Abstract
Pneumolysin (Ply) and its variants are protective against pneumococcal infections in animal models, and as a Toll-like receptor 4 agonist, pneumolysin has been reported to be a mucosal adjuvant. DnaJ has been approved as a useful candidate vaccine protein; we therefore designed novel fusion proteins of DnaJ with a form of Ply that has a deletion of A146 (ΔA146Ply-DnaJ [the C terminus of ΔA146Ply connected with the N terminus of DnaJ] and DnaJ-ΔA146Ply [the C terminus of DnaJ connected with the N terminus of ΔA146Ply]) to test whether they are protective against focal and lethal pneumococcal infections and their potential protective mechanisms. The purified proteins were used to intranasally immunize the animals without additional adjuvant. Immunization with DnaJ-ΔA146Ply or DnaJ plus ΔA146Ply (Ply with a single deletion of A146) could significantly reduce S. pneumoniae colonization in the nasopharynx and lung relative with DnaJ alone. Additionally, we observed the best protection for DnaJ-ΔA146Ply-immunized mice after challenge with lethal doses of S. pneumoniae strains, which was comparable to that achieved by PPV23. Mice immunized with DnaJ-ΔA146Ply produced significantly higher levels of anti-DnaJ IgG in serum and secretory IgA (sIgA) in saliva than those immunized with DnaJ alone. The production of IL-17A was also striking in DnaJ-ΔA146Ply-immunized mice. IL-17A knockout (KO) mice did not benefit from DnaJ-ΔA146Ply immunization in colonization experiments, and sIgA production was impaired in IL-17A KO mice. Collectively, our results indicate a mucosal adjuvant potential for ΔA146Ply and that, without additional adjuvant, DnaJ-ΔA146Ply fusion protein exhibits extensive immune stimulation and is effective against pneumococcal challenges, properties which are partially attributed to the IL-17A-mediated immune responses.
INTRODUCTION
Pneumonia remains the leading killer of children under 5 years of age, and over 90% of cases occur in developing and undeveloped countries (1). Streptococcus pneumoniae is one of the most common causes of pneumonia. As a common inhabitant of the respiratory tract, pneumococci cause many types of illnesses, including pneumonia, otitis media, meningitis, and bloodstream infections. Vaccination is an effective way to reduce the burden of pneumococcal diseases. Currently available pneumococcal vaccines are all based on the serotype-specific capsular polysaccharides. However, 93 distinct capsular serotypes have been identified so far (2). Although these polysaccharide-based vaccines have greatly decreased the burden of pneumococcal disease, the limited serotype coverage can be an issue. There is a risk of natural serotype switching, and it is believed that vaccine serotypes can be replaced by nonvaccine serotypes after vaccination (3–5). Protein-based vaccines are attractive because these antigens could avoid problems of poor polysaccharide immunogenicity in infants and elderly persons and would probably cover most pneumococcal strains. To obtain a comprehensive protection, multiprotein combination formulations against pneumococcal infections in animal models have been investigated (6–9).
Mucosal delivery is proposed to induce an effective protection against pneumococci which readily strengthens the protective immune response in the lungs and upper respiratory tract. Mucosal vaccination enhances the mucosal barriers through the important effector antigen-specific secretory IgA (sIgA), which prevents S. pneumoniae from adhering to or infecting the epithelial cells in the respiratory tracts (10–12). Also, specific effector T cells reinforce the barrier functions of mucosal sites, based on previous publications (13, 14). Despite the attractive advantages of mucosal immunity, only a few mucosal vaccines have been licensed. This is mainly due to problems with developing safe and effective mucosal adjuvants. As far as we are aware, cholera toxin (CT) and heat-labile enterotoxin (LT) are the two most important adjuvants which have been widely used in animal studies; however, they are not suitable for human use due to their toxicity (15, 16).
Pneumolysin (Ply) is an important virulence factor of S. pneumoniae and has a strong impact on the host response. Ply interacts with Toll-like receptor 4 (TLR4) (17–19) and induces the activation of the NLRP3 inflammasome independently of TLR4, thus contributing to host protection against S. pneumoniae infections (20, 21). Wild-type Ply has been suggested as a potential mucosal adjuvant for use in combination with other proteins (20, 22); nevertheless, like CT, wild-type Ply is toxic and should not be considered for human use. In previous studies, pneumococcal carriage was shown to induce production of anti-Ply antibodies (23, 24). Several Ply variants have been used as potential vaccine candidates and also as carriers for glycoconjugated vaccines in animal models (25–28); of them, ΔA146Ply (Ply with a single deletion of A146) was one of the most remarkable variants with minimal toxicity. Hence, we wanted to evaluate whether ΔA146Ply has mucosal adjuvant capacity like its wild type.
Many reports have shown that intraperitoneal or intranasal immunization with recombinant DnaJ induces a striking protective immune response and protects mice against focal and lethal infections with different serotypes of S. pneumoniae (29, 30). As a heat shock protein, DnaJ plays an important role in the pathogenesis of pneumococcal infection (31), and the antibody to DnaJ could inhibit S. pneumoniae adhesion to type II epithelial lung carcinoma cells (30). Also, it is highly conserved in prokaryotes.
In this study, we successfully overexpressed two types of ΔA146Ply and DnaJ fusion proteins and purified them by Ni2+ affinity chromatography. Their immunogenicity and protective activities were investigated by intranasal immunization and were compared with those of DnaJ or ΔA146Ply alone and an equimolar DnaJ-and-ΔA146Ply mixture in animal models. The results indicate that ΔA146Ply has potential as a mucosal adjuvant and that DnaJ-ΔA146Ply (the C terminus of DnaJ connected with the N terminus of ΔA146Ply) is a promising candidate protein vaccine against pneumococcal infections. Notably, IL-17A-mediated immune responses are important for DnaJ-ΔA146Ply-elicited protection and the production of antigen-specific sIgA.
MATERIALS AND METHODS
Bacterial strains.
Escherichia coli DH5α (Invitrogen, CA, USA) was used as the host for routing plasmid cloning. Recombinant proteins were expressed in E. coli BL21(DE3) (Novagen). Pneumococcal strain D39 (NCTC 7466, serotype 2) was purchased from the National Collection of Type Cultures (London, United Kingdom); S. pneumoniae strain TIGR4 (serotype 4) was purchased from the American Type Culture Collection (ATCC), and pneumococcal strains CMCC 31436 (serotype 3), CMCC 31207 (serotype 6B), CMCC 31614 (serotype 14), and CMCC 31693 (serotype 19F) were obtained from the China Medical Culture Collection (CMCC, Beijing, China). S. pneumoniae was routinely grown on Columbia sheep blood agar or in semisynthetic casein hydrolysate medium supplemented with 0.5% yeast extract (C+Y) medium in an atmosphere of 5% CO2 at 37°C.
Mice.
Specific-pathogen-free 6- to 8-week-old female C57BL/6 wild-type mice were purchased from and raised at Chongqing Medical University, Chongqing, China. IL-17A knockout (IL-17A KO) mice were backcrossed 10 times to a C57BL/6 background and generated as described previously (32). All animal experiments were approved by the respective ethics committees of Chongqing Medical University.
Cloning, expression, and purification of recombinant DnaJ, ΔA146Ply, DnaJ-ΔA146Ply, and ΔA146Ply-DnaJ in E. coli.
DnaJ is a recombinant antigen originating from the TIGR4 strain. The ΔA146Ply (full-length wild-type Ply with a deletion of A146) gene was constructed by using site-directed mutagenesis by overlap extension in our previous study (30). It was then cloned, expressed, and purified as described in the previous studies (26, 30). The full-length DnaJ gene was PCR amplified from the TIGR4 strain using two pairs of primers: F-DnaJ-N plus R-DnaJ-N and F-DnaJ-C plus R-DnaJ-C (Table 1). Similarly, the ΔA146Ply gene was amplified from plasmid ΔA146Ply/pW28 using two pairs of primers: F-ΔA146Ply-N plus R-ΔA146Ply-N and F-ΔA146Ply-C plus R-ΔA146Ply-C (Table 1). The DnaJ and ΔA146Ply PCR fragments were successively inserted into the expression vector pET28a (Novagen) (33). The constructs were then transformed into competent E. coli BL21(DE3), which was grown at 37°C in Luria broth (LB) supplemented with 50 μg/ml kanamycin. Correct cloning was confirmed by PCR and DNA sequencing. Induction with isopropyl-β-d-1-thiogalactopyranoside (IPTG) resulted in production of the 6×His-tagged recombinant fusion proteins DnaJ-ΔA146Ply and ΔA146Ply-DnaJ (the C terminus of ΔA146Ply connected with the N terminus of DnaJ), which were purified by Ni2+-charged column chromatography (GE). Fractions were collected and analyzed by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The recombinant proteins were dialyzed in phosphate-buffered saline (PBS; pH 7.0) to remove the imidazole. Lipopolysaccharide (LPS) contamination was then removed from the recombinant proteins using the ToxinEraser endotoxin removal kit (Genscript Biotechnology, Inc.), and the residual LPS content of the protein preparation was determined by using the endpoint chromogenic assay (ECA) kit for the detection of bacteria endotoxin (Zhanjiang A&C Biological Ltd.). Proteins were stored at −80°C before use.
TABLE 1.
Primers used for cloning
| Primer | Sequence (5′–3′)a |
|---|---|
| F- DnaJ-N | GGAATTCCATATGAACAATACTGAATTT |
| R-DnaJ-N | CGAGCTCTTCTCCATCAAAGG |
| F-DnaJ-C | CGGCGGCCGCATGAACAATACTGAATT |
| R-DnaJ-C | CCCTCGAGTTATTCTCCATCAAAGGCA |
| F-ΔA146Ply-N | GGAATTCCATATGGCA AAT AAAGCAGTA AAT |
| R-ΔA146Ply-N | CGAGCTCGTCATTTTCTACCTTATCCTCT |
| F-ΔA146Ply-C | CGGCGGCCGC ATGGCAAATAAAGCAGTAAATG |
| R-ΔA146Ply-C | CCCTCGAGTTACTAGTCATTTTCTACCTTATCCTCT |
Underlining indicates the recognition sites of restriction enzymes.
Detection of DnaJ-ΔA146Ply and ΔA146Ply-DnaJ fusion proteins by Western blotting.
The DnaJ-ΔA146Ply and ΔA146Ply-DnaJ proteins were detected by Western blotting with anti-DnaJ or anti-ΔA146Ply sera. Pooled antisera from mice immunized with DnaJ-ΔA146Ply were used to probe the recombinant DnaJ and ΔA146Ply proteins and their native versions in different S. pneumoniae strains. S. pneumoniae standard strain D39, TIGR4, and clinical isolates CMCC 31436 (serotype 3), CMCC 31207 (serotype 6B), CMCC 31614 (serotype 14), and CMCC 31693 (serotype 19F) were grown in C+Y medium and collected.
Intranasal immunization of mice.
In this study, groups of C57BL/6 mice were immunized three times at 14-day intervals with 30 μl PBS containing 8 μg DnaJ and/or 10 μg ΔA146Ply; this was calculated to ensure that the ratio of ΔA146Ply to DnaJ was identical to that present in an equivalent dose of the fusion protein. DnaJ in 1 μg CT (Sigma-Aldrich) was considered one positive control, and the 23-valent pneumococcal polysaccharide vaccine PPV23 (Chengdu Institute of Biological Products, Chengdu, China) was injected as another positive control. Intranasal immunization was carried out with the anesthetized C57BL/6 mouse held in a supine position with the head down while 30 μl of the antigen solution was delivered slowly with a micropipette onto the nares (34). Serum samples were collected from the tail vein of each animal on the day before each immunization and days 6, 13, 21, 35, and 42. The saliva was collected 7 days after the last immunization and stored at −20°C for further studies.
ELISA analysis of serum and saliva.
The levels of specific antibodies in immunized mice were determined by enzyme-linked immunosorbent assay (ELISA). The titers of anti-DnaJ specific IgG in sera were also determined by ELISA (9). Purified recombinant DnaJ (5 μg/ml) was used to coat 96-well plates and incubated at 4°C overnight. The plates were washed three times with PBS–0.1% Tween 20 (PBST) and then blocked with 2% bovine serum albumin-PBST for 2 h at 37°C. After washing, serial dilutions of serum and saliva samples were added to the plates and incubated for 1 h at 37°C. Bound immunoglobulin was detected by peroxidase-conjugated AffiniPure goat anti-mouse IgG (ZSGB-Bio, Beijing, China), followed by the substrate tetramethyl benzidine. Absorbance was measured at 450 nm. The antibody titers were expressed as the reciprocal of the highest sample dilution giving absorbance 2.1-fold higher than the background absorbance. In addition, goat anti-mouse IgA, IgG1, IgG2a, IgG2b, and IgG3 conjugated to horseradish peroxidase (HRP) (Santa Cruz) were used to analyze the distribution of sIgA in saliva and IgG subtypes in sera. The serum total IgE was measured by ELISA kits (Biolegend) following the manufacturer's recommendations.
Cytokine assays.
Spleens were removed from immunized and control mice 7 days after the last immunization. The splenocytes were then washed and resuspended in RPMI 1640 (HyClone, Barrington, IL, USA) supplemented with 10% fetal bovine serum (5 × 106 cells/ml). The cells (1 ml) were cultured in 24-well plates and then stimulated with 5 μg recombinant DnaJ protein in vitro for 72 h at 37°C in a 5% CO2 incubator. The levels of IL-4, gamma interferon (IFN-γ), IL-10, and IL-17A in the supernatants were detected by ELISA kits (Biolegend).
Histology.
For histological examination, lung samples were collected seven day after the last immunization, fixed in 4% paraformaldehyde, embedded in paraffin, and serially sectioned. Serial 5-μm tissue sections were subjected to hematoxylin-eosin (HE) staining and examined under a light microscope.
Challenge studies.
In focal infection models, C57BL/6 mice were challenged intranasally with CMCC31207 (serotype 6B, 1 × 108 CFU) or CMCC31693 (serotype 19F, 1 × 108 CFU) after immunization. Mice were sacrificed 3 days after the challenge, and nasal wash fluids were collected by flushing the nasal cavities with 300 μl of sterile PBS. The lungs were removed and homogenized in PBS immediately. Samples were serially diluted with sterile PBS, and 100 μl was plated on Columbia sheep blood agar. The colonies were counted after incubation overnight at 37°C and 5% CO2. IL-17A KO mice were challenged intranasally with CMCC31693 (serotype 19F, 1 × 107 CFU) after immunization, and then the bacteria loads in nasal wash and lung were determined. Wild-type C57BL/6 mice were used as controls.
In the lethal-infection models, intranasal challenge experiments were performed as described previously. Two weeks after the last immunization, C57BL/6 mice were anesthetized and then intranasally challenged with 30 μl of bacterial suspension containing D39 (serotype 2, 5 × 107 CFU) or CMCC31436 (serotype 3, 1.5 × 108 CFU). The challenged mice were observed twice daily by an experienced person. The survival of each mouse was monitored for consecutive 21 days.
Statistics analysis.
The Mann-Whitney U test (Prism 5; GraphPad Software, La Jolla, CA, USA) was used to compare antibody titers, numbers of pneumococci (CFU), cytokine levels, and median survival times for groups of mice. P values of <0.05 were considered to indicate significant differences.
RESULTS
Expression, purification, and characterization of recombinant proteins.
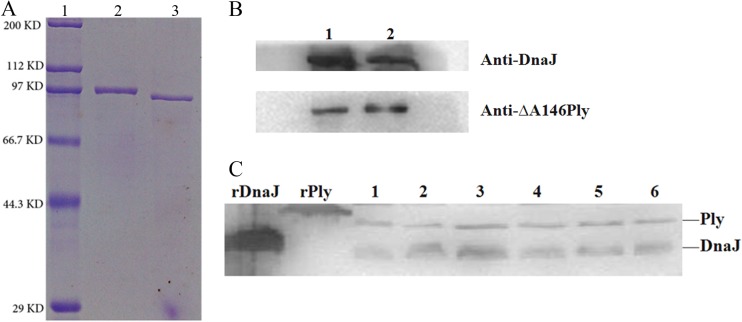
The recombinant proteins DnaJ, ΔA146Ply, DnaJ-ΔA146Ply, and ΔA146Ply-DnaJ were overexpressed and purified from E. coli. In each case, analysis by gel electrophoresis revealed a single protein of the expected size (Table 2) that reacted with antisera to either DnaJ or ΔA146Ply. Western blot analysis (Fig. 1) demonstrated the specific recognition by DnaJ-ΔA146Ply polyclonal antiserum of the recombinant DnaJ and ΔA146Ply proteins, and corresponding bands were visible in samples from different S. pneumoniae strains.
TABLE 2.
Properties of purified proteins used in this study
| Construct | Protein | Size (kDa) | Western blotting resulta |
LPS concn (EU/μg) | ||
|---|---|---|---|---|---|---|
| DnaJ | ΔA146Ply | DnaJ-ΔA146Ply | ||||
| pET28aΔA146Ply | ΔA146Ply | 53 | − | + | + | 0.0222 |
| pET28aDnaJ | DnaJ | 38 | + | − | + | 0.1 |
| pET28aDnaJ-ΔA146Ply | DnaJ-ΔA146Ply | 93 | + | + | + | 0.0223 |
| pET28aΔA146Ply-DnaJ | ΔA146Ply-DnaJ | 93 | + | + | + | 0.0092 |
| pGEX4T-2 | GST | 26 | − | − | − | 0.3598 |
−, negative; +, positive.
FIG 1.
Purification and characterization of recombinant DnaJ-ΔA146Ply and ΔA146Ply-DnaJ proteins, respectively. (A) Recombinant DnaJ-ΔA146Ply (lane 2) and ΔA146Ply-DnaJ (lane 3) proteins were cloned, expressed, and purified from E. coli. The proteins were subjected to SDS-PAGE and detected by direct staining with Coomassie brilliant blue. Lane 1, protein marker. (B) Western blotting of DnaJ-ΔA146Ply (lane 1) and ΔA146Ply-DnaJ (lane 2) using DnaJ and ΔA146Ply antisera. (C) Western blot analysis of native DnaJ (38 kDa) and Ply (53 kDa) in pneumococcal strains using DnaJ-ΔA146Ply antisera. SDS–12% PAGE gels were loaded with cell lysates obtained from pneumococcal strains, including NCTC 7466 (D39, serotype 2), CMCC 31436 (serotype 3), TIGR4 (serotype 4), CMCC 31207 (serotype 6B), CMCC 31614 (serotype 14), and CMCC 31693 (serotype 19F) (lanes 1 to 6, respectively).
As LPS is a TLR4 agonist, LPS contaminating protein preparations may act as an adjuvant. To avoid LPS contamination, a ToxinEraser endotoxin removal kit was employed to remove LPS from our protein preparations; the LPS concentration was below 0.1 endotoxin units (EU)/μg in our protein preparations. On the other hand, to test whether 0.1 EU/μg of LPS is able to induce mucosal adjuvant activity, we introduced an LPS-contaminated GST Tag protein as a control. GST protein was expressed and purified in E. coli, and LPS was not removed. The LPS concentration was 0.35 EU/μg in the purified GST preparation, which did not display any adjuvant effect despite containing significantly higher residual LPS (Fig. 2A and B). Thus, LPS contamination less than 0.1 EU/μg was considered to be insufficient to stimulate the immune system, and preparations could be safely evaluated in the following immune response and protection experiments.
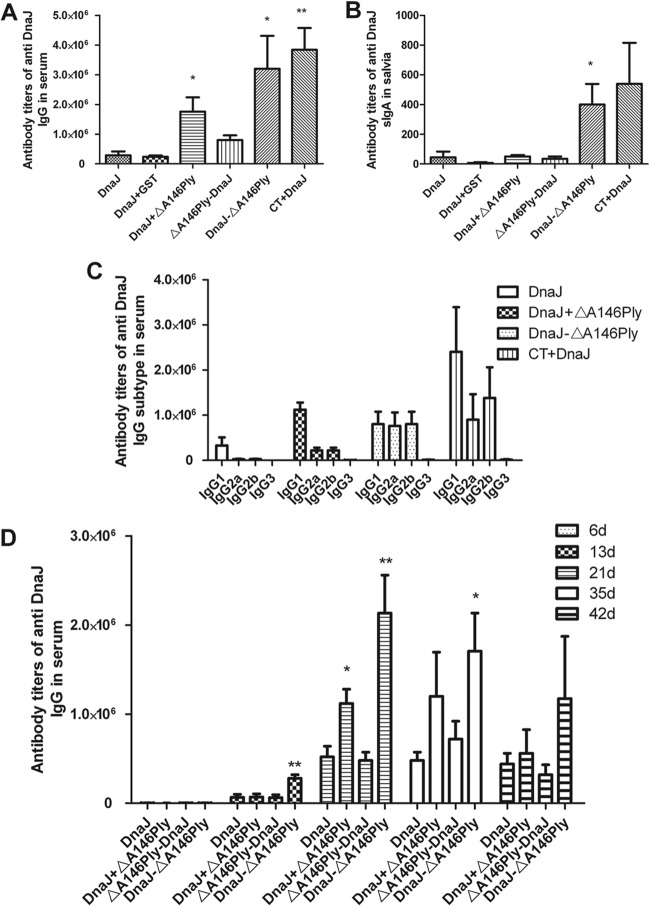
FIG 2.
Anti-DnaJ responses in the serum and saliva of intranasally immunized mice. Antibody titers were measured in serum (A) and saliva (B) taken from animals immunized as depicted on the x axis. (C) Levels IgG subclasses were detected by purified DnaJ, and IgG1, IgG2a, and IgG2b were found predominantly in sera from mice immunized intranasally with DnaJ-ΔA146Ply. (D) Time course of antibody induction in immunized mice. Data are means for 4 mice and standard deviations. The results are representative of three independent experiments. *, P < 0.05, and **, P < 0.01, compared with the DnaJ group.
ΔA146Ply has a strong mucosal adjuvant activity that induces antigen-specific antibody.
In order to assess the mucosal adjuvant activities of ΔA146Ply, DnaJ-specific IgG and IgA responses were measured in serum and saliva. Intranasal immunization with DnaJ-ΔA146Ply significantly enhanced DnaJ-specific serum IgG and saliva sIgA antibody responses (Fig. 2A and B) and led to a very rapid production of anti-DnaJ antibodies (P < 0.01) (Fig. 2D). Furthermore, to compare the relative amounts of IgG isotypes, DnaJ-specific titers were measured using isotype-specific secondary reagents. IgG1 was predominant in DnaJ-plus-ΔA146Ply and CT-plus-DnaJ groups, whereas mice vaccinated with DnaJ-ΔA146Ply produced comparatively high levels of IgG1, IgG2a, and IgG2b (Fig. 2C).
Intranasal immunization with DnaJ-ΔA146Ply or a DnaJ-and-ΔA146Ply mixture reduced S. pneumoniae colonization in nasopharynx and lung.
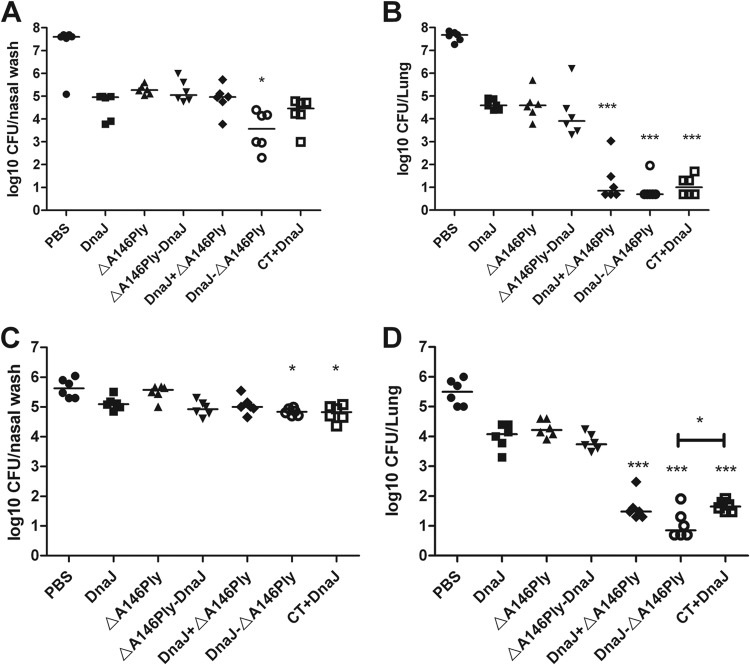
The focal pneumonia model has been successfully established using strains of two different serotypes, CMCC31207 (serotype 6B, 1 × 108CFU) and CMCC31693 (serotype 19F, 1 × 108CFU) (34, 35). In the 19F challenge model, intranasal immunization with recombinant DnaJ-ΔA146Ply reduced the bacterial load in the nasopharynx approximately 3 to 10 times relative to vaccination with DnaJ alone (P < 0.05) (Fig. 3A). Meanwhile, DnaJ-ΔA146Ply or DnaJ and ΔA146Ply mixture also resulted in significant protection against pulmonary infection, reducing the bacterial load in the lung >10-fold compared to DnaJ-immunized mice (P < 0.05) (Fig. 3B). Similar results were observed in the serotype strain 6B infection model (P < 0.05) (Fig. 3C and D). The most effective protection was achieved with the fusion protein DnaJ-ΔA146Ply, which led to a nearly 3-fold reduction in the bacterial load in nasopharynx and a >100-fold decrease in the load in lungs compared with the DnaJ control.
FIG 3.
Vaccine efficacy with regard to pneumococcal colonization in focal infection models. Groups of six C57BL/6 mice were intranasally immunized with the indicated antigens and challenged 2 weeks after the third immunization with 19F/CMCC31693 (1 × 108 CFU) (A and B) or 6B/CMCC31207 (1 × 108 CFU) (C and D). Levels of nasopharynx and lung colonization in individual mice were determined at day 4 after challenge. Each dot represents one mouse. The horizontal lines indicate the median CFU per nasopharynx or lung. The detection line is 10 CFU. *, P < 0.05, and ***, P < 0.001, compared with the DnaJ group.
Intranasal immunization with DnaJ-ΔA146Ply or DnaJ plus ΔA146Ply prevented lethal infection by two strains of S. pneumoniae.
To further evaluate the protection efficacies against different strains of S. pneumoniae, groups of mice were immunized intranasally with the recombinant protein antigens and subsequently challenged with the lethal dose of D39 (serotype 2, 5 × 107CFU) and CMCC31436 (serotype 3, 1 × 108CFU).
In a D39 challenge experiment (Fig. 4A), the median survival times for mice that received single DnaJ (P = 0.0722), ΔA146Ply (P = 0.1724), or ΔA146Ply-DnaJ (P = 0.0837) were not significantly different from those for the group that received PBS. In contrast, mice immunized with DnaJ+ΔA146Ply (P < 0.01) and DnaJ-ΔA146Ply (P < 0.001) survived significantly longer than the PBS group. A total of 83.3% of the mice immunized with the DnaJ-ΔA146Ply protein were protected, whereas only 66.7% and 58.3% of mice survived in the CT+DnaJ- and PPV23-immunized groups, but a survival difference was not noted between the groups.
FIG 4.
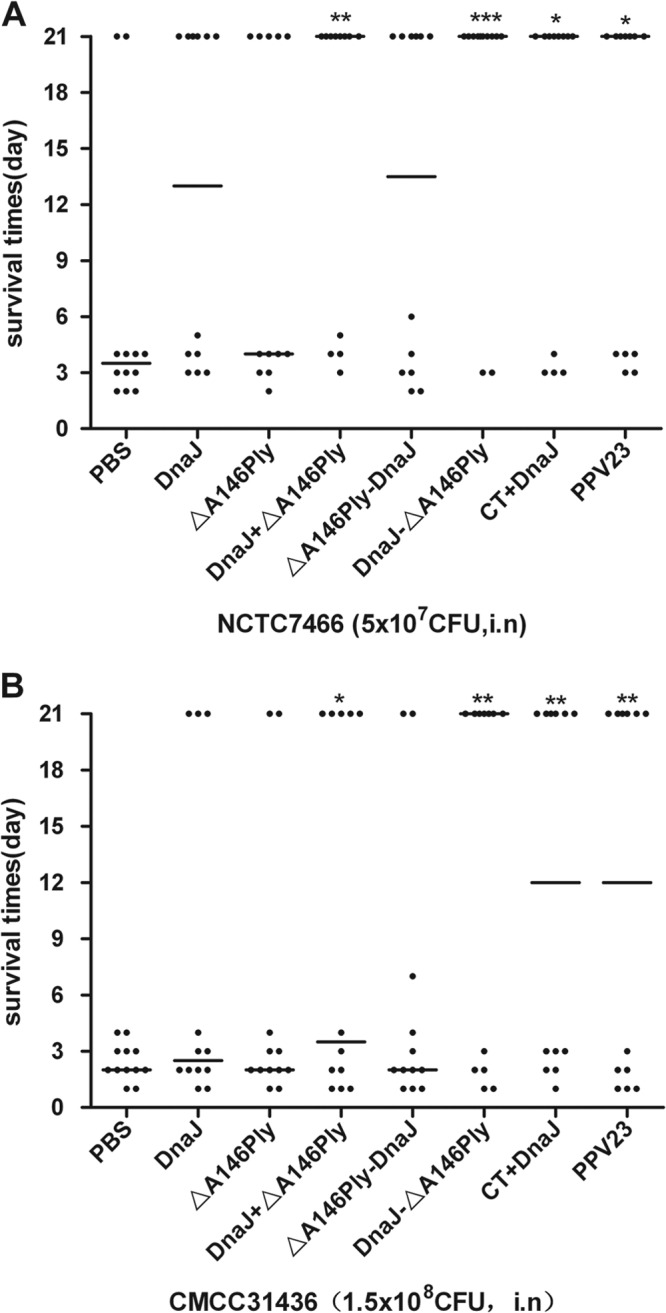
Survival times for mice after intranasal challenge. Groups of 12 C57BL/6 mice were immunized intranasally with the indicated antigens and challenged 2 weeks after the last immunization with NCTC7466 (D39, 5 × 107 CFU) (A) or CMCC31436 (serotype 3, 1 × 108 CFU) (B). Each dot represents one mouse. The horizontal lines denote the median survival time for each group. *, P < 0.05, **, P < 0.01, and ***, P < 0.001, compared with the PBS control group.
A similar result was observed in CMCC 31436 challenge experiment (Fig. 4B), the median survival times for mice that received single DnaJ (P = 0.0833), ΔA146Ply (P = 0.1878), or ΔA146Ply-DnaJ (P = 0.1555) were not significantly different from the time for the group that received PBS. In contrast, mice immunized with DnaJ+ΔA146Ply (P < 0.05) and DnaJ-ΔA146Ply (P < 0.01) survived significantly longer than PBS-treated negative-control mice. A 58.3% protection was achieved in the DnaJ-ΔA146Ply group, while a 50% protection was achieved in the PPV23 and CT+DnaJ groups.
As the positive control, immunization with CT+DnaJ or PPV23 elicited effective protection against D39 and CMCC31436. Furthermore, protection elicited by the DnaJ-ΔA146Ply protein vaccine was as effective as that resulting from PPV23 without additional adjuvant.
DnaJ-ΔA146Ply immunized animals demonstrate minor histological change in lung tissues and decreased IgE level in serum compared with CT.
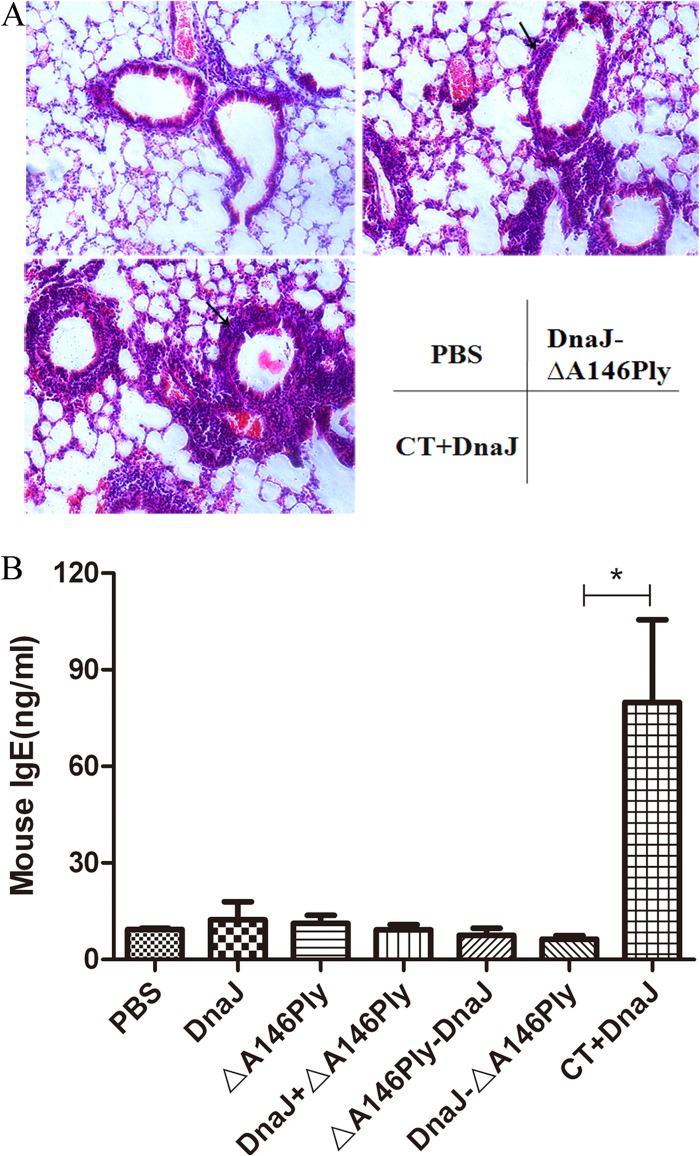
To study the histological change in animals after immunization, lung tissues were collected on day 7 after the last immunization and processed for HE staining. Representative lung sections from the three animal groups are shown in Fig. 5A. HE staining was used to assess the inflammatory response after DnaJ-ΔA146Ply and CT+DnaJ vaccination. HE-stained lung sections from DnaJ-ΔA146Ply- and CT+DnaJ-vaccinated mice showed inflammatory cell infiltration, in contrast to the PBS control. However, lungs from CT+DnaJ-vaccinated mice exhibited massive cell infiltration.
FIG 5.
(A) Pathological analysis of the lung tissues from animals immunized with PBS, DnaJ-ΔA146Ply, and DnaJ plus CT before challenge. The arrow indicates cell infiltration, in contrast to the PBS control. (B) Comparison of serum total IgE between animals (4 per group) immunized with PBS, DnaJ, or ΔA146Ply alone or with DnaJ+ΔA146Ply, DnaJ-ΔA146Ply, ΔA146Ply-DnaJ, or DnaJ plus CT. *, P = 0.0292 (DnaJ-ΔA146Ply versus CT+DnaJ).
Further experiments were carried out to detect serum IgE level by ELISA. The amount of IgE was significantly greater after vaccination with CT+DnaJ than after vaccination with DnaJ-ΔA146Ply (Fig. 6B). In contrast, the amount of serum IgE in DnaJ-ΔA146Ply-immunized mice was similar to that in PBS control mice, suggesting that DnaJ-ΔA146Ply did not induce IgE production or IgE-associated allergic and hypersensitivity reactions.
FIG 6.
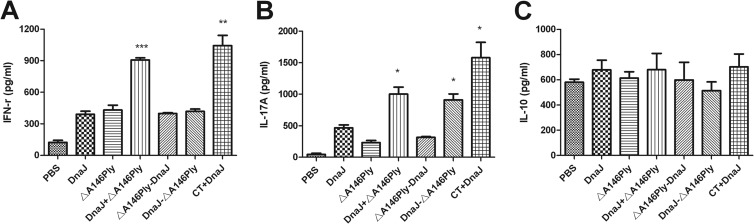
Effect of mucosal immunization with recombinant protein antigens or PBS alone on cytokine production in splenocytes. Seven days after the last immunization, the splenocytes (1 × 105 cells/well) were cultured in the presence of 5 μg of recombinant DnaJ for 72 h at 37°C. After 72 h, the culture supernatants were assayed for the levels of IFN-γ, IL-17A, and IL-10 by ELISA. *, P < 0.05, **, P < 0.01, and ***, P < 0.001, compared with the DnaJ group.
Cytokine secretion by spleen cells in immunized mice.
To evaluate the phenotype (Th1, Th2, Th17, and Treg) of the immune responses elicited by intranasal immunization with DnaJ-ΔA146Ply, suspensions of splenocytes from vaccinated and control mice were isolated and cultured in vitro and stimulated with recombinant DnaJ (5 μg/ml). The amounts of IFN-γ and IL-17A in immunized groups were greater than those in the group that had not been vaccinated (Fig. 6). CT+DnaJ-vaccinated mice produced the highest levels of IFN-γ and IL-17A, reflecting a stronger adjuvant effect for CT than ΔA146Ply. In contrast, the amounts of IFN-γ in DnaJ-ΔA146Ply- and DnaJ-vaccinated mice were not significantly different, suggesting that DnaJ-ΔA146Ply enhanced Th1 cell response only weakly, and serum IFN-γ levels were significantly higher in DnaJ+ΔA146Ply-immunized group than in the other protein-vaccinated groups. The amounts of IL-17A produced in splenocytes from DnaJ-ΔA146Ply- and DnaJ+ΔA146Ply-immunized mice were significantly higher than in those from DnaJ- and ΔA146Ply-vaccinated mice. No significant difference was found in the production of IL-10, and the IL-4 level was lower than the detection limit in this experiment (data not shown).
IL-17A participates in vaccine-mediated bacterial clearance and the production of sIgA.
Several studies have demonstrated that IL-17A mediates pneumococcal colonization in mice (36–38). In the present study, the DnaJ-ΔA146Ply fusion protein also induced significant production of IL-17A and reduced S. pneumoniae colonization in the nasopharynx and lungs. To validate the involvement of IL-17A in DnaJ-ΔA146Ply-mediated protection, IL-17A KO mice were immunized with DnaJ-ΔA146Ply, and the bacterial load in nasal washes and lungs was determined after challenge with pneumococcal strain 19F.
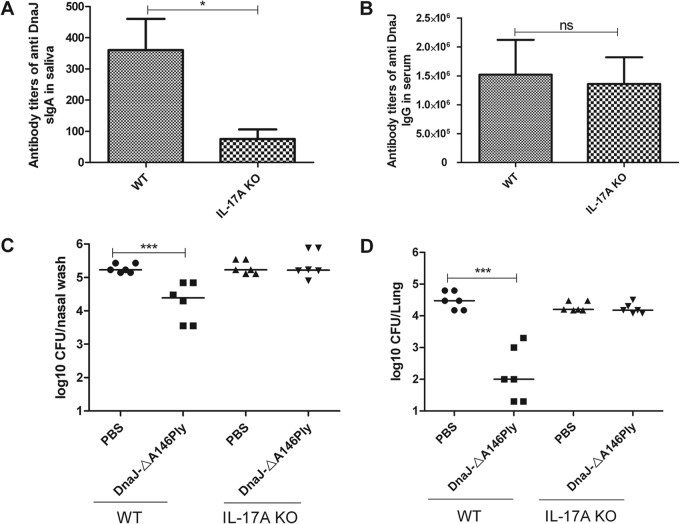
In the present study, a pronounced reduction in the level of sIgA (Fig. 7A) was observed in the saliva of IL-17A KO mice compared with wild-type mice, whereas the level of IgG (Fig. 7B) in serum was similar between wild-type and IL-17A KO mice. In the focal pneumonia model using strain 19F, the intranasal immunization of wild-type mice with recombinant DnaJ-ΔA146Ply reduced the bacterial load relative to mice vaccinated with PBS control. There was no difference in bacterial load between DnaJ-ΔA146Ply and PBS groups in the IL-17A KO mice. Together, these results demonstrate that the IL-17A-mediated immune response is important for DnaJ-ΔA146Ply-elicited production of antigen-specific sIgA and protection against pneumococcal infections.
FIG 7.
IL-17A regulates the production of sIgA and participates in bacterial clearance induced by DnaJ-ΔA146Ply. (A and B) Antibody titers were measured in serum and saliva taken from animals intranasally immunized with DnaJ-ΔA146Ply. Data are means and standard errors of the means from four mice. (C and D) Groups of six mice were intranasally immunized with the indicated antigens and intranasally challenged 2 weeks after the third immunization with 19F/CMCC31693 (1 × 107 CFU). Nasopharynx and lung colonization of individual mice was determined at day 4 after challenge. Each dot represents one mouse. The horizontal lines indicate the median CFU per nasopharynx or lung. WT, wild-type mice. *, P < 0.05; ***, P < 0.001.
DISCUSSION
As a common respiratory pathogen, S. pneumoniae initially attaches and enters the body at mucosal surfaces, and therefore mucosal immune responses function as a first line of defense to prevent pneumococci from entering the body. The protective mucosal immune response against pneumococcal infections is likely to be more effectively induced by mucosal immunization through intranasal inoculation; however, the two main types of pneumococcal vaccines currently used are administered by injection. We and other groups have previously shown that systemic immunization with DnaJ and ΔA146Ply alone is protective against pneumococcal infections (25–27, 29, 30). In this work, we investigated the protection elicited by the combination or fusion form of DnaJ and ΔA146Ply proteins by the intranasal route and whether ΔA146Ply functions as a mucosal adjuvant. The results revealed that intranasal immunization with DnaJ-ΔA146Ply significantly enhanced DnaJ-specific serum IgG and saliva sIgA antibody responses and provided striking protection against infections. ΔA146Ply modulated the production of anti-DnaJ IgG subtypes in mice given the DnaJ-ΔA146Ply fusion protein intranasally. The DnaJ-ΔA146Ply fusion protein is sufficient to elicit a protective response and is a promising pneumococcal vaccine candidate.
In general, mucosal immunization requires the coadministration of appropriate adjuvants to induce immune responses connecting innate and adaptive immunity. Vibrio cholerae cholera toxin (CT) and Escherichia coli heat-labile enterotoxin (LT) and their subunits are known to readily induce immune responses and have been widely used (39, 40). However, neither of them can be used for human vaccine in their native form because of their intrinsic toxic effect. In this study, the histological analysis and the serum total IgE assay support the advantages of ΔA146Ply over CT as an pneumococcal vaccine adjuvant. TLR agonists, including Pam3/2Cys, lipid A, flagellin, imidazoquinolines and CpG motifs, are important mucosal adjuvants (33, 41). Of these, flagellin, a TLR5 agonist, has been tested as an adjuvant against bacterial infections in animals, and human clinical trials are under way (33, 42, 43). Ply has previously been shown to interact with TLR4, resulting in changes in cellular activation (17–19, 44), suggesting that Ply has efficient adjuvant capacity. Hence, we wanted to evaluate whether the nonhemolytic Ply (ΔA146Ply) has an efficient mucosal adjuvant capacity, like wild-type Ply and other TLR agonists.
Serum passive transfer studies have revealed the protective property of antigen-specific IgG in controlling pneumococcal diseases (26, 34). Also, it is well known that IgA is an important immunoglobulin with anti-inflammatory properties (45). In this study, intranasal immunization with DnaJ-ΔA146Ply induced significantly high levels of specific anti-DnaJ IgG antibodies in serum, as well as anti-DnaJ sIgA antibodies in saliva, demonstrating the protective properties of intranasal vaccination with the combination of the two proteins.
For effective vaccination, antigens and adjuvant should be administered simultaneously and through the same pathway. This could be easily achieved by coupling the TLR ligands directly to the antigens. Compared to nonconjugated antigens, fusion antigens have several advantages, including easy antigen delivery by targeting TLRs on immune cells, enhanced cellular uptake by immune cells, and finally increased induction of immune responses. Our observation coincides with previous reports, and it is therefore reasonable that the DnaJ-ΔA146Ply fusion form is better than the mixture, which is similar to results in recent reports (20, 22).
It should be noted that the fusion of proteins affects their biological properties. ΔA146Ply-DnaJ was less potent in induction of protective immunity in vivo than DnaJ-ΔA146Ply and was less effective than the mixture of the two single antigens. Presumably, N-terminal DnaJ affects the folding property of the fusion proteins, because DnaJ is involved in protein folding by acting as a molecular chaperone (46). Additionally, the possibility cannot be ruled out that the TLR-binding domain of Ply is located in its C terminus, which would be destroyed by fusion and lead to poor immune responses. At this stage, we do not know the characteristics of the fusion proteins, and it is difficult to assess their spatial location. Nevertheless, based on the current data, it is suggested that antigens be fused to the N terminus of Ply to elicit better responses.
Intranasal delivery of recombinant DnaJ-ΔA146Ply and DnaJ+ΔA146Ply induced both mucosal and systemic immunity against S. pneumoniae, and vaccinated mice produced high levels of IFN-γ and IL-17A. Given that the Th1 cytokine IFN-γ and the Th17 cytokine IL-17A modulate protective immunity to pneumococcal infections (38, 47, 48), we propose a mixed Th1 and Th17 response to assess the effectiveness of vaccination. Interestingly, we found that sIgA in DnaJ-ΔA146Ply-vaccinated IL-17A KO mice is barely detectable. Although IL-17A-mediated production of sIgA in the small intestine and lung has been reported (49, 50), further studies are required to illuminate the relationship between IL-17A and sIgA in vaccination.
We and other groups have shown that DnaJ or ΔA146Ply protein protected against colonization and invasive pneumococcal infections where CT or alum was used as an adjuvant (25–27, 29, 30). In this study, without additional adjuvant, mucosal immunization with recombinant DnaJ-ΔA146Ply fusion protein provided the best protection against intranasal challenge with pathogenic S. pneumoniae. Protection elicited by the protein vaccine was as effective as that resulting from the PPV23 vaccine. In this formulation, ΔA146Ply plays a double role, that of a conserved pneumococcal antigen itself and that of an adjuvant for DnaJ protein.
In conclusion, this study indicates that mucosal immunization with DnaJ-ΔA146Ply fusion protein induces both mucosal and systemic immunity and protects against pneumococcal diseases. ΔA146Ply fusion proteins are promising candidates as next-generation pneumococcal vaccines.
ACKNOWLEDGMENTS
We thank Zhinan Yin (College of Life Sciences, Nankai University, Tianjin, China) and Richard A. Flavell (Yale University School of Medicine, New Haven, CT) for kindly provide the IL-17A−/− mice.
This study was supported by the National Natural Science Foundation grants of China (no. 31070819), the special fund of Chongqing Key Laboratory (CSTC), and Innovation Experiment Project Management of the Experimental Teaching Center (no. 20110315).
We declare that no competing interests exist.
Footnotes
Published ahead of print 3 February 2014
REFERENCES
- 1.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, Hib and Pneumococcal Global Burden of Disease Study Team 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902. 10.1016/S0140-6736(09)61204-6 [DOI] [PubMed] [Google Scholar]
- 2.Rodgers GL, Klugman KP. 2011. The future of pneumococcal disease prevention. Vaccine 29:C43–C48. 10.1016/j.vaccine.2011.07.047 [DOI] [PubMed] [Google Scholar]
- 3.Ginsburg AS, Nahm MH, Khambaty FM, Alderson MR. 2012. Issues and challenges in the development of pneumococcal protein vaccines. Expert Rev. Vaccines 11:279–285. 10.1586/erv.12.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jauneikaite E, Jefferies JM, Hibberd ML, Clarke SC. 2012. Prevalence of Streptococcus pneumoniae serotypes causing invasive and non-invasive disease in South East Asia: a review. Vaccine 30:3503–3514. 10.1016/j.vaccine.2012.03.066 [DOI] [PubMed] [Google Scholar]
- 5.Pitsiou GG, Kioumis IP. 2011. Pneumococcal vaccination in adults: does it really work? Respir. Med. 105:1776–1783. 10.1016/j.rmed.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 6.Min X, Zhang X, Wang H, Gong Y, Li M, Xu W, Yin Y, Cao J. 2012. Protection against pneumococcal infection elicited by immunization with glutamyl tRNA synthetase, polyamine transport protein D and sortase A. Vaccine 30:3624–3633. 10.1016/j.vaccine.2012.03.042 [DOI] [PubMed] [Google Scholar]
- 7.Harfouche C, Filippini S, Gianfaldoni C, Ruggiero P, Moschioni M, Maccari S, Pancotto L, Arcidiacono L, Galletti B, Censini S, Mori E, Giuliani M, Facciotti C, Cartocci E, Savino S, Doro F, Pallaoro M, Nocadello S, Mancuso G, Haston M, Goldblatt D, Barocchi MA, Pizza M, Rappuoli R, Masignani V. 2012. RrgB321, a fusion protein of the three variants of the pneumococcal pilus backbone RrgB, is protective in vivo and elicits opsonic antibodies. Infect. Immun. 80:451–460. 10.1128/IAI.05780-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogunniyi AD, Grabowicz M, Briles DE, Cook J, Paton JC. 2007. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 75:350–357. 10.1128/IAI.01103-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao J, Chen D, Xu W, Chen T, Xu S, Luo J, Zhao Q, Liu B, Wang D, Zhang X, Shan Y, Yin Y. 2007. Enhanced protection against pneumococcal infection elicited by immunization with the combination of PspA, PspC, and ClpP. Vaccine 25:4996–5005. 10.1016/j.vaccine.2007.04.069 [DOI] [PubMed] [Google Scholar]
- 10.Brandtzaeg P. 2010. Function of mucosa-associated lymphoid tissue in antibody formation. Immunol. Invest. 39:303–355. 10.3109/08820131003680369 [DOI] [PubMed] [Google Scholar]
- 11.Holmgren J, Czerkinsky C. 2005. Mucosal immunity and vaccines. Nature Med. 11:S45–S53. 10.1038/nm1213 [DOI] [PubMed] [Google Scholar]
- 12.Sheridan BS, Lefrancois L. 2011. Regional and mucosal memory T cells. Nat. Immunol. 12:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lycke N. 2012. Recent progress in mucosal vaccine development: potential and limitations. Nat. Rev. Immunol. 12:592–605. 10.1038/nri3251 [DOI] [PubMed] [Google Scholar]
- 14.Bevan MJ. 2011. Memory T cells as an occupying force. Eur. J. Immunol. 41:1192–1195. 10.1002/eji.201041377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 350:896–903. 10.1056/NEJMoa030595 [DOI] [PubMed] [Google Scholar]
- 16.Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, Woodrow M, Thierry-Carstensen B, Andersen P, Novicki D, Del Giudice G, Rappuoli R. 2009. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One 4:e6999. 10.1371/journal.pone.0006999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. U. S. A. 100:1966–1971. 10.1073/pnas.0435928100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoma S, Tsuchiya K, Kawamura I, Nomura T, Hara H, Uchiyama T, Daim S, Mitsuyama M. 2008. Critical involvement of pneumolysin in production of interleukin-1α and caspase-1-dependent cytokines in infection with Streptococcus pneumoniae in vitro: a novel function of pneumolysin in caspase-1 activation. Infect. Immun. 76:1547–1557. 10.1128/IAI.01269-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dogan S, Zhang Q, Pridmore AC, Mitchell TJ, Finn A, Murdoch C. 2011. Pneumolysin-induced CXCL8 production by nasopharyngeal epithelial cells is dependent on calcium flux and MAPK activation via Toll-like receptor 4. Microbes Infect. 13:65–75. 10.1016/j.micinf.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 20.McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, Moran B, Fitzgerald KA, Tschopp J, Petrilli V, Andrew PW, Kadioglu A, Lavelle EC. 2010. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 6:e1001191. 10.1371/journal.ppat.1001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witzenrath M, Pache F, Lorenz D, Koppe U, Gutbier B, Tabeling C, Reppe K, Meixenberger K, Dorhoi A, Ma J, Holmes A, Trendelenburg G, Heimesaat MM, Bereswill S, van der Linden M, Tschopp J, Mitchell TJ, Suttorp N, Opitz B. 2011. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. J. Immunol. 187:434–440. 10.4049/jimmunol.1003143 [DOI] [PubMed] [Google Scholar]
- 22.Douce G, Ross K, Cowan G, Tao J, Tim M, Mitchell J. 2010. Novel mucosal vaccines generated by genetic conjugation of heterologous proteins to pneumolysin (PLY) from Streptococcus pneumoniae. Vaccine 28:3231–3237. 10.1016/j.vaccine.2010.02.014 [DOI] [PubMed] [Google Scholar]
- 23.Simell B, Korkeila M, Pursiainen H, Kilpi TM, Käyhty H. 2001. Pneumococcal carriage and otitis media induce salivary antibodies to pneumococcal surface adhesin a, pneumolysin, and pneumococcal surface protein A in children. J. Infect. Dis. 183:887–896. 10.1086/319246 [DOI] [PubMed] [Google Scholar]
- 24.Holmlund E, Quiambao B, Ollgren J, Nohynek H, Käyhty H. 2006. Development of natural antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A and pneumolysin in Filipino pregnant women and their infants in relation to pneumococcal carriage. Vaccine 24:57–65. 10.1016/j.vaccine.2005.07.055 [DOI] [PubMed] [Google Scholar]
- 25.Kirkham L-AS, Kerr AR, Douce GR, Paterson GK, Dilts DA, Liu D-F, Mitchell TJ. 2006. Construction and immunological characterization of a novel nontoxic protective pneumolysin mutant for use in future pneumococcal vaccines. Infect. Immun. 74:586–593. 10.1128/IAI.74.1.586-593.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu K, Zhang X, Shi J, Li N, Li D, Luo M, Cao J, Yin N, Wang H, Xu W, He Y, Yin Y. 2010. Immunization with a combination of three pneumococcal proteins confers additive and broad protection against Streptococcus pneumoniae infections in mice. Infect. Immun. 78:1276–1283. 10.1128/IAI.00473-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goulart C, da Silva TR, Rodriguez D, Politano WR, Leite LC, Darrieux M. 2013. Characterization of protective immune responses induced by pneumococcal surface protein A in fusion with pneumolysin derivatives. PLoS One 8:e59605. 10.1371/journal.pone.0059605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee C-J, Wang TR, Frasch CE. 2001. Immunogenicity in mice of pneumococcal glycoconjugate vaccines using pneumococcal protein carriers. Vaccine 19:3216–3225. 10.1016/S0264-410X(01)00033-0 [DOI] [PubMed] [Google Scholar]
- 29.Khan MN, Bansal A, Shukla D, Paliwal P, Sarada SK, Mustoori SR, Banerjee PK. 2006. Immunogenicity and protective efficacy of DnaJ (hsp40) of Streptococcus pneumoniae against lethal infection in mice. Vaccine 24:6225–6231. 10.1016/j.vaccine.2006.05.074 [DOI] [PubMed] [Google Scholar]
- 30.Cui Y, Zhang X, Gong Y, Niu S, Yin N, Yao R, Xu W, Li D, Wang H, He Y, Cao J, Yin Y. 2011. Immunization with DnaJ (hsp40) could elicit protection against nasopharyngeal colonization and invasive infection caused by different strains of Streptococcus pneumoniae. Vaccine 29:1736–1744. 10.1016/j.vaccine.2010.12.126 [DOI] [PubMed] [Google Scholar]
- 31.Cui J, Zhang Q, Dong J, Jiang H, Zhou A, Dong S, Zhang X, Yin Y, Hong W. 2011. Construction of DnaJ-deficient mutant strain of Streptococcus pneumoniae and its preliminary study on virulence. J. Third Mil. Med. Univ. 33:2000–2003 [Google Scholar]
- 32.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17:375–387. 10.1016/S1074-7613(02)00391-6 [DOI] [PubMed] [Google Scholar]
- 33.Nguyen CT, Kim SY, Kim MS, Lee SE, Rhee JH. 2011. Intranasal immunization with recombinant PspA fused with a flagellin enhances cross-protective immunity against Streptococcus pneumoniae infection in mice. Vaccine 29:5731–5739. 10.1016/j.vaccine.2011.05.095 [DOI] [PubMed] [Google Scholar]
- 34.Gong Y, Xu W, Cui Y, Zhang X, Yao R, Li D, Wang H, He Y, Cao J, Yin Y. 2011. Immunization with a ZmpB-based protein vaccine could protect against pneumococcal diseases in mice. Infect. Immun. 79:867–878. 10.1128/IAI.00717-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao J, Zhang X, Gong Y, Zhang Y, Cui Y, Lai X, Yin Y, Li M, Li D, Zhang L. 2013. Protection against pneumococcal infection elicited by immunization with multiple pneumococcal heat shock proteins. Vaccine 31:3564–3571. 10.1016/j.vaccine.2013.05.061 [DOI] [PubMed] [Google Scholar]
- 36.Lu Y-J, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, Thompson CM, Harney KF, Anderson PW, Lipsitch M, Malley R. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4:e1000159. 10.1371/journal.ppat.1000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moffitt KL, Gierahn TM, Lu YJ, Gouveia P, Alderson M, Flechtner JB, Higgins DE, Malley R. 2011. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe 9:158–165. 10.1016/j.chom.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Clarke TB, Weiser JN. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Invest. 119:1899–1909. 10.1172/JCI36731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mowat AM, Donachie AM, Jägewall S, Schön K, Löwenadler B, Dalsgaard K, Kaastrup P, Lycke N. 2001. CTA1-DD-immune stimulating complexes: a novel, rationally designed combined mucosal vaccine adjuvant effective with nanogram doses of antigen. J. Immunol. 167:3398–3405 http://www.jimmunol.org/content/167/6/3398.long [DOI] [PubMed] [Google Scholar]
- 40.Peppoloni S, Ruggiero P, Contorni M, Morandi M, Pizza M, Rappuoli R, Podda A, Del Giudice G. 2003. Mutants of the Escherichia coli heat-labile enterotoxin as safe and strong adjuvants for intranasal delivery of vaccines. Expert Rev. Vaccines 2:285–293. 10.1586/14760584.2.2.285 [DOI] [PubMed] [Google Scholar]
- 41.Fujita Y, Taguchi H. 2012. Overview and outlook of Toll-like receptor ligand-antigen conjugate vaccines. Ther. Deliv. 3:749–760. 10.4155/tde.12.52 [DOI] [PubMed] [Google Scholar]
- 42.Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, Kavita U, Stanberry L, Shaw A. 2011. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine 29:5145–5152. 10.1016/j.vaccine.2011.05.041 [DOI] [PubMed] [Google Scholar]
- 43.Treanor JJ, Taylor DN, Tussey L, Hay C, Nolan C, Fitzgerald T, Liu G, Kavita U, Song L, Dark I, Shaw A. 2010. Safety and immunogenicity of a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125) in healthy young adults. Vaccine 28:8268–8274. 10.1016/j.vaccine.2010.10.009 [DOI] [PubMed] [Google Scholar]
- 44.Dessing MC, Hirst RA, de Vos AF, van der Poll T. 2009. Role of Toll-like receptors 2 and 4 in pulmonary inflammation and injury induced by pneumolysin in mice. PLoS One 4:e7993. 10.1371/journal.pone.0007993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mestecky J, Russell MW, Elson CO. 1999. Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut 44:2–5. 10.1136/gut.44.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wild J, Rossmeissl P, Walter WA, Gross CA. 1996. Involvement of the DnaK–DnaJ–GrpE chaperone team in protein secretion in Escherichia coli. J. Bacteriol. 178:3608–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundgren A, Bhuiyan TR, Novak D, Kaim J, Reske A, Lu Y-J, Qadri F, Malley R. 2012. Characterization of Th17 responses to Streptococcus pneumoniae in humans: comparisons between adults and children in a developed and a developing country. Vaccine 30:3897–3907. 10.1016/j.vaccine.2012.03.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jahn-Schmid B, Messner P, Unger FM, Sleytr UB, Scheiner O, Kraft D. 1996. Toward selective elicitation of TH1-controlled vaccination responses: vaccine applications of bacterial surface layer proteins. J. Biotechnol. 44:225–231. 10.1016/0168-1656(95)00124-7 [DOI] [PubMed] [Google Scholar]
- 49.Hirota K, Turner J-E, Villa M, Duarte JH, Demengeot J, Steinmetz OM, Stockinger B. 2013. Plasticity of TH17 cells in Peyer's patches is responsible for the induction of T cell–dependent IgA responses. Nat. Immunol. 14:372–379. 10.1038/ni.2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaffar Z, Ferrini ME, Herritt LA, Roberts K. 2009. Cutting edge: lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J. Immunol. 182:4507–4511. 10.4049/jimmunol.0900237 [DOI] [PMC free article] [PubMed] [Google Scholar]