Abstract
Passive protection, the administration of antibodies to prevent infection, has garnered significant interest in recent years as a potential prophylactic countermeasure to decrease the prevalence of hospital-acquired infections. Pili, polymerized protein structures covalently anchored to the peptidoglycan wall of many Gram-positive pathogens, are ideal targets for antibody intervention, given their importance in establishing infection and their accessibility to antibody interactions. In this work, we demonstrated that a monoclonal antibody to the major component of Enterococcus faecalis pili, EbpC, labels polymerized pilus structures, diminishes biofilm formation, and significantly prevents the establishment of a rat endocarditis infection. The effectiveness of this anti-EbpC monoclonal provides strong evidence in support of its potential as a preventative. In addition, after radiolabeling, this monoclonal identified the site of enterococcal infection, providing a rare example of molecularly specific imaging of an established bacterial infection and demonstrating the versatility of this agent for use in future diagnostic and therapeutic applications.
INTRODUCTION
Enterococci, once regarded as commensal organisms of intestinal origin with minimal clinical importance, have emerged as significant nosocomial pathogens causing endocarditis as well as bloodstream, wound, and urinary tract infections (1, 2). Recent data cite enterococci as accounting for 12% of all hospital infections, becoming the second most commonly isolated nosocomial pathogen behind staphylococci (3). The endocarditis and biofilm-associated pili (Ebp) of Enterococcus faecalis have been extensively studied for their role as virulence factors important for establishing infection. As with all orthologous pilus structures in other Gram-positive pathogens, including Corynebacterium diphtheriae, Actinomyces naeslundii, Streptococcus pyogenes (group A streptococcus [GAS]), Streptococcus agalactiae (group B streptococcus [GBS]), and Streptococcus pneumoniae (4), the Ebp pili are terminally anchored to the peptidoglycan surface of the bacterium, allowing pili to readily interact with the external environment (5). EbpA, EbpB, and EbpC are the three structural pilin components which make up the pilus unit, with EbpC being the major component of the shaft (6, 7).
As its name suggests, the presence of Ebp has been associated with enterococcal infection, specifically, with the ability of enterococci to form a biofilm or establish endocarditis infection. Biofilms, surface-adherent cells covered in a matrix of polysaccharide, DNA, and/or protein, provide protection for the microbe, making them more resistant to the immune response or antimicrobial attack. However, high-titer antibody responses to all three proteins of the Ebp have been found in patients with E. faecalis endocarditis, demonstrating that pili are readily expressed at some point during infection and can be recognized by the host immune system (8). Biofilms are associated with native valve endocarditis, in addition to assisting with the establishment of infection on artificial surfaces of a number of medical devices, such as catheters and artificial heart valves (9). In endocarditis, enterococci either adhere to the heart's valve or inner endothelial lining directly or adhere to a sterile platelet/thrombin vegetation to establish infection. The resulting infection is a cumulative attachment of microbes, fibrin, platelets, and other host cells to the infection site, which results in significant patient mortality. Adherence assays have demonstrated that disruption of the ebpA, ebpB, or ebpC gene (6) or deletion of genes which regulate the expression of Ebp (10) inhibits the ability of E. faecalis to establish a biofilm on a polystyrene surface. Furthermore, disruption of ebp genes has been shown to significantly reduce the ability of E. faecalis to form vegetations in a rat endocarditis model, which provides direct evidence of the importance of the pili in endocarditis (6). As with many gene disruption experiments in microbes evaluating their effect on pathogenicity, a logical next and often more challenging step is to identify an agent which, upon administration, can similarly cause attenuation.
Given the huge success of recombinant IgG monoclonal antibody (MAb) therapy in cancer and inflammatory/autoimmune disease (11, 12) and the increasing battle against the emergence of multiantibiotic-resistant bacteria, the use of MAb drugs for passive protection against Gram-positive nosocomial pathogens has gained considerable interest in recent years (13–20). We have previously described a panel of MAbs against the major pilin protein EbpC, from which MAb 69 was selected on the basis of its affinity to recombinant EbpC antigen and its ability to recognize surface-displayed EbpC (10). Here we demonstrate the ability of this MAb to bind to the polymer structure of the pili, functionally inhibit the establishment of E. faecalis adhesion in vitro, prevent endocarditis infection in vivo, and, using positron emission tomography (PET) molecular imaging studies of infected animals, validate that MAb 69 directly targets and recognizes pili at the site of endocarditis infection. These results identify Ebp to be a viable MAb target for preventing infection by E. faecalis. In addition, we demonstrate that our MAb localizes to the established infection. Such localization may provide a means to readily monitor drug treatment in animal models of established infection and could ultimately allow identification (via imaging) and, possibly, treatment of patient populations through MAb therapy or MAb-targeted payload delivery.
MATERIALS AND METHODS
Materials and strains.
The bacterial strains used in this study included E. faecalis strains OG1RF (wild type) and OG1RF ΔebpABC, which have been previously described (6, 10). Recombinant EbpC (rEbpC) protein was produced as previously described (9).
TEM and immunogold labeling.
Transmission electron microscopy (TEM) experiments were carried out as previously described (21). Briefly, overnight-cultured OG1RF was inoculated into 20 ml brain heart infusion (BHI) broth (Gibco) at a starting optical density at 600 nm (OD600) of 0.05 and harvested at mid-log phase. One OD600 equivalent of cells was washed once with 0.1 M NaCl before being resuspended in 1 ml phosphate-buffered saline (PBS). Immunogold labeling was performed using anti-EbpC MAb 69 (10 μg/ml) (10), followed by 12-nm-diameter gold bead-conjugated donkey anti-mouse IgG (1:20; catalog no. 115-205-071; Jackson ImmunoResearch Laboratories), using previously described methods (22). Samples were viewed in a JEOL 1400 transmission electron microscope.
In vitro biofilm inhibition assay.
Biofilm procedures were carried out as previously described (10). OG1RF cells cultured overnight in tryptic soy broth containing 0.25% glucose (TSBG) were diluted 1:100 in TSBG with serial dilutions of anti-EbpC MAb 69 or control IgG (mouse gamma globulin; catalog no. 015-000-002; Jackson ImmunoResearch Laboratories) in polystyrene 96-well microtiter plates for 24 h at 37°C. The mouse gamma globulin utilized as the control IgG was dialyzed in PBS and evaluated to confirm minimal cross-reactivity to E. faecalis surface antigens by flow cytometry (data not shown). Plates were washed with PBS, fixed with Bouin's fixative, and stained with 1% crystal violet (CV). The CV-stained cells were solubilized with 80:20 ethanol-acetone, and absorbance values were determined via a Multiskan EX plate reader and 595-nm filter.
Rat experimental endocarditis model.
Aortic valve endocarditis was produced in rats by following our previously published methods (6, 23, 24). Briefly, male Sprague-Dawley rats (weight, ∼200 g) were used for induction of endocarditis by placement (while the rats were under 2.5% isoflurane anesthesia) through a small incision of a sterile polyethylene catheter, which was advanced ∼4 cm into the left ventricle. The catheter was heat sealed and left in place during the course of passive protection and molecular imaging experiments.
Passive protection of infected animals.
For passive immunization, rats were injected intravenously (i.v.) through the tail vein with 2 mg/kg of body weight of MAb 69 or control IgG at 24 h postcatheterization and 1 h prior to bacterial inoculation. At 25 h postcatheterization, the animals were inoculated (i.v. in the tail vein) using ≥9 × 107 CFU/rat of E. faecalis OG1RF bacteria (an inoculum that represented a dose greater than or equal to the 75% infectious dose [ID75] premixed in saline) grown in brain heart infusion plus 40% serum (BHIS), and the animals were euthanized at 24 h postinfection. Two independent experiments (n = 15 each) were performed, and the results of the two experiments were combined.
Molecular imaging of MAb 69 targeting in infected animals.
For molecular imaging of MAb 69 targeting in infected animals, the route of bacterial inoculation in rats was intracardiac (via catheter), and the inoculum was administered approximately 15 min after catheter placement. Animals were inoculated with 1 × 107 CFU/rat of E. faecalis OG1RF grown in BHIS. Animals were divided into two groups that received (i) radiolabeled MAb 69 to EbpC or (ii) a previously described radiolabeled control MAb (25) which demonstrated no reactivity to E. faecalis surface antigens (data not shown). In addition, each agent was administered to one catheterized/noninfected rat to control for binding to the catheter or the site of catheter damage.
Radiolabeling and characterization of imaging agents.
1,4,7,10-Tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (DOTA) conjugation was performed by reacting 1 mg of MAb 69 or the control murine antibody with a 20-fold molar excess of DOTA–N-hydroxysuccinimide (NHS) (Macrocyclics, Dallas, TX) in sodium phosphate dibasic buffer (0.1 M, pH 8.5). The reaction mixture was kept at 4°C and stirred for 4 h or overnight. The resulting immunoconjugates were purified with a Zeba spin desalting column, collected in PBS, and stored at −80°C. The number of DOTA moieties per MAb molecule was calculated according to standardized methods (26), and potency enzyme-linked immunosorbent assay (ELISAs) were used to verify that the chemical modifications did not significantly alter biological activity. Radiolabeling with 64Cu was performed as previously described (27). Briefly, 37 MBq of 64Cu was added to 100 μg of each immunoconjugate in 0.1 M sodium acetate (pH 6), and the mixture was heated at 40°C for 1 h. Reactions were quenched with EDTA and purified with Zeba spin desalting columns. Labeling efficiency and purity were determined to be >85% by radio-thin-layer chromatography (radio-TLC) using an AR-2000 scanner (Bioscan). Radiochemical purity was >95%, as shown by radio-high-pressure liquid chromatography analysis with a TSK gel G3000SW (5 μm) column and a mobile phase of 90% buffer A (0.1 M sodium phosphate buffer [pH 7.3]) and 10% buffer B (CH3CN) (isocratic) at a flow rate of 1 ml/min. Radiolabeling and characterization of the control MAb were performed using identical procedures.
To confirm the biological activity of the prepared imaging agents, ELISA-based potency experiments were performed. ELISA procedures utilizing rEbpC and recombinant human cell adhesion molecule/Fc (rHuEpCAM/Fc) have been previously described (25). Briefly, rEbpC or rHuEpCAM/Fc was coated onto Microlon 600 ELISA plates (Greiner Bio-One). After a brief blocking step, the samples were applied at the concentrations indicated below. Following the primary antibody binding, goat anti-mouse IgG (Fc) horseradish peroxidase (HRP)-labeled secondary antibody was added to detect bound mouse IgG. Tetramethylbenzidine (TMB) substrate was used to develop the HRP. Absorbance values were determined via a Multiskan EX plate reader and 450-nm filter. For investigative purposes in the ELISA evaluation, mock radiolabeling procedures were performed as described above without the addition of the 64Cu isotope.
PET/CT imaging.
All animal studies were performed in accordance with the standards of the University of Texas Health Science Center—Houston (Houston, TX), Department of Comparative Medicine, and the Center for Molecular Imaging after review and approval of the protocol by the Institutional Animal Care and Use Committee or Animal Welfare Committee, respectively. For all imaging procedures, rats were anesthetized with 1% isoflurane. Animals received 5.5 to 7.4 MBq (normalized to 40 μg/rat) of either 64Cu-DOTA-MAb 69 or 64Cu-DOTA-control MAb via the tail vein, and PET/computed tomography (CT) imaging was performed on a Siemens Inveon μPET/CT scanner (Siemens Medical, Knoxville, TN) using instrument parameters described elsewhere (28). Images were acquired 24 h after radiotracer injection to allow sufficient clearance of radioactivity from background tissues. Region-of-interest (ROI) analysis was then conducted using the vendor software package to quantify radiotracer accumulation in the infection site and selected organs.
Posteuthanasia procedures.
After euthanasia, hearts were aseptically removed from all euthanized animals. The aortic valve vegetations were excised, weighed, and homogenized in 1 ml of saline, and dilutions were plated onto Enterococcosel agar (BD) supplemented with rifampin (100 μg/ml) for selection of inoculated E. faecalis strain OG1RF. Rats with sterile cultures of undiluted vegetation homogenates (∼1 ml) were considered to have had no induction of endocarditis.
Data analysis.
Since the entire vegetation (homogenized in 1 ml of saline and dilutions) was plated, vegetations yielding no growth were scored as sterile and were assigned the value of 1 CFU/vegetation for statistical purposes. Comparison of the results between the bacterial cultures obtained with the MAb 69 treatment group and the control IgG treatment group was done by the Mann-Whitney Wilcoxon unpaired test using Prism for Windows (version 4.00; GraphPad Software). The bacterial densities (geometric mean log10 number of CFU) in the vegetations were compared. Overall, differences were considered significant at P levels of <0.05 by use of two-tailed significance levels.
RESULTS
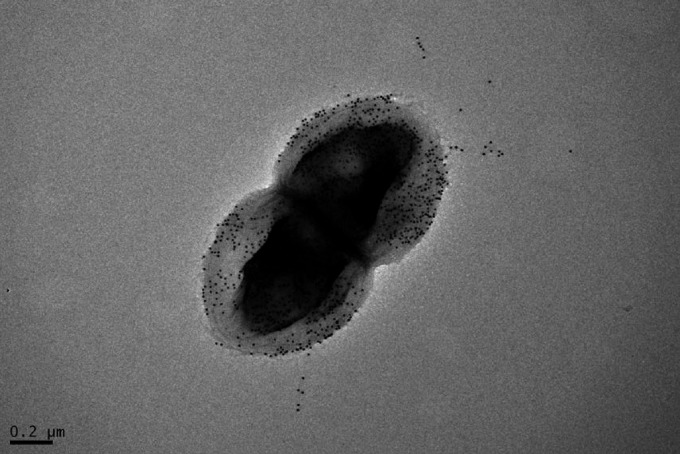
A high-affinity monoclonal antibody targeting the major pilin subunit of E. faecalis EbpC densely labels the bacterial surface and extruding pilus structures, as seen in electron microscopy.
It has been previously shown that the loss of pilin polymerization significantly impacts biofilm formation and infectivity in E. faecalis, demonstrating an important functional role for the pilus fiber in the establishment of infection (6, 29). Although we have previously demonstrated by ELISA that MAb 69 recognizes recombinant EbpC monomer and that MAb 69 can readily label in vitro-grown E. faecalis cells in all phases of growth via flow cytometry (10), this did not determine whether the antibody was interacting with polymerized EbpC extending from the cell wall since both the monomer and polymer are present on the bacterial surface (7). Given that extended pilus structures may be of importance when considering targeting EbpC antigen efficiently with MAb, we evaluated the ability to MAb 69 to bind the pilin polymer by electron microscopy (EM). As shown in Fig. 1, the cell surface and extended pilin structures were readily made visible by use of an MAb 69/secondary antibody gold bead conjugate, suggesting that the polymer form of EbpC is indeed recognized by this MAb.
FIG 1.
Detection of E. faecalis EbpC on the cell surface by immunoelectron microscopy. Anti-EbpC MAb-probed OG1RF cells grown in BHI were immunogold labeled, negatively stained with 1% uranyl acetate, and viewed by transmission electron microscopy.
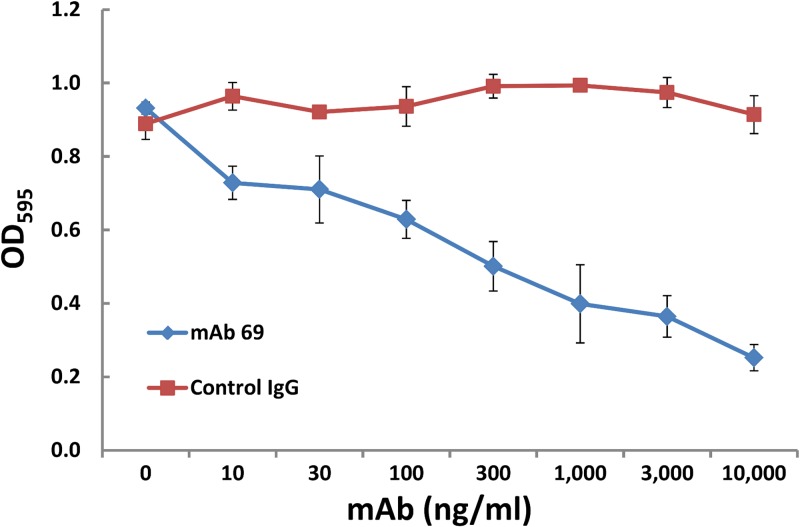
Anti-pilin MAb prevents E. faecalis adherence in a biofilm adhesion assay.
Utilizing an established polystyrene biofilm assay in which E. faecalis endocarditis isolates have been shown to produce biofilm significantly more often and to a greater degree than nonendocarditis isolates (30) and in which disruption of EbpC significantly impacts biofilm formation, we evaluated the ability of MAb 69 to prevent establishment of biofilm in an in vitro microwell assay. Our results demonstrated a clear dose-dependent inhibition of the formation of enterococcal biofilm, with significant inhibition occurring at MAb 69 concentrations as low as 10 ng/ml compared to the inhibition found for cells incubated with control IgG evaluated using the same concentrations (Fig. 2).
FIG 2.
Inhibition of in vitro biofilm formation. Inhibition was achieved by diluting an overnight culture of the wild-type OG1RF strain into 96-well polystyrene plates in the presence of either MAb 69 or control mouse IgG in triplicate wells. Static conditions were maintained for a 24-h period before crystal violet staining. The plotted data represent the average of triplicate wells and are representative of 3 independent experiments.
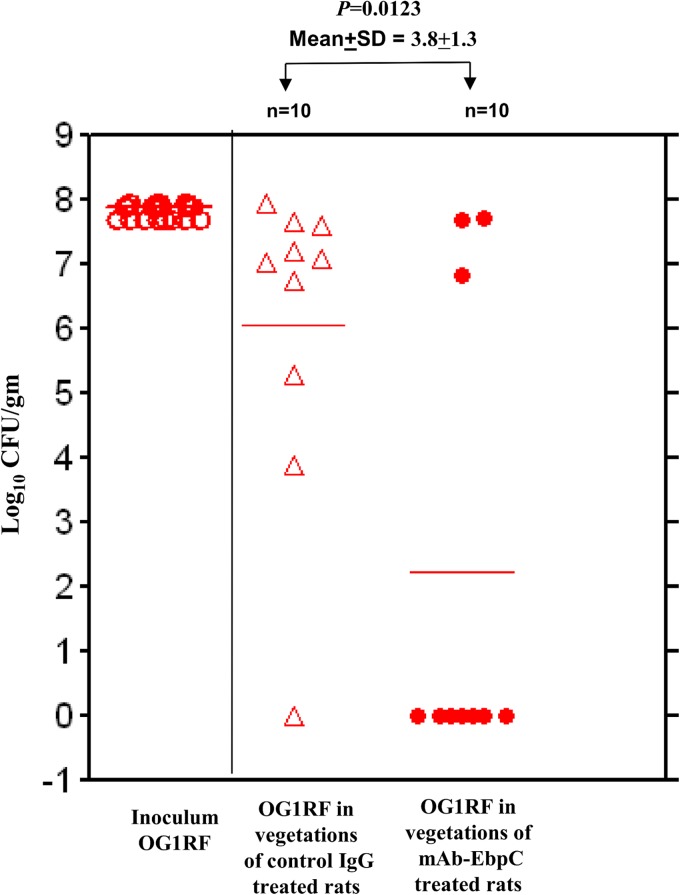
i.v. administration of anti-pilin monoclonal antibody provides passive protection against E. faecalis endocarditis.
To test whether MAb 69 could prevent the establishment of infection in vivo, we utilized an established infective endocarditis model. MAb 69 (2 mg/kg) was administered 1 h prior to E. faecalis challenge. As seen in Fig. 3, whereas 9/10 rats given control IgG developed endocarditis, only 3/10 given MAb 69 did so. This demonstrated that significant protection against the establishment of endocarditis was achieved in rats surveyed at 48 h postchallenge compared to that achieved in rats receiving a similarly administered IgG control.
FIG 3.
EbpC MAb protection against E. faecalis OG1RF infection in a rat endocarditis model.
MAb 69 targets established endocarditis infection in vivo, as shown using PET molecular imaging.
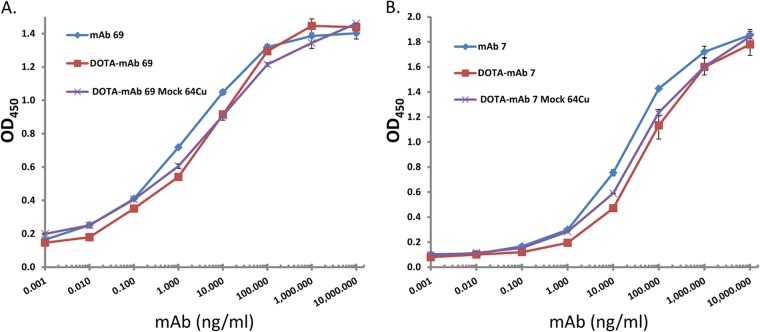
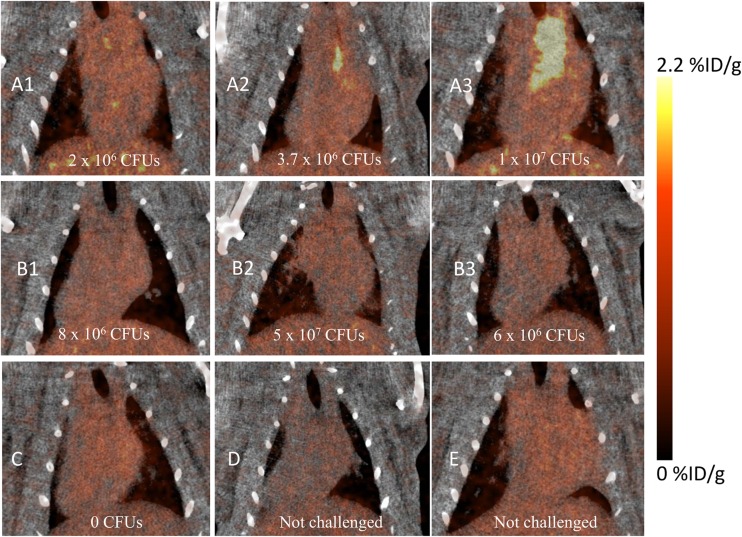
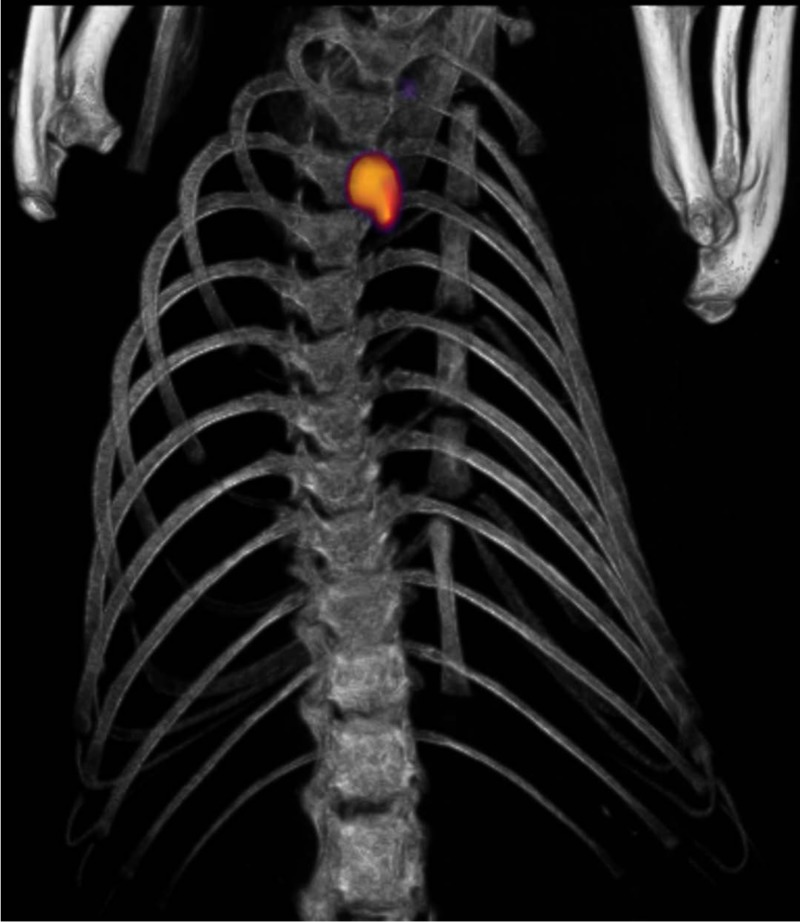
Having shown robust labeling for electron microscopy studies and flow cytometry evaluation (10), we next sought to address whether EbpC could be recognized by in vivo imaging after an infection was established. We conjugated an average of 6.5 DOTA moieties to MAb 69 for chelation of 64Cu and demonstrated via ELISA that conjugation and the radiolabeling conditions had a minimal impact on MAb binding to rEbpC (Fig. 4). To increase the likelihood of obtaining an infection that could be detected by PET, our initial study utilized a bacterial inoculum of 1 × 108 CFU/rat and allowed 72 h for establishment of infection. Of the six rats challenged, four died prior to imaging at this inoculum dosage. However, the remaining two rats tolerated the challenge and provided preliminary results to suggest that visualization of the localized infection using radiolabeled MAb 69 was feasible. As shown in Fig. S1 in the supplemental material, injection of 64Cu-DOTA-MAb 69 allowed visualization of infection with a signal correlating to the site of infection 6 times that of the background signal of heart tissue. No other thoracic tissues exhibited notable uptake. Conversely, the control imaging agent did not localize to the infection site and was at background levels in the imaging field of view. Building on these preliminary results, seven additional rats were catheterized and challenged with approximately 10-fold less E. faecalis, or 1 × 107 CFU/rat. At 48 h postinfection, four rats received radiolabeled MAb 69 and three received radiolabeled control MAb. In addition, two rats that were catheterized but that were not infected with E. faecalis received radiolabeled agent to test for nonspecific targeting to the site of catheter damage. Evaluation of rats at 72 h postinfection demonstrated that of the four infected rats which received MAb 69, two had focal uptake of labeled MAb 69 in the region of the heart that correlated with the site of infection (Fig. 5A2 and A3). Quantification of the PET signal originating from the entire infected region in these rats gave mean percent infectious dose (ID)/g values of 1.4 and 2.9 for infections with 3.7 × 106 and 1 × 107 CFU, respectively, with corresponding infection/muscle ratios of 13 and 12.9. Further examination of the infected area revealed regions with a maximum percent ID/g value of 19.2 in the rat with the largest amount of infection. Analysis of the other two rats, one with no CFU, despite receiving a challenge (Fig. 5C), and one with 2 × 106 CFU, the smallest amount of any animal evaluated (Fig. 5A1), revealed no discernible PET signal at the infection site, with the latter result suggesting a possible limit in the number of CFU for PET detection. This result was compared to that for highly infected rats which received control MAb agent and showed no signs of labeled MAb uptake (Fig. 5B1 to B3). In addition, control animals receiving no bacterial challenge demonstrated no radiolabeled MAb agent uptake at the site of catheterization (Fig. 5D and E), demonstrating the specificity of MAb 69 to E. faecalis infection and not to host tissue damage. The three-dimensional PET/CT image of the rat with the largest number of CFU clearly delineated the infection site and showed a low background signal in other tissues (Fig. 6).
FIG 4.
Imaging agent potency ELISA. MAbs underwent DOTA conjugation via NHS chemistry and subsequent mock labeling conditions without the addition of 64Cu. To ascertain the effects of MAb labeling on the ability to bind selected targets, corresponding proteins were coated on ELISA wells and allowed to react with MAbs at the indicated concentrations. The bound MAbs were visualized with HRP-labeled secondary antibodies, followed by color development with TMB substrate. The results for triplicate wells were averaged and plotted. (A) MAb 69; (B) isotype-matched control MAb 7.
FIG 5.
Coronal PET/CT images of rats with endocarditis obtained 24 h after radiotracer injection. (A) Three rats (A1 to A3) with established infections imaged with 64Cu-DOTA-MAb 69; (B) three rats (B1 to B3) with established infections imaged with 64Cu-DOTA-control MAb 7; (C) a challenged rat without an established infection imaged with 64Cu-DOTA-MAb 69; (D) a catheterized but not challenged rat imaged with 64Cu-DOTA-control MAb 7; (E) a catheterized but not challenged rat imaged with 64Cu-DOTA-MAb 69. The size of the E. faecalis population in rats with established infection was measured by postmortem analysis, and the number of CFU is noted on each image.
FIG 6.
PET/CT image of endocarditis at 72 h postinfection in a rat infected with E. faecalis obtained using 64Cu-DOTA MAb 69.
DISCUSSION
With the increased prevalence of antibiotic resistance of bacterial nosocomial infections within hospitals and health care facilities and few new antibiotics on the horizon, new approaches for prevention, such as vaccines, are needed. Unfortunately, the populations which require protection from these opportunistic infections are often the least able to mount an effective immune response, making active immunization a less viable option (13). Many at-risk patients are immunocompromised as a result of chemotherapy, underlying diseases, surgical treatments, and/or advanced age. This population is large and rapidly growing in number due to increasing life spans and the increased use of implanted foreign bodies and organ transplantation, the latter of which requires immunosuppressive drugs. Active vaccination also does little to help those individuals who are at immediate risk of infection, as in patients undergoing emergency surgery or premature neonates, where time constraints prevent a robust vaccine response for protection (18).
Passive protection through antibody administration is an alternative to active immunization, given the often suboptimal patient conditions for generating a protective immune response. MAbs targeting protein toxins have demonstrated success in a range of bacterial infections, including infections caused by Clostridium difficile (31), Bacillus anthracis (32, 33), and Shiga toxin-producing Escherichia coli (STEC) (34, 35), whereby blocking the toxin action prevented pathogenesis. However, targeting the bacterium itself, with its complex pathogenesis, has been a significant hurdle for immunoprevention. For enterococci, only a few surface antigens have been evaluated as targets of passively administered antibodies (19, 23, 36, 37). Of relevance to our work, Schlievert et al. (37) demonstrated that Fab fragments of polyclonal IgG from rabbits immunized with the surface virulence factor aggregation substance reduced E. faecalis vegetation in a rabbit endocarditis model. For this particular target, however, active immunization experiments and in vitro evaluation suggested that whole IgG, due to its bivalent nature, encouraged aggregation and actually increased the pathogenesis of enterococcal endocarditis by increasing vegetation size (37).
Antibody-mediated passive protection against bacterial infection has been studied in numerous Gram-positive pathogens (13, 17, 18, 38). Given the multitude of protein virulence factors, which are often associated with the cell surface, that are at the disposal of Gram-positive pathogens, it is not surprising that one strategy that pharmaceutical companies have pursued has been polyclonal preparations of antibodies. These pooled human immunoglobulin preparations from immunized volunteers or donors with high titers of antibodies against certain virulence factors have had limited success and have been plagued by a lack of batch-to-batch consistency and a lack of detailed knowledge of antibody-antigen specificity or the specific epitopes targeted. Many of these issues can be overcome by the use of defined monoclonal antibodies, where a detailed understanding of epitope specificity and affinity can be surmised. As seen in the exploding field of monoclonal antibody cancer therapies, targeting of multiple epitopes or multiple antigens per target cell (39) may provide a means in the future to overcome the complexities and redundancies of bacterial virulence mechanisms (20).
Despite their success in cancer and treatment of inflammatory disease, only one monoclonal against an infectious disease, palivizumab (Synagis), has been approved to date. It targets the respiratory syncytial virus (RSV) in neonates (40). Failure of a passive immunization strategy against Gram-positive pathogens (14, 18) has been blamed on not only the multifactorial nature of pathogenesis but also a lack of understanding of the target proteome during infection and how this modulates the interaction between host and microbe (18). A better understanding of how these opportunistic pathogens utilize and regulate a number of virulence factors important for pathogenesis should identify potential opportunities to prevent infection. Over the last decade, basic research into the structure and function of Gram-positive bacterial pili has laid the groundwork for exploring pili as a target for prevention and therapy. Covalently linked to and extending from the cell wall surface, pili are an attractive target for antibody-mediated prevention, and recent investigations have evaluated the effectiveness of pilus-based vaccines against S. agalactiae, S. pyogenes, and S. pneumoniae, demonstrating significant levels of protection in multiple animal models (4).
We and others have improved our understanding of Ebp pilus assembly and expression and evaluated the role of pili as an enterococcal virulence factor (6, 7, 10, 41, 42). Here we extend these findings by demonstrating that an IgG MAb selected against the major Ebp pilin protein of E. faecalis, EbpC, is sufficient to markedly decrease the establishment of enterococcal endocarditis infection in vivo, demonstrating that a high-affinity MAb targeting a single epitope can be sufficient for protection against E. faecalis and suggesting a future for the use of anti-pilin MAbs in MAb-based preventatives against enterococci as well as other pilin-producing Gram-positive pathogens. The protective dose of 2 mg/kg utilized was equivalent to or less than the concentrations of protective dosing utilized in previously described passive protection models (23, 35, 43, 44) and was well below the human dose of palivizumab of 15 mg/kg (40). By vetting EbpC as a target for monoclonal antibody-mediated protection, we have added E. faecalis to a growing list of Gram-positive pathogens against which monoclonal antibodies have been demonstrated to offer significant passive protection in animal models of infection. With our increasing understanding of virulence factors and the increasing relevance of monoclonal antibody therapy, MAbs administered for passive protection hold significant promise as future preventatives in the fight against many Gram-positive pathogens (45, 46).
This work also demonstrated that our MAb can specifically target an established endocarditis infection caused by a Gram-positive bacterium via recognition of the pilin antigen and identify the site of infection. Although early investigations using polyclonals generated against Staphylococcus aureus demonstrated the feasibility of imaging endocarditis with antibacterial antibodies (47), the limitations of polyclonal preparations likely prevented clinical implementation. More recently, Panizzi et al. took advantage of the coagulase of S. aureus to deposit a prothrombin derivative on the bacterial vegetation for visualization of endocarditis infection (48). Surprisingly, despite the multitude of Gram-positive bacterial surface proteins recently discovered and studied, including many pili and other LPXTG-anchored proteins, we could find no examples of a monoclonal antibody targeting a single protein antigen to visualize a Gram-positive bacterial infection.
Molecular targeting with the specificity and affinity of a monoclonal antibody could have both clinical and research utility. In the clinic, infective endocarditis is primarily diagnosed through the use of echocardiography and blood cultures. However, in cases of false positives found in echocardiography when valvular abnormalities unrelated to the current infection are observed or when a vegetation is present in a patient with negative blood cultures (49), there might be potential for such imaging technology to be investigated further. The utility of live-animal imaging of bacterial infection as a model system to test the efficacy of new antimicrobials also has significant research value, allowing evaluation of different stages of infection without sacrificing the animal. As discovery and understanding of bacterial surface antigens and how they are regulated continue to improve (10, 50), we expect the identification of many additional targets to which highly specific MAbs for imaging of bacterial infection can be applied.
Although established infections are often thought of as being more recalcitrant to immunological recognition or antibiotic therapy due to biofilm formation, it has previously been shown that MAbs can penetrate biofilm. Monoclonal antibodies against poly-N-acetylglucosamine (PNAG), for example, appear to traverse the biofilm (51), only to get trapped by free antigen prior to cell interaction, making them less effective in established infection than on planktonic bacteria. Although the release of pili is likely upon cell death, we have not observed proteolytic cleavage of pili, as has been seen with other enterococcal surface antigens that we have evaluated (52). However, whether our MAbs are actually binding surface Ebp on bacteria in the biofilm or binding pili in the matrix is for future study. In addition, given MAb 69's ability to recognize the EbpC polymerized structures distal from the bacterial surface, as evaluated by electron microscopy, future work will address whether visualization of infection by targeting EbpC requires the polymerized structure or whether targeting monomer EbpC would be sufficient.
In conclusion, this work has demonstrated that targeting a single epitope on Gram-positive bacterial pili with a monoclonal antibody can protect against in vitro biofilm formation and against in vivo rat enterococcal infection. Excluding S. aureus, in which no pilus structure has been identified, pili represent a prominent structure on the surface of many Gram-positive pathogens, including E. faecium, GBS, and GAS. Future work will evaluate the efficacy of MAbs against the major pilin subunit of other organisms, with aspirations of development of a pan-anti-pilus MAb cocktail for nosocomial infection prevention. Demonstration that MAbs are able to recognize pilus antigenic targets at the site of infection may also open opportunities for MAb therapy and MAb-targeted delivery of antimicrobials, which, in addition to endocarditis, could potentially be extended to treatment of other biofilm-associated infections caused by Gram-positive bacteria.
Supplementary Material
ACKNOWLEDGMENT
This work was supported in part by NIH grant R01 AI047923-14 from NIAID to B.E.M.
Footnotes
Published ahead of print 22 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01403-13.
REFERENCES
- 1.Murray BE. 1990. The life and times of the Enterococcus. Clin. Microbiol. Rev. 3:46–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray BE, Weinstock GM. 1999. Enterococci: new aspects of an old organism. Proc. Assoc. Am. Physicians 111:328–334. 10.1046/j.1525-1381.1999.99241.x [DOI] [PubMed] [Google Scholar]
- 3.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, National Healthcare Safety Network (NHSN) Team, Participating NHSN Facilities 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect. Control Hosp. Epidemiol. 34:1–14. 10.1086/668770 [DOI] [PubMed] [Google Scholar]
- 4.Hendrickx AP, Budzik JM, Oh SY, Schneewind O. 2011. Architects at the bacterial surface—sortases and the assembly of pili with isopeptide bonds. Nat. Rev. Microbiol. 9:166–176. 10.1038/nrmicro2520 [DOI] [PubMed] [Google Scholar]
- 5.Danne C, Dramsi S. 2012. Pili of gram-positive bacteria: roles in host colonization. Res. Microbiol. 163:645–658. 10.1016/j.resmic.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 6.Nallapareddy SR, Singh KV, Sillanpaa J, Garsin DA, Hook M, Erlandsen SL, Murray BE. 2006. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J. Clin. Invest. 116:2799–2807. 10.1172/JCI29021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sillanpaa J, Chang C, Singh KV, Montealegre MC, Nallapareddy SR, Harvey BR, Ton-That H, Murray BE. 2013. Contribution of individual Ebp pilus subunits of Enterococcus faecalis OG1RF to pilus biogenesis, biofilm formation and urinary tract infection. PLoS One 8:e68813. 10.1371/journal.pone.0068813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sillanpaa J, Xu Y, Nallapareddy SR, Murray BE, Hook M. 2004. A family of putative MSCRAMMs from Enterococcus faecalis. Microbiology 150:2069–2078. 10.1099/mic.0.27074-0 [DOI] [PubMed] [Google Scholar]
- 9.Mohamed JA, Huang DB. 2007. Biofilm formation by enterococci. J. Med. Microbiol. 56:1581–1588. 10.1099/jmm.0.47331-0 [DOI] [PubMed] [Google Scholar]
- 10.Gao P, Pinkston KL, Nallapareddy SR, van Hoof A, Murray BE, Harvey BR. 2010. Enterococcus faecalis rnjB is required for pilin gene expression and biofilm formation. J. Bacteriol. 192:5489–5498. 10.1128/JB.00725-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. 2010. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 9:325–338. 10.1038/nrd3003 [DOI] [PubMed] [Google Scholar]
- 12.Leavy O. 2010. Therapeutic antibodies: past, present and future. Nat. Rev. Immunol. 10:297. 10.1038/nri2763 [DOI] [PubMed] [Google Scholar]
- 13.Dunman PM, Nesin M. 2003. Passive immunization as prophylaxis: when and where will this work? Curr. Opin. Pharmacol. 3:486–496. 10.1016/j.coph.2003.05.005 [DOI] [PubMed] [Google Scholar]
- 14.Otto M. 2008. Targeted immunotherapy for staphylococcal infections: focus on anti-MSCRAMM antibodies. BioDrugs 22:27–36. 10.2165/00063030-200822010-00003 [DOI] [PubMed] [Google Scholar]
- 15.Berghman LR, Abi-Ghanem D, Waghela SD, Ricke SC. 2005. Antibodies: an alternative for antibiotics? Poult. Sci. 84:660–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisman LE. 2007. Antibody for the prevention of neonatal nosocomial staphylococcal infection: a review of the literature. Arch. Pediatr. 14(Suppl. 1):S31–S34. 10.1016/S0929-693X(07)80008-X [DOI] [PubMed] [Google Scholar]
- 17.Projan SJ, Nesin M, Dunman PM. 2006. Staphylococcal vaccines and immunotherapy: to dream the impossible dream? Curr. Opin. Pharmacol. 6:473–479. 10.1016/j.coph.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 18.Schaffer AC, Lee JC. 2009. Staphylococcal vaccines and immunotherapies. Infect. Dis. Clin. North Am. 23:153–171. 10.1016/j.idc.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 19.Theilacker C, Krueger WA, Kropec A, Huebner J. 2004. Rationale for the development of immunotherapy regimens against enterococcal infections. Vaccine 22(Suppl. 1):S31–S38. 10.1016/j.vaccine.2004.08.014 [DOI] [PubMed] [Google Scholar]
- 20.Oleksiewicz MB, Nagy G, Nagy E. 2012. Anti-bacterial monoclonal antibodies: back to the future? Arch. Biochem. Biophys. 526:124–131. 10.1016/j.abb.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 21.Chang C, Huang IH, Hendrickx AP, Ton-That H. 2013. Visualization of Gram-positive bacterial pili. Methods Mol. Biol. 966:77–95. 10.1007/978-1-62703-245-2_5 [DOI] [PubMed] [Google Scholar]
- 22.Chang C, Mandlik A, Das A, Ton-That H. 2011. Cell surface display of minor pilin adhesins in the form of a simple heterodimeric assembly in Corynebacterium diphtheriae. Mol. Microbiol. 79:1236–1247. 10.1111/j.1365-2958.2010.07515.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh KV, Nallapareddy SR, Sillanpaa J, Murray BE. 2010. Importance of the collagen adhesin Ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog. 6:e1000716. 10.1371/journal.ppat.1000716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh KV, Nallapareddy SR, Nannini EC, Murray BE. 2005. Fsr-independent production of protease(s) may explain the lack of attenuation of an Enterococcus faecalis fsr mutant versus a gelE-sprE mutant in induction of endocarditis. Infect. Immun. 73:4888–4894. 10.1128/IAI.73.8.4888-4894.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall MA, Pinkston KL, Wilganowski N, Robinson H, Ghosh P, Azhdarinia A, Vazquez-Arreguin K, Kolonin AM, Harvey BR, Sevick-Muraca EM. 2012. Comparison of mAbs targeting epithelial cell adhesion molecule for the detection of prostate cancer lymph node metastases with multimodal contrast agents: quantitative small-animal PET/CT and NIRF. J. Nucl. Med. 53:1427–1437. 10.2967/jnumed.112.106302 [DOI] [PubMed] [Google Scholar]
- 26.Hall MA, Kwon S, Robinson H, Lachance PA, Azhdarinia A, Ranganathan R, Price RE, Chan W, Sevick-Muraca EM. 2012. Imaging prostate cancer lymph node metastases with a multimodality contrast agent. Prostate 72:129–146. 10.1002/pros.21413 [DOI] [PubMed] [Google Scholar]
- 27.Ghosh SC, Ghosh P, Wilganowski N, Robinson H, Hall MA, Dickinson G, Pinkston KL, Harvey BR, Sevick-Muraca EM, Azhdarinia A. 2013. Multimodal chelation platform for near-infrared fluorescence/nuclear imaging. J. Med. Chem. 56:406–416. 10.1021/jm300906g [DOI] [PubMed] [Google Scholar]
- 28.Sampath L, Kwon S, Hall MA, Price RE, Sevick-Muraca EM. 2010. Detection of cancer metastases with a dual-labeled near-infrared/positron emission tomography imaging agent. Transl. Oncol. 3:307–317. 10.1593/tlo.10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemp KD, Singh KV, Nallapareddy SR, Murray BE. 2007. Relative contributions of Enterococcus faecalis OG1RF sortase-encoding genes, srtA and bps (srtC), to biofilm formation and a murine model of urinary tract infection. Infect. Immun. 75:5399–5404. 10.1128/IAI.00663-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamed JA, Huang W, Nallapareddy SR, Teng F, Murray BE. 2004. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 72:3658–3663. 10.1128/IAI.72.6.3658-3663.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Gerding DN, Nichol G, Thomas WD, Jr, Leney M, Sloan S, Hay CA, Ambrosino DM. 2010. Treatment with monoclonal antibodies against Clostridium difficile toxins. N. Engl. J. Med. 362:197–205. 10.1056/NEJMoa0907635 [DOI] [PubMed] [Google Scholar]
- 32.Mabry R, Rani M, Geiger R, Hubbard GB, Carrion R, Jr, Brasky K, Patterson JL, Georgiou G, Iverson BL. 2005. Passive protection against anthrax by using a high-affinity antitoxin antibody fragment lacking an Fc region. Infect. Immun. 73:8362–8368. 10.1128/IAI.73.12.8362-8368.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harvey BR, Georgiou G, Hayhurst A, Jeong KJ, Iverson BL, Rogers GK. 2004. Anchored periplasmic expression, a versatile technology for the isolation of high-affinity antibodies from Escherichia coli-expressed libraries. Proc. Natl. Acad. Sci. U. S. A. 101:9193–9198. 10.1073/pnas.0400187101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bitzan M, Poole R, Mehran M, Sicard E, Brockus C, Thuning-Roberson C, Riviere M. 2009. Safety and pharmacokinetics of chimeric anti-Shiga toxin 1 and anti-Shiga toxin 2 monoclonal antibodies in healthy volunteers. Antimicrob. Agents Chemother. 53:3081–3087. 10.1128/AAC.01661-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheoran AS, Chapman-Bonofiglio S, Harvey BR, Mukherjee J, Georgiou G, Donohue-Rolfe A, Tzipori S. 2005. Human antibody against Shiga toxin 2 administered to piglets after the onset of diarrhea due to Escherichia coli O157:H7 prevents fatal systemic complications. Infect. Immun. 73:4607–4613. 10.1128/IAI.73.8.4607-4613.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huebner J, Quaas A, Krueger WA, Goldmann DA, Pier GB. 2000. Prophylactic and therapeutic efficacy of antibodies to a capsular polysaccharide shared among vancomycin-sensitive and -resistant enterococci. Infect. Immun. 68:4631–4636. 10.1128/IAI.68.8.4631-4636.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlievert PM, Chuang-Smith ON, Peterson ML, Cook LC, Dunny GM. 2010. Enterococcus faecalis endocarditis severity in rabbits is reduced by IgG Fabs interfering with aggregation substance. PLoS One 5:e13194. 10.1371/journal.pone.0013194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casadevall A, Dadachova E, Pirofski LA. 2004. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2:695–703. 10.1038/nrmicro974 [DOI] [PubMed] [Google Scholar]
- 39.Koefoed K, Steinaa L, Soderberg JN, Kjaer I, Jacobsen HJ, Meijer PJ, Haurum JS, Jensen A, Kragh M, Andersen PS, Pedersen MW. 2011. Rational identification of an optimal antibody mixture for targeting the epidermal growth factor receptor. MAbs 3:584–595. 10.4161/mabs.3.6.17955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meissner HC, Welliver RC, Chartrand SA, Law BJ, Weisman LE, Dorkin HL, Rodriguez WJ. 1999. Immunoprophylaxis with palivizumab, a humanized respiratory syncytial virus monoclonal antibody, for prevention of respiratory syncytial virus infection in high risk infants: a consensus opinion. Pediatr. Infect. Dis. J. 18:223–231. 10.1097/00006454-199903000-00004 [DOI] [PubMed] [Google Scholar]
- 41.Nielsen HV, Flores-Mireles AL, Kau AL, Kline KA, Pinkner JS, Neiers F, Normark S, Henriques-Normark B, Caparon MG, Hultgren SJ. 2013. Pilin and sortase residues critical for endocarditis- and biofilm-associated pilus biogenesis in Enterococcus faecalis. J. Bacteriol. 195:4484–4495. 10.1128/JB.00451-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen HV, Guiton PS, Kline KA, Port GC, Pinkner JS, Neiers F, Normark S, Henriques-Normark B, Caparon MG, Hultgren SJ. 2012. The metal ion-dependent adhesion site motif of the Enterococcus faecalis EbpA pilin mediates pilus function in catheter-associated urinary tract infection. mBio 3(4):e00177–00112. 10.1128/mBio.00177-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebert T, Smith S, Pancari G, Clark D, Hampton R, Secore S, Towne V, Fan H, Wang XM, Wu X, Ernst R, Harvey BR, Finnefrock AC, Wang F, Tan C, Durr E, Cope L, Anderson A, An Z, McNeely T. 2010. A fully human monoclonal antibody to Staphylococcus aureus iron regulated surface determinant B (IsdB) with functional activity in vitro and in vivo. Hum. Antibodies 19:113–128. 10.3233/HAB-2010-0235 [DOI] [PubMed] [Google Scholar]
- 44.Senn BM, Visram Z, Meinke AL, Neubauer C, Gelbmann D, Sinzinger J, Hanner M, Lundberg U, Boisvert H, Reinscheid D, von Gabain A, Nagy E. 2011. Monoclonal antibodies targeting different cell wall antigens of group B streptococcus mediate protection in both Fc-dependent and independent manner. Vaccine 29:4116–4124. 10.1016/j.vaccine.2011.03.100 [DOI] [PubMed] [Google Scholar]
- 45.Weisman LE, Thackray HM, Steinhorn RH, Walsh WF, Lassiter HA, Dhanireddy R, Brozanski BS, Palmer KG, Trautman MS, Escobedo M, Meissner HC, Sasidharan P, Fretz J, Kokai-Kun JF, Kramer WG, Fischer GW, Mond JJ. 2011. A randomized study of a monoclonal antibody (pagibaximab) to prevent staphylococcal sepsis. Pediatrics 128:271–279. 10.1542/peds.2010-3081 [DOI] [PubMed] [Google Scholar]
- 46.Roux D, Pier GB, Skurnik D. 2012. Magic bullets for the 21st century: the reemergence of immunotherapy for multi- and pan-resistant microbes. J. Antimicrob. Chemother. 67:2785–2787. 10.1093/jac/dks335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong DW, Dhawan VK, Tanaka T, Mishkin FS, Reese IC, Thadepalli H. 1982. Imaging endocarditis with Tc-99m-labeled antibody—an experimental study: concise communication. J. Nucl. Med. 23:229–234 [PubMed] [Google Scholar]
- 48.Panizzi P, Nahrendorf M, Figueiredo JL, Panizzi J, Marinelli B, Iwamoto Y, Keliher E, Maddur AA, Waterman P, Kroh HK, Leuschner F, Aikawa E, Swirski FK, Pittet MJ, Hackeng TM, Fuentes-Prior P, Schneewind O, Bock PE, Weissleder R. 2011. In vivo detection of Staphylococcus aureus endocarditis by targeting pathogen-specific prothrombin activation. Nat. Med. 17:1142–1146. 10.1038/nm.2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Jr, Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M, Taubert KA, Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association, Infectious Diseases Society of America 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394–e434. 10.1161/CIRCULATIONAHA.105.165564 [DOI] [PubMed] [Google Scholar]
- 50.Gao P, Pinkston KL, Bourgogne A, Cruz MR, Garsin DA, Murray BE, Harvey BR. 2013. Library screen identifies Enterococcus faecalis CcpA, the catabolite control protein A, as an effector of Ace, a collagen adhesion protein linked to virulence. J. Bacteriol. 195:4761–4768. 10.1128/JB.00706-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franca A, Vilanova M, Cerca N, Pier GB. 2013. Monoclonal antibody raised against PNAG has variable effects on static S. epidermidis biofilm accumulation in vitro. Int. J. Biol. Sci. 9:518–520. 10.7150/ijbs.6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinkston KL, Gao P, Diaz-Garcia D, Sillanpaa J, Nallapareddy SR, Murray BE, Harvey BR. 2011. The Fsr quorum-sensing system of Enterococcus faecalis modulates surface display of the collagen-binding MSCRAMM Ace through regulation of gelE. J. Bacteriol. 193:4317–4325. 10.1128/JB.05026-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.