Abstract
Mycobacterium tuberculosis infection of the central nervous system is thought to be initiated once the bacilli have breached the blood brain barrier and are phagocytosed, primarily by microglial cells. In this study, the interactions of M. tuberculosis with neurons in vitro and in vivo were investigated. The data obtained demonstrate that neurons can act as host cells for M. tuberculosis. M. tuberculosis bacilli were internalized by murine neuronal cultured cells in a time-dependent manner after exposure, with superior uptake by HT22 cells compared to Neuro-2a cells (17.7% versus 9.8%). Internalization of M. tuberculosis bacilli by human SK-N-SH cultured neurons suggested the clinical relevance of the findings. Moreover, primary murine hippocampus-derived neuronal cultures could similarly internalize M. tuberculosis. Internalized M. tuberculosis bacilli represented a productive infection with retention of bacterial viability and replicative potential, increasing 2- to 4-fold within 48 h. M. tuberculosis bacillus infection of neurons was confirmed in vivo in the brains of C57BL/6 mice after intracerebral challenge. This study, therefore, demonstrates neurons as potential new target cells for M. tuberculosis within the central nervous system.
INTRODUCTION
Tuberculosis is primarily a respiratory disease that is initiated after the inhalation of only a few bacilli and subsequent phagocytosis by alveolar macrophages to establish a local infection focus. Globally, approximately 8.8 million new cases of tuberculosis were reported in 2011, and the disease was associated with 1.45 million deaths (1). Although pulmonary tuberculosis is the predominant form of infection, extrapulmonary tuberculosis constitutes up to 20% of reported cases, approximately 1 to 5% of which are attributed to tuberculosis of the central nervous system (CNS-TB) (2). CNS-TB occurs primarily in childhood but significantly increases in adults under conditions of immune suppression, which are associated with considerable morbidity and mortality (3, 4). Pathogenesis of CNS-TB is initiated as a secondary infection during hematogenous dissemination of pulmonary infection to the brain parenchyma (5). Despite its neuroprotective properties, it has been proposed that Mycobacterium tuberculosis can cross the blood brain barrier and invade the CNS as free bacilli, which is supported by studies that illustrated pathogen-specific gene upregulation associated with traversal of the blood brain barrier (6). However, the mechanisms associated with evading the protective properties of the blood brain barrier for several bacteria, including M. tuberculosis, remain primarily undefined. Initial events during CNS-TB are characterized by infection of the meninges, the establishment of localized foci, and the subsequent release of bacilli into the subarachnoid space (7).
Several studies have investigated and reported on different cell types that are targeted by M. tuberculosis bacilli for invasion (8, 9, 10, 11). Among these, macrophages are well described as preferred host cells despite their primary protective function in innate immune responses; the evolutionary development of specific immune evasion mechanisms allows M. tuberculosis to exist within what is essentially a hostile environment. Studies have also indicated that cells other than macrophages, such as dendritic cells, are infected by M. tuberculosis bacilli at a higher rate than was previously thought (11). Differential cytokine profiles produced by infected macrophages and dendritic cells in comparative studies have suggested that the functional consequences of M. tuberculosis infection of these two distinct cell types may be different (12, 13). Similarly, M. tuberculosis infection of different nonphagocytic cell types may induce responses that are variable. The diversity of cell types that can be infected by M. tuberculosis bacilli, particularly at extrapulmonary sites, suggests that latent infection may be established at such locations. Recent studies demonstrated viable bacilli present in resident macrophages and sinusoidal endothelium cells of the spleen and liver expressing a genetic profile corresponding to latent infection (14).
M. tuberculosis bacilli encode specific proteins that actively facilitate entry into cells (15, 16), thereby circumventing the requirement for cells to be phagocytic in order to establish infection. Among several intracellular bacterial species that are capable of infecting the central nervous system (17), studies have indicated that microglia are targeted by invading M. tuberculosis bacilli (18, 19), leading to a robust proinflammatory response dependent on NADPH oxidase-dependent reactive oxygen species (ROS) generation (20) and the induction of reactive nitrogen intermediates (21). Neurons have never been shown to be infected by M. tuberculosis bacilli and are not thought to be involved in the etiology of the disease. However, neural targeting by Mycobacterium leprae through binding to laminin α2 on Schwann cells has been reported (22), and the presence of M. leprae in the medulla oblongata and spinal cord of patients with lepromatous leprosy was inferred from DNA amplification studies, although the presence of bacilli within neurons was not detected (23). Nonetheless, several pathogenic species do infect neurons, including the intracellular bacterium Listeria monocytogenes, which can be controlled in a gamma interferon (IFN-γ)-dependent bactericidal manner (24, 25).
In this study, the potential of M. tuberculosis bacilli to infect neurons was investigated. Although neurons are generally regarded as nonphagocytic cells, Bowen et al. demonstrated that phagocytosis by different neuronal cell types occurs both in vitro and in vivo (26). The phagocytic capability of neurons may therefore be largely unappreciated and underinvestigated. Thus, it was hypothesized that neurons are capable of mycobacterial internalization, thereby affecting neuronal cellular responses. The results obtained in this study conclusively established that M. tuberculosis bacilli were able to infect neurons directly, as demonstrated by the intracellular location of bacilli through confocal microscopy.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were bred and maintained under specific-pathogen-free conditions at the animal facility of the University of Cape Town (South Africa). One-day-old neonates and adult mice between 8 and 12 weeks of age were used. All animal experimental protocols complied with South African regulations. Approval was obtained from the Animal Research Ethics Committee, University of Cape Town, Cape Town, South Africa (reference numbers AEC 010/017 and AEC 010/018) in accordance with the South African National Standard 10386, The Care and Use of Animals for Scientific Purposes.
Mycobacteria.
M. tuberculosis strain H37Rv (Trudeau Mycobacterial Culture Collection, New York, NY), was grown at 37°C in Middlebrook 7H9 broth (Difco Laboratories) containing 10% oleic acid-albumin-dextrose-catalase (OADC) and 0.5% Tween 80 until log phase and then aliquoted and stored at −80°C. A frozen aliquot was thawed, passed 30 times through a 29-gauge needle, and plated in 10-fold serial dilutions on Middlebrook 7H10 agar (Difco Laboratories) containing 10% OADC and 0.5% glycerol. The concentration of M. tuberculosis was then determined by counting the CFU. M. tuberculosis with green fluorescent protein (H37Rv-GFP; provided by Joel Ernst, New York University School of Medicine, USA) was prepared similarly, with 25 μg/ml kanamycin added to broth or agar (11).
Cell cultures and infection.
Primary microglia were isolated from mixed glial cultures prepared from cerebral cortices of C57BL/6 neonates of either sex, as described previously (27, 28). Cultures of mouse primary neurons were prepared from C57BL/6 day 17 embryos or neonates as described previously with modifications (29, 30, 31). Briefly, the dissected hippocampi were digested, dissociated by trituration, and then seeded at a density of 1 × 105 cells per well on poly-l-lysine-coated coverslips in 24-well plates or glass chamber slides. The primary neuronal cultures were maintained in serum-free neurobasal medium (Gibco) containing 2% B27 supplement (Gibco) at 37°C in a 5% CO2 incubator.
HT22 (a murine neuronal cell line) was obtained from the Salk Institute, San Diego, CA, USA; Neuro-2a (murine neuroblastoma) and BV2 (murine microglia) cells were obtained from the National Institute for Cancer Research, Genoa, Italy. HT22 and Neuro-2a cells were propagated in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), and BV2 cells were propagated in RPMI 1640 medium with 10% FBS. Human neuroblastoma SK-N-SH cells (a gift from A. Vahidnia, Leiden University Medical Centre, Leiden, Netherlands) were grown in DMEM supplemented with 10% FBS and differentiated for 2 weeks in culture with 10 μM retinoic acid (Sigma) (32).
To assess optimal cellular infection, neurons were seeded at 1 × 105/well, while microglia were seeded at 1 × 104/well, and cultured with M. tuberculosis at a multiplicity of infection (MOI) of 2:1, 5:1, 10:1, 30:1, 50:1, or 100:1 (ratios of M. tuberculosis bacilli to cells). Although association of M. tuberculosis with cultured neuronal cells occurred at all ratios (data not shown), an MOI of 30:1 was deemed optimal, supported by other studies using an ex vivo model of hippocampal neuronal infection with L. monocytogenes (25), while an MOI of 2:1 was found optimal for microglial infection. Thus, briefly, HT22 and Neuro-2a cells and primary hippocampal neurons were infected with H37Rv-GFP at an MOI of 30:1 and microglia at an MOI of 2:1 for 4 h in 24-well culture plates, after which the culture medium was replaced with fresh medium containing 50 μg/ml gentamicin (Sigma). For image studies, cells were fixed for staining at 6 h, 24 h, and 48 h postinfection for further analysis. For colony enumeration, neurons were extensively washed with phosphate-buffered saline (PBS) and lysed with 1% Triton X-100 in PBS, and the lysates were cultured on Middlebrook 7H10 agar (Difco) supplemented with 10% OADC, 0.5% glycerol, and 25 μg/ml kanamycin. Mycobacterial cultures were incubated inside semisealed plastic bags at 37°C for 18 to 21 days, and colony counts were registered.
Histology and immunocytochemistry of infected cultures.
For histology, H37Rv-infected cells were fixed with 4% paraformaldehyde (PFA) in PBS, washed, and subjected to Ziehl-Neelsen (ZN) staining for acid-fast bacilli. For immunohistochemical analysis, H37Rv-GFP-infected cells were fixed with 4% PFA, washed, and blocked with 1% bovine serum albumin (BSA) and then incubated with either Alexa 555-conjugated phalloidin (1:600; Invitrogen) or anti-microtubule-associated protein 2 (MAP2) (1:1,000; Abcam) and visualized with Cy3-conjugated secondary antibody (1:1,000; Jackson ImmunoResearch Laboratories). SK-N-SH cell nuclei were stained with 6-diamidino-phenylidone (DAPI) (Sigma). The immunofluorescence-labeled cultures were mounted in fluorescent mounting medium (Dako), and images were captured with a Zeiss (Oberkochen, Germany) LSM 510 confocal microscope. High-resolution confocal imaging was carried out using a 63× (1.4-numerical-aperture [NA]) oil immersion objective, and z-stacks were acquired using Nyquist sampling parameters.
The quantification of M. tuberculosis-associated cultured cells was performed using light microscopy, where 100 to 400 microglial or neuronal cells were examined under bright-field microscopy for each experiment. Cultured neurons or microglia, which associated with M. tuberculosis bacilli, were calculated as the number of cells associated with acid-fast bacilli out of the total number of cells examined.
The quantification of cells that contained internalized M. tuberculosis bacilli was performed using confocal microscopy, where 100 to 150 cells per cell type were examined for each experiment. The cells containing internalized bacilli were counted in the 3-dimensional z-stack images of 5 different fields per cell type. The percentage of internalization was calculated as the number of cells containing intracellular H37Rv-GFP bacilli out of the total number of cells counted in the z-stack images.
Intracerebral infection and immunohistochemistry of infected mice.
Six C57BL/6 female mice (8 to 12 weeks old) from 2 independent experiments were inoculated intracerebrally in the left cerebral hemisphere with 1 × 105 CFU of H37Rv-GFP using a Hamilton syringe (Gastight no. 1701; Hamilton, Bonaduz, Switzerland). At 7 and 14 days postinfection, the infected mice were deeply anesthetized and transcardially perfused with 4% PFA. The 40-μm brain sections were cut using a vibratome and then processed for immunohistochemistry. The microglia and astrocytes were labeled with CD11b (clone M1/70; 1:500; Abcam) and glial-fibrillary acidic protein (GFAP) antibody (1:1,000; Sigma), respectively. The neurons were labeled with either NeuN (clone A60; 1:100; Merck-Millipore), MAP2 (1:1,000; Abcam), or β-III-tubulin antibody (1:1,000; Abcam) and corresponding Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). The nuclei were stained with DAPI (Sigma). The immunofluorescence-labeled sections were mounted in fluorescent mounting medium and viewed with a Zeiss 510LSM unit.
Lysotracker staining.
To effectively label and track phagolysosomes in live cells, infected cultures were incubated with Lysotracker (Molecular Probes, USA) before fixation and permeabilization. Two hours prior to the experimental time points, Lysotracker (1:900) was added to the culture supernatant at 37°C. At 24 h or 48 h postinfection, the cells were fixed with 4% PFA in PBS, washed, and blocked with 1% BSA in PBS. To ensure sufficient staining, the cells were incubated again with Lysotracker (1:1,000) overnight at 4°C. Subsequently, nuclei were stained with DAPI, and the coverslips were mounted in fluorescent mounting medium (Dako, CA, USA).
Cytokine quantification.
Supernatants from neuronal cultures were frozen at −80°C until further analysis. Cytokine concentrations were determined by enzyme-linked immunosorbent assay (ELISA) using reagents from R&D Systems (Minneapolis, MN) according to the instructions of the manufacturers.
Statistical analysis.
The data are presented as means and standard deviations (SD). Statistical analysis was performed by one-way analysis of variance (ANOVA) and one-tailed t test for comparisons among the time points. For all tests, a P value of <0.05 was considered significant.
RESULTS
M. tuberculosis bacilli associate with neuronal cultures.
In initial studies, the microglial cell line BV2 and cortex-derived primary microglia were cultured in the presence of M. tuberculosis bacilli at an MOI of 2:1 for 6 h, 24 h, and 48 h as established phagocytic cells against which to measure neuron-bacillus interaction. A time-dependent association of M. tuberculosis bacilli was observed in both BV2 (Fig. 1A and B) and primary microglial (Fig. 1C and D) cultures with >30% of host cells representing microglia-bacillus interaction. Next, neuronal cultures of immortalized HT22 cells (Fig. 2A and B) and Neuro-2a cells (Fig. 2C and D) were exposed to M. tuberculosis bacilli at an MOI of 30:1 for 6 h, 24 h, and 48 h. Analysis of infected neuronal cultures showed that M. tuberculosis bacilli were associated with the soma and the neurites. Quantitatively, there was a time-dependent increase in association of M. tuberculosis bacilli with both neuronal cell lines and an overall higher number of HT22 cells than of Neuro-2a cells associated with bacilli at all time points investigated (Table 1). Although Neuro-2a cells and HT22 cells are widely used to characterize neuronal behavior and responses, in general, transformed cell lines may differ from primary cells with respect to structure, development, binding, and functional characteristics (33). Therefore, to confirm the neuronal infectivity of M. tuberculosis bacilli and to exclude the possibility that previously observed mycobacterial association was due to anomalous characteristics of neuronal cell lines, the observation was validated in primary neuronal cell cultures. Primary neuronal cell cultures were established from murine hippocampi and infected with M. tuberculosis bacilli at an MOI of 30:1 for 6 h, 24 h, and 48 h. Uptake in primary neuronal cultures was comparable to that in HT22 cells and Neuro-2a cells; M. tuberculosis bacilli displayed a time-dependent increase in association from 7.9% to 21.3% over a 48-h period (Table 1) with bacilli associated with the neurites and cell bodies (Fig. 2E and F). Therefore, for the first time, the data clearly demonstrate that M. tuberculosis is capable of direct interaction with neurons.
FIG 1.
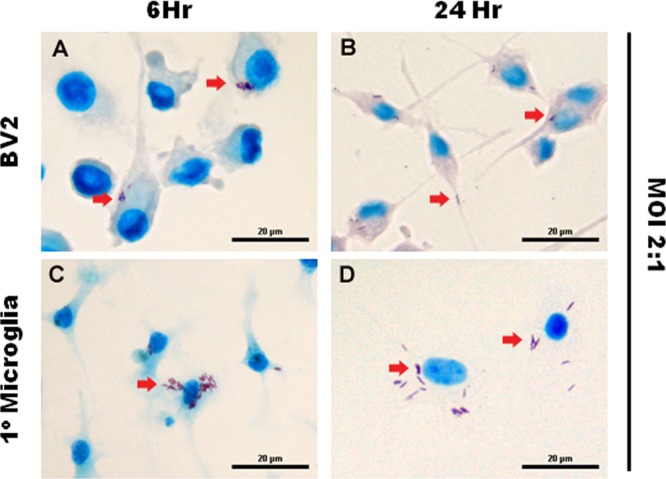
M. tuberculosis infects microglia. The microglial cell line BV2 (A and B) and primary microglia (C and D), isolated from the cortices of 1-day-old C57BL/6 neonates, were infected with M. tuberculosis at an MOI of 2:1 for 6 and 24 h. The arrows indicate the uptake of acid-fast bacilli by the microglial cells. Scale bars, 20 μm.
FIG 2.
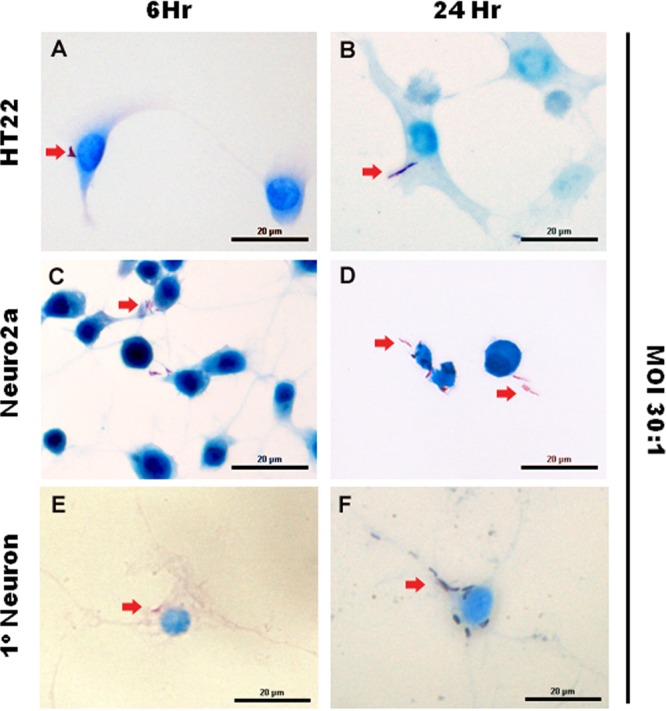
M. tuberculosis bacilli are associated with cultured neurons. Neuronal cultures—HT22, Neuro-2a, and primary neurons—established from the hippocampi of 1-day-old C57BL/6 neonates were infected with M. tuberculosis at an MOI of 30:1 for 6 and 24 h. Ziehl-Neelsen staining shows the presence of acid-fast bacilli (arrows) in the cytoplasm and neurites of neurons. Scale bars, 20 μm.
TABLE 1.
Microglial and neuronal association and internalization of M. tuberculosis bacillia
| Cell type | Bacillus/cell ratio | % cell associated |
% internalized |
Ratio (% internalized/% cell associated) |
||||
|---|---|---|---|---|---|---|---|---|
| 6 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | ||
| BV2 | 2:1 | 2.1 ± 0.92 | 31.1 ± 1.84 | 34.6 ± 4.79 | 31.0 ± 4.20 | 34.7 ± 2.50 | 1.00 | 1.00 |
| Primary microglia | 2:1 | 6.5 ± 2.23 | 31.5 ± 5.83 | 32.5 ± 0.86 | 30.8 ± 6.30 | 32.1 ± 6.56 | 0.98 | 0.99 |
| HT22 | 30:1 | 6.7 ± 1.65 | 17.2 ± 9.74 | 28.9 ± 1.00 | 11.8 ± 1.83 | 17.7 ± 1.34 | 0.68 | 0.61 |
| Neuro-2a | 30:1 | 1.9 ± 0.45 | 6.1 ± 4.03 | 20.0 ± 0.72 | 6.2 ± 1.41 | 9.8 ± 1.97 | 1.00 | 0.49 |
| Primary neurons | 30:1 | 7.9 ± 1.64 | 13.6 ± 3.30 | 21.3 ± 0.46 | 11.2 ± 0.89 | 10.1 ± 2.07 | 0.82 | 0.48 |
| SK-N-SH | 30:1 | 10.2 ± 1.47 | 17.9 ± 2.65 | 21.0 ± 2.41 | 11.9 ± 1.72 | 18.5 ± 1.18 | 0.67 | 0.88 |
Microglia and neurons were infected with M. tuberculosis, and cells that were associated with or had internalized bacilli at 6 h, 24 h, and 48 h were counted. The results represent the means ± SD of the combined data from at least 3 individual experiments.
Murine neurons internalize M. tuberculosis bacilli in vitro.
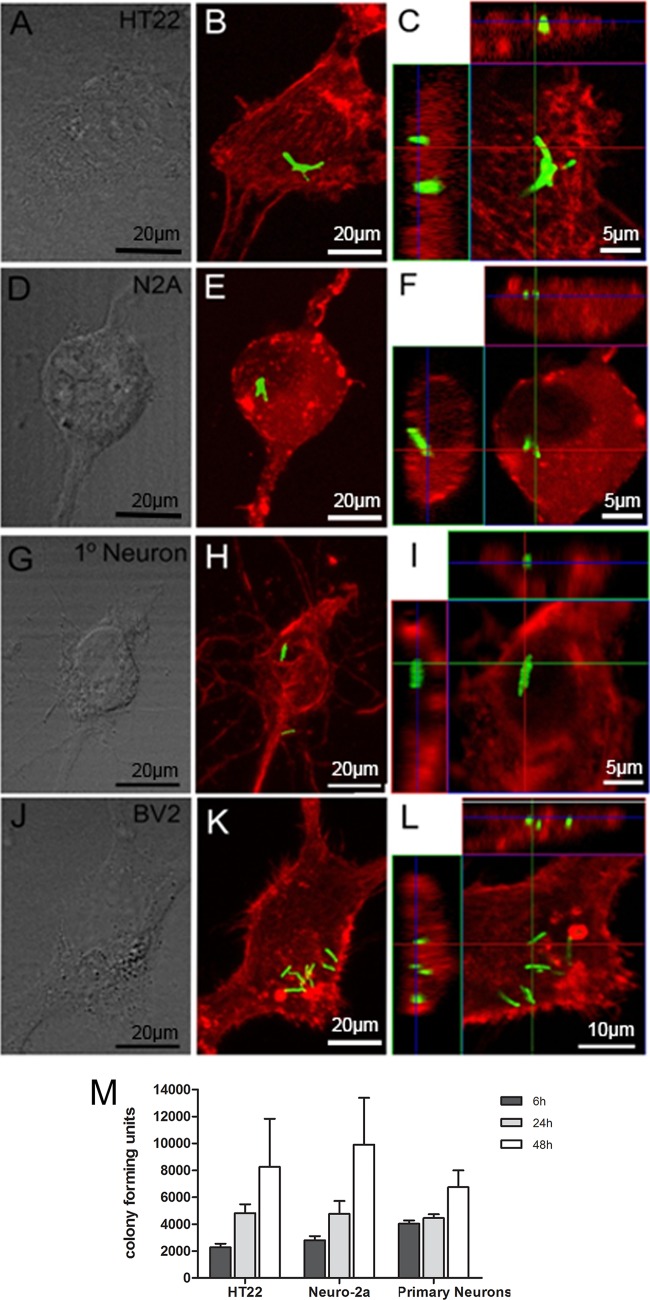
Observations of an association of bacilli with cultured neurons after Ziehl-Neelsen staining may have represented bound extracellular bacilli rather than bacteria that were intracellular. To investigate whether M. tuberculosis bacilli were indeed internalized by neurons, the neuronal cell lines HT22 (Fig. 3A to C) and Neuro-2a (Fig. 3D to F), as well as primary neuronal cultures (Fig. 3G to I), were infected with a recombinant GFP-expressing M. tuberculosis H37Rv strain at an MOI of 30:1. Similarly, BV2 microglial cells (Fig. 3J to L) were infected at an MOI of 2:1 to act as a positive-control cell strain for bacterial internalization. To confirm cytoplasmic localization of bacilli in microglia, the cultures were stained with cytofluorochrome-conjugated phalloidin, a cytoskeletal marker, and analyzed by confocal microscopy. Analysis of z-stack images showed that in most cases multiple bacilli were phagocytosed by BV2 (Fig. 3K), and orthogonal views confirmed that all bacilli were internalized (Fig. 3L). To determine whether M. tuberculosis bacilli were internalized by neurons, infected HT22 and Neuro-2a cultures were stained with cytofluorochrome-conjugated phalloidin while infected primary neurons were identified with the neuron-specific marker MAP2. The orthogonal display of the three-dimensional data demonstrated internalized M. tuberculosis bacilli in HT22 (Fig. 3C), Neuro-2a (Fig. 3F), and primary neuronal (Fig. 3I) cultures, embedded within cytoplasmic structures, as identified by the different markers.
FIG 3.
Confocal microscopy of internalized M. tuberculosis bacilli and bacterial replication in neurons. HT22 cells (A to C), Neuro-2a cells (D to F), and primary neurons (G to I), established from the hippocampi of 17-day-old C57BL/6 mouse embryos, and BV2 cells (J to L) were infected with M. tuberculosis for 24 h and then subjected to immunohistochemistry for 6 h, 24 h, and 48 h, and the numbers of CFU were determined from lysed cultures (M). The fluorescence images of phalloidin-labeled cell lines, including HT22, Neuro-2a, and BV2, and MAP2-labeled primary neurons highlight the cytoskeletal proteins (red) to demonstrate the cytosolic location of GFP-expressing bacilli (green). The internalized bacilli can be seen in the top and side images in the orthogonal views, which show the colocalization of cytoskeleton and bacilli in the x-y plane of an optical section of the z-stack. Phase-contrast images of cultured cells are presented in panels A, D, G, and J. (M) Bacterial replication in HT22 and Neuro-2a cells and primary neurons assessed at 6 h, 24 h, and 48 h. The results are the means and SD of quadruple experimental data sets and are representative of one of three similar experiments.
Quantification of the cells with bacilli showed ≥98% of microglial-cell-associated bacilli were internalized after 24 h and 48 h (Table 1). Importantly, for HT22, 11.8% and 17.7% of cultured cells internalized M. tuberculosis bacilli after 24 h and 48 h, respectively (Table 1). Although Neuro-2a internalization was approximately 2 times lower than that of HT22, significant internalization levels of 6.2% and 9.8% at 24 h and 48 h were nonetheless measured (Table 1). For primary neuronal cultures, 11.2% of cells had internalized bacilli at 24 h, which decreased slightly to 10.1% after 48 h (Table 1). Overall, the relative ratios indicated that, whereas microglial cells internalized most of the associated bacilli, murine neuronal cells internalized only 45 to 65% of associated bacilli after 48 h (Table 1). Moreover, to determine whether internalized M. tuberculosis bacilli within neurons represent a productive infection, where bacilli remain viable and retain the capacity to replicate, infected HT22 and Neuro-2a cells and primary neurons were lysed at 6 h, 24 h, and 48 h, and bacterial colonies were enumerated. The data indicated that initial uptake and internalization of mycobacteria within primary neurons may be superior to those of HT22 and Neuro-2a, with significantly higher bacillus levels at 6 h (Fig. 3M). Notably, bacillus growth occurred in all neuron cultures, with significantly higher numbers recorded at 48 h than at 6 h (Fig. 3M).
Thus, the study provided evidence that neurons are capable of internalizing M. tuberculosis bacilli that are viable and capable of replication within the neurons.
Neurons internalize M. tuberculosis bacilli in vivo.
In this study, the localization of GFP-expressing M. tuberculosis (green) in relation to two different intracellular neuron-specific markers, β-III-tubulin (red) and MAP2 (red), was analyzed, and the results were compared to microglial uptake. Orthogonal projections (Fig. 4B, D, and F) representing 3-dimensional data sets provide a view of the x and y, as well as the z, dimensions of the original z-stack. Cell nuclei were labeled with DAPI. The respective differential interference contract (DIC) images (Fig. 4A, C, and E) illustrate the position of M. tuberculosis-GFP within the tissue. From the data presented in Fig. 4B, it was clear that M. tuberculosis-GFP bacilli were contained within β-III-tubulin-positive neuronal structures (red), indicated by partial colocalization (yellow) of the red and green fluorescence signals. Similarly, bacilli resided within MAP2-positive neuronal structures (red), which resulted in a yellow colocalization signal (Fig. 4D). Green fluorescent bacilli were also present, although it was unclear whether they represented free or internalized bacilli where MAP2 did not condense around the bacillus, as limitations in optical resolution prevented the delineation of cellular structure. Microscopic observations indicated that CD11b+ microglial cells contained higher numbers of bacilli than neurons. CD11b, as a cell surface antigen, allows sufficient spatial separation of the red signal on the surface and the green signal from the internalized bacilli not to result in a colocalized (yellow) signal, as clearly evident in Fig. 4F.
FIG 4.
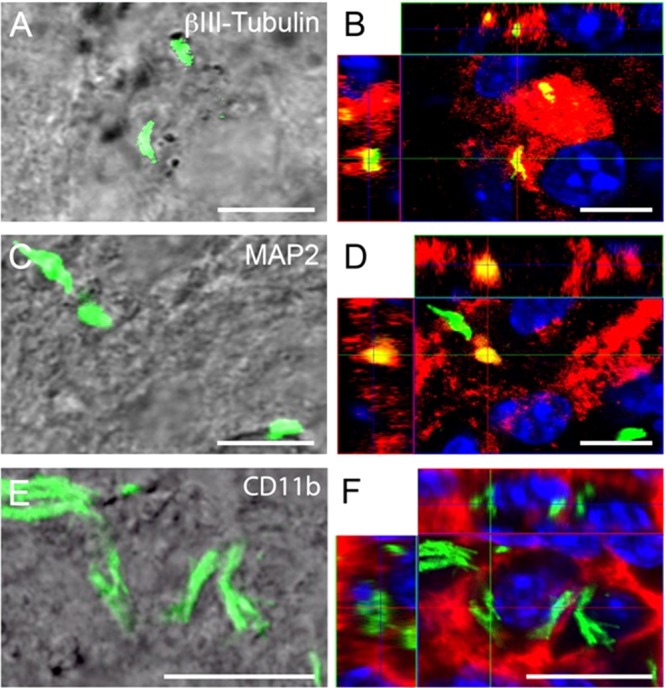
Confocal microscopy of internalized M. tuberculosis bacilli in neurons in brain sections of C57BL/6 mice 7 days after intracerebral infection. (B, D, and F) Orthogonal projections representing 3-dimensional data sets provide a view of the x-y, as well as the z, dimensions of the original z-stack. Cell nuclei are labeled with DAPI (blue). (A, C, and E) DIC images showing the tissue localization of H37Rv-GFP (green). (B) H37Rv-GFP bacilli are contained within β-III-tubulin-positive neuronal structures (red), indicated by partial colocalization (yellow) of the red and green fluorescence signals. (D) Bacilli are found within MAP2-positive neuronal structures (red, resulting in a yellow colocalization signal), but also disassociated from MAP2 (green). The latter may represent internalized or free bacilli, as it is not possible to delineate cell boundaries in these preparations. (F) Brain macrophages (identified by CD11b immunoreactivity) (red) contain large numbers of bacilli (green). CD11b is a cell surface antigen; therefore, no signal colocalization is observed. Scale bars, 10 μm.
Therefore, the data demonstrate that M. tuberculosis bacilli can be internalized by neurons under both in vitro and in vivo conditions but that neuronal uptake is subordinate to that of microglia.
M. tuberculosis bacilli encapsulated within neuronal cytoskeletal structures.
To gain additional insight into the relationship between neuronal structures and internalized bacilli, localized cytoplasmic sites were further investigated in vitro and in vivo. Bacilli may display distinct condensation of cytoskeletal elements (MAP2+) around them, which resulted in partial colocalization of the fluorescent signals (yellow) but still allowed separation of the structures in vitro (Fig. 5A). Figure 5B confirmed neuronal internalization of the bacillus, whereas closer inspection and analysis of detailed images (Fig. 5B1 to B3) revealed that the bacillus resided within a MAP2-positive capsule-like cytoplasmic neuronal structure indicative of rearrangement of the cytoskeleton during infection. Similarly, this colocalization was observed in vivo. As described above, in Fig. 4B and D, colocalization indicated M. tuberculosis infection of neurons within the CNS following intracerebral challenge. The scan resolution of these sections, due to background and tissue thickness (40 μm), was not sufficient to separate the red and green immunofluorescent signals from cytoplasmic markers and bacilli, respectively. However, the MAP2 image (Fig. 5D) showed MAP2 condensation within the area surrounding the bacillus. Therefore, it is plausible that the in vivo infection of neurons by M. tuberculosis (Fig. 5C to E) likely reflects conditions observed in vitro (Fig. 5B1 to B3).
FIG 5.
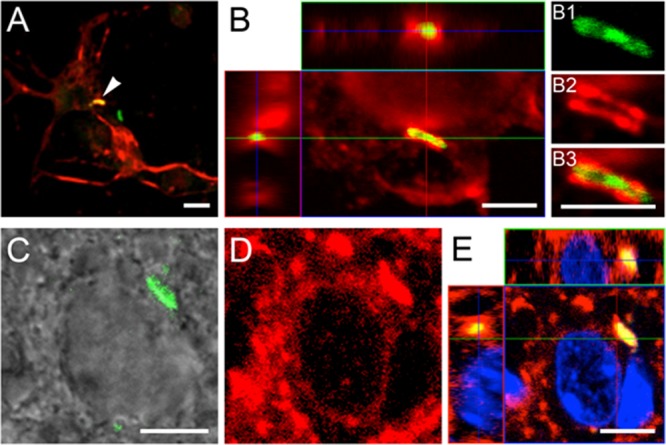
Confocal microscopy of internalized M. tuberculosis bacilli in cultured murine primary neurons and in brain sections. (B and E) Orthogonal projections representing 3-dimensional data sets provide a view of the x-y, as well as the z, dimensions of the original z-stack. Cell nuclei are labeled with DAPI (blue). (A) Overview of MAP2-positive neurons (red), established from the hippocampi of 17-day-old C57BL/6 mouse embryos, containing an H37Rv-GFP bacillus (arrowhead). (B) Bacilli (green) are found associated with MAP2-positive neuronal structures, such as the cortical cytoplasm and neurites (red), resulting in a partial colocalization signal (yellow). (B1 to B3) Detailed images reveal localization of the bacillus within the MAP2-positive neuronal structure. Panels A and B represent a 24-h time point. (C and D) In brain sections (14 days postinfection), colocalization of bacilli (green signal in DIC image) (C) and MAP2 signal (D) is evident (yellow signal in panel E), indicating internalization of bacilli by the neurons. However, it is not possible to resolve the relevant structures in brain sections at the level seen in vitro. Scale bars, 5 μm.
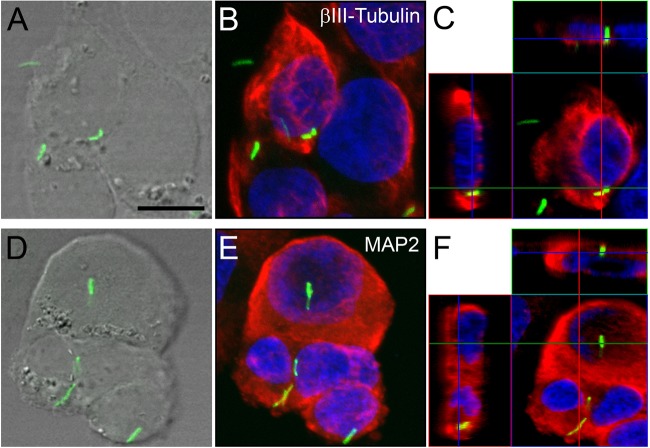
Cultured human neuronal cells internalize M. tuberculosis bacilli.
The data demonstrated M. tuberculosis internalization by murine-derived neuronal cell lines and primary murine neuronal cultures, which were confirmed by neuronal uptake in vivo. To ascertain whether these observations have clinical relevance, we infected retinoic acid-differentiated SK-N-SH human-derived neuroblastoma cell cultures with M. tuberculosis-GFP bacilli at an MOI of 30:1. The cytoplasmic localization of fluorescent bacilli within SK-N-SH cultures was confirmed through staining with the neuron-specific marker β-III-tubulin or MAP2. Similar to the observations described for murine-derived neuronal cultures, the orthogonal display of the three-dimensional data demonstrated internalized M. tuberculosis bacilli in either β-III-tubulin-positive (Fig. 6C) or MAP2-positive (Fig. 6F) cytoplasmic structures. In addition, internalization was confirmed via the colocalization of the green (bacilli) and red (β-III-tubulin or MAP2) fluorescence signals, resulting in a partially yellow fluorescence signal (Fig. 6C and F). Quantification of the percentage of SK-N-SH cells associated with bacilli showed a statistically significant increase from 10.2% to 21% over 48 h (Table 1), similar to observations in murine-derived neuronal cultures. The percentage of cells that internalized bacilli increased from 11.9% to 18.5% from 24 h to 48 h (Table 1). This study therefore confirms that human neuronal cells are capable of being infected by M. tuberculosis bacilli.
FIG 6.
Confocal microscopy of internalized M. tuberculosis bacilli in human SK-N-SH cultured neurons. (B, C, E, and F) SK-N-SH cultured neurons labeled with anti-β-III-tubulin (B and C) and anti-MAP2 (E and F) antibodies (red). (B and E) Maximum-intensity projections of z-stacks of entire cells. (C and F) Orthogonal projections representing 3-dimensional data sets provide a view of defined optical sections in the x-y, as well as the z, dimensions of the original z-stack. Cell nuclei are labeled with DAPI (blue). (A and D) DIC images showing the tissue localization of H37Rv-GFP bacilli (green). Scale bar, 20 μM. The images represent neuronal cultures at 48 h.
Limited association of M. tuberculosis bacilli with phagolysosomes during neuronal infection.
Previous studies have demonstrated that neurons are capable of phagocytosis (26), thereby introducing the possibility that M. tuberculosis bacilli may be internalized in this manner. We investigated the association of M. tuberculosis bacilli with phagolysosomes in primary neuronal cultures infected at an MOI of 30:1 for 24 h and 48 h. Analysis of confocal images showed predominant separation of the Lysotracker Red marker and green fluorescent bacilli, which indicated either arrest of phagosome-lysosome fusion during neuron infection or, alternatively, residence of bacilli within the cytosol and not within phagosomes (Fig. 7).
FIG 7.
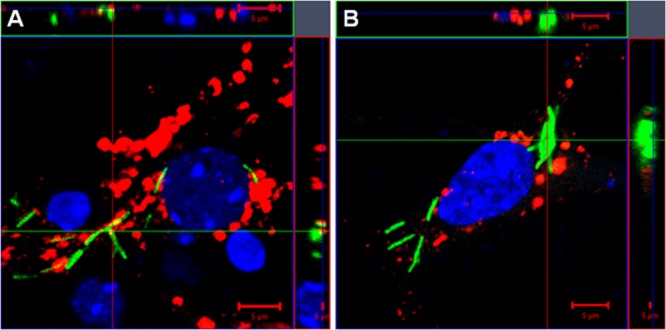
Limited association of M. tuberculosis bacilli with neuronal phagolysosomes. Murine primary neuron cultures were established from hippocampi of 17-day-old C57BL/6 embryos and infected with H37Rv-GFP bacilli (green) at a multiplicity of infection of 30:1. The neurons were stained with the phagolysosome marker Lysotracker (red) after 24 h (A) and 48 h (B). Cell nuclei were labeled with DAPI (blue) and analyzed by confocal microscopy. The images represent 3-dimensional data sets and provide a view of the x-y and z dimensions of the original z-stack. A colocalized signal (yellow) resulting from the association of the bacilli (green) with the phagolysosome, labeled with Lysotracker (red), is rarely observed in neurons at 24 h (A) and 48 h (B) postinfection, and most internalized bacilli appear disassociated from the phagolysosome. Scale bars, 5 μm.
Neurons are activated during M. tuberculosis infection.
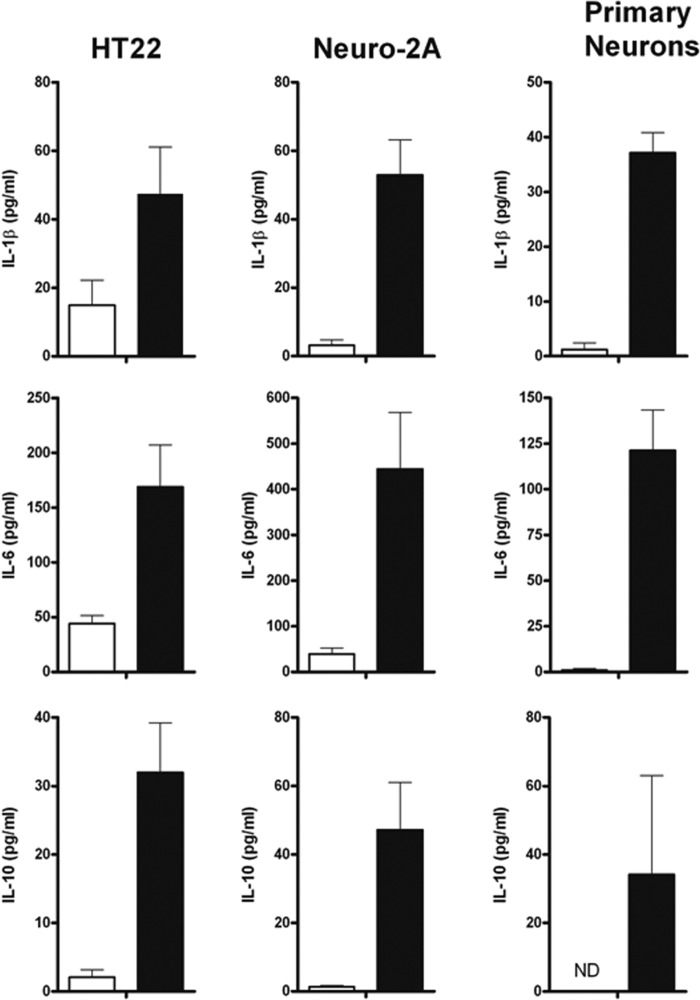
Neurons are capable of generating an immune response when confronted by various pathogens. However, neuronal activation in response to M. tuberculosis bacilli has not yet been reported. In this study, interleukin 1β (IL-1β), IL-6, and IL-10 cytokine expression was measured in M. tuberculosis-infected HT22 and Neuro-2a cells and primary neurons as an indication of functional response (Fig. 8). Both neuronal cell lines and primary neuronal cultures had significantly higher (P < 0.05) induction of the proinflammatory cytokines IL-1β and IL-6 and the anti-inflammatory cytokine IL-10 during exposure to M. tuberculosis bacilli after 48 h than uninfected cultures. IL-6 expression was higher than IL-1β or IL-10 levels measured in all cultures, with Neuro-2a cell synthesis being superior to that of HT22 or primary neuronal cultures. This study therefore, established that neurons have the capacity to induce an immune response during M. tuberculosis challenge.
FIG 8.
Neurons induce an immune response during M. tuberculosis infection. HT22, Neuro-2a, and primary neuronal cultures were infected with M. tuberculosis, and IL-1β, IL-6, and IL-10 were measured in the culture supernatants after 48 h by ELISA. The results represent the means and SD of combined data from 4 to 6 experiments; each experiment was performed in triplicate. ND, not detected.
DISCUSSION
Numerous bacterial species are able to invade the CNS to establish infection, and the mechanisms of invasion of several are known and their target cells identified (17). In contrast, the mechanisms associated with M. tuberculosis infection of the CNS and the specific cells targeted for invasion are mostly unknown. Although studies have described microglia as the preferred cell population infected by M. tuberculosis bacilli during CNS-TB pathogenesis (18, 19, 20), the ubiquitous nature of M. tuberculosis enables it to infect different cell types, which include both phagocytic and nonphagocytic cells (8, 9). Previous studies that investigated the relationship between M. tuberculosis bacilli and cells of the CNS have typically focused on microglia as the principal cellular target for infection (18, 34), while astroglia were infected to a lesser extent (19). The interaction of M. tuberculosis bacilli with neuronal cells has not yet been reported; hence, in a series of experiments, the neuronal infectivity of bacilli was investigated. This study describes the novel observation of neurons acting as additional target cells for M. tuberculosis bacilli in the CNS. It was demonstrated for the first time that, other than uptake by microglia and astrocytes, M. tuberculosis bacilli display neurotropic qualities and are internalized by neurons during CNS infection. Nonphysiological factors due to the immortalization of cell lines used in this study were excluded as contributing factors for bacillus uptake by demonstrating that de novo cultures of isolated primary neurons internalize M. tuberculosis bacilli. The physiological relevance of these findings was validated through in vivo infection studies, substantiating observations and conclusions from in vitro culture investigations.
This study demonstrates internalization of M. tuberculosis bacilli by neurons, although the manner in which pathogen recognition occurs is unknown. Neurons can be activated via innate immune pathways, commonly expressed in macrophages, where binding and recognition are mediated through cell surface-expressed molecules that include Fc receptors, mannose receptors, complement receptors, and Toll-like receptors (TLRs) (35). Here, neuronal expression of TLR2 and TLR4 (36, 37) may potentially present them as receptor candidates for bacillus recognition, as we and others have shown that both molecules are critical for protective immune function against M. tuberculosis bacilli (38, 39). However, their role is controversial, as reports have also shown redundancy for TL2 and TLR4 in mediating immune protection. Uptake of M. tuberculosis bacilli by mononuclear cells occurs primarily through phagocytosis. The phagocytic capacity of neurons is contentious, and while Bowen et al. (26) demonstrated that neurons are capable of phagocytosis, thereby elucidating a potential mechanism for neuronal entry of M. tuberculosis bacilli, other mechanisms of uptake require investigation. Our data showing that the majority of M. tuberculosis bacilli do not reside within phagolysosomes of neurons suggest that mechanisms of entry other than phagocytosis should be considered, although it cannot be excluded. Indeed, entry of M. tuberculosis bacilli into other nonphagocytic cells indicates an active mechanism of invasion induced by the organism (8). The M. tuberculosis bacillus carries four mammalian cell entry (mce) operons, with mce1A potentially mediating invasion of host cells (15, 16).
The data presented in this study do not exclude phagocytosis as a mechanism through which bacilli are internalized, but the limited colocalization with acidified vesicles would then argue for arrest of phagolysosome maturation during neuronal infection. Alternatively, bacilli may be free within the cytoplasm, either through nonphagocytic entry or subsequent to phagosome escape, though the latter remains an area of controversy (40). Whether neurons employ macropinocytosis as a mechanism for M. tuberculosis bacillus uptake, as was shown in nonphagocytic cells (8), remains to be elucidated. Nevertheless, two populations of internalized bacilli could clearly be distinguished: those that colocalized with cytoskeletal structures and those that did not. Whether these two bacterial populations reside within different cytoplasmic environments or whether they represent different stages of the same internalization process is unclear. However, bacilli that were tightly associated with cytoplasmic structures within neurons appeared to be encased in a sheath-like structure, evidence of cytoskeletal rearrangement upon infection. M. tuberculosis bacillus-induced cytoskeletal rearrangement is known and has previously been reported (41, 42), and the observations in this study suggest that similar changes in the neuronal cytoskeleton occur during bacillus invasion. Moreover, it was established that entry of M. tuberculosis bacilli into epithelial cells was dependent on microtubules, which could be inhibited by administration of colchicine and nocodazole, known to cause microtubule depolymerization (43). Our findings do not exclude cytoskeletal concentration around bacilli residing within phagosomes, as others have shown actin nucleation around mycobacterial phagosomes (44, 45). The role of cytoskeletal concentration around bacilli is controversial, and its complexity is highlighted by contrasting reports. Kolonko and colleagues (45) suggest that actin polymerization protects M. tuberculosis bacilli from delivery to a bactericidal environment, whereas Anes and colleagues (44) propose that actin assembly promotes killing of pathogenic mycobacteria.
In this study, the overall capacity of neurons to internalize M. tuberculosis bacilli was reduced compared to microglial cells, reflected in the greater number of microglia than of neurons that were infected. In addition, generally fewer bacilli were internalized by neurons than by microglia in culture, despite being exposed to higher concentrations of bacilli. This is not surprising, as neurons are not considered to be professional phagocytic cells like microglia, and under normal conditions, preferential uptake is mediated by microglia. However, it may be argued that under conditions where the growth of bacilli is promoted, such as in HIV coinfection, where immune suppression prevails, M. tuberculosis infection of neurons could occur. The difference in uptake may represent variance in recognition of bacilli when exposed to either microglia or neurons, or it may reflect differences in the phagocytic capabilities of the cell types. Nonetheless, the increased capacity of microglia to phagocytose bacilli within an environment usually regarded as immune privileged can be interpreted as a protective role for microglia over neurons during CNS-TB. Indeed, evidence from several reports points to a cooperative relationship between microglia and neurons, where neuronal function is shielded by microglial activity (46). It is therefore interesting to speculate that direct targeting of neurons for infection could potentially represent an evasive mechanism by which bacilli circumvent the bactericidal effects of microglia. The evidence provided in this study indicates that the microenvironment present within neurons is permissive for bacterial replication and could potentially present a cellular niche for persistence. For example, a key mechanism of host protection against M. tuberculosis bacilli is apoptosis of infected cells mediated by cytolytic T cells (47). Reduced expression of major histocompatibility complex (MHC) class 1 in adult neurons (48) would favor escape from CD8+ T cell-mediated apoptosis once the neurons are infected by M. tuberculosis bacilli and promote the cell type as a reservoir for persistence, as has been described for other cells in extrapulmonary tissues (14). Nonetheless, the data in this study, which show neurons to be capable of a functional cytokine response, may indicate integral involvement as part of the host collective immune reaction rather than acting as passive bystander cells during M. tuberculosis bacillus challenge.
Importantly, data that demonstrate the ability of M. tuberculosis bacilli to infect human neuronal cells provide a persuasive argument for neurons to be considered clinically important as potential host cells. Several studies have reported an increase in the prevalence of CNS-TB under conditions of immune suppression, e.g., in HIV-coinfected patients, who often present with low CD4+ T cell counts (49, 50). M. tuberculosis bacilli are more likely to be detected in the cerebrospinal fluid (CSF) of immunosuppressed patients (51, 52), possibly due to higher levels of bacilli and of dissemination. As a key requirement to control M. tuberculosis bacillus replication (53), low CD4+ T cell counts could predispose such patients to increased levels of CNS bacilli. Therefore, neuronal infection may be particularly relevant under clinical conditions where patients fail to control CNS-TB infection and the bacillus burden is substantial, possibly at specific, localized foci within the brain, and thereby potentially creates conditions for neurons to be targeted. Therefore, it is interesting to speculate whether deterioration of cognitive function and mental deterioration observed during advanced tuberculosis of the CNS is associated with impairment of neuronal function as a direct consequence of M. tuberculosis bacillus uptake.
In summary, conclusive evidence that M. tuberculosis has the necessary capacity to infect neurons is provided. The implications of neuronal infection, the effect on neuronal function, and the influence exerted on the intercellular interactions during host immune responses against tuberculosis need further investigation and should be examined.
ACKNOWLEDGMENTS
We thank Marylin Tyler and Lizette Fick for their contributions to the histopathology, Lester Davids and Toni Wiggins for their help in acquiring the SK-N-SH cell line, and especially Faried Abbass for technical support. We thank the staff of the Division of Immunology and the Animal Unit at UCT for their contributions to animal care and technical support.
This study was supported by the National Research Foundation (South Africa), Deutscher Akademischer Austausch Dienst (Germany), the Medical Research Council (South Africa), and the University of Cape Town and National Health and Laboratory Service (South Africa).
Footnotes
Published ahead of print 24 February 2014
REFERENCES
- 1.WHO. 2012. Global tuberculosis control 2011 WHO, Geneva, Switzerland [Google Scholar]
- 2.Be NA, Kim KS, Bishai WR, Jain SK. 2009. Pathogenesis of central nervous system tuberculosis. Curr. Mol. Med. 9:94–99. 10.2174/156652409787581655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kingkaew N, Sangtong B, Amnuaiphon W, Jongpaibulpatana J, Mankatittham W, Akksilp S, Sirinak C, Nateniyom S, Burapat C, Kittikraisak W, Monkongdee P, Varma JK. 2009. HIV-associated extrapulmonary tuberculosis in Thailand: epidemiology and risk factors for death. Int. J. Infect. Dis. 13:722–729. 10.1016/j.ijid.2008.11.013 [DOI] [PubMed] [Google Scholar]
- 4.Sharma SK, Mohan A, Kadhiravan T. 2005. HIV-TB co-infection: epidemiology, diagnosis, and management. Indian J. Med. Res. 121:550–567 [PubMed] [Google Scholar]
- 5.Donald PR, Schaaf HS, Schoeman JF. 2005. Tuberculous meningitis and miliary tuberculosis: the Rich focus revisited. J. Infect. 50:193–195. 10.1016/j.jinf.2004.02.010 [DOI] [PubMed] [Google Scholar]
- 6.Jain SK, Paul-Satyaseela M, Lamichhane G, Kim KS, Bishai WR. 2006. Mycobacterium tuberculosis invasion and traversal across an in vitro human blood-brain barrier as a pathogenic mechanism for central nervous system tuberculosis. J. Infect. Dis. 193:1287–1295. 10.1086/502631 [DOI] [PubMed] [Google Scholar]
- 7.Rich A, McCordick H. 1933. The pathogenesis of tuberculous meningitis. Bull. John Hopkins Hosp. 52:5–37 [Google Scholar]
- 8.Garcia-Perez BE, Mondragon-Flores R, Luna-Herrera J. 2003. Internalization of Mycobacterium tuberculosis by macropinocytosis in non-phagocytic cells. Microb. Pathog. 35:49–55. 10.1016/S0882-4010(03)00089-5 [DOI] [PubMed] [Google Scholar]
- 9.Munoz S, Rivas-Santiago B, Enciso JA. 2009. Mycobacterium tuberculosis entry into mast cells through cholesterol-rich membrane microdomains. Scand. J. Immunol. 70:256–263. 10.1111/j.1365-3083.2009.02295.x [DOI] [PubMed] [Google Scholar]
- 10.Teitelbaum R, Schubert W, Gunther L, Kress Y, Macaluso F, Pollard JW, McMurray DM, Bloom BR. 1999. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity 10:641–650. 10.1016/S1074-7613(00)80063-1 [DOI] [PubMed] [Google Scholar]
- 11.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. 2007. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 179:2509–2519 [DOI] [PubMed] [Google Scholar]
- 12.Giacomini E, Iona E, Ferroni L, Miettinen M, Fattorini L, Orefici G, Julkunen I, Coccia EM. 2001. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J. Immunol. 166:7033–7041 [DOI] [PubMed] [Google Scholar]
- 13.Hickman SP, Chan J, Salgame P. 2002. Mycobacterium tuberculosis induces differential cytokine production from dendritic cells and macrophages with divergent effects on naive T cell polarization. J. Immunol. 168:4636–4642 [DOI] [PubMed] [Google Scholar]
- 14.Barrios-Payan J, Saqui-Salces M, Jeyanathan M, Vazquez AA, Arreola MC, Rook G, Hernandez-Pando R. 2012. Extrapulmonary location of Mycobacterium tuberculosis DNA during latent infection. J. Infect. Dis. 206:1194–1205. 10.1093/infdis/jis381 [DOI] [PubMed] [Google Scholar]
- 15.Arruda S, Bomfim G, Knights R, Huima-Byron T, Riley LW. 1993. Cloning of an M. tuberculosis DNA fragment associated with entry and survival inside cells. Science 261:1454–1457. 10.1126/science.8367727 [DOI] [PubMed] [Google Scholar]
- 16.Chitale S, Ehrt S, Kawamura I, Fujimura T, Shimono N, Anand N, Lu S, Cohen-Gould L, Riley LW. 2001. Recombinant Mycobacterium tuberculosis protein associated with mammalian cell entry. Cell. Microbiol. 3:247–254. 10.1046/j.1462-5822.2001.00110.x [DOI] [PubMed] [Google Scholar]
- 17.Drevets DA, Leenen PJ, Greenfield RA. 2004. Invasion of the central nervous system by intracellular bacteria. Clin. Microbiol. Rev. 17:323–347. 10.1128/CMR.17.2.323-347.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson PK, Gekker G, Hu S, Sheng WS, Anderson WR, Ulevitch RJ, Tobias PS, Gustafson KV, Molitor TW, Chao CC. 1995. CD14 receptor-mediated uptake of nonopsonized Mycobacterium tuberculosis by human microglia. Infect. Immun. 63:1598–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rock RB, Hu S, Gekker G, Sheng WS, May B, Kapur V, Peterson PK. 2005. Mycobacterium tuberculosis-induced cytokine and chemokine expression by human microglia and astrocytes: effects of dexamethasone. J. Infect. Dis. 192:2054–2058. 10.1086/498165 [DOI] [PubMed] [Google Scholar]
- 20.Yang CS, Lee HM, Lee JY, Kim JA, Lee SJ, Shin DM, Lee YH, Lee DS, El-Benna J, Jo EK. 2007. Reactive oxygen species and p47phox activation are essential for the Mycobacterium tuberculosis-induced pro-inflammatory response in murine microglia. J. Neuroinflammation 4:27. 10.1186/1742-2094-4-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson PK, Hu S, Anderson WR, Chao CC. 1994. Nitric oxide production and neurotoxicity mediated by activated microglia from human versus mouse brain. J. Infect. Dis. 170:457–460. 10.1093/infdis/170.2.457 [DOI] [PubMed] [Google Scholar]
- 22.Rambukkana A, Salzer JL, Yurchenco PD, Tuomanen EI. 1997. Neural targeting of Mycobacterium leprae mediated by the G domain of the laminin-alpha2 chain. Cell 88:811–821. 10.1016/S0092-8674(00)81927-3 [DOI] [PubMed] [Google Scholar]
- 23.Aung T, Kitajima S, Nomoto M, En J, Yonezawa S, Arikawa I, Goto M. 2007. Mycobacterium leprae in neurons of the medulla oblongata and spinal cord in leprosy. J. Neuropathol. Exp. Neurol. 66:284–294. 10.1097/nen.0b013e31803d597e [DOI] [PubMed] [Google Scholar]
- 24.Jin Y, Dons L, Kristensson K, Rottenberg ME. 2001. Neural route of cerebral Listeria monocytogenes murine infection: role of immune response mechanisms in controlling bacterial neuroinvasion. Infect. Immun. 69:1093–1100. 10.1128/IAI.69.2.1093-1100.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin Y, Lundkvist G, Dons L, Kristensson K, Rottenberg ME. 2004. Interferon-gamma mediates neuronal killing of intracellular bacteria. Scand. J. Immunol. 60:437–448. 10.1111/j.0300-9475.2004.01500.x [DOI] [PubMed] [Google Scholar]
- 26.Bowen S, Ateh DD, Deinhardt K, Bird MM, Price KM, Baker CS, Robson JC, Swash M, Shamsuddin W, Kawar S, El-Tawil T, Roos J, Hoyle A, Nickols CD, Knowles CH, Pullen AH, Luthert PJ, Weller RO, Hafezparast M, Franklin RJ, Revesz T, King RH, Berninghausen O, Fisher EM, Schiavo G, Martin JE. 2007. The phagocytic capacity of neurones. Eur. J. Neurosci. 25:2947–2955. 10.1111/j.1460-9568.2007.05554.x [DOI] [PubMed] [Google Scholar]
- 27.Giulian D, Baker TJ. 1986. Characterization of ameboid microglia isolated from developing mammalian brain. J. Neurosci. 6:2163–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saura J, Tusell JM, Serratosa J. 2003. High-yield isolation of murine microglia by mild trypsinization. Glia 44:183–189. 10.1002/glia.10274 [DOI] [PubMed] [Google Scholar]
- 29.Ahlemeyer B, Baumgart-Vogt E. 2005. Optimized protocols for the simultaneous preparation of primary neuronal cultures of the neocortex, hippocampus and cerebellum from individual newborn (P0.5) C57Bl/6J mice. J. Neurosci. Methods 149:110–120. 10.1016/j.jneumeth.2005.05.022 [DOI] [PubMed] [Google Scholar]
- 30.Brewer GJ, Torricelli JR, Evege EK, Price PJ. 1993. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 35:567–576. 10.1002/jnr.490350513 [DOI] [PubMed] [Google Scholar]
- 31.Kaech S, Banker G. 2006. Culturing hippocampal neurons. Nat. Protoc. 1:2406–2415. 10.1038/nprot.2006.356 [DOI] [PubMed] [Google Scholar]
- 32.Jain P, Cerone MA, Leblanc AC, Autexier C. 2007. Telomerase and neuronal marker status of differentiated NT2 and SK-N-SH human neuronal cells and primary human neurons. J. Neurosci. Res. 85:83–89. 10.1002/jnr.21094 [DOI] [PubMed] [Google Scholar]
- 33.LePage KT, Dickey RW, Gerwick WH, Jester EL, Murray TF. 2005. On the use of neuro-2a neuroblastoma cells versus intact neurons in primary culture for neurotoxicity studies. Crit. Rev. Neurobiol. 17:27–50. 10.1615/CritRevNeurobiol.v17.i1.20 [DOI] [PubMed] [Google Scholar]
- 34.Shams H, Wizel B, Lakey DL, Samten B, Vankayalapati R, Valdivia RH, Kitchens RL, Griffith DE, Barnes PF. 2003. The CD14 receptor does not mediate entry of Mycobacterium tuberculosis into human mononuclear phagocytes. FEMS Immunol. Med. Microbiol. 36:63–69. 10.1016/S0928-8244(03)00039-7 [DOI] [PubMed] [Google Scholar]
- 35.Peltier DC, Simms A, Farmer JR, Miller DJ. 2010. Human neuronal cells possess functional cytoplasmic and TLR-mediated innate immune pathways influenced by phosphatidylinositol-3 kinase signaling. J. Immunol. 184:7010–7021. 10.4049/jimmunol.0904133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PJ. 2007. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J. Neurochem. 102:37–50. 10.1111/j.1471-4159.2007.04524.x [DOI] [PubMed] [Google Scholar]
- 37.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. 2007. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc. Natl. Acad. Sci. U. S. A 104:13798–13803. 10.1073/pnas.0702553104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abel B, Thieblemont N, Quesniaux VJ, Brown N, Mpagi J, Miyake K, Bihl F, Ryffel B. 2002. Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J. Immunol. 169:3155–3162 [DOI] [PubMed] [Google Scholar]
- 39.Drennan MB, Nicolle D, Quesniaux VJ, Jacobs M, Allie N, Mpagi J, Fremond C, Wagner H, Kirschning C, Ryffel B. 2004. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am. J. Pathol. 164:49–57. 10.1016/S0002-9440(10)63095-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welin A, Lerm M. 2012. Inside or outside of phagosome? The controversy of the intracellular localization of Mycobacterium tuberculosis. Tuberculosis 92:113–120. 10.1016/j.tube.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 41.Esposito C, Marasco D, Delogu G, Pedone E, Berisio R. 2011. Heparin-binding hemagglutinin HBHA from Mycobacterium tuberculosis affects actin polymerisation. Biochem. Biophys. Res. Commun. 410:339–344. 10.1016/j.bbrc.2011.05.159 [DOI] [PubMed] [Google Scholar]
- 42.Lasunskaia EB, Campos MN, de Andrade MR, Damatta RA, Kipnis TL, Einicker-Lamas M, Da Silva WD. 2006. Mycobacteria directly induce cytoskeletal rearrangements for macrophage spreading and polarization through TLR2-dependent PI3K signaling. J. Leukoc. Biol. 80:1480–1490. 10.1189/jlb.0106066 [DOI] [PubMed] [Google Scholar]
- 43.Bermudez LE, Goodman J. 1996. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect. Immun. 64:1400–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anes E, Kühnel MP, Bos E, Moniz-Pereira J, Habermann A, Griffiths G. 2003. Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat. Cell Biol. 5:793–802. 10.1038/ncb1036 [DOI] [PubMed] [Google Scholar]
- 45.Kolonko M, Geffken AC, Blumer T, Hagens K, Schaible UE, Hagedorn M. 2013. WASH-driven actin polymerization is required for efficient mycobacterial phagosome maturation arrest. Cell. Microbiol. 10.1111/cmi.12217 [DOI] [PubMed] [Google Scholar]
- 46.Polazzi E, Monti B. 2010. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog. Neurobiol. 92:293–315. 10.1016/j.pneurobio.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 47.Keane J, Remold HG, Kornfeld H. 2000. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 164:2016–2020 [DOI] [PubMed] [Google Scholar]
- 48.Neumann H, Cavalie A, Jenne DE, Wekerle H. 1995. Induction of MHC class I genes in neurons. Science 269:549–552. 10.1126/science.7624779 [DOI] [PubMed] [Google Scholar]
- 49.Berenguer J, Moreno S, Laguna F, Vicente T, Adrados M, Ortega A, Gonzalez-LaHoz J, Bouza E. 1992. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N. Engl. J. Med. 326:668–672. 10.1056/NEJM199203053261004 [DOI] [PubMed] [Google Scholar]
- 50.Silber E, Sonnenberg P, Ho KC, Koornhof HJ, Eintracht S, Morris L, Saffer D. 1999. Meningitis in a community with a high prevalence of tuberculosis and HIV infection. J. Neurol. Sci. 162:20–26. 10.1016/S0022-510X(98)00259-7 [DOI] [PubMed] [Google Scholar]
- 51.El Sahly HM, Teeter LD, Pan X, Musser JM, Graviss EA. 2007. Mortality associated with central nervous system tuberculosis. J. Infect. 55:502–509. 10.1016/j.jinf.2007.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puccioni-Sohler M, Brandao CO. 2007. Factors associated to the positive cerebrospinal fluid culture in the tuberculous meningitis. Arq. Neuropsiquiatr. 65:48–53. 10.1590/S0004-282X2007000100011 [DOI] [PubMed] [Google Scholar]
- 53.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol. 162:5407–5416 [PubMed] [Google Scholar]