Abstract
Gamma interferon (IFN-γ) is an important driver of intestinal inflammation during colitis caused by Salmonella enterica serovar Typhimurium. Here we used the mouse colitis model to investigate the cellular sources of IFN-γ in the cecal mucosa during the acute phase of an S. Typhimurium infection. While IFN-γ staining was detected in T cells, NK cells, and inflammatory monocytes at 2 days after infection, the majority of IFN-γ-positive cells in the cecal mucosa were neutrophils. Furthermore, neutrophil depletion blunted mucosal Ifng expression and reduced the severity of intestinal lesions during S. Typhimurium infection. We conclude that neutrophils are a prominent cellular source of IFN-γ during the innate phase of S. Typhimurium-induced colitis.
INTRODUCTION
Individuals with primary immunodeficiencies impairing production of gamma interferon (IFN-γ) or IFN-γ signaling present in childhood with disseminated bacterial infections. The vast majority of these infections are caused by two bacterial pathogens: nontyphoidal Salmonella serovars and weakly virulent Mycobacterium species (1). Nontyphoidal Salmonella serovars, such as S. enterica serovar Typhimurium, cause a localized gastroenteritis in immunocompetent individuals, which is characterized by acute intestinal inflammation and diarrhea (reviewed in references 2 and 3). The importance of innate immune responses during gastroenteritis is illustrated by the rapid onset of intestinal inflammation, which gives rise to symptoms within 24 h of ingesting S. Typhimurium (4). A histopathological hallmark of S. Typhimurium-induced gastroenteritis is a severe acute infiltrate of neutrophils in the ileal and colonic mucosae (5, 6). Studies using a mouse model of S. Typhimurium-induced colitis show that in animals lacking IFN-γ, the severity of intestinal inflammation is markedly attenuated (7, 8).
Conventional wisdom holds that IFN-γ is produced during the innate phase of a bacterial infection by natural killer (NK) cells and NKT cells (reviewed in reference 9) while CD4+ and CD8+ T cells become the principal cellular sources at later stages, which are governed by adaptive immune responses (reviewed in references 10 and 11). Consistent with this idea, splenic NK cells are a prominent cellular source of innate IFN-γ production in models of systemic S. Typhimurium infection (12–14). Furthermore, CD1D-restricted NKT cells contribute to innate IFN-γ production in the livers and spleens of mice with disseminated S. Typhimurium infection (15, 16). However, the identities of the innate immune cells contributing to early IFN-γ production in the intestinal mucosa have not been fully resolved. The goal of this study was to identify the cellular sources of innate IFN-γ production in the intestinal mucosa during S. Typhimurium-induced colitis.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. Typhimurium strain IR715 is a fully virulent, nalidixic acid-resistant derivative of wild-type isolate ATCC 14028 (American Type Culture Collection) (17). Bacteria were cultured overnight aerobically at 37°C in Luria-Bertani (LB) broth or on LB agar plates with 0.05 mg/ml nalidixic acid.
Animal experiments.
Mouse experiments were performed according to USDA guidelines and were approved by the Institutional Animal Care and Use Committee at the University of California at Davis. Female, 8- to 12-week-old C57BL/6 mice (Jackson Laboratory) or mice carrying an internal ribosome entry site (IRES)-enhanced cyan fluorescence protein (eYFP) reporter cassette inserted between the translational stop codon and 3′ untranslated region (UTR)/poly(A) tail of the Ifng gene (Jackson Laboratory, lab stock number 017581) were used for the mouse colitis model. In brief, mice were inoculated intragastrically with streptomycin (0.1 ml of a 200-mg/ml solution in sterile distilled water), and 24 h later mice were inoculated intragastrically either with sterile LB broth or with bacteria (0.1 ml containing between 1 × 109 and 1 × 1010 CFU/ml). For Ly6G depletion experiments, mice were given 0.5 mg/animal of either anti-Ly6G antibody (clone 1A8, Bio X Cell BE0075) or an isotype control (purified rat IgG2a, clone 2A3, Bio X Cell BE0089) in 500 μl of sterile Dulbecco phosphate-buffered saline (DPBS) intraperitoneally for 2 days. The first injection was administered concurrently with streptomycin treatment and the second at 24 h after infection. At 2 days after infection, mice were euthanized and samples of the cecum were collected for the isolation of mRNA and histopathological analysis. For bacteriological analysis, colon contents, mesenteric lymph nodes, and the liver and spleen were homogenized, and serial 10-fold dilutions were spread on agar plates containing the appropriate antibiotics. The cecum from each animal was collected to obtain cellular populations for analysis by flow cytometry.
Quantitative real-time PCR.
For quantitative analysis of mRNA levels, 1 μg of RNA from each sample was reverse transcribed in a 50-μl volume (TaqMan reverse transcription reagent; Applied Biosystems), and 4 μl of cDNA was used for each real-time reaction. Real-time PCR was performed using Sybr green (Applied Biosystems) and an Applied Biosystems ViiA 7 fast real-time PCR system. The data were analyzed using a comparative cycle threshold method (Applied Biosystems). Increases in cytokine expression in infected mice were calculated relative to the average level of the respective cytokine in four control animals from the corresponding time point after inoculation with sterile LB broth. A list of genes analyzed in this study and the respective primers is provided in Table 1.
TABLE 1.
Primers for quantitative real-time PCR
| Gene | Primer 1 | Primer 2 |
|---|---|---|
| Ifng | 5′-TGATGGCATGTCAGACAGCA-3′ | 5′-GGCACAAGTCATATAGCCTGACAC-3′ |
| Gapdh | 5′-TGTAGACCATGTAGTTGAGGTCA-3′ | 5′-AGGTCGGTGTGAACGGATTTG-3′ |
Histopathology.
Tissue samples were fixed in formalin, processed according to standard procedures for paraffin embedding, sectioned at 5 μm, and stained with hematoxylin and eosin. A veterinary pathologist performed a blinded scoring for inflammatory changes on a rating scale from 0 (not detected) to 5 (severe) for each of the following five histological parameters: neutrophil infiltration, infiltration by mononuclear cells, submucosal edema, epithelial injury, and exudate (18).
Preparation of cecal cell suspensions.
The cecum and proximal colon were collected from either C57BL/6 or eYFP-IFN-γ reporter mice (Jackson Laboratory). Fat and connective tissue were removed. Intestinal sections were cut longitudinally from the proximal colon to the tip of the cecum. The cecal content was removed by scraping gently with the flat edge of scissors. The sections were then washed in cold 1× Hanks balanced salt solution (HBSS) (Gibco catalog no. 14185) containing 0.015 M HEPES (Gibco catalog no. 15630) a total of six times to remove mucus and remaining fecal matter. Tissue was then transferred into 10 ml of prewarmed (37°C) 1× RPMI medium (Sigma catalog no. R1145) containing 10% fetal bovine serum, penicillin-streptomycin (Gibco catalog no. 15240-062), 0.015 M HEPES, and 2.9 units/ml of collagenase (FALGPA) (Sigma-Aldrich catalog no. C6885). Additionally, for cecal lymphocytes bound for intercellular staining and fluorescence-activated cell sorter (FACS) analysis, 0.15 mg of brefeldin A was added to the disassociation medium. Tissue was then processed using a protocol provided by the manufacturer. In brief, tissue was run twice on program Brain_01 on the gentleMacs Disassociator. Tissue samples were then rotated slowly at 37°C for 35 min in a hybridization oven. Samples were then disassociated using a high-speed program on the gentleMacs Disassociator as recommended by the manufacturer. The cell suspension was then strained twice through a 70-μm cell strainer or until a firm pellet was obtained. With the exception of the incubation time at 37°C, samples remained chilled on ice during all steps in tissue processing.
Flow cytometry.
A total of 4 × 106 intestinal cells were resuspended in 2 ml of DPBS and were stained with Aqua Live/Dead cell discriminator (Invitrogen no. L34597) as per the manufacturer's protocol. To distinguish cell populations shown in Fig. 1, cells were surface stained for 20 min in the dark at 4°C with optimized concentrations with anti-CD3 allophycocyanin (APC)/Cy7 (clone 17A2; Biolegend), anti-NK1.1 phycoerythrin (PE) (clone PK136; Biolegend), anti-CD11B PeCy7 (clone M1/70; eBioscience), anti-CD11C Alexa Fluor 700 (clone N418; eBioscience), anti-LY6C Pacific Blue (clone HK1.4; Biolegend), and anti-LY6G peridinin chlorophyll protein (PerCp)/Cy5.5 (clone 1A8; Biolegend). Cells were washed twice with PBS containing 1% bovine serum albumin and 1 mM EDTA (FACS buffer). After staining, cells were fixed and permeabilized using BD Cytofix/Cytoperm (BD 51-2090KZ). Permeabilized cells were then stained with anti-IFN-γ APC (clone XMG1.2; eBioscience) per the manufacturer's protocol. Cells were then washed twice with BD Perm/Wash buffer (BD 554723) and once with FACS buffer. Samples were then resuspended in 300 μl FACS buffer for analysis. To confirm neutrophil morphology, LY6G+ LY6C+ cells were sorted using a MoFlo high-speed cell sorter (Dako). Cytospin samples were prepared from the sorted cells using a Shandon cytocentrifuge (Thermo Scientific, MI) and dried prior to staining with a Diff-Quik staining kit (IMEB, Inc., San Marcos, CA).
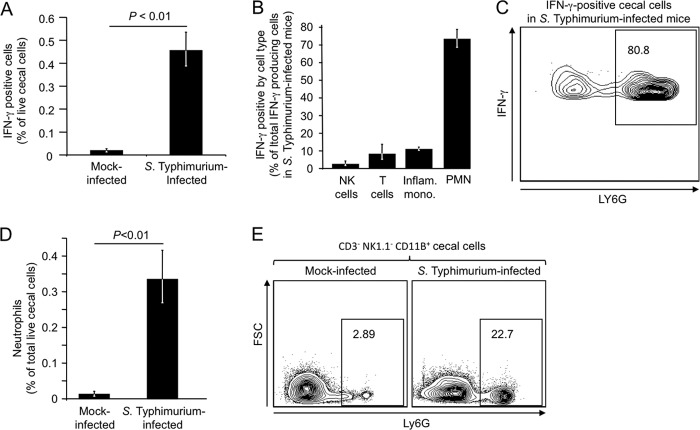
FIG 1.
Detection of IFN-γ-positive cells in the cecal mucosa by intracellular cytokine staining. Flow cytometric analysis of cecal cell suspensions generated 2 days after inoculation of streptomycin-pretreated mice (C57BL/6) with S. Typhimurium or sterile medium (mock infection) is shown. (A) Numbers of IFN-γ-positive cells in mock-infected or S. Typhimurium-infected mice are shown as a percentage of total live cecal cells. (B) Numbers of IFN-γ-positive NK cells, T cells, inflammatory monocytes (Inflam. mono.), and neutrophils (PMN) as a percentage of all live IFN-γ-positive cecal cells. (C) Representative flow cytometry image showing that a large fraction of IFN-γ-positive live cecal cells express the neutrophil marker LY6G. (D) Number of neutrophils as percentage of total live cecal cells. (E) Representative flow cytometry images from a mock-infected mouse (left panel) or an S. Typhimurium-infected mouse (right panel), showing the fraction of CD3− NK1.1− CD11B+ cells expressing the neutrophil marker LY6G. In panels A, B, and D, each bar represents the geometric mean ± standard error from flow cytometry measurements performed with 4 different animals.
To determine cellular populations for experiments using eYFP-Ifng reporter mice shown in Fig. 2, single-cell suspensions were surface stained with anti-CD3 APC (clone 17A2; Biolegend), anti-NK1.1 PECy7 (clone PK136; Biolegend), anti-CD11B APC/Cy7 (clone M1/70; eBioscience), anti-LY6C Pacific Blue ((clone HK1.4; Biolegend), and anti-LY6G PerCp/Cy5.5 (clone 1A8; Biolegend). Samples were then fixed for 1 h using BD Cytofix (BD 554714), washed twice in FACS buffer, and resuspended for FACS analysis.
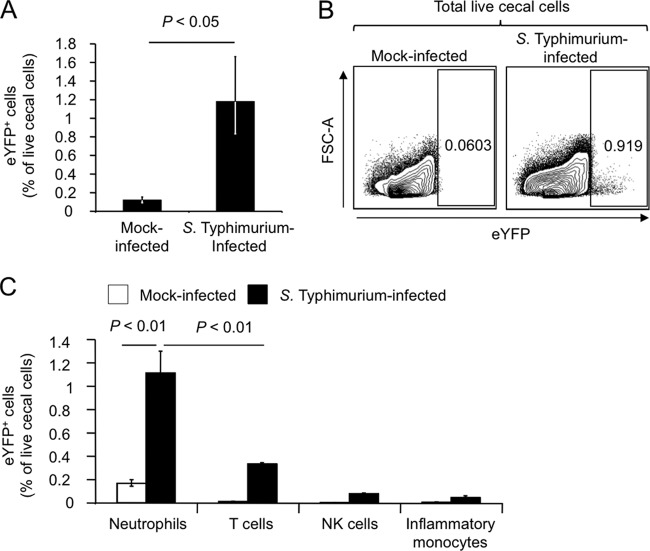
FIG 2.
Detection of Ifng expression in the cecal mucosa using an eYFP reporter. Streptomycin-pretreated mice expressing eYFP under the control of the Ifng promoter were inoculated with S. Typhimurium or sterile medium (mock infection) and cecal cell suspensions generated 2 days later. (A) Numbers of eYFP+ cells in mock-infected or S. Typhimurium-infected mice are shown as percentage of total live cecal cells. (B) Representative flow cytometry images from a mock-infected mouse (left panel) or an S. Typhimurium-infected mouse (right panel), showing eYFP+ cells among total live cecal cells. (C) Numbers of eYFP+ NK cells, eYFP+ T cells, eYFP+ inflammatory monocytes, and eYFP+ neutrophils in mock-infected or S. Typhimurium-infected mice are shown as percentage of total live cecal cells. In panels A and C, each bar represents the geometric mean ± standard error from flow cytometry measurements performed with 4 different animals.
For neutrophil depletion experiments, cell suspensions were surface stained for 20 min in the dark with anti-CD3 PE (clone 17A2; Biolegend), anti-NK1.1 PE (clone PK136; Biolegend), anti-B220 PE (clone RA3-6B2; Biolegend), anti-CD11B APC/Cy7 (clone M1/70; Biolegend), and anti-GR-1 APC (clone RB6-8C5).
Cells were washed twice, resuspended in FACS buffer, and analyzed using an LSR II (Beckman-Coulter, San Jose, CA) flow cytometer. The resulting data were analyzed using FlowJo software (TreeStar, Inc., Ashland, OR). All gates were based on fluorescence-minus-one controls.
Statistical analysis.
To determine statistical significance between treatment groups in the animal experiments, an unpaired Student t test was used. A P value of less than 0.05 was considered to be significant.
RESULTS
Neutrophils are a source of IFN-γ during S. Typhimurium colitis.
To investigate cellular sources of IFN-γ during gastroenteritis, streptomycin-pretreated mice (C57BL/6) were inoculated with S. Typhimurium or sterile medium (mock infection). In this model, mice inoculated with S. Typhimurium develop acute cecal inflammation, which recapitulates aspects of human gastroenteritis (19). We used flow cytometry to identify the innate cell types in the cecal mucosa that contributed to IFN-γ production during S. Typhimurium infection using intracellular cytokine staining. To detect cells producing IFN-γ during infection, single-cell suspensions from the cecum were generated 48 h after infection in the presence of brefeldin A, which blocks the release of IFN-γ by inhibiting protein transport from the endoplasmic reticulum to the Golgi apparatus. The 48-h time point was chosen since this is the time required for developing overt cecal inflammation after streptomycin-pretreated mice are infected with derivatives of S. Typhimurium strain ATCC 14028 (18).
Intracellular cytokine staining revealed that the fraction of cecal cells positive for IFN-γ was significantly (P < 0.01) increased in S. Typhimurium-infected mice compared to mock-infected mice (Fig. 1A). The populations of IFN-γ-positive cells in S. Typhimurium-infected mice were further differentiated using antibodies against CD3 (a T cell marker), NK1.1 (an NK cell marker), CD11B (a subunit of complement receptor [CR] 3), CD11C (a subunit of CR4), LY6C (a marker expressed by inflammatory monocytes and neutrophils), and LY6G (a neutrophil marker) (see Fig. S1 in the supplemental material). The LY6G/LY6C markers are superior to GR-1 (granulocyte differentiation antigen 1) and the mouse macrophage marker F4/80 in identifying neutrophils/monocytes (20). Several cell types, including T cells (IFN-γ+ CD3+ cells), NK cells (IFN-γ+ CD3− NK1.1+ cells), and inflammatory monocytes (IFN-γ+ CD3− NK1.1− CD11B+ LY6C+ LY6G− cells) were identified as minor contributors to IFN-γ production, with each accounting for 10% or less of the total number of IFN-γ-positive cells in the cecal mucosa (Fig. 1B; see Fig. S1 in the supplemental material). Remarkably, the vast majority of all IFN-γ-positive cells in the cecal mucosa of S. Typhimurium-infected mice were neutrophils (IFN-γ+ CD3− NK1.1− CD11B+ LY6C+ LY6G+ cells), which accounted for more than 70% of all IFN-γ-positive cells (Fig. 1B and C). CD3− B220− NK1.1− CD11B+ LY6C+ LY6G+ cells were analyzed by microscopy, which confirmed their neutrophil morphology (data not shown). We also quantified neutrophil recruitment into the cecal mucosa. This analysis suggested that S. Typhimurium infection was associated with a significant (P < 0.01) increase in the fraction of neutrophils (CD3− B220− NK1.1− CD11B+ LY6C+ LY6G+ cells) present in the live cecal cell population (Fig. 1D and E).
We next wanted to use an independent approach to test whether neutrophils are a cellular source of IFN-γ production during S. Typhimurium colitis. To this end, mice expressing an enhanced cyan fluorescence protein (eYFP) reporter cassette under the control of the Ifng promoter (21) were pretreated with streptomycin and inoculated with sterile medium (mock infection) or with S. Typhimurium. Single-cell suspensions from the cecum were generated 48 h after infection and analyzed by flow cytometry. The fraction of eYFP+ cells present in the cecal mucosa increased significantly (P < 0.05) in S. Typhimurium-infected mice compared to mock-infected mice (Fig. 2A and B). The population of eYFP+ cells was further differentiated using antibodies against relevant surface markers (CD3, NK1.1, CD11B, LY6C, and LY6G). Strikingly, the majority of eYFP+ cells in the cecal mucosa of S. Typhimurium-infected mice were neutrophils (eYFP+ CD3− NK1.1− CD11B+ LY6C+ LY6G+ cells) (Fig. 2C). Collectively, these data suggested that neutrophils recruited into the cecal mucosa during S. Typhimurium infection are a prominent early cellular source of IFN-γ.
LY6G depletion blunts early Ifng expression during S. Typhimurium colitis.
The hypothesis that neutrophils are a major cellular source of IFN-γ suggests that depletion of this cell type might result in a blunted mucosal expression of Ifng, the gene encoding IFN-γ, during S. Typhimurium infection. To test this prediction, LY6G-positive cells were depleted using intraperitoneal injection of an anti-LY6G antibody or an isotype control antibody (mock depletion) prior to pretreatment with streptomycin and inoculation with S. Typhimurium or sterile medium (mock infection). Depletion of neutrophils was confirmed using a panel of surface markers that were compatible with the anti-LY6G antibody used for depletion (CD3, NK1.1, B220, CD11B, and GR-1) (see Fig. S2 in the supplemental material). Analysis of cecal cell suspensions by flow cytometry revealed that treatment with anti-LY6G antibody significantly (P < 0.05) reduced the number of neutrophils (CD3− NK1.1− B220− CD11B+ GR-1High cells) recruited into the cecal mucosa at 48 h after S. Typhimurium infection (Fig. 3A). Compared to that in mock-depleted animals, LY6G depletion resulted in a significant (P < 0.05) increase in the bacterial load in the liver and spleen (Fig. 3B). Importantly, LY6G depletion significantly (P < 0.05) blunted Ifng expression in the cecal mucosa during S. Typhimurium infection (Fig. 3C). These data further supported the idea that neutrophils contribute to mucosal IFN-γ production during S. Typhimurium colitis.
FIG 3.
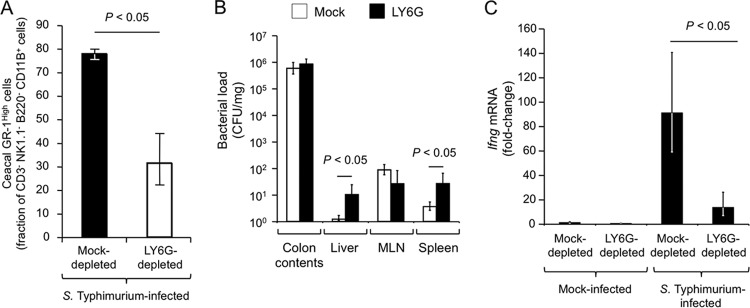
Neutrophil depletion blunts cecal Ifng expression during S. Typhimurium colitis. Streptomycin-pretreated mice were injected with an anti-LY6G antibody (LY6G depleted) or with an isotype control antibody (mock-depleted) and inoculated with S. Typhimurium or sterile medium (mock infection). Two days after infection, organs were collected for analysis. Each bar represents the geometric mean ± standard error from measurements performed with 4 different animals. (A) Number of neutrophils (GR-1High cells) as a percentage of the total number of phagocytes (CD3− NK1.1− B220− CD11B+ cells) present in live cecal cell populations. (B) Bacterial numbers recovered from cecal contents, livers, mesenteric lymph nodes (MLN), and spleens of mice. (C) Ifng expression was determined by quantitative real-time PCR. Data are expressed as fold change over transcript levels detected in mice that were mock infected and mock depleted.
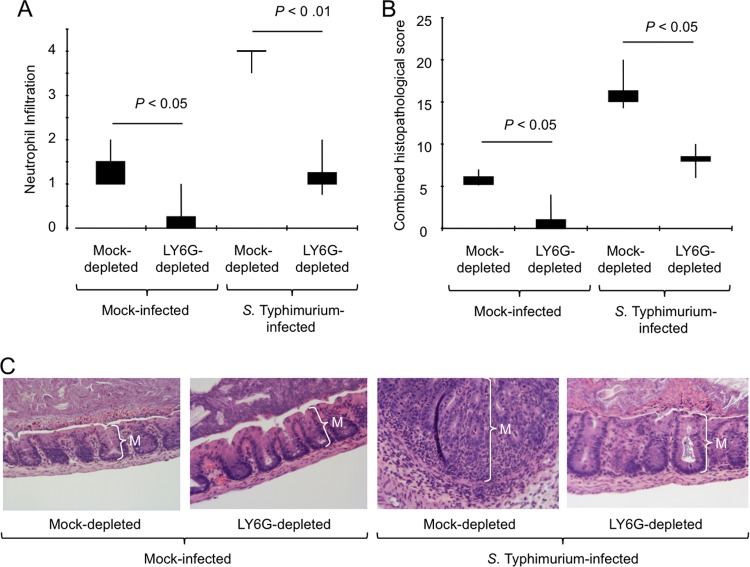
IFN-γ is an important driver of inflammation during S. Typhimurium colitis (7, 8). Depletion of a cell type contributing to mucosal IFN-γ production would thus be expected to reduce the severity of histopathological lesions induced during S. Typhimurium infection. To test this prediction, a veterinary pathologist performed blinded scoring of histopathological lesions in sections of the cecum collected from streptomycin-pretreated mock-depleted or LY6G-depleted mice at 48 h after inoculation with S. Typhimurium or sterile medium (mock infection). LY6G depletion significantly reduced the numbers of neutrophils detected in histological sections (Fig. 4A), which was consistent with results obtained by flow cytometry (Fig. 3A). Histopathological evaluation revealed epithelial erosion, inflammatory infiltrates in the mucosa, and edema in the submucosa of mock-depleted mice infected with S. Typhimurium. These pathological changes were significantly (P < 0.01) reduced in S. Typhimurium-infected LY6G-depleted mice (Fig. 4B and C).
FIG 4.
Neutrophil depletion reduces cecal pathology during S. Typhimurium colitis. Streptomycin-pretreated mice were injected with an anti-LY6G antibody (LY6G depleted) or with an isotype control antibody (mock depleted) and inoculated with S. Typhimurium or sterile medium (mock infection). (A and B) Two days after infection, organs were collected for analysis, and a veterinary pathologist performed blinded scoring of hematoxylin/eosin-stained sections from the cecal mucosa for neutrophil infiltration (A) and histopathological changes (B). Neutrophil infiltration was scored on the following scale: 0, 0 to 5 neutrophils per field; 1, 6 to 20 neutrophils per field; 2, 21 to 60 neutrophils per field; 3, 61 to 100 neutrophils per field; 4, >100 neutrophils per field. Boxes in whisker plots represent the second and third quartiles of scores, while lines indicate the first and fourth quartiles. (C) Representative images of histological sections from the ceca of mice taken at the same magnification. Note the marked thickening of the mucosa (M) due to neutrophil influx, which is observed in the section of an S. Typhimurium-infected, mock-depleted mouse.
DISCUSSION
Previous studies on S. Typhimurium-induced colitis have implicated NK cells and several subsets of T cells, including CD1D-restricted NKT cells, CD8+ T cells, and CD4+ T cells, in contributing to mucosal IFN-γ production (15, 18, 22, 23). Consistent with these reports, we detected intracellular IFN-γ staining in NK cells and in T cells isolated from the cecal mucosa. However, neutrophils comprised more than 70% of IFN-γ positive live cecal cells isolated 2 days after oral S. Typhimurium infection (Fig. 1B). Furthermore, neutrophil depletion resulted in a significant blunting of mucosal Ifng expression in the cecal mucosa 2 days after S. Typhimurium infection.
Consistent with the important role of IFN-γ in driving inflammatory changes in tissue (7, 8), the severity of histopathological lesions in the cecal mucosa was significantly reduced in S. Typhimurium-infected neutrophil-depleted mice. A marked reduction in the severity of histopathological changes in response to S. Typhimurium infection is also observed in ligated ileal loops of calves with CD18 deficiency, a primary immunodeficiency resulting in an inability to recruit neutrophils into the intestinal mucosa because CD18/CD11B (CR3) is required for neutrophil extravasation (24). However, neutrophil deficiency affects many aspects of host-microbe interaction that have to be considered when interpreting these data. While reduced levels of IFN-γ are one possible reason for diminishing the severity of intestinal lesions, it is also possible that neutrophils directly contribute to tissue damage, for example, by releasing reactive oxygen and nitrogen species. Similarly, although it is conceivable that blunted IFN-γ production contributes to an increased bacterial load in the spleens of neutrophil-depleted mice (Fig. 3B), an alternative explanation is that the bactericidal activity of neutrophils is required to prevent spread of bacteria beyond the mesenteric lymph node.
A common approach to detect cytokine expression by flow cytometry is to stimulate cell suspensions ex vivo with the protein kinase C agonist phorbol-12-myristate-13-acetate (PMA) in the presence of the ionophore ionomycin. PMA is a potent activator of the T cell antigen receptor on T cells. When cell suspensions from the cecum collected 48 h after S. Typhimurium infection are stimulated ex vivo with PMA-ionomycin prior to intracellular cytokine staining, approximately 50% of IFN-γ-positive cells are T cells (18). However, when we performed the same experiment in the absence of ex vivo PMA-ionomycin stimulation, T cells accounted for fewer than 10% of IFN-γ-positive cells (Fig. 1B). The latter result likely provides a more realistic view of cell types contributing to IFN-γ production in vivo, because the only stimulus provided was an in vivo infection with S. Typhimurium for 48 h. Importantly, PMA induces neutrophil cell death within 3 to 5 h after stimulation (25–27), thereby depleting this cell type prior to analysis of cytokine staining in live cells by flow cytometry. Thus, the widespread use of ex vivo PMA-ionomycin stimulation might be one of the reasons why neutrophils are not commonly detected as a major cellular source of IFN-γ.
While NK cells and NKT cells are commonly thought of as the primary sources of IFN-γ during the innate phase of a bacterial infection (reviewed in reference 9), there is mounting evidence that neutrophils are another important early source of this cytokine. For example, neutrophils produce IFN-γ during acute necrotizing pneumonia caused by Nocardia asteroides infection of mice (28). Neutrophils are an early source of IFN-γ after intravenous infection of mice with Listeria monocytogenes (29). Neutrophils are a cellular source of IFN-γ in splenocyte populations collected from mice 3 days after oral S. Typhimurium infection (30). Furthermore, neutrophils collected from the peritoneal cavities of mice 5 days after intraperitoneal S. Typhimurium infection produce IFN-γ (31). Our results suggest that neutrophils are also a prominent cellular source of mucosal IFN-γ production during S. Typhimurium-induced colitis. Induction of Ifng expression in the cecal mucosa at 2 days after oral S. Typhimurium infection is MYD88 dependent (18). Similarly, IFN-γ production elicited by neutrophils recovered from the peritoneal cavities of mice 5 days after intraperitoneal infection with Toxoplasma gondii is MYD88 dependent (31). Collectively, these data suggest that neutrophils are an important cellular source of IFN-γ during the innate phase of a bacterial infection.
Supplementary Material
ACKNOWLEDGMENTS
Work in A.J.B.'s laboratory was supported by Public Health Service grants AI044170, AI076246, and AI096528. D.D.K. was supported by Public Health Services grant OD11147. We also acknowledge NIH facilities infrastructure grant RR12088.
We do not declare conflicts of interest.
Footnotes
Published ahead of print 13 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01508-13.
REFERENCES
- 1.de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J, Al-Muhsen S, Janniere L, Rose Y, de Suremain M, Kong XF, Filipe-Santos O, Chapgier A, Picard C, Fischer A, Dogu F, Ikinciogullari A, Tanir G, Al-Hajjar S, Al-Jumaah S, Frayha HH, Al Sum Z, Al-Ajaji S, Alangari A, Al-Ghonaium A, Adimi P, Mansouri D, Ben-Mustapha I, Yancoski J, Garty BZ, Rodriguez-Gallego C, Caragol I, Kutukculer N, Kumararatne DS, Patel S, Doffinger R, Exley A, Jeppsson O, Reichenbach J, Nadal D, Boyko Y, Pietrucha B, Anderson S, Levin M, Schandene L, Schepers K, Efira A, Mascart F, Matsuoka M, Sakai T, Siegrist CA, Frecerova K, Bluetters-Sawatzki R, Bernhoft J, et al. 2010. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine 89:381–402. 10.1097/MD.0b013e3181fdd832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang S, Kingsley RA, Santos RL, Andrews-Polymenis H, Raffatellu M, Figueiredo J, Nunes J, Tsolis RM, Adams LG, Bäumler AJ. 2003. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhea. Infect. Immun. 71:1–12. 10.1128/IAI.71.1.1-12.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos RL, Raffatellu M, Bevins CL, Adams LG, Tukel C, Tsolis RM, Baumler AJ. 2009. Life in the inflamed intestine, Salmonella style. Trends Microbiol. 17:498–506. 10.1016/j.tim.2009.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glynn JR, Palmer SR. 1992. Incubation period, severity of disease, and infecting dose: evidence from a Salmonella outbreak. Am. J. Epidemiol. 136:1369–1377 [DOI] [PubMed] [Google Scholar]
- 5.Tsolis RM, Adams LG, Ficht TA, Baumler AJ. 1999. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 67:4879–4885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos RL, Zhang S, Tsolis RM, Bäumler AJ, Adams LG. 2002. Morphologic and molecular characterization of Salmonella typhimurium infection in neonatal calves. Vet. Pathol. 39:200–215. 10.1354/vp.39-2-200 [DOI] [PubMed] [Google Scholar]
- 7.Rhee SJ, Walker WA, Cherayil BJ. 2005. Developmentally regulated intestinal expression of IFN-gamma and its target genes and the age-specific response to enteric Salmonella infection. J. Immunol. 175:1127–1136 [DOI] [PubMed] [Google Scholar]
- 8.Awoniyi M, Miller SI, Wilson CB, Hajjar AM, Smith KD. 2012. Homeostatic regulation of Salmonella-induced mucosal inflammation and injury by IL-23. PLoS One 7:e37311. 10.1371/journal.pone.0037311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun JC, Lanier LL. 2011. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat. Rev. Immunol. 11:645–657. 10.1038/nri3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takada K, Jameson SC. 2009. Naive T cell homeostasis: from awareness of space to a sense of place. Nat. Rev. Immunol. 9:823–832. 10.1038/nri2657 [DOI] [PubMed] [Google Scholar]
- 11.O'Garra A, Robinson D. 2004. Development and function of T helper 1 cells. Adv. Immunol. 83:133–162. 10.1016/S0065-2776(04)83004-9 [DOI] [PubMed] [Google Scholar]
- 12.Ramarathinam L, Niesel DW, Klimpel GR. 1993. Salmonella typhimurium induces IFN-gamma production in murine splenocytes. Role of natural killer cells and macrophages. J. Immunol. 150:3973–3981 [PubMed] [Google Scholar]
- 13.Lapaque N, Walzer T, Meresse S, Vivier E, Trowsdale J. 2009. Interactions between human NK cells and macrophages in response to Salmonella infection. J. Immunol. 182:4339–4348. 10.4049/jimmunol.0803329 [DOI] [PubMed] [Google Scholar]
- 14.Kupz A, Scott TA, Belz GT, Andrews DM, Greyer M, Lew AM, Brooks AG, Smyth MJ, Curtiss R, III, Bedoui S, Strugnell RA. 2013. Contribution of Thy1+ NK cells to protective IFN-gamma production during Salmonella typhimurium infections. Proc. Natl. Acad. Sci. U. S. A. 110:2252–2257. 10.1073/pnas.1222047110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berntman E, Rolf J, Johansson C, Anderson P, Cardell SL. 2005. The role of CD1d-restricted NK T lymphocytes in the immune response to oral infection with Salmonella typhimurium. Eur. J. Immunol. 35:2100–2109. 10.1002/eji.200425846 [DOI] [PubMed] [Google Scholar]
- 16.Selvanantham T, Escalante NK, Cruz Tleugabulova M, Fieve S, Girardin SE, Philpott DJ, Mallevaey T. 2013. Nod1 and Nod2 enhance TLR-mediated invariant NKT cell activation during bacterial infection. J. Immunol. 191:5646–5654. 10.4049/jimmunol.1301412 [DOI] [PubMed] [Google Scholar]
- 17.Stojiljkovic I, Bäumler AJ, Heffron F. 1995. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J. Bacteriol. 177:1357–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keestra AM, Godinez I, Xavier MN, Winter MG, Tsolis RM, Bäumler AJ. 2011. Early, MyD88-dependent induction of interleukin-17A expression during Salmonella colitis. Infect. Immun. 79:3131–3140. 10.1128/IAI.00018-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839–2858. 10.1128/IAI.71.5.2839-2858.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose S, Misharin A, Perlman H. 2012. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A 81:343–350. 10.1002/cyto.a.22012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinhardt RL, Liang HE, Locksley RM. 2009. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 10:385–393. 10.1038/ni.1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrington L, Srikanth CV, Antony R, Shi HN, Cherayil BJ. 2007. A role for natural killer cells in intestinal inflammation caused by infection with Salmonella enterica serovar Typhimurium. FEMS Immunol. Med. Microbiol. 51:372–380. 10.1111/j.1574-695X.2007.00313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godinez I, Haneda T, Raffatellu M, George MD, Paixao TA, Rolan HG, Santos RL, Dandekar S, Tsolis RM, Baumler AJ. 2008. T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect. Immun. 76:2008–2017. 10.1128/IAI.01691-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunes JS, Lawhon SD, Rossetti CA, Khare S, Figueiredo JF, Gull T, Burghardt RC, Baumler AJ, Tsolis RM, Andrews-Polymenis HL, Adams LG. 2010. Morphologic and cytokine profile characterization of Salmonella enterica serovar typhimurium infection in calves with bovine leukocyte adhesion deficiency. Vet. Pathol. 47:322–333. 10.1177/0300985809358037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takei H, Araki A, Watanabe H, Ichinose A, Sendo F. 1996. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 59:229–240 [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, Namiki H. 1998. Phorbol 12-myristate 13-acetate induced cell death of porcine peripheral blood polymorphonuclear leucocytes. Cell Struct. Funct. 23:367–372. 10.1247/csf.23.367 [DOI] [PubMed] [Google Scholar]
- 27.Witting PK, Zeng B, Wong M, McMahon AC, Rayner BS, Sapir AJ, Lowe HC, Freedman SB, Brieger DB. 2008. Polymorphonuclear leukocyte phagocytic function increases in plasminogen knockout mice. Thromb. Res. 122:674–682. 10.1016/j.thromres.2007.12.013 [DOI] [PubMed] [Google Scholar]
- 28.Ellis TN, Beaman BL. 2002. Murine polymorphonuclear neutrophils produce interferon-gamma in response to pulmonary infection with Nocardia asteroides. J. Leukoc. Biol. 72:373–381 [PubMed] [Google Scholar]
- 29.Yin J, Ferguson TA. 2009. Identification of an IFN-gamma-producing neutrophil early in the response to Listeria monocytogenes. J. Immunol. 182:7069–7073. 10.4049/jimmunol.0802410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirby AC, Yrlid U, Wick MJ. 2002. The innate immune response differs in primary and secondary Salmonella infection. J. Immunol. 169:4450–4459 [DOI] [PubMed] [Google Scholar]
- 31.Sturge CR, Benson A, Raetz M, Wilhelm CL, Mirpuri J, Vitetta ES, Yarovinsky F. 2013. TLR-independent neutrophil-derived IFN-gamma is important for host resistance to intracellular pathogens. Proc. Natl. Acad. Sci. U. S. A. 110:10711–10716. 10.1073/pnas.1307868110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.