDu3 and SDu5, no significant differences were observed in their ability to colonize the spleens of infected animals. This result is consistent with those obtained by Stecher et al. (21), who reported that a nonflagellated mutant of S. Typhimurium caused significantly reduced levels of cecal inflammation but showed no differences in mesenteric lymph node (MLN), liver, and spleen colonization compared to the wild-type strain in the mouse model of colitis. These results strengthen the notion that the streptomycin-pretreated model is not suitable for studying the correlation between intestinal inflammatory responses and translocation of Salmonella to internal organs, since NTS rapidly disseminate to the liver and spleen of mice, establishing a systemic infection that resembles typhoid, regardless of the intestinal inflammatory response generated (34).
Interestingly, all aflagellate clinical isolates included in this study were recovered from invasive cases of salmonellosis (i.e., were isolated from blood), whereas all nonblood isolates harbored flagella. Thus, it is tempting to speculate that in S. Dublin, the lack of flagella promotes systemic dissemination in the human host through dampening of the gut immune response. Confirmation of such a hypothesis would require analysis of a larger collection of human isolates obtained at a worldwide level. Note that isolates SDu4, SDu7, and SDu8 were isolated from blood yet harbored flagella and were motile. However, these strains were isolated from a drug-addicted individual, a toddler, and an elderly person, respectively, suggesting that in these cases the immune status of the host may have influenced the disease outcome.
In summary, the data presented here indicate that the aflagellate phenotype is frequent among field isolates of S. Dublin and renders strains significantly less proinflammatory than flagellated ones. The fact that all aflagellate isolates recovered from humans were isolated from invasive infections suggests that this phenotype may render strains more prone to systemic dissemination in humans. Our data also indicate that the aflagellate phenotype is a consequence of abrogated fliC but not fliA transcription and is irreversible in the S. Dublin blood isolates studied here. The molecular mechanism responsible for such a phenotype is currently being investigated.
ACKNOWLEDGMENTS
We thank Manja Barthel and Bärbel Stecher (ETH, Zurich, Switzerland) for excellent training in the streptomycin-pretreated mouse model of Salmonella colitis. We also thank Jean Claude Sirard (Université Lille Nord de France) for providing cell lines and purified FliC. Gabriela Algorta and Teresa Camou are acknowledged for providing isolates from the NSC collection and the MPH, respectively.
This work was supported by Programa CSIC I+D 2010 (Universidad de la República, Uruguay).
Footnotes
Published ahead of print 13 January 2014
REFERENCES
- 1.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, Jones TF, Fazil A, Hoekstra RM. 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50:882–889. 10.1086/650733 [DOI] [PubMed] [Google Scholar]
- 2.Langridge GC, Wain J, Nair S. 2008. Invasive salmonellosis in humans. EcoSal Plus 10.1128/ecosalplus.8.6.2.2 [DOI] [PubMed] [Google Scholar]
- 3.Hendriksen RS, Vieira AR, Karlsmose S, Lo Fo Wong DM, Jensen AB, Wegener HC, Aarestrup FM. 2011. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 8:887–900. 10.1089/fpd.2010.0787 [DOI] [PubMed] [Google Scholar]
- 4.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. 2012. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379:2489–2499. 10.1016/S0140-6736(11)61752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones TF, Ingram LA, Cieslak PR, Vugia DJ, Tobin-D'Angelo M, Hurd S, Medus C, Cronquist A, Angulo FJ. 2008. Salmonellosis outcomes differ substantially by serotype. J. Infect. Dis. 198:109–114. 10.1086/588823 [DOI] [PubMed] [Google Scholar]
- 6.Wollin R. 2007. A study of invasiveness of different Salmonella serovars based on analysis of the Enter-net database. Euro Surveill. 12:E070927.3. [DOI] [PubMed] [Google Scholar]
- 7.Vugia DJ, Samuel M, Farley MM, Marcus R, Shiferaw B, Shallow S, Smith K, Angulo FJ. 2004. Invasive Salmonella infections in the United States, FoodNet, 1996–1999: incidence, serotype distribution, and outcome. Clin. Infect. Dis. 38(Suppl 3):S149–S156. 10.1086/381581 [DOI] [PubMed] [Google Scholar]
- 8.Fernandes SA, Tavechio AT, Ghilardi AC, Dias AM, Almeida IA, Melo LC. 2006. Salmonella serovars isolated from humans in Sao Paulo State, Brazil, 1996–2003. Rev. Inst. Med. Trop. Sao Paulo 48:179–184. 10.1590/S0036-46652006000400001 [DOI] [PubMed] [Google Scholar]
- 9.Uzzau S, Brown DJ, Wallis T, Rubino S, Leori G, Bernard S, Casadesus J, Platt DJ, Olsen JE. 2000. Host adapted serotypes of Salmonella enterica. Epidemiol. Infect. 125:229–255. 10.1017/S0950268899004379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang FC, Fierer J. 1991. Human infection with Salmonella dublin. Medicine (Baltimore) 70:198–207 [DOI] [PubMed] [Google Scholar]
- 11.Salmon-Ceron D, Detruchis P, Jaccard A, Leport C, Karam-Sarkis D, Rosenbaum W, Meyohas MC, Coulaud JP, Vilde JL. 1992. Non-typhic Salmonella bacteremias in HIV infections. Clinical and therapeutic data, and course in 68 patients. Presse Med. 21:847–851 [PubMed] [Google Scholar]
- 12.Levine WC, Buehler JW, Bean NH, Tauxe RV. 1991. Epidemiology of nontyphoidal Salmonella bacteremia during the human immunodeficiency virus epidemic. J. Infect. Dis. 164:81–87. 10.1093/infdis/164.1.81 [DOI] [PubMed] [Google Scholar]
- 13.Wangdi T, Winter SE, Baumler AJ. 2012. Typhoid fever: “you can't hit what you can't see.” Gut Microbes 3:88–92. 10.4161/gmic.18602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsolis RM, Young GM, Solnick JV, Baumler AJ. 2008. From bench to bedside: stealth of enteroinvasive pathogens. Nat. Rev. Microbiol. 6:883–892. 10.1038/nrmicro2012 [DOI] [PubMed] [Google Scholar]
- 15.Tsolis RM, Adams LG, Ficht TA, Baumler AJ. 1999. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 67:4879–4885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Santos RL, Tsolis RM, Stender S, Hardt WD, Baumler AJ, Adams LG. 2002. The Salmonella enterica serotype Typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect. Immun. 70:3843–3855. 10.1128/IAI.70.7.3843-3855.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hapfelmeier S, Ehrbar K, Stecher B, Barthel M, Kremer M, Hardt WD. 2004. Role of the Salmonella pathogenicity island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72:795–809. 10.1128/IAI.72.2.795-809.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coburn B, Li Y, Owen D, Vallance BA, Finlay BB. 2005. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect. Immun. 73:3219–3227. 10.1128/IAI.73.6.3219-3227.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hapfelmeier S, Stecher B, Barthel M, Kremer M, Muller AJ, Heikenwalder M, Stallmach T, Hensel M, Pfeffer K, Akira S, Hardt WD. 2005. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar Typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J. Immunol. 174:1675–1685 [DOI] [PubMed] [Google Scholar]
- 20.Coombes BK, Coburn BA, Potter AA, Gomis S, Mirakhur K, Li Y, Finlay BB. 2005. Analysis of the contribution of Salmonella pathogenicity islands 1 and 2 to enteric disease progression using a novel bovine ileal loop model and a murine model of infectious enterocolitis. Infect. Immun. 73:7161–7169. 10.1128/IAI.73.11.7161-7169.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stecher B, Hapfelmeier S, Muller C, Kremer M, Stallmach T, Hardt WD. 2004. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72:4138–4150. 10.1128/IAI.72.7.4138-4150.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt CK, Ikeda JS, Darnell SC, Watson PR, Bispham J, Wallis TS, Weinstein DL, Metcalf ES, O'Brien AD. 2001. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect. Immun. 69:5619–5625. 10.1128/IAI.69.9.5619-5625.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apel D, Surette MG. 2008. Bringing order to a complex molecular machine: the assembly of the bacterial flagella. Biochim. Biophys. Acta. 1778:1851–1858. 10.1016/j.bbamem.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 24.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng Akira JKS, Underhill DM, Aderem A. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099–1103. 10.1038/35074106 [DOI] [PubMed] [Google Scholar]
- 25.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882–1885 [DOI] [PubMed] [Google Scholar]
- 26.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. 2006. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat. Immunol. 7:569–575. 10.1038/ni1344 [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477:596–600. 10.1038/nature10510 [DOI] [PubMed] [Google Scholar]
- 28.Zeng H, Carlson AQ, Guo Y, Yu Y, Collier-Hyams LS, Madara JL, Gewirtz AT, Neish AS. 2003. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J. Immunol. 171:3668–3674 [DOI] [PubMed] [Google Scholar]
- 29.Gewirtz AT, Siber AM, Madara JL, McCormick BA. 1999. Orchestration of neutrophil movement by intestinal epithelial cells in response to Salmonella typhimurium can be uncoupled from bacterial internalization. Infect. Immun. 67:608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sierro F, Dubois B, Coste A, Kaiserlian D, Kraehenbuhl JP, Sirard JC. 2001. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 98:13722–13727. 10.1073/pnas.241308598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijay-Kumar M, Wu H, Jones R, Grant G, Babbin B, King TP, Kelly D, Gewirtz AT, Neish AS. 2006. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am. J. Pathol. 169:1686–1700. 10.2353/ajpath.2006.060345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winter SE, Thiennimitr P, Nuccio SP, Haneda T, Winter MG, Wilson RP, Russell JM, Henry T, Tran QT, Lawhon SD, Gomez G, Bevins CL, Russmann H, Monack DM, Adams LG, Baumler AJ. 2009. Contribution of flagellin pattern recognition to intestinal inflammation during Salmonella enterica serotype Typhimurium infection. Infect. Immun. 77:1904–1916. 10.1128/IAI.01341-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iqbal M, Philbin VJ, Withanage GS, Wigley P, Beal RK, Goodchild MJ, Barrow P, McConnell I, Maskell DJ, Young J, Bumstead N, Boyd Y, Smith AL. 2005. Identification and functional characterization of chicken Toll-like receptor 5 reveals a fundamental role in the biology of infection with Salmonella enterica serovar Typhimurium. Infect. Immun. 73:2344–2350. 10.1128/IAI.73.4.2344-2350.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winter SE, Winter MG, Godinez I, Yang HJ, Russmann H, Andrews-Polymenis HL, Baumler AJ. 2010. A rapid change in virulence gene expression during the transition from the intestinal lumen into tissue promotes systemic dissemination of Salmonella. PLoS Pathog. 6:e1001060. 10.1371/journal.ppat.1001060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Achtman M, Wain J, Weill FX, Nair S, Zhou Z, Sangal V, Krauland MG, Hale JL, Harbottle H, Uesbeck A, Dougan G, Harrison LH, Brisse S. 2012. Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog. 8:e1002776. 10.1371/journal.ppat.1002776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yim L, Betancor L, Martinez A, Giossa G, Bryant C, Maskell D, Chabalgoity JA. 2010. Differential phenotypic diversity among epidemic-spanning Salmonella enterica serovar Enteritidis isolates from humans or animals. Appl. Environ. Microbiol. 76:6812–6820. 10.1128/AEM.00497-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yim L, Betancor L, Martinez A, Bryant C, Maskell D, Chabalgoity JA. 2011. Naturally occurring motility-defective mutants of Salmonella enterica serovar Enteritidis isolated preferentially from nonhuman rather than human sources. Appl. Environ. Microbiol. 77:7740–7748. 10.1128/AEM.05318-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nempont C, Cayet D, Rumbo M, Bompard C, Villeret V, Sirard JC. 2008. Deletion of flagellin's hypervariable region abrogates antibody-mediated neutralization and systemic activation of TLR5-dependent immunity. J. Immunol. 181:2036–2043 [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 40.Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839–2858. 10.1128/IAI.71.5.2839-2858.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chevance FF, Hughes KT. 2008. Coordinating assembly of a bacterial macromolecular machine. Nat. Rev. Microbiol. 6:455–465. 10.1038/nrmicro1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eaves-Pyles T, Murthy K, Liaudet L, Virag L, Ross G, Soriano FG, Szabo C, Salzman AL. 2001. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I kappa B alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J. Immunol. 166:1248–1260 [DOI] [PubMed] [Google Scholar]
- 43.Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, Mak TW, Tsolis RM, Bevins CL, Solnick JV, Dandekar S, Baumler AJ. 2009. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5:476–486. 10.1016/j.chom.2009.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan Q, Zhou M, Zhu L, Zhu G. 2013. Flagella and bacterial pathogenicity. J. Basic Microbiol. 53:1–8. 10.1002/jobm.201100335 [DOI] [PubMed] [Google Scholar]
- 45.Lockman HA, Curtiss R., 3rd 1990. Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect. Immun. 58:137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt CK, Darnell SC, Tesh VL, Stocker BA, O'Brien AD. 1994. Mutation of flgM attenuates virulence of Salmonella typhimurium, and mutation of fliA represses the attenuated phenotype. J. Bacteriol. 176:368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai MA, Quarles EK, Lopez-Yglesias AH, Zhao X, Hajjar AM, Smith KD. 2013. Innate immune detection of flagellin positively and negatively regulates Salmonella infection. PLoS One 8:e72047. 10.1371/journal.pone.0072047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stecher B, Barthel M, Schlumberger MC, Haberli L, Rabsch W, Kremer M, Hardt WD. 2008. Motility allows S. Typhimurium to benefit from the mucosal defence. Cell. Microbiol. 10:1166–1180. 10.1111/j.1462-5822.2008.01118.x [DOI] [PubMed] [Google Scholar]
- 49.Betancor L, Yim L, Martinez A, Fookes M, Sasias S, Schelotto F, Thomson N, Maskell D, Chabalgoity JA. 2012. Genomic comparison of the closely related Salmonella enterica serovars Enteritidis and Dublin. Open Microbiol. J. 6:5–13. 10.2174/1874285801206010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selander RK, Smith NH, Li J, Beltran P, Ferris KE, Kopecko DJ, Rubin FA. 1992. Molecular evolutionary genetics of the cattle-adapted serovar Salmonella dublin. J. Bacteriol. 174:3587–3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franklin A, Linne T, Rehbinder V. 1990. Plasmid profile analysis and restriction enzyme fingerprinting of Salmonella DO-group strains. APMIS 98:665–668. 10.1111/j.1699-0463.1990.tb04986.x [DOI] [PubMed] [Google Scholar]
- 52.Olsen JE, Hoegh-Andersen KH, Casadesus J, Thomsen LE. 2012. The importance of motility and chemotaxis for extra-animal survival of Salmonella enterica serovar Typhimurium and Dublin. J. Appl. Microbiol. 113:560–568. 10.1111/j.1365-2672.2012.05363.x [DOI] [PubMed] [Google Scholar]
- 53.Olsen JE, Hoegh-Andersen KH, Casadesus J, Rosenkranzt J, Chadfield MS, Thomsen LE. 2013. The role of flagella and chemotaxis genes in host pathogen interaction of the host adapted Salmonella enterica serovar Dublin compared to the broad host range serovar S. Typhimurium. BMC Microbiol. 13:67. 10.1186/1471-2180-13-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomson NR, Clayton DJ, Windhorst D, Vernikos G, Davidson S, Churcher C, Quail MA, Stevens M, Jones MA, Watson M, Barron A, Layton A, Pickard D, Kingsley RA, Bignell A, Clark L, Harris B, Ormond D, Abdellah Z, Brooks K, Cherevach I, Chillingworth T, Woodward J, Norberczak H, Lord A, Arrowsmith C, Jagels K, Moule S, Mungall K, Sanders M, Whitehead S, Chabalgoity JA, Maskell D, Humphrey T, Roberts M, Barrow PA, Dougan G, Parkhill J. 2008. Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome Res. 18:1624–1637. 10.1101/gr.077404.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baumler AJ, Tsolis RM, Ficht TA, Adams LG. 1998. Evolution of host adaptation in Salmonella enterica. Infect. Immun. 66:4579–4587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaiser P, Rothwell L, Galyov EE, Barrow PA, Burnside J, Wigley P. 2000. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 146:3217–3226 [DOI] [PubMed] [Google Scholar]
- 57.de Freitas Neto OC, Setta A, Imre A, Bukovinski A, Elazomi A, Kaiser P, Berchieri A, Jr, Barrow P, Jones M. 2013. A flagellated motile Salmonella Gallinarum mutant (SG Fla+) elicits a pro-inflammatory response from avian epithelial cells and macrophages and is less virulent to chickens. Vet. Microbiol. 165:425–433. 10.1016/j.vetmic.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 58.Herrera-Leon S, McQuiston JR, Usera MA, Fields PI, Garaizar J, Echeita MA. 2004. Multiplex PCR for distinguishing the most common phase-1 flagellar antigens of Salmonella spp. J. Clin. Microbiol. 42:2581–2586. 10.1128/JCM.42.6.2581-2586.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cummings LA, Wilkerson WD, Bergsbaken T, Cookson BT. 2006. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol. Microbiol. 61:795–809. 10.1111/j.1365-2958.2006.05271.x [DOI] [PubMed] [Google Scholar]
- 60.Muñoz N, Van Maele L, Marques JM, Rial A, Sirard JC, Chabalgoity JA. 2010. Mucosal administration of flagellin protects mice from Streptococcus pneumoniae lung infection. Infect. Immun. 78:4226–4233. 10.1128/IAI.00224-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marques JM, Rial A, Munoz N, Pellay FX, Van Maele L, Leger H, Camou T, Sirard JC, Benecke A, Chabalgoity JA. 2012. Protection against Streptococcus pneumoniae serotype 1 acute infection shows a signature of Th17- and IFN-gamma-mediated immunity. Immunobiology 217:420–429. 10.1016/j.imbio.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 62.Muñoz N. 2013. Patrones moleculares asociados a patógenos (PAMPs) como base para nuevas inmunoterapias contra Streptococcus neumoniae. Ph.D. thesis Instituto de Higiene, Facultad de Medicina, Universidad de la República, Montevideo, Uruguay [Google Scholar]