Abstract
Protective immunity to Plasmodium falciparum malaria acquired after natural exposure is largely antibody mediated. IgG-specific P. falciparum EMP1 (PfEMP1) proteins on the infected erythrocyte surface are particularly important. The transient antibody responses and the slowly acquired protective immunity probably reflect the clonal antigenic variation and allelic polymorphism of PfEMP1. However, it is likely that other immune-evasive mechanisms are also involved, such as interference with formation and maintenance of immunological memory. We measured PfEMP1-specific antibody levels by enzyme-linked immunosorbent assay (ELISA) and memory B-cell frequencies by enzyme-linked immunosorbent spot (ELISPOT) assay in a cohort of P. falciparum-exposed nonpregnant Ghanaian women. The antigens used were a VAR2CSA-type PfEMP1 (IT4VAR04) with expression restricted to parasites infecting the placenta, as well as two commonly recognized PfEMP1 proteins (HB3VAR06 and IT4VAR60) implicated in rosetting and not pregnancy restricted. This enabled, for the first time, a direct comparison in the same individuals of immune responses specific for a clinically important parasite antigen expressed only during well-defined periods (pregnancy) to responses specific for comparable antigens expressed independent of pregnancy. Our data indicate that PfEMP1-specific B-cell memory is adequately acquired even when antigen exposure is infrequent (e.g., VAR2CSA-type PfEMP1). Furthermore, immunological memory specific for VAR2CSA-type PfEMP1 can be maintained for many years without antigen reexposure and after circulating antigen-specific IgG has disappeared. The study provides evidence that natural exposure to P. falciparum leads to formation of durable B-cell immunity to clinically important PfEMP1 antigens. This has encouraging implications for current efforts to develop PfEMP1-based vaccines.
INTRODUCTION
Protective immunity to Plasmodium falciparum malaria acquired after natural exposure is mediated to a large extent by IgG antibodies targeting the asexual blood stages of the parasites (reviewed in reference 1). The low rate of acquisition probably reflects the extensive clonal antigenic variation and allelic polymorphism of key antigens. However, other immune-evasive mechanisms may also be involved, such as interference with formation and maintenance of immunological memory. Indeed, it has often been speculated that such subversion is important for the slow and incomplete acquisition of clinical protection following natural exposure to P. falciparum in areas where these parasites are stably transmitted (reviewed in references 2, 3, and 4). The evidence supporting the hypothesis of a fragile or dysfunctional immunological memory to P. falciparum includes the often transient IgG responses in children with malaria (5–9), apparent interference with antigen presentation (10, 11), “masking” of surface-exposed IgG epitopes (12), and expansion of “atypical” or “exhausted” B cells after prolonged exposure to P. falciparum antigens (13, 14). Conversely, the hypothesis is challenged by recent evidence that P. falciparum-specific B-cell memory can be acquired efficiently and maintained for extended periods in the absence of exposure (15–17) and by data suggesting that “atypical” P. falciparum-specific memory B cells are not functionally “exhausted” (18).
The present study was designed to provide further and more direct evidence regarding the relationship between exposure to P. falciparum antigens, antibody levels, and immunological memory. To that end, we used an approach not previously employed. Rather than comparing individuals with and without P. falciparum exposure (which makes it difficult to control for confounders), we recruited a single cohort of nonpregnant women living in an area with stable P. falciparum transmission. Within this cohort, we compared antibody levels and memory B-cell frequencies specific for a parasite protein that is expressed only during pregnancy to those for similar antigens not restricted in this way. More specifically, we compared responses to the VAR2CSA-type P. falciparum EMP1 (PfEMP1) protein IT4VAR04 (19) and responses specific for two other PfEMP1 proteins, HB3VAR06 (20) and IT4VAR60 (also known as PAR+ or FCR3S1.2VAR2) (21). The PfEMP1 proteins constitute an ∼60-member family of clonally variant P. falciparum antigens that are expressed in a mutually exclusive manner on the surfaces of P. falciparum-infected erythrocytes (IEs), where they act as ligands for a range of host receptors in the vasculature (22–25). PfEMP1 proteins are thus responsible for the characteristic ability of IEs to adhere in various tissues and organs, which confers particular virulence to this parasite species (reviewed in reference 26). VAR2CSA-type PfEMP1 proteins mediate sequestration of IEs to chondroitin sulfate A (CSA) (27, 28), and many lines of evidence show that this type of PfEMP1 is expressed only by parasites sequestered in the placenta and that VAR2CSA is the target of acquired protective immunity to placental malaria (reviewed in reference 29). In contrast, HB3VAR06 and IT4VAR60 are both involved in rosetting, an adhesion phenotype that is linked to severe P. falciparum malaria in children (reviewed in reference 30). As a probable consequence of this, anti-rosetting IgG seems to be an important component of acquired protective immunity to severe malaria during childhood (31). Both HB3VAR6 and IT4VAR60 are encoded by typical group A var genes. It has long been recognized that certain antigenic variants are “common” and immunologically “well-recognized” (32, 33) and that this phenotype is linked to transcription of group A var genes and expression of the PfEMP1 proteins encoded by these genes (34–36). We provide direct evidence that B-cell memory to the clinically important PfEMP1 antigens is induced and can be maintained for years without reexposure (at least for VAR2CSA-type PfEMP1) and that circulating IgG is not a reliable indicator of PfEMP1-specific B-cell memory status. These findings have important implications for our understanding of immunity to P. falciparum malaria in general, and for the efforts to develop PfEMP1-based vaccines against this disease in particular.
MATERIALS AND METHODS
Study site and study participants.
The study was conducted in Assin Foso, located in a rainforest area approximately 80 km north of Cape Coast, the capital of Central Region, Ghana. Generally, transmission of P. falciparum parasites remains high in this country (37), and our study area has been characterized as having intense transmission of P. falciparum parasites, with limited seasonal variation (38, 39). Although transmission appears to have declined in recent years (40), malaria remains a serious health problem in the area.
We studied 104 adult, nonpregnant women, who consented in writing to participate after receiving an explanation of the study design and purpose. Anamnestic information (age, number of previous pregnancies, time since last pregnancy, malaria prophylaxis while pregnant, and use of insecticide-impregnated bed nets) and a venous blood sample were obtained from all participants (Table 1). Ten parturient women from the same area were included as positive controls, and 13 Danish women without visits to areas where P. falciparum is endemic were included as negative controls. The study was approved by the Institutional Review Board of Noguchi Memorial Institute for Medical Research, University of Ghana (study 038/10-11), and by the Regional Research Ethics Committees, Capital Region of Denmark (protocol H-4-2013-083).
TABLE 1.
Characteristics of study participants
| Parameter | Value |
||
|---|---|---|---|
| Exposed, nonpregnant women | Exposed, parturient women | Nonexposed, nonpregnant women | |
| No. of donors | 104 | 10 | 13 |
| Age (yr)a | 29 (17 to 54) | 29 (18 to 35) | 37 (23 to 54) |
| No. of pregnanciesa | 2 (0 to 11) | 2 (1 to 4) | 2 (0 to 4) |
| Time (yr) since last pregnancya | 2 (<1 to 20)b | 3 (<1 to 26) | |
| Proportion parasitemic at sampling | 0.12 | 0.10 | |
| Proportion with malaria during pregnancyc | 0.58b | 0.30 | |
| Proportion with malaria prophylaxis during pregnancyc | 0.66b | 0.80 | |
| Proportion with ITN usec | 0.60 | 0.60 | |
Data are medians (ranges) and were self-reported.
Excluding 22 self-reported nulligravidae.
Self-reported (for most recent pregnancy) ITN, insecticide-treated bed net.
Blood sample collection and preparation.
Venous blood samples (8 ml) from the Ghanaian donors were collected in heparinized tubes during the major rainy season (July-August) of 2011. Peripheral blood mononuclear cells (PBMC) were separated by gradient centrifugation on Lymphoprep (StemCell Technologies, Grenoble, France) and were cryopreserved in liquid nitrogen by use of a controlled-gradient freezing device as described in detail previously (41). Plasma was stored at −20°C. The presence of P. falciparum asexual-blood-stage parasitemia at the time of blood sampling was determined by microscopic examination of Giemsa-stained blood smears.
Antigens.
Recombinant proteins representing the entire ectodomains of three PfEMP1 proteins were produced in baculovirus-infected insect cells, essentially as described elsewhere (19; L. Stevenson et al., unpublished data). In brief, the ectodomain-encoding parts of the it4var04, hb3var06, and it4var60 genes (encoding amino acids Met1 to Gln2,644, Met1 to Cys2,958, and Met1 to Ser2,136, respectively) (see http://genome.cbs.dtu.dk/services/VarDom/ for sequence data) were codon optimized for insect cells by GeneArt (Life Technologies BV, Nærum, Denmark) and then were expressed in High Five cells. The full-length proteins IT4VAR04 (FV2), HB3VAR06 (FV6), and IT4VAR60 (FV60) were harvested from supernatants and purified on a Ni2+ metal-chelation agarose column (HisTrap HP; GE Healthcare). Tetanus toxoid (TT) was purchased from Statens Serum Institut (Copenhagen, Denmark) and used as a nonmalaria control antigen.
Plasma IgG antibody measurements by ELISA.
Plasma levels of IgG with specificity for FV2, FV6, and FV60 were measured by enzyme-linked immunosorbent assay (ELISA), essentially as described elsewhere (27). In brief, 96-well flat-bottomed Nunc microtiter plates (Thermo Scientific, Waltham, MA) were coated with antigen in Dulbecco's phosphate-buffered saline (PBS; Lonza, Vallensbæk Strand, Denmark) (pH 7.2, 4°C, overnight) and blocked (1 h, room temperature) with dilution buffer (29.2 g NaCl, 0.2 g KCl, 0.2 g KH2PO4, 0.92 g Na2HPO4, 10 g bovine serum albumin [BSA], 10 ml Triton X-100, 1 ml phenol red, 1 liter distilled water [pH 7.3]). Plasma samples diluted 1:400 (FV2), 1:200 (FV6), 1:120 (FV60), or 1:800 (TT) were added to triplicate wells and incubated (1 h, room temperature). Optimal plasma dilutions for detection of IgG specific for each antigen were determined from 2-fold titrations of samples with known low or high IgG reactivity. Wells were washed in washing buffer (the same as dilution buffer, but without BSA [pH 7.2]), and bound antibody was detected with horseradish peroxidase (HRP)-conjugated rabbit anti-human IgG (1:3,000) (Dako, Glostrup, Denmark) followed by o-phenylenediamine (Sigma-Aldrich, Copenhagen, Denmark). The enzymatic reaction was stopped by addition of 2.5 M H2SO4 (100 μl/well), the optical density (OD) was read at 492 nm, and the specific antibody level was calculated in arbitrary units (AU) [(ODSAMPLE − ODBLANK)/(ODPOSCTRL − ODBLANK)], essentially as described elsewhere (42). Specifically, we included samples from two individual Ghanaian donors as positive-control samples in all ELISA plates. One sample (PF1034) had high IgG reactivities to FV2, FV6, and TT, whereas the other (NF022) had high reactivity to FV60. Antibody levels above the mean level in negative-control samples plus 2 standard deviations (SD) were considered positive.
Memory B-cell frequency determination by ELISPOT assay.
Cryopreserved PBMC were thawed in a water bath (37°C), washed in complete medium (RPMI 1640 supplemented with 10% fetal bovine serum [FBS], 4 mM l-glutamine, and 20 IU/ml PenStrep), and adjusted to 2.5 × 106 cells/ml. The cells were then incubated (37°C, 5% CO2, 72 h) in complete medium in the presence of interleukin-2 (IL-2) (Ness-Ziona, Israel) and the polyclonal activator R-848 (Invivogen, Toulouse, France). Enzyme-linked immunosorbent spot (ELISPOT) assay plates (Merck Millipore, Hellerup, Denmark) were prewetted (50 μl of 70% ethanol, 30 s) and washed 5 times in distilled water. FV2, FV6, FV60, or TT (5 μg/ml, 100 μl) was added to each well, and the plates were incubated overnight (4°C). Plates were then washed 5 times in PBS and blocked with complete medium (1 h, room temperature). Precultured PBMC (see above) were washed twice in complete medium and seeded in duplicate ELISPOT assay wells (100 μl/well). The plates were incubated (37°C, 5% CO2, 24 h), washed 5 times in PBS, and developed with HRP-conjugated rabbit anti-human IgG (1:3,000; Dako, Glostrup, Denmark) followed by 3,3′,5,5′-tetramethylbenzidine (Mabtech, Nacka Strand, Sweden). The plates were finally washed under running tap water and dried in the dark. The number of spots was determined with an ImmunoSpot reader (CTL-Europe, Bonn, Germany). The limited number of cells available precluded accurate determinations of very low frequencies of antigen-specific antibody-secreting cells. We therefore used a negative cutoff of one antibody-secreting cell per million PBMC, based on the observed frequency of spots among the negative-control donors (one or two spots in ∼10% of samples, indicating a frequency of spot-forming cells in these donors of ∼2 × 10−7).
Statistical analysis.
Differences in median antibody levels between donor groups were tested by the Mann-Whitney rank sum test. Parameter associations were tested by Kruskal-Wallis one-way analysis of variance on ranks. Correlations were tested by Spearman rank-order correlation, using actual values, whether above or below the negative cutoff. Confidence intervals for proportions were calculated as described previously (43). P values of <0.05 were considered statistically significant.
RESULTS
Plasma levels of PfEMP1-specific IgG and P. falciparum exposure.
Plasma levels of antigen-specific (including PfEMP1-specific) IgG are a measure of previous exposure to that antigen or to a similar, cross-reactive antigen(s) (44, 45). In accordance with this, we found that the median levels of IgG specific for the recombinant PfEMP1 antigens FV6 and FV60 were much higher in plasmas from Ghanaian women naturally exposed to P. falciparum than in plasmas from women without exposure to these parasites (negative-control donors), indicating the regular exposure of the Ghanaian women to P. falciparum parasites (Fig. 1). Levels were unaffected by pregnancy status among the P. falciparum-exposed women (Fig. 1). Using a negative-cutoff value of the mean plus 2 standard deviations for plasma levels in the negative-control donors, 99% (95% confidence interval, 95% to 100%) and 89% (82% to 94%) of the P. falciparum-exposed nonpregnant women in our cohort had above-cutoff levels of FV6- and FV60-specific IgG, respectively.
FIG 1.
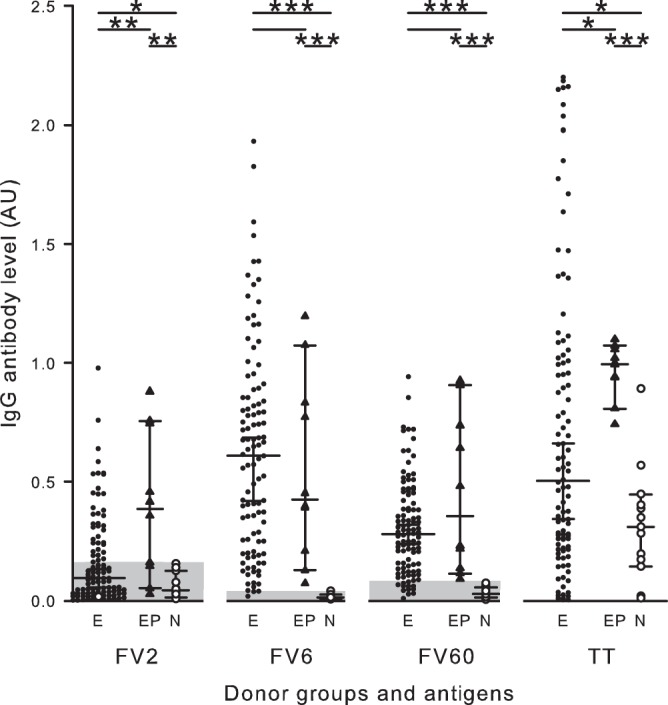
Plasma levels of antigen-specific IgG in different groups of women. Levels of antigen-specific IgG in plasmas from P. falciparum-exposed nonpregnant (●; E) and parturient (▲; EP) women and from nonexposed, nonpregnant women (○; N) are shown. Individual plasma levels of IgG with specificity for the P. falciparum antigens FV2, FV6, and FV60 and the non-P. falciparum antigen tetanus toxoid (TT) are shown. Median levels (horizontal lines) and their 95% confidence intervals (error bars) are also indicated. Values are expressed as arbitrary units (AU) (see Materials and Methods for details). Significant intergroup differences are indicated by lines along the top of the diagram. The asterisks indicate the level of statistical significance (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The x axis breaks indicate that levels of IgG specific for the different antigens are not directly comparable. Shaded areas indicate values below the negative cutoff.
In contrast, the median level of IgG specific for the VAR2CSA-type FV2 antigen was only marginally higher in plasmas from the nonpregnant cohort women than in plasmas from the negative controls (Fig. 1). The median level of FV2-specific IgG in plasma from the P. falciparum-exposed nonpregnant women was markedly lower than that in plasma from positive controls (pregnant women at delivery) (Fig. 1). Overall, only 36% (27% to 45%) of the nonpregnant women had levels above the negative cutoff, compared to 70% (40% to 89%) of the P. falciparum-exposed control women at the time of delivery. Together, these findings support earlier observations that expression of VAR2CSA-type PfEMP1 proteins is restricted to pregnancy (27, 46) and that IgG with this antigen specificity decline fairly rapid after delivery (47, 48). Two women reporting to be nulligravidae had FV2-specific IgG levels above the negative cutoff. It seems most likely that they had in fact been (unsuccessfully) pregnant previously, but they either did not know or did not remember.
The large majority of women from all three categories of women studied (P. falciparum-exposed nonpregnant women, P. falciparum-exposed pregnant women at delivery [positive controls], and nonpregnant women without P. falciparum exposure [negative controls]) had high plasma levels of IgG specific for the P. falciparum-unrelated control antigen TT (Fig. 1). The particularly high levels in the positive-control women probably reflect the fact that many had recently received the prophylactic TT booster vaccination that is a standard part of antenatal care in Ghana. These data confirm the usefulness of PfEMP1-specific IgG levels as markers of exposure (49), the existence of interclonally conserved antibody epitopes in PfEMP1 proteins from genotypically distinct P. falciparum parasites (50), and the often transient nature of PfEMP1-specific IgG responses (in the absence of regular reexposure) (5–9).
Plasma levels of PfEMP1-specific IgG, age, and time since last pregnancy.
Levels of P. falciparum-specific (including PfEMP1-specific) IgG increase with age, generally plateau at adolescence, and may even decline in older age groups (44, 49). In agreement with this, we did not find a significant correlation between PfEMP1-specific IgG levels and age in the current study, which included only adults (Fig. 2). IEs sequestered in the placenta adhere selectively to CSA in the intervillous space (51–53). This adhesion is mediated by VAR2CSA-type PfEMP1 (27, 28), and available evidence overwhelmingly supports the hypothesis that expression of this PfEMP1 type is restricted to parasites infecting pregnant women (reviewed in reference 29). In the present study, 22 of the 104 P. falciparum-exposed but nonpregnant women reported not having been pregnant previously (nulligravidae). Accordingly, these 22 women should probably be considered nonexposed with respect to FV2, but the relationship between FV2-specific IgG and age remained statistically insignificant after exclusion of nulligravidae. When we considered only the 37 women who did have positive FV2 responses, a borderline significant positive association between age and FV2-specific IgG responses was found [P(rs = 0.27) = 0.07]. It seems likely that this reflects that age is a crude marker of the number of previous pregnancies.
FIG 2.
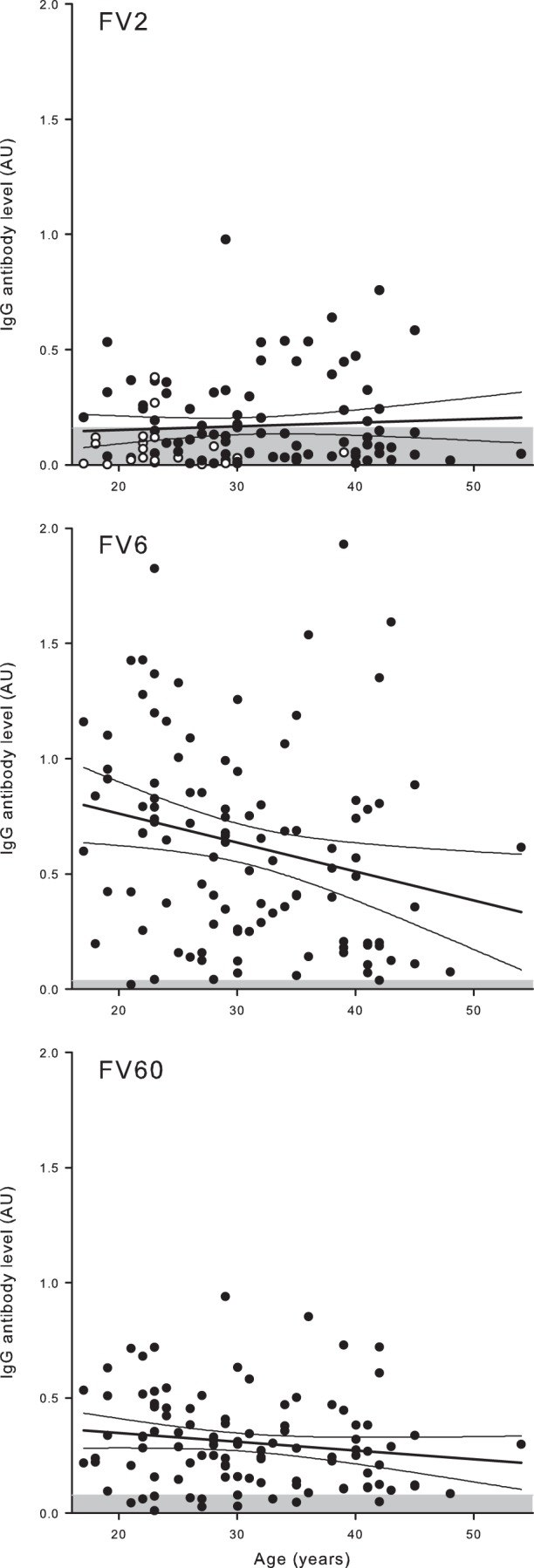
Relationship between plasma IgG levels and age in nonpregnant Ghanaian women. Ages and IgG levels specific for FV2 (top), FV6 (center), and FV60 (bottom) in individual donors are shown, as well as the linear regression lines and 95% confidence intervals for their relationship. The negative cutoff (mean plus 2 SD of levels in negative-control donors) is indicated by shading. With respect to FV2, nonexposed donors (nulligravidae) are indicated with open symbols.
In the plasmas of pregnant women, a positive association between antibody levels and parity is a well-established hallmark of IgG specific for VAR2CSA-type PfEMP1 (reviewed in reference 29). We reported previously that levels of IgG with specificity for CSA-adhering IEs decline within a few months after delivery, whereas levels of IgG specific for IEs not adhering to CSA do not (48). Supporting this observation, levels of IgG specific for the two studied PfEMP1 proteins not related to pregnancy (FV6 and FV60) were not related to time since last pregnancy (Fig. 3). However, in contrast to our expectation, we did not find a statistically significant negative association between FV2-specific IgG levels and time since last pregnancy (Fig. 3). Either our study was underpowered to detect such an association (because a proportion of the studied women might not have been infected by P. falciparum parasites during their latest pregnancy) or the rate of decline is actually lower than originally anticipated. Supporting the former hypothesis, we found that the proportion of women with measurable FV2-specific IgG did decline with time since delivery [P(rs = −0.61) = 0.03], whereas this was not the case for the pregnancy-unrelated PfEMP1 antigens FV6 and FV60 (Fig. 3).
FIG 3.
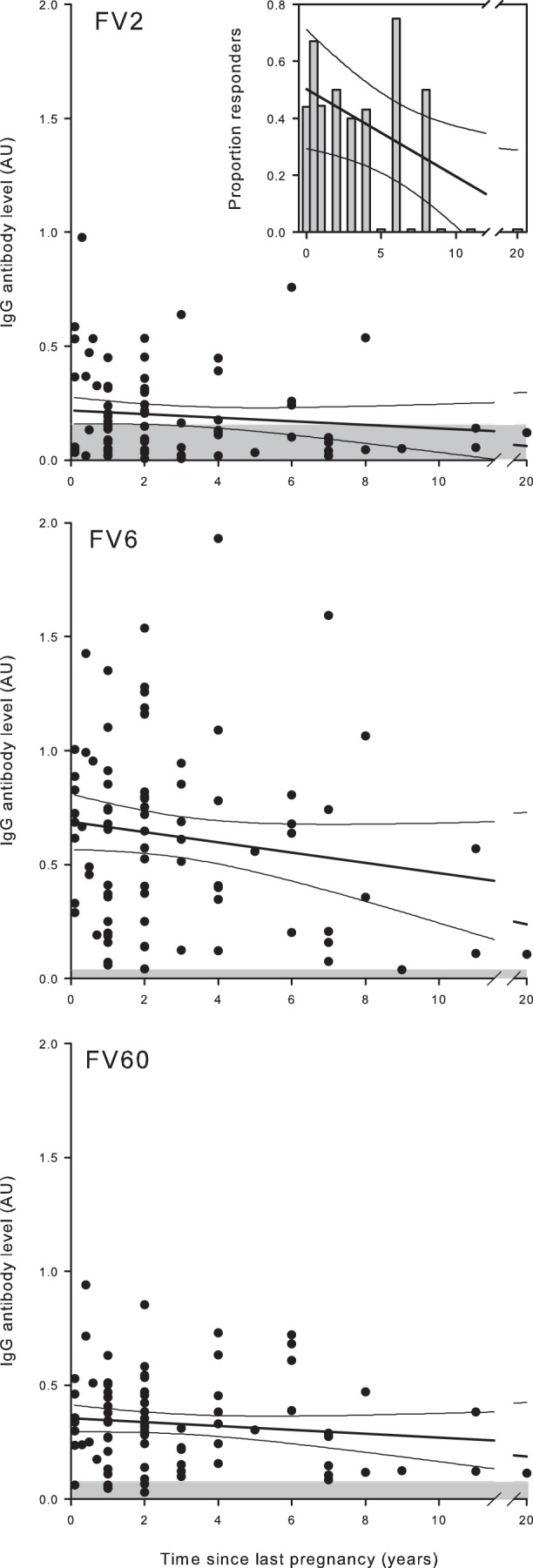
Relationship between plasma IgG levels and time since last pregnancy in nonpregnant Ghanaian women. Times since last pregnancy and IgG levels specific for FV2 (top), FV6 (center), and FV60 (bottom) in individual donors are shown, as well as the linear regression lines and 95% confidence intervals for their relationship. The negative cutoff (mean plus 2 SD of levels in negative-control donors) is indicated by shading. Note that nulligravidae were excluded from this graph. The proportions of women with FV2-specific IgG levels above the negative cutoff at different time points are shown in the inset (all time points where proportion is zero are indicated as 0.01 for visibility only).
Plasma levels of PfEMP1-specific IgG and parity.
Among P. falciparum-exposed pregnant women, there is a well-documented association between parity and levels at the time of delivery of IgG specific for the VAR2CSA-type PfEMP1 (reviewed in reference 29). We found a similar association [P(rs = 0.36) < 0.001] among the P. falciparum-exposed women studied here (Fig. 4), although they were not pregnant at the time of study, and many had not been pregnant for a long time (Fig. 3). To the best of our knowledge, such an association, which was absent for the two PfEMP1 proteins unrelated to pregnancy (Fig. 4), has not been reported before. It supports the above-mentioned hypothesis that the rate of decline in antibodies specific for VAR2CSA-type PfEMP1 following delivery is actually lower than we originally proposed (48).
FIG 4.
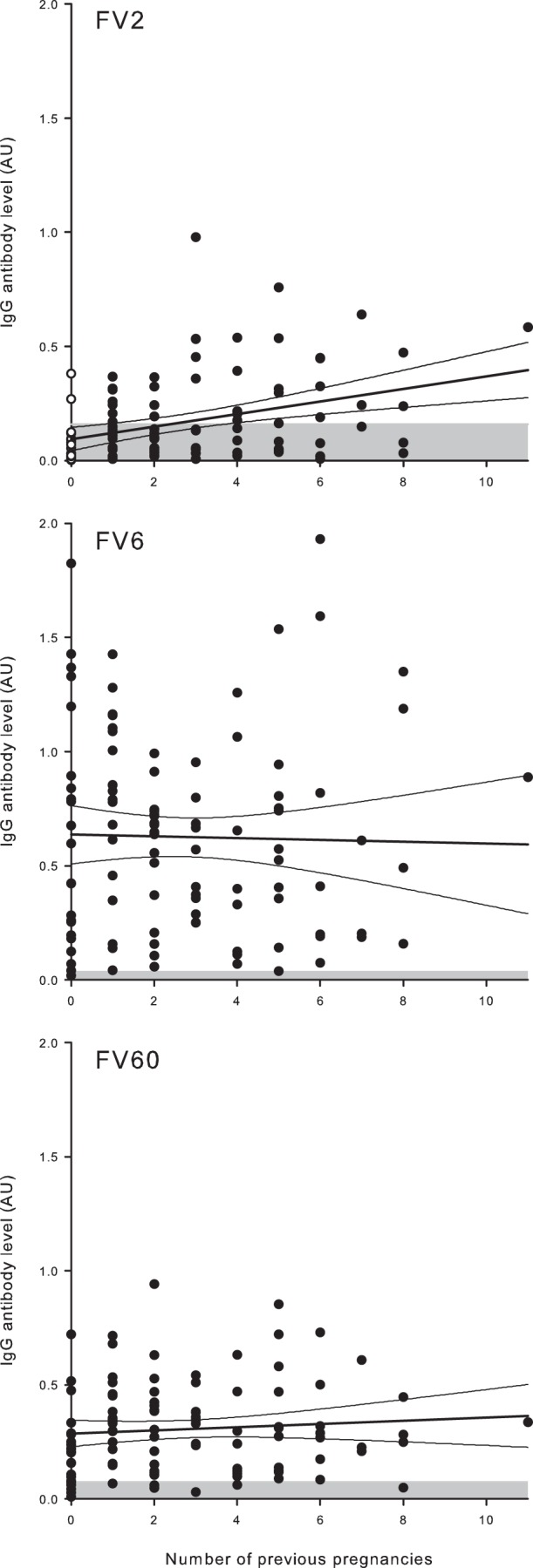
Relationship between plasma IgG levels and parity in nonpregnant Ghanaian women. Parity and IgG levels specific for FV2 (top), FV6 (center), and FV60 (bottom) in individual donors are shown, as well as the linear regression lines and 95% confidence intervals for their relationship. The negative cutoff (mean plus 2 SD of levels in negative-control donors) is indicated by shading. With respect to FV2, nonexposed donors (nulligravidae) are indicated with open symbols.
Frequencies of PfEMP1-specific memory B cells and specific antigen exposure.
It has often been speculated that P. falciparum is able to subvert the acquisition of immunological memory and that this is central to the sluggish development of clinical immunity to malaria and to the transience of P. falciparum-specific antibody responses (2–4). We found that the frequencies of memory B cells capable of transforming into antibody-secreting cells following in vitro stimulation were similar for all the studied antigens (Fig. 5). With respect to the PfEMP1 antigens, frequencies among the P. falciparum-exposed donors did not reflect whether antigen exposure was infrequent (FV2) or not (FV6 and FV60). In both cases, a sizeable proportion of the donors had frequencies below the negative cutoff. It is unlikely that this reflects a lack of exposure to P. falciparum parasites in general, since all donors were permanent residents of an area with stable parasite transmission. Instead, it may reflect a lack of exposure to the specific PfEMP1 antigens used. For FV2, this is supported by the observation that a large proportion of the donors with frequencies below the negative cutoff were nulli- or primigravidae. This explanation obviously does not apply to FV6 and FV60, which are not restricted to pregnancy. An alternative or additional possibility is that long-lived, bone-marrow-resident plasma cells are the main source of circulating antibody, rather than the circulating memory B cells we measured (54). This hypothesis is supported by our finding that many more donors had detectable FV6- and FV60-specific IgG (Fig. 1) than had detectable memory B cells specific for these antigens (Fig. 5). Finally, we set the negative cutoff based on data from only 500,000 PBMC due to the limited number of cells available for analysis. The stochastic nature of the ELISPOT assay makes it difficult to determine low frequencies of responding cells accurately. Thus, it is possible that some of the P. falciparum-exposed donors with levels below the negative cutoff did in fact have higher specific antibody-secreting cell frequencies than the unexposed control donors. With respect to TT, frequencies of memory B cells were high and similar in all donor groups.
FIG 5.
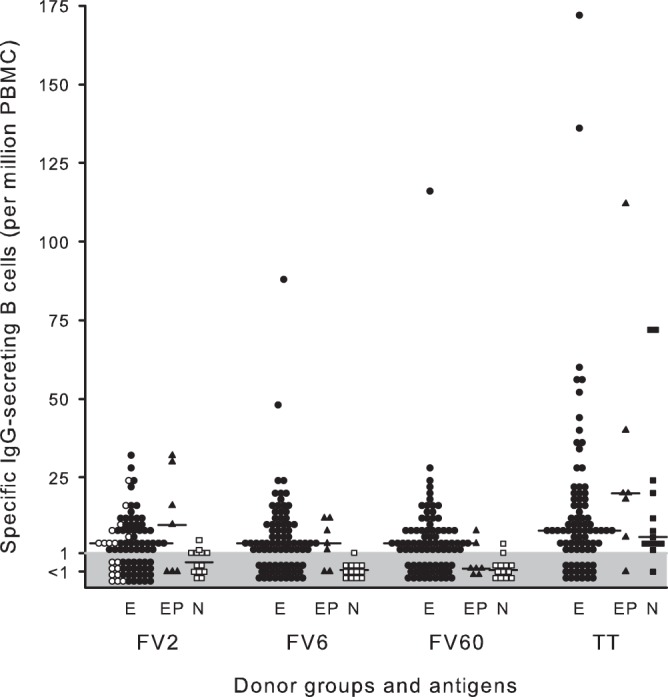
Frequencies of antigen-specific IgG-secreting B cells in different groups of women. Frequencies of IgG-secreting B cells in peripheral blood from individual P. falciparum-exposed nonpregnant (●; E) and parturient (▲; EP) women and from nonexposed, nonpregnant women (○; N) are shown. Frequencies of B cells secreting IgG specific for the P. falciparum antigens FV2, FV6, and FV60 and the non-P. falciparum antigen tetanus toxoid (TT) are shown. The negative cutoff (1 antibody-secreting cell per 1 × 106 PBMC) is indicated by shading. Median levels (horizontal lines) are also indicated.
PfEMP1-specific memory B cells and time since last antigen exposure.
Memory B cells for VAR2CSA-type PfEMP1 could be detected for up to 20 years after the last pregnancy, and the frequency of these cells remained stable over this period (Fig. 6). The average frequency of memory B cells did not increase with increasing parity for any of the PfEMP1 antigens (Fig. 7), although the proportion of women with FV2-specific memory B-cell frequencies above the negative cutoff did increase with parity [P(rs = 0.86) < 0.001]. The sustained presence of FV2-specific memory B cells many years after the last pregnancy implies that maintenance of PfEMP1-specific immunological memory does not require regular exposure to antigen, given that essentially all available evidence indicates that expression of VAR2CSA-type PfEMP1 proteins is restricted to pregnancy (29). Furthermore, the finding that the frequency of FV2-specific memory B cells did not depend on parity suggests that exposure to VAR2CSA-type PfEMP1 proteins over the course of a single pregnancy may be sufficient to induce affinity maturation, class switching, and induction of long-term sustainable immunological memory. Even though switching to VAR2CSA-type PfEMP1 proteins appears to be noncompatible with parasite survival in in nonpregnant hosts, women already primed with these antigens during earlier pregnancies may also contribute to long-term sustaining of VAR2CSA-specific immunological memory. Several women who claimed not to have been pregnant before did have levels of FV2-specific memory B cells above the negative cutoff (Fig. 7, top panel). We have no convincing explanation for this. The simplest reason is that these women had in fact been pregnant previously, but they either did not know or did not remember it.
FIG 6.
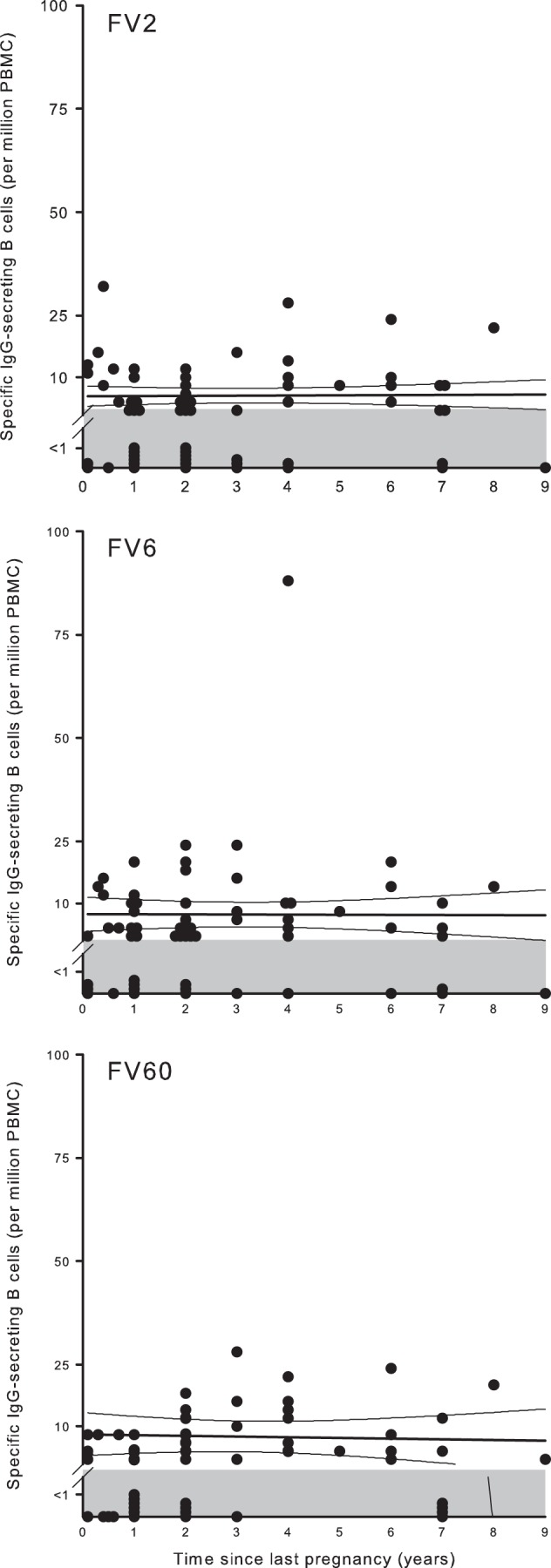
Relationship between frequency of IgG-secreting B cells and time since last pregnancy in nonpregnant Ghanaian women. Times since last pregnancy and frequencies of IgG antibody-secreting B cells specific for FV2 (top), FV6 (center), and FV60 (bottom) in individual donors are shown, as well as the linear regression lines and 95% confidence intervals for their relationship. The negative cutoff (1 antibody-secreting cell per 1 × 106 PBMC) is indicated by shading. Note that nulligravidae were excluded from this graph.
FIG 7.
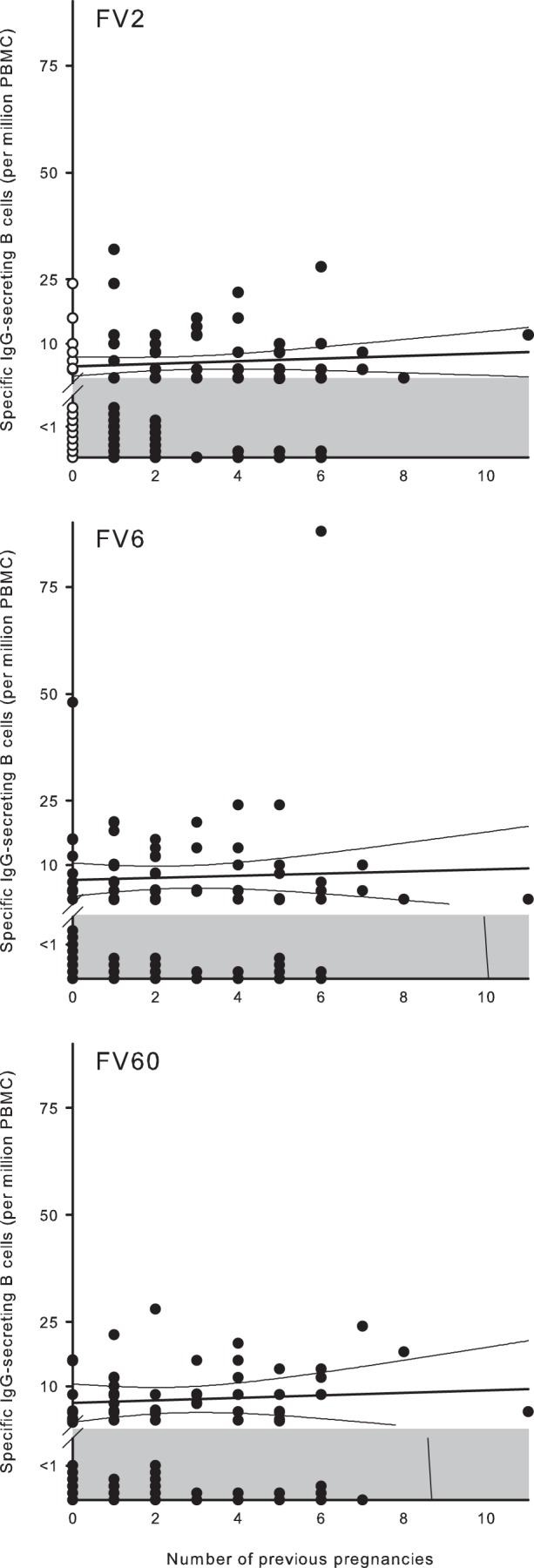
Relationship between frequency of IgG-secreting B cells and parity in nonpregnant Ghanaian women. Parity and frequencies of IgG antibody-secreting B cells specific for FV2 (top), FV6 (center), and FV60 (bottom) in individual donors are shown, as well as the linear regression lines and 95% confidence intervals for their relationship. The negative cutoff (1 antibody-secreting cell per 1 × 106 PBMC) is indicated by shading. With respect to FV2, nonexposed donors (nulligravidae) are indicated with open symbols.
Relationship between PfEMP1-specific IgG levels and memory B cells.
Previous studies on the relationship between antigen-specific IgG levels and memory B-cell frequencies, including studies of P. falciparum-specific responses, have yielded conflicting results (15, 55–57). In the present study, we did not find convincing evidence of such a relationship (Fig. 8). While we did observe a statistically significant relationship between FV6-specific IgG and the frequency of the corresponding memory B cells (Fig. 8, middle panel), this relationship depended strongly on two outlying data points, so we caution against making firm conclusions.
FIG 8.
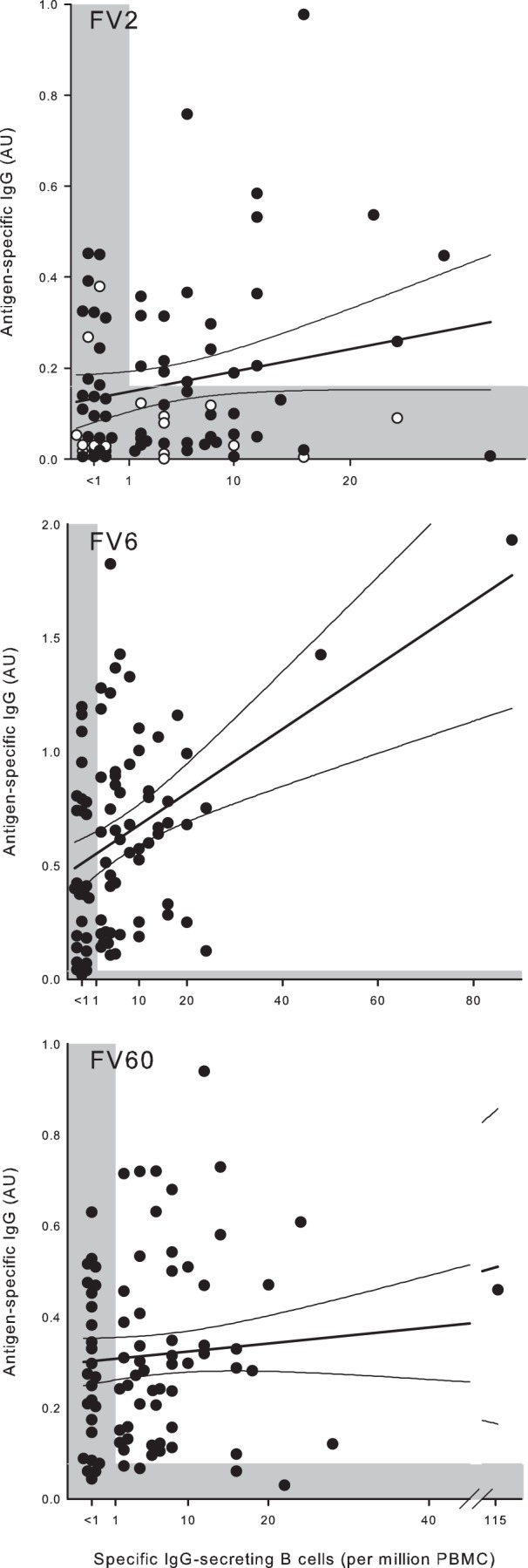
Relationship between frequency of IgG-secreting B cells and plasma IgG levels in nonpregnant Ghanaian women. Frequencies of IgG antibody-secreting B cells specific for FV2 (top), FV6 (center), and FV60 (bottom) and plasma levels of the same IgG in individual donors are shown, as well as the linear regression lines and 95% confidence intervals for their relationship. The negative cutoffs are indicated by shading. With respect to FV2, nonexposed donors (nulligravidae) are indicated with open symbols. Note the scaling of the axes.
DISCUSSION
Following exposure to blood-borne antigens, short-lived plasma cells arise rapidly in the red pulp of the spleen, secreting antibodies that may help to control the acute infection. Long-lived memory B cells appear later, as a result of the germinal center reaction of clonal expansion and somatic hypermutation, and these cells can circulate for months or longer. They act as sentinels that do not themselves secrete antibody but can initiate a rapid and high-affinity antibody response upon reinfection. They also give rise to plasma cells that migrate to the bone marrow, where they reside as nondividing, antibody-secreting plasma cells for shorter or longer periods. These terminally differentiated cells are increasingly considered the main source of specific antibody that can persist in the blood for very long periods, even in the absence of reexposure or persistent antigen (54). With respect to P. falciparum malaria, it has often been proposed that formation and maintenance of immunological B-cell memory are deficient, although the evidence is far from unequivocal (reviewed in references 2, 3, and 4). Thus, it has been speculated that continuous or regular exposure to P. falciparum antigens is required to maintain B-cell memory, but also that formation of B-cell memory is compromised in areas of intense parasite transmission (58, 59). Several recent reports indicating the formation of durable B-cell memory following natural exposure to P. falciparum antigens add to the ambiguity (15–18). If immunological memory to malaria were indeed defective, it could undermine the sustainability of recent reductions in parasite transmission, as it might leave formerly protected individuals vulnerable if transmission should reappear (60, 61). Also, it could be an understudied contributing factor to the difficulties in developing robust vaccines against the disease.
In the present study, we investigated how exposure to P. falciparum antigens, antibody levels, and immunological memory are related. We used a novel approach designed to remove some of the shortcomings in earlier studies of the relationship between P. falciparum antigen-specific antibody levels and frequencies of the corresponding memory B cells. Specifically, we studied a single group of P. falciparum-exposed women and compared their responses to three clinically important, structurally and functionally similar antigens that are exposed to the immune system in very different ways. One (FV2) is a VAR2CSA-type PfEMP1 encoded by var genes that are transcribed and translated only by parasites infecting pregnant women (62, 63). In contrast, FV6 and FV60 are encoded by var genes that are transcribed and translated independent of pregnancy, and they represent rosetting PfEMP1 proteins thought to be involved in the pathogenesis of severe malaria in childhood (31). People living in areas with stable transmission of P. falciparum parasites are thought to be exposed regularly to parasites expressing this type of PfEMP1 proteins (32, 33, 64, 65).
First, we found that levels of circulating IgG specific for the VAR2CSA-type PfEMP1 FV2 were generally low in nonpregnant, P. falciparum-exposed women, while most had significant levels of IgG specific for the non-pregnancy-restricted PfEMP1 antigens FV6 and FV60 (Fig. 1). This fits earlier observations that PfEMP1-specific antibody responses tend to be transient and require regular boosting (7) and that IgG to CSA-adherent IEs is generally absent early in pregnancy (47) and decays within a few months of delivery (48). Although other authors have reported a high durability of IgG specific for VAR2CSA-type PfEMP1, the confidence intervals of their estimates are very wide (66). Furthermore, the estimates were based on repeated measures among pregnant women only, although durability is likely to be quite different during and after pregnancy. In any case, we did not observe the significant relationship between FV2-specific IgG levels and time since last pregnancy (Fig. 3). However, this may simply reflect a lack of statistical power to detect such a relationship, as indicated by the fact that the proportion of women with measurable VAR2CSA-specific IgG did decline with time since last pregnancy.
Second, we found a significant relationship between VAR2CSA-specific IgG and the number of previous pregnancies, whereas no such relationship was seen for IgG specific for PfEMP1 proteins not related to pregnancy (Fig. 4). A relationship between VAR2CSA-specific IgG and the number of previous pregnancies among nonpregnant women has not been observed previously, although it clearly resembles that seen for pregnant women at term in several earlier reports (reviewed in reference 29). The finding is not self-evident, as the decline in VAR2CSA-specific IgG levels following delivery discussed above would be expected to make such a relationship fade within months of delivery—unless early pregnancies tend to be spaced wider than later ones (thereby leaving more time for catabolic decay of antibodies), which seems improbable. Instead, the relationship might reflect a gradually increasing longevity of plasma cells secreting IgG specific for VAR2CSA-type PfEMP1 over the course of several pregnancies, in line with the standard antibody response following vaccination and revaccination (67). Because circulating levels of antibodies mainly reflect the rate at which they are synthesized, an increased life span of IgG-secreting cells would lead to more durable antibody levels after antigen has been cleared. Indeed, the transition from short-lived IgG responses in young children (7, 8, 68, 69) to more durable antibody responses later on (5, 9) may be a more general instance of this putative change in the longevity of the antibody-secreting cells. This would provide an alternative or complementary explanation for the incremental acquisition of antibodies in areas of seasonal parasite transmission (70), which is generally attributed to a need for numerous antigen exposures before solid immunological memory is formed.
Third, we found that the frequencies of memory B cells that could give rise to antibody-secreting plasma cells following nonspecific induction in vitro were similar for all three PfEMP1 proteins studied, despite marked differences in donor exposure to these antigens (Fig. 5). This suggests that robust immunological memory can result even when antigen exposure is infrequent (as is the case for VAR2CSA). This conclusion is supported by data on acquisition of immunity in settings with a low level of endemicity, in travelers, and in experimentally infected mice (17, 59, 71). The finding that FV2-specific memory B-cell frequencies did not depend on time since last pregnancy (Fig. 6) or number of previous pregnancies (Fig. 7) is further evidence that fully formed FV2-specific B-cell memory can be formed in the course of a single pregnancy and that this memory can be maintained for many years in the absence of antigen reexposure.
Fourth, we did not find convincing correlations between the frequencies of antigen-specific memory B cells and levels of the corresponding antibody specificities in the circulation (Fig. 8). Previous reports on the relationship between memory B cells and circulating P. falciparum-specific antibody have yielded ambiguous results (15, 55–57). The underlying assumption of these studies is that memory B cells are required for antibody production, considering antibody-secreting plasma cells to be too short-lived to play a major role in sustained antibody production (reviewed in reference 72). However, long-lived, bone-marrow-resident plasma cells are increasingly regarded as the main source of circulating antibody (54), and there is direct experimental evidence that long-lived plasma cells can persist for extended periods in the absence of detectable memory B cells (54). This suggests that the relationship between circulating memory B cells and circulating antibody may not be as straightforward as sometimes assumed.
Two final points deserve mention. One is our observation that a minority of the women who reported that they had not been pregnant before had FV2-specific IgG (Fig. 2), whereas FV2-specific memory B-cell frequencies (Fig. 7) above the negative cutoff were detected in about half of these women. This was unexpected given the strong evidence that expression of VAR2CSA-type PfEMP1 is restricted to pregnancy (29). Apart from the trivial explanation of unrecognized or unremembered (unsuccessful) pregnancies mentioned above, it remains a possibility that VAR2CSA-specific immunity can occasionally appear independent of pregnancy. Indeed, there are a few reports of significant levels of IgG to VAR2CSA-type PfEMP1 among P. falciparum-exposed men and children (73). However, the strong link between parity and susceptibility to placental malaria suggests that pregnancy-independent immune responses to VAR2CSA-type PfEMP1 do not normally protect against placental malaria. Functional characterization of VAR2CSA-reactive antibodies in men, children, and nulligravidae might shed light on this issue. The other point relates to previous reports that intensive and/or repeated antigen exposure to malaria antigens can drive expansion of so-called “atypical” memory B cells (14) that may be functionally “exhausted” (13, 74, 75), as has been observed in HIV infection (76). It has been suggested that this could explain the paradoxical inverse relationship between parasite exposure and acquired immunity observed in some studies (58, 59). However, the antigen specificity of phenotypically identified “atypical”/“exhausted” memory B cells observed in these papers was not established, and other recent studies did not find evidence that B-cell memory for P. falciparum antigens is deficient (16, 17) or that “atypical” P. falciparum-specific B cells are dysfunctional (18). Careful studies of the phenotype-function relationships among B-cell subsets with known antigen specificity are clearly warranted.
In conclusion, our study provides evidence that natural exposure to P. falciparum parasites leads to induction of immunological B-cell memory for clinically important PfEMP1 antigens, even when antigen exposure is infrequent. Furthermore, we provide evidence that this memory can be maintained for long periods after antigen exposure has ceased, and after circulating antibody is no longer detectable. This bodes well for the sustainability of efforts to eliminate and eradicate P. falciparum and for current efforts to develop malaria vaccines in general, and PfEMP1-based vaccines in particular.
ACKNOWLEDGMENTS
This work was supported by the Danish Council for Independent Research (DFF-FSS; grants 11-115707 and 11-120879), the European Community's Seventh Framework Programme (FP7/2007-2013; grant 242095—EVIMalaR), The Lundbeck Foundation (grant R839-A7627), and the University of Copenhagen (Programme of Excellence in Membrane Topology and Quaternary Structure of Key Membrane Proteins Involved in Plasmodium falciparum Malaria Pathogenesis and Immunity). P.A., L.S., and L.B. were supported by the Ghana Educational Trust Fund (GetFUND), EVIMalaR, and DFF-FSS, respectively.
Ali Salanti is thanked for the it4var04 plasmid. Erik Laursen, Kirsten Pihl Zimling, and Maiken Visti are thanked for excellent technical assistance.
Footnotes
Published ahead of print 24 February 2014
REFERENCES
- 1.Hviid L. 2005. Naturally acquired immunity to Plasmodium falciparum malaria in Africa. Acta Trop. 95:270–275. 10.1016/j.actatropica.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 2.Struik SS, Riley EM. 2004. Does malaria suffer from lack of memory? Immunol. Rev. 201:268–290. 10.1111/j.0105-2896.2004.00181.x [DOI] [PubMed] [Google Scholar]
- 3.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. 2008. Immunity to malaria: more questions than answers. Nat. Immunol. 9:725–732. 10.1038/ni.f.205 [DOI] [PubMed] [Google Scholar]
- 4.Portugal S, Pierce SK, Crompton PD. 2013. Young lives lost as B cells falter: what we are learning about antibody responses in malaria. J. Immunol. 190:3039–3046. 10.4049/jimmunol.1203067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Früh K, Doumbo O, Müller H-M, Koita O, McBride J, Crisanti A, Touré Y, Bujard H. 1991. Human antibody response to the major merozoite surface antigen of Plasmodium falciparum is strain specific and short-lived. Infect. Immun. 59:1319–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavanagh D, Elhassan IM, Roper C, Robinson VJ, Giha H, Holder AA, Hviid L, Theander TG, Arnot DE, McBride JS. 1998. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein 1 in an area of unstable malaria in Sudan. J. Immunol. 161:347–359 [PubMed] [Google Scholar]
- 7.Kinyanjui SM, Bull P, Newbold CI, Marsh K. 2003. Kinetics of antibody responses to Plasmodium falciparum-infected erythrocyte variant surface antigens. J. Infect. Dis. 187:667–674. 10.1086/373994 [DOI] [PubMed] [Google Scholar]
- 8.Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. 2007. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar. J. 6:82. 10.1186/1475-2875-6-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akpogheneta OJ, Duah NO, Tetteh KK, Dunyo S, Lanar DE, Pinder M, Conway DJ. 2008. Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect. Immun. 76:1748–1755. 10.1128/IAI.01333-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urban BC, Ferguson DJP, Pain A, Willcox N, Plebanski M, Austyn JM, Roberts DJ. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73–77. 10.1038/21900 [DOI] [PubMed] [Google Scholar]
- 11.Urban BC, Willcox N, Roberts DJ. 2001. A role for CD36 in the regulation of dendritic cell function. Proc. Natl. Acad. Sci. U. S. A. 98:8750–8755. 10.1073/pnas.151028698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barfod L, Dalgaard MB, Pleman ST, Ofori MF, Pleass RJ, Hviid L. 2011. Evasion of immunity to Plasmodium falciparum malaria by IgM masking of protective IgG epitopes in infected erythrocyte surface-exposed PfEMP1. Proc. Natl. Acad. Sci. U. S. A. 108:12485–12490. 10.1073/pnas.1103708108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P, Crompton PD, Marsh K, Ndungu FM. 2013. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J. Immunol. 190:1038–1047. 10.4049/jimmunol.1202438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, Kayentao K, Ongoiba A, Doumbo OK, Pierce SK. 2009. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J. Immunol. 183:2176–2182. 10.4049/jimmunol.0901297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wipasa J, Suphavilai C, Okell LC, Cook J, Corran PH, Thaikla K, Liewsaree W, Riley EM, Hafalla JC. 2010. Long-lived antibody and B cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 6:e1000770. 10.1371/journal.ppat.1000770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ndungu FM, Olotu A, Mwacharo J, Nyonda M, Apfeld J, Mramba LK, Fegan GW, Bejon P, Marsh K. 2012. Memory B cells are a more reliable archive for historical antimalarial responses than plasma antibodies in no-longer exposed children. Proc. Natl. Acad. Sci. U. S. A. 109:8247–8252. 10.1073/pnas.1200472109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ndungu FM, Lundblom K, Rono J, Illingworth J, Eriksson S, Farnert A. 2013. Long-lived Plasmodium falciparum specific memory B cells in naturally exposed Swedish travellers. Eur. J. Immunol. 43:2919–2929. 10.1002/eji.201343630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muellenbeck MF, Ueberheide B, Amulic B, Epp A, Fenyo D, Busse CE, Esen M, Theisen M, Mordmuller B, Wardemann H. 2013. Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies. J. Exp. Med. 210:389–399. 10.1084/jem.20121970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khunrae P, Dahlbäck M, Nielsen MA, Andersen G, Ditlev SB, Resende M, Pinto VV, Theander TG, Higgins MK, Salanti A. 2010. Full-length recombinant Plasmodium falciparum VAR2CSA binds specifically to CSPG and induces potent parasite adhesion-blocking antibodies. J. Mol. Biol. 397:826–834. 10.1016/j.jmb.2010.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghumra A, Semblat JP, Ataide R, Kifude C, Adams Y, Claessens A, Anong DN, Bull PC, Fennell C, Arman M, Amambua-Ngwa A, Walther M, Conway DJ, Kassambara L, Doumbo OK, Raza A, Rowe JA. 2012. Induction of strain-transcending antibodies against group A PfEMP1 surface antigens from virulent malaria parasites. PLoS Pathog. 8:e1002665. 10.1371/journal.ppat.1002665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraemer SM, Kyes SA, Aggarwal G, Springer AL, Nelson SO, Christodoulou Z, Smith LM, Wang W, Levin E, Newbold CI, Myler PJ, Smith JD. 2007. Patterns of gene recombination shape var gene repertoires in Plasmodium falciparum: comparisons of geographically diverse isolates. BMC Genomics 8:45. 10.1186/1471-2164-8-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leech JH, Barnwell JW, Miller LH, Howard RJ. 1984. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 159:1567–1575. 10.1084/jem.159.6.1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77–87. 10.1016/0092-8674(95)90054-3 [DOI] [PubMed] [Google Scholar]
- 24.Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, Peterson DS, Pinches R, Newbold CI, Miller LH. 1995. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82:101–110. 10.1016/0092-8674(95)90056-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su X, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, Peterson DS, Ravetch JA, Wellems TE. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82:89–100. 10.1016/0092-8674(95)90055-1 [DOI] [PubMed] [Google Scholar]
- 26.Kraemer SM, Smith JD. 2006. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr. Opin. Microbiol. 9:374–380. 10.1016/j.mib.2006.06.006 [DOI] [PubMed] [Google Scholar]
- 27.Salanti A, Dahlbäck M, Turner L, Nielsen MA, Barfod L, Magistrado P, Jensen ATR, Lavstsen T, Ofori MF, Marsh K, Hviid L, Theander TG. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 200:1197–1203. 10.1084/jem.20041579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clausen TM, Christoffersen S, Dahlback M, Langkilde AE, Jensen KE, Resende M, Agerbak MO, Andersen D, Berisha B, Ditlev SB, Pinto VV, Nielsen MA, Theander TG, Larsen S, Salanti A. 2012. Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J. Biol. Chem. 287:23332–23345. 10.1074/jbc.M112.348839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hviid L. 2011. The case for PfEMP1-based vaccines to protect pregnant women against Plasmodium falciparum malaria. Expert Rev. Vaccines 10:1405–1414. 10.1586/erv.11.113 [DOI] [PubMed] [Google Scholar]
- 30.Mercereau-Puijalon O, Guillotte M, Vigan-Womas I. 2008. Rosetting in Plasmodium falciparum: a cytoadherence phenotype with multiple actors. Transfus. Clin. Biol. 15:62–71. 10.1016/j.tracli.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 31.Carlson J, Helmby H, Hill AV, Brewster D, Greenwood BM, Wahlgren M. 1990. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 336:1457–1460. 10.1016/0140-6736(90)93174-N [DOI] [PubMed] [Google Scholar]
- 32.Bull PC, Lowe BS, Kortok M, Marsh K. 1999. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect. Immun. 67:733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen MA, Staalsoe T, Kurtzhals JAL, Goka BQ, Dodoo D, Alifrangis M, Theander TG, Akanmori BD, Hviid L. 2002. Plasmodium falciparum variant surface antigen expression varies between isolates causing severe and nonsevere malaria and is modified by acquired immunity. J. Immunol. 168:3444–3450 [DOI] [PubMed] [Google Scholar]
- 34.Kraemer SM, Smith JD. 2003. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Mol. Microbiol. 50:1527–1538. 10.1046/j.1365-2958.2003.03814.x [DOI] [PubMed] [Google Scholar]
- 35.Lavstsen T, Salanti A, Jensen ATR, Arnot DE, Theander TG. 2003. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar. J. 2:27. 10.1186/1475-2875-2-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen ATR, Magistrado PA, Sharp S, Joergensen L, Lavstsen T, Chiucchiuini A, Salanti A, Vestergaard LS, Lusingu JP, Hermsen R, Sauerwein R, Christensen J, Nielsen MA, Hviid L, Sutherland C, Staalsoe T, Theander TG. 2004. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J. Exp. Med. 199:1179–1190. 10.1084/jem.20040274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. 2012. World malaria report. World Health Organization, Geneva, Switzerland [Google Scholar]
- 38.Afari EA, Akanmori BD, Nakano T, Ofori-Adjei D. 1992. Plasmodium falciparum: sensitivity to chloroquine in vivo in three ecological zones in Ghana. Trans. R. Soc. Trop. Med. Hyg. 86:231–232. 10.1016/0035-9203(92)90285-K [DOI] [PubMed] [Google Scholar]
- 39.Afari EA, Appawu M, Dunyo S, Baffoe-Wilmot A, Nkrumah FK. 1995. Malaria infection, morbidity and transmission in two ecological zones in southern Ghana. Afr. J. Health Sci. 2:312–316 [PubMed] [Google Scholar]
- 40.Ampomah P. 2008. M.Phil. thesis University of Cape Coast, Cape Coast, Ghana [Google Scholar]
- 41.Hviid L, Albeck G, Hansen B, Theander TG, Talbot A. 1993. A new portable device for automatic controlled-gradient cryopreservation of blood mononuclear cells. J. Immunol. Methods 157:135–142. 10.1016/0022-1759(93)90079-M [DOI] [PubMed] [Google Scholar]
- 42.Guitard J, Cottrell G, Magnouha NM, Salanti A, Li T, Sow S, Deloron P, Tuikue NN. 2008. Differential evolution of anti-VAR2CSA-IgG3 in primigravidae and multigravidae pregnant women infected by Plasmodium falciparum. Malar. J. 7:10. 10.1186/1475-2875-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altman DG, Machin D, Bryant TN, Gardner MJ. (ed). 2000. Statistics with confidence. BMJ Books, London, United Kingdom [Google Scholar]
- 44.Vestergaard LS, Lusingu JP, Nielsen MA, Mmbando BP, Dodoo D, Akanmori BD, Alifrangis M, Bygbjerg IC, Lemnge MM, Staalsoe T, Hviid L, Theander TG. 2008. Differences in human antibody reactivity to Plasmodium falciparum variant surface antigens are dependent on age and malaria transmission intensity in northeastern Tanzania. Infect. Immun. 76:2706–2714. 10.1128/IAI.01401-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campo JJ, Whitman TJ, Freilich D, Burgess TH, Martin GJ, Doolan DL. 2011. Toward a surrogate marker of malaria exposure: modeling longitudinal antibody measurements under outbreak conditions. PLoS One 6:e21826. 10.1371/journal.pone.0021826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salanti A, Staalsoe T, Lavstsen T, Jensen ATR, Sowa MPK, Arnot DE, Hviid L, Theander TG. 2003. Selective upregulation of a single distinctly structured var gene in CSA-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49:179–191. 10.1046/j.1365-2958.2003.03570.x [DOI] [PubMed] [Google Scholar]
- 47.O'Neil-Dunne I, Achur RN, Agbor-Enoh ST, Valiyaveettil M, Naik RS, Ockenhouse CF, Zhou A, Megnekou R, Leke R, Taylor DW, Gowda DC. 2001. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect. Immun. 69:7487–7492. 10.1128/IAI.69.12.7487-7492.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staalsoe T, Megnekou R, Fievet N, Ricke CH, Zornig HD, Leke R, Taylor DW, Deloron P, Hviid L. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum infected erythrocytes that are associated with protection against placental parasitemia. J. Infect. Dis. 184:618–626. 10.1086/322809 [DOI] [PubMed] [Google Scholar]
- 49.Cham CK, Turner L, Lusingu J, Vestergaard L, Mmbando B, Kurtis JD, Jensen AT, Salanti A, Lavstsen T, Theander TG. 2009. Sequential, ordered acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 domains. J. Immunol. 183:3356–3363. 10.4049/jimmunol.0901331 [DOI] [PubMed] [Google Scholar]
- 50.Barfod L, Dobrilovic T, Magistrado P, Khunrae P, Viwami F, Bruun J, Dahlbäck M, Bernasconi NL, Fried M, John D, Duffy PE, Salanti A, Lanzavecchia A, Lim CT, Ndam NT, Higgins MK, Hviid L. 2010. Chondroitin sulfate A-adhering Plasmodium falciparum-infected erythrocytes express functionally important antibody epitopes shared by multiple variants. J. Immunol. 185:7553–7561. 10.4049/jimmunol.1002390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogerson SJ, Chaiyaroj SC, Ng K, Reeder JC, Brown GV. 1995. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 182:15–20. 10.1084/jem.182.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fried M, Duffy PE. 1996. Adherence of Plasmodium falciparum to chondroitin sulphate A in the human placenta. Science 272:1502–1504. 10.1126/science.272.5267.1502 [DOI] [PubMed] [Google Scholar]
- 53.Muthusamy A, Achur RN, Valiyaveettil M, Botti JJ, Taylor DW, Leke RF, Gowda DC. 2007. Chondroitin sulfate proteoglycan but not hyaluronic acid is the receptor for the adherence of Plasmodium falciparum-infected erythrocytes in human placenta and IRBC adherence upregulates the receptor expression. Am. J. Pathol. 170:1989–2000. 10.2353/ajpath.2007.061238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slifka MK, Antia R, Whitmire JK, Ahmed R. 1998. Humoral immunity due to long-lived plasma cells. Immunity 8:363–372. 10.1016/S1074-7613(00)80541-5 [DOI] [PubMed] [Google Scholar]
- 55.Fievet N, Chougnet C, Dubois B, Deloron P. 1993. Quantification of antibody-secreting lymphocytes that react with Pf155/RESA from Plasmodium falciparum: an ELISPOT assay for field studies. Clin. Exp. Immunol. 91:63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Migot F, Chougnet C, Henzel D, Dubois B, Jambou R, Fievet N, Deloron P. 1995. Anti-malaria antibody-producing B cell frequencies in adults after a Plasmodium falciparum outbreak in Madagascar. Clin. Exp. Immunol. 102:529–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dorfman JR, Bejon P, Ndungu FM, Langhorne J, Kortok MM, Lowe BS, Mwangi TW, Williams TN, Marsh K. 2005. B cell memory to 3 Plasmodium falciparum blood-stage antigens in a malaria-endemic area. J. Infect. Dis. 191:1623–1630. 10.1086/429671 [DOI] [PubMed] [Google Scholar]
- 58.Weiss GE, Clark EH, Li S, Traore B, Kayentao K, Ongoiba A, Hernandez JN, Doumbo OK, Pierce SK, Branch OH, Crompton PD. 2011. A positive correlation between atypical memory B cells and Plasmodium falciparum transmission intensity in cross-sectional studies in Peru and Mali. PLoS One 6:e15983. 10.1371/journal.pone.0015983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clark EH, Silva CJ, Weiss GH, Li S, Padilla C, Crompton PD, Hernandez JN, Branch OH. 2012. Plasmodium falciparum malaria in the low transmission Peruvian Amazon is associated with immunologic memory. Infect. Immun. 80:1583–1592. 10.1128/IAI.05961-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snow RW, Omumbo JA, Lowe B, Molyneux CS, Obiero JO, Palmer A, Weber MW, Pinder M, Nahlen B, Obonyo C, Newbold C, Gupta S, Marsh K. 1997. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet 349:1650–1654. 10.1016/S0140-6736(97)02038-2 [DOI] [PubMed] [Google Scholar]
- 61.Reyburn H, Mbatia R, Drakeley C, Bruce J, Carneiro I, Olomi R, Cox J, Nkya WM, Lemnge M, Greenwood BM, Riley EM. 2005. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA 293:1461–1470. 10.1001/jama.293.12.1461 [DOI] [PubMed] [Google Scholar]
- 62.Amulic B, Salanti A, Lavstsen T, Nielsen MA, Deitsch K. 2009. An upstream open reading frame controls translation of var2csa, a gene implicated in placental malaria. PLoS Pathog. 5:e1000256. 10.1371/journal.ppat.1000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bancells C, Deitsch KW. A molecular switch in the efficiency of translation reinitiation controls expression of var2csa, a gene implicated in pregnancy associated malaria. Mol. Microbiol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lavstsen T, Magistrado P, Hermsen CC, Salanti A, Jensen ATR, Sauerwein R, Hviid L, Theander TG, Staalsoe T. 2005. Expression of Plasmodium falciparum erythrocyte membrane protein 1 in experimentally infected humans. Malar. J. 4:21. 10.1186/1475-2875-4-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cham GK, Turner L, Kurtis JD, Mutabingwa T, Fried M, Jensen AT, Lavstsen T, Hviid L, Duffy PE, Theander TG. 2010. Hierarchical, domain type-specific acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 in Tanzanian children. Infect. Immun. 78:4653–4659. 10.1128/IAI.00593-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fowkes FJ, McGready R, Cross NJ, Hommel M, Simpson JA, Elliott SR, Richards JS, Lackovic K, Viladpai-Nguen J, Narum D, Tsuboi T, Anders RF, Nosten F, Beeson JG. 2012. New insights into acquisition, boosting and longevity of immunity to malaria in pregnant women. J. Infect. Dis. 206:1612–1621. 10.1093/infdis/jis566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slifka MK, Ahmed R. 1998. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr. Opin. Immunol. 10:252–258. 10.1016/S0952-7915(98)80162-3 [DOI] [PubMed] [Google Scholar]
- 68.Giha HA, Staalsoe T, Dodoo D, Elhassan IM, Roper C, Satti GM, Arnot DE, Theander TG, Hviid L. 1999. Nine-year longitudinal study of antibodies to variant antigens on the surface of Plasmodium falciparum-infected erythrocytes. Infect. Immun. 67:4092–4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ofori MF, Dodoo D, Staalsoe T, Kurtzhals JAL, Koram K, Theander TG, Akanmori BD, Hviid L. 2002. Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens. Infect. Immun. 70:2982–2988. 10.1128/IAI.70.6.2982-2988.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, Molina DM, Burk CR, Waisberg M, Jasinskas A, Tan X, Doumbo S, Doumtabe D, Kone Y, Narum DL, Liang X, Doumbo OK, Miller LH, Doolan DL, Baldi P, Felgner PL, Pierce SK. 2010. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc. Natl. Acad. Sci. U. S. A. 107:6958–6963. 10.1073/pnas.1001323107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ndungu FM, Cadman ET, Coulcher J, Nduati E, Couper E, Macdonald DW, Ng D, Langhorne J. 2009. Functional memory B cells and long-lived plasma cells are generated after a single Plasmodium chabaudi infection in mice. PLoS Pathog. 5:e1000690. 10.1371/journal.ppat.1000690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zinkernagel RM, Bachmann MF, Kündig TM, Oehen S, Pirchet H, Hengartner H. 1996. On immunological memory. Annu. Rev. Immunol. 14:333–367. 10.1146/annurev.immunol.14.1.333 [DOI] [PubMed] [Google Scholar]
- 73.Beeson JG, Ndungu F, Persson KE, Chesson JM, Kelly GL, Uyoga S, Hallamore SL, Williams TN, Reeder JC, Brown GV, Marsh K. 2007. Antibodies among men and children to placental-binding Plasmodium falciparum-infected erythrocytes that express var2csa. Am. J. Trop. Med. Hyg. 77:22–28 [PubMed] [Google Scholar]
- 74.Traore B, Kone Y, Doumbo S, Doumtabe D, Traore A, Crompton P, Mircetic M, Huang CY, Kayentao K, Dicko A, Sagara I, Ellis RD, Miura K, Guindo A, Miller LH, Doumbo OK, Pierce SK. 2009. The TLR9 agonist CpG fails to enhance the acquisition of Plasmodium falciparum-specific memory B cells in semi-immune adults in Mali. Vaccine 27:7299–7303. 10.1016/j.vaccine.2009.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weiss GE, Traore B, Kayentao K, Ongoiba A, Doumbo S, Doumtabe D, Kone Y, Dia S, Guindo A, Traore A, Huang CY, Miura K, Mircetic M, Li S, Baughman A, Narum DL, Miller LH, Doumbo OK, Pierce SK, Crompton PD. 2010. The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog. 6:e1000912. 10.1371/journal.ppat.1000912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O'Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. 2008. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205:1797–1805. 10.1084/jem.20072683 [DOI] [PMC free article] [PubMed] [Google Scholar]


