Abstract
Production of Pfs25, a Plasmodium falciparum transmission-blocking vaccine target antigen, in functional conformation with the potential to elicit effective immunogenicity still remains a major challenge. In the current study, codon-harmonized recombinant Pfs25 (CHrPfs25) was expressed in Escherichia coli, and purified protein after simple oxidative refolding steps retained reduction-sensitive conformational epitopes of transmission-blocking monoclonal antibodies. CHrPfs25 formulated in several adjuvants elicited strong immunogenicity in preclinical studies in mice. Antibodies elicited after immunization recognized native Pfs25 on the surface of live gametes of P. falciparum and demonstrated complete malaria transmission-blocking activity. The transmission-blocking efficacy was 100% even after a 1:128 dilution of sera from immunized mice in the complete Freund's adjuvant and Montanide ISA51 groups and after a 1:16 dilution of sera from mice in the alum group. The blocking was mediated by antibodies; purified IgG at concentrations as low as 31.25 μg/ml exhibited 100% transmission blocking in membrane feeding assays employing two different species of mosquitoes, Anopheles gambiae and Anopheles stephensi. This study provides the first evidence for successful expression of biologically functional rPfs25 in E. coli. The extremely potent malaria transmission-blocking activity of antibodies elicited by immunization with purified protein provides strong support for further evaluation of E. coli-derived CHrPfs25 as a malaria transmission-blocking vaccine in human clinical trials.
INTRODUCTION
Nearly half the world's population lives in regions where malaria is endemic, and this accounts for approximately 219 million clinical cases with up to 660,000 deaths, mostly of children under five years of age (1, 2). The parasite has displayed a remarkable ability to develop resistance to almost any antimalaria drug used, and there has been a recent report on artemisinin-resistant Plasmodium falciparum in Cambodia (3). While efforts to develop vaccines targeting various life cycle stages of the parasite are under way, in both the human host and the mosquito vector, none is available currently. Vaccines targeting the transmission stages of the parasites, the sexual stages, are considered essential to achieve the goal of gradual elimination of malaria. Malaria transmission reduction can be achieved either by blocking the development of gametocytes, the sexual stages of the parasite, or by reducing further development of these transmission stages in the mosquito vector (4–6). Recent emphasis on global elimination and eradication of malaria has outlined a critical role for a malaria transmission-blocking vaccine (TBV) as an effective tool for reducing malaria transmission.
The long-term success of a TBV depends upon induction of high functional antibody titers in order to effectively block the parasite transmission cycle (7). In P. falciparum, Pfs230, Pfs48/45, and Pfs25 have been identified as target antigens, and antibodies directed against any of these antigens are capable of effectively reducing malaria transmission (8–13), with similar homologs identified in Plasmodium vivax. Malaria caused by P. falciparum and P. vivax, species that are coendemic in many areas, accounts for greater than 90% of total malaria cases, and TBVs targeting these two species can play a significant role in malaria elimination. Among these target antigens, most significant progress has been achieved with Pfs25 and the P. vivax homolog Pvs25. These studies on adjuvant-formulated recombinant Pfs25 expressed in yeast (Saccharomyces cerevisiae and Pichia pastoris) (12, 14), cell-free translation using wheat germ (15), plants (16), and an algal system (17), and DNA vaccines (18–21) have provided unequivocal support for Pfs25 as an effective target antigen. Pfs25 is a 25-kDa surface protein containing an N-terminal signal sequence followed by four epidermal growth factor (EGF)-like domains with 11 disulfide bonds and a C-terminal glycosylphosphatidylinositol (GPI) anchor sequence (11, 22). However, a phase I clinical trial with Pfs25 expressed in P. pastoris formulated in Montanide ISA51 adjuvant showed only moderate immunogenicity in human volunteers (23). While the reasons for low functional immunogenicity in phase I trials remain open to speculation, Pfs25 expressed in yeast was highly heterogeneous in nature, consisting of two major isoforms (A and B) (24, 25). Several attempts have been made to enhance the immunogenicity of yeast-derived Pfs25, including coadministration with cholera toxin as an adjuvant (26), chemical conjugation of Pfs25 linked with outer membrane protein of Neisseria meningitides serogroup B (27) or recombinant Pseudomonas aeruginosa exotoxin A (25), and use of nonconjugated or conjugated Pfs25 with lichenase carrier protein (LickM) produced in plants (16).
Despite the progress in expressing recombinant proteins, including Pfs25, in different recombinant systems, Escherichia coli still remains a preferred host for ease of use and cost-effective production and purification of recombinant proteins for use as biological products and vaccines. Recombinant expression of P. falciparum proteins in E. coli has been problematic due to codon bias and formation of aberrant disulfide bonds, resulting in an inaccurately folded and highly heterogeneous mix of oligomeric forms of the purified product. Pfs25 contains 22 conserved cysteine residues, and all 11 disulfide bonds are important for structural integrity of the molecule (11, 22). Mispairing of cysteine residues is accompanied by misfolding or aggregation of proteins, requiring solubilization and protein refolding and resulting in low yields of functional molecules (28, 29). A previous study on attempts to express Pfs25 in E. coli reaffirmed all the points described above (30). Our lab has recently revisited the issue of E. coli expression of P. falciparum proteins, especially those that require proper disulfide bond pairing. Recombinant Pfs48/45 expressed after codon harmonization (9) was found to retain functional transmission-blocking immunogenicity. In codon harmonization, synonymous codons having usage frequencies in E. coli that are equal to or less than the usage frequencies in the native expression host are replaced, including rare codons present at link/end segments (31). In the current study, we present results on the expression and purification of codon-harmonized recombinant Pfs25 (CHrPfs25) in E. coli in an appropriate monomeric conformation, which elicited highly potent (100% reduction) malaria transmission-blocking antibody (TBA) responses.
MATERIALS AND METHODS
Molecular cloning and expression of Pfs25 in E. coli.
The coding sequence of Pfs25 (accession number PF3D7_1031000, www.plasmoDB.org) was codon harmonized (31), and the CHrPfs25 sequence (lacking N-terminal signal and C-terminal GPI anchor residues) fused with 6 histidines at the 5′end was cloned in the expression vector pET (K−). E. coli BL21 cells (Invitrogen) transformed with CHrPfs25-pET (K−) were grown to an optical density (OD) of 1.00, followed by induction with 100 μM IPTG (isopropyl-β-d-thiogalactopyranoside) for an additional 3 h at 25°C.
Purification and refolding of CHrPfs25.
The cells were lysed, and after centrifugation (18,000 × g, 45 min) the cell pellet was washed with phosphate-buffered saline (PBS) (pH 7.4) and resuspended in 100 mM Tris, pH 12.0. The pH of the supernatant was adjusted to 6.8 and incubated with Ni-nitrilotriacetic acid (NTA) agarose beads in the presence of 10 mM imidazole for 6 h at 4°C. The beads were washed with Tris buffer containing 20 mM imidazole and 30 mM imidazole, and protein refolding was achieved by washing the beads with a 10:1 ratio of reduced/oxidized glutathione in Tris buffer. The bound protein was eluted with 100 mM imidazole and treated with the glutathione refolding steps, followed by dialysis using PBS (pH 6.9) at 4°C. The protein quality was assessed by SDS-PAGE and Western blotting, and the protein concentration was measured by the bicinchoninic acid (BCA) method (Thermo Scientific). Endotoxin values determined with a Limulus amoebocyte lysate (LAL) chromogenic endotoxin quantitation kit (Thermo Scientific) ranged between 0.7 and 7.2 endotoxin units (EU)/ml among >8 different batches of purified CHrPfs25.
SDS-PAGE and Western blotting.
Protein samples or purified gametes were mixed with SDS-PAGE loading buffer with or without 5% β-mercaptoethanol (Sigma-Aldrich), heated for 5 min at 100°C, and resolved on 12.5% SDS-polyacrylamide gels. After electrophoretic transfer, nitrocellulose membranes were blocked with 1% nonfat dry milk in PBS and 0.01% Tween 20. After incubation with various antibodies, membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-mouse IgG and developed using the Amersham ECL Prime Western blotting detection reagent (GE Healthcare).
Immunizations.
Female BALB/c mice (n = 5) were immunized with 10 μg of CHrPfs25 emulsified in complete Freund's adjuvant (CFA) (Sigma-Aldrich) or Montanide ISA51 (Seppic) or mixed with aluminum hydroxide (alhydrogel) (Brenntag Biosector) through the intraperitoneal (i.p.) route in a total volume of 0.1 ml/mouse. Mice received booster doses at 3-week intervals with 10 μg of protein in the same adjuvants except that incomplete Freund's adjuvant was used for mice immunized with CFA. Animals in the alum group received a fourth dose of protein vaccine. Bleeds were collected 21 days after the first dose and 10 days after each booster dose. Sera from mice immunized with PBS-adjuvant formulations only were used as negative controls in all the experiments.
Evaluation of antibody by ELISA and live immunofluorescence assay (IFA).
The antibody titers in the sera were determined by standardized enzyme-linked immunosorbent assay (ELISA) (21). ELISA plates (Immulon 2) were coated with 1.5 μg/ml CHrPfs25 in 0.1 M carbonate-bicarbonate buffer (pH 9.6). The avidity of antibodies was analyzed by ELISA as described previously (21). Extracellular gametes were purified by discontinuous Nycodenz gradient centrifugation from P. falciparum NF54 gametocyte cultures (32, 33) and incubated with mouse sera (dilutions of 1:500 and 1:1,000) for 30 min at 4°C, followed by further incubation with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG. The reactivity of antisera was analyzed using an Olympus microscope and Q color 3 camera (Olympus America Inc., PA) under a 100× oil immersion objective.
Purification of murine IgG.
Total IgG was purified using protein A-Sepharose beads. Pooled mouse sera diluted (1:1) with the binding buffer (1.5 M glycine, 3 M NaCl, pH 9.0) were incubated for 2 to 3 h at 4°C with protein A-Sepharose beads. The beads were washed with 20 ml of binding buffer and IgG eluted with 0.2 M glycine (pH 2.5) in tubes containing 1 M Tris-HCl, pH 8.0. The total IgG concentration was determined by the BCA method, quality was assessed by SDS-PAGE under reducing and nonreducing conditions, and buffer exchange was carried out using PBS (pH 7.4).
MFA.
The transmission-blocking activities of mice sera and purified total IgG were tested by standard membrane feeding assay (MFA) (21). Various test or control sera or total IgG was tested at the indicated dilution or concentration, and the parasite infectivity was measured 8 to 9 days after the blood meal by counting the number of oocysts per midgut by microscopy.
Data analysis and statistics.
Antibody endpoint titers were defined as serum dilutions giving an absorbance higher than the average OD at 405 nm of preimmune serum + 2 standard deviations (SD). Percent inhibition of oocyst development per mosquito was determined using the formula 100 × [(mean oocyst number with control sera − mean oocyst number with test sera)/(mean oocyst number with control sera)]. Statistical analysis was performed using the GraphPad Instat3 software package. The differences between experimental groups were analyzed by the one-way analysis of variance (ANOVA) Kruskal-Wallis test. P values of <0.05 were considered significant.
RESULTS
Purification, refolding, and characterization of CHrPfs25.
Pfs25 contains 11 disulfide bonds, which are absolutely critical for functional immunogenicity. Previous attempts to express properly folded Pfs25 in E. coli have been unsuccessful (30), most likely as a result of a high level of codon mismatch between P. falciparum and E. coli. We modified the Pfs25 sequence prior to cloning by harmonization of the coding sequence, and recombinant CHrPfs25 protein was successfully expressed in E. coli as shown by SDS-PAGE (Fig. 1A). The expression of the protein was confirmed by Western blotting using anti-His antibody (Fig. 1B). A multistep purification and refolding protocol was developed and standardized for the purification of CHrPfs25 from the inclusion bodies, and the protein yield varied between 9 and 13 mg/liter for 8 independent bacterial cultures. Figure 1C and D show the SDS-PAGE profile and identity of monomeric purified CHrPfs25 used for immunogenicity evaluation studies.
FIG 1.

Expression, purification, and characterization of codon-harmonized recombinant Pfs25 (CHrPfs25). (A) Expression of CHrPfs25 in E. coli. Lane 1, protein molecular mass standards; lane 2, bacterial lysate prior to induction; lanes 3 and 4, bacterial lysate induced with 100 μM IPTG. (B) Confirmation of expressed protein from bacterial lysate by Western blot analysis using anti-His antibody. (C) SDS-PAGE analysis of refolded purified protein. (D to F) Characterization of refolded protein by Western blotting using anti-His antibody (D) and Pfs25-specific monoclonal antibodies ID3 (E) and 4B7 (F). In panels E and F, protein samples were run under nonreduced (lane 1) and reduced (lane 2) conditions for analysis.
To confirm that the refolded purified protein retained the appropriate conformational epitopes, the reactivity of conformation-dependent or -independent Pfs25-specific monoclonal antibodies ID3 and MRA-4B7, respectively, was evaluated under nonreducing and reducing conditions. Both ID3 and MRA-4B7 recognized the nonreduced form of the protein (Fig. 1E and F), while the ID3 reactivity was completely abolished upon reduction of CHrPfs25 (Fig. 1E), suggesting the presence of reduction-sensitive conformational epitopes as recognized in the native protein.
Evaluation of immunogenicity and avidity of antibodies.
In order to assess the immunogenicity of CHrPfs25 expressed in E. coli, sera from mice immunized in three different adjuvants were analyzed for antibody titers by ELISA. Significant antibody titers were elicited after just a single-dose immunization, and the antibody titers increased after each booster dose, with ELISA titers reaching 640,000 in the CFA and Montanide groups and 160,000 in the alum group (Table 1). No Pfs25-specific antibodies were detected in mice immunized with adjuvants alone. Next, we compared antibody avidity in all the above-mentioned sera using various concentrations of NaSCN to disrupt the antigen-antibody association. As shown in Fig. 2, the NaSCN concentrations needed to cause 50% dissociation were comparable in all the three adjuvant groups, indicating that the antibodies elicited were not qualitatively different.
TABLE 1.
Evaluation of anti-Pfs25 antibody endpoint ELISA titers in sera from mice immunized with CHrPfs25 in different adjuvants
| Adjuvant | Anti-Pfs25 ELISA titer |
|||
|---|---|---|---|---|
| Prime | First boost | Second boost | Third boost | |
| CFA | >12,800 | 640,000 | 640,000 | |
| Montanide ISA51 | >12,800 | 640,000 | 640,000 | |
| Alum | >12,800 | 10,000 | 80,000 | 160,000 |
FIG 2.
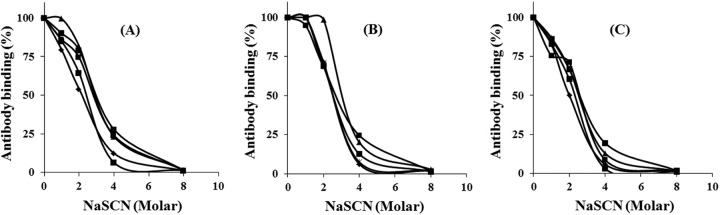
Analysis of antibody-antigen binding strength. Avidity of anti-Pfs25 was evaluated in sera from mice immunized with CHrPfs25 in different adjuvants: CFA (A), Montanide ISA51 (B), and alum (C). Sera were tested individually, and increasing concentrations of NaSCN (0, 2, 4, 6, and 8 M) were used prior to development of the ELISA reaction.
Recognition of native Pfs25 on purified P. falciparum gametes by live IFA and Western blotting.
Immune sera in all the adjuvant groups showed strong surface recognition of native Pfs25 protein in live parasites (Fig. 3I shows immunofluorescence assay [IFA] data at a 1:1,000 dilution of control and immune sera). Further evidence for the recognition of native parasite antigen was obtained by Western blot analysis using lysates of purified gametes with reducing and nonreducing SDS-PAGE. Immune sera from mice, as well as conformation-dependent Pfs25-specific monoclonal antibody ID3, recognized the nonreduced form of native protein in the parasite lysates (Fig. 3II).
FIG 3.
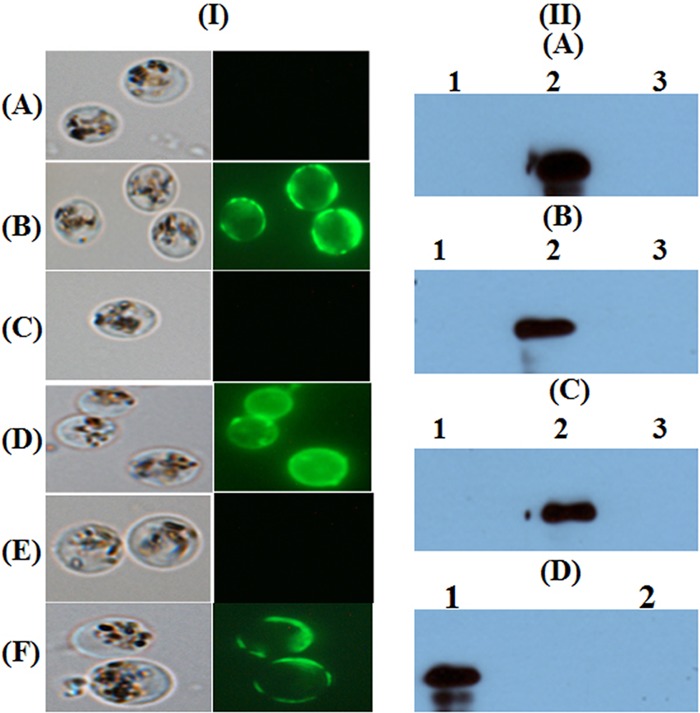
(I) Recognition of native protein on the surface of P. falciparum parasites by anti-Pfs25 antibodies using live immunofluorescence assay with the CFA (A and B), Montanide ISA51 (C and D), and alum (E and F) adjuvant groups. (A, C, and E) Control sera from the respective adjuvant-only group; (B, D, and F) immune sera (serum dilution, 1:1,000). (II) Western blot analysis. Extracts of purified gametes were separated by SDS-PAGE under nonreducing and reducing conditions. (A, B, and C) Reactivity of sera from the CFA, Montanide ISA51, and alum groups, respectively (lanes 1, control sera; lanes 2 and 3, immune sera; lanes 1 and 2, nonreduced samples; lanes 3, reduced samples). (D) Reactivity of Pfs25-specific monoclonal antibody ID3 with nonreduced (lane 1) and reduced (lane 2) protein.
Evaluation of TBA by MFA.
The transmission-blocking efficacy of antibodies was determined by MFA. Initially, individual immunized mouse sera were tested at a 1:2 dilution in MFA, along with control and normal human serum (NHS) tested in parallel. While all the immune sera showed 100% transmission-blocking activity (TBA) in Anopheles gambiae mosquitoes (data not shown), there was some level of nonspecific toxicity from sera (at a 1:2 dilution) from control mice immunized with adjuvants alone, with toxicity diminishing at further serum dilutions (1:4 onwards). In order to assess the specific transmission-blocking potency of immune sera, we next evaluated all the immune sera at various 2-fold serial dilutions (1:2 to 1:128) (Fig. 4) along with dilutions of control sera. One hundred percent TBA (not a single oocyst detected) was demonstrated in the presence of immune sera diluted 1:32 (CFA group) (Fig. 4A), 1:64 (Montanide ISA51 group) (Fig. 4B), and 1:8 (alum group) (Fig. 4C). The blocking efficacy (>98%) of sera was evident even after a 1:128 dilution of serum in the CFA and Montanide groups and after a 1:32 dilution in the alum group. These results demonstrate that sera from mice immunized with CHrPfs25 formulated in three adjuvants are extremely potent blockers of malaria transmission as assessed through mosquito membrane feeding biological assays.
FIG 4.
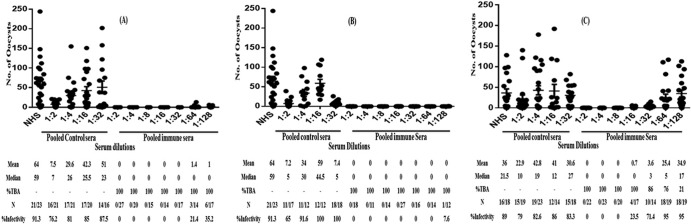
Transmission-blocking activity (TBA) and infectivity prevalence in A. gambiae mosquitoes as determined by membrane feeding assay (MFA). Mature gametocytes of P. falciparum (NF54) were mixed with various dilutions (1:2 to 1:128) of anti-Pfs25 serum. (A) Complete Freund's adjuvant (CFA); (B) Montanide ISA51 adjuvant; (C) alum adjuvant. All sera were tested at various dilutions in the same membrane feeding assay, and data points represent the number of oocysts per mosquito. Statistically significant differences in TBA based on median values between groups were analyzed with the one-way analysis of variance (ANOVA) Kruskal-Wallis test. Statistical significance was set at a P value of <0.0001. N represents infected/total mosquitoes dissected.
In order to obtain indisputable evidence that the TBA is indeed due to antibodies elicited by immunization, purified IgG was tested in MFA at various concentrations using A. gambiae. One hundred percent reduction in the development of oocysts was demonstrated with a concentration of 31.25 μg/ml of total IgG purified from sera from mice immunized with the Montanide ISA51 (Fig. 5B) and alum (Fig. 5C) formulations, while 62.5 μg/ml of total IgG from the CFA group showed 100% transmission-blocking activity (Fig. 5A). While A. gambiae is the major vector in Africa, Anopheles stephensi is well recognized as the major malaria vector in Asia. We also tested purified total IgG (125 and 62.5 μg/ml) in MFA employing A. stephensi mosquitoes. Once again, 100% transmission-blocking efficacy of purified IgG from all the three adjuvant groups was demonstrated (Fig. 6), thus ruling out any mosquito vector restriction for Pfs25 antibody-mediated transmission reduction.
FIG 5.
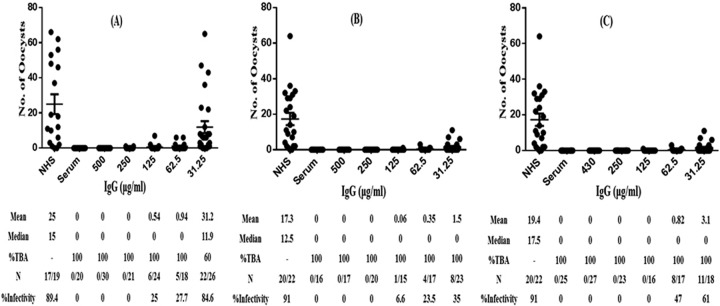
Functional activity of purified antibodies as determined by MFA. Total IgG from pooled sera was purified using a protein A-Sepharose column and tested at different concentrations (μg/ml) for evaluation of TBA and infectivity prevalence in A. gambiae mosquitos. (A) CFA; (B) Montanide ISA51; (C) alum. Pooled serum (dilution, 1:8) from CHrPfs25 formulated in the respective adjuvant group and normal human serum (NHS) were used as controls in the same membrane feeding assay. Statistically significant differences in TBA were analyzed as described for Fig. 4. Statistical significance was set at a P value of <0.0001. N represents infected/total mosquitoes dissected.
FIG 6.
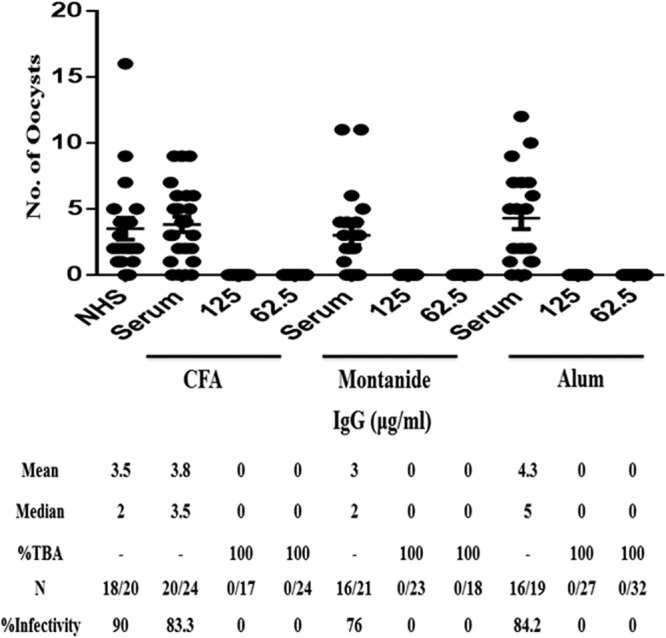
Evaluation of TBA and parasite infectivity via MFA in A. stephensi. P. falciparum NF54 gametocytes mixed with total IgG from different adjuvant groups (CFA, Montanide ISA51, and alum) at concentrations of 125 and 62.5 μg/ml were tested in MFA. NHS and pooled control serum (dilution, 1:8) from the respective adjuvant-only group were used as control sera in the same assay. Statistically significant differences in TBA were analyzed as described for Fig. 4. Statistical significance was set at a P value of <0.0001. N represents infected/total mosquitoes dissected.
DISCUSSION
Recent years have witnessed significant strides in the direction of development of malaria vaccines; however, no effective vaccine is yet available for the prevention of malaria, lessening malaria morbidity or mortality, or reducing malaria transmission. The last has been recognized to likely play a crucial role in achieving the goal of gradual elimination of malaria. Malaria transmission-blocking immunity targets the sexual development of the parasite within the mosquito midgut, and antibodies effectively compromise the survival of extracellular gametes, prevent fertilization between male and female gametes, or interfere with the subsequent development of zygotes into motile ookinetes (4, 5). Any of these outcomes will amount to reduced infectivity of sexual stages in the mosquito, which will be reflected in reduced malaria transmission and eventually ease the burden of the disease.
Of the three target antigens (Pfs230, Pfs48/45, and Pfs25) (4, 5, 9, 11, 34, 35), most significant progress has been achieved with Pfs25, including phase I clinical trials with yeast-derived Pfs25 and Pvs25. Further efforts are needed to improve the rather minimal immunogenicity observed in phase I clinical trials (16, 25–27, 36). The molecular heterogeneity of expressed Pfs25, the paucity of effective adjuvants, and retention of native conformation in the rPfs25 used for vaccine formulations are also some of the major factors contributing to poor trial outcomes.
For the production of recombinant biologics, E. coli still remains the system of choice for its ease and low overall costs involved. Previously, recombinant Pfs25-TrpE fusion protein was expressed in E. coli; however, the protein was not folded correctly, as suggested by lack of recognition by transmission-blocking monoclonal antibodies (30) and failure to elicit transmission-blocking antibodies. In the current study, we successfully expressed and purified Pfs25 in E. coli with high yield following application of codon harmonization of the coding-gene sequence. Codon harmonization not only optimizes the native sequence for expression in a heterologous expression system, it also regulates the rate of protein synthesis by controlling ribosomal pausing to facilitate simultaneous protein folding (31). While the approach was successful, the initial limitation of this approach was the expression of recombinant CHrPfs25 protein sequestered in inclusion bodies. Therefore, the protocol for protein purification was optimized to achieve conformational epitope formation while minimizing protein oligomerization and thus further improving the quality and stability of the purified monomeric protein.
High concentrations of chaotropes used for solubilization of inclusion bodies often result in suboptimal protein refolding and reduced recovery of bioactive protein molecules (29, 37). First, we opted to employ alternate ways to solubilize inclusion bodies using brief exposure to high pH (29), and the second consideration was protein refolding to obtain Pfs25 in the appropriate conformation (11, 22, 38). It is well known that the correct pairing of cysteine residues in the protein has important consequences for protein folding, protein stability, and biological function. Using an optimized ratio of reduced to oxidized glutathione (GSH to GSSG), we were successful in oxidative refolding of Pfs25 in the appropriate conformation. Oxidized and reduced forms of glutathione facilitate efficient reduction and/or isomerization of nonnative disulfide bonds, which ensures correct refolding of protein (39–41). Our protocol also took into consideration the effect of fine-tuning the pH and the presence of glycerol for disulfide interchange reactions (42) and enhanced thermodynamic stability of native disulfide bonds (43). As presented in this study, Pfs25, a highly cysteine-rich protein, was successfully folded in monomeric form using an oxidative protein refolding protocol optimized in our lab. The purified protein retained disulfide bond-dependent conformational epitopes as revealed by the recognition of purified CHrPfs25 by specific monoclonal antibodies; ID3 and MRA-4B7 recognized nonreduced CHrPfs25, and the recognition by ID3 was completely lost in reduced form, suggesting that the refolded CHrPfs25 protein contained functional conformational critical disulfide bonds.
To be an effective TBV target antigen, the next critical need is for it to elicit high antibody titers with fewer vaccine doses administered and to block the development of the parasite in the mosquito midgut. Adjuvants as immune response modifiers play an important role in the optimum immunogenicity of administered vaccine molecules, and different adjuvants and vaccine dosing schedules affect the magnitudes and longevities of immune responses. Mice immunized with CHrPfs25 formulated with CFA or Montanide ISA51 elicited stronger antibody titers (640,000) than those immunized with an alum formulation (160,000). While we observed these quantitative differences in the levels of antibody titers, the qualities of antibodies elicited with the three adjuvant formulations were quite comparable as revealed by avidity assay. These results suggested that while the magnitude of the antibody response was different among adjuvant groups, the overall antibody quality was not affected by immunization in different formulations.
The ultimate value of the research findings reported in this study lies in the functional efficacy of elicited antibodies. IFA with live purified P. falciparum gametes and Western blotting with purified lysates indicated strong reactivity of immune sera to native antigens in the parasite. Further functional assessment of immune sera tested at various dilutions in MFA demonstrated extremely potent (100%) transmission-blocking activity of antibodies, and not even a single oocyst was detected in the presence of serum dilutions of 1:32 (CFA group), 1:64 (Montanide group), and 1:8 (alum group). Even at higher dilutions of immune sera (1:128 for the CFA and Montanide ISA51 adjuvant groups and a 1:16 dilution for the alum group), there was >98% transmission-blocking efficacy. MFAs with purified total IgG from pooled immune mouse sera provided direct evidence that the transmission-blocking activity was mediated by antibodies elicited by vaccines. Purified antibodies at concentrations of 31.25 to 62.5 μg/ml exhibited 100% transmission blocking. While we do not know the levels of Pfs25-specific IgG in the total IgG, assuming a value of 1 to 5%, our studies suggest that the presence of less than 5 μg/ml of Pfs25-specific antibodies in the blood meal may be sufficient to effectively interfere with the development of the parasite in the mosquito midgut and block further transmission. Finally, we also present evidence that the transmission-blocking activity is not restricted to one species of Anopheles mosquitoes. MFA studies employing A. gambiae and A. stephensi revealed comparable efficacies of immune IgG.
In this study, we present the first evidence for successful expression and purification of CHrPfs25 in E. coli after simple oxidative refolding steps in an appropriate native conformation. Based on the high reproducibility of our optimized protein expression and purification protocol, we believe that CHrPfs25 production will be scalable for good-manufacturing-practice (GMP) production for clinical-grade preparation. Immunization with purified CHrPfs25 elicited high titers of specific antibodies capable of strong recognition of native Pfs25 protein on the surface of live P. falciparum gametes and extremely potent transmission-blocking activity. The highly reproducible Pfs25 expression, ease and low cost of protein expression, and, most importantly, potent transmission-blocking activity demonstrated by CHrPfs25 expressed in E. coli and formulated in clinically approved alum provide a compelling rationale for developing CHrPfs25 for further clinical trials in human volunteers.
ACKNOWLEDGMENTS
We thank Dibyadyuti Datta for breeding mosquitoes used in MFA and Isabella Quakyi for the ID3 monoclonal antibody.
This research was supported by NIH grants RO1AI47089 and R21AI101427.
R.K. and N.K. designed the research, R.K. conducted experiments, R.K. and N.K. analyzed the data, E.A. collaborated on codon harmonization of the sequence, N.K. conceived the idea, and R.K. and N.K. wrote the paper.
E.A. and N.K. are named as inventors on US patent 8,501,926 B2 relating to this work.
Footnotes
Published ahead of print 13 January 2014
REFERENCES
- 1.WHO. 2011. World malaria report 2011. WHO, Geneva, Switzerland [Google Scholar]
- 2.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. 2012. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379:413–431. 10.1016/S0140-6736(12)60034-8 [DOI] [PubMed] [Google Scholar]
- 3.Miotto O, Almagro-Garcia J, Manske M, Macinnis B, Campino S, Rockett KA, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Duong S, Nguon C, Chuor CM, Saunders D, Se Y, Lon C, Fukuda MM, Amenga-Etego L, Hodgson AV, Asoala V, Imwong M, Takala-Harrison S, Nosten F, Su XZ, Ringwald P, Ariey F, Dolecek C, Hien TT, Boni MF, Thai CQ, Amambua-Ngwa A, Conway DJ, Djimdé AA, Doumbo OK, Zongo I, Ouedraogo JB, Alcock D, Drury E, Auburn S, Koch O, Sanders M, Hubbart C, Maslen G, Ruano-Rubio V, Jyothi D, Miles A, O'Brien J, Gamble C, Oyola SO, Rayner JC, Newbold CI, Berriman M, Spencer CC, McVean G, Day NP, White NJ, Bethell D, Dondorp AM, Plowe CV, Fairhurst RM, Kwiatkowski DP. 2013. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat. Genet. 45:648–655. 10.1038/ng.2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaslow DC. 2002. Transmission-blocking vaccines. Chem. Immunol. 80:287–307. 10.1159/000058850 [DOI] [PubMed] [Google Scholar]
- 5.Kumar N. 2007. A vaccine to prevent transmission of human malaria: a long way to travel on a dusty and often bumpy road. Curr. Sci. 92:1535–1544 [Google Scholar]
- 6.Smith TA, Chitnis N, Briët OJ, Tanner M. 2011. Uses of mosquito-stage transmission-blocking vaccines against Plasmodium falciparum. Trends Parasitol. 27:190–196. 10.1016/j.pt.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 7.Ponnudurai T, van Gemert GJ, Bensink T, Lensen AH, Meuwissen JH. 1987. Transmission blockade of Plasmodium falciparum: its variability with gametocyte numbers and concentration of antibody. Trans. R. Soc. Trop. Med. Hyg. 81:491–493. 10.1016/0035-9203(87)90172-6 [DOI] [PubMed] [Google Scholar]
- 8.Outchkourov NS, Roeffen W, Kaan A, Jansen J, Luty A, Schuiffel D, van Gemert GJ, van de Vegte-Bolmer M, Sauerwein RW, Stunnenberg HG. 2008. Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc. Natl. Acad. Sci. U. S. A. 105:4301–4305. 10.1073/pnas.0800459105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhury DR, Angov E, Kariuki T, Kumar N. 2009. A potent malaria transmission blocking vaccine based on codon harmonized full length Pfs48/45 expressed in Escherichia coli. PLoS One 4:e6352. 10.1371/journal.pone.0006352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tachibana M, Wu Y, Iriko H, Muratova O, MacDonald NJ, Sattabongkot J, Takeo S, Otsuki H, Torii M, Tsuboi T. 2011. N-terminal prodomain of Pfs230 synthesized using a cell-free system is sufficient to induce complement-dependent malaria transmission-blocking activity. Clin. Vaccine Immunol. 18:1343–1350. 10.1128/CVI.05104-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, McCutchan TF, Miller LH. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333:74–76. 10.1038/333074a0 [DOI] [PubMed] [Google Scholar]
- 12.Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. 1991. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J. Exp. Med. 174:1203–1208. 10.1084/jem.174.5.1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gozar MM, Price VL, Kaslow DC. 1998. Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission-blocking antibodies in mice. Infect. Immun. 66:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaslow DC, Shiloach J. 1994. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Biotechnology 12:494–499. 10.1038/nbt0594-494 [DOI] [PubMed] [Google Scholar]
- 15.Tsuboi T, Takeo S, Iriko H, Jin L, Tsuchimochi M, Matsuda S, Han ET, Otsuki H, Kaneko O, Sattabongkot J, Udomsangpetch R, Sawasaki T, Torii M, Endo Y. 2008. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect. Immun. 76:1702–1708. 10.1128/IAI.01539-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrance CE, Chichester JA, Musiychuk K, Shamloul M, Rhee A, Manceva SD, Jones RM, Mamedov T, Sharma S, Mett V, Streatfield SJ, Roeffen W, van de Vegte-Bolmer M, Sauerwein RW, Wu Y, Muratova O, Miller L, Duffy P, Sinden R, Yusibov V. 2011. Antibodies to plant-produced Plasmodium falciparum sexual stage protein Pfs25 exhibit transmission blocking activity. Hum. Vaccine 7:191–198. 10.4161/hv.7.0.14588 [DOI] [PubMed] [Google Scholar]
- 17.Gregory JA, Li F, Tomosada LM, Cox CJ, Topol AB, Vinetz JM, Mayfield S. 2012. Algae-produced Pfs25 elicits antibodies that inhibit malaria transmission. PLoS One 7:e37179. 10.1371/journal.pone.0037179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobo CA, Dhar R, Kumar N. 1999. Immunization of mice with DNA-based Pfs25 elicits potent malaria transmission-blocking antibodies. Infect. Immun. 67:1688–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coban C, Philipp MT, Purcell JE, Keister DB, Okulate M, Martin DS, Kumar N. 2004. Induction of Plasmodium falciparum transmission-blocking antibodies in nonhuman primates by a combination of DNA and protein immunizations. Infect. Immun. 72:253–259. 10.1128/IAI.72.1.253-259.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeBlanc R, Vasquez Y, Hannaman D, Kumar N. 2008. Markedly enhanced immunogenicity of a Pfs25 DNA-based malaria transmission-blocking vaccine by in vivo electroporation. Vaccine 26:185–192. 10.1016/j.vaccine.2007.10.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar R, Nyakundi R, Kariuki T, Ozwara H, Nyamongo O, Mlambo G, Ellefsen B, Hannaman D, Kumar N. 2013. Functional evaluation of malaria Pfs25 DNA vaccine by in vivo electroporation in olive baboons. Vaccine 31:3140–3147. 10.1016/j.vaccine.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxena AK, Singh K, Su HP, Klein MM, Stowers AW, Saul AJ, Long CA, Garboczi DN. 2006. The essential mosquito-stage P25 and P28 proteins from Plasmodium form tile-like triangular prisms. Nat. Struct. Mol. Biol. 13:90–91. 10.1038/nsmb1024 [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, Aebig J, Orcutt A, Muratova O, Song G, Lambert L, Zhu D, Miura K, Long C, Saul A, Miller LH, Durbin AP. 2008. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One 3:e2636. 10.1371/journal.pone.0002636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai CW, Duggan PF, Shimp RL, Jr, Miller LH, Narum DL. 2006. Overproduction of Pichia pastoris or Plasmodium falciparum protein disulfide isomerase affects expression, folding and O-linked glycosylation of a malaria vaccine candidate expressed in P. pastoris. J. Biotechnol. 121:458–470. 10.1016/j.jbiotec.2005.08.025 [DOI] [PubMed] [Google Scholar]
- 25.Kubler-Kielb J, Majadly F, Wu Y, Narum DL, Guo C, Miller LH, Shiloach J, Robbins JB, Schneerson R. 2007. Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proc. Natl. Acad. Sci. U. S. A. 104:293–298. 10.1073/pnas.0609885104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arakawa T, Komesu A, Otsuki H, Sattabongkot J, Udomsangpetch R, Matsumoto Y, Tsuji N, Wu Y, Torii M, Tsuboi T. 2005. Nasal immunization with a malaria transmission-blocking vaccine candidate, Pfs25, induces complete protective immunity in mice against field isolates of Plasmodium falciparum. Infect. Immun. 73:7375–7380. 10.1128/IAI.73.11.7375-7380.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Przysiecki C, Flanagan E, Bello-Irizarry SN, Ionescu R, Muratova O, Dobrescu G, Lambert L, Keister D, Rippeon Y, Long CA, Shi L, Caulfield M, Shaw A, Saul A, Shiver J, Miller LH. 2006. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc. Natl. Acad. Sci. U. S. A. 103:18243–18248. 10.1073/pnas.0608545103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto M, Misawa S, Tsumoto K, Kumagai I, Hayashi H, Kobayashi Y. 2003. On-column refolding and characterization of soluble human interleukin-15 receptor alpha-chain produced in Escherichia coli. Protein Expr. Purif. 31:64–71. 10.1016/S1046-5928(03)00143-8 [DOI] [PubMed] [Google Scholar]
- 29.Singh SM, Upadhayay AK, Panda AK. 2008. Solubilization at high pH results in improved recovery of proteins from inclusion bodies of E. coli. J. Chem. Technol. Biotechnol. 83:1126–1134. 10.1002/jctb.1945 [DOI] [Google Scholar]
- 30.Kaslow DC, Bathurst IC, Lensen T, Ponnudurai T, Barr PJ, Keister DB. 1994. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect. Immun. 62:5576–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angov E, Hillier CJ, Kincaid RL, Lyon JA. 2008. Heterologous protein expression is enhanced by harmonizing the codon usage frequencies of the target gene with those of the expression host. PLoS One 3:e2189. 10.1371/journal.pone.0002189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ifediba T, Vanderberg JP. 1981. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature 294:364–366. 10.1038/294364a0 [DOI] [PubMed] [Google Scholar]
- 33.Vermeulen AN, Ponnudurai T, Lensen AH, Roeffen WF, Meuwissen JE. 1983. The purification of Plasmodium falciparum macrogametes and/or zygotes prepared from in vitro cultures. Trans. R. Soc. Trop. Med. Hyg. 77:753–755. 10.1016/0035-9203(83)90280-8 [DOI] [PubMed] [Google Scholar]
- 34.Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. 1987. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J. Immunol. 139:4213–4217 [PubMed] [Google Scholar]
- 35.Carter R, Graves PM, Keister DB, Quakyi IA. 1990. Properties of epitopes of Pfs 48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite Immunol. 12:587–603. 10.1111/j.1365-3024.1990.tb00990.x [DOI] [PubMed] [Google Scholar]
- 36.Gregory JA, Topol AB, Doerner DZ, Mayfield S. 2013. Alga-produced cholera toxin-pfs25 fusion proteins as oral vaccines. Appl. Environ. Microbiol. 79:3917–3925. 10.1128/AEM.00714-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patra AK, Mukhopadhyay R, Mukhija R, Krishnan A, Garg LC, Panda AK. 2000. Optimization of inclusion body solubilization and renaturation of recombinant human growth hormone from Escherichia coli. Protein Expr. Purif. 18:182–192. 10.1006/prep.1999.1179 [DOI] [PubMed] [Google Scholar]
- 38.Fries HC, Lamers MB, Smits MA, Ponnudurai T, Meuwissen JH. 1989. Characterization of epitopes on the 25 kD protein of the macrogametes/zygotes of Plasmodium falciparum. Parasite Immunol. 11:31–45. 10.1111/j.1365-3024.1989.tb00646.x [DOI] [PubMed] [Google Scholar]
- 39.Lyles MM, Gilbert HF. 1991. Catalysis of the oxidative folding of ribonuclease A by protein disulfide isomerase: dependence of the rate on the composition of the redox buffer. Biochemistry 30:613–619. 10.1021/bi00217a004 [DOI] [PubMed] [Google Scholar]
- 40.Chakravarthi S, Jessop CE, Bulleid NJ. 2006. The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep. 7:271–275. 10.1038/sj.embor.7400645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakravarthi S, Bulleid NJ. 2004. Glutathione is required to regulate the formation of native disulfide bonds within proteins entering the secretory pathway. J. Biol. Chem. 279:39872–39879. 10.1074/jbc.M406912200 [DOI] [PubMed] [Google Scholar]
- 42.Monahan FJ, German JB, Kinsella JE. 1995. Effect of pH and temperature on protein unfolding and thiol/disulfide interchange reactions during heat-induced gelation of whey protein. J. Agric. Food Chem. 43:46–52. 10.1021/jf00049a010 [DOI] [Google Scholar]
- 43.Mishra R, Seckler R, Bhat R. 2005. Efficient refolding of aggregation-prone citrate synthase by polyol osmolytes: how well are protein folding and stability aspects coupled? J. Biol. Chem. 280:15553–15560. 10.1074/jbc.M410947200 [DOI] [PubMed] [Google Scholar]


