Abstract
The type VI secretion system (T6SS) has emerged as a critical virulence factor for the group of closely related Burkholderia spp. that includes Burkholderia pseudomallei, B. mallei, and B. thailandensis. While the genomes of these bacteria, referred to as the Bptm group, appear to encode several T6SSs, we and others have shown that one of these, type VI secretion system 5 (T6SS-5), is required for virulence in mammalian infection models. Despite its pivotal role in the pathogenesis of the Bptm group, the effector repertoire of T6SS-5 has remained elusive. Here we used quantitative mass spectrometry to compare the secretome of wild-type B. thailandensis to that of a mutant harboring a nonfunctional T6SS-5. This analysis identified VgrG-5 as a novel secreted protein whose export depends on T6SS-5 function. Bioinformatics analysis revealed that VgrG-5 is a specialized VgrG protein that harbors a C-terminal domain (CTD) conserved among Bptm group species. We found that a vgrG-5 ΔCTD mutant is avirulent in mice and is unable to stimulate the fusion of host cells, a hallmark of the Bptm group previously shown to require T6SS-5 function. The singularity of VgrG-5 as a detected T6SS-5 substrate, taken together with the essentiality of its CTD for virulence, suggests that the protein is critical for the effector activity of T6SS-5. Intriguingly, we show that unlike the bacterial-cell-targeting T6SSs characterized so far, T6SS-5 localizes to the bacterial cell pole. We propose a model whereby the CTD of VgrG-5—, propelled by T6SS-5—, plays a key role in inducing membrane fusion, either by the recruitment of other factors or by direct participation.
INTRODUCTION
The saprophytic bacterium Burkholderia pseudomallei is the cause of melioidosis, a severe invasive disease in humans and animals (1, 2). Melioidosis has a high mortality rate and is the third most common cause of death from infectious diseases, after AIDS and tuberculosis, in northeast Thailand (3, 4). The disease is difficult to treat due to the intrinsic resistance of B. pseudomallei to a wide range of antibiotic classes (5). Understanding the pathogenesis of B. pseudomallei is important to define alternative therapeutic targets. However, at present, the function and mechanism of its virulence factors are not well understood.
Type VI secretion systems (T6SSs) are widespread Gram-negative bacterial multicomponent protein export machineries with components that display a structural resemblance to the T4 contractile bacteriophage tail spike complex (6). For example, the exported T6SS proteins Hcp and VgrG are structurally related to the phage tail tube protein gp19 and the membrane-piercing spike proteins gp27 and gp5, respectively (7, 8). It has been proposed that Hcp and VgrG act analogously to their phage counterparts, allowing T6SSs to breach membranes and deliver effector proteins into both prokaryotic and eukaryotic target cells (8–11). T6SS effectors active against bacteria have been characterized extensively and include cell wall-hydrolyzing enzymes, phospholipases, nucleases, and pore-forming colicin-like molecules (12–15). In contrast, relatively few eukaryotic-cell-targeting effectors are known. Interestingly, the best characterized of these, a protein with actin cross-linking activity from Vibrio cholerae, is not a canonical secretion substrate; instead, the effector is fused to the C terminus of a VgrG protein (16). Such VgrG proteins appear to serve both structural and effector functions.
B. pseudomallei is closely related to Burkholderia mallei, an obligate host-associated pathogen, and Burkholderia thailandensis, a soil saprophyte of low virulence (17, 18). These three species, referred to below as the Bptm group (19), display similar intracellular behaviors, such as the abilities to escape from endosomes and to exploit host actin for motility. Another hallmark of the Bptm group is the capacity for cell-to-cell spread via the induction of host cell fusion and multinucleated giant cell (MNGC) formation. Interestingly, the Bptm group species share multiple orthologous T6SSs. One of these, T6SS-5 (also known as T6SS-1), is strictly required for MNGC formation (20–24). Consistent with an important role of cell-to-cell spread in Bptm group pathogenesis, T6SS-5 inactivation strongly attenuates the virulence of the Bptm group in assorted animal infection models (20, 21, 25). The precise function of T6SS-5 and the identity of effector proteins secreted by the system are not known.
In this study, we sought to identify proteins secreted by Bptm group T6SS-5 using mass spectrometry-based secretomics. We identified VgrG-5 as a novel protein secreted by T6SS-5, and we provide evidence that it harbors a C-terminal domain (CTD) essential for MNGC formation and virulence in mice. Moreover, we demonstrate that T6SS-5 differs from the T6SSs involved in interbacterial interactions described to date in that it localizes to the bacterial cell pole and displays restricted dynamics. We speculate that this unique localization pattern reflects the direct involvement of T6SS-5 in mediating the fusion of host cells.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. thailandensis E264 and Escherichia coli DH5α and SM10λpir were routinely grown at 37°C in Luria-Bertani medium supplemented with 200 μg/ml trimethoprim, 50 μg/ml kanamycin, 25 μg/ml Irgasan, or 0.05% (wt/vol) rhamnose where necessary. B. thailandensis was grown on M9 minimal medium supplemented with 0.4% (wt/vol) glucose and 0.1% (wt/vol) p-chlorophenylalanine as a counterselectable marker for genetic manipulations (26). For mass spectrometric secretome analysis, B. thailandensis was cultured in Vogel-Bonner minimal medium (VBMM) supplemented with amino acids at a total concentration of 19 mM, 0.5% (wt/vol) glucose, and 1% Tween 80 as described elsewhere (27, 28).
B. thailandensis mutant construction.
For overexpression of the VirAG regulator, the coding sequences of B. thailandensis virA and virG were inserted into the pSCrhaB2 expression vector carrying the E. coli rhamnose-inducible promoter PrhaB (29). To construct an unmarked in-frame deletion of the vgrG-5 CTD (vgrG-5ΔCTD) in B. thailandensis, nucleotides 1951 through 3039 (native 3′ end) of vgrG-5 were deleted and replaced by a stop codon inserted immediately downstream of nucleotide 1950, resulting in the expression of a VgrG-5 protein encompassing amino acids 1 to 650. For this purpose, the suicide vector pJRC115 and the pheS/p-chlorophenylalanine system for counterselection were utilized as described previously (25, 26). The same technique was applied to generate chromosomal fusions of the vesicular stomatitis virus glycoprotein (VSV-G) epitope tag to the C terminus of full-length VgrG-5, of superfolder green fluorescent protein (GFP) to the C terminus of ClpV-5 (ClpV-5–GFP), and of GFP to the C terminus of ClpV-1 (ClpV-1–GFP). B. thailandensis vgrG-5ΔCTD attTn7::vgrG-5 was generated by insertion of full-length vgrG-5 into pUC18T-mini-Tn7T-Tp::PS12 (25) and subsequent transformation of vgrG-5ΔCTD with the plasmid.
Mass spectrometry.
B. thailandensis wild-type and tssK-5 mutant overexpressing virAG (wild-type–vir and ΔtssK-5–vir) cultures were harvested at an optical density at 600 nm (OD600) of 1, and supernatant proteins were prepared and analyzed as described elsewhere (28, 30). Briefly, samples of two biological replicates performed in triplicate were prepared. Proteins were precipitated and digested with trypsin, and peptide fragments were analyzed by liquid chromatography-tandem mass spectrometry (LC–MS-MS) using an LTQ Orbitrap hybrid mass spectrometer. Relative protein quantitation was performed using the label-free spectral count method developed by Liu et al. (31). To calculate the relative protein abundances of samples, the spectral counts of each protein were normalized to the total spectral counts of each sample. The secretomes of B. thailandensis wild-type–vir and ΔtssK-5–vir strains were defined using an empirically defined threshold value of 15 average spectral counts per protein.
Cell culture and infection.
The RAW 264.7 mouse macrophage and HeLa cell lines were kindly provided by Dennis Ko (Duke University School of Medicine, Durham, NC, USA). Cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (HyClone). For immunoprecipitation of VgrG-5, macrophages were grown to 80 to 90% confluence. Bacteria harvested from overnight cultures were opsonized in 10% normal mouse serum (Innovative Research) in DMEM for 30 min and were added to the cells at a multiplicity of infection (MOI) of 200. At 6 h postinfection, whole-cell lysates were prepared as described below. To analyze ClpV5–GFP production and focus formation by fluorescence microscopy, macrophages were seeded onto glass coverslips and were grown to 80% confluence. Cells were infected with opsonized B. thailandensis clpV-5–GFP for 2 and 4 h (MOI of 200) and 8 h (MOI of 50) and were prepared for microscopic examination according to the protocol described below. To analyze MNGC formation, RAW 264.7 macrophages were infected with B. thailandensis at an MOI of 10 for 1 h, washed, and incubated in DMEM supplemented with imipenem (100 μg/ml) for approximately 15 h or 24 h.
Western blotting.
Western blotting with antibodies against VSV-G and bacterial RNA polymerase was performed as described previously (32). An anti-peptide VgrG-5 antibody (GenScript) raised against a 14-amino-acid peptide (DISENEVLSTDQNT) corresponding to residues 840 to 853 of B. thailandensis VgrG-5 was used to detect the protein. For this purpose, membranes were blocked in 5% bovine serum albumin (BSA) for 30 min and were incubated with an anti-VgrG-5 antibody diluted 1:5,000 in 5% BSA for 2 h, followed by incubation with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (1:5,000) for 1 h. For β-actin detection, membranes were blocked in 5% milk for 30 min, probed with mouse anti-β-actin diluted 1:10,000 in 5% milk for 1 h, and then incubated with an HRP-conjugated goat anti-mouse antibody (1:5,000) for 1 h. Blots were developed using SuperSignal West Pico chemiluminescent substrate (Pierce) and were imaged with the FluorChem Q system (Alpha Innotech). Tween 20 was added to blocking, washing, and antibody dilution buffers at a final concentration of 0.5% (vol/vol). For reprobing of blots, membranes were incubated in a stripping buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 100 mM 2-mercaptoethanol) for 30 min.
Immunoprecipitation.
RAW 264.7 macrophages were infected with B. thailandensis containing a chromosomal fusion of vgrG-5 to the VSV-G epitope tag (VgrG-5–V) at an MOI of 200. The wild-type strain expressing untagged VgrG-5 protein was used as a control. At 6 h postinfection, infected macrophages were scraped into lysis buffer (20 mM Tris-HCl [pH 7.5], 200 mM NaCl, 1% Triton X-100, 2 mM EDTA, 5% glycerol, 2 mM phenylmethylsulfonyl fluoride [PMSF], 50 μg/μl lysozyme), incubated for 30 min at 4°C in an end-over-end shaker, and sonicated four times for 10 s each time. The lysates were centrifuged at 12,000 rpm for 20 min, and the supernatant was incubated with an agarose-conjugated anti-VSV-G antibody (Sigma-Aldrich) for 2 h at 4°C. The agarose beads were washed three times with a washing buffer (20 mM Tris-HCl [pH 7.5], 200 mM NaCl, 2% glycerol), and the samples were separated on 7.5% SDS-PAGE gels and were probed with the antibodies indicated in Fig. 2.
FIG 2.

Detection of VgrG-5 during infection of macrophages prior to the development of MNGCs. (A) Western blot of cellular fractions of B. thailandensis expressing a chromosomal vgrG-5–VSV-G fusion (vgrG-5–V) and wild type (vgrG-5) grown in LB using the indicated antibodies. RNAP, RNA polymerase. (B) Western blot of whole-cell extracts of RAW 264.7 macrophages infected with B. thailandensis wild type or vgrG-5–V. Membranes were probed with the indicated antibodies before (lysate) and after VSV-G immunoprecipitation (IP).
Fluorescence microscopy.
RAW 264.7 macrophages were infected with B. thailandensis clpV-5–GFP at an MOI of 100. At 2, 4, and 8 h postinfection, cells were washed, fixed with 4% formaldehyde, and permeabilized with 1% Triton X-100. After blocking with 2% BSA, cells were stained with Texas Red phalloidin (Invitrogen) and 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich). To analyze the subcellular localization of ClpV-5–GFP foci, HeLa cells were infected at an MOI of 50 for 17 h. For live imaging of ClpV-5–GFP foci, HeLa cells were seeded into a 24-well glass-bottom plate (MatTek) and were grown to 70 to 80% confluence. Cells were infected at an MOI of 100 for 2 h, washed with Dulbecco's phosphate-buffered saline (DPBS), and incubated in DMEM supplemented with 100 μg/ml imipenem. At approximately 17 h postinfection, the cell culture medium was replaced with HEPES-buffered and phenol red-free DMEM (Caisson Laboratories) supplemented with 100 μg/ml imipenem for imaging. To examine ClpV-5–GFP and ClpV-1–GFP foci within B. thailandensis outside of host cells, bacteria were grown to mid-log phase in LB and were transferred to 1% agarose pads supplemented with LB. Fluorescence images were acquired on a Nikon Eclipse Ti inverted microscope equipped with a temperature-controlled environmental chamber, a Nikon Plan Apo 60× or 100× oil immersion objective, and a charge-coupled device (CCD) camera (Andor). Image processing and measurement of subcellular ClpV-5–GFP and ClpV-1–GFP foci were performed with NIS-Elements software (Nikon). Images were adjusted for brightness and contrast using Adobe Photoshop CS5. The images shown in Fig. 3C were acquired on a Leica DMRE microscope using an HCX PL Apo 40× oil objective and Leica Application Suite software.
FIG 3.

Deletion of the VgrG-5 CTD abolishes MNGC formation and B. thailandensis virulence. (A) Representative fluorescence microscopy images of RAW 264.7 macrophages infected with a B. thailandensis mutant expressing only the gp27/gp5-like domains of VgrG-5 (vgrG-5ΔCTD), a complemented mutant (vgrG-5ΔCTD attTn7::vgrG-5), or the wild type (wt) for 15 h. To visualize mono- and multinucleated macrophages, the plasma membrane was stained with an Alexa Fluor 594–wheat germ agglutinin conjugate, and DNA was stained with DAPI. Bar, 10 μm. (B) Quantification of MNGC formation by macrophages infected with the strains used for panel A [expressed as a percentage, calculated as (number of nuclei within mononucleated cells)/(number of nuclei in multinucleated cells of any size) × 100]. Values are means ± standard deviations of two independent experiments performed in triplicate. N/D, not detected. **, P < 0.01 (Mann-Whitney test). (C) Representative fluorescence microscopy images of RAW 264.7 macrophages infected with the indicated B. thailandensis mutants for 24 h and stained with an Alexa Fluor 594–wheat germ agglutinin conjugate and DAPI. (D) Survival of C57BL/6 mice that were aerosol infected with ∼1 × 105 CFU/lung of B. thailandensis vgrG-5ΔCTD (n = 16) or the complemented mutant (vgrG-5ΔCTD::CTD) (n = 12). The graph shows combined data from separate vgrG-5ΔCTD and vgrG-5ΔCTD::CTD infections. Three (vgrG-5ΔCTD) and two (vgrG-5ΔCTD::CTD) independent infections were performed. ***, P < 0.001 (log rank Mantel-Cox test).
Mouse infection.
All animal experiments were performed with the approval of the University of Washington IACUC (protocol 2671-06). Mice were infected as described previously (25). Briefly, specific-pathogen-free female C57BL/6 mice (Jackson Laboratories) were aerosol infected with B. thailandensis vgrG-5ΔCTD or vgrG-5ΔCTD::CTD and monitored for survival for 14 days. Three and two independent infections per vgrG-5ΔCTD and vgrG-5ΔCTD::CTD mutant were performed, respectively.
Statistical analysis.
Statistical analyses were performed by Student's t test, Mann-Whitney U test, or log rank test. A P value of <0.05 was considered statistically significant.
RESULTS
Identification of VgrG-5 as a substrate of T6SS-5.
T6SS-5 is a major virulence determinant of the Bptm group, but the effector(s) mediating the influence of T6SS-5 on the host is not known (20, 21, 25). To identify candidate effector proteins of T6SS-5, we utilized the label-free spectral counting mass spectrometry-based approach to compare the secretome of a B. thailandensis wild-type strain with that of a T6SS-5-inactivated (ΔtssK-5) strain. Since T6SS-5 is not significantly expressed outside the host, we overexpressed virAG, a two-component transcriptional activator of the system, within these backgrounds (resulting in wild-type–vir and ΔtssK-5–vir strains) (21).
The global effects of virAG on proteins secreted by B. thailandensis are not documented. Thus, we initiated our study by examining virAG-dependent changes to the wild-type secretome. Of the 52 proteins that passed our filtering criteria, 44 (84.6%) were detected in both the wild-type and wild-type–vir samples, 3 (5.8%) were present only in the wild-type–vir samples, and 5 (9.6%) were detected only in the wild-type sample (Fig. 1A; see also Table S1 in the supplemental material). Two of the proteins found exclusively in wild-type–vir samples, Hcp-5 and VgrG-5, are T6SS-5-associated factors. The third protein, a ubiquitin-specific protease, was identified in a previous study as a virAG-inducibly secreted protein of B. mallei (33). Together, these results are consistent with known VirAG-dependent effects on protein production in the Bptm group (21, 34).
FIG 1.
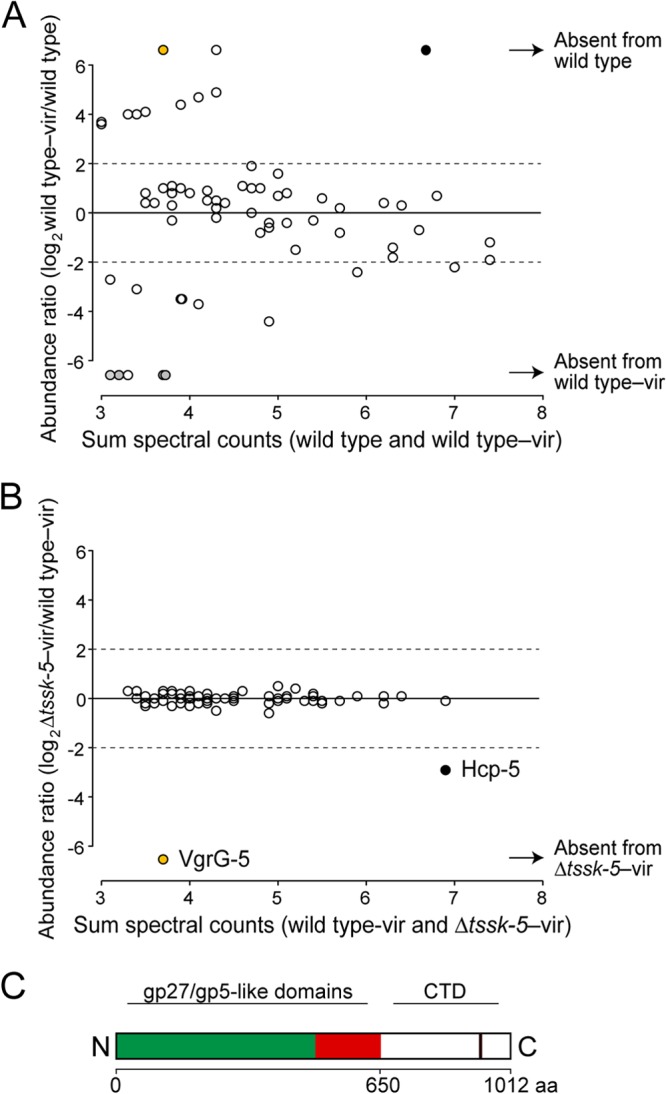
Identification of VgrG-5 as secreted by T6SS-5 by using a mass spectrometry-based screen. (A and B) Relative abundance of individual supernatant proteins of B. thailandensis wild-type–vir compared to the wild-type reference secretome (28) (A) and ΔtssK-5—vir (B). Black, orange, and light gray circles indicate the Hcp-5, VgrG-5, and T6SS-1 substrates, respectively; vir, ectopic expression of the regulator VirAG. (C) Schematic representation of VgrG-5 domains. The gp27-like (green) and gp5-like (red) domains are universally present in VgrG proteins. They share structural similarity with bacteriophage T4 proteins, which form the needle complex involved in puncturing the target membrane. VgrG-5 contains an additional C-terminal domain (CTD) (open rectangle). The solid vertical line within the CTD indicates a predicted coiled-coil region.
We also noted a trend among the proteins detected exclusively in the wild-type secretome. Three of the five such proteins we identified were previously found to be substrates of T6SS-1 of the Bptm group, which functions in interbacterial interactions (28) (see Table S1 in the supplemental material). Hence, via an unknown mechanism, host cell targeting by T6SS-5 and bacterial cell-targeting by T6SS-1 appear to be reciprocally regulated by VirAG.
We next sought to define the candidate effectors exported by T6SS-5 among the proteins found in the wild-type–vir secretome. Of the 47 proteins exported by the wild-type–vir strain, only Hcp-5 and VgrG-5 exhibited T6SS-5-dependent export (Fig. 1B; see also Table S1 in the supplemental material). Hcp-5 was previously identified as a T6SS-5 substrate; however, this family of proteins is thought to participate in structural and chaperone activities rather than to function as effectors (7, 35). In contrast, no T6SS-5-exported VgrG-like protein has been identified previously. Some VgrG proteins have been demonstrated to have both structural and effector capacities (16, 36). Therefore, given our interest in defining the effector(s) responsible for the T6SS-5-dependent virulence of the Bptm group, we focused our attention on VgrG-5.
The CTD of VgrG-5 is conserved within and across the Bptm group species.
VgrG proteins invariably contain two domains that share structural similarity with the bacteriophage tail spike complex (8, 9). However, a subset of VgrG proteins, referred to as specialized VgrG proteins, contain an additional C-terminal domain (CTD) that often harbors effector activity (36, 37). Sequence alignments comparing B. thailandensis VgrG-5 with a broad cross-section of VgrG proteins indicate that it contains a CTD of approximately 360 amino acid residues (Fig. 1C). This CTD is conserved within the apparent VgrG-5 orthologs of the Bptm group and with the related species Burkholderia oklahomensis (Table 1). Furthermore, a survey of publicly available genome sequences showed that the VgrG-5 CTD is highly conserved among isolates of B. thailandensis (85.5 to 100% identity) (n = 4), B. pseudomallei (99.7 to 100% identity) (n = 24), B. mallei (100% identity) (n = 4), and B. oklahomensis (98.1% identity) (n = 2). Together, these data suggest that the CTD is a functionally important component of the VgrG-5 protein. We were unable to identify homology between characterized proteins and the CTD, indicating that this domain may possess novel activity.
TABLE 1.
Pairwise comparison of amino acid sequence identities of VgrG-5 CTDs across Bptm group type strains
| Strain | Amino acid sequence identity of the VgrG-5 CTD (%) |
||
|---|---|---|---|
| B. pseudomallei K96243 | B. mallei ATCC 23344 | B. oklahomensis C6786a | |
| B. thailandensis E264 | 87 | 87 | 83.3 |
| B. pseudomallei K96243 | 99.6 | 83.2 | |
| B. mallei ATCC 23344 | 83.5 | ||
Infection of host cells with B. oklahomensis does not lead to MNGC formation, which is proposed to be due to an actin polymerization defect (53). In the accompanying paper, Toesca et al. show that the B. pseudomallei, B. thailandensis, B. mallei, and B. oklahomensis VgrG-5 proteins are functionally interchangeable with respect to MNGC formation (40).
VgrG-5 is produced in macrophages prior to MNGC formation.
To probe the potential for VgrG-5 to function as an effector, we first sought to measure its production during infection of host cells. To this end, we constructed a chromosomally encoded fusion of the vesicular stomatitis virus glycoprotein (VSV-G) epitope to the C terminus of VgrG-5 (VgrG-5–V) and used immunoprecipitation to monitor protein production during B. thailandensis infection of RAW 264.7 macrophages. At 6 h postinfection, 2 h before MNGC formation is observed, cells were harvested and were analyzed for the presence of VgrG-5. As shown in Fig. 2, VgrG-5–V production was specifically activated during host cell infection; its production was not evident within cells propagated in culture medium. Since vgrG-5–V is expressed under the control of its native promoter, this result implies that the production of VgrG-5 is induced by a host-derived signal. Nonetheless, the intracellular induction of VgrG-5, which has also been observed at the level of mRNA (38), does not appear to lead to an abundance of the protein, as we found that detection of VgrG-5–V was achieved only after enrichment by immunoprecipitation.
The VgrG-5 CTD is required for host cell fusion and virulence.
Given that T6SS-5 function is required for the formation of MNGCs and that VgrG-5 was the only substrate with potential effector activity identified in our secretome analysis, we investigated whether the VgrG-5 CTD is involved in mediating host cell fusion. To this end, we constructed a mutant B. thailandensis strain that expresses only the gp27- and gp5-like domains of VgrG-5 by replacing the region of the gene encoding the CTD with a stop codon on the chromosome (vgrG-5ΔCTD). To analyze the ability of the vgrG-5ΔCTD mutant to stimulate host cell fusions, we infected RAW 264.7 macrophages with B. thailandensis wild type or vgrG-5ΔCTD at an MOI of 10. At 15 h postinfection, wild-type-infected cells displayed numerous fusion events, as indicated by the presence of 43% of observable nuclei in MNGCs (Fig. 3A and B). In contrast, infection with the vgrG-5ΔCTD mutant did not induce MNGC formation (0% of nuclei present in MNGCs), even at an extended time point postinfection (Fig. 3A, B, and C). The MNGC formation defect of the vgrG-5ΔCTD mutant was partially restored by expressing vgrG-5 from a neutral chromosomal site (vgrG-5ΔCTD attTn7::vgrG-5) (Fig. 3A and B). Expression of full-length vgrG-5 in host cells was not sufficient to induce MNGC formation (data not shown).
We have shown previously that inactivation of T6SS-5 results in a loss of virulence and a survival defect for B. thailandensis in the lungs of C57BL/6 mice (25). The finding that the specialized C terminus of VgrG-5 is required for the induction of host cell fusions led us to examine whether it is similarly important for the virulence of B. thailandensis. To this end, we infected C57BL/6 mice with ∼1 × 105 CFU of the vgrG-5ΔCTD mutant via the aerosol route and monitored the survival of the mice for 14 days. At this infectious dose, mice succumb to infection with wild-type B. thailandensis by 2 to 4 days postinfection (25, 39). Similar to the phenotype previously observed for ΔT6SS-5, mice challenged with the vgrG-5ΔCTD mutant showed no outward disease symptoms, and all survived the course of infection (Fig. 4D) (25). Given the inefficiency of ectopic vgrG-5 complementation, for murine infection studies we complemented the vgrG-5ΔCTD mutant by repair of the ΔCTD lesion with the native CTD sequence (vgrG-5ΔCTD::CTD). Importantly, this strategy restored wild-type levels of virulence to the mutant (Fig. 3D). In the accompanying paper, Toesca and colleagues demonstrate that the VgrG-5 CTD is dispensable for basic T6SS-5 function (Hcp-5 secretion) (40). Therefore, collectively, these data strongly suggest that the CTD of VgrG-5 has a critical function in T6SS-5-dependent MNGC formation and virulence.
FIG 4.

Localization and dynamics of T6SS-5 within B. thailandensis cells. (A) Formation of discrete ClpV-5–GFP foci within B. thailandensis at the indicated time points after infection of RAW 264.7 macrophages. Actin and DNA were visualized with Texas Red phalloidin and DAPI, respectively. m, medium (overlay of fluorescence and phase-contrast images of bacteria grown in LB medium and transferred to an agarose pad). Bars, 5 μm. Insets, higher magnifications of the images. Bars, 1 μm. (B) Representative overlay of fluorescence and phase-contrast microscopy images of B. thailandensis clpV-1–GFP grown in LB and transferred to an agarose pad. Inset, higher magnification of the image. (C) Subcellular localization of ClpV-1–GFP and ClpV-5–GFP foci within B. thailandensis cells grown in LB and during infection of HeLa cells, respectively (ClpV-1–GFP, 99 foci; ClpV-5–GFP, 91 foci). Polar regions were defined as the outer 0 to 10% of the cell length. (D and E) Quantification of the dynamics of ClpV-1–GFP and ClpV-5–GFP foci. Twelve time-lapse images taken at 10-s intervals were used to analyze the appearance and disappearance of foci expressed as the on-off ratio, calculated as (sum of appearing and disappearing foci)/(total number of foci at time zero) (D) as well as the movements of foci expressed as the movement ratio, calculated as (sum of moving foci)/(total number of foci at time zero) (E). A total of 128 ClpV-1–GFP foci and 253 ClpV-5–GFP foci were analyzed. Values are means ± standard deviations of two independent experiments. **, P < 0.01 (unpaired t test with Welch correction). (F) Presence of ClpV-5–GFP foci in bacteria forming an actin tail versus bacteria forming no actin tail during infection of HeLa cells. Values are normalized to the total number of bacteria forming an actin tail and to the total number of bacteria forming no actin tail, respectively. Values are means ± standard deviations obtained from two independent experiments with 730 bacteria. (G) Fluorescence microscopy images showing the three most abundant patterns of ClpV-5–GFP localization within B. thailandensis during infection of HeLa cells. Actin was stained with Texas Red phalloidin. Values were obtained from two independent experiments (152 bacteria positive for ClpV-5–GFP foci).
T6SS-5 is localized to bacterial poles during host cell infection.
The T6SS apparatus can be visualized by examining the localization of its structural components fused to fluorescent reporter proteins. For example, fusion proteins of the T6SS ATPase ClpV with green fluorescent protein (ClpV–GFP) have been found to localize to dynamic foci in the bacterial cytoplasm (41). The formation and activity of these foci correlate with T6SS activity; thus, monitoring of fluorescently labeled ClpV can provide insights into the conditions and signals that stimulate or inhibit the assembly and activation of a T6SS. Importantly, the application of this approach has so far been limited to the conditions of T6SS-mediated interbacterial interactions, where bacterial strains are cocultured on agarose pads.
We sought to visualize B. thailandensis T6SS-5 during host cell infection in order to obtain insights into its activity in the intracellular environment. To this end, we generated a fusion of GFP to the C terminus of ClpV-5 (ClpV-5–GFP) encoded at the native chromosomal clpV-5 locus. Importantly, the clpV-5–GFP strain displayed the capacity to efficiently induce MNGC formation, indicating that ClpV-5 fused to the fluorescent marker retained function (see Fig. S1 in the supplemental material). In agreement with our data showing a lack of VgrG-5 expression outside of host cells, no fluorescence attributable to ClpV-5–GFP was observed in B. thailandensis clpV-5–GFP grown in culture medium (Fig. 4A). Next, we infected RAW 264.7 macrophages with the clpV-5–GFP strain and analyzed GFP fluorescence at time points before (2 h, 4 h) and after (8 h) MNGC formation. A diffuse fluorescent signal of ClpV-5–GFP was observed at 2 h postinfection; however, by 4 h postinfection, apparently assembled T6SS-5 apparatuses were observed as one or more discrete fluorescent ClpV-5–GFP foci (Fig. 4A).
T6SS-5 displays apparent polar localization within intracellular B. thailandensis cells (77%) (Fig. 4A and C). Intriguingly, subcellular localization studies of other T6SSs have not observed a bias in the localization of these systems to cell poles; rather, they appear to be distributed randomly throughout the cell body (41–43). To determine whether our observations of ClpV-5–GFP at cell poles is a specific phenomenon of T6SS-5 or a general behavior exhibited by B. thailandensis T6SSs, we next examined the localization of ClpV-1–GFP, which is linked to and specifically implicated in the function of B. thailandensis T6SS-1. In prior work, our group showed that in contrast to T6SS-5, T6SS-1 specializes in bacterial cell targeting (25, 28). Quantification of T6SS-1 localization indicated that this system, unlike T6SS-5, is distributed in a virtually random fashion along the length of the cell (Fig. 4B and C). Furthermore, time lapse fluorescence imaging revealed differences in dynamic behavior between T6SS-1 and T6SS-5. Over the course of 3 min, the numbers of appearing, disappearing, and moving foci were significantly higher for the ClpV-1–GFP strain than for the ClpV-5–GFP strain, in which foci displayed comparatively static behavior (Fig. 4D and E) (see movies S1 and S2 in the supplemental material).
Another protein that plays an important role in the intracellular life cycle of the Bptm group is BimA, which polymerizes host cell actin for intracellular motility (44). Since BimA also exhibits polar localization, we asked whether there is an association between the assembly of T6SS-5 and the localization of BimA as indicated by actin tail formation. The number of B. thailandensis bacteria showing both actin tails and assembled T6SS-5 machineries was not significantly different from the number of bacteria harboring assembled T6SS-5 that did not polymerize actin (Fig. 4F). Since actin tail formation does not appear to affect the assembly of T6SS-5, we next investigated whether there is a spatial association between T6SS-5 and BimA positioning. Interestingly, T6SS-5 can be found both at the pole where actin polymerization occurs and at the opposite pole (Fig. 4G).
DISCUSSION
T6SS-5 function is of critical importance for the Bptm group to cause lethal infections in mammalian hosts (20, 21, 25). In our previous work, we showed that B. thailandensis ΔtssK-5, containing a nonfunctional T6SS-5, displays defects in host cell fusion and in virulence (25). Here we identified VgrG-5 as a novel protein secreted by T6SS-5 and showed that the deletion of the VgrG-5 CTD recapitulates the MNGC and virulence deficiency of the ΔtssK-5 mutant. In the accompanying paper, Toesca et al. provide evidence that deletion of the CTD does not affect the secretory activity of T6SS-5 (40). Taken together, the data show that VgrG-5 CTD function is critical for the effector activity of T6SS-5.
At present, T6SS-5 is the only T6SS for which experimental data support an exclusive role in targeting eukaryotic cells (25). Bacterial protein secretion machineries dedicated to host-pathogen interactions typically secrete multiple effector proteins into host cells, which act in a coordinated fashion to execute their function (45, 46). Our finding that VgrG-5 may be the only protein with effector function secreted by T6SS-5 was therefore unexpected. Importantly, the possibility that overexpression of virAG alone is not a sufficient stimulus to trigger the export of the complete set of T6SS-5 substrates cannot be excluded, nor can we rule out the possibility that other effectors were below our detection limit. A recent study suggested that adaptor proteins may be widely utilized to facilitate the recruitment of effectors to VgrG proteins via binding at the VgrG C terminus (47). It is conceivable that one or more such proteins bind the C terminus of VgrG-5 and recruit effectors, potentially offering another explanation for the requirement for the VgrG-5 CTD.
Microscopic imaging of fluorescently labeled structural components of the T6SS apparatus has provided important insights into the export mechanism of the T6SS and its activity in the context of environmental stimuli (41, 48, 49). Furthermore, monitoring of the dynamics of fluorescently labeled T6SS proteins in combination with automated quantitative data analysis has proven to be a powerful technique for investigating the requirements for T6SS-dependent killing of bacteria (41). Here we showed that T6SS-5 localizes to the poles of B. thailandensis cells and exists predominantly in a quiescent state during the infection of host cells. The polar position of T6SS-5 is similar to that of other bacterial secretion systems interacting with host cells, such as the T3SS, T4SS, or T7SS (50–52). In contrast, the behavior of T6SS-5 differs considerably from those of B. thailandensis T6SS-1 and other bacterial-cell-targeting T6SSs characterized thus far, which display an irregular and highly dynamic localization pattern (41, 43). The differences between the host cell-targeting T6SS-5 and T6SSs involved in interbacterial interaction may reflect different strategies for the efficient delivery of substrates into profoundly different target cell types. Since T6SS-5 is, to our knowledge, the first eukaryotic-cell-targeting T6SS for which the subcellular position has been visualized, it remains to be determined whether polar localization is a common feature of T6SSs involved in host cell interactions.
It is tempting to speculate that the polar and less dynamic localization of T6SS-5 represents a specific adaptation enabling it to directly mediate the fusion of host cell membranes. Although direct evidence has yet to emerge, the T6SS is thought to function as a contact-dependent cell puncturing device. Interestingly, a previous study of B. pseudomallei showed that a mutant with a functional T6SS-5 that was unable to move inside the host cell cytoplasm was virtually incapable of inducing MNGCs (24). This indicates that the bacteria need to navigate the cytoplasm of the host cell in order to reach the subcellular site appropriate for VgrG-5 CTD function. The polar localization of T6SS-5, in combination with the requirement for intracellular motility in MNGC induction, leads us to propose that intracellular Bptm group bacteria employ the membrane-puncturing potential of T6SS-5 to insert the VgrG-5 CTD into the host cell plasma membrane. Subsequently, the VgrG-5 CTD could induce membrane fusions by interacting directly with a neighboring host cell receptor or by engaging in homotypic interactions within or between cells, as shown in the accompanying article by Toesca et al. (40).
The identification of VgrG-5 as a T6SS-5 substrate and the observation that its CTD is specifically involved in MNGC formation provide a foundation for defining the mechanism of Bptm group-induced host cell fusion. Future studies aimed at pinpointing the subcellular localization of the translocated VgrG-5 CTD and its interaction partner(s), which may be bacterial or host derived, will provide important insights into CTD function and processes that influence the progression of B. pseudomallei infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jason Smith for sharing reagents and Monika Schütz for help with microscopy.
This work was supported by NIH grants AI057141 to J.D.M and K08 HL094759 to T.E.W.
Footnotes
Published ahead of print 22 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01368-13.
REFERENCES
- 1.Limmathurotsakul D, Thammasart S, Warrasuth N, Thapanagulsak P, Jatapai A, Pengreungrojanachai V, Anun S, Joraka W, Thongkamkoon P, Saiyen P, Wongratanacheewin S, Day NP, Peacock SJ. 2012. Melioidosis in animals, Thailand, 2006–2010. Emerg. Infect. Dis. 18:325–327. 10.3201/eid1802.111347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Currie BJ, Peacock SJ. 2012. Melioidosis. N. Engl. J. Med. 367:1035–1044. 10.1056/NEJMra1204699 [DOI] [PubMed] [Google Scholar]
- 3.Chaowagul W, White NJ, Dance DA, Wattanagoon Y, Naigowit P, Davis TM, Looareesuwan S, Pitakwatchara N. 1989. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J. Infect. Dis. 159:890–899. 10.1093/infdis/159.5.890 [DOI] [PubMed] [Google Scholar]
- 4.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NP, Peacock SJ. 2010. Increasing incidence of human melioidosis in Northeast Thailand. Am. J. Trop. Med. Hyg. 82:1113–1117. 10.4269/ajtmh.2010.10-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaowagul W. 2000. Recent advances in the treatment of severe melioidosis. Acta Trop. 74:133–137. 10.1016/S0001-706X(99)00062-5 [DOI] [PubMed] [Google Scholar]
- 6.Cascales E, Cambillau C. 2012. Structural biology of type VI secretion systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367:1102–1111. 10.1098/rstb.2011.0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pell LG, Kanelis V, Donaldson LW, Howell PL, Davidson AR. 2009. The phage lambda major tail protein structure reveals a common evolution for long-tailed phages and the type VI bacterial secretion system. Proc. Natl. Acad. Sci. U. S. A. 106:4160–4165. 10.1073/pnas.0900044106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leiman PG, Basler M, Ramagopal UA, Bonanno JB, Sauder JM, Pukatzki S, Burley SK, Almo SC, Mekalanos JJ. 2009. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc. Natl. Acad. Sci. U. S. A. 106:4154–4159. 10.1073/pnas.0813360106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. 2007. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. U. S. A. 104:15508–15513. 10.1073/pnas.0706532104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hood RD, Singh P, Hsu F, Guvener T, Carl MA, Trinidad RR, Silverman JM, Ohlson BB, Hicks KG, Plemel RL, Li M, Schwarz S, Wang WY, Merz AJ, Goodlett DR, Mougous JD. 2010. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7:25–37. 10.1016/j.chom.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell AB, Hood RD, Bui NK, LeRoux M, Vollmer W, Mougous JD. 2011. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475:343–347. 10.1038/nature10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou S, Bui NK, Russell AB, Lexa KW, Gardiner TE, LeRoux M, Vollmer W, Mougous JD. 2012. Structure of a peptidoglycan amidase effector targeted to Gram-negative bacteria by the type VI secretion system. Cell Rep. 1:656–664. 10.1016/j.celrep.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koskiniemi S, Lamoureux JG, Nikolakakis KC, t'Kint de Roodenbeke C, Kaplan MD, Low DA, Hayes CS. 2013. Rhs proteins from diverse bacteria mediate intercellular competition. Proc. Natl. Acad. Sci. U. S. A. 110:7032–7037. 10.1073/pnas.1300627110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell AB, LeRoux M, Hathazi K, Agnello DM, Ishikawa T, Wiggins PA, Wai SN, Mougous JD. 2013. Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature 496:508–512. 10.1038/nature12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong TG, Ho BT, Yoder-Himes DR, Mekalanos JJ. 2013. Identification of T6SS-dependent effector and immunity proteins by Tn-seq in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 110:2623–2628. 10.1073/pnas.1222783110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma AT, McAuley S, Pukatzki S, Mekalanos JJ. 2009. Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells. Cell Host Microbe 5:234–243. 10.1016/j.chom.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brett PJ, DeShazer D, Woods DE. 1998. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 48(Part 1):317–320. 10.1099/00207713-48-1-317 [DOI] [PubMed] [Google Scholar]
- 18.Whitlock GC, Estes DM, Torres AG. 2007. Glanders: off to the races with Burkholderia mallei. FEMS Microbiol. Lett. 277:115–122. 10.1111/j.1574-6968.2007.00949.x [DOI] [PubMed] [Google Scholar]
- 19.Majerczyk CD, Greenberg EP, Chandler JR. 2013. Quorum sensing in Burkholderia, p 40–57 In Vasil ML, Darwin AJ. (ed), Regulation of bacterial virulence. ASM Press, Washington, DC [Google Scholar]
- 20.Pilatz S, Breitbach K, Hein N, Fehlhaber B, Schulze J, Brenneke B, Eberl L, Steinmetz I. 2006. Identification of Burkholderia pseudomallei genes required for the intracellular life cycle and in vivo virulence. Infect. Immun. 74:3576–3586. 10.1128/IAI.01262-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schell MA, Ulrich RL, Ribot WJ, Brueggemann EE, Hines HB, Chen D, Lipscomb L, Kim HS, Mrazek J, Nierman WC, Deshazer D. 2007. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol. Microbiol. 64:1466–1485. 10.1111/j.1365-2958.2007.05734.x [DOI] [PubMed] [Google Scholar]
- 22.Shalom G, Shaw JG, Thomas MS. 2007. In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology 153:2689–2699. 10.1099/mic.0.2007/006585-0 [DOI] [PubMed] [Google Scholar]
- 23.Burtnick MN, DeShazer D, Nair V, Gherardini FC, Brett PJ. 2010. Burkholderia mallei cluster 1 type VI secretion mutants exhibit growth and actin polymerization defects in RAW 264.7 murine macrophages. Infect. Immun. 78:88–99. 10.1128/IAI.00985-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.French CT, Toesca IJ, Wu TH, Teslaa T, Beaty SM, Wong W, Liu M, Schröder I, Chiou PY, Teitell MA, Miller JF. 2011. Dissection of the Burkholderia intracellular life cycle using a photothermal nanoblade. Proc. Natl. Acad. Sci. U. S. A. 108:12095–12100. 10.1073/pnas.1107183108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz S, West TE, Boyer F, Chiang WC, Carl MA, Hood RD, Rohmer L, Tolker-Nielsen T, Skerrett SJ, Mougous JD. 2010. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog. 6:e1001068. 10.1371/journal.ppat.1001068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandler JR, Duerkop BA, Hinz A, West TE, Herman JP, Churchill ME, Skerrett SJ, Greenberg EP. 2009. Mutational analysis of Burkholderia thailandensis quorum sensing and self-aggregation. J. Bacteriol. 191:5901–5909. 10.1128/JB.00591-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer KL, Aye LM, Whiteley M. 2007. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 189:8079–8087. 10.1128/JB.01138-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell AB, Singh P, Brittnacher M, Bui NK, Hood RD, Carl MA, Agnello DM, Schwarz S, Goodlett DR, Vollmer W, Mougous JD. 2012. A widespread bacterial type VI secretion effector superfamily identified using a heuristic approach. Cell Host Microbe 11:538–549. 10.1016/j.chom.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valvano MA, Keith KE, Cardona ST. 2005. Survival and persistence of opportunistic Burkholderia species in host cells. Curr. Opin. Microbiol. 8:99–105. 10.1016/j.mib.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 30.Wehmhoner D, Haussler S, Tummler B, Jansch L, Bredenbruch F, Wehland J, Steinmetz I. 2003. Inter- and intraclonal diversity of the Pseudomonas aeruginosa proteome manifests within the secretome. J. Bacteriol. 185:5807–5814. 10.1128/JB.185.19.5807-5814.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Sadygov RG, Yates JR., III 2004. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 76:4193–4201. 10.1021/ac0498563 [DOI] [PubMed] [Google Scholar]
- 32.Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA, Goodman AL, Joachimiak G, Ordonez CL, Lory S, Walz T, Joachimiak A, Mekalanos JJ. 2006. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312:1526–1530. 10.1126/science.1128393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanks J, Burtnick MN, Brett PJ, Waag DM, Spurgers KB, Ribot WJ, Schell MA, Panchal RG, Gherardini FC, Wilkinson KD, Deshazer D. 2009. Burkholderia mallei tssM encodes a putative deubiquitinase that is secreted and expressed inside infected RAW 264.7 murine macrophages. Infect. Immun. 77:1636–1648. 10.1128/IAI.01339-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burtnick MN, Brett PJ, Harding SV, Ngugi SA, Ribot WJ, Chantratita N, Scorpio A, Milne TS, Dean RE, Fritz DL, Peacock SJ, Prior JL, Atkins TP, Deshazer D. 2011. The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect. Immun. 79:1512–1525. 10.1128/IAI.01218-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverman JM, Agnello DM, Zheng H, Andrews BT, Li M, Catalano CE, Gonen T, Mougous JD. 2013. Haemolysin coregulated protein is an exported receptor and chaperone of type VI secretion substrates. Mol. Cell 51:584–593. 10.1016/j.molcel.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brooks TM, Unterweger D, Bachmann V, Kostiuk B, Pukatzki S. 2013. Lytic activity of the Vibrio cholerae type VI secretion toxin VgrG-3 is inhibited by the antitoxin TsaB. J. Biol. Chem. 288:7618–7625. 10.1074/jbc.M112.436725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma AT, Mekalanos JJ. 2010. In vivo actin cross-linking induced by Vibrio cholerae type VI secretion system is associated with intestinal inflammation. Proc. Natl. Acad. Sci. U. S. A. 107:4365–4370. 10.1073/pnas.0915156107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Wong J, Sun GW, Liu Y, Tan GY, Gan YH. 2011. Regulation of type VI secretion system during Burkholderia pseudomallei infection. Infect. Immun. 79:3064–3073. 10.1128/IAI.05148-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West TE, Frevert CW, Liggitt HD, Skerrett SJ. 2008. Inhalation of Burkholderia thailandensis results in lethal necrotizing pneumonia in mice: a surrogate model for pneumonic melioidosis. Trans. R. Soc. Trop. Med. Hyg. 102(Suppl 1):S119–S126. 10.1016/S0035-9203(08)70028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toesca IJ, French CT, Miller JF. Type VI secretion system 5 spike protein VgrG mediates membrane fusion during intercellular spread by Pseudomallei group Burkholderia species. Infect. Immun. 82:1436–1444. 10.1128/IAI.01367-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LeRoux M, De Leon JA, Kuwada NJ, Russell AB, Pinto-Santini D, Hood RD, Agnello DM, Robertson SM, Wiggins PA, Mougous JD. 2012. Quantitative single-cell characterization of bacterial interactions reveals type VI secretion is a double-edged sword. Proc. Natl. Acad. Sci. U. S. A. 109:19804–19809. 10.1073/pnas.1213963109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu F, Schwarz S, Mougous JD. 2009. TagR promotes PpkA-catalysed type VI secretion activation in Pseudomonas aeruginosa. Mol. Microbiol. 72:1111–1125. 10.1111/j.1365-2958.2009.06701.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basler M, Ho BT, Mekalanos JJ. 2013. Tit-for-tat: type VI secretion system counterattack during bacterial cell-cell interactions. Cell 152:884–894. 10.1016/j.cell.2013.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevens JM, Ulrich RL, Taylor LA, Wood MW, Deshazer D, Stevens MP, Galyov EE. 2005. Actin-binding proteins from Burkholderia mallei and Burkholderia thailandensis can functionally compensate for the actin-based motility defect of a Burkholderia pseudomallei bimA mutant. J. Bacteriol. 187:7857–7862. 10.1128/JB.187.22.7857-7862.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos-Morales F. 2012. Impact of Salmonella enterica type III secretion system effectors on the eukaryotic host cell. ISRN Cell Biol. 2012:78934. 10.5402/2012/787934 [DOI] [Google Scholar]
- 46.Voth DE, Broederdorf LJ, Graham JG. 2012. Bacterial type IV secretion systems: versatile virulence machines. Future Microbiol. 7:241–257. 10.2217/fmb.11.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shneider MM, Buth SA, Ho BT, Basler M, Mekalanos JJ, Leiman PG. 2013. PAAR-repeat proteins sharpen and diversify the type VI secretion system spike. Nature 500:350–353. 10.1038/nature12453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silverman JM, Austin LS, Hsu F, Hicks KG, Hood RD, Mougous JD. 2011. Separate inputs modulate phosphorylation-dependent and -independent type VI secretion activation. Mol. Microbiol. 82:1277–1290. 10.1111/j.1365-2958.2011.07889.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basler M, Pilhofer M, Henderson GP, Jensen GJ, Mekalanos JJ. 2012. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 483:182–186. 10.1038/nature10846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaumouille V, Francetic O, Sansonetti PJ, Tran Van Nhieu G. 2008. Cytoplasmic targeting of IpaC to the bacterial pole directs polar type III secretion in Shigella. EMBO J. 27:447–457. 10.1038/sj.emboj.7601976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carlsson F, Joshi SA, Rangell L, Brown EJ. 2009. Polar localization of virulence-related Esx-1 secretion in mycobacteria. PLoS Pathog. 5:e1000285. 10.1371/journal.ppat.1000285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan JK, Luedtke BE, Shaw EI. 2010. Polar localization of the Coxiella burnetii type IVB secretion system. FEMS Microbiol. Lett. 305:177–183. 10.1111/j.1574-6968.2010.01926.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wand ME, Muller CM, Titball RW, Michell SL. 2011. Macrophage and Galleria mellonella infection models reflect the virulence of naturally occurring isolates of B. pseudomallei, B. thailandensis and B. oklahomensis. BMC Microbiol. 11:11. 10.1186/1471-2180-11-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


