Abstract
Fimbria-mediated adherence to the intestinal epithelia is a key step in enteroaggregative Escherichia coli (EAEC) pathogenesis. To date, four fimbriae have been described for EAEC; aggregative adherence fimbria II (AAF/II) is the most important adherence factor for EAEC prototype strain 042. Previously, we described results showing that extracellular matrix (ECM) components might be involved in the recognition of AAF/II fimbriae by intestinal cells. In this study, we sought to identify novel potential receptors on intestinal epithelial cells recognized by the AAF/II fimbriae. Purified AafA-dsc protein, the major subunit of AAF/II fimbriae, was incubated with a monolayer of T84 cells, cross-linked to the surface-exposed T84 cell proteins, and immunoprecipitated by using anti-AafA antibodies. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of cellular proteins bound to AafA-dsc protein identified laminin (previously recognized as a potential receptor for AAF/II) and cytokeratin 8 (CK8). Involvement of the major subunit of AAF/II fimbriae (AafA protein) in the binding to recombinant CK8 was confirmed by adherence assays with purified AAF/II fimbriae, AafA-dsc protein, and strain 042. Moreover, HEp-2 cells transfected with CK8 small interfering RNA (siRNA) showed reduced 042 adherence compared with cells transfected with scrambled siRNA as a control. Adherence of 042 to HEp-2 cells preincubated with antibodies against ECM proteins or CK8 was substantially reduced. Altogether, our results supported the idea of a role of CK8 as a potential receptor for EAEC.
INTRODUCTION
Enteroaggregative Escherichia coli (EAEC) is an important cause of endemic and epidemic diarrheal disease worldwide (1). Recently, an outbreak of Shiga toxin-producing EAEC has increased the need to understand the pathogenic mechanisms employed by the microorganism to colonize and infect intestinal cells (2). In general, EAEC pathogenesis comprises colonization of the intestinal mucosa, followed by elaboration of enterotoxins and cytotoxins and release of proinflammatory cytokines from infected epithelial cells (3). EAEC adherence to intestinal cells is mediated by fimbrial adhesins, designated aggregative adherence fimbriae (AAF). To date, four variants of the AAF fimbriae have been described, all encoded by 55- to 65-MDa plasmids (pAA plasmid) (4, 5, 6, 7). Adherence of the prototype EAEC strain 042 to cells and abiotic surfaces requires the AAF pilus variant called AAF/II (6). The AAF/II organelle comprises two structural subunits: the major subunit, AafA, and the minor subunit, AafB, which is hypothesized but not proven to be located at the pilus tip. AafA is required for adhesion to epithelial cell monolayers and abiotic surfaces, whereas AafB has been associated with the release of cytokines (8).
Even though the importance of the AAF/II fimbriae in the adherence of EAEC to intestinal cells has been established, the cell receptors involved in adhesin recognition have not been fully characterized. We previously showed binding of AAF/II to extracellular matrix (ECM) proteins, such as fibronectin, laminin, and type IV collagen, which enhanced bacterial adherence to the surface of polarized intestinal monolayers (9). Using a proteomics approach, the epidermal growth factor receptor (EGFR), Thrombospondin-1 (TSP1), glucose-regulated protein (GRP-94), and fibronectin were identified as potential receptors for an AAF/II-producing strain (10). Although significant progress in the discovery of receptors for EAEC has been made, the blocking of known receptors did not cause full inhibition of bacterial binding, suggesting that other receptors for AAF/II might exist in intestinal cells.
Using a proteomic approach, here we show that cytokeratin 8 (CK8) is a potential receptor for AAF/II fimbriae in intestinal epithelial cells.
MATERIALS AND METHODS
Bacterial strains.
Prototype EAEC strain 042 (O44:H18) was originally isolated from a child with diarrhea in Lima, Peru. The EAEC 042aafA strain was constructed using the lambda red linear recombination method (11), by which the aafA region was replaced with a kanamycin (Km)-sacB cassette. This cassette was amplified from pPuc19-Km-SacB by PCR with the following primers: forward, 5′-TATTCGGAATGTAAGAAAACCTAGGAGAGGCCAGAGTGAATCCTGCTGATTTATTTCCTCCTTGAGGTTTTATCAGTAATTGACCGTGATTGCCTTCCCCTTATTTGTTAACTGTTAATTGTCCTTGTT-3′; and reverse, 5′-TCGTGATGTCAACGTTGACAGGAGCGCAAATATCGACCTGAGTTTTACTATTAGACAACCGCAACGCTGCGCTGATGCTGGTATGCGAATAAAAGCTTGGTTTCTTGAAAATTTTTTTTTTGACTCAATAT-3′. Kanamycin-resistant recombinants were selected and screened by PCR. The EAEC 042aafB strain is an isogenic mutant harboring an insertion of suicide plasmid pJP5603 into the aafB gene (8). For complementation experiments, EAEC 042aafA was transformed with a pBAD30 plasmid harboring aafDA genes under the control of the pBAD promoter (pBADaafDA). All bacteria were grown overnight in Luria-Bertani (LB) broth with the addition of ampicillin (100 μg/ml) and kanamycin (50 μg/ml) when appropriate.
Cell lines.
T84, HEp-2, and MDCK cells were routinely maintained in Dulbecco's modified Eagle's medium (DMEM), DMEM–F-12 medium, and DMEM, respectively, supplemented with 10% fetal bovine serum (FBS), penicillin (10 U/ml), and streptomycin (10 μg/ml), at 37°C in 5% CO2.
Cross-linking assay.
T84 cell monolayers grown using a 75-cm2 flask were incubated with monomeric AafA-dsc protein constructed via the donor-strand complementation (dsc) method as previously described (12). Cells were incubated with AafA-dsc protein (5 μg/ml)–phosphate-buffered saline (PBS) or with PBS alone. Bound AafA-dsc protein was cross-linked to T84 surface-exposed proteins with the addition of 1 mM 3,3′dithiobis(sulfosuccinimidylpropionate) (DTSSP; Pierce). Cells were lysed in mammalian protein extraction reagent (M-PER) (Pierce) at room temperature. Soluble proteins were incubated with anti-AafA serum for immunoprecipitation analyses using A/G agarose columns (Pierce). Immunoprecipitated proteins were visualized by silver-stained SDS-PAGE analyses, and protein bands were excised from SDS gel for matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF) analyses (Mass Spectometry Core Laboratory, University of Texas Medical Branch).
AAF/II fimbria purification.
To purify fimbria filaments, EAEC strain 042 was grown in 1 liter of DMEM (high glucose) (DMEM/HG) at 37°C with shaking until an optical density at 600 nm (OD600) of 1.0 was reached. Cells were harvested by centrifugation at 6,000 × g and resuspended in 10 ml of a solution containing 0.5 mM Tris and 75 mM NaCl and heated to 65°C for 30 min. Subsequently, cells were pelleted by centrifugation at 6,000 × g for 10 min. Supernatants were recovered and centrifuged at 21,000 × g for 30 min to remove the remaining debris. To further remove trace proteins such as dispersin and other outer membrane proteins, supernatants were precipitated with 30% ammonium sulfate saturation for 3 h with stirring at 4°C. The supernatant fraction was again adjusted to 60% ammonium sulfate saturation, and proteins were precipitated overnight with stirring. Ammonium sulfate precipitates were recovered by centrifugation at 9,000 × g for 30 min, and pellets were resuspended in ∼10 ml of 50 mM Tris–10 mM NaCl, dialyzed in PBS three times (1 h, 3 h, and overnight), filtered through a 0.2-μM-pore-size filter, and stored at −20°C. The presence of AAF/II fimbriae was confirmed by SDS-PAGE and immunoblotting. AafB protein was not detected in the AAF/II purified fimbria fraction (data not shown).
Solid-phase binding assay.
The wells of 24-well plates (Thermo Labsystems, Franklin, MA) were coated with a solution of 5 μg/ml of laminin (Sigma), cytokeratin 8 (CK8; Abcam), or bovine serum albumin (BSA; Sigma) in PBS overnight at 4°C. Unbound protein was removed by washing three times with PBS and subsequently blocked with 1% skim milk–PBS for 1 h at room temperature. The blocking buffer was removed, and the wells were washed three times prior to addition of the bacteria in a 200-μl final volume. Incubation with bacteria was performed for 2 h at 37°C. Plates were washed, rabbit anti-O44 serum (Denka Seiken Co., Ltd., Tokyo, Japan) diluted 1:200 was added to the wells, and the reaction mixture was incubated for 1 h at room temperature. Anti-rabbit horseradish peroxidase (HRP) conjugate (KPL, Gaithersburg, MD) was added following another PBS wash step. Peroxidase activity associated with each well was detected by the addition of TMB (3,3′,5,5′-tetramethylbenzidine) substrate solution (KPL). Optical densities were read at 450 nm with a 96-well plate reader (Infinite; Tecan). To analyze binding data, the background absorbance from wells in which bacteria were not added was subtracted from the absorbance in test wells. For AAF/II fimbria binding, different concentrations of purified AAF/II fimbriae were added to wells coated with laminin, CK8, or BSA and incubated for 1 h at room temperature; anti-AafA antiserum (diluted 1:1,000) raised in rabbits was used to detect the bound protein. For AafA-dsc protein binding, 10 μg/ml of Aaf-dsc protein was added to 96-well plates coated separately with CK8 or BSA and incubated for 2 h at room temperature. Binding to CK8 was determined by using anti-AafA antiserum as described above.
Quantification of bacterial binding.
The wells of microtiter plates coated with CK8 or bovine serum albumin (BSA) were incubated with 1 ml DMEM/HG containing 1 × 108 bacteria at 37°C for 4 h. After the wells were washed with PBS, the bacterial cells that had adhered to the wells were collected by scraping them into PBS; serial dilutions were plated onto LB agar plates. The number of adherent bacteria was determined by counting the resulting colonies in triplicate.
siRNA.
Subconfluent cultures (∼40% to ∼50%) of HEp-2 cells were transfected with a mixture containing 80 pmol of synthetic double-stranded RNA (small interfering RNA [siRNA]) for cytokeratin 8 (sc-72111; Santa Cruz Biotechnology) or scrambled siRNA and siRNA transfection reagent (Santa Cruz Biotechnology) in a total volume of 1 ml of DMEM/HG without either antibiotics or serum. The cells were incubated for 7 h at 37°C in 5% CO2. Then, medium was replaced by DMEM supplemented with FBS and antibiotics, and after 48 to 72 h, transfected cells were used in adherence assays. To verify reduction in CK8 expression, HEp-2 cells were lysed using radioimmunoprecipitation assay (RIPA) buffer (Pierce) and proteins were separated on 10% polyacrylamide gels and blotted onto nitrocellulose membrane. The membrane was incubated with primary antibodies against CK8 (Santa Cruz Biotechnology) and the secondary HRP-conjugated antibody. Relative CK8 and actin levels were analyzed using chemiluminescence Luminol reagent (Santa Cruz Biotechology). The resulting autoradiography was scanned and quantified with ImageJ 1.43 software (http://rsbweb.nih.gov/ij/) to determine intensity. Additionally, transfected cells were grown in an 8-well Lab-Tek Chamber slide, immunostained with the appropriated antibodies, and examined using confocal microscopy (Leica TCS SP2).
Adherence assays.
HEp-2 cells previously treated with scrambled or CK8 siRNA were incubated with 500 μl/well of DMEM/HG for 30 min. Then, the medium was aspirated and bacteria were added to the monolayer (in triplicate), with a multiplicity of infection (MOI) of 10. The plates were incubated at 37°C in 5% CO2 for 3 h and then washed three times with PBS. Cells were lysed with a solution containing 0.1% (vol/vol) Triton X-100–PBS, and serial dilutions of the lysates were plated on LB agar. The number of adherent bacteria was determined by counting CFU. For blocking experiments, HEp-2 cells were preincubated with DMEM/HG only or supplemented with (alone or in combination) anti-fibronectin, anti-laminin, anti-CK8 antibodies or with isotype IgG (rabbit) as a control (Abcam).
Fluorescence assays.
HEp-2 cells, grown in a Lab-Tek Chamber slide, were washed twice with PBS. Cells were fixed using 4% paraformaldehyde–PBS for 20 min at room temperature. The fixed cells were then not permeabilized or permeabilized by adding 0.2% Triton X-100–PBS for 5 min at room temperature. Actin filaments were visualized in the permeabilized cells by incubation with 0.05 μg/ml tetramethyl rhodamine isocyanate (TRITC)-phalloidin for 30 min at room temperature. CK8 were visualized in nonpermeabilized cells by incubation with the specified primary anti-CK8 antibody (1:100 dilution) for 1 h at room temperature, followed by incubation for 1 h at room temperature with the specified secondary antibody. The bacterial and human DNA was visualized by incubation with TO-PRO-3 (Life Technologies) for 1 h at room temperature. Slides were mounted on Gelvatol, covered with a glass coverslip, and examined under a Leica TCS SP5 confocal microscope at ×100 or ×63 magnification.
Statistical analysis.
The statistical significance of the results of comparisons between the individual groups was analyzed using the unpaired Student's t test with a threshold of P < 0.05. Values are expressed as the means ± the standard errors of the means of the results of three experiments.
RESULTS
Laminin and cytokeratin 8 are identified in pulldown assays.
To identify novel potential cell receptors for the AAF/II fimbriae, T84 cell monolayers were incubated with the AafA-dsc protein or with PBS as a control. Cross-linked complexes between AafA-dsc protein and surface-exposed proteins were obtained by immunoprecipitation with anti-AafA antibodies, and immunoprecipitated proteins were analyzed by SDS-PAGE. Compared to T84 cells incubated with PBS, several bands were observed when cells were incubated with AafA-dsc protein (Fig. 1). Ten bands were analyzed by MALDI-TOF, and two proteins gave a significant match: the β3 subunit of laminin and cytokeratin 8 (CK8). For the other 8 bands, the amount of protein was insufficient to perform the analysis.
FIG 1.
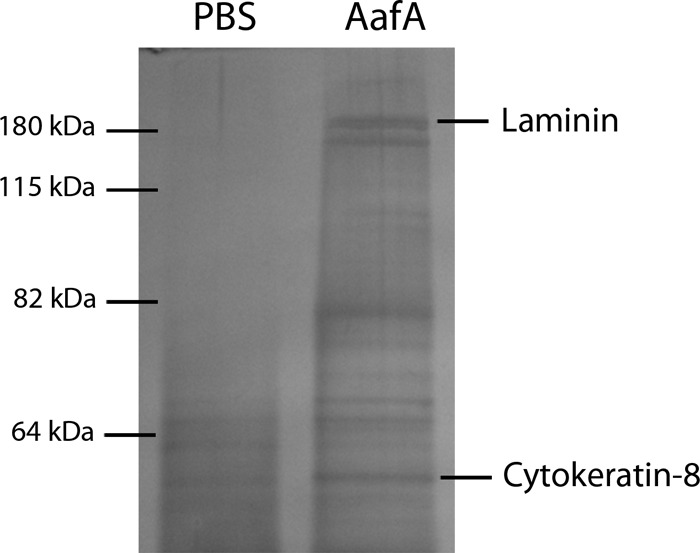
Identification of laminin and CK8 as a potential receptor for EAEC. T84 cells were incubated with 5 μg/ml of AafA-dsc protein or PBS (control), and bound AafA protein was cross-linked to T84 surface-exposed proteins with 1 mM DTSSP cross-linker (Pierce). Then, cells were lysed and cellular extracts were incubated with anti-AafA serum for immunoprecipitation analyses using A/G agarose columns. Immunoprecipitated proteins were visualized by silver-stained SDS-PAGE analyses, and protein bands were excised from the SDS gel for MALDI-TOF analyses.
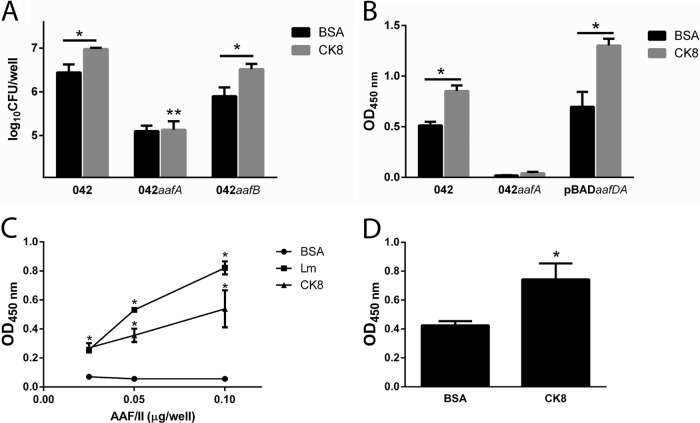
EAEC 042 binding to cytokeratin 8 is dependent on AAF/II fimbria.
We previously showed that EAEC strain 042 binds to laminin and to other ECM proteins such as fibronectin and collagen IV, a binding that is mediated by the AAF/II fimbriae (9). We quantified the adherence to recombinant purified human CK8 of the EAEC 042 mutant in the AafA or AafB subunits. Binding of EAEC 042 to CK8-coated wells was significantly higher than to the BSA-coated wells used as a negative control. Strain 042aafB exhibited binding to CK8 similar to that of its wild-type parent; in contrast, the 042aafA mutant bound significantly less to CK8 (Fig. 2A). Similar results were obtained when the ability of EAEC 042 to bind to CK8 protein was assayed by enzyme-linked immunosorbent assay (ELISA). The complemented 042aafA mutant strain expressing the aafA gene in trans was also able to bind CK8 at levels comparable to the wild-type strain levels (Fig. 2B). To demonstrate direct binding of AAF/II to laminin and CK8, we performed ELISAs with purified fimbriae. We found dose-dependent binding of AAF/II fimbriae to laminin and CK8 (Fig. 2C). Additionally, to determine direct binding of the major subunit of AAF/II fimbriae to CK8, we evaluated the binding of AafA-dsc protein to CK8 by ELISA. Binding to CK8 was significantly greater than that to BSA (Fig. 2D).
FIG 2.
EAEC 042 binding to CK8 is dependent of AAF/II fimbriae. (A) EAEC 042 and its aafA and aafB mutants were added to 24-well plates coated with 5 μg/ml of CK8 or BSA, and the levels of bacteria bound were determined by plating. (B to D) EAEC strain 042 (B), AAF/II purified fimbriae (C), or AafA-dsc protein (D) was added to CK8- and BSA-coated wells, and binding was detected by ELISA. The bars represent the means of the results from three experiments, with the error bars indicating standard deviations. *, significantly different from BSA-coated control (P < 0.05). **, significantly different from EAEC 042 and its aafB mutant binding to CK8 (P < 0.05). Lm, laminin.
CK8 gene knockdown reduces EAEC binding to epithelial cells.
To evaluate the potential involvement of CK8 as an EAEC receptor in epithelial cells, we knocked down the CK8 expression employing specific siRNA in HEp-2 cells. Using this procedure, we observed a substantial reduction of CK8 expression as determined by a Western blotting assay (Fig. 3A). This was confirmed by an immunofluorescence assay, where recognition of CK8 protein on the surface of ck8 knockdown cells was less intense than that observed in control cells (Fig. 3B).
FIG 3.
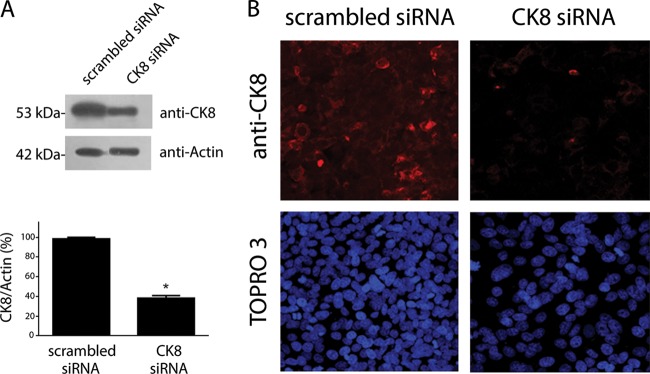
ck8 gene knockdown. HEp-2 cells were treated with scrambled or CK8 siRNA. The reduction of CK8 expression was determined by Western blot analysis (A) and visualized by immunofluorescence assays (B). *, significantly different from control treatment (P < 0.05).
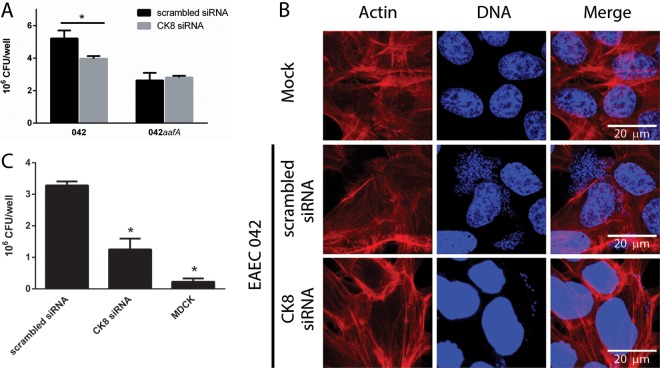
The adhesion of EAEC 042 to HEp-2 cells treated with ck8 siRNA was significantly reduced compared with adhesion to cells treated with scrambled siRNA (Fig. 4A). No difference in the adherence of the aafA mutant strain was observed in HEp-2 cells treated with ck8 or with scrambled siRNA (Fig. 4A). Additionally, an immunofluorescence assay of HEp-2 cells treated or not with ck8 siRNA infected with the EAEC strain was performed. HEp-2 cells treated with ck8 siRNA exhibited a reduced amount of adherent bacteria compared to the number of bacteria found on control cells (Fig. 4B).
FIG 4.
ck8 gene knockdown reduces EAEC binding to epithelial cells. HEp-2 cells treated with scrambled or CK8 siRNA (A and C) and MDCK cells (C) were infected by EAEC 042 or EAEC 042aafA. Numbers of adherent bacteria were determined by counting of the CFU. *, significantly different from adhesion to HEp-2 with the scrambled siRNA (P < 0.05). (B) Immunofluorescence of HEp-2 cells treated with scrambled or CK8 siRNA and infected with EAEC 042. Actin was visualized in red (TRITC-phalloidin), and bacterial or human DNA was visualized in blue (TO-PRO-3).
We also evaluated the adhesion of strain 042 to MCDK cells, a cell line that does not express CK8 on the cell surface (13). In this assay, a significant reduction in the EAEC strain 042 binding to MDCK cells was observed compared to the adherence observed to HEp-2 cells (Fig. 4C).
Participation of fibronectin, laminin, and CK8 in the EAEC adherence to epithelial cells.
We have previously shown that the ECM protein fibronectin augments the binding of 042 to epithelial cells, a process that is mediated by the AAF/II fimbriae (9). Considering the results obtained in that study, we evaluated the roles of fibronectin, laminin, and CK8 in the adherence of EAEC strain 042 to HEp-2 cells. Before infection with 042, HEp-2 cells were preincubated with antibodies against CK8 alone (Fig. 5A), CK8/laminin (Fig. 5B), and CK8/laminin/fibronectin (Fig. 5C). Compared to the observed adherence to cells incubated with the isotype control, a significant reduction in the amount of adherent bacteria was found in all cases.
FIG 5.
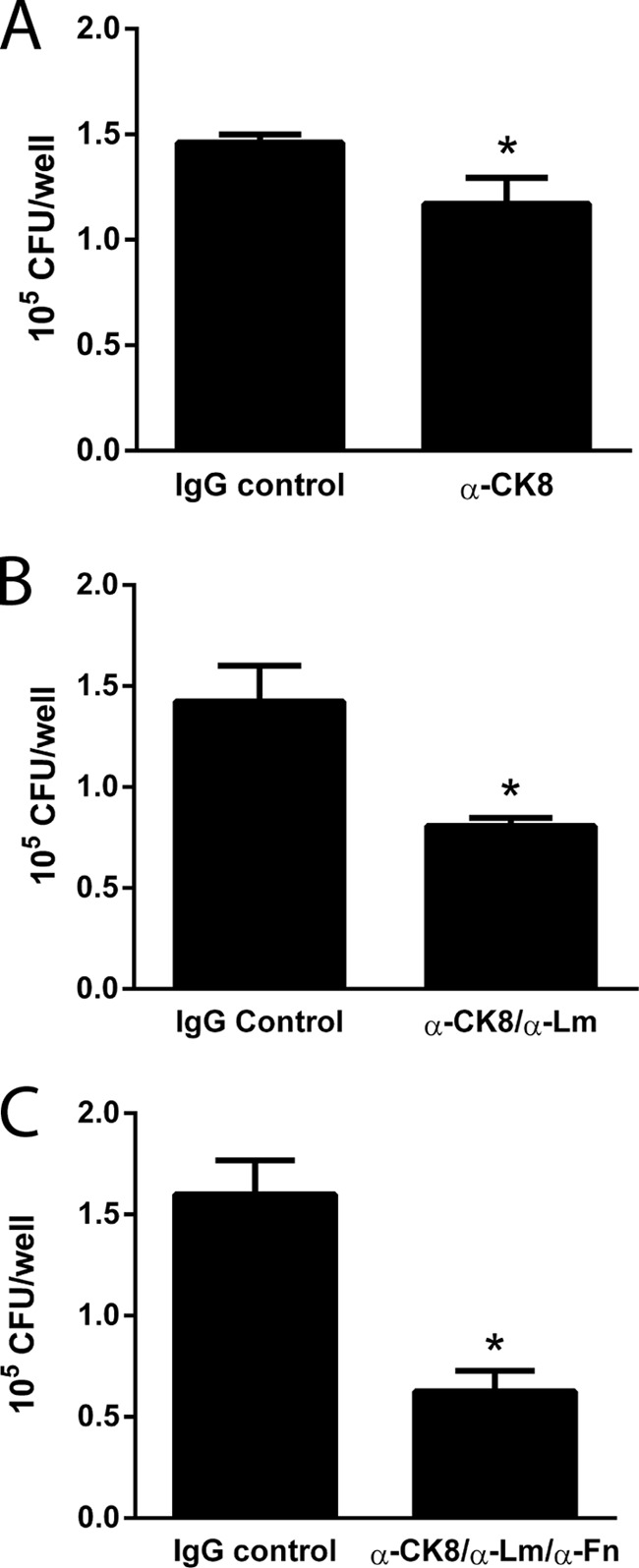
Participation of fibronectin, laminin, and CK8 in the EAEC adherence to epithelial cells. Numbers of adherent bacteria after infection by EAEC 042 of HEp-2 cells preincubated with DMEM supplemented with 1 μg of isotype IgG control or anti-CK8 antibodies (A), 2 μg of isotype IgG control or anti-CK8/laminin antibodies (1 μg each) (B), and 3 μg of isotype IgG control or anti-CK8/laminin/fibronectin antibodies (1 μg each) (C) are indicated. Numbers of adherent bacteria were determined by counting of the CFU. *, significantly different from control treatment (P < 0.05).
DISCUSSION
Adherence to intestinal cells is a key step in colonization by enteric pathogens. For EAEC, adherence to intestinal cells is attributed to the AAF fimbriae; however, the cell receptors that allow AAF recognition are not fully characterized. In the search of potential receptors for the EAEC, our group determined that EAEC strain 042 could adhere to ECM proteins laminin, type IV collagen, and fibronectin, a process mediated by the AAF/II fimbriae (9). Soon after, using an AAF/II-producing EAEC, four proteins were identified as potential cell receptors: fibronectin, EGFR, TSP1, and GRP-94 (10). Intestinal cells preincubated with antibodies against these molecules showed reduced but not abolished adherence of EAEC to cells. These data supported the idea of a role of these proteins as potential receptors for EAEC but also suggested the involvement of another receptor(s) in the recognition of this pathogen by intestinal cells. We here identify novel potential receptors for the AAF/II fimbriae. T84 cells were incubated with a purified and refolded AafA protein (AafA-dsc), and cross-linked complexes were captured by immunoprecipitation using antibodies against AafA-dsc protein. Following MALDI-TOF analysis, we identified two proteins: the β-subunit of laminin and cytokeratin 8.
Laminins are a family of glycoproteins that provide the structural scaffolding of basement membranes in almost every animal tissue. Each laminin is a heterotrimer assembled from α, β, and γ chain subunits (14). As mentioned above, we described laminin as a potential receptor for AAF/II (9). To corroborate this observation, we evaluated the adherence of the purified AAF/II fimbriae to laminin, showing a dose-dependent binding and supporting the idea of a role of this ECM protein as a potential receptor for EAEC (Fig. 2C).
Keratins or cytokeratins are the largest subgroup of intermediate filament proteins and are important constituents of the cell cytoskeleton. These proteins are important for the mechanical stability and integrity of epithelial cells and tissues. To date, 19 human cytokeratins have been identified, and all of them are expressed in epithelial cells. The composition of the cytokeratins differs among tissues, but CK8, CK18, and CK19 are common constituents of the intestinal epithelia (15). Although CK8 is an intermediate filament, it has also been found at the surface of several cell lines (16). In this work, we found that EAEC strain 042 and the purified AAF/II fimbriae have the ability to bind purified human CK8 protein, a binding that is mediated by the major subunit of AAF/II, the AafA protein (Fig. 2). In addition, attenuation in the expression of the CK8-encoding gene in HEp-2 cells ameliorated adherence of EAEC; adherence of strain 042 to MDCK cells, which have low expression of CK8, was significantly reduced compared to HEp-2 adherence (Fig. 3). All these data supported the idea of a role of CK8 as a potential receptor for EAEC.
The ability to bind to cytokeratins has been previously described for other pathogens. Porphyromonas gingivalis fimbriae bind to cytokeratin I present on epithelial cells (17). The Srr-1 surface adhesin of Streptococcus agalactiae binds to keratin 4 and promotes the adherence of the bacteria to HEp-2 cells (18). Binding to CK8 was reported for Streptococcus gallolyticus, Enterococcus faecalis, Staphylococcus aureus, Lactococcus lactis, and Streptococcus pyogenes (19, 20, 21). These observations suggest that binding to cytokeratin might be important for colonization at the epithelium, a characteristic shared by several pathogens, including EAEC. A recent report revealed that Pet toxin from EAEC has the ability to bind CK8 on epithelial cells, and such binding may be essential for its cytotoxic effects (13).
Several studies on CK8 expression have shown that this protein is overexpressed in the intestinal tissue of patients with cancer compared to normal intestinal cells (22). This observation could be a limitation in our study, considering the nature of the epithelial cells used. However, HEp-2 cells and T84 cells have been extensively used in bacterial pathogenesis studies. It would be interesting to evaluate in the future the contribution of CK8 in nontumor cells. Of course, it is possible that early adherence of EAEC to the intestinal epithelium may depend upon other proteins and may be followed by induction of a proinflammatory state, leading in turn to increased surface expression of CK8 and increased or stronger cell adherence. This hypothesis is under investigation. Of note, we have previously observed adherence of EAEC to fibronectin, secretion of which may also be enhanced in inflamed tissue (M. J. Farfan, unpublished data).
In conclusion, we report a novel potential receptor for EAEC, CK8 protein, which might help elucidate the complex molecular mechanisms involved in the fimbria-dependent adherence of EAEC to epithelial cells.
ACKNOWLEDGMENTS
This work was supported by grant 1120809 from FONDECYT to M.J.F. F.N.-G. was supported by grant 128490 from CONACYT. Work in the Nataro laboratory was supported by grant AI-033096 from the National Institutes of Health to J.P.N.
Footnotes
Published ahead of print 10 February 2014
REFERENCES
- 1.Huang DB, Mohanty A, DuPont HL, Okhuysen PC, Chiang T. 2006. A review of an emerging enteric pathogen: enteroaggregative Escherichia coli. J. Med. Microbiol. 55:1303–1311. 10.1099/jmm.0.46674-0 [DOI] [PubMed] [Google Scholar]
- 2.Rasko DA, Webster DR, Sahl JW, Bashir A, Boisen N, Scheutz F, Paxinos EE, Sebra R, Chin CS, Iliopoulos D, Klammer A, Peluso P, Lee L, Kislyuk AO, Bullard J, Kasarskis A, Wang S, Eid J, Rank D, Redman JC, Steyert SR, Frimodt-Møller J, Struve C, Petersen AM, Krogfelt KA, Nataro JP, Schadt EE, Waldor MK. 2011. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N. Engl. J. Med. 365:709–717. 10.1056/NEJMoa1106920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington SM, Dudley EG, Nataro JP. 2006. Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol. Lett. 254:12–18. 10.1111/j.1574-6968.2005.00005.x [DOI] [PubMed] [Google Scholar]
- 4.Bernier C, Gounon P, Le Bouguenec C. 2002. Identification of an aggregative adhesion fimbria (AAF) type III-encoding operon in enteroaggregative Escherichia coli as a sensitive probe for detecting the AAF-encoding operon family. Infect. Immun. 70:4302–4311. 10.1128/IAI.70.8.4302-4311.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boisen N, Struve C, Scheutz F, Krogfelt KA, Nataro JP. 2008. New adhesin of enteroaggregative Escherichia coli related to the Afa/Dr/AAF family. Infect. Immun. 76:3281–3292. 10.1128/IAI.01646-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czeczulin JR, Balepur S, Hicks S, Phillips A, Hall R, Kothary MH, Navarro-Garcia F, Nataro JP. 1997. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect. Immun. 65:4135–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nataro JP, Yikang D, Giron JA, Savarino SJ, Kothary MH, Hall R. 1993. Aggregative adherence fimbria I expression in enteroaggregative Escherichia coli requires two unlinked plasmid regions. Infect. Immun. 61:1126–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington SM, Strauman MC, Abe CM, Nataro JP. 2005. Aggregative adherence fimbriae contribute to the inflammatory response of epithelial cells infected with enteroaggregative Escherichia coli. Cell. Microbiol. 7:1565–1578. 10.1111/j.1462-5822.2005.00588.x [DOI] [PubMed] [Google Scholar]
- 9.Farfan MJ, Inman KG, Nataro JP. 2008. The major pilin subunit of the AAF/II fimbriae from enteroaggregative Escherichia coli mediates binding to extracellular matrix proteins. Infect. Immun. 76:4378–4384. 10.1128/IAI.00439-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konar M, Sachin O, Priya A, Ghosh S. 2012. Identification of key proteins of cultured human intestinal cells involved in interaction with enteroaggregative Escherichia coli. FEMS Immunol. Med. Microbiol. 66:177–190. 10.1111/j.1574-695X.2012.00998.x [DOI] [PubMed] [Google Scholar]
- 11.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Garnett JA, Matthews S. 2011. Crystallization and initial crystallographic analysis of AafA: the major adhesive subunit of the enteroaggregative Escherichia coli AAF/II pilus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 67:454–456. 10.1107/S1744309111001412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nava-Acosta R, Navarro-Garcia F. 2013. Cytokeratin 8 is an epithelial cell receptor for pet, a cytotoxic serine protease autotransporter of enterobacteriaceae. mBio 4:e00838–13. 10.1128/mBio.00838-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colognato H, Yurchenco PD. 2000. Form and function: the laminin family of heterotrimers. Dev. Dyn. 218:213–234. [DOI] [PubMed] [Google Scholar]
- 15.Ku NO, Toivola DM, Zhou Q, Tao GZ, Zhong B, Omary MB. 2004. Studying simple epithelial keratins in cells and tissues. Methods Cell Biol. 78:489–517. 10.1016/S0091-679X(04)78017-6 [DOI] [PubMed] [Google Scholar]
- 16.Hembrough TA, Vasudevan J, Allietta MM, Glass WF, II, Gonias SL. 1995. A cytokeratin 8-like protein with plasminogen-binding activity is present on the external surfaces of hepatocytes, HepG2 cells and breast carcinoma cell lines. J. Cell Sci. 108:1071–1082 [DOI] [PubMed] [Google Scholar]
- 17.Sojar HT, Sharma A, Genco RJ. 2002. Porphyromonas gingivalis fimbriae bind to cytokeratin of epithelial cells. Infect. Immun. 70:96–101. 10.1128/IAI.70.1.96-101.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samen U, Eikmanns BJ, Reinscheid DJ, Borges F. 2007. The surface protein Srr-1 of Streptococcus agalactiae binds human keratin 4 and promotes adherence to epithelial HEp-2 cells. Infect. Immun. 75:5405–5414. 10.1128/IAI.00717-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boleij A, Laarakkers CM, Gloerich J, Swinkels DW, Tjalsma H. 2011. Surface-affinity profiling to identify host-pathogen interactions. Infect. Immun. 79:4777–4783. 10.1128/IAI.05572-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haim M, Trost A, Maier CJ, Achatz G, Feichtner S, Hintner H, Bauer JW, Onder K. 2010. Cytokeratin 8 interacts with clumping factor B: a new possible virulence factor target. Microbiology 156:3710–3721. 10.1099/mic.0.034413-0 [DOI] [PubMed] [Google Scholar]
- 21.Tamura GS, Nittayajarn A. 2000. Group B streptococci and other gram-positive cocci bind to cytokeratin 8. Infect. Immun. 68:2129–2134. 10.1128/IAI.68.4.2129-2134.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polley AC, Mulholland F, Pin C, Williams EA, Bradburn DM, Mills SJ, Mathers JC, Johnson IT. 2006. Proteomic analysis reveals field-wide changes in protein expression in the morphologically normal mucosa of patients with colorectal neoplasia. Cancer Res. 66:6553–6562. 10.1158/0008-5472.CAN-06-0534 [DOI] [PubMed] [Google Scholar]