Abstract
Pneumococcal adherence to mucosal surfaces is a critical step in nasopharyngeal colonization, but so far few pneumococcal adhesins involved in the interaction with host cells have been identified. PhtA, PhtB, PhtD, and PhtE are conserved pneumococcal surface proteins that have proven promising as vaccine candidates. One suggested virulence function of Pht proteins is to mediate adherence at the respiratory mucosa. In this study, we assessed the role of Pht proteins in pneumococcal binding to respiratory epithelial cells. Pneumococci were incubated with human nasopharyngeal epithelial cells (Detroit-562) and lung epithelial cells (A549 and NCI-H292), and the proportion of bound bacteria was measured by plating viable counts. Strains R36A (unencapsulated), D39 (serotype 2), 43 (serotype 3), 4-CDC (serotype 4), and 2737 (serotype 19F) with one or more of the four homologous Pht proteins deleted were compared with their wild-type counterparts. Also, the effect of anti-PhtD antibodies on the adherence of strain 2737 to the respiratory epithelial cells was studied. Our results suggest that Pht proteins play a role in pneumococcal adhesion to the respiratory epithelium. We also found that antibody to PhtD is able to inhibit bacterial attachment to the cells, suggesting that antibodies against PhtD present at mucosal surfaces might protect from pneumococcal attachment and subsequent colonization. However, the relative significance of Pht proteins to the ability of pneumococci to bind in vitro to epithelial cells depends on the genetic background and the capsular serotype of the strain.
INTRODUCTION
Pneumococcus is a human pathogen that colonizes the nasopharynx, usually without causing symptoms, but may spread from the nasal location and cause mild mucosal infections (acute otitis media and sinusitis) or severe, invasive infections (pneumonia, meningitis, and septicemia). Mucosal surfaces of the human upper respiratory tract are the primary site of a pneumococcal infection. The initial step in pneumococcal colonization is the attachment of the bacteria to the mucosal surfaces in the nasopharynx. Aspiration of colonized bacteria may lead to pneumococcal adherence to the lower airway epithelium and bronchopulmonary infections. The progression from asymptomatic colonization to disease is largely dependent not only on host factors but also on factors characteristic of specific pneumococcal strains. Pneumococci possess several surface proteins that are important in its pathogenesis at the airway epithelia (1–7).
Pneumococcal histidine triad proteins PhtA, PhtB, PhtD, and PhtE form a group of conserved surface proteins characterized by histidine triad motifs (8). They share extensive sequence similarity, with PhtB and PhtD having the highest sequence homology (87%) (8, 9). Pht proteins have been shown to be strongly immunogenic and to induce protective humoral immunity in the host, therefore establishing Pht proteins as strong vaccine candidates. Immunization of mice with Pht proteins has been shown to induce protection against nasopharyngeal colonization (10, 11), sepsis, and pneumonia (8, 10, 12, 13). Of all the Pht proteins, immunization with PhtD is likely to elicit the broadest protection against pneumococcal infections as PhtD is present among all pneumococcal strains (8, 9). Immunization of rhesus macaques with a protein-based vaccine containing PhtD was shown to protect the animals from pneumococcal pneumonia (14). PhtD has also been included in phase I pneumococcal protein vaccine clinical studies rendering promising outcomes (15, 16).
Homologs of pht genes have been identified in the human pathogens Streptococcus pyogenes (17, 18) and Streptococcus agalactiae (19), but also among commensal streptococcal species and in some nonstreptococcal species (20). In S. pyogenes, this type of protein has been identified as an adhesin binding to extracellular matrix protein type I collagen (21). The expression of pht genes is controlled individually; phtA, phtB, and phtE each possess their own promoter, whereas phtD is under the control of an operon (22). Binding sites for transcriptional repressor AdcR have been found upstream of pht genes (22, 23). AdcR binding has been shown to be induced under conditions of high Zn2+, which results in inhibition of transcription of pht genes under its dependence (9, 23). The concentrations of ions essential to pathogen survival are carefully controlled at mucosal surfaces; the zinc concentration in bronchoalveolar lavage fluid is 5- to 10-fold lower than that in the human plasma (24, 25). In Streptococcus pyogenes, S. agalactiae, and Streptococcus equi, candidate AdcR sites were found upstream of the orthologous lmb-phtD operon (22). Lmb is a laminin-binding protein that in S. agalactiae functions in attachment to laminin and in this way contributes to bacterial colonization and translocation of bacteria into the bloodstream (26). As pneumococcal phtD is regulated as a member of lmb-phtD operon, it was suggested that the two cotranscribed genes might both be involved in the adhesion and invasion process on mucosal surfaces, where the zinc concentration is low (22).
The biological function of Pht proteins in pneumococcal virulence is still poorly understood, but roles in metal scavenging (9), complement inhibition (27, 28), and in adherence to respiratory epithelium (29) have been suggested. Our aim in this study was to further assess the possible role of Pht proteins in pneumococcal colonization by comparing the ability of wild-type and mutant pneumococcal strains lacking one or more of the Pht proteins to bind to respiratory epithelial cell lines and also to assess the possible inhibitory function of Pht antibodies in the adherence.
MATERIALS AND METHODS
Bacterial strains.
The pneumococcal strains used in this study are listed in Table 1. The adhesion properties of the wild-type strains were compared with those of the corresponding knockout mutants lacking one or more of the Pht proteins. These mutants were prepared by GlaxoSmithKline Biologicals (GSK) as previously described (27). Pneumococcal surface protein C (PspC) has been shown to contribute to nasopharyngeal colonization and pneumonia (30–32) and to promote adhesion to and translocation across epithelial layers (7, 33–37). We used a PspC-deficient mutant, D39, kindly provided by David Briles, as a control in the adhesion analyses.
TABLE 1.
Pneumococcal strains used in this study
| Serotype | Strain | Descriptiona | Reference |
|---|---|---|---|
| Rough | R36A | Wild-type, unencapsulated derivative of D39 | 55 |
| R36A PhtA−B−D−E− | R36A derivative with phtA, phtB, phtD, and phtE deletions | 27 | |
| 2 | D39 | Wild type | 55 |
| D39 PhtB− | D39 derivative with phtB deleted; Spr | This study | |
| D39 PhtD− | D39 derivative with phtD deleted; Emr | This study | |
| D39 PhtB−D− | D39 derivative with phtB and phtD deleted; Spr Emr | This study | |
| D39 PhtA−B−D−E− | D39 derivative with phtA, phtB, phtD, and phtE deletions; Spr Emr | 27 | |
| D39 PspC− | TRE108, D39 derivative with pspC deletion; Emr | 56 | |
| 3 | 3-43 | Wild-type clinical isolate, naturally lacks phtA | 27 |
| 3-43 Pht(A)B−D−E− | 3-43 derivative with phtB, phtD, and phtE deletions | 27 | |
| 4 | 4-CDC | Wild-type clinical isolate from blood | 57 |
| 4-CDC PhtB− | 4-CDC derivative with phtB deleted; Spr | This study | |
| 4-CDC PhtD− | 4-CDC derivative with phtD deleted; Emr | This study | |
| 4-CDC PhtB−D− | 4-CDC derivative with phtB and phtD deleted; Spr Emr | This study | |
| 4-CDC PhtA−B−D−E− | 4-CDC derivative with phtA, phtB, phtD, and phtE deletions; Spr Emr | 27 | |
| 19F | 2737 | Wild-type clinical isolate, naturally lacks phtA | 27 |
| 2737 PhtB− | 2737 derivative with phtB deleted; Spr | This study | |
| 2737 PhtD− | 2737 derivative with phtD deleted; Emr | This study | |
| 2737 PhtB−D− | 2737 derivative with phtB and phtD deleted; Spr Emr | This study | |
| 2737 Pht(A)B−D−E− | 2737 derivative with phtB, phtD, and phtE deletions; Spr Emr | 27 |
Emr, erythromycin resistant; Spr, spectinomycin resistant.
Eukaryotic cell lines.
The role of Pht proteins in pneumococcal adhesion to epithelial cells was studied with three different cell lines. The epithelial cells used were nasopharyngeal human carcinoma cell line Detroit 562 (ATCC CCL-138; American Type Culture Collection), human lung epithelial cell line A549 (ATCC CCL-185), and human lung mucoepidermoid carcinoma cell line NCI-H292 (ATCC CRL-1848).
Antibodies.
The immune sera and monoclonal antibodies (MAbs) that were used in the adhesion assay were provided by GSK. The polyclonal immune sera were obtained by immunizing rabbits with PhtD (anti-PhtD concentration in the serum, 9,641 μg/ml) or PspC from strain 4-CDC (anti-PspC concentration, 1,357 μg/ml). The sera used in the adhesion assay were heat inactivated by incubation at +56°C for 30 min, after which the inactivated sera were divided into small aliquots and stored at −20°C until used. The polyclonal PhtD antiserum is cross-reactive with all Pht proteins. When the role of serum anti-PhtD antibodies in the inhibition of bacterial binding was assessed, anti-PspC serum, baby rabbit complement without antibodies, and antibody-depleted human serum (Valley Biomedical) were used as controls. In addition to the polyclonal immune sera, the effect of two monoclonal anti-PhtD antibodies (MAbs) on bacterial binding was assessed: MAb6 (2,539 μg/ml) and MAb20 (3,199 μg/ml). The effect of monoclonal antibodies on bacterial binding was compared to that in bacteria incubated in buffer.
Bacterial cultivation.
Bacteria were cultured on tryptone soy agar supplemented with 5% sheep blood (TSAB) plates or on Todd-Hewitt broth supplemented with 0.5% yeast extract (THYE; pH 7.6 to 7.8) agar plates or liquid culture at +37°C in an atmosphere with 5% CO2 overnight. To test the antibody-mediated inhibition of bacterial adherence, the serotype 19F wild-type strain 2737 (hereafter, 19F-2737) was cultured in THYE supplemented with 0.25 μM TPEN [N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine; Sigma-Aldrich], a zinc chelator, to enhance the expression of Pht proteins. Addition of this zinc chelator to the culture medium has been shown to result in increased expression of the Pht proteins (9). Pneumococcal colonies were inoculated into THYE broth, and cultures were grown statically to the early logarithmic growth phase (turbidity of 25 to 30 Klett units). Strain D39 PspC− was grown in the presence of 0.3 μg/ml erythromycin. The bacterial cultures were then diluted 1:1 with fresh THYE medium containing glycerol (Sigma-Aldrich) at a final concentration of 15%. The cultures were divided into small aliquots and stored at −70°C. The concentration of the frozen bacterial stocks, as well as the proportion of bacteria binding to epithelial cells, was determined by plating viable counts. THYE plates containing antibiotics were used for selective culture of PhtB− (250 μg/ml of spectinomycin), PhtD−, and PhtB− PhtD− (hereafter, PhtB−D−) (0.2 μg/ml of erythromycin) mutant strains in the competitive adhesion assay.
Cultivation of human respiratory epithelial cell lines.
Detroit 562 cells were cultured in Eagle's minimal essential medium (EMEM; ATCC-30-2003) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco). A549 cells were cultured in Kaighn's modification of Ham's F-12 medium (F-12K; ATCC-30-2004) with 10% FBS and NCI-H292 cells in RPMI 1640 medium (ATCC-30-2001) with 10% FBS. All cell lines were cultured without antibiotics, in a concentration of 3 × 105 cells per ml at +37°C in 5% CO2. Cells attached to the bottom of culture flasks were detached with 0.25% trypsin–1 mM EDTA (Gibco). For the adhesion assay, cells were seeded on 24-well or 48-well plates (Nunclon Surface) in a concentration of 3 × 105 cells per ml and cultured for 2 days until the cells formed a confluent monolayer, the estimated final cell concentration being 5 × 105 cells per ml.
Adhesion assays. (i) Bacterial adhesion assay.
Bacterial suspensions were inoculated on the respiratory epithelial cells on three parallel wells in a concentration of 5 × 106 CFU per ml per well on a 24-well plate (for comparison of wild-type and quadruple Pht mutants) or in a concentration of 5 × 106 CFU per ml in a volume of 500 μl per well on a 48-well plate (for comparison of wild-type and single, double, and quadruple mutant strains). To sediment the bacteria into closer contact with the epithelial cells, the plates were centrifuged at 1,300 × g for 5 min (as previously described by Gould and Weiser [38]), after which the cells were incubated with the bacteria for 1 h (for comparison of wild-type and quadruple Pht mutants) or 1 h 20 min (for comparison of wild-type and single mutant strains) at +37°C in 5% CO2. The number of bacteria inoculated on the wells was verified by plating serial dilutions on duplicate plates and counting the number of CFU. After the 1-h or 1-h 20-min incubation period, unattached bacteria were removed by washing the cells with Hanks' balanced salt solution with CaCl2 and MgCl2 (HBSS++; Gibco). Attached bacteria and epithelial cells were detached from the wells with trypsin-EDTA. The number of bound bacteria was determined by plating viable counts. The number of CFU of bacteria bound to cells was compared to the CFU of bacteria in the inoculum. Each strain was analyzed in triplicate wells, and the analysis was repeated five times.
(ii) Competitive bacterial adhesion assay.
In the competitive adhesion assay, bacterial suspensions were inoculated on the cells on a 24-well plate in a concentration of 5 × 106 CFU/ml of the wild-type strain and an equal concentration of the PhtB−, PhtD−, or PhtB−D− mutant counterpart per well. The plates were centrifuged at 1,300 × g for 5 min. After an incubation period of 1 h 20 min, unbound bacteria were removed, and the number of adherent bacteria was determined by plating serial dilutions of the detached bacterial suspensions on THYE agar plates (for culture of wild-type and mutant strains) or THYE agar plates containing spectinomycin (for culture of PhtB− mutants) or erythromycin (for culture of PhtD−, PhtB−D−, or PhtB−D−E− mutants). Each strain was analyzed in triplicate wells, and serial dilutions of the detached bacteria were made from each well. Suspensions of the different dilutions were cultured on duplicate agar plates. The analysis was repeated in five separate experiments.
Antibody-mediated inhibition of bacterial adherence.
Pneumococcal wild-type strain 2737 was preopsonized with monoclonal anti-PhtD antibodies or with different polyclonal rabbit sera for 25 min at room temperature. The effect of rabbit immune sera on the inhibition of bacterial binding was compared to that in bacteria incubated with a nonimmune rabbit serum and a nonimmune human serum in order to control possible unspecific effects serum proteins might have on bacterial adhesion. PhtD MAb6 and PhtD MAb20 were used at 1:20 and 1:50 dilutions. The polyclonal anti-PhtD serum and anti-PspC serum, as well as the baby rabbit complement serum (nonimmune rabbit serum) and antibody-depleted human serum (nonimmune human serum), were diluted 1:10 and 1:20. After the preopsonization, bacterial suspensions were inoculated on Detroit 562 and A549 cells in a concentration of 5 × 106 CFU per ml in a volume of 150 μl per well on a 48-well plate. The plates were centrifuged at 1,300 × g for 5 min and incubated for 1 h 20 min at +37°C in 5% CO2. After the incubation period, unattached bacteria were removed by washing with HBSS++, and the attached bacteria were detached with trypsin-EDTA. The number of bacteria inoculated on the wells and the number of bound bacteria were verified by plating serial dilutions and counting the number of CFU on THYE plates. The analysis was repeated 5 times with both cell lines in three parallel wells in each analysis. The binding of bacteria in samples treated with monoclonal antibodies was compared to that of bacteria incubated with HBSS++. The binding of bacteria in samples treated with anti-PhtC or anti-PhtD sera was compared with that of bacterial samples incubated with heat-inactivated baby rabbit complement (rabbit serum).
Statistical methods.
The number of bacteria bound to the cells was compared to the number of bacteria inoculated (CFU), reported as binding percentages. The percentages higher than 100% adherence were due to bacterial replication while in cell culture. The binding of the quadruple Pht mutant pneumococcal strains was compared to the binding of the corresponding wild-type strains, reported as relative binding percentages. The results are expressed as the average of five different experiments with standard deviations. Student's two-tailed, paired t test was applied in comparisons of the binding of wild-type and mutant pneumococci and in comparisons of bacteria opsonized with anti-PhtD antibodies and control sera. In all analyses, P values of <0.05 were considered to indicate a statistically significant difference.
RESULTS
Adhesion of wild-type and quadruple Pht mutant strains to respiratory epithelial cells.
We first compared the ability of wild-type pneumococcal strains and strains lacking all Pht proteins to bind to respiratory epithelial cells. The encapsulated serotype 3 strain 43 had the lowest adherence capacity to the cells: 16% of the bacteria inoculated on the wells were observed to bind to Detroit 562, 32% to A549, and 7% to NCI-H292. The binding of the unencapsulated R36A was substantial as higher bacterial numbers were recovered from the cells compared to the bacterial numbers inoculated (530%, 220%, and 150% binding on Detroit 562, A549, and NCI-H292 cells, respectively). Lack of PspC significantly reduced the binding of D39 to each of the three cell lines (Fig. 1). We found that the relative adhesion of 19F-2737 was significantly reduced to the lung epithelial cells (A549 and NCI-H292) when all Pht proteins were absent. The relative binding of the quadruple mutant 2737 strain to nasopharyngeal Detroit 562 cells was only 40% compared to its wild-type counterpart, but the difference did not reach statistical significance. Lack of Pht proteins appeared to have no effect on the adherence properties of the unencapsulated strain and resulted in varied effects in the strains representing capsular serotypes 2, 3, and 4. Binding of the serotype 3 strain 43 lacking all Pht proteins was significantly reduced in the A549 cell line, and a trend of reduced binding was also seen with the Detroit 562 cell line (40%). The ability of the quadruple mutant D39 strain to bind to the NCI-H292 cell line, but not the other two cell lines, was reduced (Fig. 1).
FIG 1.
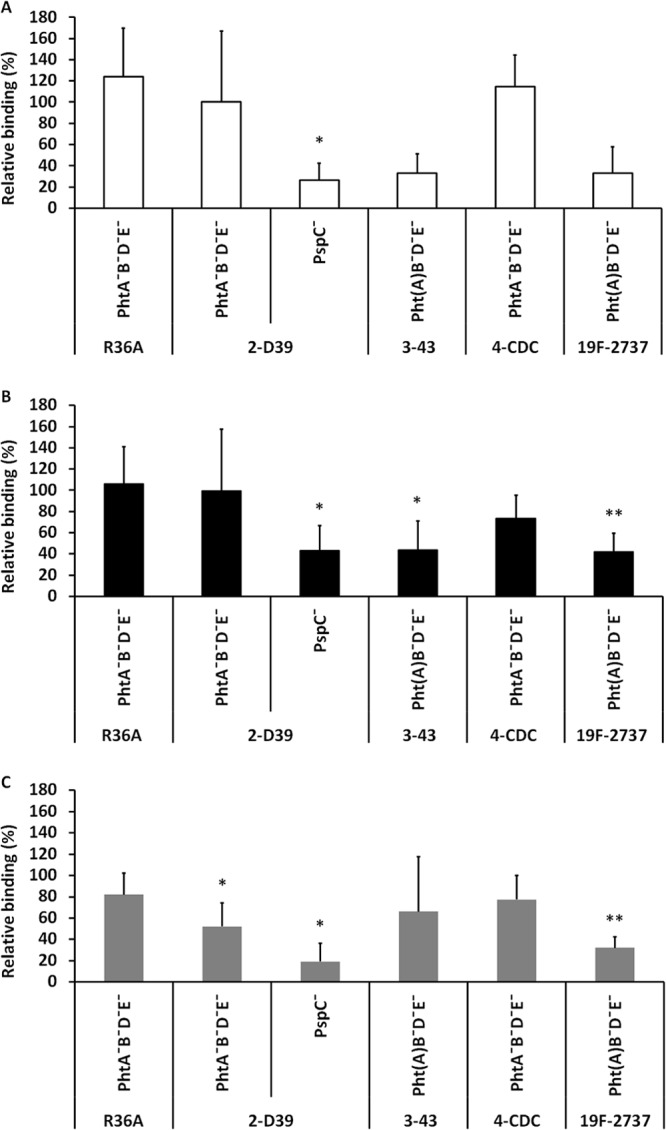
Binding of wild-type and mutant pneumococcal strains to the nasopharyngeal Detroit 562 cell line (A) and lung epithelial cell lines A549 (B) and NCI-H292 (C). The relative binding percentage indicates the adhesion of the mutant strain compared to the corresponding wild-type strain. Strains 3-43 and 19F-2737 naturally lack PhtA. The figures summarize five different experiments. Significant differences (Student's paired t test) are indicated with asterisks: *, P < 0.05; **, P < 0.01.
Adhesion of wild-type and single, double, and quadruple Pht mutant strains to respiratory epithelial cells.
To assess the relative role of single Pht proteins in pneumococcal adhesion to epithelial cells, we tested the binding of D39, 4-CDC, and 2737 wild-type and mutant strains to nasopharyngeal and lung epithelial cells. Adhesion of the quadruple Pht mutant D39 strain to each of the cell lines was slightly reduced, but the difference was statistically significant only for the A549 cell line (Fig. 2A). The binding of the mutant 4-CDC strain that lacked both PhtB and PhtD was significantly reduced compared to that of the wild-type strain in the Detroit 562 cell line (Fig. 2B). Binding of the quadruple Pht mutant 4-CDC was also reduced in the A549 cell line (Fig. 2B). In this study setting, binding of the single, double, and quadruple Pht mutant 2737 strains to all three cell lines was significantly reduced (Fig. 2C).
FIG 2.
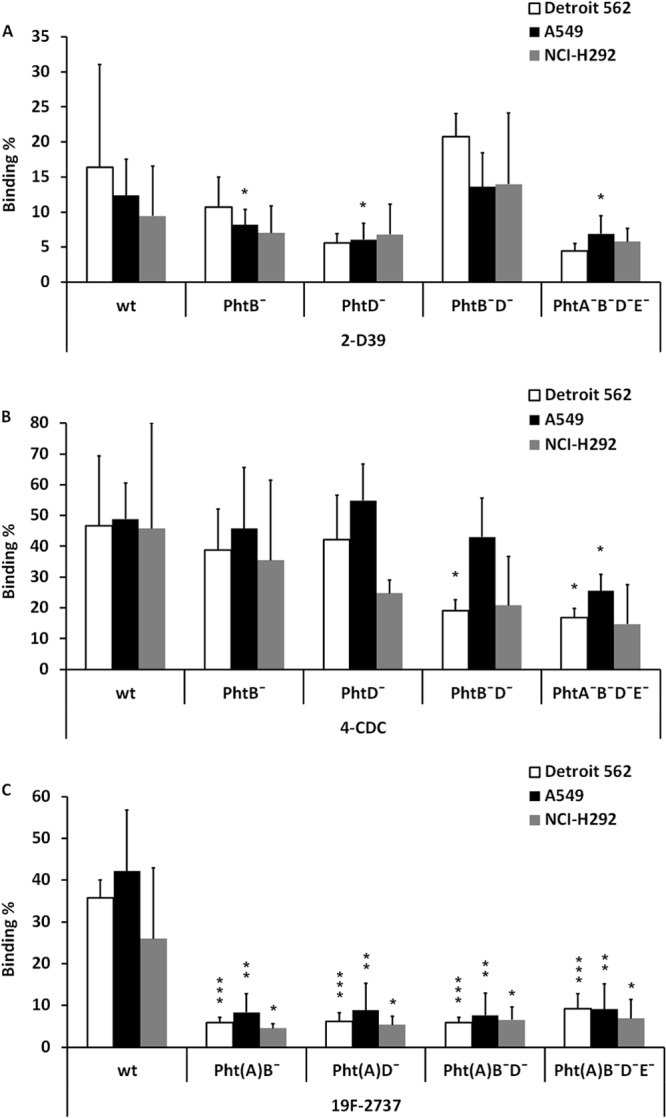
Binding of pneumococcal strains 2-D39 (A), 4-CDC (B) and 19F-2737 (C) lacking one or more Pht proteins to nasopharyngeal epithelial cells (Detroit 562) or lung epithelial cells (A549 and NCI-H292). wt, wild type. The panels summarize five different experiments. Significant differences (Student's paired t test) are indicated with asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Competitive adhesion of serotype 19F-2737 wild-type and mutant strains to respiratory Detroit 562 cells.
The binding abilities of 2737 PhtB−, PhtD−, PhtB−D−, and PhtB−D−E− mutants were compared with the wild-type strain also in a competitive adhesion assay. When the wild-type and mutant 27327 strains were inoculated on the Detroit 562 cells in a 1:1 ratio (Fig. 3A), the average binding of the mutant strains was significantly reduced compared to that in the wild-type 2737 strain (Fig. 3B). The result of the competitive adhesion experiment was in line with the results obtained by comparing the binding of the 2737 wild-type and mutant strains separately (Fig. 2C).
FIG 3.
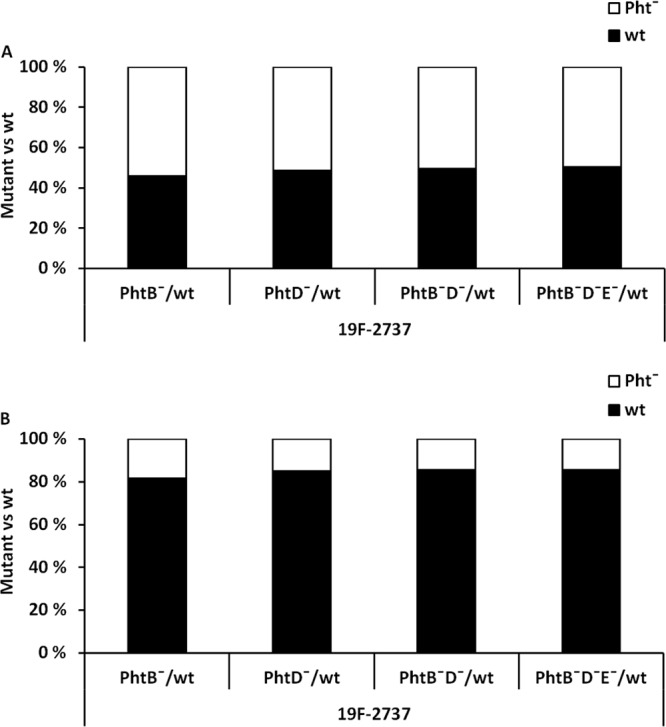
Competitive adhesion of pneumococcal wild-type (wt) and Pht− mutant 19F-2737 strains to Detroit 562 cells. The proportion of the Pht̄ mutant bacteria compared to the wild-type strain was 50% in the inoculum (A), but the proportion of Pht̄ mutant bacteria bound to the Detroit 562 cells was less than 20% (B). Each analysis was repeated in five separate experiments, and the panels summarize all five experiments. The difference in the proportions of the binding of the mutant and wild-type bacteria was statistically significant for each mutant (P < 0.001, Student's paired t test).
Inhibition of binding of the 2737 strain to respiratory epithelial cells.
Preopsonization of the 2737 strain with monoclonal anti-PhtD antibodies reduced the binding of the strain to nasopharyngeal epithelial cells (Detroit 562 cell line) compared to bacteria preopsonized with HBSS++ only (Fig. 4A). Anti-PhtD polyclonal serum, when diluted 1:10, inhibited adhesion of 2737 to Detroit 562 cells compared to the level in bacteria preopsonized with nonimmune rabbit serum (Fig. 4B). Compared to treatment with nonimmune human serum, even the dilution 1:20 reduced the binding of pneumococci to Detroit 562 cells (P < 0.05, Student's paired t test). The monoclonal anti-PhtD antibodies also reduced the binding of the 2737 strain to A549 lung epithelial cells (Fig. 5A). Polyclonal anti-PhtD and -PspC sera diluted 1:10 reduced binding of the pneumococcal strain to the A549 cells compared to treatment with nonimmune rabbit serum (Fig. 5B), whereas also the dilution 1:20 reduced binding of pneumococci to the cells, when treatment with anti-PhtD or -PspC sera was compared to treatment with nonimmune human serum (P < 0.05). With either cell line, we observed no significant differences between bacteria treated with heat-inactivated rabbit sera or human sera or between bacteria treated with HBSS++ buffer or with the nonimmune sera.
FIG 4.
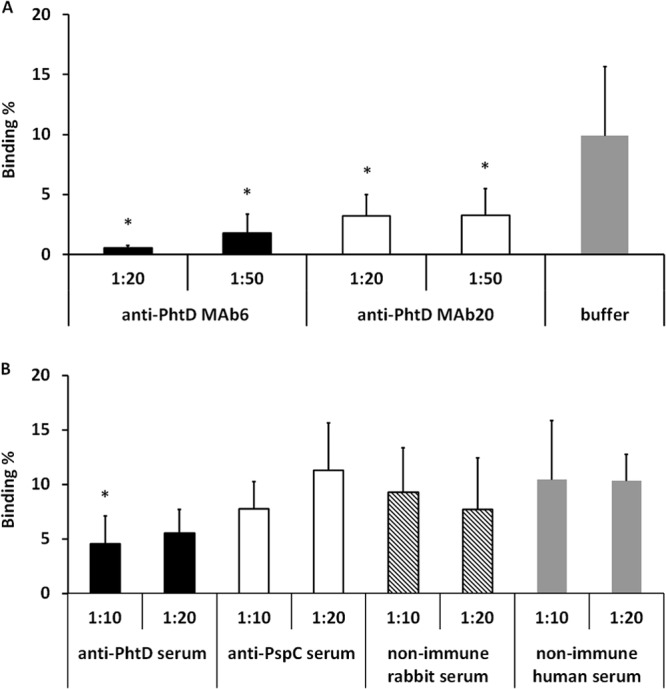
Binding of 19F-2737 to nasopharyngeal epithelial cells (Detroit 562) in the presence of monoclonal antibodies or HBSS++ buffer (A) and with immune or nonimmune sera (B). The significant differences in samples treated with monoclonal antibodies compared to buffer (A) or with anti-PhtD- or anti-PspC-specific sera compared to rabbit antiserum (B) are indicated with asterisks (P < 0.05, Student's paired t test). The panels summarize five different experiments.
FIG 5.
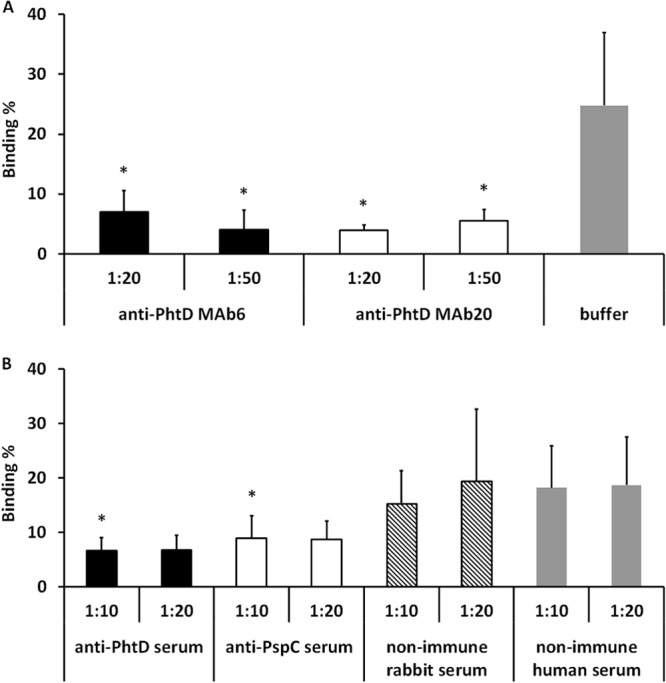
Binding of 19F-2737 to lung epithelial cells (A549) in the presence of monoclonal antibodies or HBSS++ buffer (A) and with immune or nonimmune sera (B). The significant differences in samples treated with monoclonal antibodies compared to buffer (A) or with anti-PhtD- or anti-PspC-specific sera compared to rabbit antiserum (B) are indicated with asterisks (P < 0.05, Student's paired t test). The panels summarize five different experiments.
DISCUSSION
Pneumococcal pathogenesis is a complex process in which the initial step is the attachment of the bacteria to the mucosal cell surface in the nasopharynx. Few pneumococcal surface proteins have been shown to play direct roles as adhesins, such as pneumococcal surface adhesin A (PsaA) and PspC, which bind to E-cadherin and polymeric Ig receptor, respectively (1, 5, 7). Pneumococcal pili and pneumococcal choline binding protein A (PcpA) have been shown to mediate attachment to respiratory epithelial cells, but their precise ligands are not known (2, 3, 6). Some pneumococcal proteins have been suggested to indirectly affect the adhesion process, such as pneumococcal neuraminidase A (NanA), which by cleaving terminal sialic acids from the respiratory mucosa might reveal receptors for bacterial adherence (4). One previous study has assessed the role of Pht proteins in bacterial adherence to respiratory epithelial cells (29). Khan and Pichichero studied the binding of wild-type and PhtD and PhtE mutant TIGR4 strains to the Detroit 562 and A549 cell lines by flow cytometry and found that the number of epithelial cells with adherent pneumococci was reduced when either Pht protein was missing. They were also able to show increased adherence of Escherichia coli to the epithelial cells when PhtD and PhtE were expressed on the bacterial surface. The results of our study support the previous report and indicate that Pht proteins affect the ability of different pneumococcal strains to bind to respiratory epithelial cells. These results, which suggest a role for Pht proteins in the mucosal infection stage, are in concordance with mouse studies, where deletion of Pht proteins has been shown to attenuate the virulence of the infecting strain in nasopharyngeal colonization and pneumonia (28, 39, 40).
However, the impact of Pht proteins on adhesion of pneumococci to respiratory epithelial cells is likely to depend on the background of the strain. It has been shown by capsule switching experiments that the capsular serotype expressed by a pneumococcal strain impacts the accessibility of its surface adhesins and thus affects virulence within the respiratory tract (41). Even though at the time of adhesion the capsule is partially shed and bacteria attached to the epithelium tend to be less heavily encapsulated than unattached pneumococci (42), the overall percentages of adherence of the encapsulated wild-type pneumococcal strains were moderate in the present study, as generally less than half of the inoculated bacteria were found to bind to the pharyngeal and lung epithelial cells. We found that binding of the wild-type as well as Pht mutant serotype 3 strains to the cells was very low, which could result from the thick capsule of this serotype covering the surface-exposed adhesins and inhibiting the bacterium from getting into contact with the cells. In contrast, the unencapsulated R36A bound very strongly to the epithelial cells. In setting up the bacterial binding assay, we tested different incubation periods from 15 min to up to 4 h and found that bacterial binding to the cells was increased the longer the bacteria were allowed to adhere (data not shown). However, when the incubation period was 2 h or longer, bacterial multiplication resulted in binding percentages of >100% and less repeatable results. We also tested different inoculum sizes and found that even though bacterial binding percentages were slightly increased when the inoculum size was larger, the differences between wild-type and mutant strains were not affected. Since bacteria may multiply during the incubation period, it is possible that the bacterial growth rate could affect the observed bacterial binding. In setting up the adhesion assay, we compared the number of bacteria recovered from the wells to the number of bacteria found in the cell culture supernatant as well as to the number of bacteria that were inoculated on the wells. We did not detect any differences between the mutant pneumococcal strains and the corresponding wild-type strains in bacterial growth curves in liquid culture nor in multiplication rates in cell culture supernatant during the adhesion experiment, which indicates that the differences observed in bacterial binding in the adhesion assay could not be explained by different bacterial growth rates. S. pneumoniae isolates from pneumonia patients have been shown to vary in their ability to bind to human lung epithelial cells (A549) (43), and such strain-specific binding properties are also likely to explain the differences we observed in binding of the wild-type strains.
When we first compared the binding of mutants lacking all Pht proteins to their wild-type parents, we found the greatest reduction in adhesion to the respiratory epithelial cell lines with the 19F-2737 strain, whereas with strain 4-CDC there seemed to be no significant reduction. As we next assessed the binding of single, double, and quadruple Pht mutant strains to the cell lines, the lack of all four Pht proteins appeared to result in reduced binding of the mutant in both the 19F-2737 background as well as the 4-CDC background. We conclude that this difference is likely a result of a slight change to the methodology. In the latter assay, the bacteria were incubated with the cells for a longer period (1 h 20 min compared to 1 h in the former assay), and the unbound bacteria were more stringently removed with an additional washing step. In order to assess the impact of the methodological difference on the results, the analyses with the Detroit 562 cells and 19F-2737 strains were done in parallel by removing the unbound bacteria by a light wash and a more stringent wash. We observed that the binding of the mutants was reduced compared to that of the wild type either way, but the difference between the wild type and quadruple mutant was not statistically significant when the unbound bacteria were removed only gently (data not shown). The introduction of an additional washing step to the assay thus improved the ability of the method to detect the differences between Pht mutant and wild-type strains due to better removal of unspecifically bound bacteria.
Although we found that the ability of single and double (PhtB− and PhtD−) mutant D39 and 4-CDC strains to bind to each of the three cell lines was affected to some degree, the greatest reduction was seen with the mutants lacking all Pht proteins. This observation suggests a functional overlap of Pht proteins also noted in a previous study (28). In contrast, even the deletion of a single Pht protein (PhtB or PhtD) significantly reduced the binding of the 19F-2737 to the respiratory epithelial cell lines in our study, and deletion of either PhtD or PhtE reduced binding of the TIGR4 strain in a previous report (29). The 2737 strain naturally lacks PhtA, which may partly explain the importance of PhtB and PhtD to the strain's binding capacity. The variability of the results, seen as wide standard deviation bars, was somewhat greater for the D39 and 4-CDC strains than for the 19F strain. The higher variability reduces the chance of observing significant differences in binding of the wild-type and mutant strains. Nevertheless, the relative importance of Pht proteins for pneumococcal adhesion is likely to depend on the presence of other surface proteins in the strain. A D39 mutant lacking PhtA and PhtB or all Pht proteins was significantly attenuated compared with the wild-type D39 in a pneumonia mouse model (28). In the TIGR4 background, deletion of even a single Pht protein (PhtA, PhtB, or PhtD) reduced the virulence of the strain in the lung infection of mice (39), which indicates that the presence of all Pht proteins is crucial for the virulence of this particular strain. In contrast, the lack of Pht proteins did not affect the binding ability of the unencapsulated strain R36A. It is possible that other adhesins, which are not masked by a capsule in the R36A strain, are able to compensate for the deletion of the Pht proteins. This observation with the unencapsulated strain is similar to the result reported in another study where PlcA, a pneumococcal collagen-like protein, was shown to contribute to the binding and invasion of D39 but not its unecapsulated derivative, R6, to Detroit 562 and A549 cells (44). The adhesion and invasion properties of the PlcA mutant R6 strain were unaffected, possibly due to compensating functions of other surface adhesins (44).
The expression and the role of Pht proteins in pneumococcal virulence might also depend on the stage of infection. Transcription of the pht genes in vivo is greatest for pneumococci residing in the nasopharynx or lungs compared to blood (28), suggesting that these proteins are important at the nasopharyngeal niche. Pneumococcus undergoes phase variation, which involves not only the thickness of the capsule (45, 46) but also the extent to which each surface protein is expressed (37, 47, 48), which could affect the ability of the bacterium to adhere to the epithelium. For example, PsaA was shown to contribute to pneumococcal adherence to Detroit 562 and A549 cells more in transparent or intermediate strains than in opaque strains (49, 50). We did not measure the expression levels of other surface proteins in the wild-type and Pht mutant strains in this study. However, we have previously shown that the surface expression of PspA and PspC in the quadruple Pht mutant strains was no different from that in the corresponding wild-type strains (27), which indicates that the observed reduced binding of the Pht mutant strains is unlikely to be explained by reduced expression of other surface proteins. We also found that although the lack of either PhtB or PhtD alone resulted in significantly reduced binding of the D39 strain to the A549 cell line, the binding of the PhtB−D− double mutant was not reduced. The colony morphology of the D39 PhtB−D− strain did not differ from that of the wild-type D39 strain or those of the other D39 mutants. However, there remains a possibility that the expression of other surface proteins was enhanced in the D39 PhtB−D− mutant, which would compensate for the loss of PhtB and PhtD and restore the mutant's ability to bind to the epithelial cells.
In this study, we found that PhtD antibodies are able to inhibit the binding of 19F-2737 to Detroit 562 and A549 cells. The 2737 strain was selected for the antibody inhibition assay, because the deletion of Pht proteins had the greatest impact on the adhesion capacity of this particular strain. Binding of the wild-type 2737 strain to epithelial cells was significantly reduced after preopsonization with either monoclonal anti-PhtD antibodies or polyclonal anti-PhtD rabbit sera. Khan and Pichichero recently reported that pneumococci aggregated in the presence of human serum IgG, resulting in a nonspecific drop in pneumococcal adherence to Detroit 562 cells, which led them to use IgG Fab fragments to study the functional role of PhtD- and PhtE-specific Fabs in blocking pneumococcal adherence (29). Our adhesion method is different from the one they used: we measured the binding of bacteria to epithelial cell layers and counted the number of live bacteria attached to the cells, whereas Khan and Pichichero measured the binding of fluorescently labeled bacteria to detached, floating epithelial cells and measured the fluorescence of cells by flow cytometry. It is possible that their methodology is more susceptible to aggregation, because they did not measure binding of bacteria to a cell layer.
In order to enhance the expression of Pht proteins, we cultured the bacteria in the presence of a zinc chelator, as access to zinc has been suggested to be one of the factors that control the expression of these proteins (8, 28). The pht genes are significantly upregulated in vivo compared with RNA levels in vitro (28), and this enhanced expression is likely to be important at mucosal surfaces, where the concentration of zinc is low. However, we were unable to detect a difference in binding of the strains cultured in the presence or absence of zinc chelator (data not shown), suggesting that zinc deprivation alone was not sufficient in this study setting to trigger overexpression of the Pht proteins to a level that would affect the in vitro adhesion properties of the pneumococcal strains. It is possible, that in vivo the lack of Pht proteins would have a more significant impact on the ability of the pneumococcal strains analyzed in this study to bind to the respiratory epithelium. Importantly, immunization with PhtD could lead to a very good protection in vivo, although in this study we were able to show only modest, albeit significant, inhibition of adherence to respiratory epithelial cells.
All of the Pht proteins have been shown to induce protective immunity against different pneumococcal strains in various mouse models of pneumococcal infection (8, 10–13). The ability of a vaccine to prevent nasopharyngeal carriage is an important property, given that a reduction in carriage is likely to reduce transmission of pneumococci and acquisition of pneumococcal infection. Immunization with any single one of the four Pht proteins was able to reduce the bacterial load in mice challenged with nasopharyngeal colonization, PhtD and PhtE affording the greatest reduction (10). Immunization of mice with other pneumococcal surface proteins, including PsaA (51, 52), PspA (53), and PspC (54), has also been shown to reduce nasopharyngeal colonization of pneumococci. However, previous studies assessing these potential vaccine candidates have included only one or a few pneumococcal challenge strains, typically the D39 strain. The protective ability of antibodies to protein antigens may depend on characteristics of the infective strain, as seen with mice immunized with PhtA (13), PhtB, or PhtD (8); the mice were protected from lethal sepsis against some but not all challenge strains. Of particular interest, vaccination with PhtD was able to protect mice from intranasal lethal challenge against different pneumococcal strains (D39, 43, and 4-CDC), whereas immunization with PspA or PspC only protected against the same strain, from which these antigens originated, D39 (10).
In conclusion, our findings support the previous reports, suggesting that Pht proteins play a role in pneumococcal adhesion to the respiratory epithelium and subsequent nasopharyngeal colonization (10, 29). However, pneumococcal adherence can be mediated by multiple adhesins. We found that the relative significance of Pht proteins on the ability of pneumococci to bind to epithelial cells depends on the genetic background and the capsular serotype of the strain. Other surface adhesins may compensate for the lack of a single protein. Given that the significance of Pht proteins to pneumococcal virulence may depend on the strain background, it is important to assess the role of Pht proteins in several strains with different genetic backgrounds, expressing different capsular serotypes. We also found that opsonization of a serotype 19F strain with antibody to PhtD was able to inhibit bacterial attachment to the mucosa. The anti-PhtD-mediated reduction of pneumococcal binding we observed suggests that antibodies against this protein present at mucosal surfaces might protect at the respiratory epithelium by preventing pneumococcal attachment and subsequent colonization. Further studies are required for broader assessment of the ability of antibodies to Pht proteins to inhibit the binding of different pneumococcal strains to respiratory epithelial cells.
ACKNOWLEDGMENTS
This work was supported by GlaxoSmithKline Biologicals (GSK). We thank David Briles from the University of Alabama at Birmingham for generously supplying the D39 PspC mutant strain. Statistician Mika Lahdenkari is acknowledged for advice on statistical analysis of the data at National Institute for Health and Welfare, Finland. We also acknowledge Emmanuel Di Paolo and Chistiane Feron (GSK) for support and expertise in molecular bacteriology.
P.H., P.D., and F.G. are employees of the GlaxoSmithKline group of companies. They own shares in GSK and are named inventors on several patents or patent applications related to the field of vaccine development.
Footnotes
Published ahead of print 3 February 2014
REFERENCES
- 1.Anderton JM, Rajam G, Romero-Steiner S, Summer S, Kowalczyk AP, Carlone GM, Sampson JS, Ades EW. 2007. E-cadherin is a receptor for the common protein pneumococcal surface adhesin A (PsaA) of Streptococcus pneumoniae. Microb. Pathog. 42:225–236. 10.1016/j.micpath.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 2.Bagnoli F, Moschioni M, Donati C, Dimitrovska V, Ferlenghi I, Facciotti C, Muzzi A, Giusti F, Emolo C, Sinisi A, Hilleringmann M, Pansegrau W, Censini S, Rappuoli R, Covacci A, Masignani V, Barocchi MA. 2008. A second pilus type in Streptococcus pneumoniae is prevalent in emerging serotypes and mediates adhesion to host cells. J. Bacteriol. 190:5480–5492. 10.1128/JB.00384-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan MN, Sharma SK, Filkins LM, Pichichero ME. 2012. PcpA of Streptococcus pneumoniae mediates adherence to nasopharyngeal and lung epithelial cells and elicits functional antibodies in humans. Microbes Infect. 14:1102–1110. 10.1016/j.micinf.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King SJ, Hippe KR, Gould JM, Bae D, Peterson S, Cline RT, Fasching C, Janoff EN, Weiser JN. 2004. Phase variable desialylation of host proteins that bind to Streptococcus pneumoniae in vivo and protect the airway. Mol. Microbiol. 54:159–171. 10.1111/j.1365-2958.2004.04252.x [DOI] [PubMed] [Google Scholar]
- 5.Lu L, Ma Y, Zhang JR. 2006. Streptococcus pneumoniae recruits complement factor H through the amino terminus of CbpA. J. Biol. Chem. 281:15464–15474. 10.1074/jbc.M602404200 [DOI] [PubMed] [Google Scholar]
- 6.Nelson AL, Ries J, Bagnoli F, Dahlberg S, Falker S, Rounioja S, Tschop J, Morfeldt E, Ferlenghi I, Hilleringmann M, Holden DW, Rappuoli R, Normark S, Barocchi MA, Henriques-Normark B. 2007. RrgA is a pilus-associated adhesin in Streptococcus pneumoniae. Mol. Microbiol. 66:329–340. 10.1111/j.1365-2958.2007.05908.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JR, Mostov KE, Lamm ME, Nanno M, Shimida S, Ohwaki M, Tuomanen E. 2000. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102:827–837. 10.1016/S0092-8674(00)00071-4 [DOI] [PubMed] [Google Scholar]
- 8.Adamou JE, Heinrichs JH, Erwin AL, Walsh W, Gayle T, Dormitzer M, Dagan R, Brewah YA, Barren P, Lathigra R, Langermann S, Koenig S, Johnson S. 2001. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect. Immun. 69:949–958. 10.1128/IAI.69.2.949-958.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rioux S, Neyt C, Di Paolo E, Turpin L, Charland N, Labbe S, Mortier MC, Mitchell TJ, Feron C, Martin D, Poolman JT. 2011. Transcriptional regulation, occurrence and putative role of the Pht family of Streptococcus pneumoniae. Microbiology 157:336–348. 10.1099/mic.0.042184-0 [DOI] [PubMed] [Google Scholar]
- 10.Godfroid F, Hermand P, Verlant V, Denoel P, Poolman JT. 2011. Preclinical evaluation of the Pht proteins as potential cross-protective pneumococcal vaccine antigens. Infect. Immun. 79:238–245. 10.1128/IAI.00378-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Masi AW, Barniak V, Mountzouros K, Hostetter MK, Green BA. 2001. Recombinant PhpA protein, a unique histidine motif-containing protein from Streptococcus pneumoniae, protects mice against intranasal pneumococcal challenge. Infect. Immun. 69:3827–3836. 10.1128/IAI.69.6.3827-3836.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamel J, Charland N, Pineau I, Ouellet C, Rioux S, Martin D, Brodeur BR. 2004. Prevention of pneumococcal disease in mice immunized with conserved surface-accessible proteins. Infect. Immun. 72:2659–2670. 10.1128/IAI.72.5.2659-2670.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wizemann TM, Heinrichs JH, Adamou JE, Erwin AL, Kunsch C, Choi GH, Barash SC, Rosen CA, Masure HR, Tuomanen E, Gayle A, Brewah YA, Walsh W, Barren P, Lathigra R, Hanson M, Langermann S, Johnson S, Koenig S. 2001. Use of a whole genome approach to identify vaccine molecules affording protection against Streptococcus pneumoniae infection. Infect. Immun. 69:1593–1598. 10.1128/IAI.69.3.1593-1598.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denoel P, Philipp MT, Doyle L, Martin D, Carletti G, Poolman JT. 2011. A protein-based pneumococcal vaccine protects rhesus macaques from pneumonia after experimental infection with Streptococcus pneumoniae. Vaccine 29:5495–5501. 10.1016/j.vaccine.2011.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bologa M, Kamtchoua T, Hopfer R, Sheng X, Hicks B, Bixler G, Hou V, Pehlic V, Yuan T, Gurunathan S. 2012. Safety and immunogenicity of pneumococcal protein vaccine candidates: monovalent choline-binding protein A (PcpA) vaccine and bivalent PcpA-pneumococcal histidine triad protein D vaccine. Vaccine 30:7461–7468. 10.1016/j.vaccine.2012.10.076 [DOI] [PubMed] [Google Scholar]
- 16.Seiberling M, Bologa M, Brookes R, Ochs M, Go K, Neveu D, Kamtchoua T, Lashley P, Yuan T, Gurunathan S. 2012. Safety and immunogenicity of a pneumococcal histidine triad protein D vaccine candidate in adults. Vaccine 30:7455–7460. 10.1016/j.vaccine.2012.10.080 [DOI] [PubMed] [Google Scholar]
- 17.Kunitomo E, Terao Y, Okamoto S, Rikimaru T, Hamada S, Kawabata S. 2008. Molecular and biological characterization of histidine triad protein in group A streptococci. Microbes Infect. 10:414–423. 10.1016/j.micinf.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 18.Reid SD, Montgomery AG, Voyich JM, DeLeo FR, Lei B, Ireland RM, Green NM, Liu M, Lukomski S, Musser JM. 2003. Characterization of an extracellular virulence factor made by group A Streptococcus with homology to the Listeria monocytogenes internalin family of proteins. Infect. Immun. 71:7043–7052. 10.1128/IAI.71.12.7043-7052.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waldemarsson J, Areschoug T, Lindahl G, Johnsson E. 2006. The streptococcal Blr and Slr proteins define a family of surface proteins with leucine-rich repeats: camouflaging by other surface structures. J. Bacteriol. 188:378–388. 10.1128/JB.188.2.378-388.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao ZQ, Zhang YM, Pan XZ, Wang B, Chen JQ. 2013. Insight into the evolution of the histidine triad protein (HTP) family in Streptococcus. PLoS One 8:e60116. 10.1371/journal.pone.0060116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bober M, Morgelin M, Olin AI, von Pawel-Rammingen U, Collin M. 2011. The membrane bound LRR lipoprotein Slr, and the cell wall-anchored M1 protein from Streptococcus pyogenes both interact with type I collagen. PLoS One 6:e20345. 10.1371/journal.pone.0020345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panina EM, Mironov AA, Gelfand MS. 2003. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc. Natl. Acad. Sci. U. S. A. 100:9912–9917. 10.1073/pnas.1733691100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shafeeq S, Kloosterman TG, Kuipers OP. 2011. Transcriptional response of Streptococcus pneumoniae to Zn2+ limitation and the repressor/activator function of AdcR. Metallomics 3:609–618. 10.1039/c1mt00030f [DOI] [PubMed] [Google Scholar]
- 24.Bunker VW, Hinks LJ, Lawson MS, Clayton BE. 1984. Assessment of zinc and copper status of healthy elderly people using metabolic balance studies and measurement of leucocyte concentrations. Am. J. Clin. Nutr. 40:1096–1102 [DOI] [PubMed] [Google Scholar]
- 25.Harlyk C, Mccourt J, Bordin G, Rodriguez AR, van der Eeckhout A. 1997. Determination of copper, zinc and iron in broncho-alveolar lavages by atomic absorption spectroscopy. J. Trace Elem. Med. Biol. 11:137–142. 10.1016/S0946-672X(97)80040-5 [DOI] [PubMed] [Google Scholar]
- 26.Spellerberg B, Rozdzinski E, Martin S, Weber-Heynemann J, Schnitzler N, Lutticken R, Podbielski A. 1999. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 67:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melin M, Di Paolo E, Tikkanen L, Jarva H, Neyt C, Kayhty H, Meri S, Poolman J, Vakevainen M. 2010. Interaction of pneumococcal histidine triad proteins with human complement. Infect. Immun. 78:2089–2098. 10.1128/IAI.00811-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogunniyi AD, Grabowicz M, Mahdi LK, Cook J, Gordon DL, Sadlon TA, Paton JC. 2009. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J. 23:731–738. 10.1096/fj.08-119537 [DOI] [PubMed] [Google Scholar]
- 29.Khan MN, Pichichero ME. 2012. Vaccine candidates PhtD and PhtE of Streptococcus pneumoniae are adhesins that elicit functional antibodies in humans. Vaccine 30:2900–2907. 10.1016/j.vaccine.2012.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balachandran P, Brooks-Walter A, Virolainen-Julkunen A, Hollingshead SK, Briles DE. 2002. Role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 70:2526–2534. 10.1128/IAI.70.5.2526-2534.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orihuela CJ, Gao G, Francis KP, Yu J, Tuomanen EI. 2004. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 190:1661–1669. 10.1086/424596 [DOI] [PubMed] [Google Scholar]
- 32.Orihuela CJ, Radin JN, Sublett JE, Gao G, Kaushal D, Tuomanen EI. 2004. Microarray analysis of pneumococcal gene expression during invasive disease. Infect. Immun. 72:5582–5596. 10.1128/IAI.72.10.5582-5596.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal V, Asmat TM, Luo S, Jensch I, Zipfel PF, Hammerschmidt S. 2010. Complement regulator factor H mediates a two-step uptake of Streptococcus pneumoniae by human cells. J. Biol. Chem. 285:23486–23495. 10.1074/jbc.M110.142703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammerschmidt S, Agarwal V, Kunert A, Haelbich S, Skerka C, Zipfel PF. 2007. The host immune regulator factor H interacts via two contact sites with the PspC protein of Streptococcus pneumoniae and mediates adhesion to host epithelial cells. J. Immunol. 178:5848–5858 [DOI] [PubMed] [Google Scholar]
- 35.Hammerschmidt S, Talay SR, Brandtzaeg P, Chhatwal GS. 1997. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol. Microbiol. 25:1113–1124. 10.1046/j.1365-2958.1997.5391899.x [DOI] [PubMed] [Google Scholar]
- 36.Quin LR, Onwubiko C, Moore QC, Mills MF, McDaniel LS, Carmicle S. 2007. Factor H binding to PspC of Streptococcus pneumoniae increases adherence to human cell lines in vitro and enhances invasion of mouse lungs in vivo. Infect. Immun. 75:4082–4087. 10.1128/IAI.00474-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenow C, Ryan P, Weiser JN, Johnson S, Fontan P, Ortqvist A, Masure HR. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819–829. 10.1111/j.1365-2958.1997.mmi494.x [DOI] [PubMed] [Google Scholar]
- 38.Gould JM, Weiser JN. 2002. The inhibitory effect of C-reactive protein on bacterial phosphorylcholine platelet-activating factor receptor-mediated adherence is blocked by surfactant. J. Infect. Dis. 186:361–371. 10.1086/341658 [DOI] [PubMed] [Google Scholar]
- 39.Hava DL, Camilli A. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45:1389–1406. 10.1046/j.1365-2958.2002.03106.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plumptre CD, Ogunniyi AD, Paton JC. 2013. Surface association of Pht proteins of Streptococcus pneumoniae. Infect. Immun. 81:3644–3651. 10.1128/IAI.00562-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez CJ, Hinojosa CA, Shivshankar P, Hyams C, Camberlein E, Brown JS, Orihuela CJ. 2011. Changes in capsular serotype alter the surface exposure of pneumococcal adhesins and impact virulence. PLoS One 6:e26587. 10.1371/journal.pone.0026587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammerschmidt S, Wolff S, Hocke A, Rosseau S, Muller E, Rohde M. 2005. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect. Immun. 73:4653–4667. 10.1128/IAI.73.8.4653-4667.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robson RL, Reed NA, Horvat RT. 2006. Differential activation of inflammatory pathways in A549 type II pneumocytes by Streptococcus pneumoniae strains with different adherence properties. BMC Infect. Dis. 6:71. 10.1186/1471-2334-6-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paterson GK, Nieminen L, Jefferies JM, Mitchell TJ. 2008. PclA, a pneumococcal collagen-like protein with selected strain distribution, contributes to adherence and invasion of host cells. FEMS Microbiol. Lett. 285:170–176. 10.1111/j.1574-6968.2008.01217.x [DOI] [PubMed] [Google Scholar]
- 45.Kim JO, Weiser JN. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177:368–377. 10.1086/514205 [DOI] [PubMed] [Google Scholar]
- 46.Weiser JN, Austrian R, Sreenivasan PK, Masure HR. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Overweg K, Pericone CD, Verhoef GG, Weiser JN, Meiring HD, De Jong AP, De Groot R, Hermans PW. 2000. Differential protein expression in phenotypic variants of Streptococcus pneumoniae. Infect. Immun. 68:4604–4610. 10.1128/IAI.68.8.4604-4610.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiser JN, Markiewicz Z, Tuomanen EI, Wani JH. 1996. Relationship between phase variation in colony morphology, intrastrain variation in cell wall physiology, and nasopharyngeal colonization by Streptococcus pneumoniae. Infect. Immun. 64:2240–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berry AM, Paton JC. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64:5255–5262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero-Steiner S, Pilishvili T, Sampson JS, Johnson SE, Stinson A, Carlone GM, Ades EW. 2003. Inhibition of pneumococcal adherence to human nasopharyngeal epithelial cells by anti-PsaA antibodies. Clin. Diagn. Lab. Immunol. 10:246–251. 10.1128/CDLI.10.2.246-251.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Briles DE, Ades E, Paton JC, Sampson JS, Carlone GM, Huebner RC, Virolainen A, Swiatlo E, Hollingshead SK. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796–800. 10.1128/IAI.68.2.796-800.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De BK, Sampson JS, Ades EW, Huebner RC, Jue DL, Johnson SE, Espina M, Stinson AR, Briles DE, Carlone GM. 2000. Purification and characterization of Streptococcus pneumoniae palmitoylated pneumococcal surface adhesin A expressed in Escherichia coli. Vaccine 18:1811–1821. 10.1016/S0264-410X(99)00481-8 [DOI] [PubMed] [Google Scholar]
- 53.Wu HY, Nahm MH, Guo Y, Russell MW, Briles DE. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 175:839–846. 10.1086/513980 [DOI] [PubMed] [Google Scholar]
- 54.Briles DE, Hollingshead S, Brooks-Walter A, Nabors GS, Ferguson L, Schilling M, Gravenstein S, Braun P, King J, Swift A. 2000. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine 18:1707–1711. 10.1016/S0264-410X(99)00511-3 [DOI] [PubMed] [Google Scholar]
- 55.Avery OT, Macleod CM, McCarty M. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from Pneumococcus type Iii. J. Exp. Med. 79:137–158. 10.1084/jem.79.2.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hakansson A, Roche H, Mirza S, McDaniel LS, Brooks-Walter A, Briles DE. 2001. Characterization of binding of human lactoferrin to pneumococcal surface protein A. Infect. Immun. 69:3372–3381. 10.1128/IAI.69.5.3372-3381.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]


