Abstract
Leishmaniasis is a widespread neglected tropical disease caused by parasites of the Leishmania genus. These parasites express the enzyme 3′-nucleotidase/nuclease (3′NT/NU), which has been described to be involved in parasite nutrition and infection. Bacteria that express nucleases escape the toxic effects of neutrophil extracellular traps (NETs). Hence, we investigated the role of 3′NT/NU in Leishmania survival of NET-mediated killing. Promastigotes of Leishmania infantum were cultured in high-phosphate (HP) or low-phosphate (LP) medium to modulate nuclease activity. We compared the survival of the two different groups of Leishmania during interaction with human neutrophils, assessing the role of neutrophil extracellular traps. As previously reported, we detected higher nuclease activity in parasites cultured in LP medium. Both LP and HP promastigotes were capable of inducing the release of neutrophil extracellular traps from human neutrophils in a dose- and time-dependent manner. LP parasites had 2.4 times more survival than HP promastigotes. NET disruption was prevented by the treatment of the parasites with ammonium tetrathiomolybdate (TTM), a 3′NT/NU inhibitor. Inhibition of 3′NT/NU by 3′-AMP, 5′-GMP, or TTM decreased promastigote survival upon interaction with neutrophils. Our results show that Leishmania infantum induces NET release and that promastigotes can escape NET-mediated killing by 3′-nucleotidase/nuclease activity, thus ascribing a new function to this enzyme.
INTRODUCTION
Neutrophils are short-lived cells and the most abundant leukocytes in the blood circulation; they constitute one of the first lines of defense against invading microorganisms (1). These granulocytes can kill microorganisms by phagocytosis, degranulation, and neutrophil extracellular traps (NETs). NETs are weblike structures composed of chromatin, granules, and cytoplasmic proteins that are extruded when neutrophils undergo NETosis, a unique cell death mechanism (2–5). However, recent work challenges NETosis as a cell death mechanism because live neutrophils were detected after NET extrusion in in vivo studies (6). NETs function by killing and containing pathogens, thereby preventing the pathogen's dissemination through the organism. In addition, some studies have indicated that NETs play a role in autoimmune diseases (7–10).
A diverse group of stimuli has been described as activating NETosis (5, 11). Among the parasites, Leishmania promastigotes were demonstrated to activate release of NETs (12, 13). Leishmania amazonensis promastigotes interact intimately with NETs and are killed by web-associated histones (12). However, although promastigotes of Leishmania donovani trigger NET release, these parasites escape the toxicity of NETs (13). Groups of microorganisms have evolved different mechanisms of escaping the toxic effects of NETs. Streptococcus pneumoniae, group A Streptococcus, Streptococcus agalactiae, Staphylococcus aureus, and Vibrio cholerae express endonucleases that efficiently degrade DNA filaments from NETs, allowing these bacteria to escape the toxic effects of NETs and to spread throughout the body (14–21).
Leishmaniasis comprises a group of diseases endemic in 98 countries, mostly in tropical and subtropical areas, that are caused by parasites of the Leishmania genus. Leishmania infantum is an agent of visceral leishmaniasis, a disease that is characterized by fever, weakness, weight loss, and death if not treated. More than 90% of visceral leishmaniasis cases occur in India, Bangladesh, Nepal, Sudan, and Brazil and constitute an important public health problem in these places (22).
Leishmania parasites are auxotrophic for purines, meaning that these parasites are unable to produce purines de novo. Class I nucleases are a family of enzymes present in fungi, plants, and protozoa that specifically cleave DNA and RNA. A new member of this family, a 40-kDa 3′-nucleotidase/nuclease (3′NT/NU) enzyme, was described as a membrane-anchored protein of different species of genus Leishmania, including Leishmania infantum (23–27). This enzyme was first associated with parasite nutrition because the nuclease activity can generate nucleotides and phosphate from nucleic acids (28), allowing the parasites to acquire purines. The 3′NT/NU enzyme is stage specific and is only expressed by metacyclic and procyclic promastigotes (26). Moreover, the expression and activity of this enzyme are higher if parasites are cultured in purine- or inorganic phosphate-depleted medium (26, 29, 30).
Here, we investigated whether 3′NT/NU activity could allow Leishmania to escape from NET-mediated killing. Our results demonstrate that higher nuclease activity is correlated with parasite survival during interaction with human neutrophils. We also show that 3′NT/NU allows parasites to cleave neutrophil extracellular traps and to escape NET-mediated killing.
MATERIALS AND METHODS
Parasites.
Promastigotes of Leishmania infantum (MHOM/BR/1974/PP75) were maintained in brain heart infusion (BHI) modified medium (2 g/liter glucose, 2 g/liter peptone, 2 g/liter BHI, 0.25 g/liter liver infusion tryptose, 0.4 g/liter NaCl, 4 g/liter KCl, 11.5 g/liter NaH2PO4, 3 g/liter NaOH, 10 mg/ml hemin) supplemented with 20% fetal calf serum (FCS) at 26°C. These parasites are termed high-phosphate parasites (HP) herein because they were cultured in medium containing high concentrations of phosphate (Pi). In the low-inorganic phosphate culture medium, disodium hydrogen phosphate was replaced by sodium bicarbonate (8.4 g/liter), and the resulting promastigotes are termed low-phosphate parasites (LP) herein. The pH of both media was adjusted to 7.2 with HCl. The measurement of phosphate concentration in the HP culture medium (83 mM) and LP culture medium (2 mM) was carried out according to the method of Fiske and Subbarrow (31). Leishmania amazonensis (WHOM/BR/75/Josefa) and Leishmania donovani (MHOM/IN/83/Mongi-142) were maintained in Schneider's insect medium supplemented with 10% FCS at 26°C.
Metacyclic isolation.
Metacyclic promastigotes were isolated from 5- to 6-day cultures of HP and LP parasites using a Ficoll gradient as described previously (32). After gradient centrifugation, the metacyclics were isolated from the 10% Ficoll layer and the procyclic promastigotes from the pellet. Metacyclics were characterized by their typical morphology.
Enzyme assay.
3′-Nucleotidase activity was measured as previously described (30). Briefly, intact promastigotes (1 × 106 cells) were incubated for 60 min at 30°C in a mixture containing 116 mM NaCl, 5.4 mM KCl, 5.5 mM glucose, 50 mM HEPES-Tris buffer (pH 7.0), and 3 mM 3′-AMP as a substrate. The reaction was initiated by the addition of cells and stopped by the addition of 1.0 ml of an ice-cold suspension of 25% charcoal in 0.1 M HCl. This charcoal suspension was washed with 0.1 M HCl at least 20 times before use to avoid Pi contamination (33). This procedure reduces the values of blanks, as it removes nonhydrolyzed 3′-AMP from samples that are spontaneously hydrolyzed by sulfuric acid present in the Fiske-Subbarow reactive mixture (31). Controls in which cells were added after interruption of the reaction were used as blanks. After the reaction time, tubes were centrifuged at 1,500 × g for 15 min at 4°C, and 0.5 ml of the supernatant was added to 0.5 ml of Fiske-Subbarow reactive mixture (31). The ecto-3′-nucleotidase activity was calculated by subtracting the nonspecific 3′-AMP hydrolysis measured in the absence of cells. The concentration of Pi released in the reaction mixture was determined by using a Pi standard curve for comparison.
Neutrophil purification.
Neutrophils were isolated as previously described (12). Briefly, neutrophils were isolated from buffy coats of healthy donors by Ficoll-Histopaque density gradient centrifugation (Histopaque; Sigma-Aldrich), and then contaminant red blood cells were subjected to hypotonic lysis. The isolated neutrophils were resuspended in RPMI 1640 (LGC Biotecnologia, São Paulo, Brazil) medium and kept on ice until use. All procedures were approved by the Institutional Review Board for Human Subjects (Hospital Clementino Fraga Filho, Universidade Federal do Rio de Janeiro).
Quantification and visualization of neutrophil extracellular traps.
Neutrophils (2 × 106) were incubated with or without promastigotes of Leishmania infantum at different parasite/neutrophil ratios. After different time points, restriction enzymes (EcoR1 and HindIII, 20 U/ml; BioLabs) were added and the cultures incubated for 30 min more at 35°C. NET DNA was quantified in the supernatant using the PicoGreen double-stranded DNA (dsDNA) kit (Invitrogen) according to manufacturer's instructions and previously published methods (12). In parallel, neutrophils were incubated with 3′NT/NU inhibitors or with 3′NT/NU inhibitor-pretreated parasites to evaluate their impact on NET formation.
To visualize NETs, neutrophils (1 × 105) were allowed to seed on poly-l-lysine-treated coverslips and then incubated with HP or LP promastigotes (1 × 105). After 60 min, slides were fixed with 4% paraformaldehyde and stained with 4′,6-diamidino-2-phenylindole (DAPI) (10 μg/ml; Sigma). Epifluorescence images were taken in a Zeiss Axioplan.
Neutrophil killing assay.
A neutrophil killing assay was performed as previously described (12). Neutrophils (2 × 106) were incubated with or without DNase (20 U/ml; Fermentas Life Science). After 30 min of incubation, HP or LP parasites were added in a 1:0.1 neutrophil/parasite ratio and the culture was maintained for 120 min at 35°C with 5% CO2. Subsequently, FCS was added to a final concentration of 10%, and the culture was incubated at 26°C. After 2 days, parasite viability was assessed by counting live motile parasites in a Neubauer chamber. In parallel, we pretreated 5 × 106 parasites with different concentrations of ammonium tetrathiomolybdate (TTM; Sigma) for 20 min at room temperature in 0.5 ml of RPMI. Then, we added 2 × 105 (20 μl) pretreated parasites to the neutrophils and maintained the cultures for 120 min at 35°C with 5% CO2; the remainder of the assay was as described above. Because the final volume of the neutrophil killing assay mixture was 300 μl, the maximum ammonium tetrathiomolybdate concentration in the neutrophil-parasite culture was approximately 7 μM. In another set of experiments with 3′NT/NU inhibitors, 5′-GMP (250 μM or 500 μM; Sigma) and 3′-AMP (1,000 μM; Sigma) were added to the cocultures of neutrophils at the same time as the parasites.
Cell viability assays.
Neutrophils (2 × 106) were treated with 3′NT/NU inhibitors at different concentrations for 150 min, followed by measurement of lactate dehydrogenase in the culture supernatant according to the manufacturer's directions (Promega). Briefly, 50 μl of culture supernatant was incubated with 50 μl of the substrate mixture in a 96-well plate at room temperature protected from light. After 30 min, 50 μl of stop solution was added, and the plate was read at 490 nm on a SpectraMax fluorimeter. Leishmania infantum (1 × 106) was treated with the inhibitors as described above and promastigote viability determined by daily counting of viable cells. In parallel, the TTM inhibitor toxicity for promastigotes of Leishmania infantum was assessed by propidium iodide (PI; Sigma) staining. Parasites (5 × 106) were treated with TTM (100 μM) for 20 min and stained with PI (10 μg/ml). Cells were analyzed on a FACSCalibur flow cytometer and data analysis was performed using Summit 4.3 software.
Neutrophil extracellular trap digestion.
We generated NET-enriched supernatant by activating neutrophils (8 × 106) with phorbol myristate acetate (PMA, 100 nM; Calbiochem). After 180 min of incubation, the supernatant was recovered and was kept at −80°C until use. HP and LP parasites (2 × 106), some pretreated with TTM, were incubated with supernatant from PMA-activated neutrophils (1,000 μg of DNA) at a final volume of 200 μl. After 180 min, the plates were centrifuged at 4,000 rpm for 10 min, and the supernatant was recovered and resolved on a 1% agarose gel using GelRed staining.
Statistical analysis.
Data analysis was performed with GraphPad Prism 5.03 software. Unpaired t test analysis was performed, and P values of <0.01 and <0.05 were considered significant.
RESULTS
Leishmania infantum promastigotes induce the release of neutrophil extracellular traps.
It is well established that the cultivation of Leishmania promastigotes in medium with a low concentration of inorganic phosphate (Pi) or purines increases the expression and activity of 3′NT/NU in the parasites (26, 29, 30, 34). We cultivated Leishmania infantum promastigotes in two different culture media: high-phosphate (HP; 83 mM Pi) medium and low-phosphate (LP; 2 mM Pi) medium; the promastigotes cultivated in these media are referred to herein as HP parasites and LP parasites, respectively. As expected, 3′NT/NU activity was 1.6-fold higher in LP parasites than in HP parasites (data not shown), confirming previous work showing that Pi starvation increases the enzyme activity in L. infantum promastigotes (34). The 3′NT/NU activity assays were performed with intact parasites, indicating that the enzyme is anchored to the parasite membrane (35).
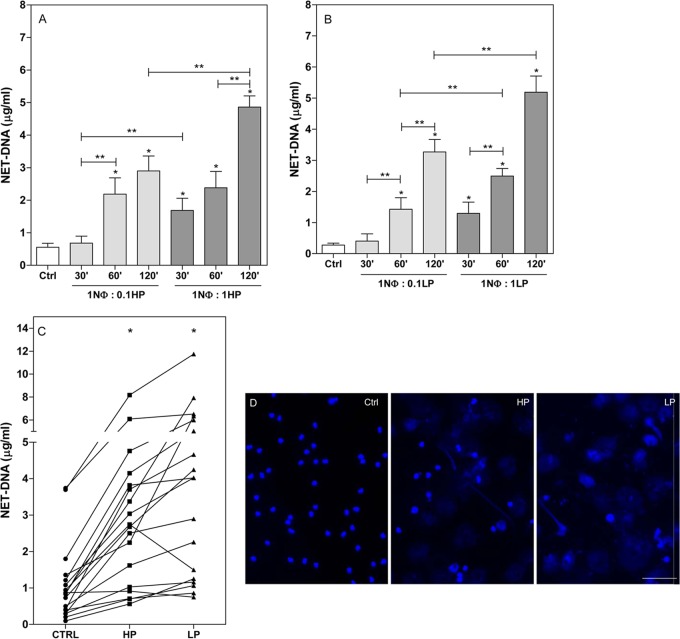
We have previously demonstrated that parasites of the genus Leishmania can activate neutrophils to release neutrophil extracellular traps (12). This finding was further corroborated by Gabriel and colleagues (13), who demonstrated that L. donovani also induces NET formation. In addition, upon interaction with neutrophils, promastigotes of Leishmania infantum activate NETosis in a time- and dose-dependent manner (Fig. 1A and B). Neutrophils start releasing traps as early as 30 min after incubation with parasites at a 1:1 neutrophil/parasite ratio (Fig. 1A and B). We performed this assay with 18 different blood donors and detected differences in the extent of response between each donor; however, all presented the same profile of response, that is, NET release upon stimulation (Fig. 1C). Fluorescence microscopy images reveal NET structures released upon 60 min of neutrophil-L. infantum interaction (Fig. 1D).
FIG 1.
Promastigotes of Leishmania infantum induce release of neutrophil extracellular traps. (A and B) Neutrophils were incubated with HP promastigotes (A) or LP promastigotes (B) of L. infantum at different cell ratios (1NΦ:0.1, 1 neutrophil-to-0.1 parasite ratio) and time points as indicated. Supernatants were recovered, and NETs were quantified as described in Materials and Methods. Controls (Ctrl) were incubated for 120 min. Results of at least 6 independent experiments are shown as means ± standard errors of the means (SEM). *, P < 0.01 for control versus experimental results; **, P < 0.05. (C) Interdonor variations in NET release. Neutrophils from different healthy blood donors were incubated with HP or LP promastigotes of L. infantum at a ratio of 1 neutrophil to 0.1 parasite. After 120 min at 35°C, supernatants were recovered and NETs quantified. The control (Ctrl) condition was the spontaneous release of NETs. Results of 18 independent experiments are shown as means ± SEM. *, P < 0.01 in relation to control. (D) Neutrophils were incubated with HP or LP promastigotes of L. infantum at a 1 neutrophil-to-0.1 parasite ratio. After 60 min, slides were fixed and stained with DAPI, and images were taken in a Zeiss Axioplan. The control condition was neutrophils incubated without parasites. Bar, 50 μm.
Neutrophil extracellular traps kill promastigotes of Leishmania infantum.
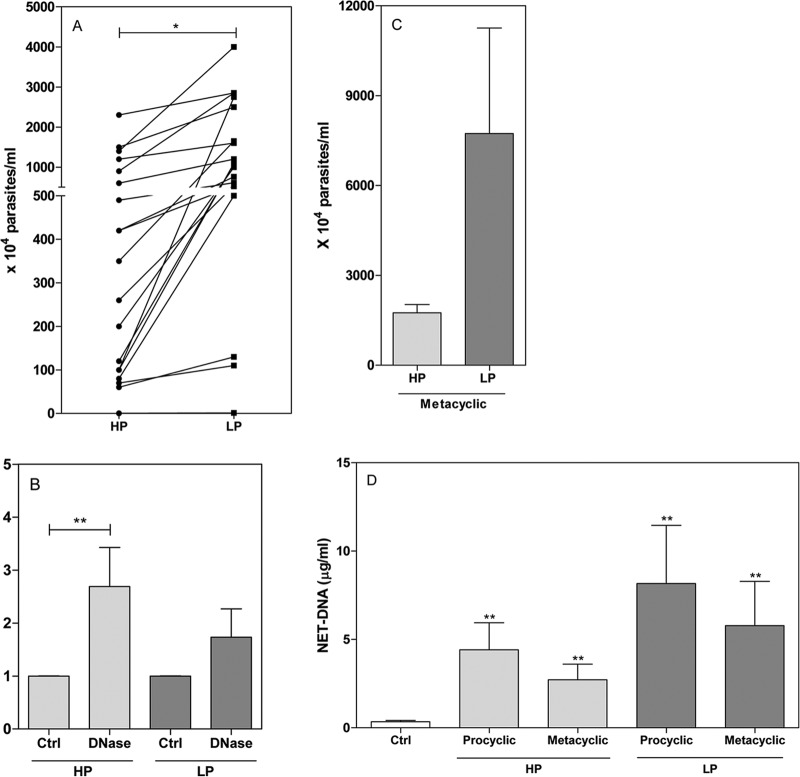
In addition to the fact that parasites are able to induce NETosis, we demonstrated that NET histones kill Leishmania amazonensis (12). Interestingly, comparing the survival characteristics of our two L. infantum populations, we found that LP parasites survived neutrophil killing at a rate 2.4 times greater than that of HP parasites (Fig. 2A). We then asked whether NETs could participate in parasite killing. The addition of DNase, an enzyme that destroys the NET DNA backbone, increased the survival of HP parasites. Upon the addition of DNase, HP and LP parasites survived, respectively, at 2.7- and 1.7-times greater rates than untreated controls (Fig. 2B). Compared to HP promastigotes, the addition of DNase did not lead to significant LP parasite survival (1.7 times greater than the survival of control parasites), perhaps because nuclease activity in these parasites may be already sufficient to cleave and escape from NETs. These results demonstrate that NETs can effectively kill Leishmania infantum parasites.
FIG 2.
Susceptibility of parasites grown in HP and LP media to neutrophil killing. Neutrophils were incubated in the absence (A, C) or presence (B) of DNase (20U/ml) for 20 min, followed by the addition of stationary-phase HP and LP promastigotes (A, B) or metacyclic promastigotes (C) (1 neutrophil-to-0.1 parasite ratio) for 2 h at 35°C. Fetal calf serum was added to the cultures to a final concentration of 10%, and live parasites were counted after 2 days at 26°C. (D) Neutrophils were incubated with HP or LP metacyclic and procyclic promastigotes at a 1 neutrophil-to-0.1 parasite ratio. After 120 min of incubation at 35°C, supernatants were recovered and NETs quantified. The control raw numbers for the experiment whose results are shown in panel B were 528.5 × 104 ± 139.5 HP promastigotes/ml and 1,288.0 × 104 ± 255.4 LP promastigotes/ml. Results are shown as means ± SEM; n = 20 (A), n = 16 (B), n = 6 (C), n = 4 (D). *, P < 0.01, and **, P < 0.05, for the statistical difference between experimental and control results.
Parasites cultivated in low concentrations of inorganic phosphate or purines undergo metacyclogenesis faster than parasites cultivated in normal conditions. Metacyclics are the infective form of Leishmania and are more resistant to the host's killing machinery than procyclic parasites. Because we used stationary promastigotes in our experiments, to exclude the higher number of metacyclics as the reason for the higher survival of LP parasites, we purified metacyclics from the two cultures and compared parasite survival. LP metacyclics had 4.4 times greater survival of NETosis than HP metacyclics (Fig. 2C). The difference was greater than when we used all stationary-phase promastigotes. This observation may be explained by the fact that 3′NT/NU activity is higher from metacyclic than from nonmetacyclic forms (34). Additionally, both metacyclic and procyclic forms of HP and LP parasites induced NET formation (Fig. 2D).
3′-Nucleotidase/nuclease activity allows parasites to escape killing by released NETs.
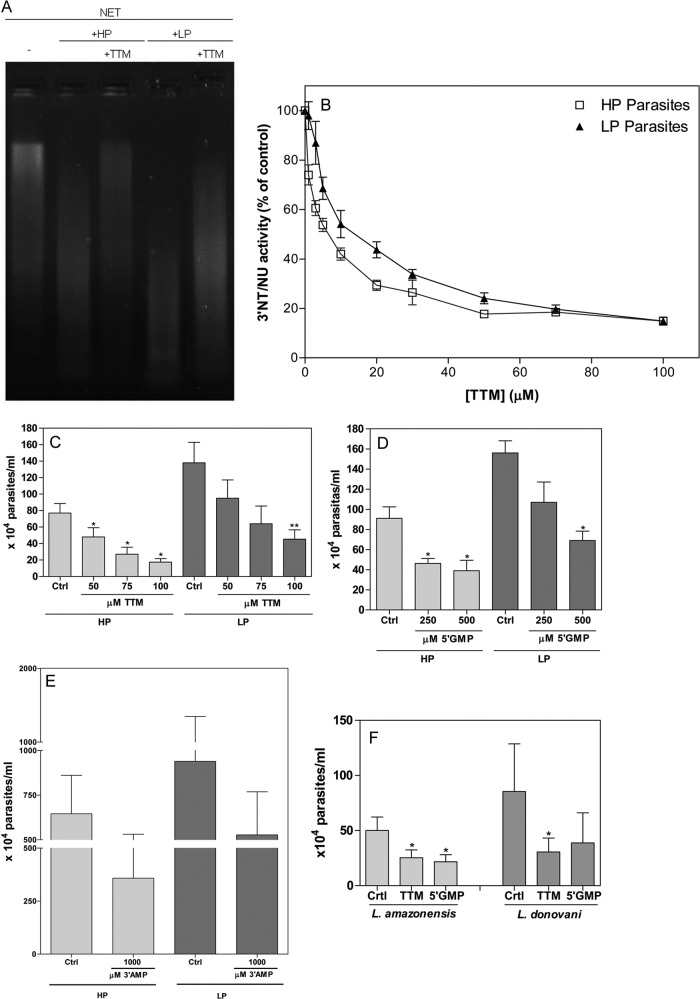
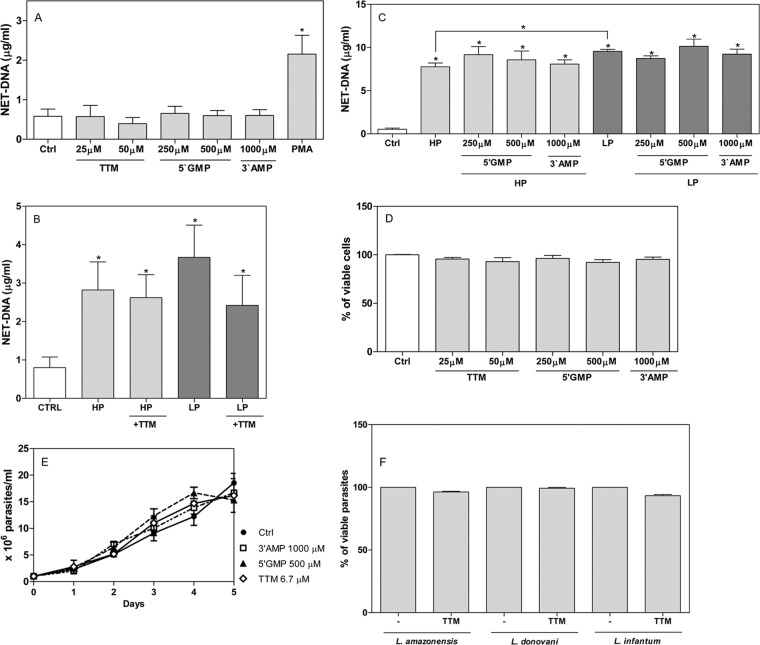
Parasites grown in low-phosphate-containing medium express higher 3′NT/NU activity and show greater survival than parasites grown in high-phosphate medium (Fig. 2A). We asked whether nuclease activity could allow parasites to escape from NETs. Thus, we generated supernatant enriched in NETs by activating neutrophils with PMA, a classic NET inducer. These supernatants were confirmed to be enriched in NETs by NET DNA measurements as described in Materials and Methods. Parasites treated with and without ammonium tetrathiomolybdate (TTM), a 3′NT/NU inhibitor (35), were incubated with NETs, and the NETs were resolved on agarose gels. LP parasites cleaved more NETs than HP parasites, and pretreatment with TTM diminished the digestion of NETs (Fig. 3A). In addition, 3′NT/NU activity was reduced by 80% when parasites were pretreated with 100 μM TTM (Fig. 3B).To assess the role of 3′NT/NU in parasite evasion of NETs, we pretreated parasites with different doses of TTM and incubated them with neutrophils. Pretreatment of parasites with TTM decreased parasite survival in a dose-dependent manner. More TTM was required to inhibit LP parasite survival (Fig. 3C). A 100 μM treatment reduced HP and LP parasite survival 4.4- and 3-fold, respectively, over the survival under control conditions (Fig. 3C). We used two other inhibitors, 5′-GMP and 3′-AMP, and found similar results (Fig. 3D and E). The addition of 250 μM 5′-GMP was sufficient to inhibit 56% of HP parasite evasion of NETs (Fig. 3D). The addition of 500 μM 5′-GMP to the coculture decreased HP and LP parasite survival by 2.3- and 2.2-fold, respectively, compared to the survival under control conditions (Fig. 3D). Again, more 5′-GMP was required to inhibit LP parasite survival. The addition of 3′-AMP to the coculture also decreased parasite survival (Fig. 3E). Furthermore, inhibition of 3′NT/NU activity of L. amazonensis and L. donovani also inhibited promastigote evasion of NET-mediated killing (Fig. 3F). Of note, none of the inhibitors induced or prevented neutrophils from releasing NETs (Fig. 4A, B, and C). Furthermore, none of the inhibitors were toxic to neutrophils (Fig. 4D) or parasites (Fig. 4E and F). Parasites (5 × 106) were pretreated with 100 μM TTM in 0.5 ml of RPMI for 20 min, and then 2 × 105 parasites (20 μl) were added to neutrophils in a final volume of 300 μl of culture. This way, the ammonium tetrathiomolybdate concentration was diluted 15 times and was approximately 7 μM. Thus, we monitored the growth of Leishmania infantum promastigotes in the presence or absence of 7 μM TTM (Fig. 4E). In parallel, cell death was assessed using propidium iodide staining and analysis by flow cytometry (Fig. 4F). None of the inhibitors were toxic to parasites under the conditions used.
FIG 3.
Leishmania 3′-nucleotidase/nuclease activity digests NETs from human neutrophils and allows the parasites to evade NETosis. (A) HP and LP parasites (2 × 106) were pretreated or not with ammonium tetrathiomolybdate (TTM, 100 μM) and incubated with NET-enriched supernatants (1,000 ng of DNA). After 180 min, tubes were centrifuged at 4,000 rpm for 10 min, and supernatants were resolved by electrophoresis in a 1% agarose gel with GelRed staining. (B) Intact HP or LP promastigotes (1 × 106) were pretreated with different concentrations of TTM and then incubated for 1 h at 30°C in NaCl (116 mM), KCl (5.4 mM), glucose (5.5 mM), and HEPES-Tris (50 mM) buffer with 3′-AMP as the substrate. Inorganic phosphate was quantified in culture supernatants as described in Materials and Methods. In the absence of TTM, considered 100% of 3′NU/NT activity, HP and LP parasites had enzymatic activities of 139.2 ± 11.8 and 220.7 ± 20.6 nmol Pi/h/106 cells, respectively. Results of 10 experiments are shown as percentages of control ± SEM. (C) Promastigotes (5 × 106 in 500 μl) were pretreated with different doses of TTM for 20 min, and then 2 × 105 (20 μl) parasites were added to 2 × 106 neutrophils and cocultured for 2 h at 35°C. FCS was added to the cultures to a final concentration of 10%, and live parasites were counted after 2 days at 26°C. (D, E) The inhibitors 3′-AMP and 5′-GMP were added to the coculture (ratio of 1 neutrophil to 0.1 parasite) together with HP and LP promastigotes. After 2 h at 35°C, FCS was added to the cultures to a final concentration of 10%, and live parasites were counted after 2 days at 26°C. Results of at least 5 independent experiments are shown as means ± SEM. *, P < 0.01; **, P < 0.05. (F) TTM and 5′-GMP were similarly tested in L. amazonensis and L. donovani promastigotes. Results from 5 independent experiments are shown as means ± SEM. *, P < 0.01.
FIG 4.
3′-Nucleotidase/nuclease inhibitors do not induce or interfere with NET formation and are not toxic to neutrophils or parasites. (A to C) Neutrophils were incubated with inhibitors for 120 min at 35°C, supernatants were recovered, and NETs were quantified. (B) HP or LP promastigotes pretreated or not with ammonium tetrathiomolybdate (TTM) were incubated with neutrophils for 120 min at 35°C, supernatants were recovered, and NETs were quantified. Results of 6 independent experiments are shown as means ± SEM. *, P < 0.01 in relation to control. (D) Neutrophils were treated with 3′NT/NU inhibitors at the indicated concentrations for 2 h at 35°, and then supernatants were recovered and the activity of lactate dehydrogenase enzyme was measured. Control neutrophils without treatment were considered 100% viable. Results are expressed as percentages of viable neutrophils and shown as means ± SEM of 4 independent experiments. (E) Growth of L. infantum cultivated as described in Materials and Methods for 5 days in the absence (closed circles) or presence of 1,000 μM 3′-AMP (open squares), 500 μM 5′-GMP (closed triangles), or 6.7 μM TTM (open diamonds). The cell proliferation was determined daily by counting cell numbers in a hemocytometer. Results are shown as means ± SEM of three experiments. (F) Parasites were treated with TTM (100 μM) for 20 min, stained with propidium iodide, and analyzed on a FACSCalibur flow cytometer. Results are expressed as percentages of viable parasites which were negative for PI staining. Results are shown as means ± SEM of 2 independent experiments performed in triplicates.
DISCUSSION
We have previously demonstrated that parasites of the genus Leishmania induce the release of neutrophil extracellular traps and interact with and are killed by these structures (12). Additionally, it was demonstrated that while Leishmania donovani triggers NET formation, it evades NET-mediated killing due to its lipophosphoglycan (LPG) (13). Here, we demonstrate that promastigotes of Leishmania infantum induce NET release in a dose- and time-dependent manner and evade NET-mediated killing through their 3′NT/NU activity.
Leishmania infection begins when an infected sand fly bites a host and, during its blood meal, inoculates metacyclic promastigotes into the skin (36–38). It is well established that neutrophils are the first cells to be recruited to the site of infection and interact with Leishmania parasites (38, 39). Thus, the study of neutrophil-Leishmania interaction could lead to a better understanding of early aspects of innate immunity to this protozoan. Among the characteristics of this interaction, NET release by neutrophils is still poorly understood.
Since the discovery of NETosis, the list of microorganisms and molecules able to trigger NET formation has increased (5, 11). To date, bacteria, fungi, viruses, and protozoan parasites have been shown to induce NETosis in neutrophils (2, 4, 12, 13, 40–43). Among parasites, Leishmania spp., Eimeria bovis, and Toxoplasma gondii were reported to activate NETosis (12, 13, 40, 41). Additionally, our group showed that Leishmania amazonensis and its LPG can activate NETosis (12). LPG is a glycolipid expressed on the promastigote membrane in all species of Leishmania, presenting high polymorphism among species and strains (44, 45). Leishmania parasites seem to induce NETs faster than other stimuli studied. Unlike the results for PMA, after 30 min of interaction with Leishmania, human neutrophils release detectable levels of NET DNA into the extracellular medium. Staphylococcus aureus also induces rapid release of NETs. Upon activation with S. aureus, neutrophils release NETs as early as 10 min later with no signs of neutrophil lysis and in a reactive oxygen species-independent way (46). Whether Leishmania parasites also activate this distinct type of NET release warrants further investigation. Promastigotes of Leishmania infantum induced NETosis in a dose- and time-dependent manner. Furthermore, NET induction seems to be conserved in different species of Leishmania (12, 13). In addition, as previously reported, human neutrophil donors differed in the magnitude of their NET response to different stimuli (3). Both HP and LP parasites activated NETosis in human neutrophils, and no differences were observed between these two populations. Furthermore, metacyclic parasites also induced NETosis.
In general, microorganisms trapped by NETs suffer the toxic effects of NET constituents (2, 12, 43), but the expression of endonucleases allows different bacteria to escape NET-mediated destruction. Streptococcus pneumoniae, group A Streptococcus, and Staphylococcus aureus express potent endonucleases that degrade DNA filaments, allowing them to escape NET-mediated killing and to disseminate throughout the body (14–18). The enzyme 3′NT/NU is a new member of the class I nuclease family. It was described in Leishmania donovani parasites as the only member of this family that is anchored to the plasma membrane (23–27, 47). Furthermore, it is conserved in different members of the Leishmania genus (L. donovani, L. infantum, L. tropica, L. major, and L. mexicana). Trypanosomatid protozoa are incapable of producing purines and are dependent on the host to supply this essential nutrient. This enzyme can provide purines by cleaving nucleotides or nucleic acids. Leishmania donovani, Leishmania chagasi, and Crithidia luciliae grown in low concentrations of purines and phosphate possess high levels of 3′NT/NU activity and expression on the parasite membrane. Accordingly, our data confirmed previous work that reported that LP parasites display a higher 3′NT/NU activity than HP parasites (34). NETs were cleaved by 3′NT/NU, as visualized by agarose gel, an activity that was reversed by treatment with the 3′NT/NU inhibitor ammonium tetrathiomolybdate. Additionally, parasite survival was decreased when 3′NT/NU inhibitors were added to the neutrophil-Leishmania coculture. Interestingly, this same mechanism of 3′NT/NU inhibition also circumvents evasion of NET-mediated killing of promastigotes from L. amazonensis, a cutaneous New World species, as well as L. donovani, a visceral Old World species. Taken together, these results clearly implicate 3′NT/NU activity as an escape strategy of Leishmania parasites to avoid NET toxicity.
Gabriel and colleagues (13) demonstrated that Leishmania donovani promastigotes evade NET toxicity due to the presence of LPG, as wild-type parasites had greater survival than LPG knockout promastigotes. Interestingly, the addition of DNase to the culture rescued LPG knockouts but not wild-type parasites from death. Similar to the results for L. donovani LPG knockout promastigotes (13), we showed that the addition of DNase clearly rescued HP and, to a smaller extent, LP parasites from NET-mediated killing. Interestingly, these two distinct mechanisms of avoiding NET-mediated killing were observed mainly in visceral leishmaniasis-causing agents. NET-associated histones were shown to mediate L. amazonensis killing (12). In an analysis of histone toxicity to L. major, a cutaneous leishmaniasis-causing agent, it was demonstrated that lpg1− mutants, which lack LPG, were equally as susceptibility to histone killing as the wild-type promastigotes (48). Also, preincubation of histone with purified L. major LPG reduced the rate of death of wild-type promastigotes without affecting lpg1− mutants (48). LPG is highly polymorphic, varying among different Leishmania species and even strains (45). Thus, the role of LPG in protecting parasites from NET killing mediated by histones associated with these traps is complex and remains to be established. Moreover, Leishmania surface metalloprotease (GP63) seems to participate in promastigote escape from NET histone killing, since it has been shown that knockdown of L. amazonensis GP63 increased parasite susceptibility to histone killing (48).
Recently, it was demonstrated that LP parasites interact more with BALB/c mouse peritoneal macrophages than do HP parasites. Moreover, the addition of 3′-AMP and adenosine to the Leishmania-macrophage coculture increases the association index (34). By cleaving NETs with 3′NT/NU, parasites could generate adenosine that may possibly increase their infection of macrophages. In addition, adenosine is well documented as an antiinflammatory agent that inactivates killing mechanisms of macrophages. Interestingly, the visceral leishmaniasis agents, Leishmania infantum and Leishmania donovani parasites, have higher 3′NT/NU activities than do cutaneous leishmaniasis agents (30). Altogether, our results clearly show that 3′-nucleotidase/nuclease activity promotes Leishmania evasion from NET-mediated trapping and killing; however, other parasite or even vector salivary molecules certainly could participate in this phenomena. We will next investigate whether 3′-nucleotidase/nuclease activity may participate in the development and establishment of Leishmania infection through facilitating parasite escape from NET-mediated trapping and killing in the in vivo murine model of leishmaniasis.
ACKNOWLEDGMENTS
We thank the Hemotherapy Service, Hospital Clementino Fraga Filho, Universidade Federal do Rio de Janeiro, for providing buffy coats.
This work was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Footnotes
Published ahead of print 10 February 2014
REFERENCES
- 1.Nathan C. 2006. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6:173–182. 10.1038/nri1785 [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 3.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176:231–241. 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. 2009. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 5:e1000639. 10.1371/journal.ppat.1000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guimarães-Costa AB, Nascimento MT, Wardini AB, Pinto-da-Silva LH, Saraiva EM. 2012. A microbicidal mechanism beyond cell death. J. Parasitol. Res. 2012:929743. 10.1155/2012/929743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, Malawista SE, de Boisfleury Chevance A, Zhang K, Conly J, Kubes P. 2012. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18:1386–1393. 10.1038/nm.2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. 2011. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3(73):73ra20. 10.1126/scitranslmed.3001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. 2011. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3(73):73ra19. 10.1126/scitranslmed.3001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. 2010. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sc. U. S. A. 107:9813–9818. 10.1073/pnas.0909927107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne HJ, Brinkmann V, Jenne DE. 2009. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 15:623–625. 10.1038/nm.1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abi Abdallah DS, Denkers EY. 2012. Neutrophils cast extracellular traps in response to protozoan parasites. Front. Immunol. 3:382. 10.3389/fimmu.2012.00382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guimarães-Costa AB, Nascimento MT, Froment GS, Soares RP, Morgado FN, Conceição-Silva F, Saraiva EM. 2009. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. U. S. A. 106:6748–6753. 10.1073/pnas.0900226106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabriel C, McMaster WR, Girard D, Descoteaux A. 2010. Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J. Immunol. 185:4319–4327. 10.4049/jimmunol.1000893 [DOI] [PubMed] [Google Scholar]
- 14.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. 2006. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr. Biol. 16:401–407. 10.1016/j.cub.2006.01.056 [DOI] [PubMed] [Google Scholar]
- 15.Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. 2006. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 16:396–400. 10.1016/j.cub.2005.12.039 [DOI] [PubMed] [Google Scholar]
- 16.Berends ETM, Horswill AR, Haste NM, Monestier M, Nizet V, von Köckritz-Blickwede M. 2010. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J. Innate Immun. 2:576–586. 10.1159/000319909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumby P, Barbian KD, Gardner DJ, Whitney AR, Welty DM, Long RD, Bailey JR, Parnell MJ, Hoe NP, Adams GG, Deleo FR, Musser JM. 2005. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc. Natl. Acad. Sci. U. S. A. 102:1679–1684. 10.1073/pnas.0406641102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker MJ, Hollands A, Sanderson-Smith ML, Cole JN, Kirk JK, Henningham A, McArthur JD, Dinkla K, Aziz RK, Kansal RG, Simpson AJ, Buchanan JT, Chhatwal GS, Kotb M, Nizet V. 2007. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat. Med. 13:981–985. 10.1038/nm1612 [DOI] [PubMed] [Google Scholar]
- 19.Thammavongsa V, Missiakas DM, Schneewind O. 2013. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 342:863–866. 10.1126/science.1242255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seper A, Hosseinzadeh A, Gorkiewicz G, Lichtenegger S, Roier S, Leitner DR, Röhm M, Grutsch A, Reidl J, Urban CF, Schild S. 2013. Vibrio cholerae evades neutrophil extracellular traps by the activity of two extracellular nucleases. PLoS Pathog. 9:e1003614. 10.1371/journal.ppat.1003614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derré-Bobillot A, Cortes-Perez NG, Yamamoto Y, Kharrat P, Couvé E, Da Cunha V, Decker P, Boissier MC, Escartin F, Cesselin B, Langella P, Bermúdez-Humarán LG, Gaudu P. 2013. Nuclease A (Gbs0661), an extracellular nuclease of Streptococcus agalactiae, attacks the neutrophil extracellular traps and is needed for full virulence. Mol. Microbiol. 89:518–531. 10.1111/mmi.12295 [DOI] [PubMed] [Google Scholar]
- 22.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, WHO Leishmaniasis Control Team 2012. Leishmaniasis Worldwide and Global estimates of its incidence. PLoS One 7:e35671. 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dwyer DM, Gottlieb M. 1984. Surface membrane localization of 3′- and 5′-nucleotidase activities in Leishmania donovani promastigotes. Mol. Biochem. Parasitol. 10:139–150. 10.1016/0166-6851(84)90002-1 [DOI] [PubMed] [Google Scholar]
- 24.Bates PA. 1993. Characterization of developmentally-regulated nucleases in promastigotes and amastigotes of Leishmania mexicana. FEMS Microbiol. Lett. 107:53–58. 10.1111/j.1574-6968.1993.tb06003.x [DOI] [PubMed] [Google Scholar]
- 25.Debrabant A, Ghedin E, Dwyer DM. 2000. Dissection of the functional domains of the Leishmania surface membrane 30-nucleotidase/nuclease, a unique member of the class I nuclease family. J. Biol. Chem. 275:16366–16372. 10.1074/jbc.M908725199 [DOI] [PubMed] [Google Scholar]
- 26.Sopwith WF, Debrabant A, Yamage M, Dwyer DM, Bates PA. 2002. Developmentally regulated expression of a cell surface class I nuclease in Leishmania mexicana. Int. J. Parasitol. 32:449–459. 10.1016/S0020-7519(01)00372-1 [DOI] [PubMed] [Google Scholar]
- 27.Joshi MB, Dwyer DM. 2007. Molecular and functional analyses of a novel class I secretory nuclease from the human pathogen, Leishmania donovani. J. Biol. Chem. 282:10079–10095. 10.1074/jbc.M610770200 [DOI] [PubMed] [Google Scholar]
- 28.Hammond DJ, Gutteridge WE. 1984. Purine and pyrimidine metabolism in the Trypanosomatidae. Mol. Biochem. Parasitol. 13:243–261. 10.1016/0166-6851(84)90117-8 [DOI] [PubMed] [Google Scholar]
- 29.Gottlieb M. 1985. Enzyme regulation in a trypanosomatid. Effect of purine starvation on levels of a 30-nucleotidase activity. Science 227:72–74 [DOI] [PubMed] [Google Scholar]
- 30.Sacci JB, Jr, Campbell TA, Gottlieb M. 1990. L. donovani: regulated changes in the level of expression of the surface 3′nucleotidase/nuclease. Exp. Parasitol. 71:158–168. 10.1016/0014-4894(90)90018-8 [DOI] [PubMed] [Google Scholar]
- 31.Fiske CH, Subbarow Y. 1925. The colorimetric determination of phosphorus. J. Biol. Chem. 66:375–400 [Google Scholar]
- 32.Yao C, Chen Y, Sudan B, Donelson JE, Wilson ME. 2008. Leishmania chagasi: homogenous metacyclic promastigotes isolated by buoyant density are highly virulent in a mouse model. Exp. Parasitol. 118:129–133. 10.1016/j.exppara.2007.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debrabant A, Gottlieb M, Dwyer DM. 1995. Isolation and characterization of the gene encoding the surface membrane 3′-nucleotidase/nuclease of Leishmania donovani. Mol. Biochem. Parasitol. 71:51–63. 10.1016/0166-6851(95)00035-Y [DOI] [PubMed] [Google Scholar]
- 34.Vieira DP, Paletta-Silva R, Saraiva EM, Lopes AH, Meyer-Fernandes JR. 2011. Leishmania chagasi: an ecto-3′-nucleotidase activity modulated by inorganic phosphate and its possible involvement in parasite-macrophage interaction. Exp. Parasitol. 127:702–707. 10.1016/j.exppara.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 35.Paletta-Silva R, Vieira DP, Vieira-Bernardo R, Majerowicz D, Gondim KC, Vannier-Santos MA, Lopes AH, Meyer-Fernandes JR. 2011. Leishmania amazonensis: characterization of an ecto-3′-nucleotidase activity and its possible role in virulence. Exp. Parasitol. 129:277–283. 10.1016/j.exppara.2011.07.014 [DOI] [PubMed] [Google Scholar]
- 36.Saraiva EM, Pimenta PFP, Brodin TN, Rowton E, Modi GB, Sacks DL. 1995. Changes in lipophosphoglycan and gene expression associated with development of Leishmania major in Phlebotomus papatasi. Parasitology 111:275–287. 10.1017/S003118200008183X [DOI] [PubMed] [Google Scholar]
- 37.Sacks D, Kamhawi S. 2001. Molecular aspects of parasite-vector and vector-host interactions in Leishmaniasis. Annu. Rev. Microbiol. 55:453–483. 10.1146/annurev.micro.55.1.453 [DOI] [PubMed] [Google Scholar]
- 38.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D. 2008. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321:970–974. 10.1126/science.1159194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribeiro-Gomes FL, Sacks D. 2012. The influence of early neutrophil-Leishmania interactions on the host immune response to infection. Front. Cell. Infect. Microbiol. 2:59. 10.3389/fcimb.2012.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abi Abdallah DS, Lin C, Ball CJ, King MR, Duhamel GE, Denkers EY. 2012. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect. Immun. 80:768–777. 10.1128/IAI.05730-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behrendt JH, Ruiz A, Zahner H, Taubert A, Hermosilla C. 2010. Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria bovis. Vet. Immunol. Immunopathol. 133:1–8. 10.1016/j.vetimm.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 42.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, Uehata T, Iwasaki H, Omori H, Yamaoka S, Yamamoto N, Akira S. 2012. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 12:109–116. 10.1016/j.chom.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 43.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. 2006. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 8:668–676. 10.1111/j.1462-5822.2005.00659.x [DOI] [PubMed] [Google Scholar]
- 44.de Assis RR, Ibraim IC, Nogueira PM, Soares RP, Turco SJ. 2012. Glycoconjugates in New World species of Leishmania: polymorphisms in lipophosphoglycan and glycoinositolphospholipids and interaction with hosts. Biochim. Biophys. Acta 1820:1354–1365. 10.1016/j.bbagen.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 45.Mahoney AB, Sacks DL, Saraiva E, Modi G, Turco SJ. 1999. Intra-species and stage-specific polymorphisms in lipophosphoglycan structure control Leishmania donovani-sand fly interactions. Biochemistry 38:9813–9823. 10.1021/bi990741g [DOI] [PubMed] [Google Scholar]
- 46.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P. 2010. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 185:7413–7425. 10.4049/jimmunol.1000675 [DOI] [PubMed] [Google Scholar]
- 47.Guilherme A, Meyer-Fernandes JR, Vieyra A. 1991. Reversible inhibition by 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid of the plasma membrane (Ca(2+)+Mg2+)ATPase from kidney proximal tubules. Biochemistry 30:5700–5706. 10.1021/bi00237a010 [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Chen Y, Xin L, Beverley SM, Carlsen ED, Popov V, Chang KP, Wang M, Soong L. 2011. Differential microbicidal effects of human histone proteins H2A and H2B on Leishmania promastigotes and amastigotes. Infect. Immun. 79:1124–1133. 10.1128/IAI.00658-10 [DOI] [PMC free article] [PubMed] [Google Scholar]