Abstract
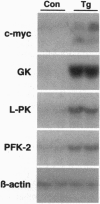
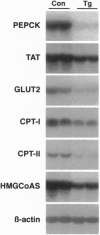
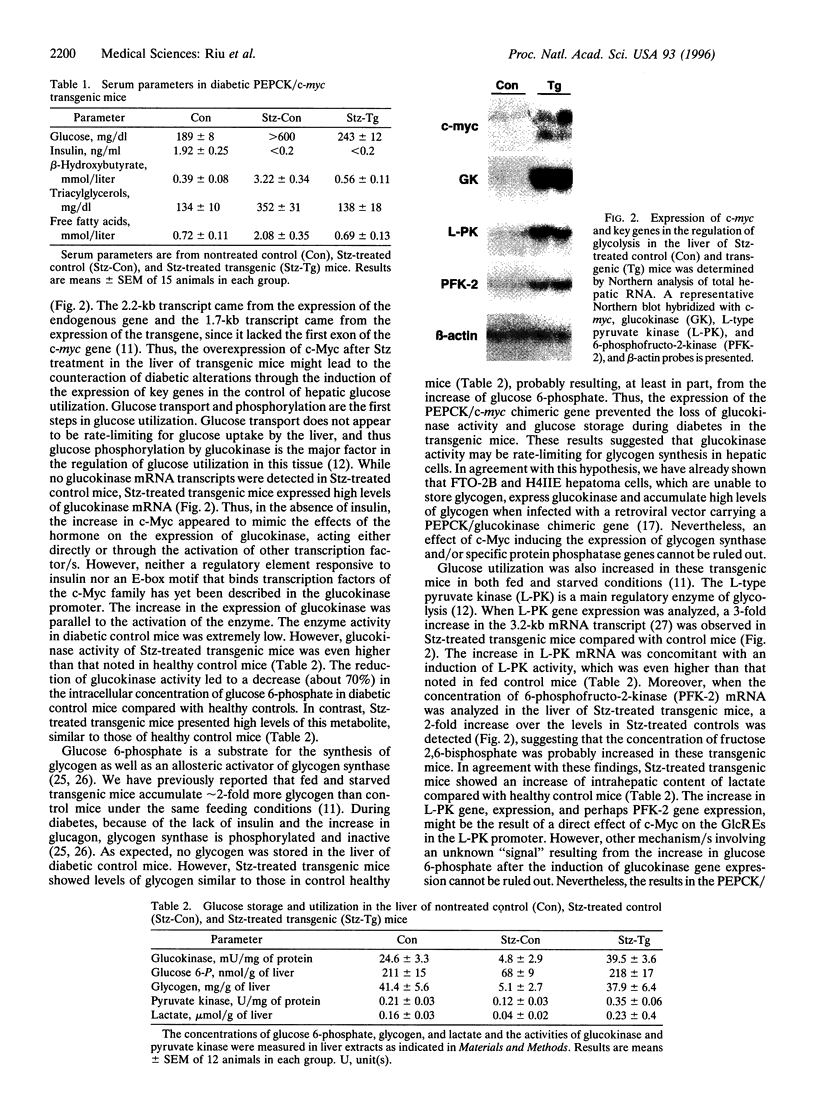
Recent studies have demonstrated that the overexpression of the c-myc gene in the liver of transgenic mice leads to an increase in both utilization and accumulation of glucose in the liver, suggesting that c-Myc transcription factor is involved in the control of liver carbohydrate metabolism in vivo. To determine whether the increase in c-Myc might control glucose homeostasis, an intraperitoneal glucose tolerance test was performed. Transgenic mice showed lower levels of blood glucose than control animals, indicating that the overexpression of c-Myc led to an increase of blood glucose disposal by the liver. Thus, the increase in c-Myc might counteract diabetic hyperglycemia. In contrast to control mice, transgenic mice treated with streptozotocin showed normalization of concentrations of blood glucose, ketone bodies, triacylglycerols and free fatty acids in the absence of insulin. These findings resulted from the normalization of liver metabolism in these animals. While low glucokinase activity was detected in the liver of diabetic control mice, high levels of both glucokinase mRNA and enzyme activity were noted in the liver of streptozotocin-treated transgenic mice, which led to an increase in intracellular levels of glucose 6-phosphate and glycogen. The liver of these mice also showed an increase in pyruvate kinase activity and lactate production. Furthermore, normalization of both the expression of genes involved in the control of gluconeogenesis and ketogenesis and the production of glucose and ketone bodies was observed in streptozotocin-treated transgenic mice. Thus, these results suggested that c-Myc counteracted diabetic alterations through its ability to induce hepatic glucose uptake and utilization and to block the activation of gluconeogenesis and ketogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayté J., Gil-Gómez G., Haro D., Marrero P. F., Hegardt F. G. Rat mitochondrial and cytosolic 3-hydroxy-3-methylglutaryl-CoA synthases are encoded by two different genes. Proc Natl Acad Sci U S A. 1990 May;87(10):3874–3878. doi: 10.1073/pnas.87.10.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergot M. O., Diaz-Guerra M. J., Puzenat N., Raymondjean M., Kahn A. Cis-regulation of the L-type pyruvate kinase gene promoter by glucose, insulin and cyclic AMP. Nucleic Acids Res. 1992 Apr 25;20(8):1871–1877. doi: 10.1093/nar/20.8.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals N., Roca N., Guerrero M., Gil-Gómez G., Ayté J., Ciudad C. J., Hegardt F. G. Regulation of the expression of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene. Its role in the control of ketogenesis. Biochem J. 1992 Apr 1;283(Pt 1):261–264. doi: 10.1042/bj2830261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cuif M. H., Cognet M., Boquet D., Tremp G., Kahn A., Vaulont S. Elements responsible for hormonal control and tissue specificity of L-type pyruvate kinase gene expression in transgenic mice. Mol Cell Biol. 1992 Nov;12(11):4852–4861. doi: 10.1128/mcb.12.11.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuif M. H., Porteu A., Kahn A., Vaulont S. Exploration of a liver-specific, glucose/insulin-responsive promoter in transgenic mice. J Biol Chem. 1993 Jul 5;268(19):13769–13772. [PubMed] [Google Scholar]
- Esser V., Britton C. H., Weis B. C., Foster D. W., McGarry J. D. Cloning, sequencing, and expression of a cDNA encoding rat liver carnitine palmitoyltransferase I. Direct evidence that a single polypeptide is involved in inhibitor interaction and catalytic function. J Biol Chem. 1993 Mar 15;268(8):5817–5822. [PubMed] [Google Scholar]
- Girard J., Perdereau D., Foufelle F., Prip-Buus C., Ferré P. Regulation of lipogenic enzyme gene expression by nutrients and hormones. FASEB J. 1994 Jan;8(1):36–42. doi: 10.1096/fasebj.8.1.7905448. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Schmid W., Schütz G. Transcriptional activation of the rat liver tyrosine aminotransferase gene by cAMP. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6637–6641. doi: 10.1073/pnas.81.21.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen H. M., Strähle U., Gloss B., Stewart F., Schmid W., Boshart M., Miksicek R., Schütz G. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell. 1987 Apr 10;49(1):29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- Kato G. J., Dang C. V. Function of the c-Myc oncoprotein. FASEB J. 1992 Sep;6(12):3065–3072. doi: 10.1096/fasebj.6.12.1521738. [DOI] [PubMed] [Google Scholar]
- Larner J. Insulin and the stimulation of glycogen synthesis. The road from glycogen structure to glycogen synthase to cyclic AMP-dependent protein kinase to insulin mediators. Adv Enzymol Relat Areas Mol Biol. 1990;63:173–231. doi: 10.1002/9780470123096.ch3. [DOI] [PubMed] [Google Scholar]
- Le Cam A., Guillouzo A., Freychet P. Ultrastructual and biochemical studies of isolated adult rat hepatocytes prepared under hypoxic conditions. Cryopreservation of hepatocytes. Exp Cell Res. 1976 Mar 15;98(2):382–395. doi: 10.1016/0014-4827(76)90448-1. [DOI] [PubMed] [Google Scholar]
- Liu J. S., Park E. A., Gurney A. L., Roesler W. J., Hanson R. W. Cyclic AMP induction of phosphoenolpyruvate carboxykinase (GTP) gene transcription is mediated by multiple promoter elements. J Biol Chem. 1991 Oct 5;266(28):19095–19102. [PubMed] [Google Scholar]
- Liu M. L., Gibbs E. M., McCoid S. C., Milici A. J., Stukenbrok H. A., McPherson R. K., Treadway J. L., Pessin J. E. Transgenic mice expressing the human GLUT4/muscle-fat facilitative glucose transporter protein exhibit efficient glycemic control. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11346–11350. doi: 10.1073/pnas.90.23.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Thompson K. S., Towle H. C. Carbohydrate regulation of the rat L-type pyruvate kinase gene requires two nuclear factors: LF-A1 and a member of the c-myc family. J Biol Chem. 1993 Jun 15;268(17):12787–12795. [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part I. Myc. Genes Dev. 1990 Dec;4(12A):2025–2035. doi: 10.1101/gad.4.12a.2025. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Bossone S. A., Patel A. J. myc function and regulation. Annu Rev Biochem. 1992;61:809–860. doi: 10.1146/annurev.bi.61.070192.004113. [DOI] [PubMed] [Google Scholar]
- Marshall B. A., Ren J. M., Johnson D. W., Gibbs E. M., Lillquist J. S., Soeller W. C., Holloszy J. O., Mueckler M. Germline manipulation of glucose homeostasis via alteration of glucose transporter levels in skeletal muscle. J Biol Chem. 1993 Sep 5;268(25):18442–18445. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992 Oct 30;258(5083):766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- Messina J. L. Inhibition and stimulation of c-myc gene transcription by insulin in rat hepatoma cells. Insulin alters the intragenic pausing of c-myc transcription. J Biol Chem. 1991 Sep 25;266(27):17995–18001. [PubMed] [Google Scholar]
- O'Brien R. M., Lucas P. C., Forest C. D., Magnuson M. A., Granner D. K. Identification of a sequence in the PEPCK gene that mediates a negative effect of insulin on transcription. Science. 1990 Aug 3;249(4968):533–537. doi: 10.1126/science.2166335. [DOI] [PubMed] [Google Scholar]
- Oka Y., Asano T., Shibasaki Y., Lin J. L., Tsukuda K., Akanuma Y., Takaku F. Increased liver glucose-transporter protein and mRNA in streptozocin-induced diabetic rats. Diabetes. 1990 Apr;39(4):441–446. doi: 10.2337/diab.39.4.441. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., Granner D. K. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- Printz R. L., Magnuson M. A., Granner D. K. Mammalian glucokinase. Annu Rev Nutr. 1993;13:463–496. doi: 10.1146/annurev.nu.13.070193.002335. [DOI] [PubMed] [Google Scholar]
- Roach P. J. Control of glycogen synthase by hierarchal protein phosphorylation. FASEB J. 1990 Sep;4(12):2961–2968. [PubMed] [Google Scholar]
- Sasaki K., Cripe T. P., Koch S. R., Andreone T. L., Petersen D. D., Beale E. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984 Dec 25;259(24):15242–15251. [PubMed] [Google Scholar]
- Shih H. M., Towle H. C. Definition of the carbohydrate response element of the rat S14 gene. Evidence for a common factor required for carbohydrate regulation of hepatic genes. J Biol Chem. 1992 Jul 5;267(19):13222–13228. [PubMed] [Google Scholar]
- Shih H., Towle H. C. Definition of the carbohydrate response element of the rat S14 gene. Context of the CACGTG motif determines the specificity of carbohydrate regulation. J Biol Chem. 1994 Mar 25;269(12):9380–9387. [PubMed] [Google Scholar]
- Stanton L. W., Fahrlander P. D., Tesser P. M., Marcu K. B. Nucleotide sequence comparison of normal and translocated murine c-myc genes. Nature. 1984 Aug 2;310(5976):423–425. doi: 10.1038/310423a0. [DOI] [PubMed] [Google Scholar]
- Valera A., Bosch F. Glucokinase expression in rat hepatoma cells induces glucose uptake and is rate limiting in glucose utilization. Eur J Biochem. 1994 Jun 1;222(2):533–539. doi: 10.1111/j.1432-1033.1994.tb18895.x. [DOI] [PubMed] [Google Scholar]
- Valera A., Fillat C., Costa C., Sabater J., Visa J., Pujol A., Bosch F. Regulated expression of human insulin in the liver of transgenic mice corrects diabetic alterations. FASEB J. 1994 Apr 1;8(6):440–447. doi: 10.1096/fasebj.8.6.8168695. [DOI] [PubMed] [Google Scholar]
- Valera A., Pelegrin M., Asins G., Fillat C., Sabater J., Pujol A., Hegardt F. G., Bosch F. Overexpression of mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase in transgenic mice causes hepatic hyperketogenesis. J Biol Chem. 1994 Mar 4;269(9):6267–6270. [PubMed] [Google Scholar]
- Valera A., Pujol A., Gregori X., Riu E., Visa J., Bosch F. Evidence from transgenic mice that myc regulates hepatic glycolysis. FASEB J. 1995 Aug;9(11):1067–1078. doi: 10.1096/fasebj.9.11.7649406. [DOI] [PubMed] [Google Scholar]
- Valera A., Rodriguez-Gil J. E., Bosch F. Vanadate treatment restores the expression of genes for key enzymes in the glucose and ketone bodies metabolism in the liver of diabetic rats. J Clin Invest. 1993 Jul;92(1):4–11. doi: 10.1172/JCI116580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaulont S., Kahn A. Transcriptional control of metabolic regulation genes by carbohydrates. FASEB J. 1994 Jan;8(1):28–35. doi: 10.1096/fasebj.8.1.8299888. [DOI] [PubMed] [Google Scholar]
- Woeltje K. F., Esser V., Weis B. C., Cox W. F., Schroeder J. G., Liao S. T., Foster D. W., McGarry J. D. Inter-tissue and inter-species characteristics of the mitochondrial carnitine palmitoyltransferase enzyme system. J Biol Chem. 1990 Jun 25;265(18):10714–10719. [PubMed] [Google Scholar]