Abstract
Infections with Streptococcus pneumoniae cause substantial morbidity and mortality, particularly in children in developing nations. Polysaccharide-conjugate vaccines provide protection against both invasive disease and colonization, but their use in developing countries is limited by restricted serotype coverage and expense of manufacture. Using proteomic screens, we recently identified several antigens that protected mice from pneumococcal colonization in a CD4+ T cell- and interleukin-17A (IL-17A)-dependent manner. Since several of these proteins are lipidated, we hypothesized that their immunogenicity and impact on colonization are in part due to activation of Toll-like receptor 2 (TLR2), a receptor for lipoproteins. Here we show that lipidated versions of the antigens elicited significantly higher activation of both human embryonic kidney cells engineered to express TLR2 (HEK-TLR2) and wild-type (WT) murine macrophages than nonlipidated mutant antigens. Lipoprotein-stimulated secretion of proinflammatory cytokines was ∼10× to ∼100× lower in murine TLR2-deficient macrophages than in WT macrophages. Subcutaneous immunization of C57BL/6 mice with protein subunit vaccines containing one or two of these lipoproteins or protein fusion constructs bearing N-terminal lipid adducts elicited a robust IL-17A response and a significant reduction in colonization compared with immunization with alum alone. In contrast, immunization of Tlr2−/− mice elicited no detectable IL-17A response and no protection against pneumococcal colonization. These experiments suggest that the lipid moieties enhance the immunogenicity and protective efficacy of pneumococcal TH17 antigens through activation of TLR2. Thus, triggering TLR2 with an antigen-specific protein subunit formulation is a possible strategy for the development of a serotype-independent pneumococcal vaccine that would reduce pneumococcal carriage.
INTRODUCTION
Streptococcus pneumoniae is a major cause of morbidity and mortality, predominantly in young children in resource-limited settings (1). While pneumococcal conjugate vaccines (PCVs) have been highly effective at reducing invasive disease due to vaccine serotypes, limitations of this approach include emergence of disease due to nonvaccine serotypes and the high cost and complexity of PCV development and manufacture (2–4). As a result, more-affordable and serotype-independent immunization approaches are being investigated. One approach includes use of well-conserved, immunogenic, noncapsular pneumococcal antigens, either as components of a subunit protein vaccine or incorporated into PCV formulations as carrier proteins (5–7). The choice of such antigens should be informed by the evolving understanding of mechanisms of immunity to the various phases of pneumococcal infection. Several studies have demonstrated the role of interleukin-17A (IL-17A) in clearance of pneumococcal carriage in mice (8, 9). The increased incidence of pneumococcal sinopulmonary infections in patients with Job's disease (10), now understood to be due to a genetic defect in the STAT3 transcription factor which is essential for differentiation of IL-17-producing TH17 cells (11), points to the importance of this effector cytokine in humans as a mechanism of protection against mucosal pneumococcal infections.
We previously performed proteomic screens to identify a panel of antigens that were recognized by CD4+ TH17 cells from mice that had been immunized with an unencapsulated killed pneumococcal whole-cell antigen (WCA) (12) and from healthy adult humans (13). Notably, several of the most immunogenic antigens identified were genetic orthologs of substrate-binding lipoproteins (LP) of ATP-binding cassette (ABC) transporter complexes. Two of these, identified by their locus within the TIGR4 sequence as SP_2108 and SP_0148, protected mice from colonization in a CD4+ T cell- and IL-17A-dependent manner when used as an intranasal vaccine (12). Given the relatively low proportion of lipoproteins within the pneumococcal proteome compared to their abundant representation within the panel of identified TH17 antigens, we hypothesized that the lipid moiety of these proteins was contributing to their immunogenicity.
Several bacterial lipoproteins have been shown to activate Toll-like receptor 2 (TLR2) (14). Bacterial lipoproteins activate TLR2 early in infection, providing costimulatory signals that initiate adaptive immune responses, including TLR2-dependent antigen-presenting-cell maturation that activates naive CD4+ T cells to differentiate into effector cells (15, 16). In animal models of pneumococcal infection using TLR2 knockout mice, TLR2 has been shown to be critical for clearance in meningitis (17) and colonization (18). TLR2- and IL-17A-mediated responses have been shown to be critical for enhanced clearance of bacteria (9); in particular, a subset of pneumococcus-specific memory TH17 cells are generated after colonization in previously exposed WT but not Tlr2−/− mice, suggesting that TLR2 activation is indeed necessary for the generation of memory CD4+ TH17 responses (9).
In the present study, we tested the hypothesis that the TH17 immunogenicity of the two lipoproteins SP_2108 and SP_0148 is dependent on the lipid adducts signaling through TLR2. We show that the lipid modification of these proteins is critical to their immunogenicity and protective efficacy in a mouse model of pneumococcal colonization. By immunizing mice with either of these lipoproteins in combination with SP_1912, an identified TH17 antigen that is not lipidated (13), we also show an in trans effect of the lipoproteins that enhances the immunogenicity of the coadministered nonlipidated (Δ lipid) antigen. Furthermore, we demonstrate that multiple formulations of parenteral vaccines containing at least one of these lipoproteins confer a significant reduction in colonization in a TLR2-dependent manner. The findings described here may inform the development of next-generation protein-based vaccines for prevention of disease caused by Streptococcus pneumoniae, in particular, the mucosal phases of infection that contribute to the reservoir of transmissible pneumococci among immunized and nonimmunized individuals.
MATERIALS AND METHODS
Generation of recombinant lipoproteins, nonlipidated mutants, and fusion constructs.
The SP_2108 and SP_0148 lipoproteins and nonlipidated SP_1912 (13) were cloned from strain TIGR4 S. pneumoniae DNA. To generate nonlipidated mutants of the lipoproteins, we performed site-directed mutagenesis of the cysteine residue within the conserved “lipobox” region of the lipidation sequence (19) to aspartate. The genetic fusion construct of SP_2108 and SP_1912, referred to here as fusion 2108-1912, was collinearly synthesized with the lipobox region at the N terminus. All constructs were synthesized with a C-terminal 6×His affinity tag and cloned into pET24b expression vector. Methods for expression of SP_2108 and SP_0148 lipoproteins have been previously described (19).
All proteins were purified using immobilized-metal affinity chromatography (IMAC). The lipoproteins, Δ lipid, and fusion 2108-1912 constructs were expressed as soluble proteins in Escherichia coli; SP_1912 was expressed in inclusion bodies. For the lipoprotein constructs, following microfluidization in the presence of 1% Tween 80, Lysonase (EMD Millipore), and protease inhibitors (Novagen), the proteins were captured on nickel-charged IMAC resin (GE Life Sciences), washed with sodium deoxycholate, and eluted by step gradient of imidazole. SP_1912 inclusion body pellets were resuspended in 6 M guanidine-HCl and purified in the presence of 6 M urea. Where necessary, endotoxin was removed either by precipitating purified lipoproteins with hexanediol and acetone, followed by overnight incubation at 4°C, centrifugation, and drying, or by the use of ActiClean Etox columns (Cyprus International) prior to dialysis, concentration, and storage. Purified lipoproteins were dialyzed into buffer containing 10 mM Tris-HCl–10 mM NaCl (pH 8). SP_1912 was dialyzed against 2 M urea and then 5 mM sodium phosphate–5% sucrose (pH 8). Proteins were concentrated to approximately 1 mg/ml and stored at −70°C. Purified proteins were routinely analyzed for purity using 4% to 20% SDS-PAGE followed by staining with Coomassie blue and gel densitometry. Identities, as well as assessment of aggregation or degradation, were determined by Western blot analysis using protein-specific antisera. Endotoxin levels were measured by the use of an Endosafe-PTS system (Charles River). All proteins were >90% pure and contained small amounts of endotoxin. Lipidation content was determined by reverse-phase high-pressure liquid chromatography (RP-HPLC) using a C8 column (Agilent 1100). Lipidated proteins were also characterized by gas chromatography-mass spectrometry (GC-MS) and LC_MS/MS to confirm the presence of acyl chain adducts (data not shown).
Mice.
For most experiments, 4-to-6-week-old C5BL/6 mice were obtained from Charles River Laboratories (C57BL/6NCrl [strain code 027]; Wilmington, MA). For experiments evaluating the role of TLR2, C57BL/6 Tlr2−/− mice (C57B6.129-Tlr2tm1Kir/J [strain code 004650]) and wild-type controls were from Jackson Laboratories (C57BL/6J [strain code 000664]; Bar Harbor, ME). Ten to 20 animals were included per immunization or control group in each experiment. All animal studies were conducted in accordance with institutional guidelines approved by the IACUC of Boston Children's Hospital, Harvard Medical School, and Genocea Biosciences.
Immunization and immunogenicity studies.
For intranasal immunizations, 4 μg of the indicated recombinant protein with 1 μg cholera toxin (CT; List Biological Laboratories Inc.) per dose was administered in a 20-μl volume in sterile phosphate-buffered saline (PBS). Animals were immunized once and then again 1 week later. For parenteral immunization, 1 to 10 μg of the indicated proteins or the fusion protein was adsorbed to 250 μg aluminum (as aluminum hydroxide [Alhydrogel; Brenntag]; referred to here as alum) per dose and administered subcutaneously in sterile saline solution in the dorsal hindquarters in 200-μl injections. Animals were immunized three times at 2-week intervals. Two to 3 weeks following the last immunization, animals were bled retro-orbitally under isoflurane anesthesia. Whole blood was stimulated with either pneumococcal WCA or the antigens of immunization as previously described (8), and the IL-17A concentration in cell supernatants was measured by enzyme-linked immunosorbent assay (ELISA) after a 6-day incubation (R&D Systems). Alternatively, 3 weeks after the last immunization, spleens were harvested and dissociated with a cell strainer into a single-cell suspension and cleared of red blood cells (ACK buffer; BioWhittaker Lonza, Walkersville, MD). Splenocytes were cultured at 200,000 cells/well and stimulated with 1 μg/ml of overlapping peptides (15-mers with 11-amino-acid overlap) spanning the vaccine antigens (JPT, Germany) in round-bottom tissue culture plates for 6 days in a final volume of 200 μl. Cell-free supernatants were collected and analyzed for IL-17A by ELISA.
Colonization.
Two weeks following the bleed for immunogenicity studies, animals were challenged with an inoculum of 2 × 107 CFU of a serotype 6B pneumococcal strain in PBS. For colonization, animals were given 20 μl inocula intranasally while gently restrained and awake. Animals were sacrificed 7 days (for intranasally immunized animals) or 10 days (for parenterally immunized mice) following challenge, and nasal washes were obtained by tracheal lavage for evaluation of the density of pneumococcal colonization as previously described (20). Prior work by our group has demonstrated an effect of intranasal CT alone at diminishing pneumococcal carriage burden compared to saline solution (20). This effect of CT causes reduced carriage burden as early as 1 week after colonization challenge; thus, evaluation of nasal washes no later than 7 days postcolonization is preferred in the intranasal immunization model.
In vitro cell stimulations.
HEK293 and HEK cells expressing human TLR2 (HEK-TLR2) were plated at 5 × 104 cells/well in a 96-well format or 5 × 105 cells/well in a 24-well format. One day following seeding, 1 to 0.001 μg/ml of lipoprotein, nonlipidated mutants, or Pam3CSK4 (a synthetic TLR2 agonist; Invivogen) was added to the wells and incubated for 18 h. Supernatants were harvested, and the concentration of IL-8, a cytokine elicited by activation of HEK cells, was measured by ELISA. Similarly, 5 × 105 macrophage cells from WT or Tlr2−/− C57BL/6 mice were plated per well and were stimulated as described for the HEK cells. Supernatants and macrophage lysates were collected after 3, 6, and 24 h of stimulation and were stored at −20°C until cytokine measurements was performed. Concentrations of tumor necrosis factor alpha (TNF-α), granulocyte colony-stimulating factor (G-CSF), and IL-6 from supernatants and of IL-1β from lysates were measured by ELISA. All ELISA reagents (human IL-8 and mouse G-CSF, TNF-α, IL-6, and IL-1β) were from R&D Systems (Minneapolis, MN). Murine macrophage cell lines were kindly provided by Douglas Golenbock (University of Massachusetts Medical School), and HEK cells were from ATCC.
Statistical analyses.
Data were analyzed and graphs were created in PRISM (Version 5.0d; GraphPad Software, Inc.). Significance was determined using the Mann-Whitney or Kruskal-Wallis test; P < 0.05 was considered significant. Spearman correlation coefficients were calculated with control animals excluded to avoid skewing of the strength of correlation.
RESULTS
Posttranslational attachment of lipid moieties is critical for TLR2 activation in vitro.
A common mechanism whereby bacteria attach acyl groups to prolipoproteins is via the enzyme prolipoprotein diacylglycerol transferase (Lgt), which attaches a diacylglycerol moiety from phosphatidylglycerol to the cysteine residue in the N-terminal lipobox sequence of predicted lipoproteins via a thioether linkage (19, 21). Additional acyl chains can be subsequently added to the amino group of cysteine (generated after signal peptide cleavage) by lipoprotein N-acyl transferase (Lnt). We identified the lipobox motif within the sequences of sp_2108 and sp_0148 and expressed recombinant versions of the proteins with a mutation encoding an aspartate in place of the cysteine residue on which the lipid moiety attachment occurs. RP-HPLC analysis of purified proteins confirmed the absence of acyl modification in the mutated proteins (Fig. 1A). These proteins, denoted Δlipid, as well as the lipidated proteins, were used to stimulate HEK cells (Fig. 1B). In these assays, native SP_2108 and SP_0148 lipoproteins (LP) elicited IL-8 from HEK-TLR2 cells in a dose-dependent fashion; 1 and 0.1 μg/ml stimulus doses elicited IL-8 secretion in the 2.6 to 3.5 ng/ml range, which was similar to the range elicited by the canonical synthetic TLR2 agonist, Pam3CSK4. Nonlipidated mutants of SP_2108 and SP_0148 elicited no measurable IL-8 response in HEK TLR2 cells. No IL-8 response was detected when HEK293 cells (which do not express TLR2) were similarly stimulated with the lipidated or nonlipidated proteins (data not shown).
FIG 1.
HPLC chromatograms and HEK-TLR2 stimulation with lipoproteins and nonlipidated proteins. (A) Overlay of RP-HPLC chromatograms. Samples were run on a C8 reverse-phase column using an acetonitrile-trifluoroacetic acid (TFA) mobile-phase system, and protein signal was detected at 280 nm using a diode array detector. The peaks between 15 and 20 min reflect the lipidated proteins. (B) HEK-TLR2 and HEK293 (expressing no TLR) cells were stimulated with decreasing doses of Pam3CSK4, SP_2108 LP (lipoprotein), SP_0148 LP, or nonlipidated (Δlipid) proteins. Following an 18-h stimulation, IL-8 was measured from the supernatants. Median values with interquartile ranges from at least 4 independent stimulations are shown. The experiments performed with all shown stimuli were repeated in HEK293 cells, and the results were at or below the limit of detection in the assay.
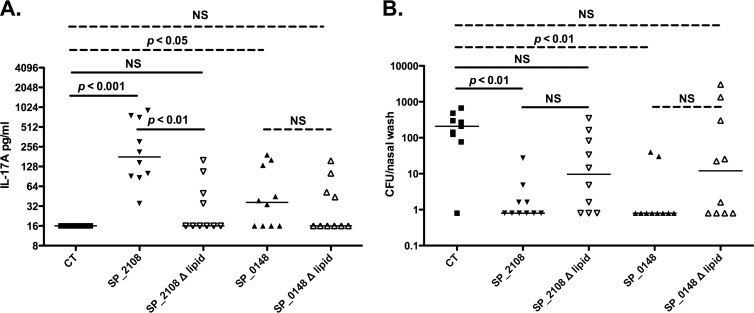
Nonlipidated mutants are significantly less immunogenic and less effective at reducing colonization than lipoproteins following intranasal immunization.
In previous studies, SP_2108 and SP_0148 elicited systemic IL-17A responses when used as intranasal immunogens in a dosing schedule similar to the one described previously (12). In order to evaluate the role of the lipid moieties attached to these proteins in eliciting IL-17A, we immunized mice intranasally with either the lipoproteins or their nonlipidated mutants with cholera toxin (CT) as an adjuvant. Whole blood from mice immunized with the nonlipidated mutants produced lower levels of IL-17A in response to stimulation with pneumococcal whole-cell antigen (WCA) than whole blood from mice immunized with the lipoproteins (compared to CT-immunized controls, P < 0.001 for SP_2108, P < 0.05 for SP_0148, and nonsignificant [NS] P for SP_2108Δlipid or SP_0148Δlipid; Fig. 2A). Similarly, while immunization with nonlipidated mutants conferred a reduction in colonization burden compared with CT immunization alone, only immunization with the lipoproteins conferred a significant reduction in carriage (P < 0.01 for SP_2108 and SP_0148; Fig. 2B). The burden of colonization inversely correlated with the concentration of IL-17A detected in whole-blood samples following stimulation with WCA: those animals with the highest IL-17A levels had the lowest nasal burden of pneumococci after exclusion of CT-immunized animals (Spearman coefficient, −0.46; 95% confidence interval [CI], −0.68 to −0.16; P = 0.003). However, in this experiment the predictive relationship between IL-17A and the colonization density is not very strong, as evidenced by the fact that 8 of 10 animals in the SP_0148-immunized group were not colonized despite their whole blood producing little IL-17A when stimulated with WCA.
FIG 2.
IL-17A responses and protection against colonization following intranasal immunization with lipidated or nonlipidated SP_2108 and SP_0148. (A) C57BL/6J mice were immunized once and then again 1 week later with 4 μg of the indicated protein and 1 μg of CT in a 10-μl volume instilled intranasally. Two weeks following their last immunization, animals were bled and whole blood was stimulated with WCA; IL-17A concentrations in cell supernatants following a 6-day incubation are shown. (B) One week following intranasal challenge, nasal washes were collected and pneumococcal CFU were counted. Bars represent median values, and P values were calculated by Kruskal-Wallis analysis of comparisons between CT, SP_21208, and SP_2108Δlipid groups (solid bars) and between CT, SP_0148, and SP_0148Δlipid groups (dashed bars).
SP_2108 and SP_0148 elicit TH17-driving cytokine responses by murine macrophages in a lipid- and TLR2-dependent manner.
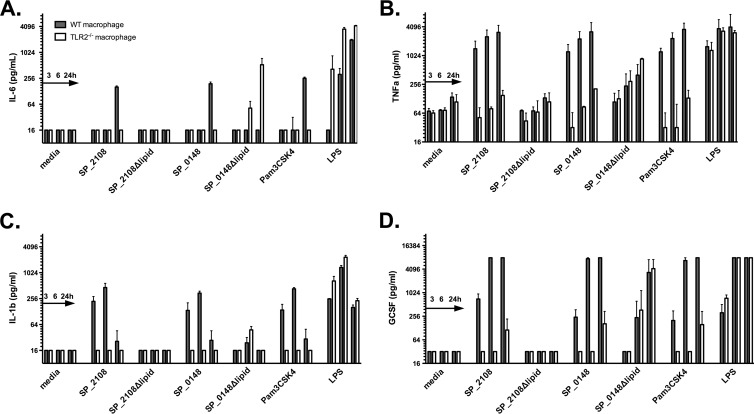
The results of the studies described above supported the hypothesis that the lipid motifs in SP_2108 and SP_0148 may be responsible for driving TH17 responses in mice. To assess this possibility further, we examined the cytokine response following stimulation of antigen-presenting cells. The recombinant lipoproteins and nonlipidated mutants were used to stimulate macrophages from WT and Tlr2−/− C57BL/6 mice. Cell supernatants were assayed for concentrations of TNF-α, G-CSF, IL-23, transforming growth factor beta (TGF-β), IL-6, and lysates for IL-1β, all proinflammatory cytokines that either promote development of adaptive TH17 cell differentiation (22, 23) or act synergistically with IL-17A (24). While IL-17A has been shown to be produced by some innate immune cells (24), there was no detectable IL-17A in these supernatants.
Stimulation of WT macrophages with SP_2108 and SP_0148 lipoproteins led to an IL-6 and IL-1β response similar in magnitude and kinetics to those seen following stimulation with Pam3CSK4 (Fig. 3A and C). In contrast, responses following stimulation with nonlipidated versions of the proteins were virtually undetectable. Tlr2−/− macrophages did not respond following stimulation with lipoproteins, indicating that the observed response was TLR2 dependent. The lipoproteins and Pam3CSK4 also stimulated TNF-α and G-CSF responses in a TLR2-dependent manner (Fig. 3B and D). IL-23 was not detectable. Concentrations of TGF-β were elevated under all stimulation conditions, including in medium-only control wells, and thus were not evaluable. Nonlipidated SP_0148 did elicit IL-6, TNF-α, and G-CSF responses in a TLR2-independent manner, perhaps suggesting different innate stimulatory properties of this protein. Heating recombinant SP_0148Δlipid to 100°C for 1 h did abrogate its proinflammatory effect (data not shown), suggesting that lipopolysaccharide contamination was an unlikely cause.
FIG 3.
In vitro proinflammatory responses to lipidated and nonlipidated proteins. WT or TLR2−/− macrophages from C57BL/6 mice were stimulated with lipidated or nonlipidated proteins, a fusion of SP_2108 and SP_1912, or Pam3CSK4, all at 1 μg/ml. LPS was used at 100 ng/ml. Values shown represent at least 4 independent stimulations harvested at 3, 6, and 24 h poststimulation. Bars and lines represent medians with interquartile ranges.
Immunogenicity and protective efficacy of parenterally administered lipoproteins.
Next, we examined whether TH17 responses to the lipoproteins could be elicited by immunizing animals parenterally without the use of CT, an adjuvant known to skew responses along the IL-17A axis (25, 26). We first confirmed the critical role of the lipid moiety in eliciting IL-17A following subcutaneous immunization with SP_2108 and SP_0148, each adsorbed onto alum. Indeed, subcutaneous immunization with the lipidated proteins conferred demonstrable IL-17A responses measured from splenocytes cultured with immunogen-specific stimuli, whereas no IL-17A was detected following immunization with nonlipidated mutants (see Fig. S1 in the supplemental material).
Next, with the goal of expanding the coverage of a protein subunit vaccine, we looked to the results of our proteomic screen using human blood samples (13), from which we had identified SP_1912, a nonlipidated protein of unknown function that conferred significant protection when administered intranasally using CT as an adjuvant (data not shown). We immunized WT mice subcutaneously three times at 2-week intervals with a mixture of SP_2108, SP_0148, and SP_1912 adsorbed onto alum. As shown in Fig. 4A, pneumococcus-specific IL-17A responses could be readily measured following immunization with the mixture of proteins. Interestingly, in similar immunization regimens, a TH17 response to SP_1912 could be detected after immunization with the mixture containing the lipoproteins but not when SP_1912 was used as a single immunogen (P = 0.0008 comparing IL-17A conferred by SP_1912 immunization alone to immunization with the lipoprotein-containing mixture; see Fig. S2A in the supplemental material). Based on these results and to reduce the number of constructs in the vaccine, we made a fusion construct of SP_2108 with SP_1912 which contains the lipid moiety of SP_2108. As shown in Fig. S3, this construct has potent TLR2-stimulating activity. Mice immunized with the genetic fusion of these two proteins developed pneumococcus-specific IL-17A responses (Fig. 4A), indicating that the IL-17A inductive capacity was retained in the fusion construct.
FIG 4.
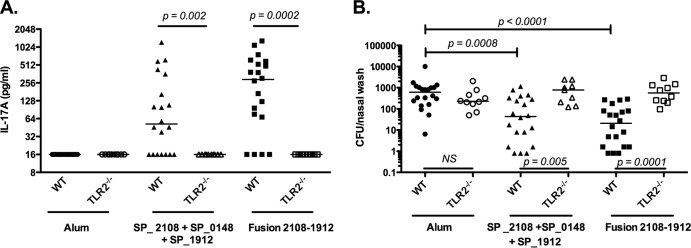
Immunization with lipoprotein-based subunit vaccine in WT and Tlr2−/− mice. WT (C57B6/J) or Tlr2−/− (C57B6.129-Tlr2tm1Kir/J) mice (n = 10 to 20/group) were immunized subcutaneously three times with alum only, 1 μg of SP_2108 and 10 μg each of SP_0148 and SP_1912 absorbed on alum, or a fusion protein of SP_2108 and SP_1912 adsorbed on alum. (A) IL-17A concentrations in cell supernatants of whole blood following a 6-day stimulation with WCA are shown. (B) Ten days following intranasal challenge, nasal washes were collected and pneumococcal CFU were counted. Bars represent medians, and P values were calculated using the Mann-Whitney test. NS, not significant.
The density of pneumococcal colonization was determined 10 days following intranasal challenge with live pneumococci. Compared to mice that received alum alone, mice immunized with the mixture of proteins or the fusion construct had a significant reduction in the density of pneumococcal carriage (P = 0.0008 or P < 0.0001, respectively; Fig. 4B).
The immunogenicity and efficacy of the lipoproteins are TLR2 dependent.
In contrast to WT mice, Tlr2−/− mice immunized with either the mixture of the three proteins or the fusion construct did not generate detectable IL-17A responses (P = 0.002 or P = 0.0002 comparing the WT mice to Tlr2−/− mice within each immunization group, respectively; Fig. 4A). Interestingly, the in trans effect, whereby TH17 responses to SP_1912 were elicited by coadministration or fusion with lipoproteins, was also found to be TLR2 dependent (see Fig. S2B in the supplemental material). Furthermore, unlike in WT mice, immunization with either the mixture of SP_2108, SP_0148, and SP_1912 or the fusion of SP_2108 and SP_1912 conferred no reduction in pneumococcal colonization density inTlr2−/− mice (P = 0.005 or P = 0.0001 comparing WT to Tlr2−/− mice within each immunization group, respectively; Fig. 4B). Importantly, alum-immunized WT and Tlr2−/− mice showed similar densities of colonization, suggesting that the lack of protection in immunized Tlr2−/− mice is not due to an inherent increased susceptibility to pneumococcal carriage due to the lack of TLR2.
DISCUSSION
Over the past several decades, there have been many efforts, both preclinical and clinical, to develop a serotype-independent pneumococcal vaccine, primarily with the goal to elicit protective antibodies to conserved proteins (5, 7, 27). With the recent discovery of T cell-mediated resistance to pneumococcal colonization in mice (28, 29), it has been proposed that a protein-based vaccine should incorporate antigens that confer both antibody- and T cell-based protection against pneumococcal disease and carriage. In this work, we explored the role of the lipid moieties of two highly immunogenic TH17 antigens previously identified by proteomic screens, SP_2108 and SP_0148, focusing on the possible role of TLR2 activation in protection against colonization.
Our understanding of the role of IL-17A-mediated immune responses to mucosal infection has expanded over the last several years, as more evidence of their importance in the defense against extracellular bacterial infections has become available (30, 31). Subsequently, a critical role of TLR2 in the generation of protective IL-17A immune responses to mucosal infections by Salmonella enterica serovar Typhimurium and Yersinia enterocolitica was demonstrated (32, 33). A requirement for TLR2 for the development of systemic pneumococcus-specific CD4+ IL-17A-secreting cells following primary pneumococcal colonization was recently demonstrated (9). Here we evaluated the role of TLR2 in the development of vaccine-induced IL-17A immune responses using two pneumococcal lipoproteins as immunogens that induce TH17 responses. Our studies show that attachment of lipid moieties is required for optimal immunogenicity of SP_2108 and SP_0148 and that this immunogenicity is TLR2 dependent. Active immunization studies using the lipoproteins as components of either mucosal or parenteral vaccines confirmed that both the lipid moieties and TLR2 enhance IL-17A immunogenicity and resistance to pneumococcal colonization following immunization.
Our findings in intranasal immunization experiments using either of the two lipoproteins or their nonlipidated counterparts alone demonstrated that, even in the presence of the potent TH17-driving adjuvant CT (25, 26), the lipid moieties enhance responses (Fig. 2B). The critical role of the lipid moieties in enhancing the IL-17A immunogenicity of SP_2108 and SP_0148 was further demonstrated in parenteral immunization experiments using alum as an adjuvant (see Fig. S1 in the supplemental material). In vitro responses from WT and Tlr2−/− macrophages demonstrated that the proinflammatory properties of these proteins are TLR2 dependent. Recent studies have suggested that dendritic cells are the primary antigen-presenting cells driving mucosal TH17 cell differentiation in vivo (34, 35). As such, evaluation of the immunogenicity of such proteins using dendritic cell lines should be considered.
We also demonstrated some differences in the immunogenicity and proinflammatory properties of SP_2108 and SP_0148. For example, in contrast to SP_2108 results, lipidation of SP_0148 did not significantly enhance the IL-17A responses to this antigen when given intranasally with CT (Fig. 2A). However, there was a much more robust increase in IL-17A responses to SP_0148 given subcutaneously with alum than to the nonlipidated protein (see Fig. S1 in the supplemental material). This might suggest that the mechanisms by which lipid moieties act as an adjuvant to the immunogenicity of a protein are dependent on many factors, including the route of immunization and characteristics of the protein itself such as the presence of particular T cell epitopes. Indeed, we note a weak correlation between SP_0148-elicited IL-17A responses and colonization burden following intranasal immunization. Beyond the possibility that protein-elicited systemic IL-17A responses are simply not very good predictors of protection against carriage in mice (unlike the case of the whole-cell pneumococcal vaccine, where the correlation is much stronger [8]), one can also speculate that immune mechanisms other than IL-17A may contribute to SP_0148 (and possibly other protein)-mediated clearance of pneumococcal carriage. Such mechanisms could include other T cell-derived cytokines such as IL-22 (36, 37), antibody-mediated clearance, or a combination of immune effectors. There is also the suggestion from in vitro studies that SP_0148 may have TLR2-independent proinflammatory properties, as seen following stimulation with the nonlipidated mutant (Fig. 3). Since this effect was abrogated following stimulation with a boiled SP_0148Δlipid preparation, it is less likely to be the result of lipopolysaccharide (LPS) contamination, but the mechanism for this stimulatory effect remains to be explained. In considering inclusion of pneumococcal proteins in next-generation vaccines, the use of proteins that are immunogens and also confer lipid-mediated TH17-promoting effects might be optimal.
Activation of TLRs expressed on macrophages and dendritic cells leads to a cascade of innate immune events, including proinflammatory cytokine release and upregulation of major histocompatibility complex (MHC) molecules and costimulatory signals, resulting in enhancement of adaptive immune responses (38). Here we show that the TLR2- and lipid-mediated adjuvant effect appears to work in trans, since the TH17 responses to SP_1912, a nonlipidated protein, could be elicited by coadministration with the two lipoproteins. Another approach is to create fusion proteins, including a lipid motif, as shown here with the fusion of SP_2108 and SP_1912. Such enhancement of immunogenicity and protection against pneumococcal colonization by fusion of proteins had been previously demonstrated with pneumolysin, a TLR4 agonist (39–41). Here we show this with a TLR2 agonist fused to the antigenic cargo, which likely enhances MHC class II (MHC-II) presentation (42).
Beyond the potential to confer serotype-independent immunity to pneumococcus, the inclusion of TLR2-activating proteins in a conjugate vaccine, either as a mixture or as carrier proteins, may enhance the immune responses to the polysaccharides. Of the three Haemophilus influenzae type B (Hib) polysaccharide conjugate vaccines that have been licensed, one uses a meningococcal outer membrane protein complex as the Hib polysaccharide carrier (Hib-OMPC) and was noted to be more immunogenic after 1 dose in infants than Hib conjugates that included either CRM197 or tetanus toxoid as carriers (43). A possible explanation for these findings was that the Hib-OMPC vaccine, unlike the other licensed Hib conjugate vaccines, activates TLR2 and that enhanced polysaccharide responses observed in mice were TLR2 dependent (44). Whether the inclusion of TLR2-activating proteins in a pneumococcal polysaccharide conjugate vaccine would similarly enhance the potency of pneumococcal polysaccharide-directed immunity deserves further investigation.
By extension, our data regarding the role of lipids and TLR2, as well as the in trans effect we noted, also suggest a possible strategy to develop vaccines directed against other mucosal pathogens for which a role of TH17 has been demonstrated, for example, Staphylococcus aureus (45), Bordetella pertussis (46), and Mycobacterium tuberculosis (47). The selection of naturally lipidated TH17 antigens from these organisms, lipidation of candidate antigens, and the inclusion of a TLR2-activating adjuvant in the formulation could all be considered in the design of a novel vaccine directed against these mucosal pathogens.
In conclusion, we demonstrate that the immunogenicity and the protective efficacy against pneumococcal colonization that is elicited following immunization with TH17 lipoproteins are dependent on their attached lipid moieties and are mediated by TLR2. These lipoproteins also enhance the immunogenicity of nonlipidated proteins included in the same vaccines. Based on their immunogenic and adjuvant properties, inclusion of these and similarly lipidated pneumococcal antigens as components of next-generation pneumococcal vaccines is worthy of further exploration. To this end, a phase 1 clinical trial of proteins described in this work is under way (ClinicalTrials.gov identifier NCT01995617).
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge PATH for supporting these studies. Further funding for this work was provided by NIH grant K08 AI095352 (K.M.). R.M. acknowledges support from the Translational Research Program at Boston Children's Hospital. M.S., L.C.G., S.M., B.D., P.G., and J.B.F. are all employees of Genocea Biosciences, Inc. T.G. is a former employee of and owns stock in Genocea Biosciences, Inc.
Footnotes
Published ahead of print 10 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01632-13.
REFERENCES
- 1.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902. 10.1016/S0140-6736(09)61204-6 [DOI] [PubMed] [Google Scholar]
- 2.Weinberger DM, Malley R, Lipsitch M. 2011. Serotype replacement in disease after pneumococcal vaccination. Lancet 378:1962–1973. 10.1016/S0140-6736(10)62225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pichichero ME, Casey JR. 2007. Evolving microbiology and molecular epidemiology of acute otitis media in the pneumococcal conjugate vaccine era. Pediatr. Infect. Dis. J. 26:S12–S16. 10.1097/INF.0b013e318154b25d [DOI] [PubMed] [Google Scholar]
- 4.McEllistrem MC, Adams JM, Patel K, Mendelsohn AB, Kaplan SL, Bradley JS, Schutze GE, Kim KS, Mason EO, Wald ER. 2005. Acute otitis media due to penicillin-nonsusceptible Streptococcus pneumoniae before and after the introduction of the pneumococcal conjugate vaccine. Clin. Infect. Dis. 40:1738–1744. 10.1086/429908 [DOI] [PubMed] [Google Scholar]
- 5.Darrieux M, Goulart C, Briles D, Leite LC. 29 July 2013. Current status and perspectives on protein-based pneumococcal vaccines. Crit. Rev. Microbiol. 10.3109/1040841X.2013.813902 [DOI] [PubMed] [Google Scholar]
- 6.Tai SS. 2006. Streptococcus pneumoniae protein vaccine candidates: properties, activities and animal studies. Crit. Rev. Microbiol. 32:139–153. 10.1080/10408410600822942 [DOI] [PubMed] [Google Scholar]
- 7.Moffitt KL, Malley R. 2011. Next generation pneumococcal vaccines. Curr. Opin. Immunol. 23:407–413. 10.1016/j.coi.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, Thompson CM, Harney KF, Anderson PW, Lipsitch M, Malley R. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4:e1000159. 10.1371/journal.ppat.1000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Clarke TB, Weiser JN. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Invest. 119:1899–1909. 10.1172/JCI36731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandesris MO, Melki I, Natividad A, Puel A, Fieschi C, Yun L, Thumerelle C, Oksenhendler E, Boutboul D, Thomas C, Hoarau C, Lebranchu Y, Stephan JL, Cazorla C, Aladjidi N, Micheau M, Tron F, Baruchel A, Barlogis V, Palenzuela G, Mathey C, Dominique S, Body G, Munzer M, Fouyssac F, Jaussaud R, Bader-Meunier B, Mahlaoui N, Blanche S, Debré M, Le Bourgeois M, Gandemer V, Lambert N, Grandin V, Ndaga S, Jacques C, Harre C, Forveille M, Alyanakian MA, Durandy A, Bodemer C, Suarez F, Hermine O, Lortholary O, Casanova JL, Fischer A, Picard C. 2012. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: molecular, cellular, and clinical features from a French national survey. Medicine 91:e1–e19. 10.1097/MD.0b013e31825f95b9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452:773–776. 10.1038/nature06764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moffitt KL, Gierahn TM, Lu YJ, Gouveia P, Alderson M, Flechtner JB, Higgins DE, Malley R. 2011. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe 9:158–165. 10.1016/j.chom.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Gierahn T, Thompson CM, Trzcinski K, Ford CB, Croucher N, Gouveia P, Flechtner JB, Malley R, Lipsitch M. 2012. Distinct effects on diversifying selection by two mechanisms of immunity against Streptococcus pneumoniae. PLoS Pathog. 8:e1002989. 10.1371/journal.ppat.1002989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736–739. 10.1126/science.285.5428.736 [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. 1999. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1–5 [PubMed] [Google Scholar]
- 16.Michelsen KS, Aicher A, Mohaupt M, Hartung T, Dimmeler S, Kirschning CJ, Schumann RR. 20 April 2001. The role of Toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells—PGN and LTA are inducers of DC maturation and require TLR2. J. Biol. Chem. 10.1074/jbc.M011615200 [DOI] [PubMed] [Google Scholar]
- 17.Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. 2002. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 186:798–806. 10.1086/342845 [DOI] [PubMed] [Google Scholar]
- 18.van Rossum AM, Lysenko ES, Weiser JN. 2005. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect. Immun. 73:7718–7726. 10.1128/IAI.73.11.7718-7726.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutcliffe IC, Harrington DJ. 2002. Pattern searches for the identification of putative lipoprotein genes in Gram-positive bacterial genomes. Microbiology 148(Pt 7):2065–2077 [DOI] [PubMed] [Google Scholar]
- 20.Malley R, Lipsitch M, Stack A, Saladino R, Fleisher G, Pelton S, Thompson C, Briles D, Anderson P. 2001. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect. Immun. 69:4870–4873. 10.1128/IAI.69.8.4870-4873.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchings MI, Palmer T, Harrington DJ, Sutcliffe IC. 2009. Lipoprotein biogenesis in Gram-positive bacteria: knowing when to hold 'em, knowing when to fold 'em. Trends Microbiol. 17:13–21. 10.1016/j.tim.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 22.Iwakura Y, Ishigame H, Saijo S, Nakae S. 2011. Functional specialization of interleukin-17 family members. Immunity 34:149–162. 10.1016/j.immuni.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 23.Bettelli E, Korn T, Oukka M, Kuchroo VK. 2008. Induction and effector functions of T(H)17 cells. Nature 453:1051–1057. 10.1038/nature07036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cua DJ, Tato CM. 2010. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10:479–489. 10.1038/nri2800 [DOI] [PubMed] [Google Scholar]
- 25.Datta SK, Sabet M, Nguyen KP, Valdez PA, Gonzalez-Navajas JM, Islam S, Mihajlov I, Fierer J, Insel PA, Webster NJ, Guiney DG, Raz E. 2010. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc. Natl. Acad. Sci. U. S. A. 107:10638–10643. 10.1073/pnas.1002348107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JB, Jang JE, Song MK, Chang J. 2009. Intranasal delivery of cholera toxin induces th17-dominated T-cell response to bystander antigens. PLoS One 4:e5190. 10.1371/journal.pone.0005190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malley R, Anderson PW. 2012. Serotype-independent pneumococcal experimental vaccines that induce cellular as well as humoral immunity. Proc. Natl. Acad. Sci. U. S. A. 109:3623–3627. 10.1073/pnas.1121383109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. 2005. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. U. S. A. 102:4848–4853. 10.1073/pnas.0501254102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trzciński K, Thompson CM, Srivastava A, Basset A, Malley R, Lipsitch M. 2008. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infect. Immun. 76:2678–2684. 10.1128/IAI.00141-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khader SA, Gaffen SL, Kolls JK. 2009. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2:403–411. 10.1038/mi.2009.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aujla SJ, Dubin PJ, Kolls JK. 28 November 2007. Th17 cells and mucosal host defense. Semin. Immunol. 10.1016/j.smim.2007.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimori JH, Newman TN, Oppong GO, Rapsinski GJ, Yen JH, Biesecker SG, Wilson RP, Butler BP, Winter MG, Tsolis RM, Ganea D, Tukel C. 2012. Microbial amyloids induce interleukin 17A (IL-17A) and IL-22 responses via Toll-like receptor 2 activation in the intestinal mucosa. Infect. Immun. 80:4398–4408. 10.1128/IAI.00911-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DePaolo RW, Kamdar K, Khakpour S, Sugiura Y, Wang W, Jabri B. 2012. A specific role for TLR1 in protective T(H)17 immunity during mucosal infection. J. Exp. Med. 209:1437–1444. 10.1084/jem.20112339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CM, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F. 2013. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38:970–983. 10.1016/j.immuni.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, Gudjonsson S, Hakansson U, Reizis B, Kotarsky K, Agace WW. 2013. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 38:958–969. 10.1016/j.immuni.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 36.Lundgren A, Bhuiyan T, Novak D, Kaim J, Reske A, Lu YJ, Qadri F, Malley R. 12 April 2012. Characterization of Th17 responses to Streptococcus pneumoniae in humans: comparisons between adults and children in a developed and a developing country. Vaccine 10.1016/j.vaccine.2012.03.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivanov S, Renneson J, Fontaine J, Barthelemy A, Paget C, Fernandez EM, Blanc F, De Trez C, Van Maele L, Dumoutier L, Huerre MR, Eberl G, Si-Tahar M, Gosset P, Renauld JC, Sirard JC, Faveeuw C, Trottein F. 2013. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J. Virol. 87:6911–6924. 10.1128/JVI.02943-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akira S, Takeda K. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511. 10.1038/nri1391 [DOI] [PubMed] [Google Scholar]
- 39.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. U. S. A. 100:1966–1971. 10.1073/pnas.0435928100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu YJ, Forte S, Thompson CM, Anderson PW, Malley R. 2009. Protection against pneumococcal colonization and fatal pneumonia by a trivalent conjugate of a fusion protein with the cell wall polysaccharide. Infect. Immun. 77:2076–2083. 10.1128/IAI.01554-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goulart C, da Silva TR, Rodriguez D, Politano WR, Leite LC, Darrieux M. 2013. Characterization of protective immune responses induced by pneumococcal surface protein A in fusion with pneumolysin derivatives. PLoS One 8:e59605. 10.1371/journal.pone.0059605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blander JM, Medzhitov R. 2006. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440:808–812. 10.1038/nature04596 [DOI] [PubMed] [Google Scholar]
- 43.Granoff DM, Holmes SJ. 1991. Comparative immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugate vaccines. Vaccine 9(Suppl):S30–S34; discussion S42–S43. 10.1016/0264-410X(91)90178-9 [DOI] [PubMed] [Google Scholar]
- 44.Latz E, Franko J, Golenbock DT, Schreiber JR. 2004. Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human Toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J. Immunol. 172:2431–2438 [DOI] [PubMed] [Google Scholar]
- 45.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. 2010. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120:1762–1773. 10.1172/JCI40891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross PJ, Sutton CE, Higgins S, Allen AC, Walsh K, Misiak A, Lavelle EC, McLoughlin RM, Mills KH. 2013. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 9:e1003264. 10.1371/journal.ppat.1003264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369–377. 10.1038/ni1449 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.